Abstract
People living with HIV face a high risk of mental illness, especially depression. We do not yet know the precise neurobiological mechanisms underlying HIV-associated depression. Depression severity in the general population has been linked to acute and chronic markers of systemic inflammation. Given the associations between depression and peripheral inflammation, and since HIV infection in the brain elicits a neuroinflammatory response, it is possible that neuroinflammation contributes to the high prevalence of depression amongst people living with HIV. The purpose of this review was to synthesise existing evidence for associations between inflammation, depression, and HIV. While there is strong evidence for independent associations between these three conditions, few preclinical or clinical studies have attempted to characterise their interrelationship, representing a major gap in the literature. This review identifies key areas of debate in the field and offers perspectives for future investigations of the pathophysiology of HIV-associated depression. Reproducing findings across diverse populations will be crucial in obtaining robust and generalisable results to elucidate the precise role of neuroinflammation in this pathophysiology.
Subject terms: Neuroscience, Molecular biology, Depression
Introduction
We are living in a global moment of transition, as AIDS-related mortality declines globally and HIV becomes a manageable chronic infection. Due to advancements in combination antiretroviral therapy (cART), the life expectancy of people living with HIV has begun to approach that of the general population [1, 2], although considerable variability remains among key population subgroups [3, 4]. Of the 1.5 million people that were diagnosed with HIV in 2020, many can expect to lead a full life [5].
There are notable clinical concerns in the management of chronic HIV infection, chief among which is the high risk for a range of mental health issues and cognitive deficits [6, 7]. Long-term HIV infection leads to neurocognitive impairment beyond that which is associated with healthy ageing [8], and distinct from that seen in neurodegenerative disorders [9], which persists in mild forms despite cART [10]. Young people living with HIV, including perinatally infected children, are also vulnerable to these cognitive deficits [11].
People living with HIV also face an alarmingly high prevalence of depression compared to the general population, with 30–50% of HIV+ participants reporting depressive symptoms in some studies [12, 13]. The risk of depression in people living with HIV is estimated to be twice that of the general population [14]. Suicide ideation is also substantially higher in people living with HIV, consistent with the observed rates of depression [15, 16]. Therefore, depressive symptomatology is a widespread and serious concern among people living with HIV.
We do not yet know the precise neurobiological mechanisms underlying HIV-associated depression. Some theories suggest that deficits in serotonergic [17] or dopaminergic [18] pathways might explain this risk of depression. Others argue that HIV-associated depression may be a consequence of inflammatory responses [19]. Inflammation, or activation of the body’s immune responses, has been correlated with depression in the general population [20, 21]. This theory is supported by overlapping somatic features such as sleep disturbance and lethargy (collectively termed “sickness behaviour”) that are shared between inflammation and depression [22]. There is growing support for an inflammatory subtype of depression, since some people with depression experience alleviation of symptoms when administered anti-inflammatory medication [23]. Additionally, there is some evidence to suggest that neuroinflammation—a collection of immune responses in the central nervous system, characterised by microglial activation and release of inflammatory cytokines—might be involved in the aetiology of depression [24]. Given the associations between depression and inflammation, and since HIV infection in the brain elicits a neuroinflammatory response [25], it may be argued that neuroinflammation contributes to HIV-associated depression. However, literature in this field is extremely limited and few studies have attempted to elucidate the relationship between neuroinflammation, depression, and HIV.
The objective of this review is to synthesise and critically appraise existing evidence which suggests that neuroinflammation may be a significant contributor to the high risk of depression amongst people living with HIV. We begin with a brief discussion of the strengths and limitations of various techniques currently in use to measure neuroinflammation in humans. We then describe evidence in the literature that points to independent relationships between HIV, depression, and inflammation, and discuss recent preclinical and clinical studies that offer preliminary evidence for an association between neuroinflammation and depression in HIV. Finally, we propose future directions for research into the contribution of neuroinflammation in HIV-associated depression.
Measuring neuroinflammation in humans
In order to identify the potential contribution of neuroinflammation in HIV-associated depression, we must first be able to accurately assess degree of neuroinflammation in humans. The most direct way to achieve this would be to assess features such as level of activation of resident immune cells or concentration of neuroinflammatory cytokines in brain tissue using brain biopsies or histopathology [26]. However, brain biopsies are highly invasive and generally cannot be justified in research studies, whereas histopathological analyses in humans are limited by availability of post-mortem brain tissue samples [27, 28]. As a result, to assess neuroinflammation in vivo, indirect markers are generally used. Methodologies for in vivo measurement of neuroinflammation have been extensively reviewed elsewhere [29], and we summarise the most widespread techniques here to aid in the consequent discussion of neuroinflammation in HIV-associated depression.
CSF and blood biomarkers
In the absence of brain tissue to directly assess glial cell activation or cytokine elevations, cerebrospinal fluid (CSF) and blood are useful tissue samples for indirect measurement of neuroinflammation. Metabolic markers in CSF have been used as an indication of neuroinflammation, since CSF has a direct interface with the brain [30]. Inflammatory cytokines such as interleukin-15 (IL-15) and monocyte chemoattractant protein-1 (MCP-1) in CSF may in fact be useful to characterise neuroinflammatory disease progression [31, 32]. However, there is considerable variability in reported CSF biomarker concentrations even within specific neurodegenerative conditions [33], and CSF biomarkers may not accurately reflect the state of cells in the brain. In addition, lumbar puncture to obtain CSF samples is quite an invasive procedure and may deter participation in studies.
Blood assays are less invasive than CSF assays, but also represent a less direct measure of inflammatory processes in the brain as there is limited exchange of molecules across the blood–brain barrier (BBB). C-Reactive Protein (CRP) is a widely-studied inflammatory cytokine whose serum concentrations have been correlated with depression severity [21]. The cytoskeletal protein neurofilament light (NFL), which is released into CSF and blood as a consequence of neuroaxonal injury, has diagnostic value in many neurological diseases [34]. In addition to being a marker of neuroaxonal injury and neurodegeneration, NFL may be a promising proxy measure of neuroinflammation as well [35–37]. Recent studies suggest that NFL levels in both CSF and plasma correlate with levels of commonly-used biomarkers of neuroinflammation such as CXCL10 and neopterin [38]. Plasma NFL is also correlated with the kynurenine-to-tryptophan ratio and kynurenine metabolites, which are both traditional measures of neuroinflammation. Finally, there are correlations between CSF and serum concentrations of both CRP and NFL [39–41], indicating that blood assays may be a preferable method of measuring inflammation to avoid highly invasive and painful procedures for participants. Taken together, these findings indicate that NFL in blood serum or plasma may be used as an indirect biomarker of neuroinflammation.
In addition to serum levels of inflammatory markers such as CRP (which represent acute inflammation), recent advancements have also allowed us to probe DNA methylation signatures of many markers of peripheral inflammation [42, 43]. These epigenetic signatures represent more stable, longitudinal measures of chronic inflammatory markers and have been calculated using data from epigenome-wide association studies (EWAS) for cytokines such as CRP and BDNF [21, 44–46]. However, DNA methylation signatures for NFL, which has only recently been recognised as a proxy measure of neuroinflammation, have not yet been studied. Calculating methylomic signatures for NFL and studying these in large cohort studies could thus be a meaningful opportunity to indirectly explore aspects of chronic neuroinflammation in humans.
Neuroimaging biomarkers
Neuroimaging techniques offer a more indirect methodology for assessment of neuroinflammation in human participants than blood or CSF assays [47]. The two most widely-used neuroimaging techniques to measure neuroinflammation are positron emission tomography (PET) and magnetic resonance spectroscopy (MRS). PET involves administration of a radioligand designed to bind to a target protein and emit radioactivity, which may be used to quantify a physiological function [48, 49]. In the context of neuroinflammation, this physiological function of interest is microglial activation, which is assessed using PET radioligands that bind specifically to the mitochondrial 18 kDa translocator protein (TSPO) [50, 51]. TSPO binding is considered a measure of microglial activation and is altered in various psychopathologies [52, 53] but not in healthy ageing [54], providing a sensitive biomarker that can be used to distinguish healthy ageing from neuropsychiatric and neurodegenerative disorders. Numerous TSPO-targeted PET radioligands have been developed in recent years, and the choice of radioligand when assessing neuroinflammation may introduce an additional confounding variable and limit comparisons across studies [55, 56]. Moreover, as PET involves injection of a radioactive tracer, use of this methodology to study neuroinflammation can be ethically contentious, especially for children or participants who are seriously ill [57, 58].
[99m]Tc-tilmanocept is another neuroimaging radiotracer which may possibly be used to measure neuroinflammation via imaging. This radiotracer binds to the CD206 receptor, which is constitutively expressed on the surface membrane of microglia and macrophages, and thus may be used as a marker of microglial activation [59]. Early studies suggest that in vivo nuclear imaging of tilmanocept can serve as a measure of BBB dysfunction [60]. Since the use of tilmanocept as a potential imaging biomarker of inflammation is fairly new, the studies that have applied this radiotracer in samples of people living with HIV so far have focused on cardiovascular disease [61, 62]. However, as tilmanocept has the potential to serve as a measure of microglial activation and BBB disruption, it may in future be used to assess neuroinflammation and neuropathology amongst people living with HIV.
MRS allows us to non-invasively identify and quantify metabolites in the brain as an additional indirect measure of neuroinflammation [63]. MRS investigates the behaviour of atoms with non-zero spin under an external magnetic field to determine the chemical composition of a localised brain region [64, 65]. Proton MRS (or 1H-MRS, targeting hydrogen atoms in brain tissue) is most commonly used in clinical studies to assess neurometabolite concentrations, which are often altered in disease states, including in HIV [66–69]. The neurometabolites myo-inositol (mI) and glutamate/glutamine (Glx) are upregulated in activated glial cells, whereas levels of N-acetyl-aspartate (NAA) and choline-containing metabolites such as glycerophosphocholine and phosphocholine (GPC + PCh) are altered when neuronal membrane integrity is compromised [70–73]. Thus, alterations in mI, Glx, NAA, or GPC + PCh concentrations may be indicative of neuroinflammation. Cerebral metabolite concentrations as measured via MRS also correlate with CSF concentrations of some chemokines amongst people living with HIV, which further supports the use of MRS as a non-invasive tool to measure neuroinflammation in this population [74]. However, due to the ubiquity of hydrogen atoms in the brain, MRS struggles with a very low signal-to-noise ratio and requires substantial signal enhancement, and often requires pre-specified Regions of Interest (ROIs) [75].
The advantages and disadvantages of the most commonly-used methodologies to measure neuroinflammation in humans discussed here are summarised in Table 1.
Table 1.
Strengths and limitations of commonly-used techniques to measure neuroinflammation in humans in the context of HIV.
| Technique | Example studies | Strengths | Limitations |
|---|---|---|---|
| Neuropathology |
Smith et al. [26] Anthony et al. [223] |
Cellular and molecular features of neuroinflammation such as microglial ramification or activation may be observed and quantified directly | Limited availability of post-mortem human brain tissue |
| Cerebrospinal fluid markers |
Cheng et al. [224] Guha et al. [225] |
Concentrations of inflammatory cytokines circulating in the central nervous system may be determined directly | High variability; lumbar puncture is an invasive and painful procedure |
| Blood biomarkers |
Lyons et al. [226] Wada et al. [227] |
Venepuncture for sample collection is a routine procedure; levels of some blood biomarkers are correlated with CSF levels | Less direct measure of CNS inflammation as there is limited exchange of molecules across the blood–brain barrier |
| PET Imaging |
Garvey et al. [228] Vera et al. [97] |
In vivo measurement of microglial and/or astroglial activation in the brain | Heterogeneity in choice of radioligands and analysis; invasive and with potential risks with injection of radioactive tracers |
| MRS Imaging |
Graham et al. [69] Cysique et al. [229] |
Non-invasive, relatively straightforward to administer; can detect changes in a range of neurometabolites in localised brain regions | Low signal-to-noise ration; requires pre-specific Regions of Interest (ROIs) |
Studies employing these techniques to assess neuroinflammation in HIV are included as examples.
Pieces of the puzzle
HIV and (neuro)inflammation
HIV is, at its core, an inflammatory condition [76]. When HIV is first detected in the body, a range of innate immune responses are activated to defend the body, including elevations in circulating cytokines and activation of Natural Killer (NK) cells [77]. These systemic changes represent features of HIV-induced inflammation [78, 79]. Since HIV targets CD4+ T cells, which are crucial for mounting an immune response in the human body, the inflammatory response to HIV is paradoxical in that level of immune activation is positively correlated with disease progression [80, 81]. This inflammation predisposes people living with HIV to a variety of physiological effects, such as cardiovascular disease [82], renal function alterations [83], increased gut permeability [84], and clusters of illnesses collectively termed “inflamm-aging” [85]. In addition to physical health effects, the inflammation elicited by chronic HIV infection has also been linked to cognitive impairment [86].
