Abstract
Introduction
Aging, genetic mutations, and other pathological conditions cause impairment of skeletal growth and bone metabolism, which affect activities of daily living and quality of life in all life stages. Although several drugs have been used in clinical settings and new drugs have been developed for the treatment of skeletal degenerative disorders such as osteoporosis and genetic disorders such as osteogenesis imperfecta (OI), there is clear demand for development of new drugs, especially orally available anabolic drugs that are applicable for a wide range of skeletal disorders.
Methods
To identify therapeutic candidates for skeletal disorders, peptide screening was performed. To validate the identified peptides, we performed a bone histomorphometric analysis with rat bone tissues and in vitro cell proliferation assays of skeletal cells. To understand the metabolism of the peptides, we performed a biochemical analysis, followed by in vitro assays for proliferation and differentiation of skeletal cells. We examined the therapeutic efficacy of the identified peptides with several mouse models representing skeletal disorders including bone fracture, osteoporosis, and osteogenesis imperfecta. In vivo therapeutic effects of the candidate were assessed with radiological analysis and mechanical property tests.
Results
We identified the egg yolk-derived functional peptide PF201. PF201 promoted in vivo bone formation in rodents and enhanced proliferation of osteoblasts and chondrocytes in vitro. D2, a metabolite of PF201, was present and circulated after digestion and absorption in the digestive tract. D2 had positive impacts on the proliferation and differentiation of mesenchymal stem cells and preosteoblasts. Oral administration of D2 accelerated bone healing in a mouse fracture model. D2 also improved bone strength and fracture healing under ovariectomy-induced osteoporotic conditions in mice, and D2 showed a therapeutic effect in a mouse OI model.
Conclusion
D2 is likely to be a candidate for an orally available therapeutic for a range of skeletal disorders.
Keywords: Egg yolk-derived peptide, Bone formation, Oral administration, Bone fracture, Osteoporosis, Osteogenesis imperfecta
1. Introduction
The skeleton functions as a framework for the mammalian body and is essential for the storage of minerals. It is formed from progenitors with two distinct modes of ossification. Intramembranous ossification directly converts condensed mesenchymal tissues to osteoblasts in generating the skull and facial bones, while endochondral ossification, in which mesenchymal cells condense and differentiate into chondrocytes to form a cartilage template that is subsequently replaced by bone and bone marrow, generates the remainder of the axial skeleton and the appendicular skeleton. Bones continuously grow in adolescent mammals, with the growth plate cartilage contributing to the length of bone. In bone tissues, functions of bone-forming osteoblasts and bone-resorbing osteoclasts are tightly coordinated via a process known as bone remodeling, which is essential to maintain the quality and quantity of bone tissues.
Aging, genetic mutations, and other pathological conditions cause impairment of skeletal growth and bone metabolism, which affect activities of daily living (ADL) and quality of life (QOL) in all life stages. Osteoporosis is a systemic metabolic disease that results in low bone mass and deterioration in skeletal strength, which causes an increased risk of bone fractures and less potency of subsequent bone healing [1]. Although osteoporosis develops gradually over years regardless of sex, postmenopausal osteoporosis in women, due to estrogen deficiency, is the most common type of the disease [2]. Several drugs have been used in clinical settings and new drugs have been developed for the treatment of skeletal degenerative disorders such as osteoporosis. Bisphosphonate and its derivatives have shown beneficial effects in preventing osteoporosis-related fractures [3]. These agents are orally administered, and selectively accumulate in bone tissues, where they inhibit osteoclast activities. However, treatments with bisphosphonates lower bone turnover, leading to a decrease of bone quality, and are known to cause medication-related osteonecrosis of the jaw (MRONJ) [4]. Parathyroid hormone (PTH) exerts an anabolic effect on bones when it is administered intermittently; its intermittent administration enhances proliferation and differentiation of osteoblastic cells, which, in turn, stimulate osteoclast activities, leading to a high bone-turnover [5]. Newly developed antibody therapeutics including an anti-RANKL (receptor activator of nuclear factor kappa-B ligand) antibody, denosumab, and an anti-sclerostin antibody, romosozumab, are also notable in terms of molecularly targeted therapies [6,7]. Injection-associated complications exist as unavoidable risks in the antibody therapies as well as PTH, as all these treatments require subcutaneous injection.
Genetic skeletal disorders tend to become apparent at a young age. Osteogenesis imperfecta (OI) is a heritable disorder characterized by bone fragility and other skeletal manifestations [8], such as short stature and bone deformities. In about 85% of cases, OI is caused by mutations in the genes encoding type I collagen (COL1A1 and COL1A2), which affect collagen quantity or structure [9]. Traditional classifications divide OI into four classes based on clinical presentation, radiographic features, and patterns of inheritance of mutations in either COL1A1 or COL1A2 [9]. The current classifications add an additional 18 types to the list based on newly identified mutations [9]. The treatment of OI is still challenging. Although bisphosphonates are available for OI patients, these do not contribute to new bone formation. PTH and antibody therapies have not yet been approved for OI treatment.
Therefore, there is clear demand for the development of new drugs, especially orally available anabolic drugs that are applicable for a wide range of skeletal disorders including osteoporosis and OI. Here we report a promising candidate for such drugs. The candidate was identified by screening of egg yolk-derived peptides; they not only promoted proliferation and differentiation of skeletal cells and their progenitors in vitro, but also promoted skeletal repair and exerted therapeutic effects on degenerative skeletal disorders in rodent models.
2. Methods
2.1. PF201 digestion with an artificial digestive juice and analysis of digestive products
Ten milligrams of PF201 were digested in 0.5% pancreatin/phosphate buffer solution at 37 °C for 16 h. Digestive products were analyzed by high-performance liquid chromatography (HPLC: Nexera XR; Shimadzu). Peptides corresponding to each peak were identified by MALDI-TOFMS (AXIMA-Performance; Shimadzu).
