Abstract
Background
Clinical findings of glucocorticoid‐deficient hypoadrenocorticism (GDH) can overlap with other diseases, presenting a diagnostic challenge.
Objectives
Describe clinicopathologic and ultrasonographic features of dogs with GDH and those suspected of having GDH that had the disease ruled out.
Animals
Six hundred twenty‐three dogs.
Methods
Records from dogs with suspected GDH between 2003 and 2018 were reviewed. Dogs with hyperkalemia or hyponatremia were excluded. Dogs were categorized as controls when the resting serum cortisol or post‐ACTH cortisol concentration were > 2 μg/dL. Clinicopathologic and ultrasonographic features were compared between groups. The optimal cut‐point, area under the receiver operating characteristic curve (AUC), sensitivity, and specificity were calculated for individual features used to detect GDH.
Results
Dogs were categorized as GDH (n = 29) or controls (n = 594). Lymphocyte count (>1750 cells/L; sensitivity, 96.6%; 95% confidence interval [CI], 82.8%‐99.8%; specificity, 60.3%; 95% CI, 56.3%‐64.1%; AUC, 0.828; 95% CI, 0.762‐0.894) and albumin/globulin ratio (<1.081; sensitivity, 86.2%; 95% CI, 69.4%‐94.5%; specificity, 78.8%; 95% CI, 75.3%‐81.9%; AUC, 0.886; 95% CI, 0.827‐0.944) had the highest discriminatory power between groups. Left adrenal gland width <0.39 cm was 80% (95% CI, 58.4%‐91.9%) sensitive and 82.4% (95% CI, 74.2‐88.4) specific for GDH. Serum cobalamin concentrations and ultrasonographic abnormalities of the GI tract were not different between groups.
Conclusion and Clinical Importance
No single variable could be used to confidently rule out GDH and obviate the need for cortisol testing in dogs with a clinical presentation consistent with GDH.
Keywords: Addison's disease, adrenal gland, atypical Addison's, cortisol
Abbreviations
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- GDH
glucocorticoid deficient hypoadrenocorticism
- GMDH
glucocorticoid and mineralocorticoid deficient hypoadrenocorticism
- ROC
receiver operating characteristic
1. INTRODUCTION
Hypoadrenocorticism in dogs results from destruction or secondary atrophy of ≥1 layers of the adrenal cortex and affects up to 0.3% of dogs. 1 , 2 , 3 Hormonal deficiencies corresponding with the affected layers of the adrenal gland result in clinical disease that has a wide variety of nonspecific presentations. Clinical signs may be indistinguishable from those of other more common diseases such as gastrointestinal (GI) or renal disease, thus presenting a substantial diagnostic challenge.
Destruction of both the zona glomerulosa and zona fasciculata layers results in glucocorticoid and mineralocorticoid‐deficient hypoadrenocorticism (GMDH). Clinical presentation may reflect these insufficiencies, with evidence of dehydration, circulatory collapse, and electrolyte abnormalities that reflect lack of aldosterone. 4 A less common presentation, encompassing up to 40% of dogs with hypoadrenocorticism, is characterized by isolated glucocorticoid deficiency (GDH) with normal serum electrolyte concentrations and vague GI clinical signs, such as lethargy, weight loss, vomiting, diarrhea, and hyporexia. 5 , 6 Hematologic and biochemical variables in GDH such as anemia, hypoalbuminemia and hypocholesterolemia also can be caused by GI disease whereas other abnormalities more specific for hypoadrenocorticism frequently are not present. 5 , 6 , 7 For example, hypoglycemia and eosinophilia are present in fewer than 33% of dogs with GDH. 5 , 7 , 8 A recent study comparing dogs that were presented for chronic GI signs did not find any significant differences in hematologic or biochemical variables between dogs with GDH and those with chronic enteropathy. 2 The overlap in clinical signs in these patients can result in a substantial diagnostic challenge for clinicians.
Abdominal ultrasonography is commonly used to evaluate patients with GI signs and those with more vague clinical signs sometimes associated with GDH. Ultrasonographic evaluation of the abdomen routinely includes assessment of the adrenal glands and in dogs with GMDH, the sensitivity and specificity of adrenal gland measurement <3.2 mm is 90% and 100% for detection of the disease, respectively. 9 However, in dogs with GDH, only 56% of dogs have adrenal glands <3.2 mm in thickness. 7 Furthermore, little information is available on the ultrasonographic appearance of the GI tract in dogs with GDH and how this information could be used to potentially differentiate dogs with GDH, from those with primary GI disease manifesting similar clinical signs.
In this retrospective study, we compared the hematologic and biochemical variables of dogs diagnosed with GDH from those that had diseases with clinical presentation similar to GDH, but in which the disease had been ruled out. In addition, we compared abdominal ultrasound examination findings between these 2 groups.
2. MATERIALS AND METHODS
2.1. Study population
Electronic medical records at the William R. Pritchard Veterinary Medical Teaching Hospital at the University of California, Davis, School of Veterinary Medicine were searched to identify a group of dogs with confirmed GDH based upon ACTH stimulation testing and a group of dogs that were tested for GDH but had the disease ruled out (control dogs). Dogs that had serum cortisol concentration measured between January 1, 2003 and December 31, 2018 were considered for inclusion. Dogs were excluded from the study if they had a history of receiving PO, topical, otic, or inhaled corticosteroids within 1 month before testing, were previously diagnosed with hyperadrenocorticism, or had previously received ketoconazole, mitotane, or trilostane.
To be included in the study, all dogs must have had a CBC (Advia 120, Siemens, Erlangen, Germany) and serum biochemistry panel (Cobas c501/6000 series, Roche Diagnostics, Indianapolis, Indiana) performed at the time of or within 1 week of being tested for GDH. Dogs were excluded from the study if hyperkalemia (serum potassium concentration >4.8 mmol/L) or hyponatremia (serum sodium concentration <143 mmol/L) was noted on serum biochemistry.
The GDH group included dogs with a 1‐hour post‐ACTH serum cortisol concentration <2 μg/dL. The control population consisted of dogs that had GDH ruled out by a resting serum cortisol or post‐ACTH serum cortisol concentration of ≥2 μg/dL.
The signalment, age, and body weight of all dogs were recorded. Serum cobalamin concentrations were recorded if assessed. The presence of GI clinical signs including vomiting, regurgitation, diarrhea, decreased appetite, anorexia, and owner‐perceived or clinically‐documented weight loss were recorded for each dog. The clinical diagnosis recorded by the attending clinician for the control group was reviewed by a single author (KR). The primary clinical diagnosis was categorized into the following categories: GI (including hepatic or pancreatic), cardiac, immune‐mediated, genitourinary, neurologic, respiratory, infectious, neoplasia outside of the GI tract, neoplasia involving the GI tract, trauma and primary surgical disease that did not involve the GI tract, trauma and primary surgical disease that did involve the GI tract (eg, GI foreign bodies, gastric dilatation, and volvulus), or undetermined.