One of the key reservoirs for latent HIV infection is the brain [87], so it is unsurprising that HIV infection also elicits a neuroinflammatory response [88, 89]. Even though HIV cannot directly infect neurons, its viral proteins (especially gp120 and tat [90, 91]) are known to induce neuronal cell death. Even non-replicating, latent proviral HIV DNA can be transcribed and translated into these neurotoxic viral proteins, which trigger inflammatory responses in the brain [92, 93]. Activation of the NLRP3 inflammasome, triggered by HIV infection and leading to the production of pro-inflammatory cytokines IL-1β and IL-18, is implicated in HIV disease progression and depletion of CD4+ T cells [94, 95]. This inflammasome activation in microglia has been shown to constitute an early and integral aspect of HIV-associated neuropathology, suggesting that HIV induces a neuroinflammatory response that may be detrimental to the central nervous system (CNS) [96].
There are three lines of evidence supporting the theory that HIV infection is linked to neuroinflammation. First, in vitro studies indicate that exposure to HIV viral proteins leads to microglial activation, a hallmark feature of neuroinflammation [25]. These findings are corroborated by clinical investigations which found that people living with HIV show global increases in microglial activation compared to negative controls [97]. Second, HIV infection induces changes in neurometabolite concentrations consistent with inflammation in several brain regions [68, 69, 98]. Finally, concentrations of NFL in both blood and CSF have been shown to be elevated in people with HIV [99–101], indicating neuronal damage, and these levels are reduced when participants start receiving cART [102]. Together, these results strongly suggest that HIV induces an neuroinflammatory response in humans.
HIV is a genetically diverse pathogen, with several subtypes and recombinant variants, including Subtype B (most common in Europe and Americas) and Subtype C (prevalent in southern Africa and India) [103]. Subtype C contains a “defective” Tat protein and has thus been hypothesised to be less neuropathogenic than Subtype B [104]. However, recent studies have shown that the (neuro)inflammatory responses elicited by these two subtypes of HIV do not differ significantly, with a wide range of CSF and serum inflammatory chemokines and cytokines being significantly elevated in individuals with Subtype B or Subtype C HIV compared to HIV-negative controls, but with no difference in the biomarker levels between the two subtypes [105]. On the contrary, an in vitro study testing inflammation and BBB dysfunction in response to Tat proteins from the HIV Subtype B and recombinant HIV CRF02_AG (prevalent in West Africa) found that Tat.B elicited a greater inflammatory response in comparison to Tat.AG [106]. Together, these results suggest that while Subtype C may not be less neuropathogenic than other subtypes as previously hypothesised, there may still be subtype-dependent variability in neuroinflammatory responses which warrant further investigation.
HIV and depression
Depression has long been known to be the most common neuropsychiatric condition associated with HIV infection [107]. Studies consistently find prevalence rates of about 30% for depression amongst people living with HIV [108], and large-scale meta-analyses estimate that this prevalence rate is twice that in the general population [14]. Amongst people living with HIV, this risk for depression seems to be somewhat dependent on sex [109], such that women are at higher risk for severe or moderate depression whereas men are at higher risk for moderate depression, and on time passed since diagnosis [110], such that individuals who have been diagnosed more recently are at higher risk for depression. Additionally, prevalence rates vary by geography and are substantially higher in the Global South than in the Global North [111], with estimated rates of 40% or higher for countries such as South Africa [112]. Combined with the fact that healthcare and research funding is concentrated in the Global North [113], this severely exacerbates the burden of depression amongst people living with HIV in the Global South.
The potential mechanisms underlying HIV-associated depression have been extensively reviewed elsewhere [19]. Briefly, possible ways in which HIV might render people susceptible to depression may include neurotoxicity leading to neurotransmitter dysfunction, metabolic dysfunction, and chronic elevations in inflammatory cytokines. Some studies have found that changes in immunometabolism, such as the ratio of kynurenine-to-tryptophan (KYN/TRP), are linked to depression amongst people living with HIV [114]. This is particularly important as ART-induced improvements in depressive symptoms seem to be mediated in part by the reversal of tryptophan catabolism [115]. There are significant overlaps between these various hypothesised mechanisms behind HIV-associated depression, for instance, as inflammatory cytokines may induce glial cell activation and an increase in tryptophan metabolism, which may further cause serotonin depletion and elicit overexpression of metabolites such as quinolinic acid [116]. Therefore, the high risk of depression amongst people living with HIV may stem from a combination of dysfunction in immunometabolism, inflammation, and neurotransmitter cascades.
Interestingly, concentrations of CSF HIV RNA, but not plasma HIV RNA, seem to predict onset of depressive symptoms [117]. This suggests that it may be necessary for HIV to interact directly with aspects of the central nervous system—whether this is metabolism, inflammation, or neurotransmitters—in order to elicit depressive behaviours, and that viral load outside the CNS may not be sufficient to explain HIV-associated depression. However, the directionality of this relationship is unclear: depressive symptoms may conversely increase susceptibility to HIV (by increasing risky behaviours) and are known to predict worse clinical outcomes in people living with HIV [118, 119]. Moreover, this relationship is further complicated when considering that antiretroviral medication may affect metabolism and inflammation [120]. Latest-generation ART, while less neurotoxic, is still associated with metabolic alterations that may contribute to chronic inflammation and immune dysfunction, which ultimately may lead to depression [121]. Conversely, while ART normalises the levels of some inflammatory cytokines in people living with HIV, concentrations of other cytokines such as IL-6 and CRP (which are strongly linked to depression) remain elevated [122, 123]. In all, the relationship between HIV and depression is multi-faceted, but seems to be dependent (at least in part) on the direct interaction of HIV with the CNS and independent of ART-induced normalisations in metabolism or inflammation.
It is important to note here that a purely neurobiological explanation will likely not be able to account for the alarmingly high risk of depression in HIV, as the aetiology of depression is complex and multi-faceted. The role of psychosocial and individual factors cannot be neglected, especially as people living with HIV are likely to experience housing or financial insecurity, discrimination on the basis of gender or sexual identity, intimate partner violence, or external and internalised stigma, all of which can contribute to depression [124, 125]. People living with HIV may also receive poor social support and experience social isolation, which may further contribute to increased inflammation and behavioural deficits [126, 127]. Moreover, some people with depression (including people living with both HIV and depression) do experience symptom relief in response to non-pharmacological interventions such as psychotherapy and social support [128], further indicating that psychosocial factors play a critical role in the progression of HIV-associated depression. Nevertheless, given that depressive symptoms do have strong biological correlates and predict negative clinical outcomes with regards to HIV, investigating the neurobiological pathogenesis of HIV-associated depression is essential in improving quality of life for people living with HIV.
Depression and (neuro)inflammation
Identifying the pathophysiology of depression is crucial to designing effective, targeted therapeutic interventions for treatment. For several decades, the prevailing theory in biological psychiatry was that depression stems from a dysfunction in serotonergic pathways [129]. This theory was supported by serendipitous clinical findings that pharmacological interventions such as monoamine oxidase inhibitors could alleviate the symptoms of depression in some patients [130]. Dysregulation of serotonin and other neurotransmitters has been strongly implicated in depression over the years [131, 132]. However, the neurotransmitter theory of depression struggles with certain key challenges, including that a third of patients do not respond to treatment with antidepressants such as selective serotonin reuptake inhibitors (SSRIs) [133, 134]. Given this, and since there is increasing evidence that depression may in fact be a constellation of symptoms, efforts have been made to classify depression into subtypes of varying aetiology, so that interventions may be precisely targeted to individuals instead of a “one-size-fits-all” approach to antidepressant treatment [135, 136].
Since there is considerable overlap between the somatic features of inflammation and depression, there is rising support for the theory that depression (or at least a subtype of depression) may be associated with inflammation [137]. In particular, behavioural changes such as fatigue, motivational anhedonia, and sleep disturbances (“sickness behaviours”) can be observed in both depression and inflammation [138], and depressive symptoms can be alleviated with anti-inflammatory medication [139]. Significant elevations in concentrations of inflammatory cytokines such as IL-1, IL-6, CRP, and Tumour Necrosis Factor (TNF-α) have consistently been observed in people with depressive symptoms [140–143]. A large meta-analysis also found that inflammation is a significant predictor of depression [144]. CRP, especially, has been the subject of much attention as a peripheral marker of inflammation that seems to be correlated with depression incidence and severity [41, 145]. Associations between CRP levels and depression remain significant even after controlling for some clinical and psychosocial factors [146], and elevations in CRP levels predict decreased functional connectivity in key brain regions [147]. While the precise nature of this association remains unknown, it is theorised that this may be a bidirectional relationship [148]. Evidence from a longitudinal twin-study suggests that different inflammatory cytokines might interact with depression differently: elevations in CRP may perhaps be a consequence of depression, while elevations in IL-6 act as a risk factor for depression [149]. Later studies have linked CRP and IL-6 to specific symptoms of depression and identified a potential causal link between IL-6 and depression [150]. NFL, which has recently gained attention as a useful biomarker of neuronal injury and neuroinflammation, has also been associated with depressive symptom severity [151]. Further genetic and immunological investigation is necessary to determine whether CRP, IL-6, NFL, or other inflammatory cytokines may have a causal relationship with depression.
The gut microbiome also interacts significantly with inflammation and immunometabolism to influence depression incidence and severity [152]. Manipulation of the gut microbiome has shown some promise in the treatment of depression, although the mechanisms underlying this “microbiota-gut-brain axis” remain unclear [153]. Recent evidence has shown that gut microbiome dysbiosis is linked to worse psychological well-being amongst people living with HIV and depression in individuals co-infected with HIV and HCV [154, 155]. While further work is necessary, these studies suggest that the gut microbiome may be an important influence in regulating the relationship between HIV and depression.
Strong evidence has been identified in recent years pointing to a link between depression and neuroinflammation [156, 157]. Elevations in neurometabolites such as mI in the brain are associated with depression incidence and severity [158]. CSF concentrations of markers such as TNF-α and IL-1β are also correlated with depression severity [159, 160], and exogenous induction of inflammation can produce some core features of depression [161]. Conversely, administration of minocycline (an anti-inflammatory drug) attenuates depressive symptoms [162], and animal models indicate that minocycline achieves this by inhibiting microglial activation [163, 164]. Indeed, Richards et al. [165] found that TSPO binding (recall that this is a marker of microglial activation) is highest in patients with depression who are not on antidepressant medication, whereas medicated participants show comparable TSPO binding to undepressed controls. These results lend support to the theory that microglial activation (a key feature of neuroinflammation) may be targeted by antidepressant therapies for treatment-resistant depression [24]. Taken together, these findings suggest that inflammation, and neuroinflammation specifically, may play a critical role in the psychopathology of depression.
Putting the pieces together
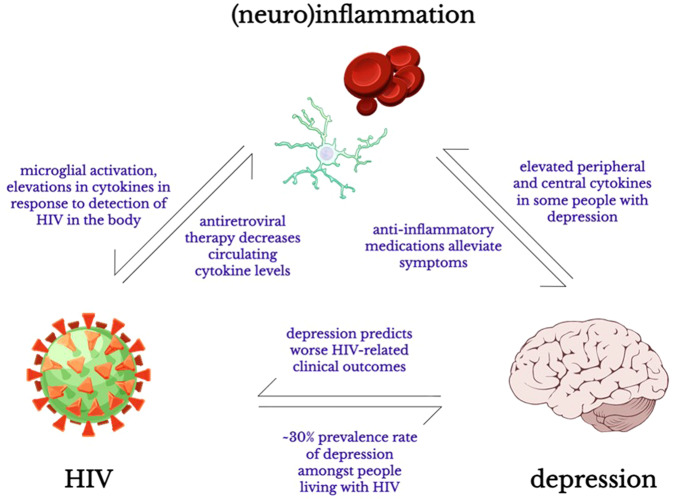
A substantial body of evidence has shown that independent associations exist between depression, neuroinflammation, and HIV (Fig. 1). However, very few studies have attempted to explore the relationship between all three conditions. The relationship between (neuro)inflammation and depression has been described in great detail in an excellent systematic review by Toenders et al. recently [148]. We point readers particularly to Fig. 3 in the review article by Toenders et al. [148] [p.321] which summarises various proposed mechanisms connecting inflammation and depression, such as metabolic dysfunction including leptin and insulin resistance and hypothalamic-pituitary axis hyperactivity. These physiological alterations have been observed with HIV infection [166, 167], and thus the molecular pathways discussed by Toenders et al. may be highly relevant to HIV-associated depression as well. Therefore, we will not discuss specific molecular or physiological pathways connecting HIV, inflammation, and depression here, and will instead focus on highlighting recent studies that have demonstrated preliminary evidence for such an association amongst people living with HIV specifically.
Fig. 1. Schematic representation of the relationship between HIV, depression, and (neuro)inflammation.
Key pieces of evidence pointing to independent associations between these conditions are summarised. Open source (CC-BY) schematic drawings via SciDraw. Microglia: 10.5281/zenodo.3926033; erythrocytes: 10.5281/zenodo.3926235; human brain: 10.5281/zenodo.3925925; virus: 10.5281/zenodo.3926017.