2.2. Biodistribution of PF201
For fluorescent labeling of PF201, PF201 was dissolved in 0.1 M NaHCO3 and then mixed with 100 mg/mL solution of Cyanine3 NHS ester (Cy3-NHS ester; Lumiprobe) at a ratio of 9:1. Following 4-h reaction under dark conditions, the Cy3-labeled PF201 solution was desalted using a Sephadex G-10 gel filtration column (GE Healthcare). The Cy3-labeled PF201 (100 mg/kg) was orally administered to 7-week-old male ddy mice (Shimizu Laboratory Supplies Co., Ltd.), and the stomach, small intestine, portal vein, and liver were collected at 1 h after the administration. The organs were homogenized in phosphate buffered saline (PBS). Extracts obtained from each organ were analyzed by HPLC (Nexera XR; Shimadzu) to detect Cy3-labeled peptides.
2.3. Cell culture and osteogenic induction
MC3T3-E1 cells and ATDC5 cells were obtained from RIKEN Cell Bank. MC3T3-E1 cells were maintained in 10% fetal bovine serum (FBS)/alpha-MEM (A10490-01; Life Technologies) supplemented with 1% penicillin/streptomycin. ATDC5 cells were maintained in 5% FBS/Dulbecco's Modified Eagle Medium (DMEM)-F12 (11039–021; Thermo Fisher) supplemented with 1% penicillin/streptomycin. Mouse mesenchymal stromal cells (MSCs) and calvarial osteoblasts were isolated as previously described [10]. Human MSCs were obtained from LONZA. Mouse and human MSCs were maintained in 10% FBS/DMEM high glucose (043–30085; Wako) supplemented with 1% penicillin/streptomycin. Osteogenic induction was performed by culturing cells with 10% FBS/DMEM high glucose supplemented with 0.1 μM dexamethasone, 10 mM β-glycerophosphate, and 50 μg/ml ascorbic acid phosphate (osteogenic medium). For osteogenic induction of hMSCs, 10 ng/ml recombinant human bone morphogenetic protein (BMP)2 (rhBMP2: 355-BM; R&D) was added to the osteogenic medium. Von Kossa staining and alizarin red staining were performed as previously described [11].
2.4. Cell proliferation assay
Cell proliferation was quantified using either Cell Counting Kit-8 (341–07761; FUJIFILM Wako Pure Chemical Corporation) or Cell Counting Kit (11647229001; Roche) according to the manufacturers’ instructions; the absorbance at 460 nm was measured after 2-h incubation with WST-8 or BrdU solution.
2.5. RT-qPCR
Total RNA was extracted and purified using ISOGEN (311–02501; Nippon Gene) and an RNeasy mini kit (74106; Qiagen), and reverse-transcribed into single-strand complementary DNA using a ReverTra Ace qPCR RT Master Mix kit (FSQ-301; Toyobo) according to the manufacturer's instructions. qPCR was performed using a FastStart Universal SYBR Green Master kit (4913850; Roche) on a 7500 Fast Real-Time PCR System (Applied Biosystems). Expression levels were calculated by the 2–ΔΔCt method. Actb (mouse) and GAPDH (human) were used as housekeeping genes for the calculation. Primers are listed in Table S1.
2.6. RNA sequencing
Human MSCs were cultured for 24 h in the presence or absence of 10 nM D2. Total RNA was isolated using ISOGEN (311–02501; Nippon Gene) and an RNeasy mini kit (74106; Qiagen). RNA-seq libraries were constructed using a Tru-Seq Illumina kit (RS-122-2001; Illumina) with some modifications. Library quality was validated using a Bioanalyzer (Agilent) before sequencing on Hiseq X (Illumina) platforms. The outputted sequence reads were aligned and mapped using Partek Flow software v2.2 (Partek). The raw reads were first subjected to pre-alignment Quality Assurance and Quality Control (QA/QC). Any base below a Phred value of 20 was trimmed from either side of the read and reads shorter than 25 nt length were removed. The processed reads were aligned by Tophat2 [12] to the GRCh38 reference genome. The mapping quality and coverage were checked by post-alignment QA/QC. Aligned reads were quantified by an expectation maximization algorithm [13], followed by a normalization of mapped reads per kilobase length of transcript per million reads (RPKM).
2.7. Animals
Wild-type (WT) C57BL/6 J mice were obtained from Charles River Japan. C57BL/6-Col1α1Mov13/J mice (Mov13 mice) were obtained from the Jackson Laboratory (JAX Stock #002197); male and female Mov13 mice and their WT littermates were used in experiments. Rats were obtained from Nihon Bioresearch Inc. All experiments were performed in accord with the protocols approved by the Animal Care and Use Committee of The University of Tokyo and Nagasaki University. Mice were kept in individual cages under controlled temperature and humidity with a 12-h circadian rhythm. They were given ad libitum access to food and water. All efforts were made to minimize the suffering of the mice. Euthanasia of mice was performed with an overdose of barbiturates. In the fracture model, ovariectomized model, and Mov 13 mice, 10 mg/kg/day D2 or vehicle was orally administered every day for the indicated periods.
2.8. Fracture model
Under general anesthesia with isofluorane in O2, the left hind limb of 8-week-old male mice was shaved and sterilized for surgery. A 15-mm incision was made longitudinally, and the muscle was bluntly dissected to expose the tibia. A transverse osteotomy was performed using disk-shaped dental steel bars at the midpoint of the tibia. The fracture was repositioned, and then the fractured bone was internally stabilized as previously described [14,15]. After irrigation with saline, the skin was closed with 4–0 nylon sutures.
2.9. Ovariectomy
Eight-week-old female mice were anesthetized by administration of 2% isoflurane at a rate of 0.2–0.3 L/min. After shaving the hair in the dorsal mid-lumbar area, the skin was cleaned and a 20-mm midline dorsal skin incision was made. After excision of a pair of ovaries, the wound was closed by suturing.
