Abstract
Background
Focal epileptic motor seizures manifested by limb contraction have been recognized anecdotally in Pomeranians.
Objectives
To investigate clinical features of idiopathic epilepsy (IE) and epilepsy of unknown cause (EUC) in Pomeranians as well as the ADAM23 haplotype frequency previously reported as a common risk haplotype for epilepsy in several breeds of dogs.
Animals
Twenty‐eight Pomeranians, including 15 with IE and 13 with EUC. Nine Pomeranians with epilepsy and 8 control Pomeranians were used for ADAM23 risk haplotype analysis.
Methods
Case series study including both retrospectively and prospectively collected cases. The ADAM23 haplotype was determined by direct sequencing of PCR amplicons. Data were analyzed descriptively.
Results
Focal epileptic seizures (FS) were the predominant type of seizure in 22 of 28 dogs (78.6%). Among these, 12 of the IE dogs (80.0%) and 10 of the EUC dogs (76.9%) showed FS. Notably, 21 of 22 Pomeranians with FS (95.5%) showed limb contraction during ictal periods. Some dogs with FS also showed immobility, generalized tremors, difficulty walking or moving, autonomic signs, orofacial automatisms or some combination during ictal events. Ten dogs with FS and limb contraction had electroencephalography (EEG) performed, and interictal epileptiform discharges were identified in 9 dogs. The haplotype frequency of ADAM23 in cases was lower (27.8%) than that of the controls (56.3%).
Conclusions and Clinical Importance
In our study, FS was the predominant type of seizure in Pomeranians, and almost all cases with FS showed limb contraction, regardless of whether having IE or EUC.
Keywords: canine, electroencephalography, focal motor seizure, seizure phenomenology, seizure semiology
Abbreviations
- ASM
antiseizure medication
- CSF
cerebrospinal fluid
- EEG
electroencephalography
- EUC
epilepsy of unknown cause
- FS
focal epileptic seizures
- FMS
focal motor seizures
- FSvGS
focal epileptic seizures evolving into generalized epileptic seizures
- GS
generalized epileptic seizures
- IE
idiopathic epilepsy
- IVETF
International Veterinary Epilepsy Task Force
- MRI
magnetic resonance imaging
1. INTRODUCTION
Epilepsy is a common functional brain disorder in dogs, and clinically is defined as ≥2 unprovoked epileptic seizures >24 hours apart. 1 According to the International Veterinary Epilepsy Task Force (IVETF) classification, 1 epileptic seizures are classified into 3 types: focal epileptic seizures (FS), generalized epileptic seizures (GS), and focal epileptic seizures evolving into generalized epileptic seizures (FSvGS). In several breeds of dogs, FS is commonly recognized in idiopathic epilepsy (IE), 2 which is characterized by a suspected genetic background or no indication of structural epilepsy with clinical onset between 6 months and 6 years of age. 1 , 3 Focal epileptic seizures represent lateralized or regional signs or both such as motor, autonomic, or behavioral signs. 1 Among these, FS characterized by elementary motor events such as stereotypical muscle contraction or automatisms also is referred to as focal motor seizures (FMS). 4
A few forms of unique stereotypical FS in specific breeds of dog have been reported with proven or suspected genetic backgrounds. 5 , 6 , 7 Focal epileptic seizures with limb contraction in Pomeranians has been sporadically recognized in Japan. However, thus far, clinical features of epilepsy in Pomeranians have not been described. Furthermore, in epilepsy in dogs, many breeds are suspected of having susceptible genetic backgrounds; 2 these are likely to be complex genetic traits for IE. 8 , 9 An ADAM23 haplotype is reportedly a common risk haplotype for epilepsy in some breeds of dogs. 9 , 10
An acknowledged hypothesis is that FS with limb contraction (a unique FMS form) is a relatively common seizure semiology in a population of Pomeranians with presumed IE or epilepsy of unknown cause (EUC, dogs with an age of seizure onset <6 months or >6 years in which the underlying cause for epilepsy is unknown) in Japan and a wide age range for clinical onset is seen.
We aimed to characterize clinical features of IE/EUC in Pomeranian dogs including seizure semiology and electroencephalography (EEG) findings, and to investigate the frequency of the common risk haplotype in the ADAM23 gene reported previously for epilepsy in dogs. 9 , 10
2. MATERIALS AND METHODS
Ours was a descriptive, case series study of both retrospectively and prospectively collected cases. Some cases also were included in a genetic investigation of the ADAM23 risk haplotype frequency in Pomeranians, which previously has been reported in IE in some breeds of dogs. 9 , 10
2.1. Study population, inclusion criteria, and exclusion criteria
Medical records from the Neurology and Neurosurgery Service of Nippon Veterinary and Life Science University (Tokyo, Japan) from April 1999 to December 2019 and those from the Neurology and Neurosurgery Service of Azabu University (Kanagawa, Japan) from June 2006 to December 2019 were searched to identify dogs that were diagnosed as “IE,” “suspected IE,” or “EUC.” Furthermore, additional cases diagnosed as “IE,” “suspected IE,” or “EUC” by a veterinarian from August 2019 to May 2020 were recruited prospectively in collaboration with veterinary neurologists in several referral practices in Japan, and clinical data were collected using a standardized questionnaire from August 2019 to May 2020.
Because most of the cases obtained retrospectively had been diagnosed before publication of IVETF consensus proposal, the diagnostic scheme and epilepsy‐specific magnetic resonance imaging (MRI) protocol proposed by IVETF in 2015 3 , 11 were not completely adopted.
We used the following inclusion criteria: history of recurrent epileptic seizures (≥2 seizures occurring >24 hours apart), normal laboratory findings (CBC and serum biochemistry with or without urinalysis), normal interictal neurologic examination, and no indication of structural epilepsy on MRI for dogs with age at clinical seizure onset <6 months or >6 years. Magnetic resonance imaging findings such as Chiari‐like malformation or relatively mild to moderate ventricular enlargement (without relevant neurological deficits other than epilepsy) were accepted, and they were not used as exclusion criteria because they have been reported to have no association with epilepsy in other breeds. 12 , 13 Some cases in our study had been diagnosed with mild hydrocephalus in addition to IE. Diagnosis of hydrocephalus is more complex than simply MRI findings of enlarged ventricles, 14 , 15 and standardized criteria for the diagnosis of hydrocephalus have not been used in the previous diagnoses for the current cases. Based on only 1 of the findings, such as narrowing of the subarachnoid space, some cases had been recorded as mild hydrocephalus in the medical records. Therefore, in our study, cases diagnosed with mild hydrocephalus together with IE on MRI were reevaluated using the recently proposed criteria, 15 which originally were derived from those described previously. 14 Namely, clinically relevant hydrocephalus was based on a ventricle/brain ratio > 0.62 and presence of ≥2 of the following morphological criteria: flattening of gyri and sulci, deformation of the interthalamic adhesion, disruption of the internal capsule, dilatation of the olfactory recess, and presence of periventricular edema. Based on the criteria, cases classified as clinically silent ventriculomegaly rather than clinically relevant hydrocephalus were included in our study.