Preclinical and post-mortem evidence
Preclinical studies of neuroinflammation in HIV-associated depression present a unique set of strengths and challenges. On the one hand, features of neuroinflammation such as microgliosis may be directly measured in animals using immunostaining of tissue samples [168] or in vivo imaging through cortical windows [169], which is seldom possible in humans as access to primary human brain tissue is not often feasible. On the other, due to the narrow host range of HIV, animal models of HIV infection cannot accurately replicate the progression of HIV infection in humans [170, 171]. Moreover, although animal models of depression have been developed to exhibit many somatic features of depression [172, 173], it remains difficult to study higher-order cognitive and emotional components of depression (such as loneliness or suicidality) in animals. For these reasons, neuroinflammation in HIV-associated depression has not been widely studied in preclinical models.
Advancements in animal models for HIV-associated depression were reviewed by Barreto et al. who suggested that it may be worthwhile to investigate the role of neuroinflammation in these animal models [174]. Since publication of their review, there have been very few attempts to explore this line of enquiry. Nemeth et al. [175] tested whether developmental expression of HIV-related proteins in transgenic rats may be linked to depressive-like behaviours and elevations in neuroinflammatory responses. They found that adolescent HIV Tg rats did exhibit depressive-like behaviours and increased concentrations of MCP-1 (a key neuroinflammatory chemokine) in the hippocampus. Treatment with meloxicam (a selective COX-2 inhibitor) successfully reduced Mcp-1 gene expression in the hippocampus, but failed to rescue depressive-like behavioural deficits in the HIV Tg rats. Their findings suggest that HIV-related proteins can trigger depressive behaviours in rats, but the expression of Mcp-1 in the hippocampus may not contribute to HIV-associated depression. The same group later found that the expression of HIV-related proteins during development induced anxiety-like behaviours in Tg rats and hyper-ramification of microglia (theorised to be involved in microglial surveillance and phagocytosis of apoptotic cells [176, 177]), but did not test for depressive-like behaviours in this instance [178]. Taken together, their results suggest that the presence of HIV viral proteins is associated with depressive-like behaviours and neuroinflammatory responses in rats, but whether HIV-induced neuroinflammation elicits these depressive-like behaviours remains unclear. Since research in this area is sparse, it is crucial for future studies to explore the role of neuroinflammation (especially biomarkers other than MCP-1) in animal models of HIV-associated depression and clarify whether blocking specific neuroinflammatory pathways may rescue HIV-related depressive-like behaviours.
Few post-mortem studies have specifically investigated the relationship between neuroinflammatory markers and depressive symptomatology in people living with HIV. One autopsy study did not find any pathological features in post-mortem brain tissues of people with HIV that could predict depression [179], whereas another found that ante-mortem plasma levels of the inflammatory biomarker sCD163 predicted post-mortem observations of microglial activation, but only included one participant with depression in their study sample and thus could not test for associations with depression [180].
Clinical studies: peripheral inflammatory biomarkers
Clinical investigations of neuroinflammation in HIV-associated depression are limited. The most closely-related studies look at markers such as CRP that may be more closely linked to peripheral inflammation (instead of neuroinflammation), but these are worthy of discussion as they provide some insights into the kind of relationship we may expect between neuroinflammation, HIV, and depression.
An early study of 23 people living with HIV measured plasma concentrations of several inflammatory cytokines (IL-15, IP-10, IL-12, and G-CSF) and found that these cytokines were significantly elevated in HIV+ individuals with depression compared to HIV+ individuals without depression [119]. Plasma cytokine levels and depressive symptom severity were significantly correlated, suggesting that peripheral inflammatory responses may predict depression severity in people living with HIV. Further studies, including a large multi-centre cohort study of 1,727 participants [181], have also observed this correlation between depression and blood biomarkers of systemic inflammation amongst people living with HIV [182, 183]. This evidence supports the hypothesis that people living with HIV may be susceptible to an inflammatory subtype of depression. Since recent evidence suggests that there is significant overlap between systemic inflammation and neuroinflammation in many disease states (contrary to our previous understanding of the CNS as isolated from the peripheral immune system) [184, 185], these trends may perhaps be reproduced when studying neuroinflammation in HIV-associated depression.
While studies generally agree that cytokines such as IL-6 and TNF-α are correlated with depression, the role of CRP (a cytokine that generally exhibits robust correlations with depression [141, 186]) specifically in HIV-associated depression is a major area of debate. Poudel-Tandukar et al. [187] saw a linear relationship between serum CRP levels and depression severity, and Memiah et al. [188] also observed significant differences in CRP levels between HIV+ participants with depressive symptoms and those without. However, several other studies have found no significant association between CRP concentrations and depression amongst people living with HIV [189–192]. (Notably, Ellis et al. [192] did not observe such a correlation for MCP-1 either, which lends support to the preclinical findings discussed previously [175].) In fact, these studies saw significant correlations between depression and other inflammatory markers such as IL-6 [189, 192] and TNF-α [190], but not CRP. Perhaps this indicates that the subtype of depression to which people living with HIV are most susceptible is mediated by other inflammatory cytokines, but not CRP? Inflammation is not the only physiological state in which cytokines such as CRP and IL-6 serve a function, which complicates any inferences we may make about their role in HIV-associated depression [193]. Further studies are necessary to resolve this debate and determine what role, if any, is played by CRP in HIV-associated depression, as this understanding may aid in developing translational tools for subtype-specific screening of depression in people living with HIV.
The inflammatory biomarkers assayed in studies of HIV-associated depression vary considerably, which limits our ability to identify strong candidates with diagnostic or therapeutic value. A recent study [194] investigated associations between levels of six cytokines (IL-1β, eotaxin, IL-15, IL-6, TNF-α, and leptin) and chronic multisite pain (CMP) in a sample of people living with HIV, but also incorporated PHQ-9 scores as a measure of depression in their regression models. They found that only one cytokine—IL-1β—out of the six analytes tested was significantly associated with CMP, and notably, exploratory analyses showed that accounting for total PHQ-9 score reduced the odds ratio of this model. Depression severity and concentrations of the biomarker IL-1β may thus be interlinked amongst people living with HIV. Memiah et al. [188] tested whether “mental health symptoms” correlated with five inflammatory biomarkers: CRP, IL-6, IL-18, soluble tumour necrosis factor receptor-I (sTNFR-I) and soluble TNFR-II. While their primary outcome measure was not specifically depression, the “self-reported mental health symptoms” in this study included many key features of depression. The authors reported that CRP and soluble TNFR-II were significantly associated with self-reported mental health issues amongst people living with HIV. Pala et al. [195] similarly sought to identify biomarkers associated with depressive symptoms amongst people living with HIV, and found that IL-6 concentrations and monocytes correlated significantly with a severe subtype of depression in their participant sample. Notably, Memiah et al. did not find a significant association between IL-6 and symptoms of depression, whereas Pala et al. did observe such a correlation. Another study found that depression, but not HIV status, was associated with the release of IL-6 [196] which aligns with the findings of Memiah et al. and suggests that the relationship between IL-6 and depressive symptoms may be independent of HIV status. Taken together, these initial findings implicate several central and peripheral inflammatory markers in depression amongst people living with HIV, but heterogeneity in results limits synthesis across studies.
Clinical studies: neuroinflammatory biomarkers
While most studies discussed in the previous section involve a broad panel of central and peripheral inflammatory biomarkers, few published studies have attempted to expressly investigate a relationship between a specific measure of neuroinflammation and HIV-associated depression. Saloner et al. [197] recently compared levels of blood biomarkers of neuroinflammation between people living with HIV and negative controls. In this study, the authors examined the associations of CSF dopaminergic biomarkers with depression severity (measured as Beck Depression Inventory [BDI-II] scores) and neuroinflammation (measured as a composite score of CSF concentrations of sCD14, MCP-1, IP-10, and neopterin) in a sample of HIV+ and HIV− participants [197]. Although their primary research question concerned the relationship between depressive symptoms and CSF dopaminergic markers, they did report a statistically non-significant trend between higher BDI-II scores and higher composite CSF neuroinflammation score [p.4] [197]. They also saw a significant correlation between BDI-II scores and CSF IP-10 concentrations specifically, but this statistical significance did not persist after FDR correction. Crucially, no such correlations were observed in the HIV- group, offering preliminary evidence that the relationship between neuroinflammation and depression may be dependent on HIV infection status.
Woods et al. [198] present the most promising results to date that implicate neuroinflammatory markers in depressive symptoms amongst people living with HIV. In this study of older adults with and without HIV, the authors found that plasma concentrations of brain-derived neurotrophic factor (BDNF) were significantly associated with depression, such that lower levels of plasma BDNF were linked to higher levels of depressive symptoms. These findings are encouraging, but isolated: substantial further work is necessary to determine the association between various neuroinflammatory markers and depression severity in HIV, and to utilise other measures of neuroinflammation (such as neuroimaging) in this direction. It will also be crucial to replicate these findings in samples with distinct demographic profiles, as no effort has yet been directed at determining the interactions, if any, of psychosocial factors with neuroinflammation in HIV-associated depression.
The way forward
Clinical studies of neuroinflammation and depression in HIV
Given the strong body of evidence linking neuroinflammation, depression, and HIV, and yet the dearth of studies investigating the relationship between the three, the vital first step towards the future is to conduct clinical studies of neuroinflammation in HIV-associated depression. Through these clinical studies, we may begin to resolve the major areas of debate in the field that have been identified through this critical review:
Is there a reliable association between peripheral concentrations of CRP and depression amongst people living with HIV?
Are people living with HIV susceptible to a subtype of depression mediated by specific cytokines (such as IL-6) and not others (CRP), for which we can develop subtype-specific therapeutic interventions?
Is the relationship between neuroinflammation and depression dependent on HIV infection status, and is there subgroup variability or differences in brain structure and function within this population?
Although research in this field is quite sparse, some efforts are underway. Notably, an ongoing clinical trial recruiting HIV+ participants in Uganda is investigating the potential for group psychotherapy to improve the severity of depression and whether depression is linked to inflammation in this group [199]. Results from this clinical trial will shed further light on the complex interactions between neuroinflammation, depression, and HIV.
A range of research methodologies are available to neurovirologists to measure neuroinflammation in people living with HIV, and these were summarised in this review. We particularly draw the readers’ attention to neurofilament light (NFL) as a blood biomarker of great potential in assessing degree of neuroinflammation. Although NFL is a marker of neuroaxonal injury, given the evidence discussed here which points to strong correlations between NFL and “traditional” biomarkers of neuroinflammation, and since there is considerable overlap between neurodegeneration and neuroinflammation, plasma or serum NFL may in future prove to be a useful indirect measure of neuroinflammation for which sensitive assays are already available. NFL concentrations in blood correlate with CSF levels, indicating a reliable measure of CNS inflammatory responses, and can be measured with an assay that is considerably less painful for participants than lumbar puncture, which may help boost participant willingness to contribute to these studies. Furthermore, calculating methylomic signatures for NFL using EWAS data (as has been done previously for CRP [42, 44]) might allow us to assess chronic elevations in NFL and offer a longitudinal measure of neuroinflammation, provided that we obtain relevant and sufficiently-powered discovery data. These serum and epigenetic NFL markers may allow us to study short- and long-term changes in NFL levels, which, combined with longitudinal data on depression severity and HIV viral loads, may elucidate the relationship between neuroinflammation and depression through the course of chronic HIV infection.
There are considerable differences in methodology, statistical approach, and reporting of effect sizes in the literature in the field. Consider three representative studies of inflammation and cognition amongst people living with HIV. Anderson et al. [102] report a slope of −11.5 for their multivariate mixed-effects model between plasma NFL concentrations and composite cognitive scores, indicating that cognition worsened by 11.5 points for each logarithmic unit increase in plasma NFL. Saloner et al. [183] report an unstandardised regression estimate of −0.025 for their cross-level interaction between plasma CRP and average depression scores on global cognition, which suggests that global cognition worsened by 0.025 points for each unit increase in CRP for participants with a specific average depression score. Imp et al. [200] report a standardised beta weight of −0.30 for their regression model testing associations between plasma sCD163 levels and global cognitive scores, indicating that global cognition worsened by 0.30 points for each unit increase in plasma sCD163 concentration. This heterogeneity in methodology and statistical reporting limits synthesis of results across studies. Variability in reporting effect sizes also restricts our ability to carry out appropriate sample size estimations for future studies. Many studies in the field, especially those involving PET, struggle with small sample sizes due to resource constraints, and accurate power analyses (involving standardised effect sizes) are crucial in ensuring that the conclusions drawn from these studies are placed in the appropriate statistical context. Thus, there is a need to standardise statistical approaches in the field to allow for robust experimental design and meta-analysis of findings.
Therefore, methodological approaches to measuring (neuro)inflammation and reporting of effect sizes in these studies are quite variable. Nevertheless, it is possible to make some estimation of the effect size for this relationship with two principles in mind: (1) our measures of neuroinflammation in humans are indirect and imprecise; and (2) neuroinflammation is only one of several biological and psychosocial factors that may be relevant to HIV and depression. In that light, we would reasonably expect neuroinflammation to have a small contribution to the pathogenesis of HIV-associated depression when correctly estimated. Therefore, going forward, researchers should clearly report standardised estimates of effect sizes and exercise caution when reviewing reports of large effects in this context [201].
