Abstract
Objective
To evaluate the effects of a home-based respiratory muscle training programme (inspiratory [IMT] or inspiratory/expiratory muscles [RMT]) supervised by telerehabilitation on quality of life and exercise tolerance in individuals with long-term post-COVID-19 symptoms. The secondary objective was to evaluate the effects of these programmes on respiratory muscle function, physical and lung function, and psychological state.
Methods
88 individuals with long-term symptoms of fatigue and dyspnoea after COVID-19 diagnosis were randomly (1:1 ratio) assigned to IMT, IMTsham, RMT or RMTsham groups for an 8-week intervention (40min/day, 6 times/week). Primary outcomes were quality of life (EuroQol-5D questionnaire) and exercise tolerance (Ruffier test). Secondary outcomes were respiratory muscle function (inspiratory/expiratory muscle strength; inspiratory muscle endurance), physical function (lower and upper limb strength [1-min Sit-to-Stand and handgrip force]), lung function (forced spirometry), and psychological status (anxiety/depression levels and post-traumatic stress disorder). All outcomes were measured pre-, intermediate- (4th week), and post-intervention.
Results
At post-intervention, there was a statistically significant and large (d>0.90) improvement in quality of life, but not in exercise tolerance, in the RMT group compared with the RMTsham group. Both of the real training groups produced a statistically significant and large increase in inspiratory muscle strength and endurance (d≥0.80) and in lower limb muscle strength (d≥0.77) compared with the 2 sham groups. Expiratory muscle strength and peak expiratory flow showed a statistically significant and large (d≥0.87) increase in the RMT group compared with the other 3 groups.
Conclusion
Only an 8-week supervised home-based RMT programme was effective in improving quality of life, but not exercise tolerance, in individuals with long-term post-COVID-19 symptoms. In addition, IMT and RMT programmes were effective in improving respiratory muscle function and lower limb muscle strength, but had no impact on lung function and psychological status.
Keywords: SARS-CoV-2, Respiratory muscle training, Quality of life, Maximal respiratory pressures, Telerehabilitation
Abbreviations: HRQoL, health-related quality of life; IMT, inspiratory muscle training; RMT, inspiratory/expiratory muscle training; VAS, visual analogue scale; MIP, maximum inspiratory pressure; MEP, maximum expiratory pressure; IME, inspiratory muscle endurance; 1-min STS, 1-min sit-to-stand; PTSD, post-traumatic stress disorder; PCL-C, post-traumatic stress disorder Check List-Civilian; PEF, peak expiratory flow
Introduction
After COVID-19 infection, up to 60% of survivors experience symptoms not explained by an alternative diagnosis [1], and these individuals are considered to have long-term post-COVID-19 symptoms when their duration persists for more than 3 months [1,2]. These symptoms include, but are not limited to, fatigue, dyspnoea, pain, muscle weakness, limited exercise capacity, depression, confusion, memory problems, difficulty concentrating (“brain fog”), neurological symptoms, smell/taste disorders, impaired lung function, and poor health-related quality of life (HRQoL) [3,4].
The pathogenesis of these persistent symptoms is largely unknown, although various hypotheses have been proposed, including the presence of hypoxia and hypoxic tissue injury induced by COVID-19-associated pneumonia and the subsequent low pulmonary diffusing capacity, ventilation-perfusion mismatching, and fibrosis [5]. As a result of increased ventilatory requirements, it is possible that symptoms such as fatigue, dyspnoea, and limited exercise tolerance are associated with diaphragm fatigue and an increase in the concentration of metabolites that activate the so-called “metaboreflex”, with subsequent reduced exercise tolerance by causing a systemic vasoconstrictor response in the limb skeletal muscle [6]. Thus, exaggerated metaboreflex activation could explain the reduced quality of life and functional capacity observed in people with inspiratory muscle weakness [7].
Respiratory muscle training has been shown to attenuate the metaboreflex in healthy individuals [8] and in individuals with chronic heart failure [9]. The beneficial effects of respiratory muscle training also include increased HRQoL, exercise tolerance, respiratory muscle strength and endurance, and lung function, and also reduced fatigue and dyspnoea levels [10,11]. Accordingly, exercise interventions designed to improve HRQoL, exercise tolerance, respiratory muscle strength and pulmonary function could be beneficial for individuals with long-term post-COVID-19 symptoms. In addition, respiratory muscle training can be implemented through telerehabilitation, thus reducing healthcare and respecting socially implemented measures due to the COVID-19 pandemic [12].
This study evaluated the effects of a home-based respiratory muscle training programme (inspiratory [IMT] or inspiratory/expiratory muscles [RMT]) supervised by telerehabilitation on HRQoL and exercise tolerance in individuals with long-term post-COVID-19 symptoms. The secondary objective was to evaluate the effects of these programmes on respiratory muscle function, physical and lung function, as well as on the psychological state of these individuals.
Methods
Study design
A parallel 4-arm, double-blinded, randomised controlled trial was conducted according to the Consolidated Standards of Reporting Trials (CONSORT) 2017 Statement for Randomized Trials of Nonpharmacologic Treatments. All of the study procedures were conducted in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of Hospital Clínico San Carlos (20/715-E_BS). The study was also registered in the United States Clinical Trials Registry (NCT04734561).
Participants
Participants were recruited from two non-profit organisations for the support of individuals with long-term post-COVID-19 symptoms (Long Covid ACTS [Autonomous Communities Together Spain] and Covid persistente España [Persistent COVID Spain]) via flyers, social networks, and internet platforms. Individuals interested in participating contacted an external researcher by email, who corroborated their potential eligibility and arranged a face-to-face visit for them to undergo the baseline assessment. At the baseline visit, the remaining face-to-face visits were scheduled for follow-up assessments. The study included COVID-19 survivors aged over 18 years who presented long-term post-COVID-19 symptoms of fatigue and dyspnoea for at least 3 months after the COVID-19 diagnosis confirmed by positive reverse-transcription-polymerase chain reaction (RT-PCR) SARS-CoV-2 test from a nasopharyngeal or oropharyngeal swab or serological tests positive for SARS-CoV-2 antibodies. Candidates were excluded if they presented 1) a diagnosis of progressive respiratory, neuromuscular or neurological disorders and/or psychiatric or cognitive conditions that hindered their ability to cooperate; 2) any contraindication to respiratory muscle training treatment; 3) lack of internet access; and 4) previous inclusion in a rehabilitation programme for their long-term post-COVID-19 symptoms. Participants who missed more than 15% of the treatment sessions were also excluded.
Randomization and blinding
Using a computer-generated randomization list (GraphPad Software©), an external researcher who was blinded to the participants’ identities independently performed a block randomization (block size of 8). Allocation concealment was conducted using opaque sealed envelopes, which were prepared by this external researcher. Participants were randomly assigned to one of 4 parallel groups, in a 1:1 ratio: 1) IMT group; 2) IMTsham group; 3) RMT group; and 4) RMTsham group. A second external researcher provided the participants with the training device (sham or real) assigned to their group in a face-to-face visit and indicated when they should start the home-based respiratory muscle training (always 3–7 days after randomization). In addition, this second external researcher sent e-mails to the physiotherapist in charge of administering the training indicating whether the participants should perform IMT or RMT. Thus, although the participants and therapist knew the training modality (IMT or RMT), they were all blinded to whether the training was real or sham, given that the external appearance of the training devices was identical. The assessor was also blinded to the treatment allocation, and participants were specifically asked not to discuss their intervention with the assessor. At the end of the last evaluation, all participants were encouraged to state which treatment group they believed they had been allocated to, to assess whether the participant blinding was effective.
Outcome measures
Outcome measures were assessed at baseline, at the end of the 4th week of intervention, and at postintervention (8 weeks), except for cognitive status (baseline and postintervention). At baseline, individuals were weighed and measured and their age, sex, smoking habits, COVID-19-related medical history and clinical characteristics (dichotomous response for the presence of dyspnoea, fatigue, chest pain, etc.) were recorded by clinical interview and medical history. Data on self-perceived dyspnoea and fatigue when performing activities of daily living were also collected at each follow-up period. The primary and secondary outcome measures of the study are briefly presented below. For a detailed description of these outcomes and the measurement process, see Appendix A.
Primary outcomes
-
1
Health-related quality of life. The EuroQol-5D questionnaire (EQ-5D-5L) [13] was employed to measure HRQoL. Additionally, the participants had to rate their current overall health on a visual analogue scale (VAS).
-
2
Exercise tolerance. Cardiorespiratory fitness was assessed by the Ruffier test according to a standardized protocol [14].