2.10. Histological analysis
PF201 was orally administered to 4-week-old male Crlj rats for 7 days (Day 1 to Day 7). Tetracycline and calcein were administered on Days 4 and 6, respectively, and the rats were sacrificed on Day 8. After fixation with 70% ethanol, the left tibias were stained with Villanueva bone stain, dehydrated with ethanol, embedded into methyl methacrylate (MMA), and subjected to histological analysis. Bone histomorphometric analysis was performed with a fluorescence microscope (BX-53; Olympus) and Histometry RT system (System Supply Inc.), basically according to the standard method [16].
2.11. Mechanical property test
Both a three-point bending test and a compression test were carried out to evaluate the maximum load before fracture using a Sun RHEO meter system. In the three-point bending test, a sample stage supported both ends of the tibia, and then a load was applied to the center (fracture site) to investigate deformation and strength. In the compression test, a load was applied to the lumbar vertebral body fixed on a sample stage in the cranio-caudal direction of the vertebral body. A load–deformation curve was obtained by measuring the relationship between the load value and the deformation amount.
2.12. Radiological analysis
Micro-computed tomography (CT) scanning of the harvested tibias and lumbar vertebral bodies was performed using a micro focus X-ray CT system SMX-90CT (Shimadzu) under the following conditions: tube voltage, 90 kV; tube current, 110 mA; layer thickness, 5.3, 7.1, and 4.0 mm; and field of view, 10.4, 13.1, and 7.2 mm for observation of the tibia and lumbar vertebral body, respectively. The resolution of one CT slice was 512 × 512 pixels. The three-dimensional construction software package TRI/3D-BON (Ratoc System Engineering) was used for quantitative analysis.
2.13. Biochemical blood test
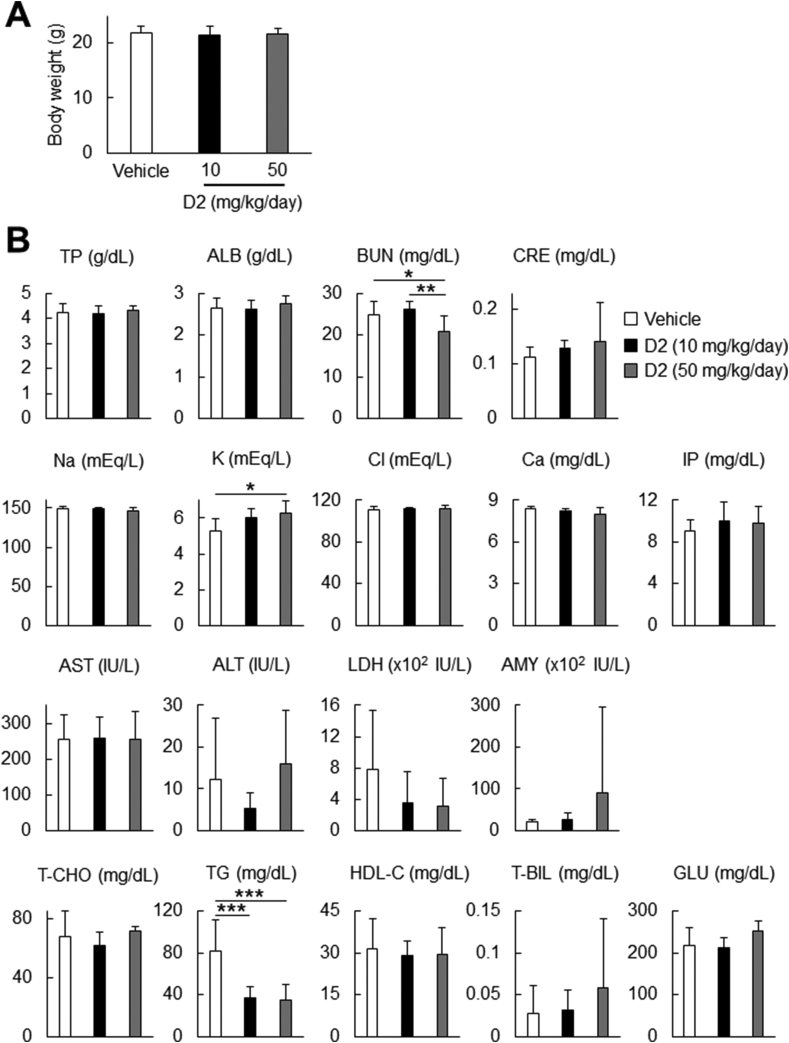
Six-week-old male C57BL/6 mice were subjected to 2-wk oral administration of D2 (6 days per week; 10 or 50 mg/kg/day). Blood samples were collected from the heart ventricle of the mice under terminal anesthesia; the samples were centrifuged for serum separation (390 g for 15 min at room temperature). The sera were analyzed by Oriental Yeast Co., Ltd.
2.14. Enzyme-linked immunosorbent assay (ELISA)
Primary osteoblasts were isolated from calvarias of P2 WT or Mov13 mice. The cells were cultured in 10% FBS/alpha-MEM supplemented with 1% penicillin/streptomycin until reaching confluency. The cells were then cultured for 24 h in DMEM high glucose under a starvation condition, followed by 48-h treatment with D2 (10 nM). The amount of mouse pro-collagen I alpha 1/pro-collagen I N-terminal propeptide in the medium was determined by a Mouse Pro-collagen I alpha 1 ELISA Kit (ab210579; Abcam) according to the manufacturer's instruction.
2.15. Statistical analysis
Data are expressed as the mean ± standard deviation (SD). Student's t-test was applied for comparisons between two groups; the Steel–Dwass test was used for multiple comparisons between groups; and Dunnett's test was used for comparisons between experimental groups and a control group. In the analysis of body weight and blood test data (Fig. 6), the means of groups were compared by one-way ANOVA and analyzed by Tukey–Kramer test. The tests used for statistical analysis in each experiment are described in the figure legends. Values of p < 0.05 were considered to indicate significant differences (∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001).
Fig. 6.
