Abstract
Background:
Multiple sclerosis (MS) is a neurological disorder marked by accumulating immune-mediated damage to the central nervous system. The dysregulated immune system in MS combined with immune effects of disease-modifying therapies (DMTs) used in MS treatment could alter responses to infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19). Most of the literature on immune response to SARS-CoV-2 infection and COVID-19 vaccination, in both the general population and patients with MS on DMTs, has focused on humoral immunity. However, immune response to COVID-19 involves multiple lines of defense, including T cells.
Objective and Methods:
We review innate and adaptive immunity to COVID-19 and expand on the role of T cells in mediating protective immunity against SARS-CoV-2 infection and in responses to COVID-19 vaccination in MS.
Results:
Innate, humoral, and T cell immune responses combat COVID-19 and generate protective immunity. Assays detecting cytokine expression by T cells show an association between SARS-CoV-2-specific T cell responses and milder/asymptomatic COVID-19 and protective immune memory.
Conclusion:
Studies of COVID-19 immunity in people with MS on DMTs should ideally include comprehensive assessment of innate, humoral, and T cell responses.
Keywords: COVID-19, disease-modifying therapy, multiple sclerosis, SARS-CoV-2, T cell
Introduction
Multiple sclerosis (MS) is an immune-mediated central nervous system (CNS) disorder marked by chronic inflammation and demyelination, and loss of neurons that causes motor, sensory, and cognitive disabilities. The dysregulated immune system in MS, combined with the wide variety of immunomodulatory effects of MS disease-modifying therapies (DMTs), could affect the host response to infections, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes coronavirus disease 2019 (COVID-19). Comprehensive understanding of the immunology of the disease and vaccine response will allow clinicians to inform patients and help guide their decisions about vaccines and treatment.
Risk factors associated with COVID-19 clinical severity include age, comorbidities, and extent of MS disability; MS itself does not appear to be a risk factor. 1 MS DMTs may, in a modality-dependent fashion, affect the immune response to SARS-CoV-2 infection and COVID-19 vaccination. The immune response to SARS-CoV-2 infection involves various cell types beyond virus-specific, antibody-producing B cells.2,3 Most of the literature on COVID-19 vaccine responses has focused on humoral (i.e., antibody) responses, due to the accessibility and feasibility of serologic tests to assess antiviral immunity. However, cell-mediated responses (e.g., CD4 and CD8 T cells) and innate immunity (neutrophils, macrophages, and natural killer cells) are integral to fighting infection and the development of protective immunity. 2 In this review, we provide a brief overview of innate and adaptive immune responses against the SARS-CoV-2 virus, with a focus on SARS-CoV-2-specific T cell immunity in COVID-19 and responses to COVID-19 vaccination in MS.
Immune responses to COVID-19
Innate immunity against COVID-19
The innate immune system is the first line of defense against pathogens. Upon pathogen entry, host pattern recognition receptors (PRRs) expressed by innate immune cells rapidly recognize pathogen-associated molecular patterns. Activation of PRRs induces the production of interferons (IFNs) and other cytokines by innate immune cells (e.g., plasmacytoid dendritic cells, monocytes/macrophages, and natural killer cells) for pathogen elimination. 4 The large SARS-CoV-2 genome encodes multiple viral proteins that facilitate evasion of host innate immunity, speeding infection of host cells, viral replication, and spread to other cells. 5 During severe COVID-19, patients exhibit delayed and impaired type I and III IFN responses, yet elevated pro-inflammatory cytokine (e.g., interleukin [IL]-6 and IL-12) levels, generating a “cytokine storm” and respiratory tract inflammation. 5 Dysregulated innate responses to SARS-CoV-2 in turn do not effectively prime adaptive immune responses to clear the virus, and hence reduce protective immune memory. 3
Humoral immunity against COVID-19
Adaptive immunity, which includes humoral and cell-mediated responses, generates pathogen-specific responses and memory of the infection. Humoral immunity is mediated by B cells that produce antibodies to combat infection by circulating pathogens. 4 Following exposure to an antigen during infection/immunization, naïve B cells differentiate into effector B cells (plasma cells) or memory B cells. Plasma cells secrete antibodies (primary immunity); memory B cells differentiate into antigen-specific plasma cells upon re-exposure to the antigen (secondary immunity). Immunoglobulin G (IgG) and M (IgM) antibodies bind to the SARS-CoV-2 receptor-binding domain (RBD) of the spike or nucleocapsid proteins. 3 In most SARS-CoV-2-infected individuals, development of neutralizing antibodies, predominantly anti-spike, occurs within 2 weeks. 6 The presence and titer of anti-spike neutralizing antibodies are inversely associated with COVID-19 infection rate in vaccinated patients.7–9 However, there is heterogeneity in the kinetics and magnitude of both virus- and vaccine-induced antibody responses, and SARS-CoV-2-specific antibody titers often wane 5–8 months after symptom onset. 10