The exclusion criteria were as follows: history suggesting other intracranial diseases, indication of structural epilepsy by MRI or cerebrospinal fluid (CSF) analysis or both, and seizure semiology that did not correspond to epileptic seizures and indicated other causes such as syncope, vestibular disease, paroxysmal dyskinesia, narcolepsy, idiopathic head tremor, or compulsive disorder. 16
Finally, cases that met the criteria were assigned to IE or EUC: IE included dogs with age of onset between 6 months and 6 years old whereas EUC included dogs with age of onset <6 months or >6 years old and with MRI that showed no indication of structural epilepsy, in accordance with the age range proposed by IVETF. 3 Dogs were not required to have CSF analysis to be diagnosed with EUC for our study. With regard to the terminology mentioned above, the IVETF proposal classifies IE into 3 subclasses: IE (genetic), IE (suspected genetic), and IE (unknown cause). 1 Because no reports about confirmed or suspected genetic background for IE in Pomeranian dogs have been published to date, IE in Pomeranians was classified as IE (unknown cause) and was simply referred to as IE in our study. Based on the terminology proposed by IVETF, 1 unknown cause (not either IE or structural epilepsy) was referred to as EUC in our study, as mentioned earlier.
2.2. Clinical data collection
The data of Pomeranian dogs with IE or EUC that met the criteria were further analyzed. Based on the medical records, seizure types were re‐classified based on the IVETF proposal. 1 Collected data included the following: signalment (i.e., age, sex, and neuter status), body weight, diagnostic interventions, details of seizure information such as age at onset, seizure type classification, detailed seizure description, monthly seizure day frequency (i.e., number of days in a month that a dog has at least 1 seizure), seizure duration, preictal or postictal events, history of cluster seizures or status epilepticus at the time of diagnosis, and clinical response to antiseizure medications (ASMs). Clinical response to ASM was re‐evaluated based on the IVETF proposal. 17 Follow‐up was performed to categorize outcome as seizure‐free (3 times the longest pretreatment interictal interval and at least 3 months), partial therapeutic success (decrease in seizure frequency or clusters and status epilepticus), no response, and undetermined. Furthermore, videos of ictal events (when available) provided by dog owners were used to confirm seizure semiology. The EEG findings were used as supportive data for the characterization when available.
2.3. Diagnostic intervention method
Magnetic resonance imaging acquisition was performed using a 1.5 or 3.0‐Tesla MRI scanner except for 3 dogs that underwent MRI using a 0.3, 0.4, or 1.5‐Tesla MRI scanner at other institutes.
Scalp EEG examinations were carried out using a digital electroencephalogram device in either of the channel montages derived from previous literature 18 , 19 for 15 to 40 minutes. In short, the average potential reference (6 dogs) and the referential derivation (4 dogs) were used for EEG monitoring. Sedation was performed using medetomidine (6 dogs) or dexmedetomidine (2 dogs) administration or sevoflurane inhalation (2 dogs). Detailed information of EEG examinations is summarized in Appendix S1.
Cerebrospinal fluid analysis, including at least cell count and total protein concentration, was evaluated when available. When a sufficient amount of CSF was collected, cytology also was performed. Fasting and postprandial serum bile acid concentrations also were retrieved from the medical records when available.
2.4. ADAM23 haplotype analysis
Ethylenediaminetetraacetic acid blood samples from Pomeranians diagnosed with IE, EUC, or other diseases, were archived or buccal swabs were collected and submitted by owners in accordance with an approved Institutional Animal Care and Use Committee protocol of Nippon Veterinary and Life Science University (accession number: H30‐3) or an approved Biobank protocol of Azabu University. Genomic DNA was extracted from whole blood using commercially available column‐based DNA extraction kits.
Six variants on the risk haplotype in ADAM23 previously reported 9 , 10 were amplified by PCR and determined by direct sequencing, as previously described. 20 Primer sequences and PCR condition are summarized in Table S1.
2.5. Data analysis
Data are presented descriptively. Categorical data are presented with frequencies and percentages when relevant. Descriptive statistics presented as median and range were used. Data analyses and visualizations were performed using R (version 4.1.2) software. 21
3. RESULTS
3.1. Study Population
Data from 37 Pomeranians with a history of seizures and that were previously diagnosed with IE, suspected IE, or EUC were retrospectively collected. Furthermore, 3 Pomeranians with a history of seizures that were diagnosed with IE, suspected IE, or EUC by a veterinarian also were prospectively recruited for data collection. Therefore, data from 40 Pomeranians were obtained. Five dogs with epileptic seizures, which were diagnosed with ventriculomegaly or mild hydrocephalus, were considered clinically silent ventriculomegaly as described above and included in the study. Twelve dogs that did not meet the criteria were excluded (Figure 1).
FIGURE 1.
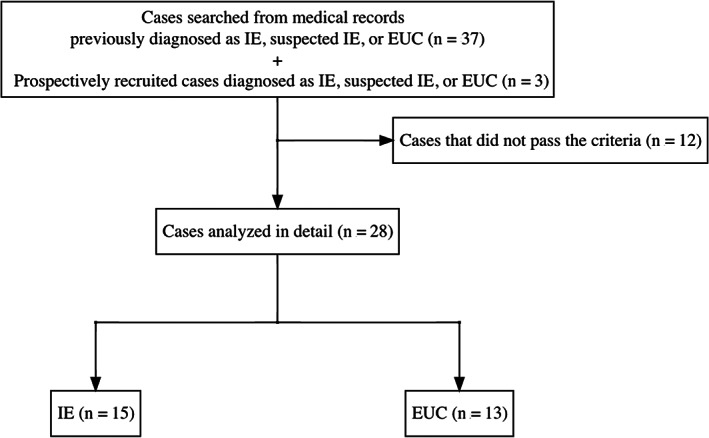
Case selection flow diagram of the current study. EUC, epilepsy of unknown causes; IE, idiopathic epilepsy
Ultimately, 28 cases met the criteria, comprising 15 IEs and 13 EUCs, with demographic data summarized in Table 1. Distribution of age at the onset of IE and EUC dogs is shown in Figure 2.
TABLE 1.