Systemic effects of D2 administration. (A) Body weight after 2-wk administration of D2 (10 mg/kg/day, N = 8; 50 mg/kg/day, N = 8) or vehicle (N = 9) in C57BL/6 mice. (B) Biochemical blood tests after 2-wk administration of D2 (10 mg/kg/day, N = 8; 50 mg/kg/day, N = 8) or vehicle (N = 9) in C57BL/6 mice. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001 (Tukey–Kramer test). TP, total protein; ALB, albumin; BUN, blood urea nitrogen; CRE, creatinine; IP, inorganic phosphate; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; AMY, amylase; T-CHO, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; T-BIL, total bilirubin; GLU, glucose.
3. Results
3.1. The egg yolk-derived peptide PF201 promotes skeletal cell proliferation and in vivo bone formation in rodents
We used peptide engineering to identify the egg yolk-derived functional peptide PF201 (see patent WO2018003817: PREVENTATIVE AND/OR THERAPEUTIC AGENT FOR OSTEOGENESIS IMPERFECTA AND THE LIKE; Fig. 1A). As shown in Fig. 1B and C, PF201 significantly promoted proliferation of the preosteoblastic cell line MC3T3-E1 and the chondrogenic cell line ATDC5 in a dose-dependent manner. To evaluate its in vivo osteogenic effects, we next administered PF201 to 3-week-old rats for 7 days. In bone histomorphometric analysis, parameters for bone formation and osteoblasts were significantly increased in the rats treated with PF201 (Fig. 1D). In contrast, PF201 did not alter osteoclast activities (Fig. 1E). These data suggest that orally administered PF201 has an anabolic function in postnatal bone tissues in rodents.
Fig. 1.
Identification of functional peptide sequences in skeletal cells in vitro and in vivo. (A) The chemical structure of PF-201. (B and C) Effects of PF201 on cell proliferation in MC3T3E1 cells (B) and ATDC5 cells (C). Cells were treated with the indicated concentration of PF201 for 24 h, and then subjected to cell proliferation assays after 2 h incubation with BrdU solution. Data are means ± SDs (n = 8). ∗p < 0.05 vs. the control (Dunnett's test). (D) Histomorphometric analyses on bone formation parameters with distal femurs in rats orally treated with PF201 for 7 days from 3 weeks of age. The left panels show the result of calcein staining (upper and lower) and H-E staining (middle). The right panels show the result of bone volume per tissue volume (BV/TV), trabecular thickness (Tb.Th), osteoblast surface per bone surface (Ob.S/BS), mineral apposition rate (MAR), and bone formation rate per bone surface (BFR/BS). Arrows indicate osteoblasts. Data are means ± SDs (n = 10). ∗p < 0.05 vs. the control (Dunnett's test). The scale bar in the top panel is 500 μm, and those in the middle and bottom panels are 10 μm. (E) Histomorphometric analyses of bone resorption parameters in the distal femurs of rats orally treated with PF201 for 7 days from 3 weeks of age. H-E staining and osteoclast surface per bone surface (Oc.S/BS) are shown. Data are means ± SDs (n = 10). No statistically significant differences were detected by Dunnett's test between the experimental groups and control group. Scale bars, 10 μm.
3.2. In vitro effects of D2, a metabolite of PF201, on preosteoblasts and mesenchymal stromal cells (MSCs)
Our biochemical analysis showed that PF201 was digested into three types of truncated peptides in an artificial digestive juice: D1 composed of an N-terminal 6 mer fragment, D2 composed of an N-terminal 4 mer fragment, and D3 composed of a C-terminal 4 mer fragment (Fig. 2A, left). An in vivo biodistribution assay with labeled peptides showed that D2, but not other truncated forms, was present in small intestine, portal vein, and liver, whereas RF201 as well as D2 were detected in stomach (Fig. 2A, right), suggesting that among the PF201 metabolites, only D2 was present and circulated after digestion and absorption. This finding led us to test the biological activities of D2 in skeletal cells and their precursors. D2 as well as PF201 promoted proliferation of osteoblast progenitors and chondrocyte progenitors in vitro (data not shown). In addition, D2 promoted osteoblast differentiation of MC3T3-E1 cells, as shown by upregulation of osteoblast marker genes: runt-related transcription factor (Runx2), alkaline phosphatase (Alpl), type I collagen α1 chain (Col1a1), and bone gamma-calboxyglutamate protein (Bglap) (Fig. 2B). The osteogenic effect of D2 was also confirmed in human and mouse MSCs; D2 induced the expression of osteoblast marker genes and calcification under osteogenic conditions (Fig. 2C). As MSCs are characterized by multipotency and a capacity for self-renewal, we then investigated the effect of D2 on the proliferation of human and mouse MSCs. D2 enhanced their proliferation at a level comparable to that by FBS (Fig. 2 D). Dose dependency was not observed from 1 nM to 1 μM of D2, suggesting that 1 nM D2 may exert a maximized effect on cell proliferation (Fig. 2 D). To gain insight into the biological activity of D2, we performed RNA-sequencing in human MSCs cultured for 24 h with or without D2. We first identified differentially expressed genes (DEGs) between the control and D2-treated MSCs according to expression levels and statistical values (see the Methods section for details), as shown by heatmap (Fig. 2E, left). Gene ontology analysis of the sets of DEGs indicated that D2 elicited diverse biological responses in MSCs (Fig. 2E, right); it is worth noting that the term “negative regulation of cell proliferation” is enriched in the set of down-regulated genes, which may partly account for the positive effect of D2 on proliferation of MSCs.
Fig. 2.
