Abstract

Single diastereomers of 4-hetaryl-2-pyrrolidone-3(5)-carbo- and 2-[4-hetaryl-2-pyrrolidon-1-yl]acetohydrazides were used in reactions with 2,4-pentanedione, providing (3R*,4S*)-3-, (4R*,5R*)-5-(3,5-dimethyl-1H-pyrazole-1-carbonyl)- and 1-[2-(3,5-dimethyl-1H-pyrazol-1-yl)-2-oxoethyl]-4-hetaryl-2-pyrrolidones. The structures of the synthesized compounds were confirmed by spectral methods and X-ray structural analysis. Some of the obtained compounds were shown to possess nootropic and anxiolytic activity.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10593-022-03140-4.
Keywords: carbohydrazides; 3,5-dimethylpyrazole; hydrazides; 2-pyrrolidone; biological activity; racetams; X-ray structural analysis
γ-Lactams and pyrazoles represent nitrogen-containing heterocyclic compounds that form the key pharmacophoric motifs in a significant number of natural and synthetic compounds characterized by a broad spectrum of biological activity.1–6 For example, the active pharmaceutical ingredients of many synthetic nootropic drugs known as racetams (piracetam, Phenotropil, levetiracetam, rolipram, and others) and detoxifiers or enterosorbents (polyvinyl-pyrrolidone, Hemodez, Enterodez) contain the structural units of 2-pyrrolidone1,7–10 (Fig. 1). Pyrrolidone ring forms the structural basis of several effective investigational drug molecules (PF-07321332 (nirmatrelvir), GC376, AG-7088 (rupinavir, Rupintrivir)) applied for the treatment and prevention of COVID-19 coronavirus infection11–13 (Fig. 1). The examples of currently approved synthetic drugs containing pyrazole rings include the anti-inflammatory drug celecoxib, the weight loss medication rimonabant, as well as fomepizole (an antidote against toxic alcohols) and others4,6,7 (Fig. 1).
Figure 1.

The structures of some active pharmaceutical ingredients containing lactam and pyrazole rings.
Thus, functionalized 2-pyrrolidones containing in their molecules hetaryl substituents along with two pharmacophoric moieties – lactam and pyrazole heterocycles can be considered as key structures for targeted synthesis of pharmacological agents with various types of activity.
The synthesis of such substituted 2-pyrrolidones has been described only in a very limited number of literature sources: 1-hetaryl-4-(pyrazol-1-ylcarbonyl)-2-pyrrolidones were obtained in good yields by cyclocondensation of 1-hetaryl-2-oxopyrrolidine-4-carbohydrazides with 2,4-pentanedione (acac) in i-PrOH medium in the presence of a catalytic amount of hydrochloric acid14–19 (Scheme 1).
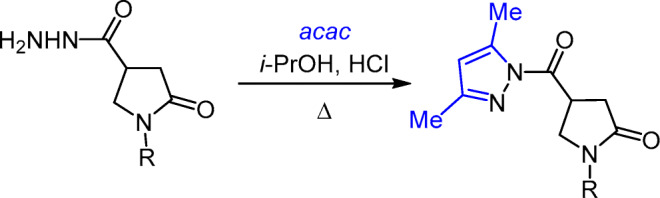
Scheme 1
For these reasons, we were interested in studying the reactions of diastereomerically pure (3R*,4S*)-4-hetaryl-2-oxopyrrolidine-3-carbohydrazides 1a–e, (4R*,5R*)-4-hetaryl-2-oxopyrrolidine-5-carbohydrazides 2a–g, and 2-(4-aryl-2-oxopyrrolidin-1-yl)acetohydrazides 3a–d with 2,4-pentanedione. The starting materials 1a–e, 2a–g, and 3a–d were synthesized according to our previously developed procedures.20–22
The successful completion of these reactions was found to substantially depend on the catalyst that was used. For example, the interaction of hydrazides 1a–с with 2,4-pentanedione under the conditions described in earlier publications14–19 (catalyst hydrochloric acid, solvent i-PrOH) was accompanied by rapid hydrolysis of compounds 1а–с, followed by esterification of the intermediate pyrrolidone carboxylic acids and resulting in the isolation of the respective isopropyl esters 4a–c (Scheme 2).
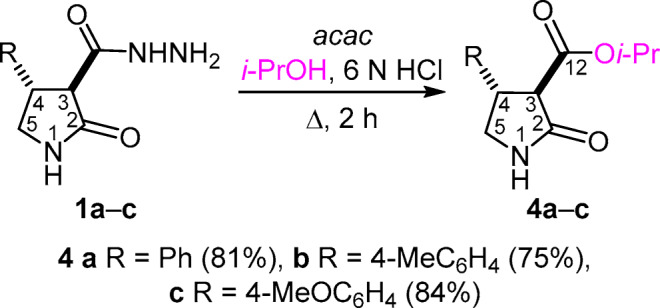
Scheme 2
The use of p-toluenesulfonic acid (TsOH) as catalyst allowed to successfully accomplish the reactions of hydrazides 1a–e, 2a–g, 3a–d with 2,4-pentanedione under identical conditions: catalyst TsOH and heating at reflux in MeOH for 30 min. The target 2-pyrrolidone pyrazole-carbonyl derivatives 5a–e, 6a–g, 7a–d were obtained in good yields (61–84%) (Schemes 3–5).
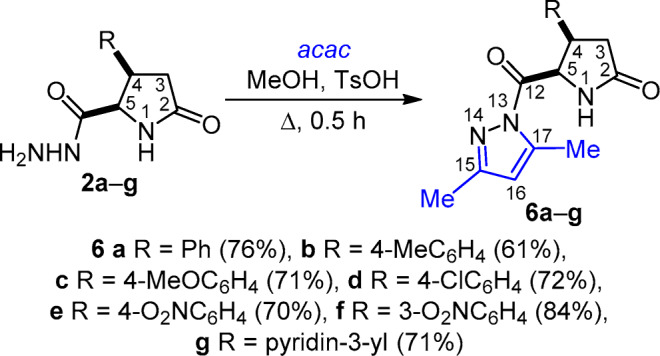
Scheme 4
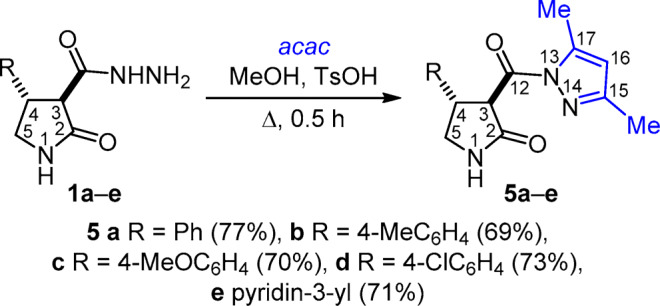
Scheme 3
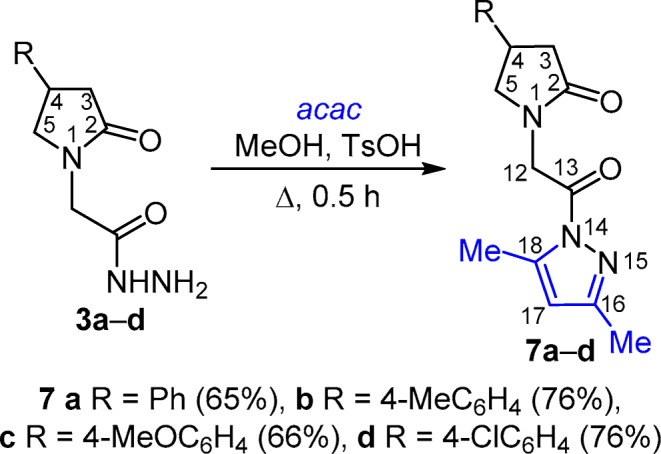
Scheme 5
Compounds 5a–e, 6a–g, and 7a–d were isolated as stable colorless crystals with clearly identifiable melting points. Their structures were confirmed by physicochemical methods of analysis (IR spectroscopy, one-dimensional 1H, 13С NMR spectroscopy, two-dimensional 1H–13C HMQC, 1H–13C HMBC experiments, as well as by X-ray structural analysis. IR spectra of all compounds 5a–e, 6a–g, and 7a–d had similar features and were in good agreement between themselves, containing strong broadened absorption bands of carbonyl groups (at the ranges of 1727–1719 and 1700–1685 cm–1), while IR spectra of compounds 5a–e, 6a–g showed the absorption bands of amide NH groups (at the ranges of 3219–3193 and 3114–3080 cm–1).
1Н and 13C NMR spectra of compounds 5a–e, 6a–g featured one set of proton and 13C signals for all structural units, pointing to the presence of only one diastereomer. For example, in 1Н NMR spectra of compounds 5a, 6a there were the methine proton signals of chiral centers: a 3-СН proton doublet at 5.17 ppm (3J3-4 = 11.1 Hz), a 4-СН proton multiplet at 4.01–4.11 ppm (compound 5a), split doublets of 5-СН proton at 5.71 ppm (3J4-5 = 8.4 Hz) and 4-СН proton at 4.07 ppm (J = 8.7 Hz, compound 6a). The downfield region in 1Н NMR spectra of compounds 5a–e, 6a–g showed characteristic singlet signals of pyrrolidine ring 1-NH protons at 8.17–8.23 ppm (compounds 5a–e) and 8.00–8.14 ppm (compounds 6a–g).