2.2. Abdominal ultrasound examination review
Archived ultrasound images were reviewed by a board‐certified radiologist (EM) using a digital imaging and communications in medicine viewer (Agfa HealthCare, Mortsel, Belgium). Static images of small intestine, colon, stomach, mesenteric lymph nodes, and adrenal glands were reviewed when available. Images of the small intestine were evaluated for changes in echogenicity, wall layering, corrugation, and dilatation, and a 2‐point scoring system was adapted from previous studies (Table 1). 10 A score of 1 was assigned if the variable was normal. Abnormal variables were assigned a score of 2. Gastrointestinal masses, colonic abnormalities, gastric abnormalities, abnormal jejunal lymph node echogenicity or shape, abnormal adrenal echogenicity or shape, and peritoneal effusion were designated as either absent (score of 1) or present (score of 2) if images of these structures were available for review. Adrenal gland width and jejunal lymph node width also were recorded. To detect a 25% difference in frequency of abnormalities of the GI tract between the GDH group and the control group with >80% power, ultrasound studies from all GDH dogs and 113 control dogs were selected for review. Dogs in the control group that were noted to have an abdominal ultrasound examination in their medical record were randomly chosen for review using a random number generator (numbergenerator.org). The radiologist reviewing the images was blinded to the diagnosis (GDH vs control). Results were included if, at minimum, static images were available for the small intestine.
TABLE 1.
Small intestinal ultrasound scoring system
| Parameter | Normal (1) | Abnormal (2) |
|---|---|---|
| Echogenicity | Normal hypoechoic mucosa and muscularis, hyperechoic submucosa and serosa | Mucosal echogenicity is increased (more echogenic than muscularis) and/or a linear hyperechoic line parallel to the wall layers within the mucosa (“fourth line”) is present or there are hyperechoic perpendicular lines consistent with lacteal dilatation |
| Wall layering | Distinct wall layering with normal relative thickness of wall layers | Submucosa or muscularis layer thickness >25% of mucosal thickness |
| Corrugation | No corrugation | Corrugation present |
| Dilatation | Empty or containing minimal or transient gas/fluid | Dilatation present |
2.3. Statistical analysis
A Shapiro‐Wilk test was used to determine if the data were normally distributed. Continuous data were compared using Mann‐Whitney U tests. The P‐values for comparisons of the hematologic and biochemical variables were corrected for multiple comparisons using a Bonferroni‐Dunn correction. Statistical significance was defined as an adjusted P < .05. Receiver operating characteristic (ROC) curves were plotted for continuous data with significant differences between GDH and control populations. The area under the curve (AUC) was obtained and the optimal cut‐point was determined by calculating Youden's J statistic. The sensitivity and specificity for detection of GDH were reported at the optimal cut‐points. Categorical variables were compared using a Fisher's exact test. Correlation between body weight and left adrenal gland width was assessed using a Pearson's test for correlation for the GDH and control groups. Statistical analyses were performed using GraphPad Prism (Version 9.3.0). Hematologic and biochemical variables with P < .05 in the univariable analysis were assessed using a backwards stepwise multivariable logistic regression analysis (statsmodel, Python3). 11
3. RESULTS
3.1. Population description
The study population included 29 dogs with GDH and 594 control dogs. The dogs with GDH had a median age of 7 years (range, 2‐15.2 years) and median body weight of 23.2 kg (range, 3.6‐85 kg). The GDH group consisted of 11 spayed female, 1 intact female, 15 neutered male, and 2 intact male dogs. Mixed breed dogs predominated in the GDH group, with 7 dogs being mixed breed. The GDH group also included 2 Australian shepherds, 2 West Highland white terriers, and 2 Labrador retrievers. There were 16 other purebred dogs with 1 dog each with GDH. In the GDH group, at least 1 of the evaluated GI clinical signs was reported in 24/29 dogs. These signs included diarrhea in 17 dogs, decreased appetite in 15 dogs, vomiting or regurgitation in 10 dogs, and weight loss in 6 dogs. All dogs in this group were diagnosed with GDH, treated with glucocorticoids, and did not receive mineral corticoid replacement at the time of diagnosis.
The control group included 594 dogs that had a median age of 6.3 years (range, 0.4‐16.4 years) and median body weight of 18.6 kg (range, 1‐80.6 kg). The control group consisted of 270 spayed female, 40 intact female, 228 neutered male, and 56 intact male dogs. 137 of the 594 dogs were mixed breed dogs. The most common purebred dogs were 41 Labrador retrievers, 29 standard poodles, and 19 Yorkshire terriers. 106 other breeds were represented in the control population. In the control group, at least 1 of the evaluated GI clinical signs was reported in 547/594 dogs. These signs included diarrhea in 300 dogs, decreased appetite in 335 dogs, vomiting or regurgitation in 375 dogs, and weight loss in 151 dogs. The primary clinical diagnosis was determined to be GI in nature for 426 dogs, which included 10 dogs with GI neoplasia and 8 with GI‐related primarily surgical disease. Genito‐urinary disease was diagnosed in 29 dogs, non‐GI neoplasia in 22 dogs, immune‐mediated disease in 20 dogs, infectious disease in 20 dogs, respiratory disease in 19 dogs, neurologic disease in 18 dogs, cardiac disease in 5 dogs, non‐GI trauma and primary surgical disease in 3 dogs, and undetermined in 32 dogs.
3.2. Hematologic and biochemical variables
Of the measured hematologic variables, 5 were significantly different between the GDH and the control groups. These included hematocrit, hemoglobin concentration, eosinophil count, lymphocyte count, and neutrophil/lymphocyte ratio (Table 2). The differences in population distributions for each of these hematologic variables is presented in Figure 1. No other significant differences were noted in hematologic variables between the study populations.
TABLE 2.