Once a link between neuroinflammation and HIV-associated depression has been established through future studies, the exact nature of this relationship may be investigated. It is probable that HIV and depression interact actively with each other and co-morbidity of the two conditions would necessitate more concerted effort than treating each condition [202] If neuroinflammation is indeed linked with depression in HIV, what is the direction and causality of this relationship? It is possible that HIV elicits a neuroinflammatory response, which then leads to depressive symptoms. However, it is equally likely that an HIV infection (through biological or psychosocial pathways) causes depression, which then leads to neuroinflammation. Which pathway represents the true mechanism underlying this association will impact the development of targeted therapeutics: if HIV-associated neuroinflammation causes depression, then we may use anti-inflammatory medication to treat this depression, but if HIV-associated depression manifests as neuroinflammation, then anti-inflammatory medication might not attenuate depressive symptoms. Therefore, it is crucial to determine the precise relationship between neuroinflammation, HIV, and depression.
Reproducing findings across diverse populations
Some of the incongruity in evidence relating to neuroinflammation, depression and HIV may be attributed to experimental variability, but the role of participant profiles must also be taken into account when interpreting these findings [203]. Social, economic, cultural, and structural factors can confound results and contribute to heterogeneity in findings across studies [204]. Moreover, the burden of depression is not universal, and there is substantial variability in the prevalence of depressive symptoms and accessibility of mental healthcare for certain population subgroups [205, 206]. Finally, replication of findings instills greater confidence in the generalisability of results and aids in effective translation to clinical interventions [207, 208]. For these reasons, future work into the role of neuroinflammation in HIV-associated depression must attempt to replicate findings across diverse populations.
In particular, we recommend that researchers and funding bodies prioritise these investigations in three key populations. First, there is a meaningful opportunity to focus on HIV-associated depression amongst children and adolescents. Not only is there strong evidence for the burden of mental health challenges faced by this population, but exploring neuroinflammation as a specific contributor to HIV-associated depression in longitudinal studies might help us develop distinct neuroinflammatory profiles for healthy development and HIV-induced developmental changes [209, 210]. Indeed, such investigations offer a unique opportunity to determine what effects, if any, perinatal HIV infection has on neuroinflammation, whether these early neuroinflammatory responses can predict risk for depression later in life, and whether there may be a critical neurodevelopmental window for interventions to minimise long-term depression trajectories [40, 69]. Second, participants who use psychoactive drugs should be included in studies of neuroinflammation and HIV. Drugs such as methamphetamine or cocaine may elicit inflammatory responses in the brain [211], and there is overlap between depression risk, drug use, and HIV [212]. However, well-designed preclinical and clinical experiments may distinguish between HIV-associated neuroinflammation and drug-associated neuroinflammation, while helping us understand the interactions between drug use, HIV, neuroinflammation, and risk for depression. Such studies may improve our ability to screen for and treat depression for HIV+ individuals who use drugs, but this is not possible if people who use drugs continue to be automatically excluded from participating.
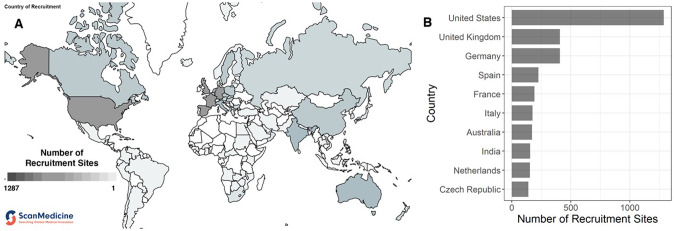
Finally, the greatest burden of HIV infection lies in the Global South, where access to latest-generation cART and psychological healthcare is also limited [213, 214]. However, a majority of neurovirology research is carried out in the Global North, with over 80% of research outputs concerning HIV/AIDS being produced by researchers from North America and Europe (González-Alcaide et al. provide an excellent summary of these concerns [215]). These trends are reflected in research specific to HIV-associated depression, as well. We ran a systematic search for all clinical trials in the 11 registries catalogued in the new open source database ScanMedicine using the keywords “HIV+ depression” (https://www.scanmedicine.com/) and examined the distribution of country of recruitment for these trials (Fig. 2). 4471 clinical trials met the search criteria. These trials recruited participants across 5619 total recruitment sites, of which 1287 (22.9%) were located in the US. The top five countries by number of registered clinical trials investigating HIV and depression, representing nearly half (n = 2518, 44.7%) of all trials, were the US, Germany, the UK, Spain, and France. In fact, only two countries (India and China) outside of North America, Europe, or Australia were in the top 20 countries by number of trials (see Supplementary Materials). This preliminary data is presented here to illustrate the fact that, given the overwhelming concentration of research funding in affluent nations, these studies often involve participant groups whose physical and mental health, socioeconomic backgrounds, and institutional access may not reflect those of the majority of people who bear the burden of HIV-associated depression (for instance, see ref. [216]).
Fig. 2. Global distribution of recruitment sites by country for clinical trials investigating HIV and depression.
We ran a search for clinical trials catalogued in 11 registries using the keywords “HIV+ depression” in the open source database ScanMedicine. A Map of the world with number of clinical trial recruitment sites for HIV-associated depression. Darker shading represents greater number of recruitment sites in that country. B Distribution of recruitment sites in the top 10 countries. The 4471 clinical trials that met the search criteria recruited participants across 5619 total recruitment sites, of which 1287 (22.9%) were in the US. Nearly half (n = 2518, 44.7%) of all recruitment sites were located in the US, Germany, the UK, Spain, and France. The global distribution of clinical trials of depression and HIV is skewed, with few studies involving participants from the Global South, where there is a substantial burden of HIV-associated depression. World map data visualisation adapted from: ScanMedicine, NIHR Innovation Observatory (NIHRIO), Newcastle University (scanmedicine.com).
Moreover, the majority of these studies involve HIV+ participants who are virally-suppressed and receiving cART [181, 217]. Only a few - those primarily recruiting participants in the Global South - include ART-naïve participants [189]. Antiretroviral drugs are known to contribute to neuropsychiatric effects such as sleep disturbances and depressive symptoms [218]. This makes it difficult to ascertain whether inflammatory responses or depressive symptoms observed in these studies arose as a result of HIV infection or ART. Studies involving HIV + individuals who are not yet receiving cART will be essential in delineating the individual contributions of HIV itself and of cART to these neuropsychiatric complications. Since it is difficult to recruit ART-naïve participants in the cART era in the Global North, such studies are more feasible in the Global South. For these reasons, investigations of HIV-associated depression involving participant cohorts in the Global South - especially southern Africa, which is fast becoming a hub for HIV/AIDS research [219]—must remain a focus for funding bodies in order to better understand confounding influences on findings, prioritise global health equity, and improve the generalisability of our findings. Involving participants from these underserved research populations using co-production or participatory research methods may also provide insight into which research questions and outcomes should be a focus for future work and ways to engage stakeholders more broadly [220].
Attempting to reproduce findings across diverse populations will allow us to parse out the complex interactions between neurobiological, psychosocial, and institutional factors that give rise to HIV-associated depression [221]. No single mechanism can sufficiently explain the aetiology of HIV-associated depression, but if we are intentional in our efforts to study these phenomena in populations from distinct clinical, socioeconomic, and cultural backgrounds, we may better understand the individual contributions of each of these factors to this overarching burden of depression. By leveraging this understanding to develop targeted mental health interventions, we may be able to reduce transmission of HIV via “positive prevention” [222]. Therefore, by investigating the risk of depression faced by people living with HIV, we may make substantive progress towards the global goal of HIV/AIDS eradication.
Conclusion
In this critical review, we synthesised evidence for independent associations between HIV, depression, and (neuro)inflammation. Preliminary preclinical and clinical evidence lends support to the hypothesis that neuroinflammation may play an important role in HIV-associated depression, although data on this relationship is extremely sparse. We also identified some key areas of debate where further work is necessary to reconcile contradictory findings. Future investigations should focus on further characterising this relationship. Studies with robust experimental design that attempt to reproduce findings in key populations must be a priority in order to approach an accurate and generalisable understanding of the contribution of neuroinflammation to HIV-associated depression.
Supplementary information
Author contributions
AMRL conceptualised the review, performed the literature search, and wrote the paper. HCW, JHV, and SRC critically reviewed the paper.
Funding
AMRL is funded by the Wellcome Trust (Grant Number 218493/Z/19/Z, Translational Neuroscience Ph.D. programme).
Data availability
Data from the ScanMedicine clinical trial registry search is available in Supplementary Materials for this paper. No other new data was created or analysed in this project.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41380-022-01619-2.
References
- 1.Vittinghoff E, Scheer S, O’Malley P, Colfax G, Holmberg SD, Buchbinder SP. Combination antiretroviral therapy and recent declines in AIDS incidence and mortality. J Infect Dis. 1999;179:717–20. doi: 10.1086/314623. [DOI] [PubMed] [Google Scholar]
- 2.Collaboration ATC Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372:293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155:209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 4.van Sighem A, Gras L, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. Aids. 2010;24:1527–35. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. Fact sheet—latest global and regional statistics on the status of the AIDS epidemic. 2021.