Effects of D2 on proliferation and differentiation of skeletal cells and their precursors. (A) Luminescence intensity in each organ at 15 min after oral administration of the labeled PF201 (10 mg/kg). After administration of Cy3 (cy NHS ester)-modified PF-201 (100 mg/kg) to ddy mice with a gastric tube, the indicated organs were collected. Their tissue extracts were subjected to liquid chromatography. (B) Effects of D2 on osteoblast differentiation in MC3T3-E1 cells. Cells were treated with 100 nM D2 for either 2 weeks or 4 weeks. Data are means ± SDs (n = 3). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.005 vs. the control (Student's t-test). (C) Effects of D2 on osteoblast differentiation in mouse mesenchymal stromal cells (MSCs) (upper) and human MSCs (lower). Upper: mouse MSCs were treated with 100 nM D2 for 4 weeks; the results of von Kossa staining and mRNA expression analysis are shown. Lower: cells were treated with 100 nM D2 with or without 10 ng/ml BMP2. Data are means ± SDs (n = 3). ∗p < 0.05 vs. cells treated with BMP2 (Student's t-test). (D) Effects of D2 on proliferation of mouse and human MSCs. Cells were cultured in serum-free DMEM with the indicated concentrations of D2 for 24 h. As positive controls, 10% and 1% FBS were used for mouse and human MSCs, respectively. Data are means ± SDs (n = 8). ∗p < 0.05, ∗∗p < 0.01, vs. control (Dunnett's test). (E) RNA-sequencing analysis in human MSCs treated with 100 nM D2 for 24 h. Hierarchical clustering and heatmap analysis revealed differentially expressed genes (DEGs) (rpkm>2 and p-value<0.05). The top 6 most enriched terms in the gene ontology analysis of DEGs are also shown.
3.3. Acceleration of fracture healing by D2
We investigated the therapeutic efficacy of D2 using several mouse models representing bone-related diseases. We first tested the efficacy in fracture healing. D2 was orally administered for 56 days after tibial fractures were created. Micro-CT analysis of the callus demonstrated that the callus volume of the D2-administered group was significantly higher during the initial 4 weeks than that of the control group, although there was no significant difference in callus volume during the remodeling phase at 6–8 weeks after fracture (Fig. 3A). When we assessed the quality of the callus based on its bone mineral density (BMD), the callus with high BMD was significantly increased in the D2-administered group compared to the control group (Fig. 3B). Both endochondral ossification and intramembranous ossification contribute to bone repair [14]; endochondral bones are likely to show relatively low BMD, whereas intramembranous bones show higher BMD, at an early phase of the repair process. The increase in the high BMD area seems to be associated with intramembranous bones, which mainly contribute to mechanical properties of repaired bones. Thus, the increase in whole callus volumes and selective increase in high BMD callus in the D2-administered group suggested that the fractured sites were more rigidly fixed by D2 administration, which likely contributed to the successful fracture repair. Consistent with this notion, a three-point bending test revealed that the mechanical strength of the fracture sites was significantly increased in the D2-administered group compared to the control group (Fig. 3C).
Fig. 3.
Effects of D2 on fracture healing in a mouse model. (A) Callus volume determined by 3D micro-CT analysis on male mice orally administered D2 or vehicle. Ten mg/kg/day D2 or vehicle was orally administered every day from 1 to 56 days after making the bone fracture. Micro-CT images of callus formation are also shown. Data are means ± SDs (n = 8). ∗p < 0.05 vs. vehicle (Student's t-test). (B) Callus volume calculated at bins of different levels of bone mineral density (BMDs) in mice orally administered D2 or vehicle. ∗p < 0.05 vs. vehicle (Student's t-test). (C) Mechanical properties of fractured sites in mice orally administered D2 or vehicle. Load and deformation were measured by three-point binding tests on healthy and fractured bones at 14 days after fractures (left). The maximum load is shown in the right panel. We used the same batch but different mice for this experiment from mice used in panels A and B. Data are means ± SDs (n = 7). ∗p < 0.05 vs. vehicle (Student's t-test).
3.4. Improvement of bone strength and fracture healing by D2 under ovariectomy-induced osteoporotic conditions
We next investigated the therapeutic effects of D2 on osteoporosis using ovariectomized (OVX) mice representing a postmenopausal osteoporotic condition. Beginning at 4 weeks after ovariectomy, we daily administered D2 for 3 weeks (Fig. 4A). Morphometry analysis of the micro-CT data showed that: (1) OVX decreased the bone volume of the lumbar vertebral body (L3) and (2) D2 administration partially recovered the bone volume (Fig. 4B). In a compression test of L3, OVX also decreased the mechanical property of L3, which was again partially recovered by D2 administration (Fig. 4C). Given that postmenopausal osteoporosis is known to increase the risk of fractures, we tested the effects of D2 administration on tibial fractures under the ovariectomy-induced osteoporotic condition. Tibial fractures were created at 4 weeks after ovariectomy, and the mice were then subjected to 3 weeks of daily D2 administration (Fig. 4D). OVX mice showed a trend toward decreased callus volume; the volume was partially recovered by D2 administration (Fig. 4E). Thus, D2 is likely to ameliorate osteoporosis and compromised fracture healing under osteoporotic conditions.
Fig. 4.
Effects of D2 on bone strength and fracture healing in ovariectomized (OVX) mice. (A) Schematic schedule of ovariectomy and subsequent administration of D2. (B) Bone volume of L3 vertebral bodies of the control mice and OVX mice orally administered D2 or vehicle. 3D micro-CT images (left) and BV/TV (right) are shown. Data are means ± SD (n = 8). ∗p < 0.05 (Steel–Dwass test). (C) Mechanical properties of L3 vertebral bodies of the control mice and OVX mice orally administered D2 (10 mg/kg/day) or vehicle. Load and deformation were measured by compression tests (left). The maximum load is shown in the right panel. Data are means ± SDs (n = 13 to 15). ∗p < 0.05 (Steel–Dwass test). (D) Schematic schedule of ovariectomy, fracture operation, and subsequent administration of D2. (E) Callus volume determined by 3D micro-CT analysis on the control mice and OVX mice orally administered D2 or vehicle. 3D micro-CT images (left) and quantitative analysis of the callus volume in the distal femurs (right) are shown. Data are means ± SDs (n = 5). ∗p < 0.05 (Steel–Dwass test).