Taking into account the fact that the chiral centers in the diastereomerically pure carbohydrazides 1a–e and 2a–g were not affected during their reactions with 2,4-pentanedione, the obtained compounds 5a–e had (3R*,4S*) configuration, while compounds 6a–g had (4R*,5R*) configuration. Indeed, the spin-spin coupling constants of 3,4-CH methine protons (3J3-4 = 10.7–11.2 Hz) in 1Н NMR spectra of compounds 5a–e and 4,5-CH methine protons (3J4-5 = 8.2–8.7 Hz) in the spectra of compounds 6a–g indicated their trans and cis orientation relative to the lactam ring plane. This orientation corresponded to (3R*,4S*) and (4R*,5R*) configurations of the chiral centers and was in good agreement with the spin-spin coupling соnstants established by us for the starting carbohydrazides 1a–e, 2a–g,20–22 as well as the literature data available for trans- and cis-isomers of similar molecules.23–25
The formation of pyrazole ring was confirmed by the presence of characteristic =CH proton quartet signals in 1Н NMR spectra of compounds 5a–e, 6a–g, and 7a–d in the region of 5.88–6.20 ppm. These quartets showed a long range spin-spin coupling (4J = 0.5–0.7 Hz) to the pyrazole ring methyl group protons manifested as a doublet in the region of 1.98–2.44 ppm. The methylene and methine proton signals were assigned to the pyrrolidone and pyrazole rings of compounds 5a–e, 6a–g, and 7a–d according to the results of 1H–13C HMQC experiment.
The assignment of C-2,12,13 carbon signals belonging to C=O groups, as well as the 3,4,5-СH proton signals of pyrrolidone ring and =CH proton signals of pyrazole ring in the molecules of compounds 5a–e, 6a–g, and 7a–d was performed on the basis of 1Н–13С HMBC data. For example, in the spectrum of compound 5a, the signal assignment relied on the correlations between 1-NH proton and С-2 carbon atom (8.19/172.1 ppm), 3-CH proton and C-12 carbon atom (5.17/170.8 ppm), as well as other spectral features (Fig. 2). Analogously, 1H–13С HMВC spectrum of compound 7a was interpreted on the basis of cross peaks between the 3-СH2 protons and С-2 carbon atom (2.42 ppm, 2.73/174.3 ppm), as well as the 12-СH2 protons and С-13 carbon atom (4.74 ppm, 4.79/168.7 ppm) (Fig. 2).
Figure 2.
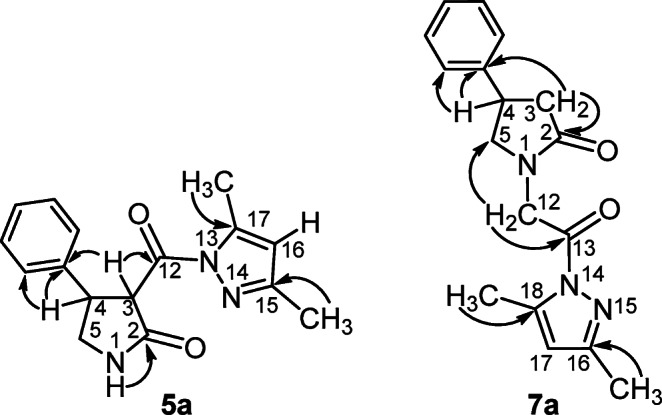
The main (reference) correlations in 1Н–13С HMBC spectra of compounds 5a and 7a.
The most downfield signals in 13C NMR spectra of compounds 5a–e, 6a–g, and 7a–d were assigned to the carbonyl carbon atoms of lactam groups (172–177 ppm) and the side chain (169–171 ppm).
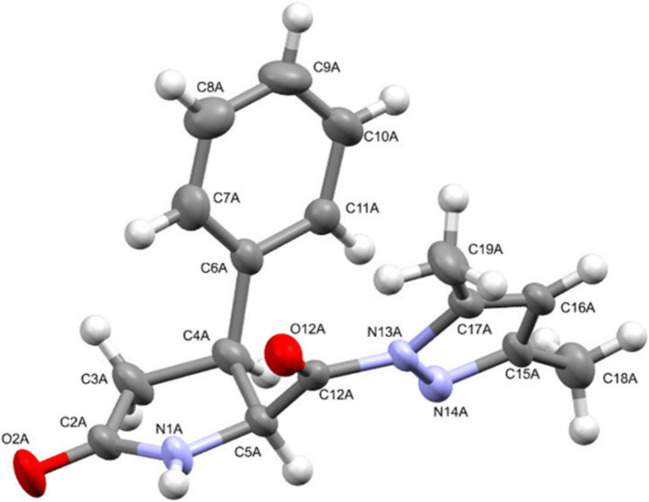
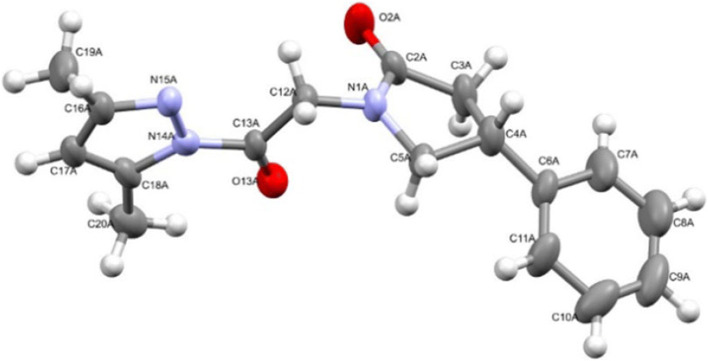
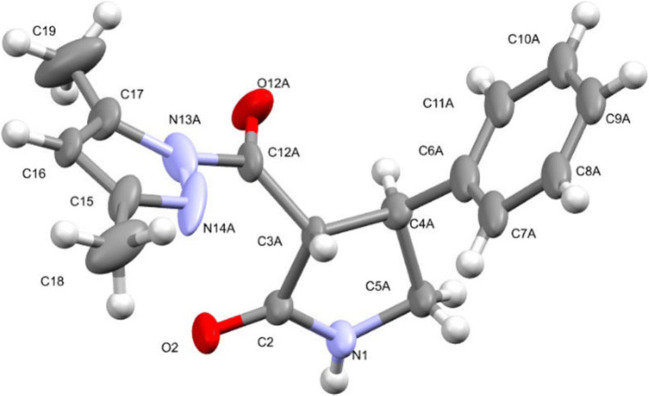
The spatial structure of the obtained compounds 5a–e, 6a–g, and 7a–d was examined by X-ray sructural analysis in the case of compounds 5–7 a (Figs. 3–7). According to the results of X-ray structural analysis, compounds 5–7 a crystallized in the form of racemates in centrosymmetric triclinic crystals.
Figure 4.
The geometry of independent molecule А of compound 6a in the crystal structure. The anisotropic displacement ellipsoids are shown at the 50% probability level.
Figure 5.
The geometry of independent molecule А of compound 7a in the crystal structure. The anisotropic displacement ellipsoids are shown at the 50% probability level.
Figure 3.
The molecular geometry of compound 5a in crystal (the predominant positions of disordered atoms are denoted with letter A). The anisotropic displacement ellipsoids are shown at the 50% probability level.
Figure 7.
A fragment of crystal packing observed for compound 7a. Projection along the а axis.
The crystal structure of compound 5a contained a single molecule in the independent part of the unit cell, in which the pyrrolidone ring together with substituents at the С(3) and С(4) atoms were significantly disordered between two positions. The disordered atoms have been denoted with letters А and В. The relative configuration of the two chiral centers C(3) and С(4) in compound 5a was R* and S* (molecule А) or S* and R* (molecule В), respectively. Thus, the crystal of compound 5a can hold both enantiomers (RS/SR) or pair of diastereomers at the same position.
In the crystal structures of compounds 6a and 7a, the independent part of the unit cell contained two independent molecules of enantiomers А and В. For compound 6a, the chiral atoms С(4А) and С(5А) had S configuration, while C(4B) and C(5B) had R configuration. In the case of compound 7a, the chiral С(4А) atoms had R configuration and С(4В) had S configuration.
The lactam rings in molecules 5–7 a had the same С(4)-envelope conformation with the С(4) atom deviating from the common plane defined by the С(3)–С(2)–N(1)–C(5) atom chain. At the same time, in the disordered molecules А and В of compound 5а, the С(4) atom deviated from the С(3)–С(2)–N(1)–C(5) plane in different directions by approximately equal distances: by 0.21 Å in molecule A and by 0.28 Å in molecule B.
The phenyl and carbonylpyrazole substituents at the C(3) and С(4) chiral atoms of compound 5a assumed equatorial positions and had an anticlinal conformation relative to each other (torsion angle С(6)–С(4)–С(3)–С(12) –99(1)°), while in the molecules of compound 6a the substituents at the C(4) and C(5) atoms were in axial and equatorial positions, respectively, and assumed an eclipsed conformation (the torsion angle С(6)–С(4)–С(5)–С(12) was equal to –10.0(4)° and –24.1(4)° in the molecules of compounds А and В, respectively). In the molecules of compound 7a, the substituents at the С(4) and N(1) atoms occupied equatorial positions.