Comparison of clinical data between idiopathic epilepsy (IE) cases and epilepsy of unknown cause (EUC) cases
| Variables | Overall (n = 28) | IE (n = 15) | EUC (n = 13) |
|---|---|---|---|
| Sex | |||
| Male (neutered) | 12 (4) | 5 (2) | 7 (2) |
| Female (neutered) | 16 (9) | 10 (6) | 6 (3) |
| Body weight (kg), median (range) | 2.6 (0.7‐8.2) | 3.5 (1.9‐8.2) | 2.4 (0.7‐4.4) |
| Age at onset (months) | |||
| Median (range) | 40.5 (2‐120) | 43.0 (9‐67) | Seizure onset <6 months old: 4.0 (2–5) |
| Seizure onset >6 years (74 months) old: 92.5 (77‐120) | |||
| Seizure type | |||
| FS | 22 (78.6%) | 12 (80.0%) | 10 (76.9%) |
| FSvGS | 1 (3.6%) | 0 | 1 (7.7%) |
| GS | 4 (14.3%) | 2 (13.3%) | 2 (15.4%) |
| GS with unknown onset | 1 (3.6%) | 1 (6.7%) | 0 |
| The presence of limb contraction among FS | 21 (95.5%) | 11 (91.7%) | 10 (100%) |
| History of cluster seizures | 13 (46.4%) | 9 (60.0%) | 4 (30.8%) |
| History of status epilepticus | 2 (7.1%) | 1 (6.7%) | 1 (7.7%) |
Note: Data were presented as count (percentage) of cases unless otherwise indicated.
Abbreviations: EUC, epilepsy of unknown cause; FS, focal epileptic seizures; FSvGS, focal epileptic seizures that evolved into generalized epileptic seizures; GS, generalized epileptic seizures; IE, idiopathic epilepsy.
FIGURE 2.
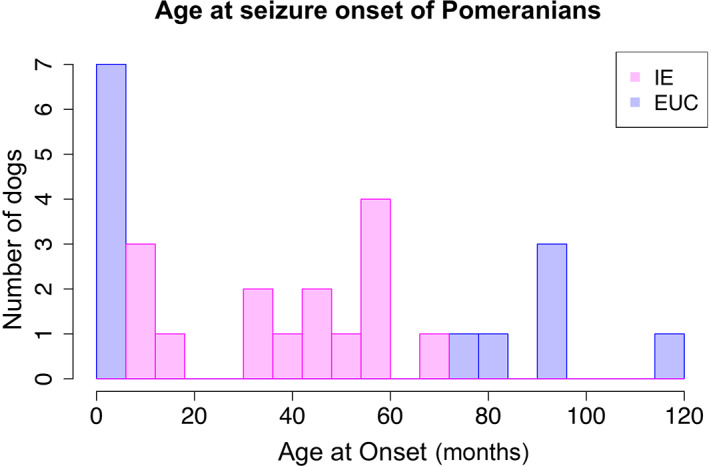
Owner‐reported age at seizure onset in 15 Pomeranians with idiopathic epilepsy (IE) and 13 Pomeranians with epilepsy of unknown cause (EUC). Each bar width of the histogram represents 6 months. Note that age at onset among the 5 dogs was approximated by owners (ie, about 1‐year‐old, 3‐year‐old [for 2 dogs], 5‐year‐old, and 8‐year‐old)
In total, 22 (78.6%) of 28 dogs including 9 IEs (60.0%) and 13 EUCs (100%) underwent MRI.
Cerebrospinal fluid analysis, including cytology, was performed in 2 dogs (7.1%; 1 IE and 1 EUC), with no clinically relevant findings in both dogs. Bile acid stimulation testing was performed in 2 dogs (7.1%; 1 IE and 1 EUC), and results were normal.
3.2. Seizure semiology
Clinical data of all 28 dogs with IE and EUC are summarized in Table 1. Focal onset seizure (ie, FS and FSvGS) accounted for 82.1% of all cases. Of note, in 22 dogs (78.6%) with FS, 21 dogs (95.5%) showed FS with limb contraction. Focal epileptic seizures with limb contraction were characterized by unilateral limb contraction (typically thoracic limb, Figure 3), and other limb(s) followed the initial limb's involvement in some dogs (Video S1 ). In 21 dogs showing FS with limb contraction, it was reported that only 1 limb was affected in 10 dogs, ≥2 limbs were involved in 9 dogs, and 2 dogs had incomplete information about affected limbs; 1 or 2 forelimbs were affected with no record of the affected side(s).
FIGURE 3.

Frames captured from videos of focal seizure with limb contraction in Pomeranian dogs with idiopathic epilepsy or epilepsy of unknown cause. (A‐C) Pomeranians displaying abnormal contraction of a left thoracic limb that causes short‐term posturing. (A) Left‐sided forced head turn (versive head movement) also was observed. (B) This dog showed left thoracic limb tonic posturing that was sustained for approximately 15 seconds. (C) Short‐term posturing of a left thoracic limb. The left‐sided lip seems to be upturned because the dog showed licking before and after this frame was captured. Ictal videos of these 3 dogs are available as Video S1. All 3 dogs showed concurrent symptoms such as immobility, generalized tremors, orofacial signs, and difficulty walking or moving during the ictal event, which also can be seen in Video S1
Focal epileptic seizures with limb contraction in Pomeranians included tonic or clonic movement with abnormal limb posture (i.e., sustained or intermittent involuntary limb muscle contractions). Some dogs also reportedly showed immobility, generalized tremors, and difficulty walking or moving during the ictal event (Video S1). Furthermore, autonomic signs or automatisms such as urination (6 dogs), vomiting (5 dogs), defecation (3 dogs), mydriasis (2 dogs), salivation (2 dogs), or licking (2 dogs) were reported in 14 dogs (Video S1: Cases 1 and 3). Seizure semiology also was confirmed by videos of ictal events that owners provided in 10 cases, including 6 IEs and 4 EUCs. Information about consciousness was available in 23 dogs, and 16 dogs appeared to be aware of their surroundings during the FS period. However, the presence of autonomic signs or consciousness were not always specified in the medical records in the retrospective cases.
Seizure types of 15 IE and 13 EUC cases are summarized and compared in Table 1. Eleven of 12 IE dogs with FS (91.7%) and all 10 EUC dogs with FS showed FS with limb contraction. One IE dog with FS, that did not show FS with the limb contraction, showed difficulty walking, difficulty standing up, and tremor as its seizure semiology. One dog with EUC showing FSvGS had generalized tremors as a part of its seizure semiology, but limb contraction was not reported. Furthermore, clinical data of EUC cases with age at clinical seizure onset <6 months or >6 years are summarized and compared in Table S2.
In 6 IE or EUC dogs showing FS with limb contraction, seizure semiology could be confirmed again at the time of our study using videos provided by the owners. Reviewing those videos of 6 dogs indicated that limb contraction itself did not last for the entire seizure duration. Limb contraction and abnormal limb posturing lasted from a few seconds to approximately 15 seconds and occurred repeatedly during an ictal event. Limb contraction was characterized by sustained or repetitive, tonic or flexed, limb posturing (Figure 3; Video S1).
In our study, monthly seizure day frequency only was determined for dogs that were observed for a minimum of 1 month before initiating ASM. There were 23 dogs, including 12 IEs and 11 EUCs, that had an observation period ≥1 month for increased seizure day frequency before initiating ASM (median, 2 months; range, 1‐6 months). The other dogs had insufficient durations of observation to determine monthly seizure day frequency. The median monthly seizure day frequency (range) before initiating ASM was 1.3 (range, 0.3‐7.0) for the entire study population, 1.0 (range, 0.3‐7.0) for IE dogs and 1.5 (range, 0.3‐4.0) for EUC dogs.
