Abstract
Background
The association of microbiota with clinical outcomes and the taxa associated with colitis in horses remains generally unknown.
Objectives
Describe the fecal microbiota of horses with colitis and investigate the association of the fecal microbiota with the development of laminitis and survival.
Animals
Thirty‐six healthy and 55 colitis horses subdivided into laminitis (n = 15) and non‐laminitis (n = 39, 1 horse with chronic laminitis was removed from this comparison) and survivors (n = 27) and nonsurvivors (n = 28).
Methods
Unmatched case‐control study. The Illumina MiSeq platform targeting the V4 region of the 16S ribosomal RNA gene was used to assess the microbiota.
Results
The community membership (Jaccard index) and structure (Yue and Clayton index) were different (analysis of molecular variance [AMOVA]; P < .001) between healthy and colitis horses. The linear discriminant analysis effect size (LEfSe; linear discriminant analysis [LDA] >3; P < .05) and random forest analyses found Enterobacteriaceae, Lactobacillus, Streptococcus, and Enterococcus enriched in colitis horses, whereas Treponema, Faecalibacterium, Ruminococcaceae, and Lachnospiraceae were enriched in healthy horses. The community membership and structure of colitis horses with or without laminitis was (AMOVA; P > .05). Enterobacteriaceae, Streptococcus, and Lactobacillus were enriched in horses with laminitis (LDA > 3; P < .05). The community membership (AMOVA; P = .008) of surviving and nonsurviving horses was different. Nonsurviving horses had an enrichment of Enterobacteriaceae, Pseudomonas, Streptococcus, and Enterococcus (LDA >3; P < .05).
Conclusion and Clinical Importance
Differences in the microbiota of horses with colitis that survive or do not survive are minor and, similarly, the microbiota differences in horses with colitis that do or do not develop laminitis are minor.
Keywords: Clostridium, diarrhea, Enterobacteriaceae, Lactobacillus, Streptococcus
Abbreviations
- AMOVA
analysis of molecular variance
- DMM
Dirichlet‐multinomial model
- LDA
linear discriminatory analysis
- LEfSe
linear discriminant analysis effect size
- NNET
neural networks analysis
- PCoA
principal coordinates analysis
- RF
random forest
- ROC
receiver operator curve
- SIRS
systemic inflammatory response syndrome
- SVM
support vector machine classifier
1. INTRODUCTION
Colitis is a major cause of morbidity and mortality in horses worldwide. Colitis is inflammation of a horse's colon and cecum resulting in watery diarrhea. 1 Clinical pathological abnormalities can include tachycardia, fever, abnormal color of the mucous membranes, leukopenia, hypoproteinemia, and electrolyte abnormalities. 2 , 3 Known infectious agents in adult horses that cause colitis include Salmonella spp., Clostridioides difficile, Clostridium perfringens, Neorickettsia risticii, equine coronavirus, and small strongyles. 2 Noninfectious causes consist of antimicrobial drugs associated diarrhea, sand impaction, dietary imbalances, neoplasia, and toxicities (eg, nonsteroidal antiinflammatory drugs). 1 Regardless of the cause of diarrhea, alteration of the gastrointestinal microbiota (also known as dysbiosis) has been reported in horses. 4 , 5 , 6 In healthy horses, the gastrointestinal microbiota is comprised of a high abundance of bacteria from the phylum Firmicutes and Bacteriodetes, with minor abundance of Fibrobacter and Proteobacteria. 4 During diarrhea, the abundance of Firmicutes, Clostridia, Ruminococcaceae, and Lachnospiraceae decreases, whereas Enterobacteriaceae, Lactobacillus, and Fusobacteria increase. 4 , 5 , 6 Three studies have reported changes in the microbiota of diarrheic horses, but included small sample sizes (6‐10 horses with diarrhea). 4 , 5 , 6 Therefore, the impact on clinical outcome (e.g., survival, development of laminitis) and key bacterial groups associated with health and disease remain generally unknown.
The proportion of horses surviving colitis varies depending on the causative agent, geographic location, and the development of complications such as endotoxemia, sepsis, or laminitis. 7 , 8 , 9 , 10 , 11 , 12 , 13 Horses with colitis that develop laminitis have an increased risk of mortality compared with those without laminitis. 14 Laminitis has been associated with alteration of the gastrointestinal microbiota and absorption of microbial byproducts through permeable inflamed intestinal mucosa. 15 , 16 Several experimental studies have established a correlation between certain groups of bacteria (ie, Lactobacillus and Streptococcus) and the development of laminitis induced by administration of high concentrations of dietary carbohydrates (starch or oligofructose). 17 , 18 , 19 , 20 However, studies investigating whether similar microbial changes occur in horses with colitis that develop laminitis are lacking.
In critically ill human patients after trauma, burn, or spinal cord injury, an association between gastrointestinal microbiota, bacteremia, and mortality has been identified. 21 , 22 , 23 , 24 , 25 , 26 , 27 Humans with severe systemic inflammatory response syndrome (SIRS) or bacteremia have fewer total obligate anaerobes, Bifidobacterium and Lactobacillus, which are predictive of mortality. 21 , 28 , 29 , 30 A lower fecal relative abundance of Firmicutes is reported in surviving critically ill humans, whereas, in nonsurvivors, a higher fecal relative abundance of Proteobacteria occurs. 29 , 30 These findings indicate that the alteration of the gastrointestinal microbiota can be a potential prognostic factor in critically ill animals. Our main objective was to describe the fecal microbiota of a large cohort of horses with colitis and to evaluate the changes in bacterial communities of colitis horses that develop laminitis. Our second objective was to investigate the fecal microbiota of surviving and nonsurviving horses with colitis.
2. MATERIALS AND METHODS
2.1. Ethic statement
This study was approved by the University of Guelph Animal Care Committee (AUP#4540).
2.2. Horses with colitis
Fecal samples from horses >1 year of age diagnosed with colitis at the Ontario Veterinary College Health Sciences Centre (OVC‐HSC) admitted between January 2014 and December 2019 were collected on admission and immediately stored at −80°C until processing. All fecal samples were collected via transrectal palpation by lubrication and disposable rectal sleeves or immediately after defecation during the initial physical examination and before any treatment. The type housing, diet, travel history, and treatment with gastroprotectants before admission were not consistently recorded for this group of horses.
Colitis was defined as a horse with acute onset diarrhea (<24 hours before admission) that had fever or leukopenia, or both. Horses were excluded if they were <1 year of age, had received antimicrobial drugs in the month before admission, had received PO medication or product (eg, di‐tri‐octahedral smectite, fecal transplantation, probiotics) for treatment of the current episode of acute diarrhea. Horses that received nonsteroidal anti‐inflammatory drugs before admission for the current episode of colitis were included in the study.