Secondary outcomes
-
1
Respiratory muscle function. Maximum static inspiratory and expiratory pressures (MIP and MEP) at the mouth were recorded using a digital mouth pressure meter (MicroRPMTM; Carefusion, San Diego, CA, USA), according to the American Thoracic Society/European Respiratory Society (ATS/ERS) guidelines [15]. Inspiratory muscle endurance (IME) was measured during a constant load breathing test using the POWERbreathe KH1© device (POWERbreathe International Ltd., Southam, UK) according to a published protocol [16].
-
2
Peripheral muscle strength. Lower limb muscle strength was determined using the 1-min sit-to-stand (1-min STS) test according to a standardized protocol [17]. Upper limb muscle strength (handgrip force) was assessed using a hand-held dynamometer (Jamar®, Patterson Medical, IL, USA) according to a standardized protocol [18].
-
3
Lung function. Forced spirometry measurements were assessed using a portable spirometer (Spirobank II USB®, MIR, Rome, Italy) according to ATS/ERS guidelines [19].
-
4
Cognitive and psychological status. The Montreal Cognitive Assessment was used as a screening test to estimate the severity of the participants’ global cognitive impairment [20]. Anxiety/depression levels were evaluated with the Hospital Anxiety and Depression Scale [21]. Post-traumatic stress disorder (PTSD) was evaluated using the PTSD CheckList-Civilian Version (PCL-C) self-rating questionnaire [22].
Interventions
Participants undertook a home-based respiratory muscle training programme using a threshold pressure device. The regimen was 40 min/day, split into two 20-min sessions (morning and afternoon), 6 times per week, over 8 weeks. The training load, regardless of whether participants performed IMT or RMT (real or sham), was individually tailored and increased according to the same distribution schedule for both inspiratory and expiratory muscle training. The only difference between the real and sham groups was the device. Specifically, the sham groups received a device without resistance (0 cm H2O) because it lacked a threshold valve. A complete description of the respiratory muscle training programme based on the Template for Intervention Description and Replication (TIDieR checklist) can be found in Appendix B.
All evening weekly sessions of the study were supervised by a physiotherapist through a virtual platform, who was blinded to the group allocation. The participants allocated to the sham group shared the training sessions with the participants allocated to the experimental group, receiving the same training programme to avoid bias related to the amount of attention.
Sample size calculation
To detect between-group differences for primary outcomes (HRQoL and exercise tolerance), we chose a repeated measures analysis of variance (ANOVA) with an interaction within-between factors, given that the main factor of interest was the group × time interaction (4 groups and 3 measurement times). According to the criteria established by Cohen [23], an effect size of moderate magnitude is required to detect clinically relevant differences. A small-moderate effect size (f=0.20) was chosen because the differences for primary outcomes between the two actual training groups were likely to be small [24] and to increase the study's power. This theoretical model was chosen because of the lack of previous information on the effects of interventions on individuals with long-term post-COVID-19 symptoms. Thus, applying a significance level of 5% (α error) and a power of 90% (β error = 10%), a sample size of 76 participants was established. Assuming a 15% dropout rate, the study sample size was set at least at 88 participants (22 per group).
Data analysis
The Statistical Package for Social Sciences 21 (SPSS Inc., Chicago, IL USA) software was employed to analyse the data. A p-value <0.05 was considered statistically significant, and an intention-to-treat analysis was performed. The Kolmogorov-Smirnov test and normal probability Q-Q plots showed that the underlying residuals of the variables had a reasonably normal distribution. Missing data were imputed by the missForest (sequential random forest) multiple imputation method using the “missForest” package of R software (version 4.1.2). This method has been shown to produce the lowest imputation error for continuous and categorical variables [25].
The effects of the interventions on the quantitative variables were assessed by a separate 4 × 3 mixed-model ANOVA. The group × time interaction was considered the main hypothesis of interest. Partial eta-squared (η2 p) was calculated as a measure of effect size (strength of association) for the interaction in the ANOVAs. Multiple comparisons with Bonferroni adjustment were performed in the case of significant ANOVA findings. Following Vicker's recommendations [26], between-group differences after the interventions were compared using an analysis of covariance adjusting for the values of the respective outcomes at baseline, given that this analysis has the highest statistical power [26]. Between-group effect sizes (Cohen's d) were classified as small (<0.5), medium (0.5–0.7) or large (≥0.8) [23].
Lastly, the effects of the interventions on the qualitative variables (dyspnoea and fatigue) at the end of treatment were assessed with binary logistic regression analysis.
Results
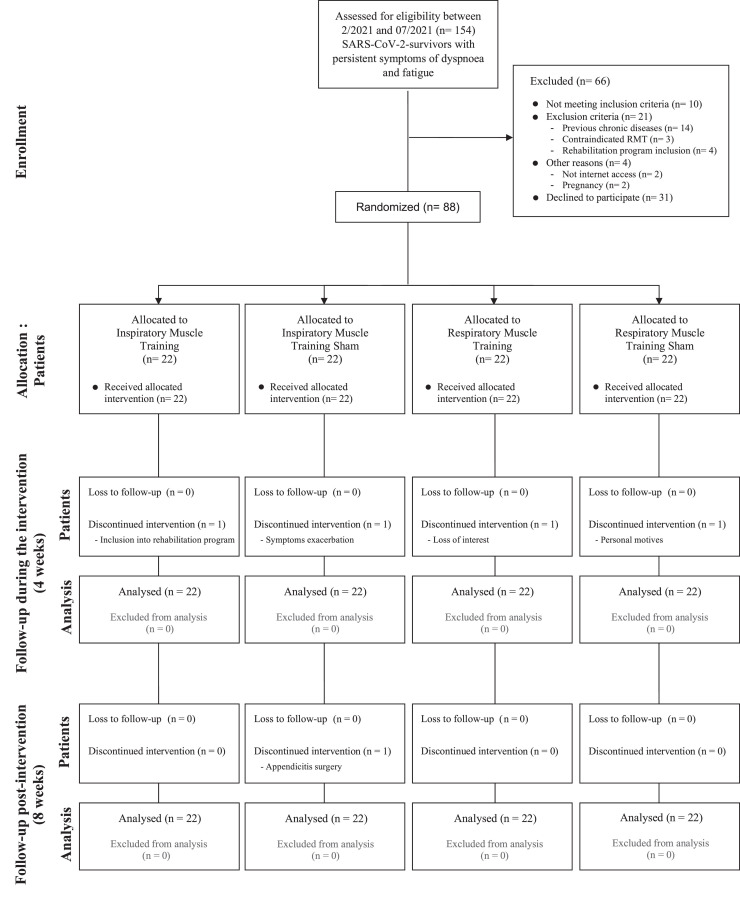
A total of 154 individuals were screened between February 2021 and July 2021 for study participation. Fig. 1 shows the flow diagram of the participants through the study. The baseline characteristics of the included individuals did not differ significantly between the 4 groups (Table 1 ). Five individuals dropped out of the study for various reasons (Fig. 1), and only 1 person presented adverse effects during the study (symptom exacerbation); however, this person belonged to the IMTsham group, so the worsening was not considered due to the intervention. Analyses of the training session compliance diaries revealed that the participants in all groups completed more than 95% of the training. Most of the participants felt that they had undergone real training, with only 8 (20%) of them believing that they had undergone sham training (3 from the IMTsham group and 5 from the RMTsham group).
Fig. 1.
Flowchart.
Table 1.
Descriptive data on anthropometry, smoking habits, COVID-19 related medical history and clinical characteristics of the sample. Values are mean (SD) and n (%).