3.5. Therapeutic effects of D2 on OI conditions
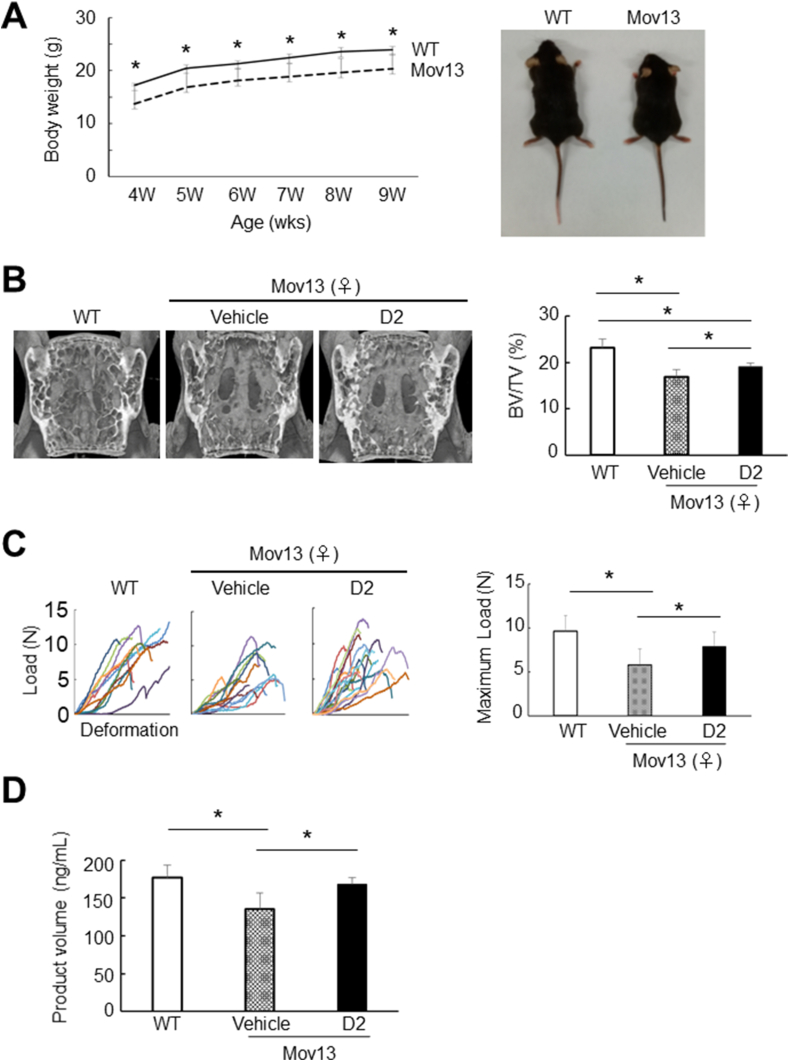
Our above data suggest that D2 can augment the activities of osteogenic cells and their precursors, and thereby accelerate bone healing and ameliorate disease conditions via an improvement of bone quality. Thus, we next asked whether D2 would ameliorate pathological conditions in osteogenesis imperfecta (OI), a bone-related genetic disease. We used the Mov13 mouse line as a model of type I OI, which was characterized by a decrease in type I collagen gene dosage due to heterozygous mutation [17,18]. The growth of Mov13 mice was significantly impaired compared to that of their WT counterparts (Fig. 5A). Beginning at the age of 4 weeks, we administered D2 or vehicle to Mov13 mice for 4 weeks. We performed the administration in drinking water, because administration with sonde caused fractures in the Mov13 mice. We confirmed that bone volume was decreased in Mov13 mice compared to WT mice, and found that D2 administration significantly increased bone volume in the Mov13 mice (Fig. 5B). A compression test of L3 revealed that its mechanical property was significantly impaired in Mov13 mice, and the property was fully recovered by D2 administration (Fig. 5C). Lastly. We examined whether synthesis of type I collagen protein was improved by D2 in Mov13 osteoblasts. The results showed that type I collagen synthesis was decreased in primary osteoblasts of Mov13 calvarias compared to that of WT ones; two-day D2 treatment recovered the amount of type I collagen in the cultured Mov13 osteoblasts (Fig. 5D). These data suggest that oral administration of D2 is a promising therapy for OI.
Fig. 5.
Effects of D2 on bone volume and its mechanical properties in a mouse model of osteogenesis imperfecta (OI). (A) Comparison of postnatal growth (left) and gross appearance (right) between wild-type (WT) and Mov13 male mice. ∗p < 0.05 (Student's t-test). (B) Bone volume of L3 vertebral bodies of WT and Mov13 female mice orally administered D2 (10 mg/kg/day) or vehicle. 3D micro-CT images (left) and BV/TV (right) of the L3 vertebrae are shown for WT and Mov13 female mice at the age of 8 weeks (after 4-week D2 administration to Mov13 mice). Data are means ± SD (n = 4 to 5). ∗p < 0.05 (Steel–Dwass test). (C) Mechanical properties of L3 vertebral bodies of WT and Mov13 female mice orally administered D2 or vehicle. Load and deformation were measured by compression tests (left). The maximum load is shown in the right panel. Data are means ± SDs (n = 11). ∗p < 0.05 (Steel–Dwass test). (D) Production of type I collagen in primary osteoblasts isolated from either WT or Mov13 mouse calvaria at P1. Cells were treated with or without 10 nM D2 for 2 days. The cellular production of type I collagen was measured by ELISA. Data are means ± SDs (n = 6). ∗p < 0.05, ∗∗p < 0.01 (Steel–Dwass test).