Horses with colitis (n = 55) were further divided into the following groups: nonsurvivors (n = 28/55; 51%) and survivors (n = 27/55; 49%) to hospital discharge and between horses that developed laminitis (C‐L; n = 15/54; 28%) during hospitalization and those that did not (C‐NL; n = 39/54; 72%). Surviving horses were defined as horses that were discharged from the hospital. One horse with chronic laminitis before admission was removed from the laminitis analysis. Laminitis was defined as lameness, increased digital pulses, positive response to hoof testers, and radiographic evidence of rotation or distal displacement of the third phalanx.
2.3. Healthy horses
Three groups of healthy horses were included in the control group (n = 36). Healthy horses were >1 year of age and considered healthy based on physical examination. Horses were excluded if they had a gastrointestinal disease within 3 months before sample collection or if they had received antimicrobial drugs or gastroprotectants in the previous 6 months. Fifteen client‐owned horses from the state of Florida, (H‐l), 11 healthy horses belonging to the teaching herd of the University of Florida's College of Veterinary Medicine Equine Research Program Shared Herd (TH‐l), and 10 healthy horses of the Ontario Veterinary College teaching herd (TH‐II) were used as healthy controls. The H‐l horses were fed hay (coastal Bermuda) and various types and amounts of grain, TH‐l horses were kept on pasture supplemented with coastal Bermuda hay and grain (1 pound per day), whereas TH‐II horses were kept in a free‐range stable and fed Timothy hay diet with no grain supplementation. The diets of all 3 groups of healthy horses were consistent during the 3 months before sampling. All fecal samples were collected from the rectum using lubrication and disposable rectal sleeves or from feces immediately after defecation. Samples were refrigerated and stored at −80°C within 1 hour after collection until processing. All samples from healthy horses were collected during the summer of 2019.
2.4. Sample size calculation
Sample size was calculated using a Dirichlet‐multinomial model (DMM) with an expected number of 20 000 sequence reads (per animal) available for comparison, and an alpha and power of 5% and 80%, respectively. The calculation provided a minimum of 15 horses per group (eg, healthy horses and horses with colitis). 31
2.5. Sample processing
Once thawed, bacterial DNA was extracted from 200 mg fecal samples using the E.Z.N.A Stool DNA Kit (Omega Bio‐TEK, GA; Catalogue no 101319‐056) and performed according to the manufacturer's protocol. Then, the V4 region of the 16s rRNA gene was amplified using the modified primers 515‐F and 806‐R 32 as previously described. 33 The PCR products were purified using Mag Bind RXNPure Plus Beads (Omega Bio‐TEK, GA) according to the manufacturer's instructions. Sequence analysis was performed using Illumina MiSeq (Illumina RTA v1.17.28; MCS v2.2) for 250 cycles from each end at Guelph's Agriculture and Food Laboratory.
2.6. Bioinformatic and statistical analysis
The software Mothur (1.45.2) was used to complete the bioinformatic analysis following the standard operating procedure. 34 , 35 Sequences that passed quality control were identified using ribosomal database project classifier, clustered at the genus level (97% similarity), and binned into phylotypes. Sub‐sampling was performed based on the sample with the minimum number of reads. Good's coverages were evaluated to ensure sub‐sampling representation. 36 The Chao‐1 (richness), inverse Simpson's (diversity), and Shannon's evenness (evenness) indices were used to assess the alpha diversity of the fecal microbiota. Comparison between healthy and colitis groups was performed using the Wilcoxon test and the healthy, laminitis, non‐laminitis, and healthy, and survival and nonsurvival comparisons were performed using the nonparametric Steel Dwass test for multiple comparisons (JMP 16, SAS Institute). Beta diversity was assessed using the Jaccard (community membership) and Yue and Clayton (community structure) indices. 37 , 38 Analysis of molecular variance (AMOVA) was used to investigate differences between groups. Clustering between groups was represented using principal coordinates analysis (PCoA) on Jaccard and Yue and Clayton indices. Assessment of the number of different meta‐communities into which the data could be clustered was completed using the Dirichlet multinomial mixtures (DMM) method. 39 Relative abundances of the main phyla, classes, orders, families, and genera were calculated and compared between groups using the Wilcoxon test or the nonparametric Steel Dwass test for multiple comparisons. Benjamini and Hochberg's false discovery rate analysis 40 was used to adjust P values for multiple comparisons (R! Core Team, 2013). Linear discriminant analysis effect size (LefSe) analysis was completed to determine the taxa enriched in the different groups based on a P < .05 and LDA score >3.5. 41 Machine learning algorithms (i.e., random forests [RF], neural networks [NNET], LDA, and support vector machine classifier [SVMC]) were used to determine the ability of the fecal microbiota to differentiate groups (i.e., healthy vs colitis) and predict outcomes (ie, laminitis and survival), and to identify microbial taxa associated with each outcome. 42 Initially, all sequences were grouped at genus level and zero and near‐zero variance predictors were removed from downstream analysis. Each machine learning algorithm was trained for each outcome. Classifiers were built using 100 repeats of 10‐fold cross‐validation. Random forests were built with 1001 trees, and all other model variables were optimized for area under the curve with the “caret” R package v. 6.0.86. Model performance was assessed and compared using the area under the receiver operator characteristics (ROC) curves. 42 Variable importance was calculated for the top‐performing model to identify taxa associated with disease status. Analysis for the machine learning was completed using R v. 3.5.3.
3. RESULTS
3.1. Horses with colitis
Horses with colitis were between 1 and 26 years of age (median, 8 years) with 28 (51%) being mares, 26 (47%) geldings, and 1 (2%) a stallion. The breeds of horses were as follows: Thoroughbred (n = 12), Warmblood (n = 10), Quarter Horse (n = 6), Standardbred (n = 6), pony (n = 5), mixed breed (n = 2), Canadian Sport Horse (n = 2), Arabian (n = 2), Appaloosa (n = 2), Holsteiner (n = 1), Morgan (n = 1), Belgian (n = 1), Miniature Horse (n = 1), Paint (n = 1), Zangersheide (n = 1), Hanoverian (n = 1), and Friesian (n = 1). Data regarding admission physical examination, CBC, serum biochemical profile, and venous blood gas analysis of horses with colitis are presented in Table 1. Thirty‐nine of 55 (71%) horses with colitis were tested for Salmonella spp. using 1 fecal culture, 22 with 2 cultures, 9 with 3 cultures, 4 with 1 culture, and 5 with 1 culture. Fifteen (27%) horses with colitis were tested for Neorickettsia risticii using a PCR test performed using blood or feces or both. Eleven (7%) colitis horses were tested for Clostridioides difficile toxins A and B using an ELISA assay and 5 (9%) horses were tested for equine coronavirus using a PCR test of feces. Four horses tested positive for Neorickettsia risticii, 4 for Clostridioides difficile toxins A and B, 3 for Salmonella spp. on a culture of intestinal contents, and 1 for equine coronavirus. All 3 horses positive for Salmonella spp., 3/4 for N. risticci, and 3/4 for C. difficile died or were euthanized during hospitalization. Two of 3 horses diagnosed with Salmonella spp. and 2/4 horses positive for N. risticci developed laminitis. Of the 55 horses, 15 developed laminitis, 39 did not, and 1 horse was removed from the analysis because it had chronic laminitis. Twenty‐seven horses survived to hospitalization and 28 did not survive. Of the 28 nonsurvivors, 9 (32%) horses had laminitis related to colitis, whereas 6/27 (22%) survivors had laminitis (P = .5).