Preictal information was available for 9 dogs and postictal information was available for 12 dogs. Two dogs showed signs of anxiety such as attention seeking or clinging to the owner and 1 dog also showed restless wandering as a preictal phenomenon. One dog had a pelvic limb limp on the affected side and 1 experienced vomiting as a postictal phenomenon.
Cluster seizures were seen in 46.4% of Pomeranians with IE and EUC. However, status epilepticus was not a common finding (only 2 cases) in our study (Table 1).
3.3. EEG analysis
Electroencephalographic data was available for 10 (35.7%) of 28 dogs, including 4 IEs and 6 EUCs. All 10 dogs had FS with limb contraction. In 9 of 10 dogs, EEG showed interictal epileptiform discharges (IEDs) in the form of spikes, sharp waves, polyspikes or some combination of these (Figure 4). These were detected in a single or some combination of the following regions: frontal (5 dogs), central/parietal (3 dogs), temporal (1 dog), or occipital region (2 dogs). In 3 of 9 dogs with EEG abnormalities, the IEDs were contralateral to the side where FS with initial limb contraction occurred. One dog showed partial contralaterality between IEDs site and FMS because FMS started on the contralateral side and progressed to the ipsilateral side. Two dogs had IEDs on both cerebral hemispheres whereas the FMS was observed unilaterally. Another dog showed partial contralaterality between IEDs site and FMS because FMS started on the same side and progressed to the contralateral side. Two dogs had IEDs ipsilateral to the side on which FS started. Detailed seizure characteristics and EEG findings for each dog are compared in Table S3 for 10 dogs that underwent EEG.
FIGURE 4.
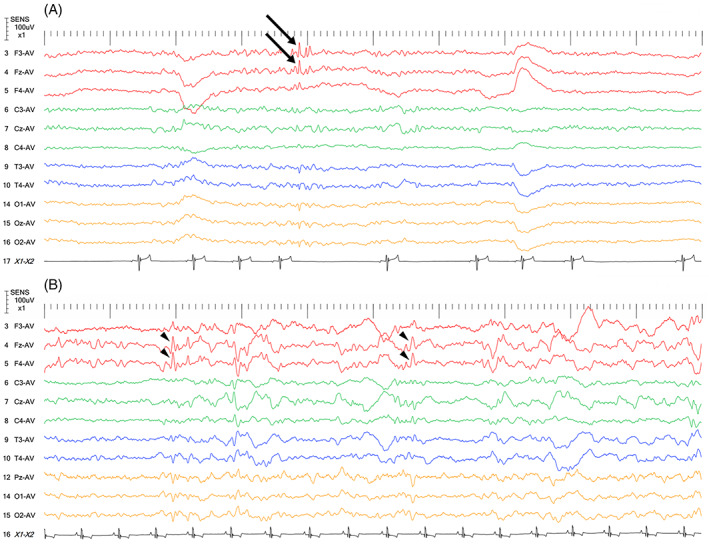
Interictal EEGs from 2 Pomeranians that were diagnosed as IE (A, B). Both had focal seizures with limb contraction. (A) Spikes (A, arrows) and sharp waves (B, arrowheads) are seen in the frontal lobe. Note that Pz‐AV in (B) is compatible with Oz‐AV in (A). The EEG montage used for these 2 dogs was as previously described. 18 For these cases, the average reference method was used. Other recording conditions were as follows: sampling frequency = 1000 Hz; high frequency filter = 60 Hz; time constant = 0.1; sensitivity = 10 μV/mm; AC cut‐off notch filter = ON; and tracing speed = 10 s/view
3.4. ASM outcome
Antiseizure medication responsiveness could be evaluated in 12 dogs, including 5 IEs and 7 EUCs. Nine of 12 dogs (3 IEs and 6 EUCs) had monotherapy (zonisamide [7 dogs], phenobarbital [2 dogs]), and 3 dogs (2 IEs and 1 EUC) had dual therapy (combinations of phenobarbital and potassium bromide, zonisamide and potassium bromide, or levetiracetam and potassium bromide, respectively).
Complete seizure freedom for at least 1 year was observed in 5 dogs (41.7%; 1 IE and 4 EUCs) with monotherapy (4 dogs) or dual therapy (1 dog; median, 24 months; range, 12‐72 months). Another IE dog had a decrease of seizure day frequency to 1 seizure day per year with monotherapy. This dog had presented with a seizure day frequency of 1 seizure day for approximately 10 days just before ASM initiation. In 2 dogs (1 IE and 1 EUC) receiving monotherapy, seizures were not observed after initiation of ASM for 4 or 8 months, respectively, but further follow‐up information was not available. Two dogs (1 IE receiving dual therapy and 1 EUC receiving monotherapy) had partial therapeutic success in observation periods of 30 months and 6 months, respectively. The monthly seizure day frequency of the former decreased from 1 to 0.5, and that of the latter decreased from 5.4 to 1.8. One EUC dog receiving monotherapy showed no seizures for 1 month, but this dog was considered undetermined because of a follow‐up period <3 months. The remaining IE dog receiving dual therapy also was considered undetermined because a few seizures occurred and abnormal behavior episodes were witnessed after 1.5 years of freedom from seizures, and thus it was difficult to evaluate ASM responsiveness appropriately.
3.5. ADAM23 haplotype analysis
The DNA from 9 of the study dogs was available for ADAM23 haplotype analysis, including 4 dogs with IE that had FS, and 5 dogs with EUC, 4 that had FS, and 1 that had GS. Eight Pomeranian dogs diagnosed with neurological diseases other than IE or EUC were genotyped as controls. The frequency of the risk haplotype was 27.8% in 9 cases and 56.3% in 8 controls. Clinical information included for the ADAM23 haplotype analysis and results are summarized in Table S4.
4. DISCUSSION
A high proportion (78.6%) of Pomeranian dogs with IE or EUC had FS, although the sample size was small. In our study, FS was considered the predominant epileptic seizure type in Pomeranians. Focal onset seizures (i.e., FS and FSvGS) were found in 82.1% of all IE and EUC cases and it was a major seizure onset form, as reported in other breeds of dogs. 22 , 23 , 24 , 25 , 26 , 27 , 28 Notably, the extremely high proportion of limb contraction in FS (95.5%; 21 of 22 FS dogs) is an important finding in our study and highlights this unique epileptic seizure semiology in Pomeranian dogs. Furthermore, EEG detected IEDs in 9 of 10 dogs showing FS with limb contraction, supporting the fact these seizures in Pomeranian dogs were epileptic in nature.