3.6. Systemic effects of D2 administration on mice
To gain insight into systemic effects of D2, we analyzed weights and biochemical parameters of blood in C57BL/6 mice that were orally administered D2 or vehicle for 2 weeks. We tested two doses of D2: 10 mg/kg/day and 50 mg/kg/day. Two-week D2 administration did not cause significant weight changes compared to vehicle administration (Fig. 6A). Biochemical blood data showed no dramatic difference between the two groups in most of parameters we tested, although levels of triglycerides and blood urea nitrogen were significantly downregulated in the D2-administered group compared to the control group (Fig. 6B); gamma-glutamyl transpeptidase (γ-GT) values were below detection limit in all groups (data not shown). In addition, D2-administered mice did not show any abnormalities in their behaviors compared to control mice during the administration period. Thus, D2 is less likely to have systemic adverse effects and toxicity when orally administered.
4. Discussion
In this study, we have four major findings. First, the egg yolk-derived peptide PF201 promoted in vivo bone formation in rodents. PF201 also enhanced proliferation of osteoblasts and chondrocytes in vitro. Second, D2, a metabolite of PF201, was present and circulated after digestion and absorption. D2 had positive impacts on proliferation and differentiation of MSCs and preosteoblasts. Third, oral administration of D2 accelerated the bone healing in a mouse fracture model. D2 also improved bone strength and fracture healing under ovariectomy-induced osteoporotic conditions in mice. Lastly, D2 showed a therapeutic effect in a mouse OI model. Although there were several reports showing positive impacts of egg yolk and its peptides on bone formation and osteoblasts [19,20], biologically-active single peptide sequences had not been identified in egg yolk. In this study, we have identified such peptide sequences and propose that D2 is a candidate for an orally available therapeutic for a range of skeletal disorders.
In bone fracture experiments, micro-CT analysis showed that D2 increased callus volumes during the bone repair process; in vitro data suggest that accelerated proliferation and differentiation of osteoblasts may underlie the phenomenon. We think that the increased callus volume contributes to increased stability of fracture sites, leading to early fracture repair. Importantly, the mechanical strength of the fracture sites was significantly increased in the D2-administered group compared to the control group. Thus, D2 may not only accelerate the fracture healing, but also decrease the risk of defective healing, such as nonunion or malunion.
Our data obtained in mouse models support the therapeutic efficacies of orally administered D2 in several skeletal degenerative disorders, also suggesting that PF201 and its metabolite D2 may at least partly overcome the limitations of currently available drugs. Orally administered PF201 did not affect osteoclast activities in rats; in vitro data further indicated that both D2 and PF201 had a positive impact on osteoblasts. Therefore, unlike antiresorptive agents such as bisphosphonate (and its derivatives) and denosumab, D2 and PF201 might avoid low bone turnover-related issues [21]. PTH and romosozumab are anabolic agents. These molecularly targeted agents, as well as denosumab, have a risk of injection-associated complications. Romosozumab was also reported to have a cardiovascular risk [22]. These risks are likely reduced in PF201 and its metabolite D2, given that they are orally available anabolic agents originated from a biological component, i.e., egg yolk.
In this study, we used Mov13 mice as a model of type I OI, which is caused by an insufficient quantity of type I collagen. D2 ameliorated OI phenotypes with increased bone strength and rescued the COL1A1 protein expression in vitro. However, the effects of D2 on other types of OI remain to be clarified. Different types of OI are caused by abnormalities at different steps of type I collagen production: construction of primary structures, post-translational modifications, protein folding, intracellular transport, and matrix incorporation [23]. It has not yet been investigated whether D2 engages in these steps and exerts therapeutic effects on other types of OI. To answer these questions, further in vitro and in vivo analyses will be needed in the future.
The source protein of PF201 peptide has been identified. The peptide sequence of PF201 is matched with amino acids of vitellogenin-2 in positions 1060 to 1067. Vitellogenin-2 is a precursor of egg yolk proteins, which is enzymatically cleaved into four yolk proteins, lipovitellin-1, lipovitellin-2, phosvitin, and YGP40, during egg formation [24]. In egg yolk, lipovitellin-1 includes the PF201 peptide sequence (see patent US10538564B2; WO2015129726A1: PEPTIDES HAVING OSTEOBLAST GROWTH-PROMOTING ACTIVITY AND USE THEREOF).
A limitation of this study is that direct targets of D2, i.e., D2 binding proteins, have not been identified yet. Because D2 has only four peptides, it remains to be clarified whether or not D2 has a specific three-dimensional structure and how D2 specifically binds to the target proteins. Further structural studies and in silico prediction for the peptide-binding protein will help to address these issues in the future. In addition, effects of D2 on MSC differentiation into other cell lineages including adipocytes and chondrocytes need to be addressed in the future. Such studies will be a key to further development of the D2-derived therapeutic drugs for skeletal disorders.
Declaration of Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Shinsuke Ohba reports financial support was provided by Japan Society for the Promotion of Science. Hironori Hojo reports financial support was provided by Japan Society for the Promotion of Science. Ung-il Chung reports financial support was provided by Japan Society for the Promotion of Science. Shinsuke Ohba reports financial support was provided by Japan Agency for Medical Research and Development.
Acknowledgements
We thank Denise Zujur, Kiyoshi Miwa, Chihiro Suzuki, Takanari Shigemitsu, and Masayoshi Aosasa for supporting the identification and characterization of functional peptides derived from chicken egg yolk; Nihon Bioresearch Inc. (Gifu, Japan) for technical support with the animal experiments; Oriental Yeast Co., Ltd. (Tokyo, Japan) for help with blood tests; and Akemi Ito and laboratory staff at the Ito Bone Histomorphometry Institute (Niigata, Japan) for help with the histology preparations and suggestions for bone histomorphometry. We are also grateful to Akiko Nakamichi, Asuka Miyoshi, Aiko Kuroda, Kasumi Noguchi, and Nozomi Nagumo for providing technical assistance. This study was supported by Grants-in-Aid for Science Research from the Japan Society for the Promotion of Science (JSPS) (20H03885 to H.H.; 16H06312 to U.C.; and 17H04403 to S.O.) and the Japan Agency for Medical Research and Development (AMED; 20ek0109321h9903 to S.O.). This work utilized the core research facility of the Center for Disease Biology and Integrative Medicine at the Graduate School of Medicine, The University of Tokyo.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2022.11.002.