Population blood work parameters
| GDH group (n = 29) | Control group (n = 594) | |||||||
|---|---|---|---|---|---|---|---|---|
| Characteristics | Reference range | Median (range) | No. (%) below reference range | No. (%) above reference range | Median (range) | No. (%) below reference range | No. (%) above reference range | Adjusted P‐value |
| Hematologic parameters | ||||||||
| Hematocrit (%) | 40‐55 | 40 (27‐53) | 12 (41.4%) | 0 (0.0%) | 46 (6‐76) | 121 (20.4%) | 36 (6 1%) | .02 |
| Hemoglobin (gm/dL) | 14‐19 | 13 (9.4‐19) | 17 (58.6%) | 0 (0.0%) | 16 (1.7‐25) | 162 (27.3%) | 33 (5.6%) | .05 |
| MCV (fL) | 65‐75 | 68 (54‐77) | 4 (13.8%) | 1 (3.4%) | 69 (52‐95) | 65 (10.9%) | 29 (4.9%) | 1 |
| White blood cells (/μL) | 6 000‐13 000 | 11 800 (4 900‐28 000) | 2 (6.9%) | 10 (34.5%) | 9 850 (700‐65 200) | 65 (10.9%) | 170 (28.6%) | .9 |
| Neutrophils (/μL) | 3 000‐10 500 | 10 390 (2 000‐23 200) | 1 (3.4%) | 7 (24.1%) | 6 800 (0‐6,000) | 22 (3.7%) | 143 (24.1%) | 1 |
| Lymphocytes (/μL) | 1 000‐4 000 | 4113 (1 000‐7 500) | 0 (0.0%) | 7 (24.1%) | 1 600 (100‐7 700) | 109 (18.4%) | 21 (3.5%) | .003 |
| Neutrophil/lymphocyte ratio | 2.3 (0.9‐12) | 4.7 (0‐122) | .003 | |||||
| Monocytes (/μL) | 150‐1200 | 857 (100‐1 400) | 1 (3.4%) | 3 (10.3%) | 500 (0‐5 200) | 10 (1.7%) | 85 (14.3%) | 1 |
| Eosinophils (/μL) | 0‐1500 | 1247 (0‐6 296) | 3 (10.3%) | 300 (0‐15 500) | 19 (3.2%) | .003 | ||
| Platelets (/μL) | 150 000‐400 000 | 322 000 (132 000‐497 000) | 1 (3.4%) | 4 (13.8%) | 262 000 (3 000‐822 000) | 69 (11.6%) | 82 (13.8%) | 1 |
| Biochemical parameters | ||||||||
| Sodium (mmol/L) | 143‐151 | 147 (143‐155) | 3 (10.3%) | 146 (143‐162) | 32 (5.4%) | 1 | ||
| Potassium (mmol/L) | 3.6‐4.8 | 4.4 (3.3‐4.8) | 4 (13.8%) | 4.2 (2.6‐4.8) | 33 (5.6%) | 1 | ||
| Chloride (mmol/L) | 108‐116 | 114 (106‐136) | 1 (3.4%) | 10 (34.5%) | 111 (91‐139) | 88 (14.8%) | 43 (7.2%) | .003 |
| Bicarbonate (mmol/L) | 20‐29 | 20 (13‐25) | 12 (41.4%) | 0 (0.0%) | 21 (8‐34) | 182 (30.6%) | 3 (0.5%) | 1 |
| Phosphorus (mg/dL) | 2.6‐5.2 | 4.2 (1.7‐7.1) | 2 (6.9%) | 5 (17.2%) | 3.9 (0.8‐28) | 48 (8.1%) | 84 (14.1%) | 1 |
| Calcium (mg/dL) | 9.6‐11.2 | 10 (8.2‐12) | 7 (24.1%) | 2 (6.9%) | 10 (4‐18) | 143 (24.1%) | 53 (8.9%) | 1 |
| BUN (mg/dL) | 11‐33 | 17 (6‐47) | 5 (17.2%) | 3 (10.3%) | 15 (3‐270) | 147 (24.7%) | 49 (8.2%) | 1 |
| Creatinine (mg/dL) | 0.8‐1.5 | 1.1 (0.6‐2.1) | 3 (10.3%) | 3 (10.3%) | 0.9 (0.1‐16) | 208 (35%) | 57 (9.6%) | .003 |
| Bilirubin (mg/dL) | 0.0‐0.2 | 0.2 (0.1‐0.4) | 3 (10.3%) | 0.2 (0‐23) | 61 (10.3%) | 1 | ||
| Glucose (mg/dL) | 86‐118 | 84 (36‐401) | 16 (55.2%) | 2 (6.9%) | 97 (7‐307) | 127 (21.4%) | 53 (8.9%) | .003 |
| Albumin (g/dL) | 3.4‐4.3 | 2.4 (1.3‐3.4) | 28 (96.6%) | 0 (0.0%) | 3.4 (0.7‐5) | 253 (42.6%) | 16 (2.7%) | .003 |
| Globulin (g/dL) | 2.9 (1.6‐4.4) | 1 (3.4%) | 11 (37.9%) | 2.4 (0.7‐6.4) | 39 (6.6%) | 82 (13.8%) | .003 | |
| Albumin/globulin ratio | 0.7 (0.4‐1.6) | 1.4 (0.3‐3) | .003 | |||||
| ALT (IU/L) | 21‐72 | 52 (18‐195) | 1 (3.4%) | 8 (27.6%) | 45 (2‐3 923) | 50 (8.4%) | 168 (28.3%) | 1 |
| AST (IU/L) | 20‐49 | 66 (27‐240) | 0 (0.0%) | 18 (62.1%) | 34 (13‐13 312) | 30 (5.1%) | 154 (25.9%) | .003 |
| ALP (IU/L) | 14‐91 | 41 (14‐2184) | 0 (0.0%) | 3 (10.3%) | 58 (7‐4 282) | 17 (2.9%) | 196 (33%) | .2 |
| GGT (IU/L) | 0‐5 | 3 (0‐7) | 2 (6.9%) | 3 (0‐113) | 103 (17.3%) | 1 | ||
| Cholesterol (mg/dL) | 139‐353 | 100 (45‐230) | 24 (82.8%) | 0 (0.0%) | 190 (45‐978) | 132 (22.2%) | 23 (3.9%) | .003 |
| Cobalamin (ng/L) a | 271‐875 | 423 (184‐927) | 2 (18.2%) | 1 (9.1%) | 410 (150‐2 965) | 93 (28.3%) | 29 (8.8%) | 1 |
Sample size for cobalamin measurements are n = 11 GDH dogs and n = 329 control dogs.
Bolded P‐values correspond with statiscitally significant findings.
FIGURE 1.