TABLE 1.
Selected physical examination findings, complete blood cell count, serum biochemical profile, and venous blood gas analysis of 55 diarrheic horses admitted to a tertiary teaching hospital
| Variable | Laminitis, n = 15 | Non‐laminitis, n = 39 | Survival, n = 27 | Nonsurvival, n = 28 | Reference ranges 3 |
|---|---|---|---|---|---|
| Attitude | |||||
| Bright | 5 (30%) | 12 (30%) | 9 (33%) | 8 (29%) | N/A |
| Obtunded | 10 (70%) | 28 (70%) | 18 (67%) | 20 (71%) | N/A |
| Colic | 5 (36%) | 13 (33%) | 6 (22%) | 12 (43%) | N/A |
| Laminitis | 15 (100%) | 0 (0%) | 6/27 (22%) | 7/28 (25%) | N/A |
| Temperature (Celsius) | 38 [37‐39] | 38 [36‐40] | 38 [36‐40] | 38 [36‐40] | 37.2‐38.3 |
| Heart rate (bpm) | 58 [36‐88] | 48 [36‐100] | 44 [36‐78] | 60 [40‐100] | 28‐44 |
| Resp. rate (rpm) | 24 [12‐60] | 24 [12‐60] | 20 [12‐60] | 24 [12‐60] | 10‐24 |
| PCV (%) | 46 [33‐70] | 45 [25‐73] | 38 [25‐66] | 51 [5‐73] | 36‐44 |
| WBC (×109/L) | 4 [1‐13] | 5 [2‐14] | 6 [2‐14] | 4 [1‐14] | 5.1‐11.0 |
| Neutrophils (×109/L) | 1 [0.2‐12] | 2 [0.05‐11] | 3 [0.5‐12] | 1 [0.05‐10] | 2.8‐7.7 |
| Bands (×109/L) | 0.2 [0.03‐0.8] | 0.2 [0.02‐2] | 0.2 [0.04‐10] | 0.3 [0.02‐2] | < 0.2 |
| pH | 7.4 [7.3‐7.4] | 7.4 [7.0‐7.5] | 7.4 [7.2‐7.5] | 7.4 [7.0‐7.5] | 7.32‐7.44 |
| HCO3 − (mmol/L) | 25 [18‐29] | 26 [15‐38] | 27 [20‐31] | 24 [15‐38] | 24‐28 |
| PvCO2 (mmHg) | 40 [32‐60] | 43 [30‐59] | 45 [30‐59] | 40 [32‐60] | 38‐49 |
| Na+ (mmol/L) | 131[119‐141] | 132 [118‐141] | 133 [120‐141] | 129 [118‐139] | 136‐144 |
| K+ (mmol/L) | 3 [2‐5] | 3 [1.3‐5] | 3 [2‐4] | 3 [1.3‐5] | 3.1‐4.3 |
| Cl− (mmol/L) | 98 [82‐112] | 98 [78‐107] | 100 [81‐112] | 96 [78‐107] | 95‐104 |
| L‐Lactate (mmol/L) | 3 [0.7‐7] | 2 [0.4‐14] | 2 [0.4‐10] | 3 [1‐14] | 0‐2 |
| Creatinine (μmol/L) | 131 [60‐378] | 127 [71‐445] | 120 [60‐349] | 153 [108‐445] | 80‐130 |
| Total protein (g/L) | 58 [38‐66] | 56 [40‐92] | 60 [42‐78] | 52 [38‐92] | 58‐75 |
Note: One horse had chronic laminitis before admission was removed from the laminitis analysis.
Abbreviations: bpm, beats per minute; PCV, packet cell volume; PvCO2, partial pressure of carbon dioxide; rpm, respiration per minute; WBC, white blood cells.
3.2. Healthy horses
Healthy horses consisted of 22 mares and 14 geldings, and the range of age was 4 to 23 years old. The following breeds were represented: Arabian (n = 1), Connemara (n = 1), Quarter Horse (n = 1), American Paint Horse (n = 2), Warmblood (n = 2), Appaloosa (n = 3), mixed breed (n = 4), Thoroughbred (n = 12), and Standardbred (n = 10).
3.3. Sequence analysis
Overall, 20 504 022 raw sequences were obtained, after filtering and cleaning 12 896 405 were available for analysis (mean, 141 718 ± 27 518; median, 139 464; range, 71 138‐222 987). Subsampling was performed at 70 000 sequences per sample. Subsampling was considered adequate based on a good coverage index value of 99.9%. In total, 28 phyla, 55 classes, 96 orders, 215 families, and 554 genera, respectively, were identified.
3.3.1. Fecal microbiota of healthy horses and horses with colitis
All 3 alpha diversity indices were similar between healthy horses and those with colitis (Table S1). Figure 1A depicts the relative abundance for the most abundant taxa identified in feces of healthy horses and horses with colitis. Significant differences in the community membership (Jaccard Index; AMOVA P < .001) and structure (Yue and Clayton Index; AMOVA P < .001) were detected between the healthy horses and horses with colitis. These differences were evident by the clustering of samples in the PCoA plots (Figure 1B,D). The LEfSe analysis indicated that samples from the healthy group were enriched with genera from the phyla Bacteroidetes, Firmicutes, Verrucomicrobia, Spirochaetes, and Fibrobacteres, whereas genera from the phyla Firmicutes, Proteobacteria, Actinobacteria, and Verrucomicrobia were enriched in horses with colitis (Figure 1C). In healthy horses, Treponema, Ruminococcus, Prevotella (P < .001), and an unclassified genus from the order Bacteroidales (P < .001), and families Lachnospiraceae (P = .03), Ruminococcaceae (P < .001), and Prevotellacease (P = .005) were enriched. The genera Streptococcus (P < .001), Enterococcus (P < .001), Lactobacillus (P = .003), and Enterobacteriaceae family (P < .001) were enriched in horses with colitis (Figure 1C). Using DMM, samples from healthy horses and horses with colitis were classified into 2 different metacommunities. Specifically, 88% (35/40) of samples in community 1 were from healthy horses and 12% (5/40) of samples were from horses with colitis, whereas 98% (50/51) of samples in community 2 were from horses with colitis and 2% (1/51) of samples were from healthy horses. The results of the random forest (RF) analysis showed that the genera Treponema, Fibrobacter, and an unclassified genus of the class Alphaproteobacteria and order Bacterioidales were more abundant in healthy horses than in horses with colitis. In horses with colitis, Enterococcus, Methylobacterium, and an unclassified genus from the class Betaproteobacteria were highly prevalent (Figure 1F). The RF analysis showed the highest predictive value (AUC ROC = 0.98). The machine learning models SVM and NNET also showed excellent ability to differentiate healthy horses from those with colitis based on the microbiota profile (AUC ROC > 0.90) (Figure 1F). The LDA analysis showed an acceptable predictive value to differentiate healthy from colitis horses (AUC ROC = 0.78).