| Muscle Training Groups |
||||
|---|---|---|---|---|
| Inspiratory | Inspiratorysham | Respiratory | Respiratorysham | |
| (n = 22) | (n = 22) | (n = 22) | (n = 22) | |
| Anthropometric characteristics | ||||
| Age (years) | 48.9 (8.3) | 45.3 (12.8) | 46.5 (9.6) | 45 (10.2) |
| Sex (female) | 17 (77 %) | 16 (73 %) | 14 (64 %) | 16 (73 %) |
| Height (m) | 1.6 (0.1) | 1.7 (0.1) | 1.7 (0.1) | 1.7 (0.1) |
| Weight (Kg) | 71.7 (15.5) | 77.2 (17) | 76 (17.6) | 78.1 (19.1) |
| Body mass index (Kg/m2) | 26.6 (6.6) | 27.1 (5.1) | 27 (5.2) | 27.1 (6) |
| Smoking habits | ||||
| Non-smoker | 10 (45 %) | 11 (50 %) | 11 (50 %) | 13 (59 %) |
| Ex-smoker | 6 (27 %) | 8 (36 %) | 6 (27 %) | 7 (32 %) |
| Smoker | 6 (27 %) | 3 (14 %) | 5 (23 %) | 2 (9 %) |
| Cumulative amount of smoking (in pack-years) | 8.3 (15.5) | 6.8 (12.7) | 4.6 (7.4) | 5.8 (12.9) |
| COVID-19 related medical history | ||||
| Time from COVID-19 diagnosis (days) | 349 (86) | 334 (107) | 364 (67) | 356 (95) |
| Pneumonia | 8 (36 %) | 9 (41 %) | 9 (41 %) | 9 (41 %) |
| Hospital admission | 8 (36 %) | 7 (32 %) | 6 (27 %) | 7 (32 %) |
| Intensive care unit admission | 1 (5 %) | 0 (0 %) | 2 (9 %) | 2 (9 %) |
| Clinical characteristics | ||||
| Dyspnoea | 22 (100 %) | 22 (100 %) | 22 (100 %) | 22 (100 %) |
| Fatigue | 22 (100 %) | 22 (100 %) | 22 (100 %) | 22 (100 %) |
| Chest pain | 13 (59 %) | 12 (55 %) | 12 (55 %) | 15 (68 %) |
| Joint pain | 8 (36 %) | 8 (36 %) | 9 (41 %) | 7 (32 %) |
| Muscle pain | 7 (32 %) | 8 (36 %) | 8 (36 %) | 8 (36 %) |
| Headache | 10 (45 %) | 6 (27 %) | 8 (36 %) | 9 (41 %) |
| Brain fog | 13 (59 %) | 10 (45 %) | 13 (59 %) | 13 (59 %) |
| Insomnia | 11 (50 %) | 8 (36 %) | 8 (36 %) | 8 (36 %) |
| Tachycardia | 6 (27 %) | 7 (32 %) | 6 (27 %) | 5 (23 %) |
| Tingling | 7 (32 %) | 7 (32 %) | 6 (27 %) | 4 (18 %) |
| Anosmia | 3 (14 %) | 3 (14 %) | 1 (5 %) | 3 (14 %) |
| Dysphonia | 2 (9 %) | 1 (5 %) | 1 (5 %) | 4 (18 %) |
| Medications | ||||
| Corticosteroids | 1 (5%) | 2 (9%) | 1 (5%) | 1 (5%) |
| Muscle relaxant | 1 (5%) | 2 (9%) | 1 (5%) | 3 (14%) |
| Sedatives | 4 (18%) | 2 (9%) | 2 (9%) | 4 (18%) |
| Analgesics | 5 (23%) | 4 (18%) | 3 (14%) | 3 (14%) |
| Antidepressants | 1 (5%) | 2 (9%) | 2 (9%) | 2 (9%) |
| Antiepileptics | - | - | 2 (9%) | 1 (5%) |
Health-related quality of life and exercise tolerance
The results of the HRQoL and exercise tolerance outcomes are presented in Table 2 . There were statistically significant interactions between the time and group factors for HRQoL outcomes [EQ-5D-5L, index (F=2.459; p=0.031; η2=0.081) and VAS (F=3.373; p=0.004; η2=0.108)]. There were no statistically significant between-group differences in terms of quality of life at the 4-week follow-up. At post-intervention, there was statistically significant improvement in HRQoL with a large magnitude (d>0.9) in the RMT group compared with the RMTsham group. The VAS-reported quality of life showed moderate-high improvements (d≈0.7) but did not reach statistical significance in the IMT and RMT groups compared with the RMTsham and IMTsham groups, respectively. In addition, all groups showed significantly improved quality of life after the intervention with respect to baseline values (IMT, p<0.001; IMTsham, p=0.003; RMT, p<0.001), except for the RMTsham group.
Table 2.
Change in health-related quality of life and physical function outcomes.
| Outcome | Muscle Training Groups |
Mean (SD) |
|||
|---|---|---|---|---|---|
| Baseline | 4-weeks | Post-intervention | |||
| HRQoL, EQ-5D-5L (index) | Inspiratory | 0.583 (0.204) | 0.686 (0.215)a | 0.746 (0.199)a | |
| Inspiratorysham | 0.622 (0.201) | 0.739 (0.148)a | 0.742 (0.193)a | ||
| Respiratory | 0.615 (0.253) | 0.725 (0.237)a | 0.827 (0.203)a,b | ||
| Respiratorysham | 0.628 (0.180) | 0.701 (0.194) | 0.684 (0.212) | ||
| EQ-5D-5L (VAS) | Inspiratory | 52.5 (15.3) | 63.6 (16.8)a | 68.4 (14.7)a | |
| Inspiratorysham | 58.9 (16.3) | 65.0 (17.2) | 68.1 (17.6)a | ||
| Respiratory | 60.2 (15.5) | 65.7 (17.3) | 79.9 (14.3)a,b | ||
| Respiratorysham | 55.3 (15.6) | 60.9 (17.9) | 60.1 (21.7) | ||
| CRF (Ruffier index) | Inspiratory | 8.3 (2.8) | 9.4 (3.6) | 7.7 (3.3)b | |
| Inspiratorysham | 8.5 (3.7) | 9.6 (2.8) | 8.8 (3.6) | ||
| Respiratory | 9.2 (2.6) | 9.7 (2.8) | 7.6 (2.4)a,b | ||
| Respiratorysham | 8.9 (2.5) | 9.1 (2.6) | 8.7 (2.7) | ||
| 1-min STS (n of squats) | Inspiratory | 33.9 (7.8) | 39.4 (7.4)a | 46.0 (8.2)a,b | |
| Inspiratorysham | 35.3 (8.1) | 37.2 (7.7)a | 38.6 (8.6) | ||
| Respiratory | 34.5 (7.3) | 39.3 (7.3)a | 45.5 (7.8)a,b | ||
| Respiratorysham | 35.9 (7.3) | 38.1 (8.4) | 39.4 (8.8) | ||
| Handgrip (Kg) | Inspiratory | 26.3 (8.6) | 28.0 (7.6) | 29.2 (9.5)a | |
| Inspiratorysham | 26.0 (8.2) | 28.1 (8.0) | 29.8 (9.0)a | ||
| Respiratory | 31.4 (10.5) | 32.6 (11.1) | 34.0 (10.0)a | ||
| Respiratorysham | 30.0 (7.4) | 30.7 (9.0) | 32.3 (8.7)a | ||
| Outcome | Comparisons between Muscle Training Groups |
Adjusted difference (95%CI); Cohen's d |
|||
| At 4-weeks | At Post-intervention | ||||
| HRQoL, EQ-5D-5L (index) | Inspiratory vs Inspiratorysham | -0.027 (-0.144; 0.090); d=0.1 | 0.029 (-0.094; 0.151); d=0.3 | ||
| Inspiratory vs Respiratory | -0.017 (-0.134; 0.100); d=0.1 | -0.060 (-0.182; 0.063); d=0.1 | |||
| Inspiratory vs Respiratorysham | 0.015 (-0.102; 0.132); d=0.4 | 0.091 (-0.032; 0.214); d=0.7 | |||
| Inspiratorysham vs Respiratory | 0.010 (-0.107; 0.127); d=0.1 | -0.089 (-0.211; 0.034); d=0.5 | |||
| Inspiratorysham vs Respiratorysham | 0.042 (-0.075; 0.159); d=0.3 | 0.062 (-0.060; 0.184); d=0.4 | |||
| Respiratory vs Respiratorysham | 0.032 (-0.085; 0.149); d=0.3 | 0.151 (0.029; 0.273); d=1⁎⁎ | |||
| EQ-5D-5L (VAS) | Inspiratory vs Inspiratorysham | 3.8 (-5.9; 13.5); d=0.5 | 4.4 (-7.1; 15.9); d=0.5 | ||
| Inspiratory vs Respiratory | 4.2 (-5.6; 14.0); d=0.5 | -6.2 (-17.8; 5.4); d=0.3 | |||
| Inspiratory vs Respiratorysham | 5.1 (-4.6; 14.7); d=0.5 | 10.1 (-1.4; 21.5); d=0.7 | |||
| Inspiratorysham vs Respiratory | 0.4 (-9.2; 10.1); d=0.1 | -10.6 (-22.1; 0.8); d=0.7 | |||
| Inspiratorysham vs Respiratorysham | 1.3 (-8.4; 10.9); d<0.1 | 5.7 (-5.8; 17.1); d=0.3 | |||
| Respiratory vs Respiratorysham | 0.8 (-8.9; 10.5); d<0.1 | 16.3 (4.8; 27.8); d=0.9⁎⁎ | |||
| CRF (Ruffier index) | Inspiratory vs Inspiratorysham | Group*time interaction not statistically significant | |||
| Inspiratory vs Respiratory | |||||
| Inspiratory vs Respiratorysham | |||||
| Inspiratorysham vs Respiratory | |||||
| Inspiratorysham vs Respiratorysham | |||||
| Respiratory vs Respiratorysham | |||||
| 1-min STS (n of squats) | Inspiratory vs Inspiratorysham | 2.8 (-2.6; 8.3); d=0.4 | 8.0 (1.6; 14.4); d=1.1⁎⁎ | ||
| Inspiratory vs Respiratory | 0.3 (-5.1; 5.8); d=0.1 | 0.7 (-5.6; 7.1); d=0.1 | |||
| Inspiratory vs Respiratorysham | 2.3 (-3.2; 7.8); d=0.4 | 7.4 (1.0; 13.8); d=1.0* | |||
| Inspiratorysham vs Respiratory | -2.5 (-8.0; 3.0); d=0.4 | -7.2 (-13.6; -0.9); d=0.8* | |||
| Inspiratorysham vs Respiratorysham | -0.5 (-6.0; 4.9); d<0.1 | -0.6 (-6.9; 5.8); d<0.1 | |||
| Respiratory vs Respiratorysham | 2.0 (-3.5; 7.5); d=0.4 | 6.7 (0.3; 13.1); d=0.8* | |||
| Handgrip (Kg) | Inspiratory vs Inspiratorysham | Group*time interaction not statistically significant | |||
| Inspiratory vs Respiratory | |||||
| Inspiratory vs Respiratorysham | |||||
| Inspiratorysham vs Respiratory | |||||
| Inspiratorysham vs Respiratorysham | |||||
| Respiratory vs Respiratorysham | |||||
Statistically significant differences with respect to baseline values p<0.05.