Although clinical response to ASM was evaluated only in some of our cases (42.9%; 12 of 28 dogs) and sufficient post‐treatment observation was not available for some dogs, complete seizure freedom for at least 1 year was achieved in 5 dogs (median, 24 months; range, 12‐72 months).
Although only some dogs underwent EEG (10 of 28 dogs; 35.7%), IEDs (including spikes, sharp waves, and polyspikes) were detected in 9 of 10 dogs and they were mainly seen in a single or some combination of frontal (5 dogs), central/parietal (3 dogs), temporal (1 dogs), or occipital (2 dogs) regions (Table S3). All of the dogs that underwent EEG showed FS characterized by limb contraction. This finding suggested that the episodes with limb contraction seen in Pomeranian dogs in our study were epileptic in nature. Although the irritative (abnormal EEG) zone was variable, the irritative zone in half of dogs that underwent EEG included the frontal lobe (Table S3). Generally, the locations of IEDs and the epileptogenic zone (including the irritative zone) occurred in the cerebral hemisphere contralateral to the side where FMS signs were seen. However, the locations of IEDs observed in some dogs did not match the regions indicated by the seizure semiology (Table S3). There are several possible reasons for this finding. First, the video the owners provided may not have captured the actual initial clinical features, and the recordings may have been started when the epileptic discharge had moved to the contralateral side. The owners may not have been aware of their dog's actual initial clinical signs and the seizure semiology they reported may not have been complete in dogs without video documentation. Second, IEDs may have been detected bilaterally because the epileptic focus was in the medial part of the cerebral hemisphere. Third, bilateral independent interictal epileptic discharges have been reported in some human patients with temporal lobe epilepsy, some of whom had normal MRI findings. 29 , 30 Furthermore, generation of secondary epileptogenesis outside of the primary focus has been described in human patients, some of whom did not have an unequivocal lesion on MRI, 31 as well as in experimental animal models of epilepsy. 32 Therefore, a dog could have multiple irritative zones other than the seizure focus predicted by seizure semiology. To confirm accurate location of IEDs or the irritative zone associated with ictal signs, long term video‐EEG monitoring is required.
In human medicine, dystonic posturing is defined as forced, unnatural posturing of an arm or leg on 1 side of the body. 33 Some dogs showed sustained, unnatural posturing that resembled the dystonic posturing recognized in humans with epilepsy. 33 , 34 , 35 In human medicine, dystonic posturing of an upper limb is a common clinical feature in temporal lobe epilepsy, and usually is considered to be contralateral to the epileptogenic focus. 33 , 34 This unilateral dystonic posturing also is observed in frontal lobe epilepsy. 35 Ictal dystonic posturing in temporal lobe epilepsy is considered to be caused by propagation of the ictal discharge into the basal ganglia, 33 , 36 as well as in extratemporal lobe epilepsy such as frontal lobe epilepsy. 35 Involvement of the basal ganglia in the generation of ictal dystonic posturing has been suggested by studies using single photon emission computed tomography (SPECT) 36 , 37 , 38 and positron emission tomography (PET). 39 , 40 These techniques may be useful in elucidating the underlying mechanisms of FS with limb contraction in Pomeranians.
Dystonia is characterized by sustained or intermittent involuntary muscle contractions that cause abnormal, often repetitive, movements, postures, or both. 41 , 42 In the proposal of IVETF, 1 the term dystonic was defined as: “Sustained contractions of both agonist and antagonist muscles producing athetoid or twisting movements which when prolonged may produce abnormal postures.” Dystonic seizure is included in the category of motor tonic seizure and is an adjunctive term used for epileptic seizures. 1 On the other hand, in the consensus statement of the International Veterinary Canine Dyskinesia Task Force, 41 dystonic movements also are mentioned, which indicates a wide variety of speed (from slow to shock‐like) and duration (from a flash to hours). Some Pomeranians in our study showed dystonic seizures, that is, tonic seizures with abnormal postures (Video S1, Case 2); some showed clonic or myoclonic seizures of the limb(s) (i.e., dystonic spasms or myoclonic dystonia) and abnormal postures with the limb flexed (i.e., dystonic movements, Video S1, Cases 1 and 3). Therefore, the term dystonic seizure only was applicable to FS with tonic limb dystonia. Eventually, the term FS with limb contraction was considered appropriate to describe this type of seizure seen in Pomeranians.
Paroxysmal dyskinesia is a group of involuntary movement disorders characterized by self‐limiting episodes including dystonia and other abnormal involuntary, nonstereotypical movements. 41 , 42 Importantly, sudden dystonia (i.e., sustained muscle contraction causing abnormal movements and postures of limbs, trunk, head or some combination of these) is a main phenomenon reported in paroxysmal dyskinesia in some breeds of dogs. 43 , 44 , 45 , 46 , 47 , 48 , 49 A similar abnormal posture, limb dystonia, also was a common finding in the Pomeranians in our study. As for some episodes seen in veterinary clinical practice, it may be difficult to differentiate between epileptic and nonepileptic paroxysms. 50 However, clinical characteristics of FS include decreased or absent conscious responses; autonomic signs such as urination, defecation, or salivation; orofacial movements (automatism) including mastication, lip smacking, licking, chewing, and yawning; and, pre‐ or postictal abnormalities or both, whereas paroxysmal dyskinesia does not include such clinical characteristics. 51
In our study, owner‐reported information about consciousness during the ictal phase was available for 23 dogs. Sixteen dogs were reported to be aware during the ictal event, which may suggest that the majority of IE or EUC in Pomeranians with FS may represent focal aware motor seizures that sometimes may be confused with paroxysmal dyskinesia. Although information about consciousness and the presence of autonomic or orofacial signs was not always specified in the medical records of the retrospective cases, 14 dogs showed autonomic signs or orofacial automatism. Furthermore, 9 of 10 dogs that underwent EEG showed IEDs. Our study also emphasizes the importance of EEG when events are not easily distinguished between epileptic seizures or other nonepileptic episodes such as paroxysmal dyskinesia. Information regarding the clinical response to ASM was available in 12 dogs, and a good response overall was reported. Five of 12 dogs had a complete seizure‐free outcome for at least 1 year (median, 24 months; range, 12‐72 months). Although paroxysmal dyskinesias are commonly self‐limiting and have a benign course, 51 the higher rates of good ASM response also supports the diagnostic likelihood of epilepsy. The evidence (including EEG abnormalities, ASM response, and homogeneous and short seizure duration ≤5 minutes) reinforced the conclusion that the limb contraction episodes seen in these dogs were epileptic in nature rather than representing paroxysmal dyskinesia.
Our study included Pomeranian dogs with IE or EUC in Japan. Therefore, predisposition to this syndrome in Pomeranians might reflect a higher allele frequency of variant(s) associated with this syndrome in a population of Pomeranians in Japan. However, by reviewing several ictal videos posted by owners in countries other than Japan using the online video sharing platform YouTube with the search terms: “pomeranian” AND “seizure” OR “epilepsy”, 52 Pomeranians exhibiting FS appeared to be found in other parts of the world.