FIGURE 1.
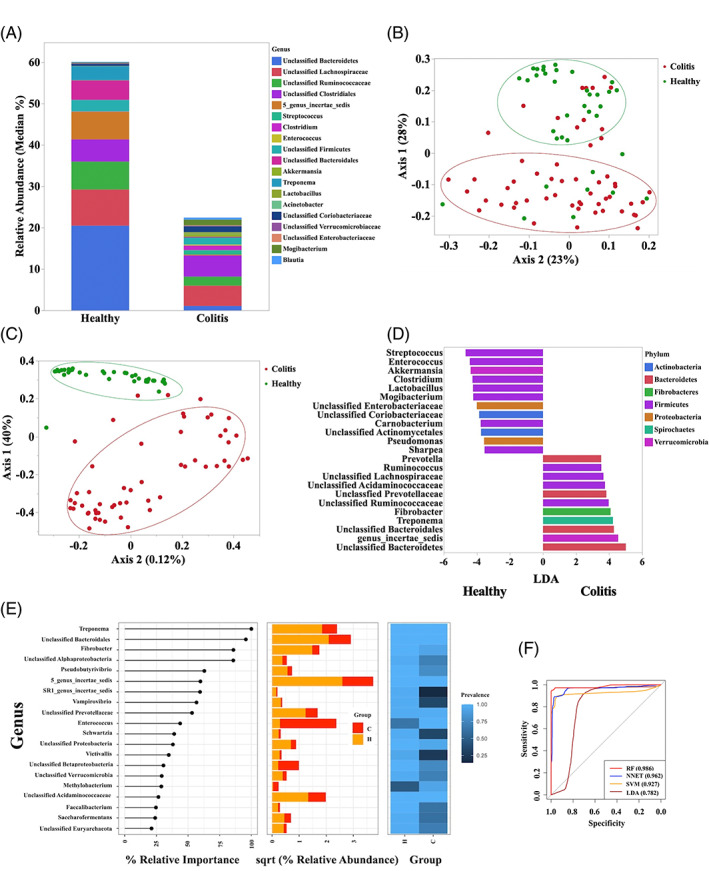
(A) Relative abundance (median) of the more abundant genera identified in healthy horses (n = 36) and horses with colitis (n = 55). (B) Principal coordinate analysis (PCoA) based on Jaccard index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from healthy horses (n = 36) and horses with colitis (n = 55). (C) Principal coordinates analysis (PCoA) based on Yue and Clayton index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from healthy horses (n = 36) and horses with colitis (n = 55). (D) Plot from LEfSe analysis indicating enriched taxa in fecal samples of healthy horses (n = 36) and horses with colitis (n = 55). Linear discriminant analysis (LDA) cut off >3.5 and P < .05. (E) Plot from random forest analysis showing the relative importance, abundance, and prevalence of taxa differentiating the fecal microbiota of healthy horses and horses with colitis. (F) Area under the receiving operator curves of the random forest (RF), neural network (NNET), support vector machine (SVM), and linear discriminate analysis (LDA) for prediction of diseases state (healthy vs colitis) based on the fecal microbiota profile
3.3.2. Laminitic vs non‐laminitic horses with colitis
The alpha diversity indices were similar between laminitic and non‐laminitic horses (Table S2). The relative abundances of the most abundant taxa identified in feces of laminitic and non‐laminitic horses are presented in Figure 2A and Table S3. The community membership (Jaccard index; AMOVA P = .5) and structure (Yue and Clayton index; AMOVA P = .16) were not significantly different (Figure 2C,D). The LEfSe analysis (P < .05 and LDA > 3) of laminitis subgroups and healthy horses indicated that in laminitic horses, the genera Streptococcus and Lactobacillus, from phylum Firmicutes, and an unclassified genus of the family Enterobacteriaceae were enriched, whereas in the non‐laminitic group, Enterococcus, Sharpea, and Clostridium sensu stricto were enriched (Figure 2E,B). Using DMM, samples from laminitic and non‐laminitic horses with colitis were classified into a single metacommunity. The RF, SVM, LDA, and NNET analyses showed no discriminatory ability of the models to predict laminitis in horses with colitis based on microbiota profile (AUC ROC < 0.70; Figure 2F).
FIGURE 2.
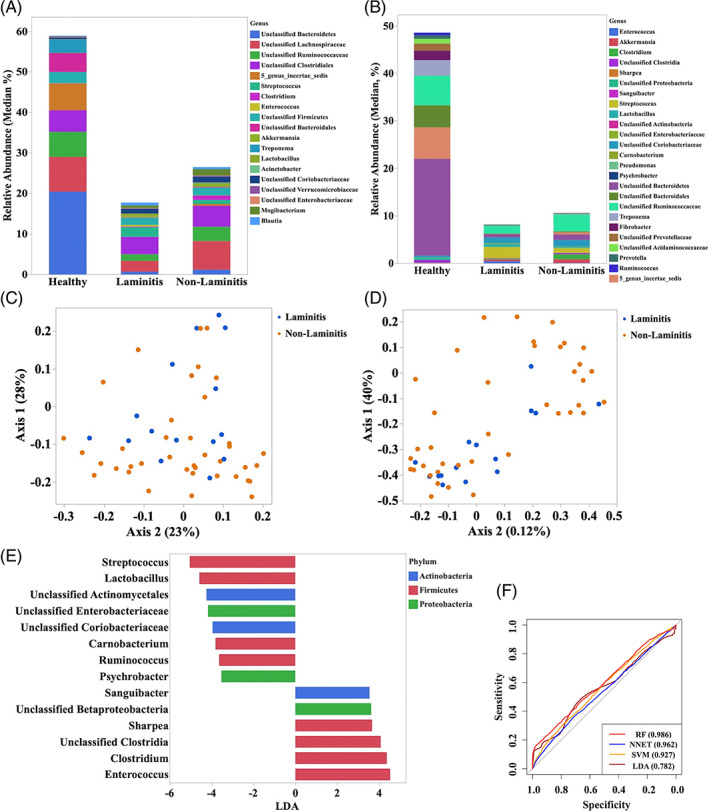
(A) Relative abundance (median) of the more abundant genera identified in horses with colitis with (n = 15) and without laminitis (n = 39) after removing one horse with chronic laminitis. (B) Principal coordinates analysis (PCoA) based on Jaccard index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from horses with colitis with (n = 15) and without laminitis (n = 39). (C) Principal coordinates analysis (PCoA) based on Yue and Clayton index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from horses with colitis with (n = 15) and without laminitis (n = 39). (D) Relative abundance analysis of the genera identified in the LEfSe analysis to be enriched in horses with colitis with (left, n = 15) and without (right, n = 39) laminitis. (E) Plot from LEfSe analysis indicating enriched taxa in fecal samples from horses with colitis with (n = 15) and without laminitis (n = 39). Linear discriminant analysis (LDA) cut off >3.5 and P < .05. (F) Area under the receiving operator curves of the random forest (RF), neural network (NNET), support vector machine (SVM), and linear discriminate analysis (LDA) for prediction of diseases state (laminitis vs non‐laminitis) based on the fecal microbiota profile
3.3.3. Surviving vs nonsurviving horses with colitis
No significant differences were identified in alpha diversity measurements between surviving and nonsurviving horses with colitis (Table S2). The relative abundance of the most abundant taxa identified in feces of survivors and nonsurvivors is presented in Figure 3A and Table S4. Community membership (Jaccard index; AMOVA P = .7) and structure (Yue and Clayton index; AMOVA P = .4) were not significantly different between survivors and nonsurvivors (Figure 3C,D). The LEfSe analysis (P < .05 and LDA > 3) indicated that the fecal microbiota of surviving horses was enriched with Enterococcus, Mogibacterium, Sharpea, Pseudomonas, Enterobacteriaceae, and an unclassified genus of the class Betaproteobacteria, whereas nonsurviving horses with colitis had enrichment of Streptococcus, Clostridium sensu stricto, Lactobacillus, Actinomycetales, and Carnobacterium (Figure 3B,E). Using DMM, samples from surviving and nonsurviving horses with colitis were classified into a single metacommunity. The RF, SVM, LDA, and NNET analysis showed no discriminatory capability of the models to predict survival of horses with colitis based on the admission fecal microbiota profile (AUC ROC < 0.70; Figure 3F).