The molecules of compounds 5а, 6а in crystalline state formed centrosymmetric dimers linked by N–H···O hydrogen bonds. There were ordinary van der Waals contacts between the dimers (Fig. 6). In the crystal of compound 7а, С–Н···О type bonds were observed, mediated by dispersion interactions (Fig. 7).
Figure 6.
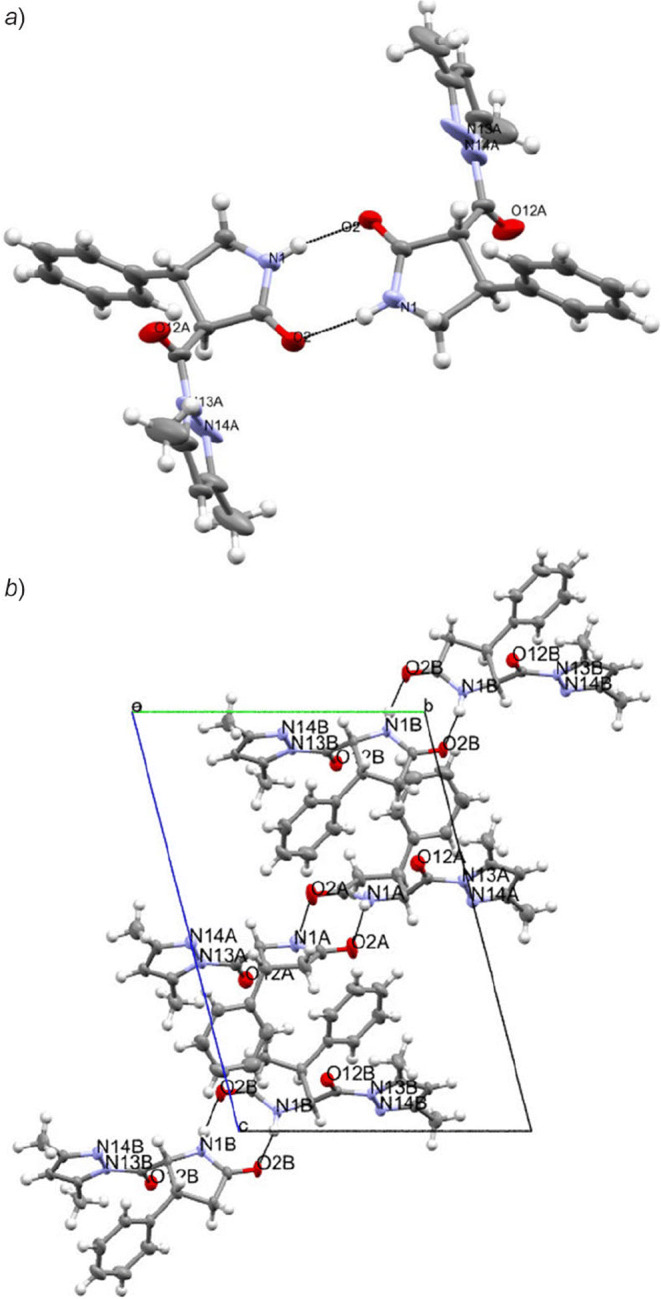
The hydrogen bond network in the crystals of compounds а) 5a and b) 6a. The formation of centrosymmetric dimers via N–H···O hydrogen bonds was demonstrated in these crystals.
Compounds 5a,c, 6a,b,d, 7a,c representing each group of the obtained products were tested as potentially biologically active compounds, anticipated to have psychotropic properties. Standard pharmacological testing methods were used, which were appropriate for the given type of activity: “open field” (OF), “elevated plus maze” (EPM), “conditioned passive avoidance reflex” (CPAr), “extrapolation avoidance” (EA), and Vogel conflict test (VCT).26 The experiments were performed with male Wistar rats in accordance with the animal testing regulations set by the legislation of the Russian Federation and the EASC technical standards for Good Laboratory Practice (GOST R 53434-2009 and GOST R 51000.4-2011).
Compounds 5a,c, 6a,b,d, 7a,c were injected into the animals intraperitoneally (IP, physiological saline) in equimolar amounts as a single dose equal to 1/10 of the molecular mass, 30 min prior to the test. The acute toxicity level (LD50, mg/kg) of compounds 5a,c, 6a,b,d, and 7a,c was evaluated from the survival rate of white mice and exceeded 2000 mg/kg, allowing to recognize them as relatively harmless materials, belonging to the IV class of toxicity. The selected reference drug was phenibut (20 mg/kg, IP), which possesses anxiolytic and nootropic activity and is prescribed for the treatment of anxiety, cognitive, and asthenic disorders.7
Statistical processing of the test results was performed using the Prism 6 program according to Shapiro–Wilk, Kruskal–Wallis, and Dunn’s criteria.
The OF test characterizes the locomotor activity (by the number of traversed squares) and orienting-exploratory activity of the animals (by the sum of rearing and head-dipping acts). As shown in Table 1, compounds 5a,c, 6a,b,d, 7a,c did not produce a significant effect on the motor activity of animals in this test, pointing to the absence of psychostimulant or sedative activity associated with these compounds. It should be noted that, after the injection of compound 7c (32 mg/kg), the animals of experiment group showed a statistically significant increase in rearing and head-dipping behaviors, as well as the number of visits to the brightly lit central area (aversive for nocturnal rodents), pointing to the prevalence of orienting-exploratory activity by the animals over the aversion toward their environment (Table 1). Thus, compound 7c in the OF test showed anxiolytic activity, comparable to that of phenibut.
Table 1.
The effect of compounds 5a,c, 6a,b,d, 7a,c on animal behavior in OF, EPM, VCT, CPAR, and EA tests (M ± m)
| Compound | OF | EPM | VCT | CPAR | EA | ||
|---|---|---|---|---|---|---|---|
| MA* | EB** | OA*** – frequency of entry | OA – residence time, s | Number of punished approaches | Residence time in closed arm, s. | Problem solving time, s. | |
| Control | 44.0 ± 3.2 | 13.3 ± 0.9 | 1.2 ± 0.4 | 12.4 ± 1.8 | 3.5 ± 0.7 | 126.4 ± 13.4 | 6.8 ± 1.7 |
| 5a | 46.0 ± 3.5 | 14.0 ± 2.1 | 2.0 ± 0.5 | 24.0 ± 5.2 | 180.0 ± 0.0*4 | 2.5 ± 0.5*4 | |
| 5c | 35.0 ± 10.0 | 17.0 ± 10.0 | 1.5 ± 0.5 | 15.2 ± 9.4 | 180.0 ± 0.0*4 | 2.0 ± 1.0*4 | |
| 6a | 41.0 ± 0.9 | 13.3 ± 1.0 | 1.6 ± 0.5 | 8.9 ± 1.2 | 137.5 ± 24.0 | 4.5 ± 1.0 | |
| 6b | 35.6 ± 2.6 | 11.0 ± 0.6 | 2.6 ± 0.4*4 | 31.9 ± 3.3*4 | 7.3 ± 0.6*4 | 145.8 ± 22.6 | 4.3 ± 0.7 |
| 6d | 38.1 ± 2.3 | 13.0 ± 0.9 | 2.1 ± 0.6 | 22.0 ± 3.0 | 140.9 ± 25.8 | 4.3 ± 0.7 | |
| 7a | 42.5 ± 9.5 | 14.5 ± 2.5 | 1.5 ± 0.5 | 10.0 ± 6.7 | 103.8 ± 24.7 | 3.5 ± 1.3 | |
| 7c | 48.5 ± 12.5 | 19.0 ± 3.5*4 | 3.0 ± 0.5*4 | 49.0 ± 9.0*4 | 5.5 ± 1.5 | 180.0 ± 0.0*4 | 3.8 ± 1.8 |
| Phenibut | 41.6 ± 4.0 | 19.0 ± 2.8*4 | 3.0 ± 0.9*4 | 45.4 ± 14.5*4 | 9.3 ± 1.2*4 | 180.0 ± 0.0*4 | 2.6 ± 0.4*4 |
* MA – motor activity (number of squares traversed in the experimental apparatus).
** EB – exploratory behavior (total number rearing and head-dipping acts).
*** OA – the open arm of the EPM apparatus.
*4 The differences were statistically significant compared to the control group, at p < 0.05.
The EPM test was used to evaluate the anxiety level in animals by recording the total time spent, time spent in the open arm, and the number of excursions into the open arm. After a single injection of compounds 5a,c, 6a,b,d, 7a,c, only compounds 6b and 7c statistically significantly increased the number and duration of excursions into the open arms of the apparatus, amount of rearing and head-dipping behaviors in the open arms among the experiment group compared to the control group of animals. This indicated a reduction in the level of anxiety (Table 1). The anxiolytic effect of compound 7c surpassed the activity of compound 6b and was comparable to that of phenibut.
The activity of the most promising compounds 6b, 7c was also studied according to the Vogel conflict test, which is a highly specific test for evaluating the anxiolytic action of new compounds. It was established from this test that, after the injection of compounds 6b, 7c, the number of animals in the experimental group punished by withdrawal of water was statistically significantly higher compared to the control group (Table 1). Remarkably, the anxiolytic activity of compound 6b exceeded that of compound 7c, the activity of which in the EPM test was higher than in the case of compound 6b and comparable to the activity of phenibut.