Humoral responses to SARS-CoV-2 do not fully reflect protective immunity against the virus.11–13 Ig levels are not the sole determinants of protective immunity, but easily accessible serologic tests (e.g., enzyme-linked immunosorbent assays [ELISAs]) are often the only tests used to assess antiviral immunity.10,13 Low/undetectable levels of SARS-CoV-2-specific antibodies in some recovered patients suggest that a minimum protective response involves other components of immunity. 3 In support, circulating antibody levels may not predict the quantity of SARS-CoV-2-specific T cell responses, highlighting the importance of other cells involved in fighting the infection. 10
Cell-mediated immunity against COVID-19
Cell-mediated immunity, enacted by T cells, helps clear intra- and extracellular pathogens once infection has occurred. Cell-mediated immunity controls ongoing infections, rather than preventing their occurrence. On encountering an antigen, naïve CD8 and CD4 T cells proliferate and differentiate into effector or memory cells. 4 CD8 cytotoxic T cells kill cytosolic pathogens in infected cells. Naïve CD4 T cells can differentiate into multiple subsets of helper T cells (Th), including Th1, Th17, Th2, and induced regulatory T (Treg) cells, each of which have distinct cytokine profiles. 14 Th1 cells activate CD8 cytotoxic T cells, macrophages, and B cells; Th2 cells activate naïve B cells and inhibit Th1 cells. 4 Upon antigen re-exposure, quiescent CD4 and CD8 memory T cells rapidly generate effector T cells to combat the pathogen (secondary immunity, recall response). 4 A third subset, Treg, inhibits proliferation of cytotoxic CD4 T cells and cytokine production, preventing pathogen-induced autoimmunity and unbridled inflammation. 4 Finally, mature, or antigen-experienced, CD4 T follicular helper (Tfh) cells migrate to germinal centers in lymph nodes and the spleen, and help cognate B cells to generate high-affinity, neutralizing antibodies. 4
The balance of T cell subsets is crucial. Increased cytotoxic CD4 Th1 and Tfh cells and reduced SARS-CoV-2-reactive Tregs are associated with severe COVID-19 disease and hospitalization. 15 In some severe cases, the naïve/memory Th ratio increases, elevating pro-inflammatory serum cytokines. 16 The decrease in regulatory and memory T cells and subsequent cytokine storm are associated with severe COVID-19 and tissue damage, 17 including neurovascular inflammation in immunocompromised patients and patients with MS. 18
SARS-CoV-2-specific T cell response
T cells broaden the antiviral defense against COVID-19 and the response to vaccination. 19 SARS-CoV-2-specific CD8 and CD4 T cells help control COVID-19 severity and the development of antiviral immunity. Although complex and seldom commercially available, diagnostic tests can detect the magnitude of T cell responses at different phases of SARS-CoV-2 infection and following vaccination (Table 1).
Table 1.
Functions and methods of T cell detection assays.
| Assay (References) | Function/method | Notes |
|---|---|---|
| ELISpot20–24 | Quantify the frequency of isolated T cells secreting cytokines (i.e., functional T cells) in response to stimulation with SARS-CoV-2-specific peptide pools. Chromogenic detection of cytokine “spots” correlates with individual-activated T cells. T cell subsets can be identified by their cytokine signature (e.g., Th1 cells secrete IFN-γ, IL-2, and TNF-α) | • In vitro stimulation of PBMCs using SARS-CoV-2 peptide pools revealed
pre-existing memory CD4 T cells cross-reactive to SARS-CoV-2 and common cold
HCoVs • SARS-CoV-2 peptide pools mapped T cell responses to individual SARS-CoV-2 epitopes, which were predominantly S-specific |
| Whole-blood cytokine release19,20 | Detect secreted cytokines from natural SARS-CoV-2 infection- and COVID-19 vaccine-induced T cells in peptide-stimulated whole blood | • Facilitated rapid quantification of secreted IFN-γ and IL-2 in whole blood
stimulated with S-specific peptide pools from BNT162b2-vaccinated individuals,
and from convalescent asymptomatic and symptomatic COVID-19
patients • IL-2 demonstrated better sensitivity than IFN-γ in detecting S-specific T cell responses 2–3 months post-vaccination and 12 months post-infection in convalescent COVID-19 patients |
| ICS23,25–27 | Quantify frequency of T cells secreting cytokines in response to stimulation with SARS-CoV-2-specific peptide pools. Immunostaining of cytokines enables quantification by FACS | • Levels of IFN-γ-secretion of SARS-CoV-2-specific memory CD4 and CD8 T
cells were greater in recovered COVID-19 patients than in their close contacts
(i.e., exposed to SARS-CoV-2, but lacking detectable
infection) • Polyfunctionality (the ability to secrete multiple cytokines, such as IFN-γ, IL-2, or TNF-a) was observed in the SARS-CoV-2-specific CD4 (25%–40%) and CD8 (30%–50%) T cells of recovered patients 2 months post-symptom onset, and was maintained for ~9 months post-symptom onset |