FIGURE 3.
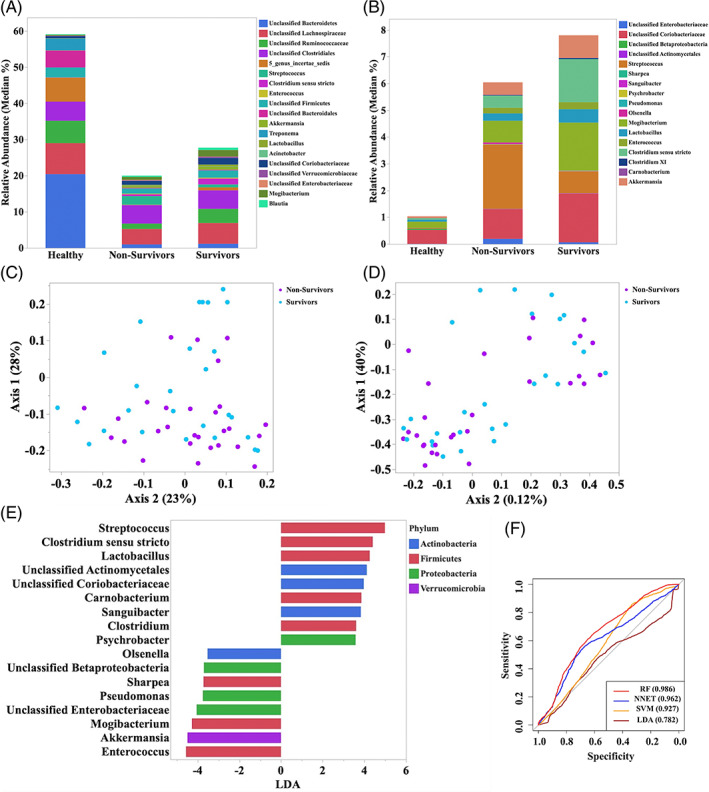
(A) Relative abundance (median) of the more abundant genera identified in surviving (n = 27) and nonsurviving (n = 28) horses with colitis. (B) Principal coordinates analysis (PCoA) based on Jaccard index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 27) and nonsurviving (n = 28) horses with colitis. (C) Principal coordinates analysis (PCoA) based on Yue and Clayton index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 27) and nonsurviving (n = 28) horses with colitis. (D) Relative abundance analysis of the genera identified in the LEfSe analysis to be enriched in surviving (n = 27) and nonsurviving (n = 28) horses with colitis. (E) Plot from LEfSe analysis indicating enriched taxa in fecal samples from surviving (n = 27) and nonsurviving (n = 28) horses with colitis. Linear discriminant analysis (LDA) cut off >3.5 and P < .05. (F) Area under the receiving operator curves of the random forest (RF), neural network (NNET), support vector machine (SVM), and linear discriminate analysis (LDA) for prediction of outcome (surviving versus nonsurviving) based on the fecal microbiota profile
A survival analysis was performed after removing horses that developed laminitis (n = 15) from the surviving (n = 6) and nonsurviving groups (n = 9). One surviving horse that had chronic laminitis at admission also was removed from this analysis. Statistical differences in the alpha diversity (richness, evenness and diversity; P > .05, for all comparisons) and beta diversity (Jaccard and Yue & Clayton indices) were not identified (AMOVA > .05 for both comparisons). The LEfSe analysis showed that Lactobacillus was no longer enriched in either group (Figure S1).
4. DISCUSSION
In our study, the differences in community membership and structure indicated that dysbiosis of the gastrointestinal tract was present in horses with colitis. This dysbiosis was associated with an enrichment in facultative anaerobes (ie, Streptococcus, Lactobacillus, Enterococcus, and Enterobacteriaceae) in diarrheic horses, whereas feces of healthy horses were enriched with obligate anaerobes such as Treponema, Ruminococcaceae, and Lachnospiraceae families. Alteration of the gastrointestinal microbiota has been reported in horses with diarrhea 4 , 5 , 6 characterized by a decrease in the relative abundance of Firmicutes, Clostridia, Ruminococcaceae, and Lachnospiraceae. These results may suggest that a shift from obligate to facultative anaerobic bacteria, which is regarded as a marker of dysbiosis of the gastrointestinal tract in humans, calves, and horses, 43 , 44 , 45 could occur in horses with colitis. Reasons for this shift are not completely understood, but during colitis intestinal contents are usually watery and in some cases hemorrhagic with marked hyperemia, ulceration, and hemorrhage of the intestinal mucosa. 2 It has been proposed that an inflammatory process of the gastrointestinal tract could result in the release of hemoglobin carrying oxygen to the lumen of the colon and resulting in increased oxygen concentrations. An increase in oxygen concentrations in the mucosa and lumen of the intestine would benefit the proliferation of facultative anaerobes and a decrease in oxygen‐sensitive obligate anaerobes (i.e., Ruminococcaceae and Lachnospiraceae) the so‐called “oxygen hypothesis.” 43 , 44 Although this hypothesis is still under investigation, an increase in oxygen concentrations in the mucosa and lumen of the intestine could explain, at least in part, our findings. The production of short‐chain fatty acids (especially butyrate) by bacterial communities of the gastrointestinal tract maintains low oxygen and nitrate concentrations in the lumen of the gastrointestinal tract. 44 Therefore, decreases in butyrate‐producing bacteria (eg, Lachnospiraceae, Ruminococcus, and Faecalibacterium) could have contributed to and facilitated proliferation of facultative anaerobes in horses with colitis. Luminal pH and nutrient availability (e.g., simple sugars) also play important roles in bacterial growth and metabolism. 46 , 47 Fecal pH and metabolite contents were not determined in our study, but it is likely that alteration in luminal pH and nutrient limitation during colonic inflammation contributed to the changes in bacterial communities during colitis. Alterations in gastrointestinal microbiota, especially the increased abundance of Lactobacillus, Enterococcus, and Enterobacteriaceae in horses with colitis, is of interest because proliferation of these taxa can enhance and perpetuate intestinal inflammation, mucosal damage, and facilitate translocation of bacteria and their by‐products. 43 , 44 Translocation of bacteria and their by‐products could explain some of the clinicopathological findings in the horses with colitis (e.g., tachycardia, fever, leukopenia).