Statistically significant differences with respect to the 4-weeks values p<0.05.
Statistically significant differences p<0.05.
Statistically significant differences p<0.01.
CRF, cardiorespiratory fitness; HRQoL, health-related quality of life; STS, sit-to-stand; VAS, visual analogue scale.
There were no statistically significant interactions between the time and group factors for exercise tolerance. There were no statistically significant between-group differences for exercise tolerance. With respect to baseline values, however, exercise tolerance improved only in the RMT group (p=0.047).
Long-term post-COVID-19 symptoms and respiratory muscle function
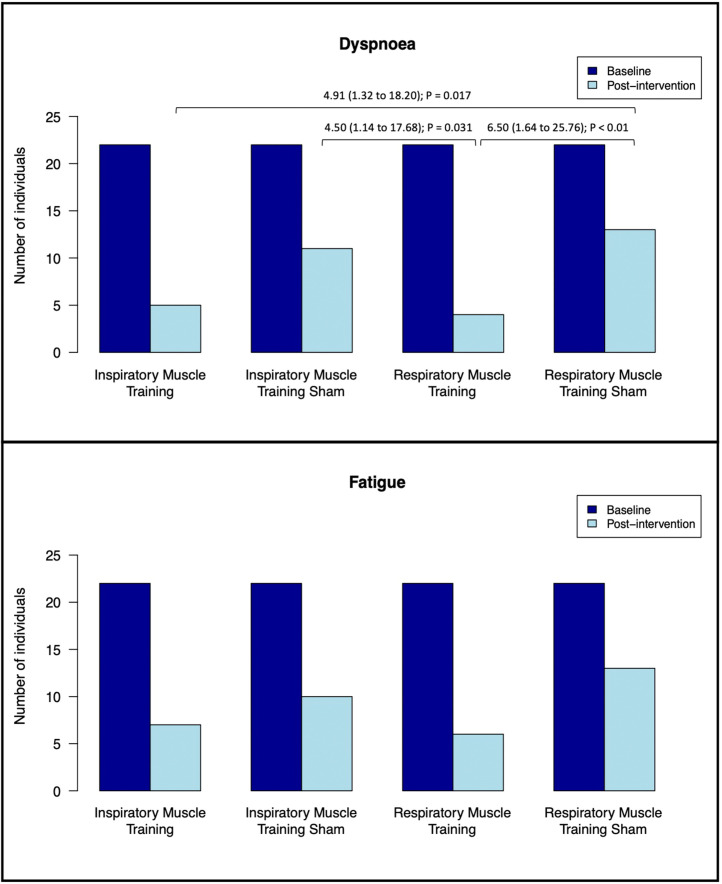
The binary logistic regression analysis revealed statistically significant between-group differences only for long-term post-COVID-19 dyspnoea after the intervention (Wald test=10.6; p=0.014) and not for long-term post-COVID-19 fatigue (Wald test=5.4; p=0.142). Specifically, the RMT group had an approximately 5–6-fold greater reduction in dyspnoea than the 2 sham groups (IMTsham, p=0.031; RMTsham, p=0.008), whereas the IMT group had an approximately 5-fold greater reduction in dyspnoea than the RMTsham group (p=0.017) (Fig. 2 ). There were no statistically significant differences among the other groups.
Fig. 2.
Change in dyspnoea and fatigue outcomes. Statistically significant associations are presented as Odds Ratios (95%CI); p-value.
The results of the respiratory muscle function outcomes are presented in Table 3 . There were statistically significant interactions between the time and group factors for respiratory muscle function outcomes (MIP: F=5.797; p<0.001; η2=0.172; MEP: F=6.840; p<0.001; η2=0.196; and IME: F=5.231; p<0.001; η2=0.157). Both real training groups showed a statistically significant and large (d>0.8) increase in inspiratory muscle strength and endurance compared with the 2 sham groups at the postintervention. Expiratory muscle strength showed a statistically significant and large (d=0.9–1.4) increase in the RMT group compared with the other 3 groups, both at 4-week follow-up and at the postintervention. In addition, all groups significantly improved MIP (IMT and RMT, p<0.001; IMTsham, p=0.004; RMTsham, p=0.006), MEP (IMT, RMT and RMTsham, p<0.001; IMTsham, p=0.032), and IME (IMT, IMTsham and RMT, p<0.001; RMTsham, p=0.003) after the intervention with respect to baseline.
Table 3.
Change in respiratory muscle function outcomes.
| Outcome | Muscle Training Groups |
Mean (SD) |
|||
|---|---|---|---|---|---|
| Baseline | 4-weeks | Post-intervention | |||
| MIP (cmH2O) (% pred) |
Inspiratory | 77.8 (21.6) 79 (19) |
99.1 (13.6)a 101 (14) |
109.5 (17.6)a,b 111 (18) |
|
| Inspiratorysham | 85.2 (22.8) 79 (20) |
93.5 (23.6) 87 (21) |
98.6 (21.9)a 91 (18) |
||
| Respiratory | 90.0 (22.1) 81 (17) |
110.7 (25.3)a 99 (18) |
123.5 (28.1)a,b 111 (21) |
||
| Respiratorysham | 91.4 (27.2) 86 (22) |
102.4 (30.3)a 96 (24) |
104.1 (27.5)a 98 (24) |
||
| MEP (cmH2O) (% pred) |
Inspiratory | 102.8 (28.6) 73 (18) |
113.1 (18.2) 81 (13) |
123.9 (24.9)a,b 88 (16) |
|
| Inspiratorysham | 104.9 (35.6) 69 (20) |
108.2 (30.6) 71 (20) |
116.0 (35.2)a,b 75 (22) |
||
| Respiratory | 113.1 (32.0) 71 (20) |
143.5 (37.2)a 90 (17) |
154.9 (38.7)a,b 97 (17) |
||
| Respiratorysham | 109.1 (39.9) 70 (23) |
122.1 (36.1)a 78 (19) |
127.1 (34.0)a 81 (19) |
||
| IME (sec) | Inspiratory | 198.0 (101.1) | 400.4 (184.2)a | 456.7 (143.8)a | |
| Inspiratorysham | 191.7 (99.3) | 233.9 (115.4) | 322.6 (174.8)a,b | ||
| Respiratory | 180.8 (96.9) | 352.4 (129.9)a | 459.3 (175.4)a,b | ||
| Respiratorysham | 194.8 (108.5) | 288.6 (145.1)a | 308.0 (130.6)a | ||
| Outcome | Comparisons between Muscle Training Groups |
Adjusted difference (95%CI); Cohen's d |
|||
| At 4-weeks | At Post-intervention | ||||
| MIP (cmH2O) | Inspiratory vs Inspiratorysham | 11.5 (-1.1; 24.0); d=0.9 | 16.2 (1.8; 30.7); d=1.0* | ||
| Inspiratory vs Respiratory | -1.9 (-14.6; 10.8); d<0.1 | -5.3 (-19.8; 9.3); d=0.2 | |||
| Inspiratory vs Respiratorysham | 7.5 (-5.3; 20.3); d=0.6 | 15.1 (0.5; 29.7); d=1.0* | |||
| Inspiratorysham vs Respiratory | -13.3 (-25.9; -0.8); d=0.8* | -21.5 (-35.9; -7.2); d=1.0⁎⁎ | |||
| Inspiratorysham vs Respiratorysham | -3.9 (-16.5; 8.6); d=0.2 | -1.1 (-15.5; 13.2); d<0.1 | |||
| Respiratory vs Respiratorysham | 9.4 (-3.1; 21.9); d=0.5 | 20.4 (6.0; 34.7); d=1.0⁎⁎ | |||
| MEP (cmH2O) | Inspiratory vs Inspiratorysham | 6.5 (-8.4; 21.4); d=0.3 | 9.7 (-5.8; 25.1); d=0.5 | ||
| Inspiratory vs Respiratory | -22.6 (-37.6; -7.6); d=1.0⁎⁎ | -22.6 (-38.1; -7.0); d=0.9⁎⁎ | |||
| Inspiratory vs Respiratorysham | -4.2 (-19.2; 10.7); d=0.1 | 2.0 (-13.5; 17.5); d=0.1 | |||
| Inspiratorysham vs Respiratory | -29.1 (-44.1; -14.2); d=1.4⁎⁎ | -32.3 (-47.8; -16.8); d=1.4⁎⁎ | |||
| Inspiratorysham vs Respiratorysham | -10.7 (-25.7; 4.2); d=0.5 | -7.7 (-23.2; 7.8); d=0.3 | |||
| Respiratory vs Respiratorysham | 18.4 (3.5; 33.3); d=0.9⁎⁎ | 24.6 (9.1; 40.0); d=1.0⁎⁎ | |||
| IME (sec) | Inspiratory vs Inspiratorysham | 161.9 (58.4; 265.5); d=1.2⁎⁎ | 130.9 (9.2; 252.5); d=0.8* | ||
| Inspiratory vs Respiratory | 35.6 (-68.2; 139.3); d=0.2 | -11.5 (-133.3; 110.3); d=0.1 | |||
| Inspiratory vs Respiratorysham | 109.4 (5.9; 213.0); d=0.7* | 147.0 (25.4; 268.6); d=1.0⁎⁎ | |||
| Inspiratorysham vs Respiratory | -126.4 (-230.0; -22.8); d=1.2⁎⁎ | -142.4 (-264.1; -20.6); d=0.9* | |||
| Inspiratorysham vs Respiratorysham | -52.5 (-156.0; 51.0); d=0.5 | 16.1 (-105.5; 137.8); d=0.1 | |||
| Respiratory vs Respiratorysham | 73.9 (-29.8; 177.5); d=0.6 | 158.5 (36.7; 280.3); d=1.1⁎⁎ | |||
Statistically significant differences with respect to baseline values p<0.05.