| AIM10,23,24 | Detect natural SARS-CoV-2 infection- and COVID-19 vaccine-induced T cells by measuring upregulation of TCRs upon stimulation with antigen-specific peptide libraries followed by FACS | • AIM assays using SARS-CoV-2 peptide pools (S and non-S) and markers for
CD4 (e.g., CD137+ OX40+) or CD8
(e.g., CD137+ CD69+) T cells
identified SARS-CoV-2-specific CD4 and CD8 T cells in recovered
patients • Memory CD4 T cells of individuals unexposed to SARS-CoV-2 are cross-reactive to homologous SARS-CoV-2 and HCoV peptide pools |
| MHC Multimer Staining23,28,29 | Detect T cells expressing TCRs capable of binding specific complexes of SARS-CoV-2 epitopes and multimers of MHC/HLA class I or II molecules by FACS | • SARS-CoV-2-specific MHC-I multimer staining detected subsets of stem cell-like memory T cells in recovered individuals, peaking ~4 months post-symptom onset |
| Single-cell immune profiling: scRNA-seq and scTCR-seq 29 | Elucidate gene expression profiles of FACS-sorted, SARS-CoV-2-specific T cells by next-generation sequencing and analysis of the single-cell transcriptome (scRNA-seq) or TCR sequence (scTCR-seq) | • A combination of MHC multimer staining, scRNA-seq, and scTCR-seq using pools of 18 DNA-barcoded MHC-I multimers revealed similar levels of S-specific T cell responses in individuals after natural SARS-CoV-2 infection and mRNA COVID-19 vaccination, though the target antigens differ. These T cell responses were boosted in recovered, vaccinated individuals |
AIM: activation-induced marker; COVID-19: coronavirus disease 2019; ELISpot: enzyme-linked immune absorbent spot; FACS: fluorescence-activated cell sorting; HCoV: human coronavirus; HLA: human leukocyte antigen; IFN: interferon; IL: interleukin; ICS: intracellular cytokine staining; MHC: major histocompatibility complex; PMBC: peripheral blood mononuclear cell; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; scRNA-seq: single-cell RNA sequencing; scTCR-seq: single-cell T cell receptor sequencing; S: spike; TCR: T cell receptor; Th1: T helper cell type 1; TNF: tumor necrosis factor.
T cell response is associated with milder COVID-19 disease and improved outcome. Asymptomatic SARS-CoV-2-infected individuals mount a more robust virus-specific T cell response, with increased IFN-γ and IL-2 secretion, than patients with symptomatic disease. 20 These functional SARS-CoV-2-specific CD4 and CD8 T cells induced during the initial phase of SARS-CoV-2 infection are associated with mild COVID-19 and rapid viral clearance. 21 Immunosuppressed patients on chemotherapy or B cell-depleting therapy who are hospitalized for COVID-19 have better COVID-19 outcomes if early T cell responses are strong. 22 Patients with hematologic cancer and impaired humoral immunity have higher COVID-19 mortality rates than those with solid cancer and without any cancer. However, patients with hematologic malignancies have improved survival if they have high levels of SARS-CoV-2-specific CD8 T cells. 22 These studies show that SARS-CoV-2-specific T cell responses diminish acute SARS-CoV-2 infection.
After infection, some recovered COVID-19 patients develop functional SARS-CoV-2-specific CD4 and CD8 memory T cells, irrespective of symptoms or degree of SARS-CoV-2-specific serology. 25 SARS-CoV-2-specific memory T cell responses are sustained up to 8 months post-infection 10 and maintained for 10 months regardless of COVID-19 severity. 23 These recovered patients develop SARS-CoV-2-specific polyfunctional stem cell-like memory T cells with multiple, simultaneous effector functions and antigen-induced recall responses. 23 Given the longevity of this virus-specific T cell memory, and the observation that human T cells recognize more than 1400 SARS-CoV-2 epitopes, T cell responses should be included in evaluation of COVID-19 vaccine efficacy. 28
T cells induced by mRNA COVID-19 vaccines mediate protection against COVID-19. The Pfizer-BioNTech BNT162b2 vaccine elicits similar, multi-specific T cell responses in COVID-19-recovered patients and uninfected COVID-19-naïve individuals. 29 However, compared with natural infection alone, T cell responses are boosted in COVID-19-recovered individuals receiving vaccine, and pre-existing virus-specific T cells can respond rapidly upon antigen re-exposure. 29 Likewise, the Moderna mRNA-1273 vaccine generates spike-specific CD8 and memory CD4 T cells 6 months post-immunization, at similar levels to natural SARS-CoV-2 infection. 26 Interestingly, vaccinated individuals with pre-existing cross-reactive CD4 T cell memory to coronaviruses have higher vaccine-induced CD4 T cell responses. 26 Similarly, BNT162b2 and mRNA-1273 induce memory CD4 and CD8 T cells 6 months post-vaccination, regardless of prior infections, with only a slight boost in the memory T cell responses of individuals with prior exposure to SARS-CoV-2. 30 Thus, mRNA vaccination induces primary protection and often enhances recall T cell response to SARS-CoV-2, supporting revaccination to boost protective T cell immunity. Importantly, antibodies can have lower affinity for emerging SARS-CoV-2 strains, but viral epitopes recognized by T cells remain as targets, as evidenced by virus- and vaccine-induced T cells with cross-reactivity to the initial Omicron variant. 27