Laminitis has been associated with an alteration of the gastrointestinal microbiota and absorption of microbial byproducts through permeable inflamed intestinal mucosa, 15 but this association only has been established in experimental studies. Our study showed that the feces of horses with colitis that develop laminitis was enriched in Lactobacillus, Streptococcus, and Enterobacteriaceae. Laminitis induced by exposure to high amounts of dietary carbohydrates (starch or oligofructose) also results in an increase in Lactobacillus and Streptococcus. 17 , 18 , 19 , 20 These changes correlated significantly with a decrease in cecal fluid pH and an increase in luminal lactate concentrations. 17 , 18 , 19 , 20 During the development of laminitis, Enterobacteriaceae populations initially increase but then decrease significantly. 15 The decrease in Enterobacteriaceae is attributed to the reduction in cecal fluid pH, causing lysis of Enterobacteriaceae and consequent endotoxin release into the cecum. Along with an acidic colonic environment, these changes cause mucosal damage and facilitate translocation of bacteria, endotoxin, and bacterial by‐products (e.g., histamine, tryptamine, phenylethylamine, 5‐hydroxytryptamine). 48 , 49 These alterations are hypothesized to induce lamellar separation and consequent laminitis. 15 , 49 Our study was limited because fecal and blood metabolomic analyses were not available to determine correlation between bacteria and by‐products associated with the development of laminitis. These data could have helped confirm whether vasoactive amines and other bacterial by‐products produced in the lumen of the colon and absorbed into systemic circulation contribute to the development of laminitis in horses with colitis. The relative increased abundance of Lactobacillus in horses with colitis that develops laminitis raises the question of whether administering probiotics containing lactic acid‐producing bacteria could be harmful for horses with acute diarrhea.
Analysis of the fecal microbiota of surviving and nonsurviving horses with colitis, including horses that developed laminitis, indicated an enrichment of Streptococcus and Lactobacillus from the phylum Firmicutes. Critically ill humans with SIRS have higher total facultative anaerobe counts and lower total anaerobic bacterial counts compared with healthy individuals. 51 The reasons for the association between changes in the gastrointestinal microbiota and mortality in the horses in our study is unknown, but could be related to the effect of the microbiota on the immune response. 50 It is also possible that enrichment of facultative anaerobes (eg, Lactobacillus and Streptococcus) in nonsurviving horses indeed indicates a more severe inflammatory process in the gastrointestinal tract. These facilitate anaerobes in turn can result in more critical damage to the intestinal mucosa, decreased colonization resistance, and subsequent absorption of bacteria, toxins, and vasoactive amines, 30 , 48 , 49 , 51 , 52 leading to an increased risk of mortality. 8 , 12 Removal of laminitis horses from the survival analysis confirmed that enrichment of Streptococcus was associated with nonsurvival of horses with colitis, suggesting a potential role of this bacterium in the severity of the intestinal inflammation and subsequent development of systemic clinical signs. 15 , 16 , 18 , 19 , 53
The main limitation of our study was that the environmental and management practices of horses with colitis did not match those of the healthy group. Studies have shown that factors such as stress, diet, exercise, drugs, and disease may influence the gastrointestinal microbiota. 54 However, the PCoA plots showed a differential clustering between healthy and colitis horses indicating that, despite differences in environment and management practices, healthy horses shared certain taxa that differentiated them from horses with colitis. Future studies including control farm horses are warranted to validate our results. Another limitation of our study was selection of a group of critically ill horses with colitis for the fecal microbiota analysis. The results could be biased toward horses with critical disease because severely affected horses are more likely to be referred to a tertiary hospital. Therefore, caution should be exercised in extrapolating our results to a different population. However, the horses admitted to our hospital are likely similar to those admitted to other tertiary hospitals. The approach used to define laminitis in the horses in our study is also a limitation. A horse with prodromic signs of laminitis or with laminitis but with radiographs that were normal or not performed would have been missed. The inclusion criteria aimed to eliminate subjectivity in the diagnosis of laminitis. An additional limitation of our study is that changes in housing, diet, or travel history before admission of horses with colitis were not consistently recorded. Changes in diet 55 and transport 56 , 57 can alter the fecal microbiota of healthy horses, and such changes could have impacted the results of our study. Finally, most horses included in our study were not tested for all of the etiologic agents known to cause acute diarrhea in horses. This lack of testing prevented us from determining associations between specific pathogens and outcomes (e.g., laminitis and survival) and changes in the gastrointestinal microbiota.
In summary, we showed that dysbiosis occur in horses with colitis with an enrichment of facultative anaerobes and a decrease in some obligate anaerobic bacteria. Although we failed to detect differences in the community structure between laminitic and non‐laminitic horses or between surviving and nonsurviving horses with colitis, specific taxa were enriched in horses with colitis that developed laminitis or died. These findings suggest that the gastrointestinal microbiota could play an important role in the development of laminitis and the severity of disease in horses with colitis.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Approved by University of Guelph Animal Care Committee (AUP#4540).
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Figure S1 A. Relative abundance (median) of the more abundant genera identified in surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses that developed laminitis associated with colitis (n = 15) and one horse with chronic laminitis. B. Plot from LEfSe analysis indicating enriched taxa in fecal samples from surviving (n = 27) and non‐surviving (n = 28) horses with colitis after removing horses with laminitis. Linear discriminant analysis (LDA) cut off > 3.5 and P < .05. C. Principal coordinates analysis (PCoA) based on Jaccard index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses with laminitis. D. Principal coordinates analysis (PCoA) based on Yue and Clayton index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses with laminitis.