Idiopathic epilepsy cases among most breeds of dogs are considered to have a complex inheritance pattern, 8 , 9 and underlying genetic variants remain to be elucidated. A risk haplotype in ADAM23 has been identified in epilepsy in some breeds. 9 , 10 Unlike some breeds that have been reported to have a risk haplotype for IE in ADAM23, 9 , 10 the frequency of the reported risk haplotype was lower in our cases than in controls. Because the number of cases and controls genotyped in our study was small (9 and 8 dogs, respectively), our results are preliminary. Further genomic investigation will be required to investigate the underlying genetics of IE and EUC in Pomeranian dogs.
Our study had some limitations. First, it included only a small number of cases, which may cause random error. For that reason, the prevalence of FS with limb contraction in Pomeranians could change when evaluated on a larger scale with more cases. Second, selection bias may have occurred if patients with atypical seizure types such as FS with limb contraction (as compared to GS) may have been selectively referred. Third, there is the possibility of bias in collection of data retrospectively from medical records. Because the data for many cases was collected from existing medical records, some data with respect to preictal or postictal events was missing. Conversely, because seizure semiology has been confirmed by veterinarians only in cases for which video was provided, there is a possibility that in other cases, the information described in the medical records was influenced by information bias. Fourth, it was not possible to apply the criteria for all cases included in our study because data for most cases were obtained retrospectively and a majority of the cases were diagnosed before the IVETF consensus guidelines were published. In particular, in our study, it is a shortcoming that the majority of the presumed EUC cases did not undergo CSF analysis. In a previous report, 24% of dogs with inflammatory CSF (6 of 25 dogs) showed normal MRI findings, but the clinical signs of dogs with normal MRI findings were not described. 53 We acknowledge the possibility that presumptive IE or EUC cases without CSF analysis might have had inflammatory CSF findings although brain MRI findings obtained in all EUCs and in 9 of 15 IEs were considered normal. However, although the duration of the observation period varied, 7 of 13 EUCs and 5 of 15 IEs for which follow‐up information was available did not develop any other neurological signs during the follow‐up period. Furthermore, serum biochemistry showed no clinically relevant abnormalities in any of the dogs, and abnormal MRI or EEG findings suggestive hepatic encephalopathy 54 , 55 were not detected, although bile acid stimulation testing was completed in very few dogs. Fifth, there still is a chance of epileptic seizure occurrence in dogs with clinically silent hydrocephalus because seizures have been long recognized in dogs with internal hydrocephalus. 56 , 57 However, a recent study reported only 2 dogs having seizures in 98 dogs diagnosed with internal hydrocephalus. 15 Furthermore, in another recent study, no dog developed epileptic seizures in 44 dogs with internal hydrocephalus that underwent ventriculoperitoneal shunting. 58 Hydrocephalus itself was considered most unlikely to be epileptogenic. 15 Sixth, although attention was given not to include cases that may have had paroxysmal dyskinesia in our study, a slight possibility of misdiagnosis remains. Finally, the number of samples used for the ADAM23 haplotype analysis was small and pedigree information was not available, leaving the genetic basis of remaining cases unknown.
5. CONCLUSION
In conclusion, we described the clinical characterization including seizure semiology of IE, EUC, or both in Pomeranian dogs in Japan, although sample size was small. Of note, 78.6% of Pomeranian dogs with IE and EUC in our study showed FS, and FS with limb contraction accounted for 95.5% of FS. Therefore, FS with limb contraction (ie, FMS) may be a major epileptic seizure type in Pomeranian dogs. Additional studies would be necessary to elucidate the prevalence as well as mechanism of FS with limb contraction and to determine the underlying genetics of IE, EUC or both in Pomeranian dogs.
CONFLICT OF INTEREST
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The study was approved by the ethics committees of Nippon Veterinary and Life Science University (accession number: H30‐3). Archiving of blood samples at Azabu University was undertaken in accordance with an approved Biobank protocol of Azabu University.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Appendix S1. Detailed information of electroencephalography examination.
Table S1. Primer sequences and detailed PCR condition.
Table S2. Clinical data of epilepsy of unknown cause cases whose age at onset was <6 months old or >6 years old.
Table S3. Comparison of detailed seizure characteristics and electroencephalographic data.
Table S4. Details of Pomeranians included for the ADAM23 haplotype analysis and results.
Video S1: Focal seizures with limb contraction in Pomeranian dogs. In addition to abnormal contraction of a left thoracic limb, 3 dogs presented with the following clinical signs: dog (A) showed immobility, generalized tremors, licking, and left‐sided forced head turn (versive head movement) during ictal event; dog (B) also showed immobility, generalized tremors, and difficulty in walking or moving; and dog (C) showed difficulty in walking or moving and licking. It was difficult to evaluate the awareness of dog (A). Dog (A) did not show mydriasis while the eyes were being captured. The owners of dogs reported that awareness was not impaired during the ictal phase, respectively (B, C).
ACKNOWLEDGMENT
This study was partially supported by The Science Research Promotion Fund from Promotion and Mutual Aid Corporation for Private School of Japan (PMAC). The authors thank the gene bank (Biobank, Azabu University, Kanagawa, Japan), which was a MEXT‐Supported Program for the Strategic Research Foundation at Private Universities (2011‐2015), for providing the DNA samples of Pomeranians with or without epilepsy (ie, cases or controls) for the preliminary genetic analysis, some of which were also included and analyzed in the current descriptive study. The authors also thank Dr Naoaki Matsuki and other colleagues for their help in collecting clinical data as well as the dog owners for provided videos.