Vaccine response in patients with MS on DMTs
Patients with MS mount variable humoral immune responses to natural SARS-CoV-2 infection and COVID-19 vaccination. Data on T cell responses have, until recently, been limited. MS DMTs generally exert their therapeutic effect by downregulating cytotoxic T cell function and pro-inflammatory cytokine secretion. IFN-β and glatiramer acetate (GA), both of which induce anti-inflammatory CD4 and CD8 T cells, appear to decrease the risk of getting, or having severe COVID-19.31–33 IFN-β is a potent antiviral agent with effects that are enhanced by vitamin D. 34 It activates antiviral T cell and monocyte responses to prevent infection, and could also reduce mid-stage COVID-19 disease severity by reducing pro-inflammatory cytokine levels and dysregulated genes in MS, and inducing multiple immunoregulatory pathways.35–37 GA shifts T cell phenotypes from pro-inflammatory (e.g., Th1 and Th17) to anti-inflammatory and regulatory (e.g., Th2, CD8 Treg), 38 perhaps reducing the virus-induced cytokine storm to avoid severe COVID-19.
Other DMTs reduce or temper the activation of pro-inflammatory subsets of B and T cells that may be exacerbated in COVID-19. Fingolimod, ozanimod, ponesimod, and siponimod reduce circulating numbers of naïve and central memory CD4 T cells.39–42 Teriflunomide reduces proliferation of activated T and B cells. 43 Cladribine depletes peripheral B cells (and less so CD4 and CD8 T cells). 44 Alemtuzumab induces prolonged depletion of memory B and CD4 and CD8 T cells. 45 In contrast, natalizumab (anti-VLA4 monoclonal antibody [mAb]) is not associated with lymphopenia, and instead sequesters effector memory CD4 and CD8 T cells away from the CNS to the peripheral blood, where they may engage with pathogens at sites of entry. 46 Post-vaccination studies of patients with MS treated with all of these therapies, excepting sphingosine 1-phosphate (S1P) modulators in some studies, show that robust SARS-CoV-2-specific T cell responses are generated after COVID-19 mRNA vaccination (Supplementary Table 1).
The various mAbs directed against CD20 are of potential concern because CD20 is a surface marker expressed on pre-B cells, naïve B cells, and memory B cells, and at low levels on a small subset of T cells. Anti-CD20 mAbs strongly deplete B cells. Their ability to reduce relapses in MS derives from depletion of putatively pathogenic B cells and possibly loss of B cell collaboration with T cells. B cell depletion by the anti-CD20 mAb, rituximab (RTX), limits the abnormal activation of pro-inflammatory CD4 and CD8 T cells. 47 The anti-CD20 mAbs, RTX, ocrelizumab (OCR), and ofatumumab (OMB) spare the majority of antibody-secreting plasma cells but B cell depletion reduces new antibody responses to SARS-CoV-2 infection. Buffering the poor antibody response, the anti-CD20s do not appear to compromise T cell responses to SARS-CoV-2 infection in MS.48–51 SARS-CoV-2-specific CD4 and CD8 T cell responses are detected for at least 1 year after infection, similar to healthy recovered controls. 48
COVID-19 outcomes in patients treated with anti-CD20 mAbs have varied. Treatment with RTX or OCR is associated with an increased incidence of severe COVID-19 (including hospitalization) in patients with MS. 1 Conversely, in a recent study of OMB-treated patients with relapsing MS with 210 confirmed and 35 suspected cases of COVID-19, 90.6% were of mild or moderate severity, and 9% were severe or life-threatening. 52 At study cutoff, 98.4% of patients had recovered or were recovering, with 23 patients being hospitalized and 2 deaths. This represents a case fatality rate that compares favorably with the general population statistics for most nations. 53
With mRNA COVID-19 vaccination, T cell and humoral responses of patients with MS may vary between treatments (Supplementary Table 1; Figure 1).49–51,54–63 Patients treated with B cell-depleting anti-CD20 therapy have lower antibody responses to mRNA COVID-19 vaccines, for months after the last anti-CD20 treatment.49–51,64 However, with OMB, a third booster vaccination can mitigate the decreased antibody responses and allow elevation of titers.49–51 In addition, SARS-CoV-2-specific T cell responses after full-course vaccination can occur in patients treated with anti-CD20 therapy (Supplementary Table 1). SARS-CoV-2-specific memory T cell responses are comparable in BNT162b2-vaccinated healthy controls, untreated patients with MS, and OCR-treated patients with MS when assessed 4.3 months (median) after last DMT treatment. 55 SARS-CoV-2-specific CD4 and CD8 T cell responses are not reduced after mRNA or viral vector COVID-19 vaccination in any MS treatment group compared with untreated patients with MS; rather, IFN-γ+ CD8 T cell responses are increased in RTX-treated patients. 58 After a full course of mRNA or viral vector COVID-19 vaccination, patients with MS on RTX, OCR, or non-S1P-modulating oral therapies have higher levels of IFN-γ-producing T cells than patients not on DMTs 8 weeks after vaccination. 56 Thus, anti-CD20 mAbs may enhance vaccine-induced T cell responses in patients with MS.