Table S1 Alpha diversity comparisons of the Chao, Shannon Evenness, and Inverse Simpson's indices of healthy horses and horses with colitis.
Table S2 Alpha diversity comparisons of the Chao, Shannon Evenness, and Inverse Simpson's indices of laminitis and non‐laminitis horses and surviving and nonsurviving horses
Table S3 Relative abundance (median and range) of the most abundant taxa identified in healthy horses and diarrheic horses with or without laminitis
Table S4 Relative abundance (median [%] and range) of the most abundant taxa identified in healthy horses and survival and nonsurviving diarrheic horses.
ACKNOWLEDGMENT
Funding provided by Equine Guelph (number 055097). Present in part as a research abstract poster presentation at the 2021 ACVIM Forum.
Ayoub C, Arroyo LG, MacNicol JL, Renaud D, Weese JS, Gomez DE. Fecal microbiota of horses with colitis and its association with laminitis and survival during hospitalization. J Vet Intern Med. 2022;36(6):2213‐2223. doi: 10.1111/jvim.16562
Funding information Equine Guelph, Grant/Award Number: 055097
REFERENCES
- 1. Shaw SD, Stämpfli H. Diagnosis and treatment of undifferentiated and infectious acute diarrhea in the adult horse. Vet Clin North Am Equine Pract. 2018;34:39‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Uzal FA, Arroyo LG, Navarro MA, Gomez DE, Asin J, Henderson E. Bacterial and viral enterocolitis in horses: a review. J Vet Diag Investig. 2022;34(3):354‐375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gomez DE, Arroyo LG, Stämpfli HR, Cruz LE, Oliver OJ. Physicochemical interpretation of acid‐base abnormalities in 54 adult horses with acute severe colitis and diarrhea. J Vet Intern Med. 2013;27:548‐553. [DOI] [PubMed] [Google Scholar]
- 4. Costa MC, Arroyo LG, Allen‐Vercoe E, et al. Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3‐V5 region of the 16S rRNA gene. PLoS One. 2012;7(7):e41484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arroyo LG, Rossi L, Santos BP, Gomez DE, Surette MG, Costa MC. Luminal and mucosal microbiota of the cecum and large colon of healthy and diarrheic horses. Animals (Basel). 2020;10(8):1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez C, Taminiau B, Brévers B, et al. Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC Microbiol. 2015;16(15):181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Berryhill EH, Magdesian KG, Aleman M, Pusterla N. Clinical presentation, diagnostic findings, and outcome of adult horses with equine coronavirus infection at a veterinary teaching hospital: 33 cases (2012‐2018). Vet J. 2019;248:95‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen ND, Woods AM. Characteristics and risk factors for failure of horses with acute diarrhea to survive: 122 cases (1990‐1996). J Am Vet Med Assoc. 1999;214:382‐390. [PubMed] [Google Scholar]
- 9. Magdesian KG, Dujowich M, Madigan JE, Hansen LM, Hirsh DC, Jang SS. Molecular characterization of Clostridium difficile isolates from horses in an intensive care unit and association of disease severity with strain type. J Am Vet Med Assoc. 2006;228(5):751‐755. [DOI] [PubMed] [Google Scholar]
- 10. Weese JS, Staempfli HR, Prescott JF. A prospective study of the roles of clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhoea. Equine Vet J. 2001;33(4):403‐409. [DOI] [PubMed] [Google Scholar]
- 11. Parsons CS, Orsini JA, Krafty R, Capewell L, Boston R. Risk factors for development of acute laminitis in horses during hospitalization: 73 cases (1997‐2004). J Am Vet Med Assoc. 2007;230:885‐889. [DOI] [PubMed] [Google Scholar]
- 12. Groover ES, Woolums AR, Cole DJ, LeRoy BE. Risk factors associated with renal insufficiency in horses with primary gastrointestinal disease: 26 cases (2000‐2003). J Am Vet Med Assoc. 2006;228(4):572‐577. [DOI] [PubMed] [Google Scholar]
- 13. Elliott J, Bailey SR. Gastrointestinal derived factors are potential triggers for the development of acute equine laminitis. J Nutr. 2006;136(7 Suppl):2103‐2107. [DOI] [PubMed] [Google Scholar]
- 14. Luethy D, Feldman R, Stefanovski D, Aitken MR. Risk factors for laminitis and nonsurvival in acute colitis: retrospective study of 85 hospitalized horses (2011‐2019). J Vet Intern Med. 2020;35(4):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Milinovich GJ, Klieve AV, Pollitt CC, Trott DJ. Microbial events in the hindgut during carbohydrate‐induced equine laminitis. Vet Clin North Equine Pract. 2010;26:79‐94. [DOI] [PubMed] [Google Scholar]
- 16. Bailey SR, Menzies‐Gow NJ, Marr CM, Elliott J. The effects of vasoactive amines found in the equine hindgut on digital blood flow in the normal horse. Equine Vet J. 2004;36:267‐272. [DOI] [PubMed] [Google Scholar]
- 17. Garner HE, Moore JN, Johnson JH, et al. Changes in the caecal flora associated with the onset of laminitis. Equine Vet J. 1978;10(4):249‐252. [DOI] [PubMed] [Google Scholar]
- 18. Milinovich GJ, Burrell PC, Pollitt CC, et al. Microbial ecology of the equine hindgut during oligofructose‐induced laminitis. ISME J. 2008;2(11):1089‐1100. [DOI] [PubMed] [Google Scholar]
- 19. Milinovich GJ, Trott DJ, Burrell PC, et al. Fluorescence in situ hybridization analysis of hindgut bacteria associated with the development of equine laminitis. Environ Microbiol. 2007;9(8):2090‐2100. [DOI] [PubMed] [Google Scholar]
- 20. Tuniyazi M, He J, Guo J, et al. Changes of microbial and metabolome of the equine hindgut during oligofructose‐induced laminitis. BMC Vet Res. 2021;17(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nicholson SE, Merrill D, Zhu C, et al. Polytrauma independent of therapeutic intervention alters the gastrointestinal microbiome. Am J Surg. 2018;216(4):699‐705. [DOI] [PubMed] [Google Scholar]
- 22. Houlden A, Goldrick M, Brough D, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang G, Sun K, Yin S, et al. Burn injury leads to increase in relative abundance of opportunistic pathogens in the rat gastrointestinal microbiome. Front Microbiol. 2017;8:1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nicholson SE, Burmeister DM, Johnson TR, et al. A prospective study in severely injured patients reveals an altered gut microbiome is associated with transfusion volume. J Trauma Acute Care Surg. 2019;86(4):573‐582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Waligora‐Dupriet AJ, Lafleur S, Charrueau C, et al. Head injury profoundly affects gut microbiota homeostasis: results of a pilot study. Nutrition. 2018;45:104‐107. [DOI] [PubMed] [Google Scholar]