Statistically significant differences with respect to the 4-weeks values p<0.05.
Statistically significant differences p<0.05.
Statistically significant differences p<0.01.
IME, inspiratory muscle endurance; MIP, maximal inspiratory pressure; MEP, maximal expiratory pressure; % pred, percentage of predicted value.
Peripheral muscle strength
The results of the peripheral muscle strength outcomes are presented in Table 2. The only physical function variable that showed a statistically significant group × time interaction was lower limb muscle strength (1-min STS: F=3.833; p=0.001; η2=0.120). There were no statistically significant between-group differences in lower limb muscle strength at the 4-week follow-up. At the postintervention, both real training groups showed a statistically significant and large (d≥0.8) increase in lower limb muscle strength compared with the 2 sham groups, especially the RMT group (d≥1). Only the real training groups showed a significant increase in lower limb muscle strength after the intervention over baseline (IMT and RMT, p<0.001). There were no statistically significant between-group differences for upper limb muscle strength. With respect to baseline values, however, upper limb muscle strength increased significantly in all groups (IMT, p=0.002; IMTsham, p<0.001; RMT, p=0.008; RMTsham, p=0.023).
Lung function
The results of the lung function outcomes are presented in Table 4 . The only lung function variable that showed a statistically significant group × time interaction was peak expiratory flow (PEF; F=3.612; p=0.003; η2=0.114). The RMT group showed a statistically significant and large (d≥0.9) increase in PEF compared with the other 3 groups at the postintervention. In fact, only the RMT group significantly increased PEF after the intervention with respect to baseline (p<0.001).
Table 4.
Change in pulmonary function outcomes.
| Outcome | Muscle Training Groups |
Mean (SD) |
|||
|---|---|---|---|---|---|
| Baseline | 4-weeks | Post-intervention | |||
| FVC (liters) (% pred) |
Inspiratory | 3.8 (0.7) 118 (16) |
3.9 (0.8) 121 (17) |
3.9 (0.8) 120 (18) |
|
| Inspiratorysham | 4.0 (1.0) 108 (16) |
4.0 (0.9) 109 (14) |
4.0 (1.0) 110 (14) |
||
| Respiratory | 4.1 (0.9) 113 (17) |
4.2 (0.9) 117 (17) |
4.2 (1.0) 118 (20) |
||
| Respiratorysham | 4.0 (0.7) 110 (20) |
4.1 (0.8) 112 (19) |
4.1 (0.8) 112 (16) |
||
| FEV1 (liters) (% pred) |
Inspiratory | 2.9 (0.5) 107 (15) |
3.0 (0.6) 109 (17) |
3.0 (0.6) 109 (17) |
|
| Inspiratorysham | 3.1 (0.9) 101 (17) |
3.1 (0.8) 101 (15) |
3.0 (0.9) 100 (16) |
||
| Respiratory | 3.1 (0.8) 100 (19) |
3.1 (0.7) 103 (16) |
3.1 (0.8) 104 (19) |
||
| Respiratorysham | 3.1 (0.6) 99 (19) |
3.1 (0.6) 102 (18) |
3.1 (0.6) 102 (15) |
||
| FEV1/FVC (%) | Inspiratory | 77 (5) | 76 (6) | 76 (4) | |
| Inspiratorysham | 78 (6) | 78 (6) | 76 (6) | ||
| Respiratory | 74 (8) | 74 (4) | 74 (6) | ||
| Respiratorysham | 76 (6) | 76 (7) | 76 (8) | ||
| PEF (liters) (% pred) |
Inspiratory | 6.5 (1.3) 98 (19) |
6.5 (1.2) 97 (16) |
6.5 (1.1) 98 (18) |
|
| Inspiratorysham | 7.1 (2.1) 96 (25) |
7.0 (2.0) 97 (23) |
6.8 (2.2) 94 (24) |
||
| Respiratory | 6.6 (1.8) 89 (20) |
7.3 (2.2)a 100 (25) |
7.6 (1.8)a 104 (20) |
||
| Respiratorysham | 7.2 (1.7) 98 (24) |
7.4 (1.6) 102 (20) |
7.3 (1.6) 101 (20) |
||
| Outcome | Comparisons between Muscle Training Groups |
Adjusted difference (95%CI); Cohen's d |
|||
| At 4-weeks | At Post-intervention | ||||
| FVC (liters) | Inspiratory vs Inspiratorysham | Group*time interaction not statistically significant | |||
| Inspiratory vs Respiratory | |||||
| Inspiratory vs Respiratorysham | |||||
| Inspiratorysham vs Respiratory | |||||
| Inspiratorysham vs Respiratorysham | |||||
| Respiratory vs Respiratorysham | |||||
| FEV1 (liters) | Inspiratory vs Inspiratorysham | Group*time interaction not statistically significant | |||
| Inspiratory vs Respiratory | |||||
| Inspiratory vs Respiratorysham | |||||
| Inspiratorysham vs Respiratory | |||||
| Inspiratorysham vs Respiratorysham | |||||
| Respiratory vs Respiratorysham | |||||
| FEV1/FVC (%) | Inspiratory vs Inspiratorysham | Group*time interaction not statistically significant | |||
| Inspiratory vs Respiratory | |||||
| Inspiratory vs Respiratorysham | |||||
| Inspiratorysham vs Respiratory | |||||
| Inspiratorysham vs Respiratorysham | |||||
| Respiratory vs Respiratorysham | |||||
| PEF (liters) | Inspiratory vs Inspiratorysham | -0.1 (-1.0; 0.8); d<0.1 | 0.1 (-0.7; 0.8); d=0.1 | ||
| Inspiratory vs Respiratory | -0.7 (-1.6; 0.1); d=0.6 | -1.1 (-1.9; -0.3); d=1.2⁎⁎ | |||
| Inspiratory vs Respiratorysham | -0.4 (-1.3; 0.5); d=0.3 | -0.3 (-1.1; 0.5); d=0.3 | |||
| Inspiratorysham vs Respiratory | -0.6 (-1.5; 0.2); d=0.7 | -1.2 (-1.9; -0.4); d=1.2⁎⁎ | |||
| Inspiratorysham vs Respiratorysham | -0.3 (-1.2; 0.6); d=0.3 | -0.4 (-1.2; 0.4); d=0.4 | |||
| Respiratory vs Respiratorysham | 0.3 (-0.5; 1.2); d=0.4 | 0.8 (0.01; 1.6); d=0.9* | |||
Statistically significant differences with respect to baseline values p<0.05.