Yu Y, Hasegawa D, Kanazono S, Saito M. Clinical characterization of epileptic seizures in Pomeranians with idiopathic epilepsy or epilepsy of unknown cause. J Vet Intern Med. 2022;36(6):2113‐2122. doi: 10.1111/jvim.16578
Funding information The Science Research Promotion Fund from the Promotion and Mutual Aid Corporation for Private School of Japan (PMAC), Grant/Award Number: 2022‐16
REFERENCES
- 1. Berendt M, Farquhar RG, Mandigers PJJ, et al. International Veterinary Epilepsy Task Force consensus report on epilepsy definition, classification and terminology in companion animals. BMC Vet Res. 2015;11:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hülsmeyer VI, Fischer A, Mandigers PJJ, et al. International veterinary epilepsy task force's current understanding of idiopathic epilepsy of genetic or suspected genetic origin in purebred dogs. BMC Vet Res. 2015;11:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. De Risio L, Bhatti S, Muñana K, et al. International Veterinary Epilepsy Task Force consensus proposal: diagnostic approach to epilepsy in dogs. BMC Vet Res. 2015;11:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chandler K. Canine epilepsy: what can we learn from human seizure disorders? Vet J. 2006;172:207‐217. [DOI] [PubMed] [Google Scholar]
- 5. Jokinen TS, Metsähonkala L, Bergamasco L, et al. Benign familial juvenile epilepsy in Lagotto Romagnolo dogs. J Vet Intern Med. 2007;21:464‐471. [DOI] [PubMed] [Google Scholar]
- 6. Seppälä EH, Jokinen TS, Fukata M, et al. LGI2 truncation causes a remitting focal epilepsy in dogs. PLoS Genet. 2011;7:e1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stassen QEM, Grinwis GCM, van Rhijn NC, Beukers M, Verhoeven‐Duif NM, Leegwater PAJ. Focal epilepsy with fear‐related behavior as primary presentation in Boerboel dogs. J Vet Intern Med. 2019;33:694‐700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lequarré AS, Andersson L, André C, et al. LUPA: a European initiative taking advantage of the canine genome architecture for unravelling complex disorders in both human and dogs. Vet J. 2011;189:155‐159. [DOI] [PubMed] [Google Scholar]
- 9. Koskinen LLE, Seppälä EH, Weissl J, et al. ADAM23 is a common risk gene for canine idiopathic epilepsy. BMC Genet. 2017;18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koskinen LLE, Seppälä EH, Belanger JM, et al. Identification of a common risk haplotype for canine idiopathic epilepsy in the ADAM23 gene. BMC Genomics. 2015;16:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rusbridge C, Long S, Jovanovik J, et al. International veterinary epilepsy task force recommendations for a veterinary epilepsy‐specific MRI protocol. BMC Vet Res. 2015;11:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Driver CJ, Chandler K, Walmsley G, Shihab N, Volk HA. The association between Chiari‐like malformation, ventriculomegaly and seizures in cavalier King Charles spaniels. Vet J. 2013;195:235‐237. [DOI] [PubMed] [Google Scholar]
- 13. Watson F, Coppi AA, Volk HA, Packer RMA, Tauro A, Rusbridge C. Comparison of volume of the forebrain, subarachnoid space and lateral ventricles between dogs with idiopathic epilepsy and controls using a stereological approach: Cavalieri's principle. Canine Med Genet. 2021;8:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laubner S, Ondreka N, Failing K, Kramer M, Schmidt MJ. Magnetic resonance imaging signs of high intraventricular pressure ‐ comparison of findings in dogs with clinically relevant internal hydrocephalus and asymptomatic dogs with ventriculomegaly. BMC Vet Res. 2015;11:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Farke D, Kolecka M, Czerwik A, et al. Prevalence of seizures in dogs and cats with idiopathic internal hydrocephalus and seizure prevalence after implantation of a ventriculo‐peritoneal shunt. J Vet Intern Med. 2020;34:1986‐1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Volk HA. Fit, collapse or strange episodes. In: Maddison JE, Volk HA, Church DB, eds. Clinical Reasoning in Small Animal Practice. West Sussex, UK: John Wiley & Sons; 2015. [Google Scholar]
- 17. Potschka H, Fischer A, Löscher W, et al. International Veterinary Epilepsy Task Force consensus proposal: outcome of therapeutic interventions in canine and feline epilepsy. BMC Vet Res. 2015;11:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hasegawa D. Diagnostic techniques to detect the epileptogenic zone: pathophysiological and presurgical analysis of epilepsy in dogs and cats. Vet J. 2016;215:64‐75. [DOI] [PubMed] [Google Scholar]
- 19. Utsugi S, Saito M, Sato T, Kunimi M. Relationship between interictal epileptiform discharges under medetomidine sedation and clinical seizures in canine idiopathic epilepsy. Vet Rec. 2020;187:67. [DOI] [PubMed] [Google Scholar]
- 20. Yu Y, Hasegawa D, Chambers JK, et al. Magnetic resonance imaging and histopathologic findings from a standard poodle with neonatal encephalopathy with seizures. Front Vet Sci. 2020;7:578936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. R Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. https://www.R-project.org/; 2021. [Google Scholar]
- 22. Berendt M, Gredal H, Pedersen LG, Alban L, Alving J. A cross‐sectional study of epilepsy in Danish Labrador retrievers: prevalence and selected risk factors. J Vet Intern Med. 2002;16:262‐268. [DOI] [PubMed] [Google Scholar]
- 23. Berendt M, Gulløv CH, Christensen SLK, et al. Prevalence and characteristics of epilepsy in the Belgian shepherd variants Groenendael and Tervueren born in Denmark 1995–2004. Acta Vet Scand. 2008;50:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Berendt M, Gulløv CH, Fredholm M. Focal epilepsy in the Belgian shepherd: evidence for simple Mendelian inheritance. J Small Anim Pract. 2009;50:655‐661. [DOI] [PubMed] [Google Scholar]
- 25. Patterson EE, Mickelson JR, Da Y, et al. Clinical characteristics and inheritance of idiopathic epilepsy in Vizslas. J Vet Intern Med. 2003;17:319‐325. [DOI] [PubMed] [Google Scholar]
- 26. Licht BG, Lin S, Luo Y, et al. Clinical characteristics and mode of inheritance of familial focal seizures in Standard Poodles. J Am Vet Med Assoc. 2007;231:1520‐1528. [DOI] [PubMed] [Google Scholar]
- 27. Hülsmeyer V, Zimmermann R, Brauer C, et al. Epilepsy in Border Collies: clinical manifestation, outcome, and mode of inheritance. J Vet Intern Med. 2010;24:171‐178. [DOI] [PubMed] [Google Scholar]
- 28. Gulløv CH, Toft N, Baadsager MMN, Berendt M. Epilepsy in the Petit Basset Griffon Vendeen: prevalence, semiology, and clinical phenotype. J Vet Intern Med. 2011;25:1372‐1378. [DOI] [PubMed] [Google Scholar]
- 29. Ergene E, Shih JJ, Blum DE, So NK. Frequency of Bitemporal independent interictal epileptiform discharges in temporal lobe epilepsy. Epilepsia. 2000;41:213‐218. [DOI] [PubMed] [Google Scholar]
- 30. Asadi‐Pooya AA, Farazdaghi M, Shahpari M. Clinical significance of bilateral epileptiform discharges in temporal lobe epilepsy. Acta Neurol Scand. 2021;143:608‐613. [DOI] [PubMed] [Google Scholar]