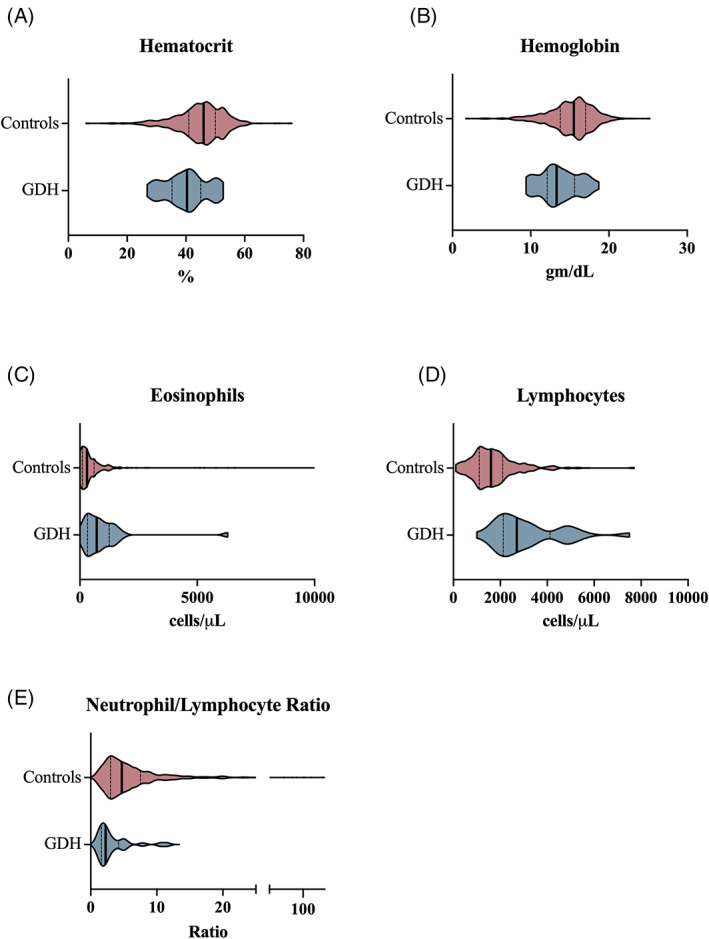
Hematologic variables that differed between a cohort of dogs with GDH versus a cohort of control dogs. Violin plots compare hematologic variables (A, hematocrit; B, hemoglobin concentration; C, eosinophil count; D, lymphocyte count; E, neutrophil/lymphocyte ratio) for the control population (red) and GDH population (blue). The bold vertical line represents the population median and thin vertical lines represent the lower and upper quartiles
Hematologic variables were evaluated for discriminatory power between the GDH and control populations. A lymphocyte count >1750 cells/μL had the highest discriminatory power with a sensitivity of 96.6%, specificity of 60.3%, and AUC of 0.828 to detect GDH in this population as compared to the gold standard of serum cortisol concentrations (Table 3). In this population, the negative predictive value for a lymphocyte count >1750 cells/μL was 99.7%. The optimal cut‐points and their associated sensitivity, specificity, and AUC are presented in Table 3. The ROC curves created for these hematologic variables are presented in Figure 2.
TABLE 3.
Performance of clinicopathologic data for detection of GDH
| Clinicopathologic parameter | Optimal cut point | Sensitivity % (95% CI) | Specificity % (95% CI) | AUC (95% CI) |
|---|---|---|---|---|
| Resting cortisol (μg/dL) | <0.85 | 96.2 (81.2‐99.8) | 98.2 (96.8‐99.0) | 0.988 (0.978‐0.999) |
| Albumin (g/dL) | <2.950 | 89.7 (73.6‐96.4) | 77.4 (73.9‐80.6) | 0.882 (0.839‐0.925) |
| Albumin/globulin ratio | <1.081 | 86.2 (69.4‐94.5) | 78.8 (75.3‐81.9) | 0.886 (0.827‐0.944) |
| AST (IU/L) | >44.50 | 79.3 (61.6‐90.1) | 70.7 (66.9‐74.2) | 0.772 (0.703‐0.840) |
| Chloride (mmol/L) | >111.5 | 86.2 (69 4‐94.5) | 53.5 (49.5‐57.4) | 0.749 (0.660‐0.837) |
| Cholesterol (mg/dL) | <133.5 | 82.8 (65.5‐92.4) | 81.3 (78.0‐84.2) | 0.878 (0.814‐0.942) |
| Cobalamin (ng/L) | >318.5 | 81.8 (52.3‐96.8) | 35.3 (30.3‐40.6) | 0.531 (0.378‐0.681) |
| Creatinine (mg/dL) | >0.95 | 82.8 (65.5‐92.4) | 60.6 (56.6‐64.5) | 0.718 (0.639‐0.797) |
| Eosinophils (/μL) | >403.5 | 72.4 (54.3‐85.3) | 67.3 (63.5‐71.0) | 0.734 (0.643‐0.825) |
| Globulin (g/dL) | >2.450 | 82.8 (65.5‐92.4) | 55.6 (51.5‐59.5) | 0.723 (0.631‐0.815) |
| Glucose (mg/dL) | <89.50 | 69.0 (50.5‐82.7) | 70.9 (67.1‐74.4) | 0.705 (0.597‐0.812) |
| Hematocrit (%) | <42.60 | 69.0 (50.8‐82.7) | 68.0 (64.2‐71.6) | 0.683 (0.587‐0.778) |
| Hemoglobin (g/dL) | <14.15 | 62.1 (44.0‐77.3) | 70.2 (66.4‐73.7) | 0.674 (0.575‐0.773) |
| Neutrophil/lymphocyte ratio | <2.659 | 65.5 (47.4‐80.1) | 81.4 (78.1‐84.3) | 0.755 (0.660‐0.850) |
| Lymphocyte (/μL) | >1750 | 96.6 (82.8‐99.8) | 60.3 (56.3‐64.1) | 0.828 (0.762‐0.894) |
FIGURE 2.
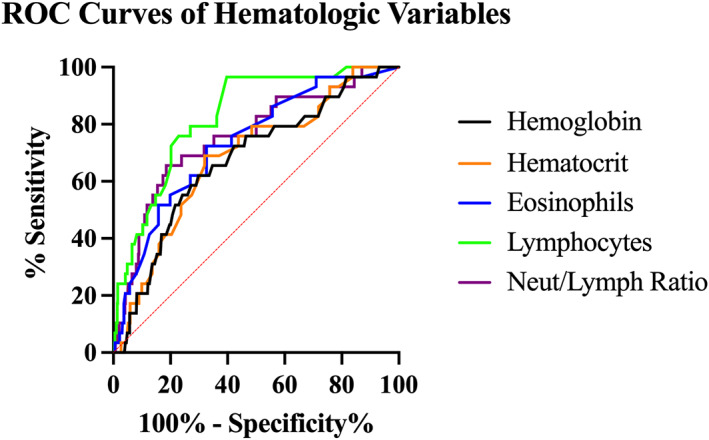
Receiver operator characteristic curves of hematologic variables discriminating between a cohort of dogs with GDH and a cohort of control dogs as compared to serum cortisol concentration measurement. Hemoglobin (gray), hematocrit (orange), lymphocyte count (green), and eosinophil count (blue), neutrophil/lymphocyte ratio (purple)
Of the measured biochemical variables, 8 were significantly different between the 2 populations, including serum creatinine, glucose, albumin and globulin concentrations, albumin/globulin ratio, aspartate transaminase (AST) activity, and serum cholesterol and chloride concentrations (Table 2). The differences in population distributions for each of these biochemical variables are presented in Figure 3.
FIGURE 3.