Contributor Information
Hironori Hojo, Email: hojo@g.ecc.u-tokyo.ac.jp.
Shinsuke Ohba, Email: ohba.shinsuke.dent@osaka-u.ac.jp.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- 1.Estell E.G., Rosen C.J. Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis. Nat Rev Endocrinol. 2021;17:31–46. doi: 10.1038/s41574-020-00426-5. [DOI] [PubMed] [Google Scholar]
- 2.Eastell R., O'Neill T.W., Hofbauer L.C., Langdahl B., Reid I.R., Gold D.T., et al. Postmenopausal osteoporosis. Nat Rev Dis Prim. 2016;2 doi: 10.1038/nrdp.2016.69. [DOI] [PubMed] [Google Scholar]
- 3.Bastounis A., Langley T., Davis S., Paskins Z., Gittoes N., Leonardi-Bee J., et al. Assessing the effectiveness of bisphosphonates for the prevention of fragility fractures: an updated systematic review and network meta-analyses. JBMR Plus. 2022;6 doi: 10.1002/jbm4.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuroshima S., Sasaki M., Sawase T. Medication-related osteonecrosis of the jaw: a literature review. J Oral Biosci. 2019;61:99–104. doi: 10.1016/j.job.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 5.Silva B.C., Bilezikian J.P. Parathyroid hormone: anabolic and catabolic actions on the skeleton. Curr Opin Pharmacol. 2015;22:41–50. doi: 10.1016/j.coph.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosman F., Crittenden D.B., Adachi J.D., Binkley N., Czerwinski E., Ferrari S., et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375:1532–1543. doi: 10.1056/NEJMoa1607948. [DOI] [PubMed] [Google Scholar]
- 7.Deeks E.D. Denosumab: a review in postmenopausal osteoporosis. Drugs Aging. 2018;35:163–173. doi: 10.1007/s40266-018-0525-7. [DOI] [PubMed] [Google Scholar]
- 8.Ben Amor I.M., Roughley P., Glorieux F.H., Rauch F. Skeletal clinical characteristics of osteogenesis imperfecta caused by haploinsufficiency mutations in COL1A1. J Bone Miner Res. 2013;28:2001–2007. doi: 10.1002/jbmr.1942. [DOI] [PubMed] [Google Scholar]
- 9.Marini J.C., Forlino A., Bächinger H.P., Bishop N.J., Byers P.H., Paepe A., et al. Osteogenesis imperfecta. Nat Rev Dis Prim. 2017;3 doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 10.Ogata N., Chikazu D., Kubota N., Terauchi Y., Tobe K., Azuma Y., et al. Insulin receptor substrate-1 in osteoblast is indispensable for maintaining bone turnover. J Clin Invest. 2000;105:935–943. doi: 10.1172/JCI9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kanke K., Masaki H., Saito T., Komiyama Y., Hojo H., Nakauchi H., et al. Stepwise differentiation of pluripotent stem cells into osteoblasts using four small molecules under serum-free and feeder-free conditions. Stem Cell Rep. 2014;2:751–760. doi: 10.1016/j.stemcr.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S.L. TopHat 2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing Y., Yu T., Wu Y.N., Roy M., Kim J., Lee C. An expectation-maximization algorithm for probabilistic reconstructions of full-length isoforms from splice graphs. Nucleic Acids Res. 2006;34:3150–3160. doi: 10.1093/nar/gkl396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitaura Y., Hojo H., Komiyama Y., Takato T., Chung U.I., Ohba S. Gli 1 haploinsufficiency leads to decreased bone mass with an uncoupling of bone metabolism in adult mice. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kashiwagi M., Hojo H., Kitaura Y., Maeda Y., Aini H., Takato T., et al. Local administration of a hedgehog agonist accelerates fracture healing in a mouse model. Biochem Biophys Res Commun. 2016;479:772–778. doi: 10.1016/j.bbrc.2016.09.134. [DOI] [PubMed] [Google Scholar]
- 16.Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., et al. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harbers K., Kuehn M., Delius H., Jaenisch R. Insertion of retrovirus into the first intron of alpha 1(I) collagen gene to embryonic lethal mutation in mice. Proc Natl Acad Sci U S A. 1984;81:1504–1508. doi: 10.1073/pnas.81.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonadio J., Saunders T.L., Tsai E., Goldstein S.A., Morris-Wiman J., Brinkley L., et al. Transgenic mouse model of the mild dominant form of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1990;87:7145–7149. doi: 10.1073/pnas.87.18.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Q., Li C., Geng F., Huang X., Ma M. Hen egg yolk phosvitin stimulates osteoblast differentiation in the absence of ascorbic acid. J Sci Food Agric. 2017;97:4532–4538. doi: 10.1002/jsfa.8320. [DOI] [PubMed] [Google Scholar]
- 20.Kim H.K., Lee S., Leem K.H. Protective effect of egg yolk peptide on bone metabolism. Menopause. 2011;18:307–313. doi: 10.1097/gme.0b013e3181f31b1f. [DOI] [PubMed] [Google Scholar]
- 21.Ural A. Biomechanical mechanisms of atypical femoral fracture. J Mech Behav Biomed Mater. 2021;124 doi: 10.1016/j.jmbbm.2021.104803. [DOI] [PubMed] [Google Scholar]
- 22.Bovijn J., Krebs K., Chen C.Y., Boxall R., Censin J.C., Ferreira T., et al. Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci Transl Med. 2020:12. doi: 10.1126/scitranslmed.aay6570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forlino A., Cabral W.A., Barnes A.M., Marini J.C. New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol. 2011;7:540–557. doi: 10.1038/nrendo.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H., Zhang S. Functions of vitellogenin in eggs. Results Probl Cell Differ. 2017;63:389–401. doi: 10.1007/978-3-319-60855-6_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.