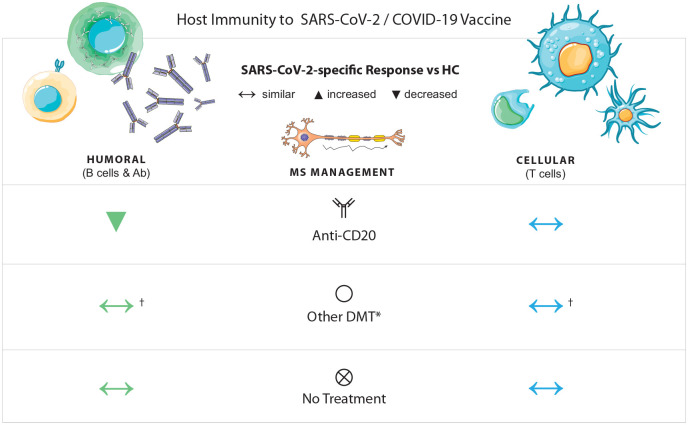
Figure 1.
SARS-CoV-2-specific humoral and T cell responses in patients with MS.
*Other DMT refers to GA, IFN-β, teriflunomide, fumarates, cladribine, natalizumab, alemtuzumab, and S1P modulators.
In patients with MS, humoral and cellular immune responses to SARS-CoV-2 infection and COVID-19 vaccination depend on the DMT. Patients with MS on RTX, OCR, OMB, or S1P receptor modulators exhibit decreased SARS-CoV-2-specific humoral responses compared with healthy controls. RTX-, OCR-, and OMB-treated patients still mount SARS-CoV-2-specific T cell responses, but S1P-treated patients may have reduced T cell responses. Patients with MS on other DMTs, including GA, IFN-β, teriflunomide, fumarates, cladribine, natalizumab, and alemtuzumab, also generate virus-specific T cell responses. Treatment with RTX, OCR, OMB, or non-S1P-modulating oral therapies was associated with enhanced T cell response after a two-dose vaccination with a mRNA COVID-19 vaccine.
Ab: antibody; COVID-19: coronavirus disease 2019; DMT: disease-modifying therapy; GA: glatiramer acetate; HC: healthy control; IFN: interferon; OCR: ocrelizumab; OMB: ofatumumab; RTX: rituximab; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; S1P: sphingosine 1-phosphate.
Treatment with S1P receptor modulators is associated with impaired humoral response to vaccination.58,61,63 In one prospective study, 51.4% of patients receiving S1P receptor modulators (35 received fingolimod and 1 received siponimod) seroconverted 30–90 days post-vaccination versus > 92.0% with other DMTs. 61 In addition, there are reduced SARS-CoV-2-specific CD4 T cell responses in patients with MS receiving S1P therapies versus both alternatively treated and untreated patients, likely due to CD4 T cell lymphopenia or altered T and B cell interactions in lymph nodes (Supplementary Table 1).58,61 Studies of COVID-19 outcomes, and variation between different S1P modulators, in S1P modulator-treated patients are urgently required.
In summary, COVID-19 infection is less severe in patients with MS receiving IFN-β and GA, but slightly more severe with anti-CD20 therapies. Responses to COVID-19 vaccination are normal with most DMTs, but some anti-CD20 therapies reduce B cell responses, and some S1P modulators reduce B and T cell responses.
The role and impact of SARS-CoV-2-specific T cell responses in patients with MS after natural or post-vaccination infection and responses to COVID-19 vaccination need further investigation to fully understand the mechanisms behind the host response to natural infection in patients treated with DMTs. Several ongoing studies will delineate the degree of protective effect of the SARS-CoV-2-specific T cell immunity mounted by patients with MS on B cell-depleting therapies, including RTX (NCT04877496), OCR (NCT04843774), and OMB (NCT04869358, NCT04486716). Other studies include a broad MS DMT spectrum (NCT05121662, NCT04796584, NCT05060354).
Perspectives and concluding remarks
The ongoing COVID-19 pandemic raises concerns over the risk of infection and disease severity in individuals with dysregulated immune systems. Patients with MS may have other risk factors associated with poorer COVID-19 outcomes, and certain DMTs could further affect immune responses to SARS-CoV-2 and COVID-19 vaccines. 1 However, innate, humoral, and T cell immune responses all combat COVID-19 and generate protective immunity.