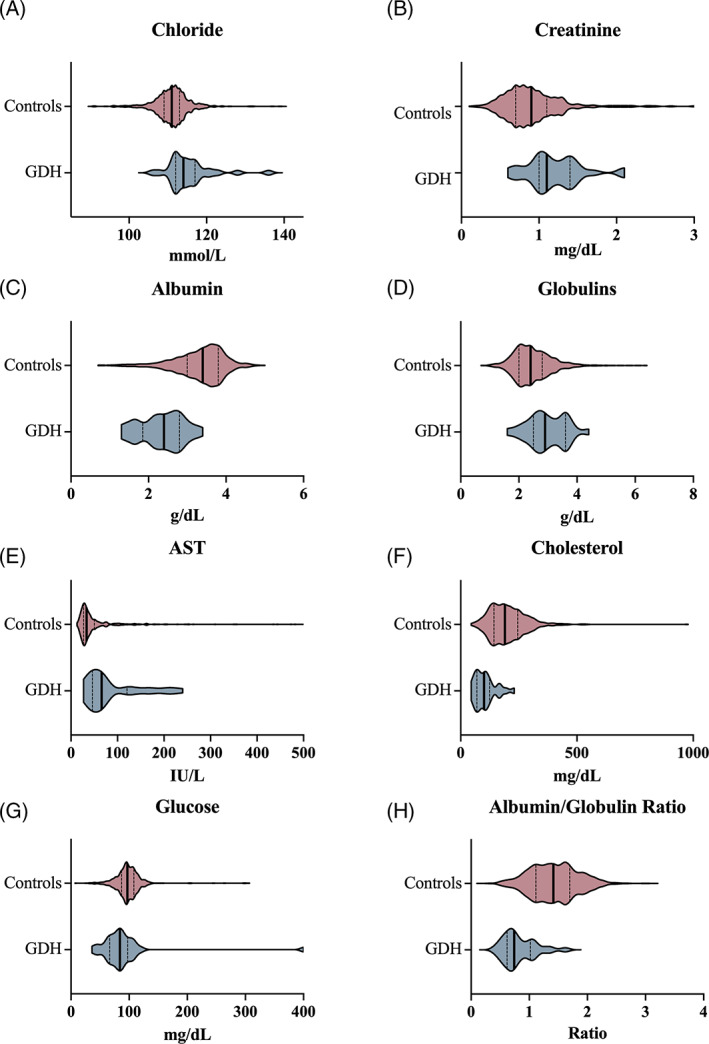
Biochemical variables that differed between a cohort of dogs with GDH versus a cohort of control dogs. Violin plots compare variables (A, chloride; B, creatinine; C, albumin; D, globulins; E, AST; F, cholesterol; G, glucose; H, albumin/globulin ratio) in the control population (red) and GDH groups (blue). The bold vertical line represents the population median and thin vertical lines represent the lower and upper quartiles
The biochemical variables that were different between the populations were evaluated for discriminatory power between the GDH and control populations. A serum albumin concentration <2.95 g/dL had the highest discriminatory power of the biochemical variables with a sensitivity of 89.7%, specificity of 77.4%, and AUC of 0.886 to detect GDH in this population as compared to the gold standard of serum cortisol concentration (Table 3). The negative predictive value for a serum albumin concentration <2.95 g/dL in this population was 99.4%. Receiver operator characteristic curves for these biochemical variables are presented in Figure 4.
FIGURE 4.
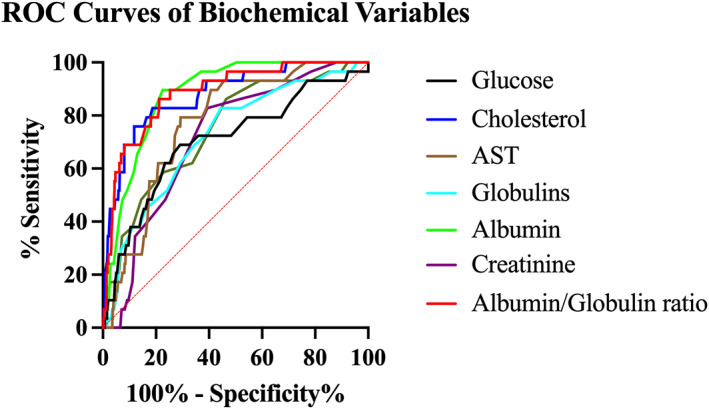
Receiver operator characteristic curves of biochemical variables discriminating between a cohort of dogs with GDH and a cohort of control dogs as compared to serum cortisol concentration measurement. Glucose (gray), cholesterol (blue), AST (brown), globulins (light blue), albumin (green), creatinine (purple), albumin/globulin ratio (red)
Backwards stepwise logistic regression modeling was performed using all hematologic and biochemical variables that were significantly different between the GDH and control groups. The final model retained serum albumin (β = −1.38, P < .001), globulin (β = 0.94, P < .001), and cholesterol (β = −0.02, P < .001) concentrations and lymphocyte count (β = 0.0007, P < .001) as significant predictors of GDH with an AUC of 0.948.
3.3. Cortisol testing
Dogs in the GDH group all had post‐ACTH stimulation serum cortisol concentrations <2 μg/dL. A resting serum cortisol concentration was available for 26/29 dogs with GDH, and the median resting cortisol concentration was 0.25 μg/dL (range, 0.2‐1.8 μg/dL) in this group. All dogs in the control group had a resting serum cortisol concentration measured, and 102/594 dogs had an ACTH stimulation test performed. The median resting serum cortisol concentration was 3.9 μg/dL (range, 0.2‐43 μg/dL). The resting serum cortisol concentration was ≥2 μg/dL in 509/594 (85.7%) of the dogs. The resting serum cortisol concentration was significantly different between the GDH and control populations (P < .0001). Resting serum cortisol concentration had high discriminatory power between the 2 groups (AUC, 0.988) and at the optimal cut‐point of <0.85 μg/dL, sensitivity and specificity were 96.2% (95% CI, 81.2%‐99.8%) and 98.2% (95% CI, 96.8%‐99%), respectively (Table 3).
3.4. Serum cobalamin concentrations
Serum cobalamin concentration was measured in 11/29 (38%) dogs with GDH and in 329/594 (55.4%) control dogs. The median (range) cobalamin concentration in the dogs with GDH was 423 ng/L (184‐927 ng/L) and 410 ng/L (150‐2965 ng/L) in the control population (Figure 5). No difference in serum cobalamin concentrations was found between these 2 populations (adjusted P = 1). In the GDH group, 2/11 (18.2%) dogs had a serum cobalamin concentration below the reference range (271‐875 ng/L), whereas a higher percentage of the control dogs (28.3%; 93/329) had hypocobalaminemia (P = .01). Serum cobalamin concentration however had poor predictive capability for detecting GDH with an AUC of 0.531 (Table 2).
FIGURE 5.