- 6.Hoare J, Sevenoaks T, Mtukushe B, Williams T, Heany S, Phillips N. Global systematic review of common mental health disorders in adults living with HIV. Current HIV/AIDS Rep. 2021;18:1–12. doi: 10.1007/s11904-021-00583-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: a comprehensive review. AIDS. 2019;33:163. doi: 10.1097/QAD.0000000000001796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schouten J, Cinque P, Gisslen M, Reiss P, Portegies P. HIV-1 infection and cognitive impairment in the cART era: a review. Aids. 2011;25:561–75. doi: 10.1097/QAD.0b013e3283437f9a. [DOI] [PubMed] [Google Scholar]
- 9.Rubin LH, Sundermann EE, Moore DJ. The current understanding of overlap between characteristics of HIV-associated neurocognitive disorders and Alzheimer’s disease. J Neurovirol. 2019;25:661–72. doi: 10.1007/s13365-018-0702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–85. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 11.van Wyhe KS, Laughton B, Cotton MF, Meintjes EM, van der Kouwe AJ, Boivin MJ, et al. Cognitive outcomes at ages seven and nine years in South African children from the children with HIV early antiretroviral (CHER) trial: a longitudinal investigation. J Int AIDS Soc. 2021;24:e25734. doi: 10.1002/jia2.25734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Too EK, Abubakar A, Nasambu C, Koot HM, Cuijpers P, Newton CR, et al. Prevalence and factors associated with common mental disorders in young people living with HIV in sub‐Saharan Africa: a systematic review. J Int AIDS Soc. 2021;24:e25705. doi: 10.1002/jia2.25705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woollett N, Cluver L, Bandeira M, Brahmbhatt H. Identifying risks for mental health problems in HIV positive adolescents accessing HIV treatment in Johannesburg. J Child Adolesc Ment Health. 2017;29:11–26. doi: 10.2989/17280583.2017.1283320. [DOI] [PubMed] [Google Scholar]
- 14.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001;158:725–30. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- 15.Catalan J, Harding R, Sibley E, Clucas C, Croome N, Sherr L. HIV infection and mental health: suicidal behaviour—systematic review. Psychol Health Med. 2011;16:588–611. doi: 10.1080/13548506.2011.582125. [DOI] [PubMed] [Google Scholar]
- 16.Schadé A, van Grootheest G, Smit JH. HIV-infected mental health patients: characteristics and comparison with HIV-infected patients from the general population and non-infected mental health patients. Bmc Psychiatry. 2013;13:1–10. doi: 10.1186/1471-244X-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shah S, Sinharay S, Matsuda K, Schreiber-Stainthorp W, Muthusamy S, Lee D, et al. Potential mechanism for HIV-associated depression: upregulation of serotonin transporters in SIV-infected macaques detected by 11C-DASB PET. Front Psychiatry. 2019;10:362. doi: 10.3389/fpsyt.2019.00362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLaurin KA, Harris M, Madormo V, Harrod SB, Mactutus CF, Booze RM. HIV-associated apathy/depression and neurocognitive impairments reflect persistent dopamine deficits. Cells. 2021;10:2158. doi: 10.3390/cells10082158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Guerra F, Fonseca J, Figueiredo V, Ziff E, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol. 2013;19:314–27. doi: 10.1007/s13365-013-0177-7. [DOI] [PubMed] [Google Scholar]
- 20.Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green C, Shen X, Stevenson AJ, Conole EL, Harris MA, Barbu MC, et al. Structural brain correlates of serum and epigenetic markers of inflammation in major depressive disorder. Brain Behav Immun. 2021;92:39–48. doi: 10.1016/j.bbi.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, et al. Effect of anti-inflammatory treatment on depression, depressive symptoms, and adverse effects: a systematic review and meta-analysis of randomized clinical trials. JAMA Psychiatry. 2014;71:1381–91. doi: 10.1001/jamapsychiatry.2014.1611. [DOI] [PubMed] [Google Scholar]
- 24.Brites D, Fernandes A. Neuroinflammation and depression: microglia activation, extracellular microvesicles and microRNA dysregulation. Front Cell Neurosci. 2015;9:476. doi: 10.3389/fncel.2015.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamik MK, Hui E, Branton WG, McKenzie BA, Chisholm J, Cohen EA, et al. HIV-1 viral protein R activates NLRP3 inflammasome in microglia: implications for HIV1 associated neuroinflammation. J Neuroimmune Pharmacol. 2017;12:233–48. doi: 10.1007/s11481-016-9708-3. [DOI] [PubMed] [Google Scholar]
- 26.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20:28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 27.Frugier T, Morganti-Kossmann MC, O’Reilly D, McLean CA. In situ detection of inflammatory mediators in post mortem human brain tissue after traumatic injury. J Neurotrauma. 2010;27:497–507. doi: 10.1089/neu.2009.1120. [DOI] [PubMed] [Google Scholar]
- 28.Gomez-Nicola D, Boche D. Post-mortem analysis of neuroinflammatory changes in human Alzheimer’s disease. Alzheimers Res Ther. 2015;7:1–8. doi: 10.1186/s13195-015-0126-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Monnet‐Tschudi F, Defaux A, Braissant O, Cagnon L, Zurich MG. Methods to assess neuroinflammation. Curr Protoc Toxicol. 2011;50:12.19.11–20. doi: 10.1002/0471140856.tx1219s50. [DOI] [PubMed] [Google Scholar]
- 30.Yan J, Kuzhiumparambil U, Bandodkar S, Dale RC, Fu S. Cerebrospinal fluid metabolomics: detection of neuroinflammation in human central nervous system disease. Clin Transl Immunol. 2021;10:e1318. doi: 10.1002/cti2.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Popp J, Oikonomidi A, Tautvydaitė D, Dayon L, Bacher M, Migliavacca E, et al. Markers of neuroinflammation associated with Alzheimer’s disease pathology in older adults. Brain Behav Immun. 2017;62:203–11. doi: 10.1016/j.bbi.2017.01.020. [DOI] [PubMed] [Google Scholar]
- 32.Abu-Rumeileh S, Steinacker P, Polischi B, Mammana A, Bartoletti-Stella A, Oeckl P, et al. CSF biomarkers of neuroinflammation in distinct forms and subtypes of neurodegenerative dementia. Alzheimers Res Ther. 2020;12:1–15. doi: 10.1186/s13195-019-0562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattsson N. CSF biomarkers in neurodegenerative diseases. Clin Chem Lab Med. 2011;49:345–52. doi: 10.1515/CCLM.2011.082. [DOI] [PubMed] [Google Scholar]
- 34.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–89. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 35.Rohrer JD, Woollacott IO, Dick KM, Brotherhood E, Gordon E, Fellows A, et al. Serum neurofilament light chain protein is a measure of disease intensity in frontotemporal dementia. Neurology. 2016;87:1329–36. doi: 10.1212/WNL.0000000000003154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao H, Mo M, Miao C, Li L, Yang H, Liu Y, et al. Association of serum biomarker neurofilament light concentration with post-stroke depression: a preliminary study. Gen Hosp Psychiatry. 2020;64:17–25. doi: 10.1016/j.genhosppsych.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Gisslén M, Price RW, Andreasson U, Norgren N, Nilsson S, Hagberg L, et al. Plasma concentration of the neurofilament light protein (NFL) is a biomarker of CNS injury in HIV infection: a cross-sectional study. EBioMedicine. 2016;3:135–40. doi: 10.1016/j.ebiom.2015.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosadas C, Zetterberg H, Heslegrave A, Haddow J, Borisova M, Taylor GP. Neurofilament light in CSF and plasma is a marker of neuronal damage in HTLV-1-associated myelopathy and correlates with neuroinflammation. Neurol Neuroimmunol Neuroinflammation. 2021;8:e1090. doi: 10.1212/NXI.0000000000001090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bergman J, Dring A, Zetterberg H, Blennow K, Norgren N, Gilthorpe J, et al. Neurofilament light in CSF and serum is a sensitive marker for axonal white matter injury in MS. Neurol Neuroimmunol Neuroinflammation. 2016;3:e271. doi: 10.1212/NXI.0000000000000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le ND, Muri L, Grandgirard D, Kuhle J, Leppert D, Leib SL. Evaluation of neurofilament light chain in the cerebrospinal fluid and blood as a biomarker for neuronal damage in experimental pneumococcal meningitis. J Neuroinflammation. 2020;17:1–9. doi: 10.1186/s12974-020-01966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felger JC, Haroon E, Patel TA, Goldsmith DR, Wommack EC, Woolwine BJ, et al. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol Psychiatry. 2020;25:1301–11. doi: 10.1038/s41380-018-0096-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stenvinkel P, Karimi M, Johansson S, Axelsson J, Suliman M, Lindholm B, et al. Impact of inflammation on epigenetic DNA methylation–a novel risk factor for cardiovascular disease? J Intern Med. 2007;261:488–99. doi: 10.1111/j.1365-2796.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- 43.Stevenson AJ, McCartney DL, Hillary RF, Campbell A, Morris SW, Bermingham ML, et al. Characterisation of an inflammation-related epigenetic score and its association with cognitive ability. Clin Epigenet. 2020;12:1–11. doi: 10.1186/s13148-020-00903-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ligthart S, Marzi C, Aslibekyan S, Mendelson MM, Conneely KN, Tanaka T, et al. DNA methylation signatures of chronic low-grade inflammation are associated with complex diseases. Genome Biol. 2016;17:1–15. doi: 10.1186/s13059-016-1119-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conole EL, Stevenson AJ, Maniega SM, Harris SE, Green C. Hernández MdCV, et al. DNA methylation and protein markers of chronic inflammation and their associations with brain and cognitive aging. Neurology. 2021;97:e2340–52. doi: 10.1212/WNL.0000000000012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheleznyakova GY, Cao H, Schiöth HBBDNF. DNA methylation changes as a biomarker of psychiatric disorders: literature review and open access database analysis. Behav Brain Funct. 2016;12:1–14. doi: 10.1186/s12993-016-0101-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Albrecht DS, Granziera C, Hooker JM, Loggia ML. In vivo imaging of human neuroinflammation. ACS Chem Neurosci. 2016;7:470–83. doi: 10.1021/acschemneuro.6b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berger A. How does it work?: positron emission tomography. BMJ Br Med J. 2003;326:1449. doi: 10.1136/bmj.326.7404.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ziegler SI. Positron emission tomography: principles, technology, and recent developments. Nucl Phys A. 2005;752:679–87. [Google Scholar]
- 50.Boerwinkle A, Ances BM. Molecular imaging of neuroinflammation in HIV. J Neuroimmune Pharmacol. 2019;14:9–15. doi: 10.1007/s11481-018-9823-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Crawshaw AA, Robertson NP. The role of TSPO PET in assessing neuroinflammation. J Neurol. 2017;264:1825–7. doi: 10.1007/s00415-017-8565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guilarte TR. TSPO in diverse CNS pathologies and psychiatric disease: a critical review and a way forward. Pharmacol Ther. 2019;194:44–58. doi: 10.1016/j.pharmthera.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tournier BB, Tsartsalis S, Ceyzériat K, Garibotto V, Millet P. In vivo TSPO signal and neuroinflammation in Alzheimer’s disease. Cells. 2020;9:1941. doi: 10.3390/cells9091941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suridjan I, Rusjan P, Voineskos AN, Selvanathan T, Setiawan E, Strafella AP, et al. Neuroinflammation in healthy aging: a PET study using a novel Trans-locator Protein 18 kDa (TSPO) radioligand,[18F]-FEPPA. Neuroimage. 2014;84:868–75. doi: 10.1016/j.neuroimage.2013.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Hu K, Shao T, Hou L, Zhang S, Ye W, et al. Recent developments on PET radiotracers for TSPO and their applications in neuroimaging. Acta Pharm Sin B. 2021;11:373–93. doi: 10.1016/j.apsb.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vera JH, Ridha B, Gilleece Y, Amlani A, Thorburn P, Dizdarevic S. PET brain imaging in HIV-associated neurocognitive disorders (HAND) in the era of combination antiretroviral therapy. Eur J Nucl Med Mol Imaging. 2017;44:895–902. doi: 10.1007/s00259-016-3602-3. [DOI] [PubMed] [Google Scholar]
- 57.Radiation safety with positron emission tomography and computed tomography. Proceedings of the Seminars in Ultrasound, CT and MRI. Vol. 31 Elsevier; 2010. pp. 39–45. 10.1053/j.sult.2009.09.005. [DOI] [PubMed]
- 58.Munson S, Eshel N, Ernst M. Ethics of PET research in children. In: Charron M, editor. Pediatric PET imaging. NY: Springer; 2006, pp. 72–91. 10.1007/0-387-34641-4_7.
- 59.Zrzavy T, Höftberger R, Berger T, Rauschka H, Butovsky O, Weiner H, et al. Pro‐inflammatory activation of microglia in the brain of patients with sepsis. Neuropathol Appl Neurobiol. 2019;45:278–90. doi: 10.1111/nan.12502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen W, Barback CV, Wang S, Hoh CK, Chang EY, Hall DJ, et al. A receptor-binding radiopharmaceutical for imaging of traumatic brain injury in a rodent model:[99mTc] Tc-tilmanocept. Nucl Med Biol. 2021;92:107–14. doi: 10.1016/j.nucmedbio.2020.02.013. [DOI] [PubMed] [Google Scholar]
- 61.Zanni MV, Toribio M, Wilks MQ, Lu MT, Burdo TH, Walker J, et al. Application of a novel CD206+ macrophage-specific arterial imaging strategy in HIV-infected individuals. J Infect Dis. 2017;215:1264–9. doi: 10.1093/infdis/jix095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grinspoon S. Novel mechanisms and anti-inflammatory strategies to reduce cardiovascular risk in human immunodeficiency virus. Trans Am Clin Climatol Assoc. 2018;129:140. [PMC free article] [PubMed] [Google Scholar]