- 31. Gollwitzer S, Scott CA, Farrell F, et al. The long‐term course of temporal lobe epilepsy: from unilateral to bilateral interictal epileptiform discharges in repeated video‐EEG monitorings. Epilepsy Behav. 2017;68:17‐21. [DOI] [PubMed] [Google Scholar]
- 32. Shen Y, Gong Y, Ruan Y, Chen Z, Xu C. Secondary epileptogenesis: common to see, but possible to treat? Front Neurol. 2021;12:747372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kotagal P, Luders H, Morris HH, et al. Dystonic posturing in complex partial seizures of temporal lobe onset: a new lateralizing sign. Neurology. 1989;39:196‐201. [DOI] [PubMed] [Google Scholar]
- 34. Bleasel A, Kotagal P, Kankirawatana P, Rybicki L. Lateralizing value and semiology of ictal limb posturing and version in temporal lobe and extratemporal epilepsy. Epilepsia. 1997;38:168‐174. [DOI] [PubMed] [Google Scholar]
- 35. Bonelli SB, Lurger S, Zimprich F, Stogmann E, Assem‐Hilger E, Baumgartner C. Clinical seizure lateralization in frontal lobe epilepsy. Epilepsia. 2007;48:517‐523. [DOI] [PubMed] [Google Scholar]
- 36. Newton MR, Berkovic SF, Austin MC, Reutens DC, McKay WJ, Bladin PF. Dystonia, clinical lateralization, and regional blood flow changes in temporal lobe seizures. Neurology. 1992;42:371‐377. [DOI] [PubMed] [Google Scholar]
- 37. Joo EY, Hong SB, Lee EK, et al. Regional cerebral hyperperfusion with ictal dystonic posturing: ictal‐Interictal SPECT subtraction. Epilepsia. 2004;45:686‐689. [DOI] [PubMed] [Google Scholar]
- 38. Mizobuchi M, Matsuda K, Inoue Y, et al. Dystonic posturing associated with putaminal hyperperfusion depicted on subtraction SPECT. Epilepsia. 2004;45:948‐953. [DOI] [PubMed] [Google Scholar]
- 39. Dupont S, Semah F, Baulac M, Samson Y. The underlying pathophysiology of ictal dystonia in temporal lobe epilepsy: an FDG‐PET study. Neurology. 1998;51:1289‐1292. [DOI] [PubMed] [Google Scholar]
- 40. Rusu V, Chassoux F, Landre E, et al. Dystonic posturing in seizures of mesial temporal origin: electroclinical and metabolic patterns. Neurology. 2005;65:1612‐1619. [DOI] [PubMed] [Google Scholar]
- 41. Cerda‐Gonzalez S, Packer RA, Garosi L, et al. International veterinary canine dyskinesia task force ecvn consensus statement: terminology and classification. J Vet Intern Med. 2021;35:1218‐1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richter A, Hamann M, Wissel J, et al. Dystonia and paroxysmal dyskinesias: under‐recognized movement disorders in domestic animals? A comparison with human dystonia/paroxysmal dyskinesias. Front Vet Sci. 2015;2:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Packer RA, Patterson EE, Taylor JF, Coates JR, Schnabel RD, O'Brien DP. Characterization and mode of inheritance of a paroxysmal dyskinesia in Chinook dogs: paroxysmal dyskinesia. J Vet Intern Med. 2010;24:1305‐1313. [DOI] [PubMed] [Google Scholar]
- 44. Black V, Garosi L, Lowrie M, Harvey RJ, Gale J. Phenotypic characterisation of canine epileptoid cramping syndrome in the Border Terrier. J Small Anim Pract. 2014;55:102‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. De Risio L, Forman OP, Mellersh CS, et al. Paroxysmal dyskinesia in Norwich Terrier dogs. Mov Disord Clin Pract. 2016;3:573‐579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lowrie M, Garosi L. Natural history of canine paroxysmal movement disorders in Labrador retrievers and Jack Russell terriers. Vet J. 2016;213:33‐37. [DOI] [PubMed] [Google Scholar]
- 47. Polidoro D, Van Ham L, Santens P, et al. Phenotypic characterization of paroxysmal dyskinesia in Maltese dogs. J Vet Intern Med. 2020;34:1541‐1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Packer RA, Wachowiak I, Thomovsky SA, Berg J, Vasquez L, O'Brien DP. Phenotypic characterization of PIGN‐associated paroxysmal dyskinesia in soft‐coated wheaten terriers and preliminary response to acetazolamide therapy. Vet J. 2021;269:105606. [DOI] [PubMed] [Google Scholar]
- 49. Whittaker DE, Volk HA, De Decker S, et al. Clinical characterisation of a novel paroxysmal dyskinesia in Welsh terrier dogs. Vet J. 2022;281:105801. [DOI] [PubMed] [Google Scholar]
- 50. Packer RMA, Berendt M, Bhatti S, et al. Inter‐observer agreement of canine and feline paroxysmal event semiology and classification by veterinary neurology specialists and non‐specialists. BMC Vet Res. 2015;11:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lowrie M, Garosi L. Classification of involuntary movements in dogs: paroxysmal dyskinesias. Vet J. 2017;220:65‐71. [DOI] [PubMed] [Google Scholar]
- 52. YouTube . https://www.youtube.com/. Accessed December 13, 2021.
- 53. Lamb CR, Croson PJ, Cappello R, Cherubini GB. Magnetic resonance imaging findings in 25 dogs with inflammatory cerebrospinal fluid. Vet Radiol Ultrasound. 2005;46:17‐22. [DOI] [PubMed] [Google Scholar]
- 54. Torisu S, Washizu M, Hasegawa D, et al. Brain magnetic resonance imaging characteristics in dogs and cats with congenital portosystemic shunts. Vet Radiol Ultrasound. 2005;46:447‐451. [DOI] [PubMed] [Google Scholar]
- 55. Wrzosek M, Płonek M, Nicpoń J. Electroencephalographic features of metabolic encephalopathy in dogs. Med Weter. 2015;71:100‐103. [Google Scholar]
- 56. Lavely JA. Pediatric seizure disorders in dogs and cats. Vet Clin North Am Small Anim Pract. 2014;44:275‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Estey CM. Congenital hydrocephalus. Vet Clin North Am Small Anim Pract. 2016;46:217‐229. [DOI] [PubMed] [Google Scholar]
- 58. Schmidt MJ, Hartmann A, Farke D, Failling K, Kolecka M. Association between improvement of clinical signs and decrease of ventricular volume after ventriculoperitoneal shunting in dogs with internal hydrocephalus. J Vet Intern Med. 2019;33:1368‐1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Detailed information of electroencephalography examination.
Table S1. Primer sequences and detailed PCR condition.
Table S2. Clinical data of epilepsy of unknown cause cases whose age at onset was <6 months old or >6 years old.
Table S3. Comparison of detailed seizure characteristics and electroencephalographic data.
Table S4. Details of Pomeranians included for the ADAM23 haplotype analysis and results.
Video S1: Focal seizures with limb contraction in Pomeranian dogs. In addition to abnormal contraction of a left thoracic limb, 3 dogs presented with the following clinical signs: dog (A) showed immobility, generalized tremors, licking, and left‐sided forced head turn (versive head movement) during ictal event; dog (B) also showed immobility, generalized tremors, and difficulty in walking or moving; and dog (C) showed difficulty in walking or moving and licking. It was difficult to evaluate the awareness of dog (A). Dog (A) did not show mydriasis while the eyes were being captured. The owners of dogs reported that awareness was not impaired during the ictal phase, respectively (B, C).