Statistically significant differences p<0.05.
Statistically significant differences p<0.01.
FEV1, force expiratory volume 1st second; FVC, force vital capacity; PEF, peak expiratory flow; % pred, percentage of predicted value.
Cognitive and psychological status
The results of the cognitive and psychological status outcomes are presented in Appendix C. There were no statistically significant interactions between the time and group factors for the cognitive and psychological status outcomes. There were no statistically significant differences between the groups for the cognitive and psychological status. However, all groups significantly improved their cognitive function (IMT and RMTsham, p<0.001; IMTsham, p=0.022; RMT, p=0.011) and reduced their distress (anxiety and depression; IMT and IMTsham, p<0.001; RMT, p=0.005) and post-traumatic stress (IMT and RMT, p<0.001; IMTsham, p=0.004; RMTsham, p=0.017) after the intervention with respect to baseline, except for the distress of the RMTsham (p=0.083).
Discussion
This study assessed the effects of a home-based respiratory muscle training programme monitored by videoconferencing on the HRQoL and exercise tolerance in individuals with long-term post-COVID-19 symptoms. The respiratory muscle training programme was effective in improving HRQoL, but not exercise tolerance, only when combined inspiratory and expiratory muscle training was performed. The participants complied with all interventions and achieved large and clinically important improvements in lower limb muscle strength, inspiratory muscle strength and endurance regardless of the muscle group trained. The RMT group (inspiratory/expiratory muscles) achieved higher improvements in expiratory muscle function (i.e., strength and PEF) compared with the 2 sham training groups. With regard to the comparison between the 2 real training groups (combined training vs. IMT in isolation), the RMT group had superior results for expiratory muscle function (i.e., strength and PEF) to those of the IMT group.
To our knowledge, no previous studies have investigated the clinical effects of a home-based respiratory muscle training programme on individuals with long-term post-COVID-19 symptoms. Therefore, the results are discussed considering other respiratory conditions with similar features, while recognizing the many differences between them. Only one study has investigated the effects of a 6-week respiratory rehabilitation programme including respiratory muscle training on elderly people with COVID-19 [27]. However, the findings should be interpreted with caution due to the very low methodological quality of the study, which might have biased its results and conclusions. For this reason, those results should not be compared with ours. Another pilot study [28] involved individuals who had recovered from COVID-19 after weaning from mechanical ventilation. The participants performed a 2-week IMT programme and showed similar improvements in HRQoL, dyspnoea and functional performance, except for lung function, although the two samples of individuals (theirs and ours) are not in fact comparable. Specifically, the study by Abodonya et al. [28] consisted mainly of men who had required intensive care unit admission and with reduced lung function. However, our sample consisted mostly of young women (∼50 years mean age) who had not required mechanical ventilation and with preserved lung function, but with long-term post-COVID-19 symptoms. Hence, our sample reinforces findings that middle-aged women have a higher risk of long-term post-COVID-19 symptoms [29]. The relevance of respiratory muscle training in severe COVID-19 has already been proven; however, our findings provide new evidence for the effects of this intervention on individuals with long-term post-COVID-19 symptoms.
Health-related quality of life and exercise tolerance
Only the RMT group showed a large and clinically important increase in HRQoL compared with the RMTsham group, with no differences in the other between-group comparisons. This improvement could be associated with an increase in the metaboreflex activation threshold, improving functional capacity, which could have an impact on constructs such as mobility and daily life activities, improving individual-reported outcomes related to the physical component. In addition, the RMT group was the only one that could be considered to have had a clinical improvement in quality of life compared with both sham training groups, given that the effect size with respect to the IMTsham group was large and exceeded the minimum clinically important difference established for the VAS of the EQ-5D-5L [30]. The coordinated action of the diaphragm and the abdominal muscles is essential for trunk stabilisation to perform functional activities [31]. Therefore, when individuals improve the strength of their expiratory muscles, they will also increase their ability to perform activities that require spinal stability, such as numerous activities of daily living. This aspect could explain the superiority observed in the RMT group (and not in the IMT group) compared with the sham training groups. However, it should be emphasised that the RMT group did not show statistically significant differences in quality of life compared to the IMTsham, so our hypothesis and results should be interpreted with caution. In the temporary situation of uncertainty and social distancing in which the study took place, it is possible that the fact of receiving care, as well as an individualised training programme by health professionals, could generate a hormonal and immunological response in our participants that was responsible for their therapeutic improvement [32]. However, although the placebo effect would also affect the real training groups, our results seem to show that respiratory muscle training provides a greater improvement in quality of life than is attributable to the placebo effect alone. Further research involving a larger training duration is needed to further substantiate these results.
We hypothesized that, as has been observed in other diseases [33,34], respiratory muscle training could increase exercise tolerance by attenuating the inspiratory metaboreflex [8]. However, this hypothesis was not confirmed, as the actual training of the respiratory muscles was not superior to the placebo training. The lack of effect on cardiorespiratory fitness could be because the respiratory muscle training intervention is not a dynamic exercise and therefore does not produce an increased workload on the cardiovascular system. Larger studies are needed to examine the effectiveness of the combined intervention (RMT group) proposed in this study as an adjunct to another exercise programme (i.e., aerobic exercises) on the functional exercise tolerance of individuals with long-term post-COVID-19 symptoms.
Long-term post-COVID-19 symptoms and respiratory muscle function
With respect to the long-term post-COVID-19 symptoms, the RMT group had an approximately 5–6-fold greater reduction in their dyspnoea than both sham groups after the intervention, whereas the IMT group had an approximately 5-fold grater reduction in their dyspnoea than the RMTsham group. Changes in fatigue failed to reach statistical significance but resulted in improvement. These results might be explained by the fact that respiratory muscle training can decrease the neural respiratory drive by improving respiratory mechanics [35] and increasing MIP, thereby reducing the ratio of inspiratory effort (oesophageal pressure expressed as a fraction of maximal oesophageal pressure at isovolume; Pes/MIP) [36], which, in turn, reduces the sensation of dyspnoea. The lack of effect on fatigue would suggest that other types of training should be applied for this long-term post-COVID-19 symptom.
Both real training groups produced a large increase (d>0.8) in inspiratory muscle strength and endurance compared with both sham groups at the postintervention assessment. The improvement in MIP could be considered clinically relevant because the effect size was large, especially because it exceeded the recently redefined minimum clinically important difference of 17 cmH2Ocm 2O in other populations [37,38]. Our results are in line with a case series that demonstrated that moderate intensity respiratory muscle training could lead to greater improvements and are perfectly tolerated by individuals with COVID-19, even by those who had been admitted to intensive care units [39]. Our findings would reinforce evidence supporting that improvements in inspiratory muscle function depend on the magnitude of the inspiratory load, with more substantial gains achieved by individuals training at higher loads [33]. Furthermore, the minimum clinically important difference for inspiratory endurance time (261 s) [34] was exceeded by the RMT group and was approached by the IMT group. This result is supported by a study of individuals with chronic obstructive pulmonary disease that showed significant increases in the proportion of type I fibres and the size of type II fibres in the external intercostal muscles after IMT [40]. Expiratory muscle strength showed a large increase (d=0.88–1.42) in the RMT group compared with the other 3 groups, which was to be expected.
Peripheral muscle strength
The respiratory muscle training, regardless of the modality, increased the muscle strength in the lower body. In fact, both real training groups showed a large effect size compared with the sham training groups and exceeded the minimum clinically important difference established for the 1-min STS test [41], so this increase could be considered clinically relevant. This finding is supported by a previous study [28] that showed similar improvements in functional performance after an IMT programme for individuals who had recovered from COVID-19. A potential explanation for this improvement in limb strength following the respiratory muscle training programme could be the attenuation of the inspiratory metaboreflex and, consequently, the larger proportion of cardiac output that can be redirected to peripheral muscles [8], as has been observed in other diseases [33,34]. Thus, the improvement in lower limb strength would be related to an indirect effect of the training programme, although the influence of other factors such as dyspnoea and/or fatigue during the test cannot be ruled out. In fact, the reduction in dyspnoea could be responsible for the increase in strength, as the training of the respiratory muscles reduced dyspnoea. However, upper limb grip strength did not increase. Grip strength requires less recruitment of muscle groups and therefore a lower energy cost than performing squats for 1 min [42]. In addition, a few seconds of isometric grip strength will result in less blood flow restriction than that caused by the eccentric/concentric contraction required for the 1-min STS. These reasons could explain why improvements would be obtained only in lower limb muscle strength, given that the metaboreflex would indirectly affect grip strength to a lesser extent.