Within the framework of studying compounds 5a,c, 6a,b,d, and 7a,c with regard to their psychotropic activity, their effects on the learning and memory (formation and retrieval of memory trace) were also characterized using animals in CPAR and EA tests. During the CPAR test, the animals treated with compounds 5a,c, 7c prior to the training retained the memory trace and did not enter the closed arm with electrode floor at 7 days after the training (Table 1). Along with that, the animals treated with compounds 5a,c solved the extrapolation task significantly faster during the EA test compared to the control group (Table 1). Thus, according to the results of both tests, from the series of compounds 5a,c, 6a,b,d, and 7a,c we selected compounds 5a,c that improved the formation and retrieval of memory trace in experimental animals and showed nootropic effects.
In conclusion, the results of this study allowed us to develop an effective method for the preparation of previously unknown diastereomerically pure 3(5)-(3,5-dimethyl-1H-pyrazole-1-carbonyl)-4-hetaryl-2-pyrrolidones and 4-aryl-1-[2-(3,5-dimethyl-1H-pyrazol-1-yl)-2-oxoethyl]-2-pyrrolidones – new members of the racetam family containing the pharmacophoric lactam and pyrazole rings in their molecules, along with hetaryl substituents. Some of these compounds were shown to possess nootropic and anxiolytic activity. The obtained data open further possibilities for more detailed study of these compounds as promising lead compounds in the drug development targeted toward the prevention and treatment of anxiety disorders.
Experimental
IR spectra were recorded on a Shimadzu IR Prestige-21 FT-IR spectrometer for samples in KBr pellets. One-dimensional 1Н, 13С, 15N NMR spectra and two-dimensional 1H–13C HMQC and 1H–13C HMBC spectra were acquired on a Jeol ECX400A spectrometer at the working frequencies of 400 MHz (for 1H nuclei), 100 MHz (for 13С nuclei), and 40 MHz (for 15N nuclei) for samples in DMSO-d6 solutions. The residual non-deuterated solvent signals (for 1Н nuclei) or deuterated solvent signals (for 13С nuclei) were used as standards, while 15N NMR chemical shifts were recorded relative to MeNO2. Elemental analysis was performed on a EuroVector EA3000 (CHN Dual) analyzer. The reaction progress and purity of the obtained compounds were controlled by TLC on Silufol UV-254 plates using 9:1:2 i-PrOH–NH4OH–H2O mobile phase and visualization under UV light (λ = 254 nm).
The synthesis of (3R*,4S*)-4-hetaryl-2-pyrrolidone-3-carbohydrazides 1a–e, (4R*,5R*)-4-hetaryl-2-pyrrolidone-5-carbohydrazides 2a–g, and 2-[4-aryl-2-pyrrolidon-1-yl]acetohydrazides 3a–d was accomplished by following published procedures.20–22
Synthesis of isopropyl (3 R* ,4 S* )-4-aryl-2-oxopyrrolidine-3-carboxylates 4a–c (General method). A mixture of the appropriate 4-aryl-2-oxopyrrolidine-3-carbohydrazide 1а–с (5 mmol), 2,4-pentanedione (15 mmol), and 6 N HCl (0.25 ml) in i-PrOH (20 ml) was heated at reflux for 2 h. The solvent was evaporated at reduced pressure (15–20 mmHg) by 2/3 of the initial volume, and the residue was treated with H2O (4 ml). The crystalline product was filtered off, air-dried, and recrystallized from MeOH.
Isopropyl (3 R* ,4 S* )-2-oxo-4-phenylpyrrolidine-3-carboxylate (4a) was obtained from carbohydrazide 1a (1095 mg, 5 mmol). Yield 1000 mg (81%), colorless crystals, mp 124– 125°С (MeOH). IR spectrum, ν, cm–1: 1729, 1689 (С=О), 3209, 3100 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.08 (3H, d, 3J = 6.2, CH3); 1.14 (3H, d, 3J = 6.2, CH3); 3.22 (1H, t, J = 9.4, 5-CH2); 3.54 (1H, d, 3J = 11.1, 3-CH); 3.59 (1H, t, J = 8.9, 5-CH2); 3.80–3.90 (1H, m, 4-CH); 4.88 (1H, sept, 3J = 6.2, ОCH); 7.18–7.25 (1H, m, H-4 Ph); 7.26–7.33 (4H, m, H-2,3,5,6 Ph); 8.11 (1H, s, NH). 13C NMR spectrum, δ, ppm: 22.0 (CH3); 22.1 (CH3); 45.5 (C-4); 47.1 (C-5); 55.7 (C-3); 68.7 (ОCH); 127.7 (C-4 Ph); 127.8 (C-2,6 Ph); 129.2 (C-3,5 Ph); 140.3 (C-1 Ph); 169.8 (C-12); 171.9 (C-2). Found, %: С 68.07; Н 7.08; N 5.64. C14H17NO3. Calculated, %: C 68.00; H 6.93; N 5.66.
Isopropyl (3 R* ,4 S* ) - 4-(4-methylphenyl)-2-oxopyrrolidine-3-carboxylate (4b) was obtained from carbohydrazide 1b (1165 mg, 5 mmol). Yield 980 mg (75%), colorless crystals, mp 114–116°С (MeOH). IR spectrum, ν, cm–1: 1734, 1700 (С=О), 3215, 3112 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.08 (3H, d, 3J = 6.2, CH3); 1.14 (3H, d, 3J = 6.2, CH3); 2.23 (3H, s, CH3 Ph); 3.17 (1H, t, J = 9.5, 5-CH2); 3.48 (1H, d, 3J = 11.1, 3-CH); 3.51–3.58 (1H, m, 5-CH2); 3.74–3.84 (1H, m, 4-CH); 4.86 (1H, sept, 3J = 6.2, ОCH); 7.10 (2H, d, J = 8.0, H Ph); 7.17 (2H, d, J = 8.0, H Ph); 8.07 (1H, s, NH). 13C NMR spectrum, δ, ppm: 21.1 (CH3 Ph); 22.0 (CH3); 22.1 (CH3); 45.1 (C-4); 47.1 (C-5); 55.8 (C-3); 68.7 (CH); 127.6 (CН Ph); 129.7 (CН Ph); 136.8 (C Ph); 137.2 (C Ph); 169.9 (C-12); 171.9 (C-2). Found, %: C 68.72; H 7.22; N 5.38. C15H19NO3. Calculated, %: C 68.94; H 7.33; N 5.36.
Isopropyl (3 R* ,4 S* ) - 4-(4-methoxyphenyl)-2-oxopyrrolidine-3-carboxylate (4c) was obtained from carbohydrazide 1с (1245 mg, 5 mmol). Yield 1165 mg (84%), colorless crystals, mp 110–112°С (MeOH). IR spectrum, ν, cm–1: 1733, 1699 (С=О), 3215, 3110 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.08 (3H, d, 3J = 6.2, CH3); 1.14 (3H, d, 3J = 6.2, CH3); 3.17 (1H, t, J = 9.5, 5-CH2); 3.47 (1H, d, 3J = 11.1, 3-CH); 3.50–3.56 (1H, m, 5-CH2); 3.69 (3H, s, CH3O); 3.72–3.82 (1H, m, 4-CH); 4.86 (1H, sept, 3J = 6.2, ОCH); 6.85 (2H, d, J = 8.7, H-3,5 Ph); 7.21 (2H, d, J = 8.7, H-2,6 Ph); 8.06 (1H, s, NH). 13C NMR spectrum, δ, ppm: 22.0 (CH3); 22.1 (CH3); 44.9 (C-4); 47.3 (C-5); 55.6 (CH3O); 55.9 (C-3); 68.7 (CH); 114.5 (C-3,5 Ph); 128.9 (C-2,6 Ph); 132.1 (C-1 Ph); 158.9 (C-4 Ph); 169.9 (C-12); 171.9 (C-2). Found, %: C 64.81; H 6.79; N 5.38. C15H19NO4. Calculated, %: C 64.97; H 6.91; N 5.05.
Synthesis of 3(5)-(3,5-dimethyl-1 H -pyrazole-1-carbonyl)-4-hetarylpyrrolidin-2-ones 5a–e, 6a–g and aryl-1-[2-(3,5-dimethyl-1 H -pyrazol-1-yl)-2-oxoethyl]-4-pyrrolidin-2-ones 7a–d (General method). A mixture of the appropriate compound 2a–g, 3a–d (5 mmol), 2,4-pentanedione (15 mmol), and p-toluenesulfonic acid (1 mmol) in MeOH (15 ml) was heated at reflux for 30 min. The solvent was evaporated at reduced pressure (15–20 mmHg) by 2/3 of the initial volume, and the residue was treated with H2O (4 ml). The crystalline product was filtered off and air-dried.