- 63.Quarantelli M. MRI/MRS in neuroinflammation: methodology and applications. Clin Transl Imaging. 2015;3:475–89. doi: 10.1007/s40336-015-0142-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tognarelli JM, Dawood M, Shariff MI, Grover VP, Crossey MM, Cox IJ, et al. Magnetic resonance spectroscopy: principles and techniques: lessons for clinicians. J Clin Exp Hepatol. 2015;5:320–8. doi: 10.1016/j.jceh.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Buonocore MH, Maddock RJ. Magnetic resonance spectroscopy of the brain: a review of physical principles and technical methods. Rev Neurosci. 2015;26:609–32. doi: 10.1515/revneuro-2015-0010. [DOI] [PubMed] [Google Scholar]
- 66.Bertholdo D, Watcharakorn A, Castillo M. Brain proton magnetic resonance spectroscopy: introduction and overview. Neuroimaging Clin. 2013;23:359–80. doi: 10.1016/j.nic.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 67.van der Graaf M. In vivo magnetic resonance spectroscopy: basic methodology and clinical applications. Eur Biophysics J. 2010;39:527–40. doi: 10.1007/s00249-009-0517-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dahmani S, Kaliss N, VanMeter JW, Moore DJ, Ellis RJ, Jiang X. Alterations of brain metabolites in adults with HIV: a systematic meta-analysis of magnetic resonance spectroscopy studies. Neurology. 2021;97:e1085–96. doi: 10.1212/WNL.0000000000012394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Graham AS, Holmes MJ, Little F, Dobbels E, Cotton MF, Laughton B, et al. MRS suggests multi-regional inflammation and white matter axonal damage at 11 years following perinatal HIV infection. NeuroImage Clin. 2020;28:102505. doi: 10.1016/j.nicl.2020.102505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chang L, Munsaka SM, Kraft-Terry S, Ernst T. Magnetic resonance spectroscopy to assess neuroinflammation and neuropathic pain. J Neuroimmune Pharmacol. 2013;8:576–93. doi: 10.1007/s11481-013-9460-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vesce S, Rossi D, Brambilla L, Volterra A. Glutamate release from astrocytes in physiological conditions and in neurodegenerative disorders characterized by neuroinflammation. Int Rev Neurobiol. 2007;82:57–71. doi: 10.1016/S0074-7742(07)82003-4. [DOI] [PubMed] [Google Scholar]
- 72.Zhu H, Barker PB. MR spectroscopy and spectroscopic imaging of the brain. Magn Reson Neuroimaging. 2011;711:203–26. doi: 10.1007/978-1-61737-992-5_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Soares D, Law M. Magnetic resonance spectroscopy of the brain: review of metabolites and clinical applications. Clin Radiol. 2009;64:12–21. doi: 10.1016/j.crad.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Letendre SL, Zheng JC, Kaul M, Yiannoutsos CT, Ellis RJ, Taylor MJ, et al. Chemokines in cerebrospinal fluid correlate with cerebral metabolite patterns in HIV-infected individuals. J Neurovirol. 2011;17:63–9. doi: 10.1007/s13365-010-0013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ahmed OA. New denoising scheme for magnetic resonance spectroscopy signals. IEEE Trans Med Imaging. 2005;24:809–16. doi: 10.1109/TMI.2004.828350. [DOI] [PubMed] [Google Scholar]
- 76.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity. 2013;39:633–45. doi: 10.1016/j.immuni.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McMichael AJ, Borrow P, Tomaras GD, Goonetilleke N, Haynes BF. The immune response during acute HIV-1 infection: clues for vaccine development. Nat Rev Immunol. 2010;10:11–23. doi: 10.1038/nri2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep. 2012;9:139–47. doi: 10.1007/s11904-012-0118-8. [DOI] [PubMed] [Google Scholar]
- 79.Connolly NC, Riddler SA, Rinaldo CR. Proinflammatory cytokines in HIV disease-a review and rationale for new therapeutic approaches. AIDS Rev. 2005;7:168–80. [PubMed] [Google Scholar]
- 80.Appay V, Sauce D. Immune activation and inflammation in HIV‐1 infection: causes and consequences. J Pathol. 2008;214:231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 81.Kinter A, Arthos J, Cicala C, Fauci AS. Chemokines, cytokines and HIV: a complex network of interactions that influence HIV pathogenesis. Immunol Rev. 2000;177:88–98. doi: 10.1034/j.1600-065x.2000.17708.x. [DOI] [PubMed] [Google Scholar]
- 82.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLOS ONE. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–95. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Siedner MJ, Zanni M, Tracy RP, Kwon DS, Tsai AC, Kakuhire B, et al. Increased systemic inflammation and gut permeability among women with treated HIV infection in rural Uganda. J Infect Dis. 2018;218:922–6. doi: 10.1093/infdis/jiy244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Babu H, Ambikan AT, Gabriel EE, Svensson Akusjärvi S, Palaniappan AN, Sundaraj V, et al. Systemic inflammation and the increased risk of inflamm-aging and age-associated diseases in people living with HIV on long term suppressive antiretroviral therapy. Front Immunol. 2019;10:1965. doi: 10.3389/fimmu.2019.01965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rubin LH, Benning L, Keating SM, Norris PJ, Burke-Miller J, Savarese A, et al. Variability in C-reactive protein is associated with cognitive impairment in women living with and without HIV: a longitudinal study. J Neurovirol. 2018;24:41–51. doi: 10.1007/s13365-017-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lutgen V, Narasipura SD, Barbian HJ, Richards M, Wallace J, Razmpour R, et al. HIV infects astrocytes in vivo and egresses from the brain to the periphery. PLoS Pathog. 2020;16:e1008381. doi: 10.1371/journal.ppat.1008381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Thompson KA, Cherry CL, Bell JE, McLean CA. Brain cell reservoirs of latent virus in presymptomatic HIV-infected individuals. Am J Pathol. 2011;179:1623–9. doi: 10.1016/j.ajpath.2011.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Churchill M, Nath A. Where does HIV hide? A focus on the central nervous system. Curr Opin HIV AIDS. 2013;8:165. doi: 10.1097/COH.0b013e32835fc601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.King J, Eugenin E, Buckner C, Berman J. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–57. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 91.Bansal AK, Mactutus CF, Nath A, Maragos W, Hauser KF, Booze RM. Neurotoxicity of HIV-1 proteins gp120 and Tat in the rat striatum. Brain Res. 2000;879:42–9. doi: 10.1016/s0006-8993(00)02725-6. [DOI] [PubMed] [Google Scholar]
- 92.Adle‐Biassette H, Levy Y, Colornbel M, Poron F, Natchev S, Keohane C, et al. Neuronal apoptosis in HIV infection in adults. Neuropathol Appl Neurobiol. 1995;21:218–27. doi: 10.1111/j.1365-2990.1995.tb01053.x. [DOI] [PubMed] [Google Scholar]
- 93.Zheng J, Thylin MR, Ghorpade A, Xiong H, Persidsky Y, Cotter R, et al. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J Neuroimmunol. 1999;98:185–200. doi: 10.1016/s0165-5728(99)00049-1. [DOI] [PubMed] [Google Scholar]
- 94.Zhang C, Song J-W, Huang H-H, Fan X, Huang L, Deng J-N, et al. NLRP3 inflammasome induces CD4+ T cell loss in chronically HIV-1–infected patients. J Clin Invest. 2021;131:e138861. doi: 10.1172/JCI138861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Leal VNC, Reis EC, Pontillo A. Inflammasome in HIV infection: lights and shadows. Mol Immunol. 2020;118:9–18. doi: 10.1016/j.molimm.2019.12.001. [DOI] [PubMed] [Google Scholar]
- 96.Walsh JG, Reinke SN, Mamik MK, McKenzie BA, Maingat F, Branton WG, et al. Rapid inflammasome activation in microglia contributes to brain disease in HIV/ AIDS. Retrovirology. 2014;11:1–18. doi: 10.1186/1742-4690-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: a TSPO PET study. Neurology. 2016;86:1425–32. doi: 10.1212/WNL.0000000000002485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Biljon N, Robertson F, Holmes M, Cotton MF, Laughton B, van der Kouwe A, et al. Multivariate approach for longitudinal analysis of brain metabolite levels from ages 5-11 years in children with perinatal HIV infection. NeuroImage. 2021;237:118101. doi: 10.1016/j.neuroimage.2021.118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gisslén M, Heslegrave A, Veleva E, Yilmaz A, Andersson L-M, Hagberg L, et al. CSF concentrations of soluble TREM2 as a marker of microglial activation in HIV1 infection. Neurol Neuroimmunol Neuroinflammation. 2019;6:e512. doi: 10.1212/NXI.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gisslen M, Keating SM, Spudich S, Arechiga V, Stephenson S, Zetterberg H, et al. Compartmentalization of cerebrospinal fluid inflammation across the spectrum of untreated HIV-1 infection, central nervous system injury and viral suppression. Plos ONE. 2021;16:e0250987. doi: 10.1371/journal.pone.0250987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guha D, Mukerji SS, Chettimada S, Misra V, Lorenz DR, Morgello S, et al. Cerebrospinal fluid extracellular vesicles and neurofilament light protein as biomarkers of central nervous system injury in HIV-infected patients on antiretroviral therapy. AIDS. 2019;33:615. doi: 10.1097/QAD.0000000000002121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anderson AM, Easley KA, Kasher N, Franklin D, Heaton RK, Zetterberg H, et al. Neurofilament light chain in blood is negatively associated with neuropsychological performance in HIV-infected adults and declines with initiation of antiretroviral therapy. J Neurovirol. 2018;24:695–701. doi: 10.1007/s13365-018-0664-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bbosa N, Kaleebu P, Ssemwanga D. HIV subtype diversity worldwide. Curr Opin HIV AIDS. 2019;14:153–60. doi: 10.1097/COH.0000000000000534. [DOI] [PubMed] [Google Scholar]
- 104.Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, et al. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78:2586–90. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.De Almeida SM, Rotta I, Jiang Y, Li X, Raboni SM, Ribeiro CE, et al. Biomarkers of chemotaxis and inflammation in cerebrospinal fluid and serum in individuals with HIV-1 subtype C versus B. J Neurovirol. 2016;22:715–24. doi: 10.1007/s13365-016-0437-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bhargavan B, Kanmogne GD. Differential mechanisms of inflammation and endothelial dysfunction by HIV-1 subtype-B and recombinant CRF02_AG tat proteins on human brain microvascular endothelial cells: implications for viral Neuropathogenesis. Mol Neurobiol. 2018;55:1352–63. doi: 10.1007/s12035-017-0382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arseniou S, Arvaniti A, Samakouri M. HIV infection and depression. Psychiatry Clin Neurosci. 2014;68:96–109. doi: 10.1111/pcn.12097. [DOI] [PubMed] [Google Scholar]
- 108.Gokhale RH, Weiser J, Sullivan PS, Luo Q, Shu F, Bradley H. Depression prevalence, antidepressant treatment status, and association with sustained HIV viral suppression among adults living with HIV in care in the United States, 2009–2014. AIDS Behav. 2019;23:3452–9. doi: 10.1007/s10461-019-02613-6. [DOI] [PubMed] [Google Scholar]
- 109.Kelso-Chichetto N, Okafor C, Cook R, Abraham A, Bolan R, Plankey M. Association between depressive symptom patterns and clinical profiles among persons living with HIV. AIDS Behav. 2018;22:1411–22. doi: 10.1007/s10461-017-1822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gianella S, Saloner R, Curtin G, Little SJ, Heaton A, Montoya JL, et al. A cross-sectional study to evaluate the effects of age and duration of HIV infection on anxiety and depression in cisgender men. AIDS Behav. 2021;26:1–8. doi: 10.1007/s10461-021-03373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rezaei S, Ahmadi S, Rahmati J, Hosseinifard H, Dehnad A, Aryankhesal A, et al. Global prevalence of depression in HIV/AIDS: a systematic review and metaanalysis. BMJ Support Palliat care. 2019;9:404–12. doi: 10.1136/bmjspcare-2019-001952. [DOI] [PubMed] [Google Scholar]
- 112.Redman K, Karstaedt A, Scheuermaier K. Increased CD4 counts, pain and depression are correlates of lower sleep quality in treated HIV positive patients with low baseline CD4 counts. Brain Behav Immun. 2018;69:548–55. doi: 10.1016/j.bbi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 113.Evans JA, Shim J-M, Ioannidis JP. Attention to local health burden and the global disparity of health research. PloS ONE. 2014;9:e90147. doi: 10.1371/journal.pone.0090147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Keegan MR, Chittiprol S, Letendre SL, Winston A, Fuchs D, Boasso A, et al. Tryptophan metabolism and its relationship with depression and cognitive impairment among HIV-infected individuals. Int J Tryptophan Res. 2016;9. 10.4137/IJTR.S36464. [DOI] [PMC free article] [PubMed]
- 115.Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, et al. Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr. 2014;65:456. doi: 10.1097/QAI.0000000000000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Steiner J, Walter M, Gos T, Guillemin GJ, Bernstein H-G, Sarnyai Z, et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: evidence for an immune-modulated glutamatergic neurotransmission? J Neuroinflammation. 2011;8:1–9. doi: 10.1186/1742-2094-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hammond ER, Crum RM, Treisman GJ, Mehta SH, Clifford DB, Ellis RJ, et al. Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms; a prospective cohort study. J Neurovirol. 2016;22:479–87. doi: 10.1007/s13365-015-0416-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hartzell JD, Janke IE, Weintrob AC. Impact of depression on HIV outcomes in the HAART era. J Antimicrobial Chemother. 2008;62:246–55. doi: 10.1093/jac/dkn193. [DOI] [PubMed] [Google Scholar]