Lung function
Changes in lung function failed to reach statistically significant between-group differences in line with meta-analyses in individuals with other respiratory diseases [43,44]. The intrinsic factors of airway limitation might be more determinant for spirometric values than the increase in respiratory muscle strength provided by the respiratory muscle training. Only the PEF value improved in the RMT group, given that expiratory muscle contraction is necessary to build up high positive intrapleural and intra-airway pressures for developing the PEF rate [45].
Cognitive and psychological status
The cognitive and psychological status of the individuals with long-term post-COVID-19 symptoms improved over the 8-week period, irrespective of group. One possible explanation for these slight improvements might be that the supervision and reinforcement provided by the physiotherapist during the training sessions facilitated the participants’ progression [46]. Although our intervention was not focused on “group therapy”, the participants addressed personal issues during the sessions that were able to influence these outcomes [46], especially when dealing with new, destabilizing conditions. In addition, according to the cut-off scores [47,48], our cohort reported low levels of anxiety, depression and post-traumatic stress, which implies that individuals with long-term post-COVID-19 symptoms were still experiencing a deficit in their emotional aspects months after the acute infection. These results reinforce the literature reporting that infection-related psychiatric symptoms persist long after recovery [49]. Based on these findings, we hypothesise that emotional state might be one of the most important determinants for the development of long-term post-COVID-19 symptoms, as our sample had no other risk factors for their development (i.e. hypertension, obesity, psychiatric or immunosuppressive condition) [29]. In addition, it makes sense that individuals who had a more severe acute phase requiring hospital admission, another risk factor that may predispose a person to develop long-term post-COVID-19 symptoms [29], may also present more psychological impairment. Further studies focused on psychological interventions to treat these aspects in individuals with long-term post-COVID-19 symptoms are needed.
Limitations
The present study has certain limitations. First, conventional cardiopulmonary exercise testing was not used in this study to assess exercise tolerance. However, the field test employed has been demonstrated as reliable and valid for assessing cardiorespiratory fitness [14]. Second, the presence of dyspnoea and fatigue symptoms were collected as dichotomous variables; the effects of the intervention for varying levels of dyspnoea and fatigue could therefore not be calculated. Third, this study exclusively assessed the effects of respiratory muscle training, with greater improvements likely to be obtained when that training is combined with other exercise modalities such as cardiorespiratory and/or endurance exercise. In addition, although a sample size calculation was performed, the number of individuals per group was small, so extrapolation of the results to clinical practice should be done with caution. Lastly, the medium and long-term effects of the interventions were not evaluated because we considered it relevant to provide timely evidence-based data on the effects of respiratory muscle training in individuals with long-term post-COVID-19 symptoms, to show the advantages and challenges of rehabilitation, and to address the effects of this new condition that has radically changed our existence. Further studies with a more pragmatic approach should explore the full potential of various programmes in these individuals’ recovery and should include a longer follow-up and longer training durations.
Clinical implications
Therapies focused on respiratory muscle training could be included in treatments to improve inspiratory muscle strength and endurance in individuals with long-term post-COVID-19 symptoms, especially individuals with high levels of dyspnoea. After COVID-19 infection, individuals appear to have impaired respiratory muscle strength [50]; these improvements might therefore be of clinical relevance to these individuals. To obtain the greatest improvements in HRQoL and expiratory muscle function, the use of combined training (inspiratory/expiratory muscles) is recommended over their application in isolation. Remote supervision has the potential to improve access to rehabilitation programmes and help improve individuals’ adherence to training, which could increase the motivation of healthcare providers to prescribe and provide the intervention.
Conclusions
An 8-week home-based respiratory muscle training programme, supervised by videoconferencing, was effective in improving HRQoL, but not exercise tolerance, only when combined inspiratory and expiratory muscle training in individuals with long-term post-COVID-19 symptoms. The home-based respiratory muscle training programme was effective in improving inspiratory muscle strength/endurance and lower limb muscle strength regardless of the muscle group trained. The combined training was also more effective in improving expiratory muscle function (i.e., strength and PEF) than IMT and the 2 sham training modalities. However, respiratory muscle training had no impact on exercise tolerance, lung function, and cognitive and psychological status.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Funding
The study was supported by grant from “Premio ayudas a la investigación en fisioterapia y covid-19” by the Illustrious Professional Association of Physiotherapists of the Community of Madrid, Spain. This funding source had no role in the design of this study and had no role during its execution, statistical analysis, interpretation of the data, or decision to submit results.
Acknowledgements
The authors thank the participants who took part in this study, the Long Covid ACTS and Covid persistente España (Persistent COVID Spain) for their help and especially Elizabeth Semper.
Footnotes
Clinical Trials Registry. (ClinicalTrials.gov: NCT04734561)
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.rehab.2022.101709.
Appendix. Supplementary materials
References
- 1.Fernández-de-las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Florencio LL, Cuadrado ML, Plaza-Manzano G, et al. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur J Intern Med. 2021;92:55–70. doi: 10.1016/J.EJIM.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. 2021;9:129. doi: 10.1016/S2213-2600(21)00031-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raman B, Cassar MP, Tunnicliffe EM, Filippini N, Griffanti L, Alfaro-Almagro F, et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalMedicine. 2021;31 doi: 10.1016/J.ECLINM.2020.100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miskowiak KW, Johnsen S, Sattler SM, Nielsen S, Kunalan K, Rungby J, et al. Cognitive impairments four months after COVID-19 hospital discharge: Pattern, severity and association with illness variables. Eur Neuropsychopharmacol. 2021;46:39–48. doi: 10.1016/J.EURONEURO.2021.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittal J, Ghosh A, Bhatt SP, Anoop S, Ansari IA, Misra A. High prevalence of post COVID-19 fatigue in patients with type 2 diabetes: A case-control study. Diabetes Metab Syndr. 2021;15 doi: 10.1016/J.DSX.2021.102302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ, Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/J.1469-7793.2001.0277K.X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ribeiro JP, Chiappa GR, Callegaro CC. The contribution of inspiratory muscles function to exercise limitation in heart failure: pathophysiological mechanisms. Rev Bras Fisioter. 2012;16:261–267. doi: 10.1590/S1413-35552012005000034. [DOI] [PubMed] [Google Scholar]
- 8.Shei RJ. Recent Advancements in Our Understanding of the Ergogenic Effect of Respiratory Muscle Training in Healthy Humans: A Systematic Review. J Strength Cond Res. 2018;32:2665–2676. doi: 10.1519/JSC.0000000000002730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno AM, Toledo-Arruda AC, Lima JS, Duarte CS, Villacorta H, Nóbrega ACL. Inspiratory Muscle Training Improves Intercostal and Forearm Muscle Oxygenation in Patients With Chronic Heart Failure: Evidence of the Origin of the Respiratory Metaboreflex. J Card Fail. 2017;23:672–679. doi: 10.1016/J.CARDFAIL.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JP, Chiappa GR, Neder AJ, Frankenstein L. Respiratory muscle function and exercise intolerance in heart failure. Curr Heart Fail Rep. 2009;6:95–101. doi: 10.1007/S11897-009-0015-7. [DOI] [PubMed] [Google Scholar]
- 11.Beaumont M, Forget P, Couturaud F, Reychler G. Effects of inspiratory muscle training in COPD patients: A systematic review and meta-analysis. Clin Respir J. 2018;12:2178–2188. doi: 10.1111/CRJ.12905. [DOI] [PubMed] [Google Scholar]