(3 R* ,4 S* ) - 3-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)- 4-phenylpyrrolidin-2-one (5a) was obtained from carbohydrazide 1a (1095 mg, 5 mmol). Yield 1090 mg (77%), colorless crystals, mp 143–145°С (i-PrOH). IR spectrum, ν, cm–1: 1722, 1700 (С=О), 3202, 3107 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.11 (3H, s, 15-CH3); 2.42 (3H, d, 4J = 0.8, 17-CH3); 3.33 (1H, t, J = 9.4, 5-CH2); 3.66 (1H, ddd, J = 9.4, J = 8.2, J = 1.2, 5-CH2); 4.01–4.11 (1H, m, 4-CH); 5.17 (1H, d, 3J = 11.1, 3-CH); 6.19 (1H, q, 4J = 0.8, 16-CH); 7.17–7.23 (1H, m, H-4 Ph); 7.24–7.29 (2H, m, H-3,5 Ph); 7.30–7.34 (2H, m, H-2,6 Ph); 8.19 (1H, s, NH). 13C NMR spectrum, δ, ppm: 14.0 (15-CH3); 14.7 (17-CH3); 45.3 (C-4); 47.2 (C-5); 54.1 (C-3); 112.8 (C-16); 127.7 (C-4 Ph); 127.8 (C-2,6 Ph); 129.2 (C-3,5 Ph); 140.3 (C-1 Ph); 144.0 (C-17); 152.6 (C-15); 170.8 (C-12); 172.1 (C-2). Found, %: С 67.76; Н 5.97; N 14.81. C16H17N3O2. Calculated, %: C 67.83; H 6.05; N 14.83.
(3 R* ,4 S* ) - 3-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(4-methylphenyl)pyrrolidin-2-one (5b) was obtained from hydrazide 1b (1165 mg, 5 mmol). Yield 1025 mg (69%), colorless crystals, mp 141–143°С (i-PrOH). IR spectrum, ν, cm–1: 1724, 1699 (С=О), 3206, 3109 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.11 (3H, s, 15-CH3); 2.19 (3H, s, CH3 Ph); 2.41 (3H, d, 4J = 0.5, 17-CH3); 3.30 (1H, t, J = 9.4, 5-CH2); 3.62 (1H, ddd, J = 9.4, J = 8.4, J = 0.8, 5-CH2); 3.97–4.07 (1H, m, 4-CH); 5.15 (1H, d, 3J = 11.1, 3-CH); 6.17 (1H, q, 4J = 0.5, 16-CH); 7.07 (2H, d, 3J = 8.0, H Ph); 7.20 (2H, d, 3J = 8.0, H Ph); 8.19 (1H, s, NH). 13C NMR spectrum, δ, ppm: 14.0 (CH3); 14.7 (CH3); 21.1 (CH3 Ph); 45.0 (C-4); 47.3 (C-5); 54.2 (C-3); 112.8 (C-16); 127.7 (CН Ph); 129.8 (CН Ph); 136.9 (C Ph); 137.2 (C Ph); 143.9 (C-17); 152.5 (C-15); 170.8 (C-12); 172.2 (C-2). Found, %: С 68.26; Н 6.46; N 13.65. C17H19N3O2. Calculated, %: C 68.67; H 6.44; N 14.13.
(3 R *,4 S *) - 3-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(4-methoxyphenyl)pyrrolidin-2-one (5c) was obtained from hydrazide 1c (3735 mg, 15 mmol). Yield 3286 mg (70%), colorless crystals, mp 147–149°С (i-PrOH). IR spectrum, ν, cm–1: 1727, 1691 (С=О), 3209, 3108 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.11 (3H, s, 15-CH3); 2.41 (3H, d, 4J = 0.7, 17-CH3); 3.29 (1H, t, J = 9.4, 5-CH2); 3.61 (1H, ddd, J = 9.4, J = 8.2, J = 1.1, 5-CH2); 3.66 (3H, s, CH3O); 3.95–4.05 (1H, m, 4-CH); 5.13 (1H, d, 3J = 11.2, 3-CH); 6.18 (1H, q, 4J = 0.7, 16-CH); 6.83 (2H, d, 3J = 8.7 H-3,5 Ph); 7.25 (2H, d, 3J = 8.7, H-2,6 Ph); 8.17 (1H, s, NH). 13C NMR spectrum, δ, ppm: 14.0 (15-CH3); 14.7 (17-CH3); 44.7 (C-4); 47.4 (C-5); 54.4 (C-3); 55.6 (CH3O Ph); 112.8 (C-16); 114.6 (C-3,5 Ph); 128.9 (C-2,6 Ph); 132.1 (C-1 Ph); 144.0 (C-17); 152.5 (C-15); 158.9 (C-4 Ph); 170.9 (C-12); 172.2 (C-2). Found, %: C 65.07; H 6.22; N 13.33. C17H19N3O3. Calculated, %: C 65.16; H 6.11; N 13.41.
(3 R *,4 S * )- 4-(4-Chlorophenyl)-3-(3,5-dimethyl-1 H -pyrazole-1-carbonyl)pyrrolidin-2-one (5d) was obtained from hydrazide 1d (1775 mg, 7 mmol). Yield 1622 mg (73%), colorless crystals, mp 139–141°С (MeOH). IR spectrum, ν, cm–1: 1719, 1689 (С=О), 3198, 3097 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.10 (3H, s, 15-CH3); 2.41 (3H, br. s, 17-CH3); 3.31 (1H, t, J = 9.0, 5-CH2); 3.57–3.68 (1H, m, 5-CH2); 3.98–4.12 (1H, m, 4-CH); 5.14 (1H, d, 3J = 10.7, 3-CH); 6.17 (1H, br. s, 16-CH); 7.32 (2H, d, 3J = 8.2, H Ph); 7.36 (2H, d, 3J = 8.2, H Ph); 8.19 (1H, s, NH). 13C NMR spectrum, δ, ppm: 14.0 (CH3); 14.6 (CH3); 44.7 (C-4); 47.1 (C-5); 54.1 (C-3); 112.8 (C-16); 129.1 (CН Ph); 129.8 (CН Ph); 132.4 (C Ph); 139.3 (C Ph); 144.0 (C-17); 152.6 (C-15); 170.6 (C-12); 171.9 (C-2). Found, %: C 60.33; H 5.01; N 13.20. C16H16N3O2Cl. Calculated, %: C 60.47; H 5.08; N 13.22.
(3 R* ,4 S* ) - 3-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(pyridin-3-yl)pyrrolidin-2-one (5e) was obtained from hydrazide 1e (1100 mg, 5 mmol). Yield 1008 mg (71%), colorless crystals, mp 148–150°С (i-PrOH). IR spectrum, ν, cm–1: 1725, 1686 (С=О), 3219, 3091 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.10 (3H, s, 15-CH3); 2.42 (3H, d, 4J = 0.6, 17-CH3); 3.38 (1H, t, J = 9.3, 5-CH2); 3.67 (1H, ddd, J = 9.3, J = 8.3, J = 1.0, 5-CH2); 4.03–4.14 (1H, m, 4-CH); 5.21 (1H, d, 3J = 11.0, 3-CH); 6.18 (1H, q, 4J = 0.6, 16-CH); 7.33 (1H, dd, J = 7.8, J = 4.7, H-5' pyridine); 7.84 (1H, dt, J = 7.8, J = 1.9, H-4' pyridine); 8.23 (1H, s, NH); 8.42 (1H, dd, J = 4.7, J = 1.5, H-6' pyridine); 8.52 (1H, d, J = 1.9, H-2' pyridine). 13C NMR spectrum, δ, ppm: 14.0 (CH3); 14.6 (CH3); 43.0 (C-4); 46.9 (C-5); 53.9 (C-3); 112.8 (C-16); 124.3 (CН pyridine); 135.7 (CН pyridine); 135.9 (C-3' pyridine); 144.0 (C-17); 148.9 (CН pyridine); 149.3 (CН pyridine); 152.7 (C-15); 170.6 (C-12); 171.9 (C-2). Found, %: C 63.12; H 5.59; N 19.54. C15H16N4O2. Calculated, %: C 63.37; H 5.67; N 19.71.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)- 4-phenylpyrrolidin-2-one (6a) was obtained from carbohydrazide 2a (1095 mg, 5 mmol). Yield 1075 mg (76%), colorless crystals, mp 186–187°С (i-PrOH). IR spectrum, ν, cm–1: 1737, 1699 (С=О), 3211, 3111 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.98 (3H, d, 4J = 0.7, 17-CH3); 2.09 (3H, s, 15-CH3); 2.43 (1H, dd, 2J = 16.6, 3J = 6.9, 3-CH2); 2.66 (1H, dd, 2J = 16.6, 3J = 9.0, 3-CH2); 4.07 (1H, dt, J = 8.7, J = 6.9, 4-CH); 5.71 (1H, dd, J = 8.4, J = 0.5, 5-CH2); 5.92 (1H, q, 4J = 0.7, 16-CH); 6.88–6.93 (2H, m, H Ph); 7.03–7.09 (3H, m, H Ph); 8.06 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.8 (17-CH3); 13.9 (15-CH3); 36.7 (С-3); 43.5 (C-4); 60.2 (C-5); 111.6 (C-16); 127.5 (C-4 Ph); 127.9 (CН Ph); 128.2 (CН Ph); 139.2 (C-1 Ph); 143.5 (C-17); 152.4 (C-15); 171.3 (C-12); 177.0 (C-2). 15N NMR spectrum, δ, ppm: –267.9 (N-1); –147.4 (N-13); –79.6 (N-14). Found, %: С 67.82; Н 6.10; N 14.81. C16H17N3O2. Calculated, %: C 67.83; H 6.05; N 14.83.