Assays detecting cytokine expression by T cells show an association between SARS-CoV-2-specific T cell responses and milder/asymptomatic COVID-19 and protective immune memory.10,23,25,26,28–30 Several DMTs suppress excessive pro-inflammatory T cell responses in MS, yet patients on these DMTs mount robust SARS-CoV-2-specific T cell responses after mRNA COVID-19 vaccination.54–58 The extent to which these T cell responses compensate for attenuated humoral responses in conferring protection against COVID-19 remains to be determined, but some studies provide reassurance. Studies of COVID-19 vaccine response and post-COVID-19 immunity in people with MS on DMTs should ideally include comprehensive assessment of innate, humoral, and T cell responses.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585221134216 for T cell responses to COVID-19 infection and vaccination in patients with multiple sclerosis receiving disease-modifying therapy by Anthony T Reder, Olaf Stuve, Stephanie K Tankou and Thomas P Leist in Multiple Sclerosis Journal
Acknowledgments
Medical writing support was provided by Juliel Espinosa, PhD, of Alphabet Health (New York, NY), funded by Novartis Pharmaceuticals Corporation. This manuscript was developed in accordance with Good Publication Practice (GPP3) guidelines.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.T.R. is a consultant for or has received unrestricted research support from Bayer, Biogen, Bristol Myers Squibb, Genentech/Roche, NKMax America, Mallinckrodt, Merck Serono, Novartis, and TG Therapeutics.
O.S. serves on the editorial board of Therapeutic Advances in Neurological Disorders and has served on data monitoring committees for Genentech/Roche, Pfizer, Novartis, and TG Therapeutics without monetary compensation. He has advised Celgene, EMD Serono, Genentech, Genzyme, and TG Therapeutics, and currently receives grant support from EMD Serono and Exalys. S.K.T. has no disclosures. T.P.L. serves as site investigator for Biogen, Bristol Myers Squibb, EMD Serono, Genentech/Roche, Janssen, Novartis, and Sanofi. He has advised Biogen, Genentech/Roche, Horizon, Janssen, and Novartis.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Novartis Pharmaceuticals Corporation. The authors had full control of the content and made the final decision on all aspects of this publication.
ORCID iD: Olaf Stuve  https://orcid.org/0000-0002-0469-6872
https://orcid.org/0000-0002-0469-6872
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Anthony T Reder, Department of Neurology, University of Chicago Medicine, Chicago, IL, USA.
Olaf Stuve, Peter O’Donnell Jr. Brain Institute, UT Southwestern Medical Center, Dallas, TX, USA; VA North Texas Health Care System, Dallas VA Medical Center, Dallas, TX, USA.
Stephanie K Tankou, Mount Sinai Health System, New York, NY, USA.
Thomas P Leist, Department of Neurology, Thomas Jefferson University, Philadelphia, PA, USA.
References
- 1.Salter A, Fox RJ, Newsome SD, et al. Outcomes and risk factors associated with SARS-CoV-2 infection in a North American registry of patients with multiple sclerosis. JAMA Neurol 2021; 78: 699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hasan A, Al-Ozairi E, Al-Baqsumi Z, et al. Cellular and humoral immune responses in Covid-19 and immunotherapeutic approaches. Immunotargets Ther 2021; 10: 63–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sette A, Crotty S.Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021; 184: 861–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy K, Weaver C.Janeway’s immunobiology. 9th ed.New York: Garland Science, 2017. [Google Scholar]
- 5.Kasuga Y, Zhu B, Jang K-J, et al. Innate immune sensing of coronavirus and viral evasion strategies. Exp Mol Med 2021; 53: 723–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Liang B, Chen C, et al. SARS-CoV-2 infection induces sustained humoral immune responses in convalescent patients following symptomatic COVID-19. Nat Comm 2021; 12: 1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: A meta-analysis. Lancet Microbe 2022; 3(1): e52–e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27(7): 1205–1211. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert PB, Montefiori DC, McDermott AB, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. Science 2022; 375: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371: eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woopen C, Schleußner K, Akgün K, et al. Approach to SARS-CoV-2 vaccination in patients with multiple sclerosis. Front Immunol 2021; 12: 701752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matko S, Akgun K, Tonn T, et al. Antigen-shift in varicella-zoster virus-specific T-cell immunity over the course of Fingolimod-treatment in relapse-remitting multiple sclerosis patients. Mult Scler Relat Disord 2020; 38: 101859. [DOI] [PubMed] [Google Scholar]