- 12.Turolla A, Rossettini G, Viceconti A, Palese A, Geri T. Musculoskeletal Physical Therapy During the COVID-19 Pandemic: Is Telerehabilitation the Answer? Phys Ther. 2020;100:1260–1264. doi: 10.1093/PTJ/PZAA093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual Life Res. 2011;20:1727–1736. doi: 10.1007/S11136-011-9903-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sartor F, Bonato M, Papini G, Bosio A, Mohammed RA, Bonomi AG, et al. A 45-Second Self-Test for Cardiorespiratory Fitness: Heart Rate-Based Estimation in Healthy Individuals. PLoS One. 2016;11 doi: 10.1371/JOURNAL.PONE.0168154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166:518–624. doi: 10.1164/RCCM.166.4.518. [DOI] [PubMed] [Google Scholar]
- 16.Langer D, Charususin N, Jácome C, Hoffman M, McConnell A, Decramer M, et al. Efficacy of a Novel Method for Inspiratory Muscle Training in People With Chronic Obstructive Pulmonary Disease. Phys Ther. 2015;95:1264–1273. doi: 10.2522/PTJ.20140245. [DOI] [PubMed] [Google Scholar]
- 17.Núñez-Cortés R, Rivera-Lillo G, Arias-Campoverde M, Soto-García D, García-Palomera R, Torres-Castro R. Use of sit-to-stand test to assess the physical capacity and exertional desaturation in patients post COVID-19. Chron Respir Dis. 2021;18 doi: 10.1177/1479973121999205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peolsson A, Hedlund R, Oberg B. Intra- and inter-tester reliability and reference values for hand strength. J Rehabil Med. 2001;33:36–41. doi: 10.1080/165019701300006524. [DOI] [PubMed] [Google Scholar]
- 19.Miller MR, Crapo R, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. General considerations for lung function testing. Eur Respir J. 2005;26:153–161. doi: 10.1183/09031936.05.00034505. [DOI] [PubMed] [Google Scholar]
- 20.Delgado C, Araneda A, Behrens MI. Validation of the Spanish-language version of the Montreal Cognitive Assessment test in adults older than 60 years. Neurologia. 2019;34:376–385. doi: 10.1016/J.NRL.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 21.Herrero MJ, Blanch J, Peri JM, de Pablo J, Pintor L, Bulbena A. A validation study of the hospital anxiety and depression scale (HADS) in a Spanish population. Gen Hosp Psychiatry. 2003;25:277–283. doi: 10.1016/S0163-8343(03)00043-4. [DOI] [PubMed] [Google Scholar]
- 22.Miles JNV, Marshall GN, Schell TL. Spanish and English versions of the PTSD Checklist-Civilian version (PCL-C): testing for differential item functioning. J Trauma Stress. 2008;21:369–376. doi: 10.1002/JTS.20349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. 2nd Edition. Routledge; New York: 1988. Statistical power analysis for the Behavioral Sciences. [Google Scholar]
- 24.Fabero-Garrido R, del Corral T, Angulo-Díaz-Parreño S, Plaza-Manzano G, Martín-Casas P, Cleland JA, et al. Respiratory muscle training improves exercise tolerance and respiratory muscle function/structure post-stroke at short term: A systematic review and meta-analysis. Ann Phys Rehabil Med. 2021;65 doi: 10.1016/J.REHAB.2021.101596. [DOI] [PubMed] [Google Scholar]
- 25.Alsaber A, Al-Herz A, Pan J, AL-Sultan AT, Mishra D. Handling missing data in a rheumatoid arthritis registry using random forest approach. Int J Rheum Dis. 2021;24:1282–1293. doi: 10.1111/1756-185X.14203. [DOI] [PubMed] [Google Scholar]
- 26.Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001;1:6. doi: 10.1186/1471-2288-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu K, Zhang W, Yang Y, Zhang J, Li Y, Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement Ther Clin Pract. 2020;39 doi: 10.1016/J.CTCP.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abodonya AM, Abdelbasset WK, Awad EA, Elalfy IE, Salem HA, Elsayed SH. Inspiratory muscle training for recovered COVID-19 patients after weaning from mechanical ventilation: A pilot control clinical study. Medicine. 2021;100:e25339. doi: 10.1097/MD.0000000000025339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crook H, Raza S, Nowell J, Young M, Edison P. Long covid-mechanisms, risk factors, and management. BMJ. 2021;374 doi: 10.1136/BMJ.N1648. [DOI] [PubMed] [Google Scholar]
- 30.Nolan CM, Longworth L, Lord J, Canavan JL, Jones SE, Kon SSC, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax. 2016;71:493–500. doi: 10.1136/THORAXJNL-2015-207782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saunders SW, Rath D, Hodges PW. Postural and respiratory activation of the trunk muscles changes with mode and speed of locomotion. Gait Posture. 2004;20:280–290. doi: 10.1016/J.GAITPOST.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 32.Czerniak E, Davidson M. Placebo, a historical perspective. European Neuropsychopharmacology. 2012;22:770–774. doi: 10.1016/j.euroneuro.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Gomes Neto M, Ferrari F, Helal L, Lopes AA, Carvalho VO, Stein R. The impact of high-intensity inspiratory muscle training on exercise capacity and inspiratory muscle strength in heart failure with reduced ejection fraction: a systematic review and meta-analysis. Clin Rehabil. 2018;32:1482–1492. doi: 10.1177/0269215518784345. [DOI] [PubMed] [Google Scholar]
- 34.Gosselink R, de Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37:416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 35.Jensen D, Schaeffer MR, Guenette JA. Pathophysiological mechanisms of exertional breathlessness in chronic obstructive pulmonary disease and interstitial lung disease. Curr Opin Support Palliat Care. 2018;12:237–245. doi: 10.1097/SPC.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 36.O'Donnell DE, Bertley JC, Chau LKL, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/AJRCCM.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 37.Balbás-Álvarez L, Candelas-Fernández P, del Corral T, la Touche R, López-de-Uralde-Villanueva I. Effect of Manual Therapy, Motor Control Exercise, and Inspiratory Muscle Training on Maximum Inspiratory Pressure and Postural Measures in Moderate Smokers: A Randomized Controlled Trial. J Manipulative Physiol Ther. 2018;41:372–382. doi: 10.1016/J.JMPT.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Iwakura M, Okura K, Kubota M, Sugawara K, Kawagoshi A, Takahashi H, et al. Estimation of minimal clinically important difference for quadriceps and inspiratory muscle strength in older outpatients with chronic obstructive pulmonary disease: a prospective cohort study. Phys Ther Res. 2020;24:35–42. doi: 10.1298/PTR.E10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li L, Yu P, Yang M, Xie W, Huang L, He C, et al. Physical Therapist Management of COVID-19 in the Intensive Care Unit: The West China Hospital Experience. Phys Ther. 2021;101:pzaa198. doi: 10.1093/PTJ/PZAA198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramírez-Sarmiento A, Orozco-Levi M, Güell R, Barreiro E, Hernandez N, Mota S, et al. Inspiratory muscle training in patients with chronic obstructive pulmonary disease: structural adaptation and physiologic outcomes. Am J Respir Crit Care Med. 2002;166:1491–1497. doi: 10.1164/RCCM.200202-075OC. [DOI] [PubMed] [Google Scholar]
- 41.Vaidya T, de Bisschop C, Beaumont M, Ouksel H, Jean V, Dessables F, et al. Is the 1-minute sit-to-stand test a good tool for the evaluation of the impact of pulmonary rehabilitation? Determination of the minimal important difference in COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:2609–2616. doi: 10.2147/COPD.S115439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White C, Dixon K, Samuel D, Stokes M. Handgrip and quadriceps muscle endurance testing in young adults. Springerplus. 2013;2:451. doi: 10.1186/2193-1801-2-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva IS, Fregonezi GAF, Dias FAL, Ribeiro CTD, Guerra RO, Ferreira GMH. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. 2013;2013 doi: 10.1002/14651858.CD003792.PUB2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee EN, Kim MJ. Meta-analysis of the Effect of a Pulmonary Rehabilitation Program on Respiratory Muscle Strength in Patients with Chronic Obstructive Pulmonary Disease. Asian Nurs Res (Korean Soc Nurs Sci) 2019;13:1–10. doi: 10.1016/J.ANR.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 45.McCool FD. Global physiology and pathophysiology of cough: ACCP evidence-based clinical practice guidelines. Chest. 2006;129:48S–53S. doi: 10.1378/CHEST.129.1_SUPPL.48S. [DOI] [PubMed] [Google Scholar]
- 46.Boutevillain L, Dupeyron A, Rouch C, Richard E, Coudeyre E. Facilitators and barriers to physical activity in people with chronic low back pain: A qualitative study. PLoS One. 2017;12 doi: 10.1371/JOURNAL.PONE.0179826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeager DE, Magruder KM, Knapp RG, Nicholas JS, Frueh BC. Performance characteristics of the posttraumatic stress disorder checklist and SPAN in Veterans Affairs primary care settings. Gen Hosp Psychiatry. 2007;29:294–301. doi: 10.1016/J.GENHOSPPSYCH.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 48.Singer S, Kuhnt S, Götze H, Hauss J, Hinz A, Liebmann A, et al. Hospital anxiety and depression scale cutoff scores for cancer patients in acute care. Br J Cancer. 2009;100:908–912. doi: 10.1038/sj.bjc.6604952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mak IWC, Chu CM, Pan PC, Yiu MGC, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31:318–326. doi: 10.1016/J.GENHOSPPSYCH.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Boutou AK, Georgopoulou A, Pitsiou G, Stanopoulos I, Kontakiotis T, Kioumis I. Changes in the respiratory function of COVID-19 survivors during follow-up: A novel respiratory disorder on the rise? Int J Clin Pract. 2021;75:e14301. doi: 10.1111/IJCP.14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.