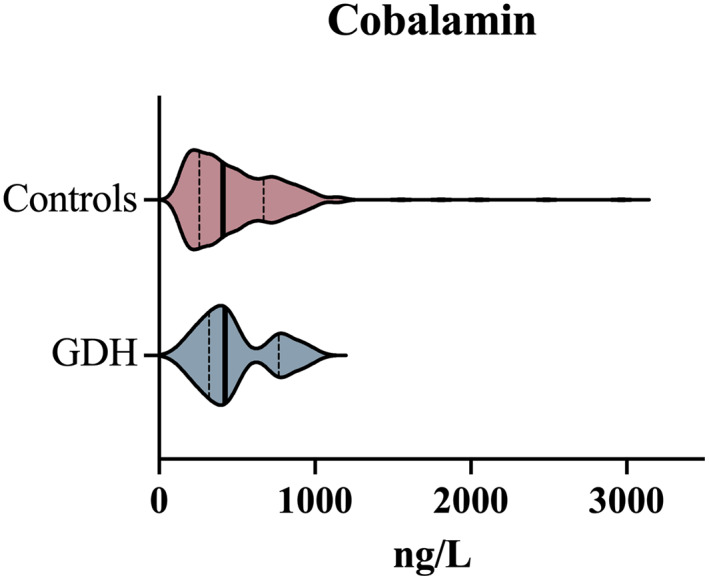
Comparison of serum cobalamin concentrations between a cohort of dogs with GDH and a cohort of control dogs. Violin plots compare the control population (red) and GDH population (blue). The bold vertical line represents the population median and thin vertical lines represent the lower and upper quartiles
3.5. Abdominal ultrasound examination
Abdominal ultrasound images were available for complete review in 20 GDH dogs and 113 control dogs (Table 4). The median ultrasonographic score assigned to the small intestine (SI) was 4 in both groups (P = 1). In the dogs with GDH, 5/20 (25%) had at least 1 SI abnormality (SI score > 4), whereas 32/113 (28.3%) of the control dogs had at least 1 abnormality (P = 1). No differences in proportions of dogs with GI mass, ulcerations, peritoneal effusion, and colonic, stomach, or jejunal lymph node abnormalities were noted between the 2 groups (Table 4). At least 1 adrenal gland width was measured in 20 dogs with GDH and 108 control dogs. The median (range) adrenal gland width of the left and right adrenal glands in dogs with GDH was 0.29 cm (0.14‐0.9 cm) and 0.34 cm (0.16‐0.63 cm), respectively. In the control population, the median (range) of the left and right adrenal glands was 0.48 cm (0.29‐1.5 cm) and 0.51 cm (0.27‐0.9 cm), respectively (Figure 6). The width of the adrenal glands differed significantly in the dogs with GDH as compared to control dogs on both the left (P < .0001) and right (P < .0001) glands. The width of the adrenal glands was a predictor of a diagnosis of GDH in this population with AUC of 0.874 (95% CI, 0.769‐0.979) and 0.836 (95% CI, 0.743‐0.928) for the left and right adrenal gland width, respectively. The optimal cut‐point for the diagnosis of GDH for the left adrenal gland width was <0.39 cm, which yielded a sensitivity of 80% (95% CI, 58.4%‐91.9%) and specificity of 82.4% (95% CI, 74.2‐88.4). The optimal cut‐point for the right adrenal gland was <0.43 cm and resulted in a sensitivity of 90% (95% CI, 69.9%‐98.2%) and specificity of 69.7% (95% CI, 60.1%‐77.9%). Of the GDH dogs with adrenal gland measurements, 15/19 of the left adrenal glands measured <0.39 cm and 16/19 of the right adrenal glands measured <0.43 cm. A significant correlation between body weight and left adrenal gland width was noted in the control population (r = 0.35; 95% CI, 0.17‐0.5; P = .0002) but not in the GDH dogs (r = 0.37; 95% CI, −0.09 to 0.7; P = .11).
TABLE 4.
Abdominal ultrasound findings
| Characteristics | GDH (n = 20) | Controls (n = 113) | P‐value |
|---|---|---|---|
| Small intestines | |||
| Echogenicity | 1 | ||
| Normal (1) | 18 | 103 | |
| Abnormal (2) | 2 | 10 | |
| Wall layering | .7 | ||
| Normal (1) | 17 | 100 | |
| Abnormal (2) | 3 | 13 | |
| Corrugation | .7 | ||
| Normal (1) | 20 | 106 | |
| Abnormal (2) | 0 | 7 | |
| Dilatation | ND | ||
| Normal (1) | 20 | 110 | |
| Abnormal (2) | 0 | 3 | |
| Total score (median, range) | 4 (4‐5) | 4 (4‐7) | 1 |
| GI masses | ND | ||
| Absent | 20 | 112 | |
| Present | 0 | 1 | |
| Ulceration | 1 | ||
| Absent | 20 | 111 | |
| Present | 0 | 2 | |
| Colon | 1 | ||
| Normal | 10 | 71 | |
| Abnormal | 2 | 20 | |
| Stomach | .3 | ||
| Normal | 12 | 90 | |
| Abnormal | 4 | 15 | |
| Jejunal lymph nodes | .6 | ||
| Normal | 13 | 58 | |
| Abnormal | 0 | 5 | |
| Peritoneal effusion | .5 | ||
| Absent | 15 | 93 | |
| Present | 5 | 20 | |
| Adrenal gland width (cm) (median, range) | |||
| Left | 0.29 (0.14‐0.9) | 0.48 (0.29‐1.5) | <.0001 |
| Right | 0.34 (0.16‐0.6) | 0.51 (0.27‐0.9) | <.0001 |
Note: Control group: left adrenal n = 108, right adrenal n = 99. ND = not determined.
Bolded P‐values correspond with statiscitally significant findings.
FIGURE 6.
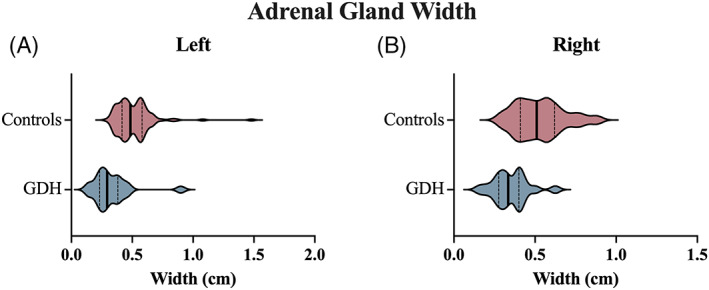
Comparison of adrenal gland width measured ultrasonographically between a cohort of dogs with GDH and a cohort of control dogs. Violin plots compare the control population (red) and GDH population (blue) for the left (A) and right (B) adrenal glands. The bold vertical line represents the population median and thin vertical lines represent the lower and upper quartiles
4. DISCUSSION
Recognition of GDH as a potential cause of disease in a patient is notoriously challenging. This difficulty is emphasized by our findings comparing clinicopathologic and ultrasonographic variables in 2 groups of dogs, those diagnosed with GDH and a control population that was suspected of GDH by the attending clinician but had the disease ruled out by measurement of serum cortisol concentrations. Our study identified laboratory and ultrasonographic variables that were significantly different between the study groups, but substantial overlap was observed among hematologic, biochemical, and ultrasonographic findings indicating that gold standard serum cortisol concentrations should be determined in dogs with a compatible clinical presentation regardless of clinicopathologic or ultrasonographic abnormalities. Our findings extend those of previous studies that have not identified reliable predictors of GDH in clinical signs or clinicopathologic variables by including ultrasonographic findings and serum cobalamin concentrations. 2 , 7 , 8
Dogs in our study were categorized as having GDH if they did not exhibit clinicopathologic evidence of mineralocorticoid deficiency including hyponatremia or hyperkalemia. In addition, none of the dogs were treated with mineralocorticoids at the time of initial diagnosis. Aldosterone deficiency was not ruled out directly in our study population, and it is possible that relative deficiency of this hormone was present in some dogs, but not evident from the available diagnostic test results. 12 Nevertheless, these variables were utilized because they most accurately reflect the diagnostic information available to clinicians when trying to establish a diagnosis of GDH. Importantly, the possibility that some GDH dogs might have had a component of aldosterone deficiency does not affect the diagnostic challenge faced by clinicians and the likelihood that such GDH cases would be overlooked and not tested for hypoadrenocorticism.