- 13.Misra A, Theel ES.Immunity to SARS-CoV-2: What do we know and should we be testing for it? J Clin Microbiol 2022; 60: e0048221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu J, Yamane H, Paul WE.Differentiation of effector CD4 T cell populations. Annu Rev Immunol 2010; 28: 445–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meckiff BJ, Ramírez- Suástegui C, Fajardo V, et al. Imbalance of regulatory and cytotoxic SARS-CoV-2-reactive CD4+ T cells in COVID-19. Cell 2020; 183: 1340–1353e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis 2020; 71: 762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schoeman D, Fielding BC.Human coronaviruses: Counteracting the damage by storm. Viruses 2021; 13: 1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dziedzic A, Saluk-Bijak J, Miller E, et al. The impact of SARS-CoV-2 infection on the development of neurodegeneration in multiple sclerosis. Int J Mol Sci 2021; 22: 1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan AT, Lim JME, Le Bert N, et al. Rapid measurement of SARS-CoV-2 spike T cells in whole blood from vaccinated and naturally infected individuals. J Clin Investig 2021; 131: e152379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Bert N, Clapham HE, Tan AT, et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J Exp Med 2021; 218: e20202617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan AT, Linster M, Tan CW, et al. Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep 2021; 34: 108728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bange EM, Han NA, Wileyto P, et al. CD8+ T cells contribute to survival in patients with COVID-19 and hematologic cancer. Nat Med 2021; 27: 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung JH, Rha M-S, Sa M, et al. SARS-CoV-2-specific T cell memory is sustained in COVID-19 convalescent patients for 10 months with successful development of stem cell-like memory T cells. Nat Comm 2021; 12: 4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mateus J, Grifoni A, Tarke A, et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science 2020; 370: 89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Yang X, Zhong J, et al. Exposure to SARS-CoV-2 generates T cell memory in the absence of a detectable viral infection. Nat Comm 2021; 12: 1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mateus J, Dan JM, Zhang Z, et al. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021; 374: eabj9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keeton R, Tincho MB, Ngomti A, et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022; 603: 488–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grifoni A, Sidney J, Vita R, et al. SARS-CoV-2 human T cell epitopes: Adaptive immune response against COVID-19. Cell Host Microbe 2021; 29: 1076–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minervina AA, Pogorelyy MV, Kirk AM, et al. Convergent epitope-specific T cell responses after SARS-CoV-2 infection and vaccination. MedRxiv. Epub ahead of print 27 July 2021. DOI: 10.1101/2021.07.12.21260227. [DOI] [Google Scholar]
- 30.Goel RR, Painter MM, Apostolidis SA, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021; 374: abm0829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reder AT, Centonze D, Naylor ML, et al. COVID-19 in patients with multiple sclerosis: Associations with disease-modifying therapies. CNS Drugs 2021; 35(3): 317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louapre C, Collongues N, Stankoff B, et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol 2020; 77: 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sormani MP, Salvetti M, Labauge P, et al. DMTs and Covid-19 severity in MS: A pooled analysis from Italy and France. Ann Clin Transl Neurol 2021; 8(8): 1738–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng X, Wang Z, Howlett-Prieto Q, et al. Vitamin D enhances responses to interferon-β in MS. Neurol Neuroimmunol Neuroinflamm 2019; 6(6): e622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng X, Bao R, Li L, et al. Interferon-β corrects massive gene dysregulation in multiple sclerosis: Short-term and long-term effects on immune regulation and neuroprotection. EBioMedicine 2019; 49: 269–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolumam GA, Thomas S, Thompson LJ, et al. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med 2005; 202: 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang N, Zhan Y, Zhu L, et al. Retrospective multicenter cohort study shows early interferon therapy is associated with favorable clinical responses in COVID-19 patients. Cell Host Microbe 2020; 28: 455.e2–464.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lalive PH, Neuhaus O, Benkhoucha M, et al. Glatiramer acetate in the treatment of multiple sclerosis. CNS Drugs 2011; 25: 401–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris S, Tran JQ, Southworth H, et al. Effect of the sphingosine-1-phosphate receptor modulator ozanimod on leukocyte subtypes in relapsing MS. Neurol Neuroimmunol Neuroinflamm 2020; 7(5): e839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Q, Mills EA, Wang Q, et al. Siponimod enriches regulatory T and B lymphocytes in secondary progressive multiple sclerosis. JCI Insight 2020; 5: e134251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song ZY, Yamasaki R, Kawano Y, et al. Peripheral blood T cell dynamics predict relapse in multiple sclerosis patients on fingolimod. PLoS One 2014; 10(4): e0124923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Ambrosio D, Steinmann J, Brossard P, et al. Differential effects of ponesimod, a selective S1P1 receptor modulator, on blood-circulating human T cell subpopulations. Immunopharmacol Immunotoxicol 2015; 37(1): 103–109. [DOI] [PubMed] [Google Scholar]