- 119.Rivera-Rivera Y, García Y, Toro V, Cappas N, López P, Yamamura Y, et al. Depression correlates with increased plasma levels of inflammatory cytokines and a dysregulated oxidant/antioxidant balance in HIV-1-infected subjects undergoing antiretroviral therapy. J Clin Cell Immunol. 2014;5:e1000276. doi: 10.4172/2155-9899.1000276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr Hiv/Aids Rep. 2017;14:93–100. doi: 10.1007/s11904-017-0356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ahmed D, Roy D, Cassol E. Examining relationships between metabolism and persistent inflammation in HIV patients on antiretroviral therapy. Mediators Inflamm. 2018;2018:e6238978. doi: 10.1155/2018/6238978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sereti I, Krebs SJ, Phanuphak N, Fletcher JL, Slike B, Pinyakorn S, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64:124. doi: 10.1093/cid/ciw683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Regidor DL, Detels R, Breen EC, Widney DP, Jacobson LP, Palella F, et al. Effect of highly active antiretroviral therapy on biomarkers of B-lymphocyte activation and inflammation. AIDS. 2011;25:303. doi: 10.1097/QAD.0b013e32834273ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Frew PM, Parker K, Vo L, Haley D, O’Leary A, Diallo DD, et al. Socioecological factors influencing women’s HIV risk in the United States: qualitative findings from the women’s HIV SeroIncidence study (HPTN 064) BMC Public Health. 2016;16:1–18. doi: 10.1186/s12889-016-3364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Karamouzian M, Akbari M, Haghdoost A-A, Setayesh H, Zolala F. “I am dead to them”: HIV-related stigma experienced by people living with HIV in Kerman, Iran. J Assoc Nurses AIDS Care. 2015;26:46–56. doi: 10.1016/j.jana.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 126.Ellis RJ, Iudicello J, Sun-Suslow N, Grelotti D, Cherner M, Morgan E, et al. Social isolation is linked to inflammation in aging people with HIV and uninfected individuals. JAIDS J Acquir Immune Defic Syndr. 2021;86:600–6. doi: 10.1097/QAI.0000000000002596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mudra Rakshasa A, Tong MT. Making “good” choices: social isolation in mice exacerbates the effects of chronic stress on decision making. Front Behav Neurosci. 2020;14:81. doi: 10.3389/fnbeh.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chaudhury S, Bakhla AK, Saini R. Prevalence, impact, and management of depression and anxiety in patients with HIV: A review. Neurobehavioral HIV Med. 2016;7:15–30. [Google Scholar]
- 129.Cowen PJ, Browning M. What has serotonin to do with depression? World Psychiatry. 2015;14:158. doi: 10.1002/wps.20229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yohn CN, Gergues MM, Samuels BA. The role of 5-HT receptors in depression. Mol Brain. 2017;10:1–12. doi: 10.1186/s13041-017-0306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Godfrey KE, Gardner AC, Kwon S, Chea W, Muthukumaraswamy SD. Differences in excitatory and inhibitory neurotransmitter levels between depressed patients and healthy controls: a systematic review and meta-analysis. J Psychiatr Res. 2018;105:33–44. doi: 10.1016/j.jpsychires.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 132.López-Muñoz F, Alamo C. Monoaminergic neurotransmission: the history of the discovery of antidepressants from 1950s until today. Curr Pharm Des. 2009;15:1563–86. doi: 10.2174/138161209788168001. [DOI] [PubMed] [Google Scholar]
- 133.Jeon SW, Kim YK. Neuroinflammation and cytokine abnormality in major depression: cause or consequence in that illness? World J Psychiatry. 2016;6:283. doi: 10.5498/wjp.v6.i3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ruberto VL, Jha MK, Murrough JW. Pharmacological treatments for patients with treatment-resistant depression. Pharmaceuticals. 2020;13:116. doi: 10.3390/ph13060116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Beijers L, Wardenaar KJ, van Loo HM, Schoevers RA. Data-driven biological subtypes of depression: systematic review of biological approaches to depression subtyping. Mol Psychiatry. 2019;24:888–900. doi: 10.1038/s41380-019-0385-5. [DOI] [PubMed] [Google Scholar]
- 136.Lichtenberg P, Belmaker R. Subtyping major depressive disorder. Psychother Psychosom. 2010;79:131–5. doi: 10.1159/000286957. [DOI] [PubMed] [Google Scholar]
- 137.Dantzer R, O’connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Irwin MR. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr Clin. 2011;34:605–20. doi: 10.1016/j.psc.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Köhler‐Forsberg O, Lydholm N, Hjorthøj C, Nordentoft C, Mors M, Benros O. M. Efficacy of anti‐inflammatory treatment on major depressive disorder or depressive symptoms: meta‐analysis of clinical trials. Acta Psychiatr Scand. 2019;139:404–19. doi: 10.1111/acps.13016. [DOI] [PubMed] [Google Scholar]
- 140.Haapakoski R, Mathieu J, Ebmeier KP, Alenius H, Kivimäki M. Cumulative metaanalysis of interleukins 6 and 1β, tumour necrosis factor α and C-reactive protein in patients with major depressive disorder. Brain Behav Immun. 2015;49:206–15. doi: 10.1016/j.bbi.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Valkanova V, Ebmeier KP, Allan CL. CRP, IL-6 and depression: a systematic review and meta-analysis of longitudinal studies. J Affect Disord. 2013;150:736–44. doi: 10.1016/j.jad.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 142.Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 143.Lee ST. Inflammation, depression, and anxiety disorder: a population-based study examining the association between Interleukin-6 and the experiencing of depressive and anxiety symptoms. Psychiatry Res. 2020;285:112809. doi: 10.1016/j.psychres.2020.112809. [DOI] [PubMed] [Google Scholar]
- 144.Colasanto M, Madigan S, Korczak DJ. Depression and inflammation among children and adolescents: a meta-analysis. J Affect Disord. 2020;277:940–8. doi: 10.1016/j.jad.2020.09.025. [DOI] [PubMed] [Google Scholar]
- 145.Khan A, Leonard D, Defina L, Barlow CE, Willis B, Brown ES. Association between C reactive protein and depression in a population of healthy adults: the Cooper Center Longitudinal Study. J Investig Med. 2020;68:1019–23. doi: 10.1136/jim-2019-001254. [DOI] [PubMed] [Google Scholar]
- 146.Pitharouli MC, Hagenaars SP, Glanville KP, Coleman JR, Hotopf M, Lewis CM, et al. Elevated C-reactive protein in patients with depression, independent of genetic, health, and psychosocial factors: results from the UK Biobank. Am J Psychiatry. 2021 doi: 10.1176/appi.ajp.2020.20060947. [DOI] [PubMed] [Google Scholar]
- 147.Mehta ND, Haroon E, Xu X, Woolwine BJ, Li Z, Felger JC. Inflammation negatively correlates with amygdala-ventromedial prefrontal functional connectivity in association with anxiety in patients with depression: preliminary results. Brain Behav Immun. 2018;73:725–30. doi: 10.1016/j.bbi.2018.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Toenders YJ, Laskaris L, Davey CG, Berk M, Milaneschi Y, Lamers F, et al. Inflammation and depression in young people: a systematic review and proposed inflammatory pathways. Mol Psychiatry. 2021;27:1–13. doi: 10.1038/s41380-021-01306-8. [DOI] [PubMed] [Google Scholar]
- 149.Huang M, Su S, Goldberg J, Miller AH, Levantsevych OM, Shallenberger L, et al. Longitudinal association of inflammation with depressive symptoms: a 7-year cross-lagged twin difference study. Brain Behav Immun. 2019;75:200–7. doi: 10.1016/j.bbi.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Milaneschi Y, Kappelmann N, Ye Z, Lamers F, Moser S, Jones PB, et al. Association of inflammation with depression and anxiety: evidence for symptom-specificity and potential causality from UK Biobank and NESDA cohorts. Mol Psychiatry. 2021;26:7393–402. doi: 10.1038/s41380-021-01188-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chen M-H, Liu Y-L, Kuo H-W, Tsai S-J, Hsu J-W, Huang K-L, et al. Neurofilament light chain is a novel biomarker for major depression and related executive dysfunction. Int J Neuropsychopharmacol. 2021;25:99–105. doi: 10.1093/ijnp/pyab068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Peirce JM, Alviña K. The role of inflammation and the gut microbiome in depression and anxiety. J Neurosci Res. 2019;97:1223–41. doi: 10.1002/jnr.24476. [DOI] [PubMed] [Google Scholar]
- 153.Capuco A, Urits I, Hasoon J, Chun R, Gerald B, Wang JK, et al. Current perspectives on gut microbiome dysbiosis and depression. Adv Ther. 2020;37:1328–46. doi: 10.1007/s12325-020-01272-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Pérez-Santiago J, Marquine MJ, Cookson D, Giraud-Colón R, Heaton RK, Grant I, et al. Gut microbiota dysbiosis is associated with worse emotional states in HIV infection. J Neurovirol. 2021;27:228–38. doi: 10.1007/s13365-020-00933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Taylor BC, Weldon KC, Ellis RJ, Franklin D, Groth T, Gentry EC, et al. Depression in individuals coinfected with HIV and HCV is associated with systematic differences in the gut microbiome and metabolome. Msystems. 2020;5:e00465–20. doi: 10.1128/mSystems.00465-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nettis MA, Pariante CM. Is there neuroinflammation in depression? Understanding the link between the brain and the peripheral immune system in depression. Int Rev Neurobiol. 2020;152:23–40. doi: 10.1016/bs.irn.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 157.Troubat R, Barone P, Leman S, Desmidt T, Cressant A, Atanasova B, et al. Neuroinflammation and depression: a review. Eur J Neurosci. 2021;53:151–71. doi: 10.1111/ejn.14720. [DOI] [PubMed] [Google Scholar]
- 158.Lirng J-F, Chen H-C, Fuh J-L, Tsai C-F, Liang J-F, Wang S-J. Increased myo-inositol level in dorsolateral prefrontal cortex in migraine patients with major depression. Cephalalgia. 2015;35:702–9. doi: 10.1177/0333102414557048. [DOI] [PubMed] [Google Scholar]
- 159.Furtado M, Katzman MA. Examining the role of neuroinflammation in major depression. Psychiatry Res. 2015;229:27–36. doi: 10.1016/j.psychres.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 160.Rossi S, Studer V, Motta C, Polidoro S, Perugini J, Macchiarulo G, et al. Neuroinflammation drives anxiety and depression in relapsing-remitting multiple sclerosis. Neurology. 2017;89:1338–47. doi: 10.1212/WNL.0000000000004411. [DOI] [PubMed] [Google Scholar]
- 161.Dooley LN, Kuhlman KR, Robles TF, Eisenberger NI, Craske MG, Bower JE. The role of inflammation in core features of depression: Insights from paradigms using exogenously-induced inflammation. Neurosci Biobehav Rev. 2018;94:219–37. doi: 10.1016/j.neubiorev.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Nettis MA, Lombardo G, Hastings C, Zajkowska Z, Mariani N, Nikkheslat N, et al. Augmentation therapy with minocycline in treatment-resistant depression patients with low-grade peripheral inflammation: results from a double-blind randomised clinical trial. Neuropsychopharmacology. 2021;46:939–48. doi: 10.1038/s41386-020-00948-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang L, Zhang J, You Z. Switching of the microglial activation phenotype is a possible treatment for depression disorder. Front Cell Neurosci. 2018;12:306. doi: 10.3389/fncel.2018.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Han Y, Zhang L, Wang Q, Zhang D, Zhao Q, Zhang J, et al. Minocycline inhibits microglial activation and alleviates depressive-like behaviors in male adolescent mice subjected to maternal separation. Psychoneuroendocrinology. 2019;107:37–45. doi: 10.1016/j.psyneuen.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 165.Richards EM, Zanotti-Fregonara P, Fujita M, Newman L, Farmer C, Ballard ED, et al. PET radioligand binding to translocator protein (TSPO) is increased in unmedicated depressed subjects. EJNMMI Res. 2018;8:1–9. doi: 10.1186/s13550-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Veloso S, Escoté X, Ceperuelo-Mallafré V, López-Dupla M, Peraire J, Viladés C, et al. Leptin and adiponectin, but not IL18, are related with insulin resistance in treated HIV-1-infected patients with lipodystrophy. Cytokine. 2012;58:253–60. doi: 10.1016/j.cyto.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 167.Zapanti E, Terzidis K, Chrousos G. Dysfunction of the hypothalamic-pituitaryadrenal axis in HIV infection and disease. Hormones. 2008;7:205–16. doi: 10.14310/horm.2002.1200. [DOI] [PubMed] [Google Scholar]
- 168.Choi D-Y, Liu M, Hunter RL, Cass WA, Pandya JD, Sullivan PG, et al. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS ONE. 2009;4:e5482. doi: 10.1371/journal.pone.0005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marker DF, Tremblay M-E, Lu S-M, Majewska AK, Gelbard HA. A thin-skull window technique for chronic two-photon in vivo imaging of murine microglia in models of neuroinflammation. J Vis Exp. 2010;43:e2059. doi: 10.3791/2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hatziioannou T, Evans DT. Animal models for HIV/AIDS research. Nat Rev Microbiol. 2012;10:852–67. doi: 10.1038/nrmicro2911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Policicchio BB, Pandrea I, Apetrei C. Animal models for HIV cure research. Front Immunol. 2016;7:12. doi: 10.3389/fimmu.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Abelaira HM, Réus GZ, Quevedo J. Animal models as tools to study the pathophysiology of depression. Braz J Psychiatry. 2013;35:S112–20. doi: 10.1590/1516-4446-2013-1098. [DOI] [PubMed] [Google Scholar]
- 173.Wang Q, Timberlake MA, II, Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2017;77:99–109. doi: 10.1016/j.pnpbp.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barreto ICG, Viegas P, Ziff EB, Konkiewitz EC. Animal models for depression associated with HIV-1 infection. J Neuroimmune Pharmacol. 2014;9:195–208. doi: 10.1007/s11481-013-9518-9. [DOI] [PubMed] [Google Scholar]