The control population included dogs that had clinical presentations that overlapped with GDH enough to prompt the attending clinician to measure serum cortisol concentrations. A heterogenous group of disease processes is represented in this group, emphasizing the diverse clinical presentation that can be associated with GDH. However, GI disease was the most prevalent clinical diagnosis in the control population, indicating substantial overlap in clinical presentation for dogs with primary GI disease and GDH. A clinician may be prompted to test for GDH if clinicopathologic or ultrasonographic findings such as hypocholesterolemia, lymphocytosis, or small adrenal glands on abdominal ultrasound are observed. 2 , 5 , 7 , 8 , 9 , 13 Given our results, reliance on any of these findings may be problematic, because substantial overlap was observed in these variables between dogs with GDH and controls.
Although GI signs predominate in GDH, panhypoproteinemia, a hallmark of protein‐losing enteropathy, is rare in GDH. In a small case series of 4 dogs diagnosed with GDH that had clinical disease mimicking protein‐losing enteropathy, only 2 dogs were reported as being hypoglobulinemic. 14 We found the albumin/globulin ratio to be the clinicopathologic feature with the highest discriminatory power between dogs with GDH and controls. Dogs with GDH had significantly lower serum albumin and higher globulin concentrations than did the controls, with over >33% of GDH dogs having hyperglobulinemia. The albumin/globulin ratio for dogs with GDH was significantly lower than that of the control population, and a ratio of <1.08 had good sensitivity (86%) and specificity (79%) to detect GDH. The mechanism of hypoalbuminemia in GDH is not known, but it may be the result of decreased production of albumin, loss secondary to disruption of GI barrier function, intestinal hemorrhage, or a combination of these factors. 15 The reason for the discrepancies in the magnitude of serum albumin and globulin concentration changes in GDH is not known, but suggest greater importance of decreased production rather than loss through the GI tract which should affect both albumin and globulins similarly. Over 33% of the dogs with GDH were hyperglobulinemic, which may reflect dehydration. Although a low albumin/globulin ratio can be suggestive of GDH, substantial overlap occurred between the 2 groups, and this ratio should not be relied upon solely to make the decision to measure serum cortisol concentrations.
Serum cholesterol concentrations were highly discriminatory between dogs with GDH and the controls, with GDH dogs having significantly lower serum cholesterol concentrations compared to controls. This finding is common in dogs with GDH, and the frequency of hypocholesterolemia in the GDH population in our study is similar to previous findings. 7 , 8 , 14 Hypocholesterolemia in dogs with GDH may be secondary to decreased intestinal fat absorption or decreased mobilization. 16 , 17 In this population, a cholesterol concentration of <133 mg/dL was 83% sensitive and 81% specific for the detection of GDH. We believe all dogs with hypocholesterolemia should be evaluated for GDH. A normal serum cholesterol concentration was found in almost 20% of the dogs with GDH, and therefore lack of hypocholesterolemia should not preclude measuring serum cortisol concentration in a dog that has other consistent clinical features.
The lymphocyte count also was identified as a strong discriminatory factor between GDH dogs and controls, as has been described previously. 4 , 13 Dogs with GDH had higher lymphocyte counts as compared to the controls, but the majority of dogs with GDH had lymphocyte counts within the normal reference range. This finding is similar to previous reports, and the mechanism is related to the lack of glucocorticoids that are responsible for a physiologic stress response in sick animals and subsequent lymphopenia. 13 In this population, a lymphocyte count >1750 cells/μL was highly sensitive (97%) for the detection of GDH, but lacked specificity (60%). This lack of specificity is likely a result of selection bias, because dogs in the control population may have been tested for GDH because of a normal or increased lymphocyte count that was ultimately a result of another disease process.
Other biochemical and hematologic variables were noted to be significantly different between the 2 groups, including hematocrit, serum chloride concentration, and AST, but they had moderate to weak discrimination between groups. When all significantly different results were assessed together, albumin, globulin, cholesterol, and lymphocyte counts were retained as significant predictors of GDH. Therefore, dogs with alterations in the concentrations of these variables should have a higher index of suspicion for GDH.
Serum cobalamin concentration often is measured in dogs with clinical signs of GI disease, because cobalamin may be depleted in dogs with defective absorption in the ileum. 18 , 19 Hypocobalaminemia has been reported in dogs with hypoadrenocorticism previously, but a mechanism has not been proposed. 2 In our study, no differences were found in serum concentrations of cobalamin or in the proportion of dogs with hypocobalaminemia, and considerable overlap of serum cobalamin concentrations was found between groups. Taken together, low serum cobalamin concentration is a poor predictor of GDH. This finding may indicate that dogs with GDH could have concurrent primary GI disease decreasing cobalamin absorption, or that in some instances GDH may result in hypocobalaminemia. The finding of hypocobalaminemia should not preclude testing for GDH in dogs presenting with signs of GI disease.
We found no difference in the proportion of dogs with GDH or control dogs with ultrasonographic abnormalities in the GI tract. Some dogs with GDH have ultrasonographically apparent GI abnormalities, including loss of normal architecture of the small intestinal wall or changes in echogenicity. Therefore, alterations to the small intestinal wall noted on abdominal ultrasound examination should not preclude further investigation of GDH. Causes of these abnormalities in dogs with GDH may be related to disruptions to the epithelial barrier of the GI tract as a result of cortisol deficiency or concurrent primary GI disease. Further evaluation of these changes in dogs with GDH should be considered. The proportion of dogs with peritoneal effusion did not differ between those with GDH and the control population. Peritoneal effusion has been reported previously in dogs with GDH, and may be related to low colloidal oncotic pressure secondary to GI loss of protein. 14
The width of the adrenal glands previously has been described as a potential marker of hypoadrenocorticism in dogs. 7 , 9 In our study, adrenal gland width was significantly smaller in dogs with GDH than in control dogs, and the optimal discriminator for left adrenal gland width was 0.39 cm, which is similar to previous reports that suggested 0.32 cm as a cut‐point. 9 However, nearly 20% of dogs that were diagnosed with GDH had adrenal glands that measured within or above the normal range. Therefore, normal adrenal gland size should not be used to rule out GDH as a differential diagnosis. Furthermore, a correlation was noted between body weight and left adrenal gland width in the control dogs as has been previously observed. 20 Therefore, adrenal gland size should be interpreted in the context of patient size.