- 43.Bar-Or A, Pachner A, Menguy-Vacheron F, et al. Teriflunomide and its mechanism of action in multiple sclerosis. Drugs 2014; 74(6): 659–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giovannoni G.Cladribine to treat relapsing forms of multiple sclerosis. Neurotherapeutics 2017; 14(4): 874–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker D, Herrod SS, Alvarez-Gonzalez C, et al. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol 2017; 74: 961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaufmann M, Haase R, Proschmann U, et al. Real-world lab data in natalizumab treated multiple sclerosis patients up to 6 years long-term follow up. Front Neurol 2018; 9: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bar-Or A, Fawaz L, Fan B, et al. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS. Ann Neurol 2010; 67(4): 452–461. [DOI] [PubMed] [Google Scholar]
- 48.Furlan R, Mandelli A, Guerrera G, et al. Treatment with ocrevus does not inhibit antiviral T-cell responses in persons with MS after SARS-CoV-2 infection. Mult Scler 2021; 27: 925. [Google Scholar]
- 49.Ziemssen T, Bopp T, Ettle B, et al. KYRIOS clinical trial: Tracking the immune response to SARS-CoV-2 mRNA vaccines in an open-label multicenter study in participants with relapsing multiple sclerosis treated with ofatumumab s.c. In: 8th Congress of the European Academy of Neurology (EAN)—Europe, 2022, Vienna, 25–28 June 2022. [Google Scholar]
- 50.Cross AH, Chinea A, Hendin B, et al. Interim results of an open-label study to assess humoral immune response to COVID-19 mRNA vaccine in participants with relapsing multiple sclerosis treated with ofatumumab. In: Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum 2022, West Palm Beach, FL, 24–26 February 2022. [Google Scholar]
- 51.Sabatino JJ, Jr, Mittl K, Rowles WM, et al. Longitudinal analysis of adaptive immunity following additional SARS-CoV-2 vaccination in MS patients on anti-CD20 therapies and sphingosine-1-phosphate receptor modulators. In: Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum 2022, West Palm Beach, FL, 24–26 February 2022. [Google Scholar]
- 52.Cross AH, Delgado S, Habek M, et al. COVID-19 outcomes and vaccination in people with relapsing multiple sclerosis treated with ofatumumab. Neurol Ther 2022; 11: 741–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johns Hopkins University & Medicine Coronavirus Resource Center. Mortality analyses: Mortality in the most affected countries, https://coronavirus.jhu.edu/data/mortality (2022, accessed 5 July 2022).
- 54.Asplund Högelin K, Ruffin N, Pin E, et al. Development of humoral and cellular immunological memory against SARS-CoV-2 despite B cell depleting treatment in multiple sclerosis. iScience 2021; 24: 103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients following PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J Neuroimmunol 2021; 361: 577746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gadani SP, Reyes-Mantilla M, Jank L, et al. Discordant humoral and T cell immune responses to SARS-CoV-2 vaccination in people with multiple sclerosis on anti-CD20 therapy. EBioMedicine 2021; 73: 103636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Apostolidis SA, Kakara M, Painter MM, et al. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat Med 2021; 27(11): 1990–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sabatino JJ, Jr, Mittl K, Rowles WM, et al. Multiple sclerosis therapies differentially impact SARS-CoV-2 vaccine-induced antibody and T cell immunity and function. JCI Insight 2022; 7: e156978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kister I, Patskovsky Y, Voloshyna I, et al. Antibody and T-cell responses to SARS CoV-2 vaccines in patients with MS on ocrelizumab and other disease-modifying therapies: Preliminary results of an ongoing, prospective study. Mult Scler 2021; 27: 926. [Google Scholar]
- 60.Repovic P, Choi J, Hong S, et al. Temporal trends in humoral and cellular immune responses to SARS-CoV-2 mRNA vaccines among multiple sclerosis patients treated with natalizumab, ocrelizumab or fumarates. In: Consortium of Multiple Sclerosis Centers (CMSC) 2021 Annual Meeting, Orlando, FL, 25–28 October 2021. [Google Scholar]
- 61.Zabalza A, Arrambide G, Otero-Romero S, et al. Is humoral and cellular response to SARS-CoV-2 vaccine modified by DMT in patients with multiple sclerosis and other autoimmune diseases. Mult Scler 2022; 28(7): 1138–1145. [DOI] [PubMed] [Google Scholar]
- 62.Zabalza A, Arrambide G, Tagliani P, et al. Humoral and cellular responses to SARS-CoV-2 in convalescent COVID-19 patients with multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2022; 9(2):e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milo R, Staun-Ram E, Karussis D, et al. Humoral and cellular immune responses to SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis: An Israeli multi-center experience following 3 vaccine doses. Front Immunol 2022; 13: 868915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Achtnichts L, Jakopp B, Oberle M, et al. Humoral immune response after the third SARS-CoV-2 mRNA vaccination in CD20 depleted people with multiple sclerosis. Vaccines (Basel) 2021; 9: 1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585221134216 for T cell responses to COVID-19 infection and vaccination in patients with multiple sclerosis receiving disease-modifying therapy by Anthony T Reder, Olaf Stuve, Stephanie K Tankou and Thomas P Leist in Multiple Sclerosis Journal