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(4-methylphenyl)pyrrolidin-2-one (6b) was obtained from hydrazide 2b (1165 mg, 5 mmol). Yield 906 mg (61%), colorless crystals, mp 172–174°С (i-PrOH). IR spectrum, ν, cm–1: 1725, 1699 (С=О), 3205, 3114 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 1.99 (3H, d, 4J = 0.5, 17-CH3); 2.11 (3H, s, 15-CH3); 2.12 (3H, s, CH3 Ph); 2.38 (1H, dd, 2J = 16.6, 3J = 6.5, 3-CH2); 2.67 (1H, dd, 2J = 16.6, 3J = 9.0, 3-CH2); 4.02 (1H, dt, J = 8.5, J = 6.5, 4-CH); 5.67 (1H, br. d, 3J = 8.2, 5-CH); 5.96 (1H, q, 4J = 0.5, 16-CH); 6.76 (2H, d, 3J = 8.0, H-2,6 Ph); 6.86 (2H, d, 3J = 8.0, H-3,5 Ph); 8.04 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.8 (17-CH3); 13.9 (15-CH3); 21.0 (CH3 Ph); 36.9 (С-3); 43.2 (C-4); 60.4 (C-5); 111.6 (C-16); 127.7 (C-2,6 Ph); 128.7 (C3,5 Ph); 136.3 (C-1 Ph); 136.5 (C-4 Ph); 143.6 (C-17); 152.4 (C-15); 171.3 (C-12); 177.0 (C-2). Found, %: С 68.52; Н 6.33; N 14.12. C17H19N3O2. Calculated, %: C 68.67; H 6.44; N 14.13.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(4-methoxyphenyl)pyrrolidin-2-one (6c) was obtained from hydrazide 2c (249 mg, 1 mmol). Yield 222 mg (71%), colorless crystals, mp 172–174°С (i-PrOH). IR spectrum, ν, cm–1: 1723, 1696 (С=О), 3208, 3106 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.02 (3H, d, 4J = 0.7, 17-CH3); 2.10 (3H, s, 15-CH3); 2.38 (1H, dd, 2J = 16.6, 3J = 6.9, 3-CH2); 2.63 (1H, dd, 2J = 16.6, 3J = 9.0, 3-CH2); 3.59 (3H, s, CH3O); 4.02 (1H, dt, J = 8.5, J = 6.9, 4-CH); 5.66 (1H, dd, J = 8.2, J = 0.5, 5-CH); 5.95 (1H, q, 4J = 0.7, 16-CH); 6.62 (2H, d, 3J = 8.7, H-3,5 Ph); 6.81 (2H, d, 3J = 8.7, H-2,6 Ph); 8.00 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.86 (17-CH3); 13.91 (15-CH3); 36.8 (С-3); 42.8 (C-4); 55.6 (CH3O Ph); 60.3 (C-5); 111.6 (C-16); 113.6 (C-3,5 Ph); 128.9 (C-2,6 Ph); 131.0 (C-1 Ph); 143.6 (C-17); 152.4 (C-15); 158.7 (C-4 Ph); 171.4 (C-12); 177.0 (C-2). Found, %: С 64.85; Н 6.06; N 13.36. C17H19N3O3. Calculated, %: C 65.16; H 6.11; N 13.41.
(4 R* ,5 R* ) - 4-(4-Chlorophenyl)-5-(3,5-dimethyl-1 H -pyrazole-1-carbonyl)pyrrolidin-2-one (6d) was obtained from hydrazide 2d (1268 mg, 5 mmol). Yield 1143 mg (72%), colorless crystals, mp 210–212°С (i-PrOH). IR spectrum, ν, cm–1: 1720, 1699 (С=О), 3201, 3094 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.04 (3H, d, 4J = 0.7, 17-CH3); 2.09 (3H, s, 15-CH3); 2.41 (1H, dd, 2J = 16.6, 3J = 7.1, 3-CH2); 2.66 (1H, dd, 2J = 16.6, 3J = 9.0, 3-CH2); 4.10 (1H, dt, J = 8.5, J = 7.1, 4-CH); 5.69 (1H, dd, J = 8.4, J = 0.5, 5-CH); 5.97 (1H, q, 4J = 0.7, 16-CH); 6.94 (2H, d, 3J = 8.5, H Ph); 7.13 (2H, d, 3J = 8.5, H Ph); 8.07 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.9 (15,17-CH3); 36.5 (С-3); 42.9 (C-4); 60.1 (C-5); 111.8 (C-16); 128.1 (CН Ph); 129.8 (CН Ph); 132.1 (C Ph); 138.2 (C Ph); 143.6 (C-17); 152.6 (C-15); 171.2 (C-12); 176.8 (C-2). Found, %: С 60.10; Н 4.93; N 13.28. C16H16N3O2Cl. Calculated, %: C 60.47; H 5.08; N 13.22.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(4-nitrophenyl)pyrrolidin-2-one (6e) was obtained from hydrazide 2e (1056 mg, 4 mmol). Yield 918 mg (70%), colorless crystals, mp 197–199°С (MeOH). IR spect- rum, ν, cm–1: 1722, 1696 (С=О), 1519, 1347 (NO2), 3193, 3087 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.03 (3H, d, 4J = 0.6, 17-CH3); 2.06 (3H, s, 15-CH3); 2.49 (1H, dd, 2J = 16.7, 3J = 7.5, 3-CH2); 2.70 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 4.24–4.33 (1H, m, 4-CH); 5.73 (1H, dd, J = 8.5, J = 0.5, 5-CH); 5.93 (1H, q, 4J = 0.6, 16-CH); 7.23 (2H, d, 3J = 8.8, H-2,6 Ph); 7.95 (2H, d, 3J = 8.8, H-3,5 Ph); 8.14 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.8 (17-CH3); 13.9 (15-CH3); 36.2 (С-3); 43.4 (C-4); 60.0 (C-5); 111.9 (C-16); 123.2 (CН Ph); 129.5 (CН Ph); 143.7 (C-17); 146.9 (C Ph); 147.3 (C Ph); 152.8 (C-15); 171.1 (C-12); 176.5 (C-2). Found, %: C 58.36; H 5.06; N 17.04. C16H16N4O4. Calculated, %: C 58.53; H 4.91; N 17.06.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(3-nitrophenyl)pyrrolidin-2-one (6f) was obtained from hydrazide 2f (1320 mg, 5 mmol). Yield 1378 mg (84%), colorless crystals, mp 178–180°С (i-PrOH). IR spectrum, ν, cm–1: 1732, 1700 (С=О), 1529, 1348 (NO2), 3193, 3080 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.01 (3H, d, 4J = 0.7, 17-CH3); 2.03 (3H, s, 15-CH3); 2.57 (1H, dd, 2J = 16.7, 3J = 8.3, 3-CH2); 2.66 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 4.29–4.38 (1H, m, 4-CH); 5.75 (1H, dd, J = 8.7, J = 0.5, 5-CH); 5.89 (1H, q, 4J = 0.7, 16-CH); 7.40 (2H, t, J = 7.9, H-5' Ph); 7.47 (1H, dt, J = 7.9, J = 1.2, H-6' Ph); 7.83 (1H, t, J = 1.9, H-2' Ph); 7.92 (1H, ddd, J = 7.9, J = 1.9, J = 1.2, H-4 Ph); 8.11 (1H, s, NH). 13C NMR spectrum, δ, ppm: 13.7 (17-CH3); 13.8 (15-CH3); 35.6 (С-3); 43.1 (C-4); 59.8 (C-5); 111.8 (C-16); 122.5 (CН Ph); 123.3 (CН Ph); 129.8 (CН Ph); 134.5 (CН Ph); 141.1 (C Ph); 143.7 (C-17); 147.4 (C Ph); 152.9 (C-15); 171.3 (C-12); 176.7 (C-2). Found, %: С 58.47; Н 4.93; N 17.01. C16H16N4O4. Calculated, %: C 58.53; H 4.91; N 17.06.
(4 R* ,5 R* ) - 5-(3,5-Dimethyl-1 H -pyrazole-1-carbonyl)-4-(pyridin-3-yl)pyrrolidin-2-one (6g) was obtained from hydrazide 2g (1100 mg, 5 mmol). Yield 1008 mg (71%), colorless crystals, mp 205–207°С (i-PrOH). IR spectrum, ν, cm–1: 1730, 1699 (С=О), 3195, 3094 (NH). 1H NMR spectrum, δ, ppm (J, Hz): 2.03 (3H, d, 4J = 0.7, 17-CH3); 2.07 (3H, s, 15-CH3); 2.48 (1H, dd, 2J = 16.7, 3J = 7.4, 3-CH2); 2.67 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 4.11–4.19 (1H, m, 4-CH); 5.71 (1H, dd, J = 8.4, J = 0.5, 5-CH); 5.94 (1H, q, 4J = 0.7, 16-CH); 7.10 (1H, dd, J = 7.9, J = 4.7, H-5' pyridine); 7.36 (1H, dt, J = 7.9, J = 1.9, H-4' pyridine); 8.10 (1H, s, NH); 8.12 (1H, d, J = 1.9, H-2' pyridine); 8.23 (1H, dd, J = 4.7, J = 1.5, H-6' pyridine). 13C NMR spectrum, δ, ppm: 13.9 (2CH3); 36.0 (С-3); 41.2 (C-4); 60.0 (C-5); 111.9 (C-16); 123.3 (C-5' pyridine); 134.6 (C-3' pyridine); 135.0 (C-4' pyridine); 148.8 (C-6' pyridine); 149.6 (C-2' pyridine); 143.6 (C-17); 152.8 (C-15); 171.2 (C-12); 176.7 (C-2). Found, %: C 58.31; H 4.78; N 19.26. C15H16N4O2. Calculated, %: C 58.53; H 4.91; N 19.71.