Our study had several limitations. In this retrospective study, the rationale for pursuing cortisol testing was not always evident, and the primary clinical diagnosis in the control population was not necessarily pursued by gold standard testing. Our patient population represented a large, heterogenous group of dogs that were tested for GDH by the attending clinician, allowing for robust comparisons between the 2 groups. These limitations prevent comparisons between presenting clinical signs in the 2 groups. There is inherent bias, in the control population, because dogs may have been tested for GDH because of the clinicopathologic or ultrasonographic findings that were presented here. Furthermore, as previously discussed, the dogs in the GDH group did not have aldosterone measured. Therefore, we cannot be certain that the included dogs did not have some degree of aldosterone deficiency. Serum aldosterone concentrations are not routinely utilized in the diagnosis of GDH, and we do not feel this limitation adversely affected our results. Given the retrospective nature of our study, it is possible that some dogs that had been treated with corticosteroids were inadvertently included in the GDH group, and such treatment could have suppressed the pituitary adrenal axis and resulted in false positive ACTH stimulation test results. Additionally, abdominal ultrasound examinations were not performed in every patient and images were not saved for all organs in some studies. To mitigate this shortcoming, a radiologist, blinded to the final diagnosis and previous ultrasound reports, reviewed all images from a random subset of patients included in the study. Lastly, the study period spanned 16 years, and during this time analytical methods and the quality of ultrasonographic imaging may have changed, potentially leading to changes in the quality of data over time.
In summary, our study describes the clinicopathologic and ultrasonographic features including albumin/globulin ratio, lymphocyte count, serum cholesterol concentration, and adrenal gland width that can be utilized to increase the index of suspicion for GDH. However, none of these markers should be relied upon in isolation to rule out GDH.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflicts of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off label antimicrobial use.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
No funding was received for this study.
Reagan KL, McLarty E, Marks SL, Sebastian J, McGill J, Gilor C. Characterization of clinicopathologic and abdominal ultrasound findings in dogs with glucocorticoid deficient hypoadrenocorticism. J Vet Intern Med. 2022;36(6):1947‐1957. doi: 10.1111/jvim.16564
REFERENCES
- 1. Kelch WJ. Canine Hypoadrenocorticism (Canine Addison's Disease): History, Contemporary Diagnosis by Practicing Veterinarians, and Epidemiology. Knoxville, TN: The University of Tennessee; 1996. [Google Scholar]
- 2. Hauck C, Schmitz SS, Burgener IA, et al. Prevalence and characterization of hypoadrenocorticism in dogs with signs of chronic gastrointestinal disease: a multicenter study. J Vet Intern Med. 2020;34:1399‐1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hanson J, Tengvall K, Bonnett B, et al. Naturally occurring adrenocortical insufficiency–an epidemiological study based on a Swedish‐insured dog population of 525,028 dogs. J Vet Intern Med. 2016;30:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dogs with hypoadrenocorticism: 225 cases (1979‐1993). J Am Vet Med Assoc. 1996;208:85‐91. [PubMed] [Google Scholar]
- 5. Borin‐Crivellenti S, Garabed RB, Moreno‐Torres KI, Wellman ML, Gilor C. Use of a combination of routine hematologic and biochemical test results in a logistic regression model as a diagnostic aid for the diagnosis of hypoadrenocorticism in dogs. Am J Vet Res. 2017;78:1171‐1181. [DOI] [PubMed] [Google Scholar]
- 6. Reagan KL, Reagan BA, Gilor C. Machine learning algorithm as a diagnostic tool for hypoadrenocorticism in dogs. Domest Anim Endocrinol. 2020;72:106396. [DOI] [PubMed] [Google Scholar]
- 7. Wakayama J, Furrow E, Merkel L, et al. A retrospective study of dogs with atypical hypoadrenocorticism: a diagnostic cut‐off or continuum? J Small Anim Pract. 2017;58:365‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson AL, Scott‐Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985‐2005). J Am Vet Med Assoc. 2007;230:1190‐1194. [DOI] [PubMed] [Google Scholar]
- 9. Wenger M, Mueller C, Kook PH, Reusch CE. Ultrasonographic evaluation of adrenal glands in dogs with primary hypoadrenocorticism or mimicking diseases. Vet Rec. 2010;167:207‐210. [DOI] [PubMed] [Google Scholar]
- 10. Gaschen L, Kircher P, Stüssi A, et al. Comparison of ultrasonographic findings with clinical activity index (CIBDAI) and diagnosis in dogs with chronic enteropathies. Vet Radiol Ultrasound. 2008;49:56‐64. [DOI] [PubMed] [Google Scholar]
- 11. Seabold S, Perktold J. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference. Austin, TX; 2010:61. [Google Scholar]
- 12. Baumstark M, Sieber‐Ruckstuhl NS, Müller C, et al. Evaluation of aldosterone concentrations in dogs with hypoadrenocorticism. J Vet Intern Med. 2014;28:154‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seth M, Drobatz K, Church D, et al. White blood cell count and the sodium to potassium ratio to screen for hypoadrenocorticism in dogs. J Vet Intern Med. 2011;25:1351‐1356. [DOI] [PubMed] [Google Scholar]
- 14. Lyngby JG, Sellon RK. Hypoadrenocorticism mimicking protein‐losing enteropathy in 4 dogs. Can Vet J. 2016;57:757‐760. [PMC free article] [PubMed] [Google Scholar]
- 15. Fischer A, Gluth M, Weege F, et al. Glucocorticoids regulate barrier function and claudin expression in intestinal epithelial cells via MKP‐1. Am J Physiol Gastrointest Liver Phys Ther. 2014;306:G218‐G228. [DOI] [PubMed] [Google Scholar]
- 16. Lifton S, King L, Zerbe C. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986‐1995). J Am Vet Med Assoc. 1996;209:2076‐2081. [PubMed] [Google Scholar]
- 17. Feldman EC, Nelson RW, Reusch C, et al. Canine and Feline Endocrinology. 4th ed. St. Louis, MO: Elsevier Saunders; 2014. [Google Scholar]
- 18. Berghoff N, Suchodolski JS, Steiner JM. Association between serum cobalamin and methylmalonic acid concentrations in dogs. Vet J. 2012;191:306‐311. [DOI] [PubMed] [Google Scholar]
- 19. Heilmann RM, Steiner JM. Clinical utility of currently available biomarkers in inflammatory enteropathies of dogs. J Vet Intern Med. 2018;32:1495‐1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Soulsby SN, Holland M, Hudson JA, Behrend EN. Ultrasonographic evaluation of adrenal gland size compared to body weight in normal dogs. Vet Radiol Ultrasound. 2015;56:317‐326. [DOI] [PubMed] [Google Scholar]