- 175.Nemeth CL, Glasper ER, Harrell CS, Malviya SA, Otis JS, Neigh GN. Meloxicam blocks neuroinflammation, but not depressive-like behaviors, in HIV-1 transgenic female rats. PloS ONE. 2014;9:e108399. doi: 10.1371/journal.pone.0108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7:483–95. doi: 10.1016/j.stem.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gemma C, Bachstetter AD. The role of microglia in adult hippocampal neurogenesis. Front Cell Neurosci. 2013;7:229. doi: 10.3389/fncel.2013.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rowson SA, Harrell CS, Bekhbat M, Gangavelli A, Wu MJ, Kelly SD, et al. Neuroinflammation and behavior in HIV-1 transgenic rats exposed to chronic adolescent stress. Front Psychiatry. 2016;7:102. doi: 10.3389/fpsyt.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Everall I, Vaida F, Khanlou N, Lazzaretto D, Achim C, Letendre S, et al. Cliniconeuropathologic correlates of human immunodeficiency virus in the era of antiretroviral therapy. J Neurovirol. 2009;15:360–70. doi: 10.3109/13550280903131915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bryant AK, Moore DJ, Burdo TH, Lakritz JR, Gouaux B, Soontornniyomkij V, et al. Plasma soluble CD163 is associated with postmortem brain pathology in human immunodeficiency virus infection. AIDS. 2017;31:973. doi: 10.1097/QAD.0000000000001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lu H, Surkan PJ, Irwin MR, Treisman GJ, Breen EC, Sacktor N, et al. Inflammation and risk of depression in HIV: prospective findings from the Multicenter AIDS Cohort Study. Am J Epidemiol. 2019;188:1994–2003. doi: 10.1093/aje/kwz190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Rubin LH, Langenecker SA, Phan KL, Keating SM, Neigh GN, Weber KM, et al. Remitted depression and cognition in HIV: The role of cortisol and inflammation. Psychoneuroendocrinology. 2020;114:104609. doi: 10.1016/j.psyneuen.2020.104609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Saloner R, Paolillo EW, Heaton RK, Grelotti DJ, Stein MB, Miller AH, et al. Chronically elevated depressive symptoms interact with acute increases in inflammation to predict worse neurocognition among people with HIV. J NeuroVirology. 2021;27:160–7. doi: 10.1007/s13365-020-00925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bendorius M, Po C, Muller S, Jeltsch-David H. From systemic inflammation to neuroinflammation: the case of neurolupus. Int J Mol Sci. 2018;19:3588. doi: 10.3390/ijms19113588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bäckryd E, Tanum L, Lind A-L, Larsson A, Gordh T. Evidence of both systemic inflammation and neuroinflammation in fibromyalgia patients, as assessed by a multiplex protein panel applied to the cerebrospinal fluid and to plasma. J Pain Res. 2017;10:515. doi: 10.2147/JPR.S128508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Osimo EF, Baxter LJ, Lewis G, Jones PB, Khandaker GM. Prevalence of low-grade inflammation in depression: a systematic review and meta-analysis of CRP levels. Psychological Med. 2019;49:1958–70. doi: 10.1017/S0033291719001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Poudel-Tandukar K, Bertone-Johnson ER, Palmer PH, Poudel KC. C-reactive protein and depression in persons with human immunodeficiency virus infection: the Positive Living with HIV (POLH) Study. Brain Behav Immun. 2014;42:89–95. doi: 10.1016/j.bbi.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 188.Memiah P, Nkinda L, Majigo M, Humwa F, Haile Z, Muthoka K, et al. Mental health symptoms and inflammatory markers among HIV infected patients in Tanzania. BMC Public Health. 2021;21:1–11. doi: 10.1186/s12889-021-11064-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Musinguzi K, Obuku A, Nakasujja N, Birabwa H, Nakku J, Levin J, et al. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 2018;19:1–7. doi: 10.1186/s12865-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zuñiga JA, Harrison ML, Henneghan A, García AA, Kesler S. Biomarkers panels can predict fatigue, depression and pain in persons living with HIV: A pilot study. Appl Nurs Res. 2020;52:151224. doi: 10.1016/j.apnr.2019.151224. [DOI] [PubMed] [Google Scholar]
- 191.Derry HM, Johnston CD, Burchett CO, Brennan-Ing M, Karpiak S, Zhu Y-S, et al. Links between inflammation, mood, and physical function among older adults with HIV. J Gerontol Ser B. 2021;52:151224. doi: 10.1093/geronb/gbab027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ellis RJ, Letendre SL, Atkinson JH, Clifford D, Collier AC, Gelman BB, et al. Higher levels of plasma inflammation biomarkers are associated with depressed mood and quality of life in aging, virally suppressed men, but not women, with HIV. Brain Behav Immun Health. 2020;7:100121. doi: 10.1016/j.bbih.2020.100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Del Giudice M, Gangestad SW. Rethinking IL-6 and CRP: Why they are more than inflammatory biomarkers, and why it matters. Brain Behav Immun. 2018;70:61–75. doi: 10.1016/j.bbi.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 194.Merlin JS, Westfall AO, Heath SL, Goodin BR, Stewart JC, Sorge RE, et al. IL-1β levels are associated with chronic multisite pain in people living with HIV. J Acquir Immune Defic Syndr. 2017;75:e99. doi: 10.1097/QAI.0000000000001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pala AN, Steca P, Bagrodia R, Helpman L, Colangeli V, Viale P, et al. Subtypes of depressive symptoms and inflammatory biomarkers: an exploratory study on a sample of HIV-positive patients. Brain Behav Immun. 2016;56:105–13. doi: 10.1016/j.bbi.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bekhbat M, Mehta CC, Kelly SD, Vester A, Ofotokun I, Felger J, et al. HIV and symptoms of depression are independently associated with impaired glucocorticoid signaling. Psychoneuroendocrinology. 2018;96:118–25. doi: 10.1016/j.psyneuen.2018.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Saloner R, Cherner M, Grelotti DJ, Paolillo EW, Moore DJ, Heaton RK, et al. Lower CSF homovanillic acid relates to higher burden of neuroinflammation anddepressioninpeoplewithHIV disease. BrainBehav Immun. 2020;90:353–63. doi: 10.1016/j.bbi.2020.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Woods SP, Babicz M, Shahani L, Colpo GD, Morgan EE, Teixeira AL. Brain-derived neurotrophic factor (BDNF) is associated with depressive symptoms in older adults with HIV disease. J Neurovirol. 2021;27:70–9. doi: 10.1007/s13365-020-00916-2. [DOI] [PubMed] [Google Scholar]
- 199.Lofgren S. Neuroinflammation and modulating factors in depression and HIV [ongoing clinical trial]. ClinicalTrialsgov. 2020. Clinical Trial Identifier: NCT04286282.
- 200.Imp BM, Rubin LH, Tien PC, Plankey MW, Golub ET, French AL, et al. Monocyte activation is associated with worse cognitive performance in HIV-infected women with virologic suppression. J Infect Dis. 2017;215:114. doi: 10.1093/infdis/jiw506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Funder DC, Ozer DJ. Evaluating effect size in psychological research: sense and nonsense. Adv Methods Pract Psychol Sci. 2019;2:156–68. [Google Scholar]
- 202.Neigh GN, Rhodes ST, Valdez A, Jovanovic T. PTSD co-morbid with HIV: separate but equal, or two parts of a whole? Neurobiol Dis. 2016;92:116–23. doi: 10.1016/j.nbd.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Blaney NT, Fernandez MI, Ethier KA, Wilson TE, Walter E, Koenig LJ, et al. Psychosocial and behavioral correlates of depression among HIV-infected pregnant women. AIDS Patient Care STDs. 2004;18:405–15. doi: 10.1089/1087291041518201. [DOI] [PubMed] [Google Scholar]
- 204.Ranjit N, Diez-Roux AV, Shea S, Cushman M, Seeman T, Jackson SA, et al. Psychosocial factors and inflammation in the multi-ethnic study of atherosclerosis. Arch Intern Med. 2007;167:174–81. doi: 10.1001/archinte.167.2.174. [DOI] [PubMed] [Google Scholar]
- 205.Bigna JJ, Tounouga DN, Kenne AM, Djikeussi TK, Foka AJ, Um LN, et al. Epidemiology of depressive disorders in people living with HIV in Africa: a systematic review and meta-analysis: Burden of depression in HIV in Africa. Gen Hosp Psychiatry. 2019;57:13–22. doi: 10.1016/j.genhosppsych.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 206.Williams DW, Li Y, Dastgheyb R, Fitzgerald KC, Maki PM, Spence AB, et al. Associations between antiretroviral drugs on depressive symptomatology in homogenous subgroups of women with HIV. J Neuroimmune Pharmacol. 2021;16:181–94. doi: 10.1007/s11481-019-09899-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Wingen T, Berkessel JB, Englich B. No replication, no trust? How low replicability influences trust in psychology. Soc Psychol Personal Sci. 2020;11:454–63. [Google Scholar]
- 208.Glasgow RE, Klesges LM, Dzewaltowski DA, Bull SS, Estabrooks P. The future of health behavior change research: what is needed to improve translation of research into health promotion practice? Ann Behav Med. 2004;27:3–12. doi: 10.1207/s15324796abm2701_2. [DOI] [PubMed] [Google Scholar]
- 209.Vreeman RC, McCoy BM, Lee S. Mental health challenges among adolescents living with HIV. J Int AIDS Soc. 2017;20:21497. doi: 10.7448/IAS.20.4.21497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Copelyn J, Thompson LC, Le Prevost M, Castro H, Sturgeon K, Rowson K, et al. Self-harm in young people with perinatal HIV and HIV negative young people in England: cross sectional analysis. BMC Public Health. 2019;19:1–11. doi: 10.1186/s12889-019-7424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol. 2012;3:121. doi: 10.3389/fphar.2012.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Bartoli F, Carrà G, Brambilla G, Carretta D, Crocamo C, Neufeind J, et al. Association between depression and non-fatal overdoses among drug users: a systematic review and meta-analysis. Drug Alcohol Depend. 2014;134:12–21. doi: 10.1016/j.drugalcdep.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 213.Brandt R. The mental health of people living with HIV/AIDS in Africa: a systematic review. Afr J AIDS Res. 2009;8:123–33. doi: 10.2989/AJAR.2009.8.2.1.853. [DOI] [PubMed] [Google Scholar]
- 214.Collins PY, Holman AR, Freeman MC, Patel V. What is the relevance of mental health to HIV/AIDS care and treatment programs in developing countries? A systematic review. AIDS. 2006;20:1571. doi: 10.1097/01.aids.0000238402.70379.d4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.González-Alcaide G, Menchi-Elanzi M, Nacarapa E, Ramos-Rincón J-M. HIV/AIDS research in Africa and the Middle East: participation and equity in North-South collaborations and relationships. Global Health. 2020;16:1–18. doi: 10.1186/s12992-020-00609-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Bagkeris E, Burgess L, Mallon PW, Post FA, Boffito M, Sachikonye M, et al. Cohort profile: the pharmacokinetic and clinical observations in PeoPle over fiftY (POPPY) study. Int J Epidemiol. 2018;47:1391–2e. doi: 10.1093/ije/dyy072. [DOI] [PubMed] [Google Scholar]
- 217.Hakkers CS, Hermans AM, van Maarseveen EM, Teunissen CE, Verberk IM, Arends JE, et al. High efavirenz levels but not neurofilament light plasma levels are associated with poor neurocognitive functioning in asymptomatic HIV patients. J Neurovirol. 2020;26:572–80. doi: 10.1007/s13365-020-00860-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Treisman GJ, Soudry O. Neuropsychiatric effects of HIV antiviral medications. Drug Saf. 2016;39:945–57. doi: 10.1007/s40264-016-0440-y. [DOI] [PubMed] [Google Scholar]
- 219.Menchi-Elanzi M, Pinargote-Celorio H, Nacarapa E, González-Alcaide G, Ramos-Rincón J-M. Scientific HIV research in Africa and the Middle East: a socioeconomic demographic analysis. Afr J AIDS Res. 2021;20:1–5. doi: 10.2989/16085906.2020.1830133. [DOI] [PubMed] [Google Scholar]
- 220.Rycroft-Malone J, Burton CR, Bucknall T, Graham ID, Hutchinson AM, Stacey D. Collaboration and co-production of knowledge in healthcare: opportunities and challenges. Int J Health Policy Manag. 2016;5:221. doi: 10.15171/ijhpm.2016.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Senn TE, Greenwood GL, Rao VR. Global mental health and HIV care: gaps and research priorities. J Int AIDS Soc. 2021;24:e25714. doi: 10.1002/jia2.25714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sikkema KJ, Watt MH, Drabkin AS, Meade CS, Hansen NB, Pence BW. Mental health treatment to reduce HIV transmission risk behavior: a positive prevention model. AIDS Behav. 2010;14:252–62. doi: 10.1007/s10461-009-9650-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Anthony I, Ramage S, Carnie F, Simmonds P, Bell J. Influence of HAART on HIV-related CNS disease and neuroinflammation. J Neuropathol Exp Neurol. 2005;64:529–36. doi: 10.1093/jnen/64.6.529. [DOI] [PubMed] [Google Scholar]
- 224.Cheng D, Luo Z, Fu X, Stephenson S, Di Germanio C, Norris PJ, et al. Elevated cerebrospinal fluid anti-CD4 autoantibody levels in HIV associate with neuroinflammation. Microbiol Spectr. 2022;10:e01975–21. doi: 10.1128/spectrum.01975-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Guha D, Lorenz DR, Misra V, Chettimada S, Morgello S, Gabuzda D. Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment. J Neuroinflammation. 2019;16:1–19. doi: 10.1186/s12974-019-1617-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lyons JL, Uno H, Ancuta P, Kamat A, Moore DJ, Singer EJ, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371. doi: 10.1097/QAI.0b013e3182237e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463. doi: 10.1097/QAD.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. Aids. 2014;28:67–72. doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- 229.Cysique LA, Moffat K, Moore DM, Lane TA, Davies NW, Carr A, et al. HIV, vascular and aging injuries in the brain of clinically stable HIV-infected adults: a 1H MRS study. PloS ONE. 2013;8:e61738. doi: 10.1371/journal.pone.0061738. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the ScanMedicine clinical trial registry search is available in Supplementary Materials for this paper. No other new data was created or analysed in this project.