1-[2-(3,5-Dimethyl-1 H -pyrazol-1-yl)-2-oxoethyl]-4-phenylpyrrolidin-2-one (7a) was obtained from acetohydrazide 3a (699 mg, 3 mmol). Yield 579 mg (65%), colorless crystals, mp 120–122°С (i-PrOH). IR spectrum, ν, cm–1: 1741, 1684 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.16 (3H, s, 16-CH3); 2.42 (1H, dd, 2J = 16.7, 3J = 8.7, 3-CH2); 2.44 (3H, br. s, 18-CH3); 2.73 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 3.44 (1H, dd, J = 8.9, J = 7.8, CH2); 3.57–3.68 (1H, m, 4-CH); 3.80 (1H, t, J = 8.6, 5-CH2); 4.74 (1H, d, 2J = 18.3, 12-CH2); 4.79 (1H, d, 2J = 18.3, 12-CH2); 6.20 (1H, br. s, 17-CH); 7.18–7.26 (1H, m, H-4 Ph); 7.28–7.36 (4H, m, H-2,6,3,5 Ph). 13C NMR spectrum, δ, ppm: 14.0 (16-CH3); 14.3 (18-CH3); 37.4 (С-4); 38.5 (C-3); 46.0 (C-12); 54.9 (C-5); 111.9 (C-17); 127.3 (C-4 Ph); 127.5 (C-2,6 Ph); 129.2 (C-3,5 Ph); 143.3 (C-1 Ph); 144.2 (C-18); 153.0 (C-16); 168.7 (C-13); 174.3 (C-2). Found, %: C 68.52; H 6.57; N 14.17. C17H19N3O2. Calculated, %: C 68.67; H 6.44; N 14.13.
1-[2-(3,5-Dimethyl-1 H -pyrazol-1-yl)-2-oxoethyl]-4-(4-methylphenyl)pyrrolidin-2-one (7b) was obtained from hydrazide 3b (247 mg, 1 mmol). Yield 236 mg (76%), colorless crystals, mp 144–146°С (i-PrOH). IR spectrum, ν, cm–1: 1743, 1687 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.16 (3H, s, 16-CH3); 2.24 (3H, s, CH3 Ph); 2.38 (1H, dd, 2J = 16.7, 3J = 8.7, 3-CH2); 2.44 (3H, d, 4J = 0.7, 18-CH3); 2.70 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 3.41 (1H, dd, J = 9.1, J = 7.5, 5-CH2); 3.52–3.64 (1H, m, 4-CH); 3.77 (1H, dd, J = 9.1, J = 8.4, 5-CH2); 4.72 (1H, d, 2J = 18.3, 12-CH2); 4.78 (1H, d, 2J = 18.3, 12-CH2); 6.20 (1H, q, 4J = 0.7, 17-CH); 7.11 (2H, d, J = 8.0, H Ph); 7.19 (2H, d, J = 8.0, H Ph). 13C NMR spectrum, δ, ppm: 14.0 (16-CH3); 14.3 (18-CH3); 21.1 (CH3 Ph); 37.0 (С-4); 38.6 (C-3); 46.0 (C-12); 54.9 (C-5); 111.9 (C-17); 127.3 (CН Ph); 129.7 (CН Ph); 136.3 (C Ph); 140.2 (C Ph); 144.2 (C-18); 152.9 (C-16); 168.7 (C-13); 174.4 (C-2). Found, %: С 69.14; Н 6.85; N 13.59. C18H21N3O2. Calculated, %: C 69.43; H 6.80; N 13.49.
1-[2-(3,5-Dimethyl-1 H -pyrazol-1-yl)-2-oxoethyl]-4-(4-methoxyphenyl)pyrrolidin-2-one (7c) was obtained from hydrazide 3c (789 mg, 3 mmol). Yield 647 mg (66%), colorless crystals, mp 125–127°С (i-PrOH). IR spectrum, ν, cm–1: 1740, 1684 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.16 (3H, s, 16-CH3); 2.38 (1H, dd, 2J = 16.6, 3J = 8.8, 3-CH2); 2.44 (3H, d, 4J = 0.5, 18-CH3); 2.68 (1H, dd, 2J = 16.6, 3J = 9.0, 3-CH2); 3.39 (1H, dd, 5-CH2); 3.50–3.62 (1H, m, 4-CH); 3.70 (3H, s, CH3O Ph); 3.76 (1H, dd, J = 9.1, J = 8.5, 5-CH2); 4.72 (1H, d, 2J = 18.3, 12-CH2); 4.78 (1H, d, 2J = 18.3, 12-CH2); 6.20 (1H, q, 4J = 0.5, 17-CH); 6.87 (2H, d, J = 8.7, H-3,5 Ph); 7.23 (2H, d, J = 8.7, H-2,6 Ph). 13C NMR spectrum, δ, ppm: 14.0 (16-CH3); 14.3 (18-CH3); 36.7 (С-4); 38.7 (C-3); 45.9 (C-12); 55.1 (C-5); 55.6 (CH3O Ph); 111.9 (C-17); 114.5 (C-3,5 Ph); 128.5 (C-2,6 Ph); 135.1 (C-1 Ph); 144.2 (C-18); 152.9 (C-16); 158.6 (C-4 Ph); 168.7 (C-13); 174.4 (C-2). Found, %: С 65.68; Н 6.47; N 12.81. C18H21N3O3. Calculated, %: C 66.04; H 6.47; N 12.84.
4-(4-Chlorophenyl)-1-[2-(3,5-dimethyl-1 H -pyrazol-1-yl)-2-oxoethyl]pyrrolidin-2-one (7d) was obtained from hydrazide 3d (267 mg, 1 mmol). Yield 252 mg (76%), colorless crystals, mp 127–129°С (i-PrOH). IR spectrum, ν, cm–1: 1740, 1676 (С=О). 1H NMR spectrum, δ, ppm (J, Hz): 2.16 (3H, s, 16-CH3); 2.40 (1H, dd, 2J = 16.7, 3J = 8.5, 3-CH2); 2.43 (3H, d, 4J = 0.5, 18-CH3); 2.73 (1H, dd, 2J = 16.7, 3J = 9.0, 3-CH2); 3.41 (1H, dd, J = 9.3, J = 7.3, 5-CH2); 3.59–3.69 (1H, m, 4-CH); 3.79 (1H, dd, J = 9.3, J = 8.5, 5-CH2); 4.73 (1H, d, 2J = 18.3, 12-CH2); 4.78 (1H, d, 2J = 18.3, 12-CH2); 6.20 (1H, q, 4J = 0.5, 17-CH); 7.35 (2H, d, J = 9.0, H Ph); 7.37 (2H, d, J = 9.0, H Ph). 13C NMR spectrum, δ, ppm: 14.0 (16-CH3); 14.3 (18-CH3); 36.7 (С-4); 38.4 (C-3); 46.0 (C-12); 54.7 (C-5); 111.9 (C-17); 129.1 (CН Ph); 129.5 (CН Ph); 131.8 (C Ph); 142.4 (C Ph); 144.2 (C-18); 153.0 (C-16); 168.7 (C-13); 174.2 (C-2). Found, %: C 61.33; H 5.91; N 12.69. C17H18N3O2Cl. Calculated, %: C 61.54; H 5.47; N 12.66.
Single crystal X-ray structural analysis of compounds 5–7 a was performed on a Bruker Kappa APEX II CCD automatic diffractometer (graphite monochromator, λ(MoKα) 0.71073 Å, temperature 293(2)K, ω-scanning). Crystals of compounds 5–7 a suitable for X-ray structural analysis were obtained by crystallization from i-PrOH. Data collection and indexation, the determination and refinement of unit cell parameters were performed by using the APEX2 software suite.27 Empirical correction for the absorption derived from crystal shape, additional spherical correction, and accounting for systematic errors was accomplished with the SADABS program.28 The structures were solved by direct method using the SHELXT program29 and refined by full matrix method of least squares by F2 with the SHELXL program.30
A disordered pyrrolidone ring position along with the substituents at С(3) and С(4) atoms were observed in the crystal structure of compound 5а. The disordered atoms were denoted with letters А and В. The population of atomic positions А was 61%, while the population of atomic positions В was 39%. The moderate precision of structure 5а resulted from the small number of reflections observed due to the poor quality of the disordered crystal. For compounds 6а and 7а, the independent part of the crystals contained two molecules of these compounds (independent molecules А and В). The non-hydrogen atom positions were refined in anisotropic approximation. The hydrogen atom positions at the carbon atoms were calculated from stereochemical criteria and refined according to the riding model. The analysis of intra- and intermolecular interactions, as well as graphic presentation were accomplished using the PLATON31 and Mercury 2020.3 programs.32
The complete X-ray structural analysis dataset has been deposited at the Cambridge Crystallographic Data Center (deposits CCDC 2157868 (compound 5a), CCDC 2157855 (compound 6a), and CCDC 2157869 (compound 7a)).