- 26. Kigerl KA, Hall JC, Wang L, et al. Gut dysbiosis impairs recovery after spinal cord injury. J Exp Med. 2016;213(12):2603‐2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Earley ZM, Akhtar S, Green SJ, et al. Burn injury alters the intestinal microbiome and increases gut permeability and bacterial translocation. PLoS One. 2015;10(7):e0129996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Burmeister DM, Johnson TR, Lai Z, et al. The gut microbiome distinguishes mortality in trauma patients upon admission to the emergency department. J Trauma Acute Care Surg. 2020;88(5):579‐587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vaishnavi C. Translocation of gut flora and its role in sepsis. Indian J Med Microbiol. 2013;31:334‐342. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu K, Ogura H, Hamasaki T, et al. Altered gut flora are associated with septic complications and death in critically ill patients with systemic inflammatory response syndrome. Dig Dis Sci. 2011;56(4):1171‐1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. La Rosa PS, Brooks JP, Deych E, et al. Hypothesis testing and power calculations for taxonomic‐based human microbiome data. PLoS One. 2012;7(12):e52078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Klindworth A, Pruesse E, Schweer T, et al. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next‐generation sequencing‐based diversity studies. Nucleic Acids Res. 2013;41(1):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walters W, Hyde ER, Berg‐Lyons D, et al. Improved bacterial 16S rRNA gene (V4 and V4‐5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2015;1(1):e00009‐e00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schloss PD, Westcott SL, Ryabin T, et al. Introducing mothur: open‐source, platform‐independent, community‐supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75(23):7537‐7541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. Development of a dual‐index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol. 2013;79:5112‐5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Good IJ. The population frequencies of species and the estimation of population parameters. Biometrika. 1953;40:237‐264. [Google Scholar]
- 37. Jaccard P. Nouvelles recherches sur la distribution florale. Nature. 1908;44:223‐270. [Google Scholar]
- 38. Yue JC, Clayton MK. Similarity measure based on species proportions. Commun Stat Methods. 2005;34:2123‐2131. [Google Scholar]
- 39. Holmes I, Harris K, Quince C. Dirichlet multinomial mixtures: generative models for microbial metagenomics. PLoS One. 2012;7:e30126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. 1995;57:289‐300. [Google Scholar]
- 41. Segata N, Izard J, Waldron L, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pasolli E, Truong DT, Malik F, Waldron L, Segata N. Machine learning meta‐analysis of large metagenomic datasets: tools and biological insights. PLoS Comput Biol. 2016;12:1‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rigottier‐Gois L. Dysbiosis in inflammatory bowel diseases: the oxygen hypothesis microbe‐microbe and microbe‐host interactions. ISME J. 2013;7:1256‐1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Stecher B. The roles of inflammation, nutrient availability and the commensal microbiota in enteric pathogen infection. Microbiol Spectrum. 2015;3:3. [DOI] [PubMed] [Google Scholar]
- 45. Shin NR, Whon TW, Bae JW. Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015;33:496‐503. [DOI] [PubMed] [Google Scholar]
- 46. Ilhan ZE, Marcus AK, Kang DW, Rittmann BE, Krajmalnik‐Brown R. pH‐mediated microbial and metabolic interactions in fecal enrichment cultures. mSphere. 2017;2(3):e00047‐e00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sorbara MT, Pamer EG. Interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol. 2019;12:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bailey SR, Baillon ML, Rycroft AN, Harris PA, Elliott J. Identification of equine cecal bacteria producing amines in an in vitro model of carbohydrate overload. Appl Environ Microbiol. 2003;69:2087‐2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bailey SR, Marr CM, Elliott J. Current research and theories on the pathogenesis of acute laminitis in the horse. Vet J. 2004;167:129‐142. [DOI] [PubMed] [Google Scholar]
- 50. Peled JU, Gomes ALC, Devlin SM, et al. Microbiota as predictor of mortality in allogeneic hematopoietic‐cell transplantation. N Engl J Med. 2020;382(9):822‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shimizu K, Ogura H, Goto M, et al. Altered gut flora and environment in patients with severe SIRS. J Trauma. 2006;60(1):126‐133. [DOI] [PubMed] [Google Scholar]
- 52. Yang XJ, Liu D, Ren HY, Zhang XY, Zhang J, Yang XJ. Effects of sepsis and its treatment measures on intestinal flora structure in critical care patients. World J Gastroenterol. 2021;27(19):2376‐2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Milinovich GJ, Burrell PC, Pollitt CC, Bouvet A, Trott DJ. Streptococcus henryi sp. nov. and Streptococcus caballi sp. nov., isolated from the hindgut of horses with oligofructose‐induced laminitis. Int J Syst Evol Microbiol. 2008;2008(58):262‐266. [DOI] [PubMed] [Google Scholar]
- 54. Garber A, Hastie P, Murray JA. Factors influencing equine gut microbiota: current knowledge. J Equine Vet Sci. 2020;88:102943. [DOI] [PubMed] [Google Scholar]
- 55. Collinet A, Grimm P, Julliand S, Julliand V. Sequential modulation of the equine fecal microbiota and fibrolytic capacity following two consecutive abrupt dietary changes and bacterial supplementation. Animals (Basel). 2021;11(5):1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Szemplinski KL, Thompson A, Cherry N, et al. Transporting and exercising unconditioned horses: effects on microflora populations. J Equine Vet Sci. 2020;90:102988. [DOI] [PubMed] [Google Scholar]
- 57. Schoster A, Mosing M, Jalali M, Staempfli HR, Weese JS. Effects of transport, fasting and anaesthesia on the faecal microbiota of healthy adult horses. Equine Vet J. 2016;48(5):595‐602. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 A. Relative abundance (median) of the more abundant genera identified in surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses that developed laminitis associated with colitis (n = 15) and one horse with chronic laminitis. B. Plot from LEfSe analysis indicating enriched taxa in fecal samples from surviving (n = 27) and non‐surviving (n = 28) horses with colitis after removing horses with laminitis. Linear discriminant analysis (LDA) cut off > 3.5 and P < .05. C. Principal coordinates analysis (PCoA) based on Jaccard index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses with laminitis. D. Principal coordinates analysis (PCoA) based on Yue and Clayton index analysis of the bacterial 16S rRNA gene sequence data for fecal samples collected from surviving (n = 20) and non‐surviving (n = 19) horses with colitis after removing horses with laminitis.
Table S1 Alpha diversity comparisons of the Chao, Shannon Evenness, and Inverse Simpson's indices of healthy horses and horses with colitis.
Table S2 Alpha diversity comparisons of the Chao, Shannon Evenness, and Inverse Simpson's indices of laminitis and non‐laminitis horses and surviving and nonsurviving horses
Table S3 Relative abundance (median and range) of the most abundant taxa identified in healthy horses and diarrheic horses with or without laminitis
Table S4 Relative abundance (median [%] and range) of the most abundant taxa identified in healthy horses and survival and nonsurviving diarrheic horses.