Supplementary information file containing IR spectra, 1H and 13C NMR spectra of all synthesized compounds 5a–e, 6a–g, 7a–d, as well as 1H–13С HMQC and 1H–13С HMBC spectra of compounds 5а,c, 6a,b, 7a and crystallographic data of structures 5–7 a is available at the journal website http://link.springer.com/journal/10593.
This study received funding from the Ministry of Education of the Russian Federation (project No. FSZN-2020-0026) and Russian Science Foundation (project No. 21-15-00192).
The spectral characterization and elemental analysis of the synthesized compounds were performed using the equipment at the Center for Collective Use “Physicochemical methods for the study of nitro compounds, coordination compounds, biologically active substances, and nanostructured materials” of the Interdisciplinary Resource Center for Collective Use “Contemporary physicochemical methods of formation and research of materials for the needs of industry, science, and education” of the Herzen Russian State Pedagogical University.
The X-ray structural analysis was performed at the Collective Use Center for Spectral Analysis of Structure, Properties, and Composition of Compounds and Materials at the Federal Research Center “Kazan Scientific Center of the Russian Academy of Sciences”. This study was performed within the framework of State contract No. 122011800131-8 with the Federal Research Center “Kazan Scientific Center of the Russian Academy of Sciences”.
Supplementary Information
(PDF 2338 kb)
Footnotes
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2022, 58(11), 598–607
Contributor Information
Igor A. Litvinov, Email: litvinov@iopc.ru
Ivan N. Tyurenkov, Email: fibfuv@mail.ru
Sergey V. Makarenko, Email: kohrgpu@yandex.ru
References
- 1.Berestovitskaya, V. M.; Tyurenkov, I. N.; Vasilyeva, O. S.; Perfilova, V. N.; Ostroglyadov, E. S.; Bagmetova, V. V. Ratsetamy: metody sinteza i biologicheskaya aktivnost'. (Racetams: methods of synthesis and biological activity [in Russian]); Asterion: St. Petersburg, 2016.
- 2.Küçükgüzel ŞG, Şenkardeş S. Eur. J. Med. Chem. 2015;97:786. doi: 10.1016/j.ejmech.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Caruano J, Muccioli GG, Robiette R. Org. Biomol. Chem. 2016;14:10134. doi: 10.1039/C6OB01349J. [DOI] [PubMed] [Google Scholar]
- 4.Mert S, Kasımoğulları R, İça T, Çolak F, Altun A, Ok S. Eur. J. Med. Chem. 2014;78:86. doi: 10.1016/j.ejmech.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 5.Faria JV, Vegi PF, Miguita AGC, dos Santos MS, Boechat N, Bernardino AMR. Bioorg. Med. Chem. 2017;25:5891. doi: 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman New J. Chem.2017, 1, 16.
- 7.Mashkovskiy, M. D. Lekarstvennye sredstva (Drugs [in Russian]); Novaya Volna: Moscow, 2012, 16th Ed.
- 8.(a) Granik, V. G. Lekarstva (farmakologicheskii, biokhimi- cheskii i khimicheskii aspekty) (Drugs (pharmacological, biochemical and chemical aspects) [in Russian]); Vuzovskaya Kniga: Moscow, 2006, 2nd Ed., p. 149. (b) Granik, V. G. Lekarstva (farmakologicheskii, biokhimicheskii i khimicheskii aspekty) (Drugs (pharmacological, biochemical and chemical aspects) [in Russian]); Vuzovskaya Kniga: Moscow, 2006, 2nd Ed., p. 153. (c) Granik, V. G. Lekarstva (farmakolo- gicheskii, biokhimicheskii i khimicheskii aspekty) (Drugs (pharmacological, biochemical and chemical aspects) [in Russian]); Vuzovskaya Kniga: Moscow, 2006, 2nd Ed., p. 360.
- 9.Gouliaev AH, Monster JB, Vedso M, Senning A. Org. Prep. Proced. Int. 1995;27:273. doi: 10.1080/00304949509458465. [DOI] [Google Scholar]
- 10.Malykh AG, Sadaie MR. Drugs. 2010;70:287. doi: 10.2165/11319230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman, R. L.; Kania, R. S.; Brothers, M. A.; Davies, J. F.; Ferre, R. A.; Gajiwala, K. S.; He, M.; Hogan, R. J.; Kozminski, K.; Li, L. Y.; Lockner, J. W.; Lou, J.; Marra, M. T.; Mitchell, L. J., Jr.; Murray, B. W.; Nieman, J. A.; Noell, S.; Planken, S. P.; Rowe, T.; Ryan, K.; Smith III, G. J.; Solowiej, J. E.; Steppan, C. M.; Taggart, B. J. Med. Chem.2020, 63, 12725. [DOI] [PMC free article] [PubMed]
- 12.Vandyck K, Deval J. Curr. Opin. Virol. 2021;49:36. doi: 10.1016/j.coviro.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Lin D, Kusov Y, Nian Y, Ma Q, Wang J, von Brunn A, Leyssen P, Lanko K, Neyts J, de Wilde A, Snijder EJ, Liu H, Hilgenfeld R. J. Med. Chem. 2020;63:4562. doi: 10.1021/acs.jmedchem.9b01828. [DOI] [PubMed] [Google Scholar]
- 14.Vaickelioniene R, Mickevicius V. Chem. Heterocycl. Compd. 2006;42:753. doi: 10.1007/s10593-006-0157-4. [DOI] [Google Scholar]
- 15.Mickevičius V, Vaickelioniené R. Chem. Heterocycl. Compd. 2008;44:170. doi: 10.1007/s10593-008-0031-7. [DOI] [Google Scholar]
- 16.Brokaite K, Mickevicius V, Mikulskiene G. Chem. Heterocycl. Compd. 2006;42:1158. doi: 10.1007/s10593-006-0220-1. [DOI] [Google Scholar]
- 17.Sapijanskaite B, Mickevičius V. Chem. Heterocycl. Compd. 2008;44:807. doi: 10.1007/s10593-008-0113-6. [DOI] [Google Scholar]
- 18.Mickevičius V, Vaickelioniené R, Jonuškienė I, Mikulskienė G, Kantminienė K. Monat. Chem. 2009;140:1513. doi: 10.1007/s00706-009-0201-z. [DOI] [Google Scholar]
- 19.Anusevičius K, Jonuškienė I, Sapijanskaitė B, Kantminienė K, Mickevičius V. Res. Chem. Intermed. 2016;42:6975. doi: 10.1007/s11164-016-2510-2. [DOI] [Google Scholar]
- 20.Gorodnicheva NV, Vasil'eva OS, Ostroglyadov ES, Baichurin RI, Makarenko SV, Karamov FA, Lodochnikova OA, Litvinov IA. Russ. Chem. Bull. 2020;69:470. doi: 10.1007/s11172-020-2786-7. [DOI] [Google Scholar]
- 21.Gorodnicheva NV, Ostroglyadov ES, Vasil'eva OS, Pelipko VV, Gurzhii VV, Berestovitskaya VM, Lipina ES. Rus. J. Org. Chem. 2016;52:1616. doi: 10.1134/S1070428016110129. [DOI] [Google Scholar]
- 22.Vasil'eva OS, Berestovitskaya BM, Tyurenkov IN, Ostroglyadov ES, Perfilova VN, Gorodnicheva NV, Yaremchuk AI. Russ. Chem. Bull. 2017;66:1491. doi: 10.1007/s11172-017-1913-6. [DOI] [Google Scholar]
- 23.Montoya-Balbás IJ, Valentín-Guevara B, López-Mendoza E, Linzaga-Elizalde I, Ordoñez M, Román-Bravo P. Molecules. 2015;20:22028. doi: 10.3390/molecules201219830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pachaly P. Chem. Ber. 1971;104:412. doi: 10.1002/cber.19711040209. [DOI] [Google Scholar]
- 25.Pachaly P. Chem. Ber. 1971;104:429. doi: 10.1002/cber.19711040211. [DOI] [Google Scholar]
- 26.Klimochkin YN, Tkachenko IM, Reznikov AN, Shiryaev VA, Kazachkova MS, Kovalev NS, Bakulin DA, Abrosimova EE, Kurkin DV, Tyurenkov IN. Russ. J. Bioorg. Chem. 2021;47:1276. doi: 10.1134/S1068162021060108. [DOI] [Google Scholar]
- 27.APEX2 (Version 2.1); Bruker AXS, Inc.: Madison, 2006.
- 28.SADABS; Bruker AXS, Inc.: Madison, 1997.
- 29.Sheldrick, G. Acta Crystallogr., Sect. A: Found. Adv.2015, A71, 3.
- 30.Sheldrick, G. Acta Crystallogr., Sect. C: Struct. Chem.2015, C71, 3. [DOI] [PMC free article] [PubMed]
- 31.Spek, A. Acta Crystallogr., Sect. A: Found. Crystallogr.1990, A46, 34.
- 32.Farrugia L. J. Appl. Crystallogr. 2012;45:849. doi: 10.1107/S0021889812029111. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 2338 kb)