Abstract
Background and purpose
Mechanisms underlying acute brain injury in SARS-CoV-2 patients remain poorly understood. A better characterization of such mechanisms remains essential to preventing long-term neurological sequelae. Our present aim was to study a panel of biomarkers of neuroinflammation and neurodegeneration in the cerebrospinal fluid (CSF) of NeuroCOVID patients.
Methods
We retrospectively collected clinical and CSF biomarkers data from 24 NeuroCOVID adults seen at the University Hospital of Guadeloupe between March and June 2021.
Results
Among 24 NeuroCOVID patients, 71% had encephalopathy and 29% meningoencephalitis. A number of these patients also experienced de novo movement disorder (33%) or stroke (21%). The CSF analysis revealed intrathecal immunoglobulin synthesis in 54% of NeuroCOVID patients (two with a type 2 pattern and 11 with a type 3) and elevated neopterin levels in 75% of them (median 9.1 nM, IQR 5.6–22.1). CSF neurofilament light chain (NfL) was also increased compared to a control group of non-COVID-19 patients with psychiatric illnesses (2905 ng/L, IQR 1428–7124 versus 1222 ng/L, IQR 1049–1566). Total-tau was elevated in the CSF of 24% of patients, whereas protein 14-3-3, generally undetectable, reached intermediate levels in two patients. Finally, CSF Aß1-42 was reduced in 52.4% of patients (median 536 ng/L, IQR 432–904) with no change in the Aß1-42/Aß1-40 ratio (0.082, IQR 0.060–0.096).
Conclusions
We showed an elevation of CSF biomarkers of neuroinflammation in NeuroCOVID patients and a rise of CSF NfL, evocative of neuronal damage. However, longitudinal studies are needed to determine whether NeuroCOVID could evolve into a chronic neurodegenerative condition.
Keywords: Encephalitis, Encephalopathy, COVID-19, Neuroinflammation, Neuronal injury, CSF biomarker
1. Introduction
The pathogenesis of nervous system damage associated with SARS-CoV-2 infection (NeuroCOVID) remains poorly known. Putative non-exclusive mechanisms at the origin of NeuroCOVID comprise:
-
•
brain invasion by retrograde progression of the virus along cranial nerve pathways [1], [2] or through brain-blood barrier (BBB) disruption;
-
•
deleterious systemic immune response or compartmentalized immune response within the central nervous system (CNS) [3], [4], [5].
Unraveling these mechanisms is critical to identifying optimal therapeutic targets and guiding our strategy to prevent long-term neurological sequelae in NeuroCOVID patients [6], [7].
NeuroCOVID might be associated with an increased risk of developing a neurodegenerative disorder or might hasten its progression [8], [9]. Triggering a neurodegenerative cascade in NeuroCOVID patients might involve the combination of several factors, including:
-
•
a specific vulnerability of some brain regions such as the hippocampus or the midbrain to SARS-CoV-2 infection [10];
-
•
gut microbiome dysregulation induced by SARS-CoV-2 infection and its possible consequences on the brain through the gut-brain axis [11], [12], [13];
-
•
SARS-CoV-2-induced dysregulation of genes critical for neuronal survival [14].
Some undefined factors associated with an extended stay in an intensive care unit (ICU) [15], a depressive state, post-traumatic distress [10], or severe sepsis [16], [17] could also influence the risk of cognitive decline after severe SARS-CoV-2 infection.
Here, the consequences of CNS SARS-CoV-2 infection were monitored by measuring different cerebrospinal fluid (CSF) parameters. Previous CSF studies performed in adults with NeuroCOVID – Virhammar et al. (n = 19) [18]; Edén et al. (n = 6) [19]; Espindola et al. (n = 58) [20]; Garcia et al. (n = 18) [21]; Paterson et al. (n = 21) [22]; Alexopoulos et al. (n = 8) [23]; Ziff et al. (n = 21) [24]); Guasp et al. (n = 60) [25]; Edén et al. (n = 23) [26] – reported changes in biomarkers of neuroinflammation [18], [19], [20], [21], [23], [24], [25], [26], astrocytic injury although this point remains debated [18], [22], [24], [26], neuronal injury [18], [19], [20], [21], [22], [23], [24], [25], [26], as well as alterations in amyloid processing [22], [24]. Most of the previous studies focused on either neuroinflammation, acute neuronal injury, or neurodegeneration, but only a small number analyzed together biomarkers characterizing these different mechanisms. Thus, additional CSF studies in large samples of well-characterized NeuroCOVID patients are needed to further delineate the pathogenesis of CNS damage and prevent its occurrence. Here, we studied a variety of biomarkers associated with neuroinflammation, neuronal injury, and neurodegeneration in the CSF of 24 NeuroCOVID adults with a CNS syndrome during the acute phase of the infection.
2. Methods
2.1. Patients and study design
We enrolled patients with neurological manifestations of a confirmed COVID-19 infection between March 2020 and June 2021 at the University Hospital of Guadeloupe (French West Indies). Patients were considered to have confirmed COVID-19 when real-time protein chain reaction (RT-PCR) for SARS-CoV-2 was positive, either in a nasopharyngeal swab or bronchoalveolar lavage. During the hospital stay, we collected data on medical history and performed clinical (including a detailed neurological examination), biological (including detailed CSF analysis) and neuroradiological (brain and spinal magnetic resonance imaging [MRI], brain computed tomography [CT]) investigations, as well as neurophysiological (electroencephalogram [EEG] and electromyogram [EMG]) recordings. Two types of brain injuries were reported:
-
•
encephalopathy defined as an altered mental status lasting ≥ 24 hours (impaired awareness, confusion, delirium with or without hallucinations, cognitive and behavioral disorder) that could be associated with seizure and focal neurologic symptoms, or with electroencephalographic criteria, in the absence of criteria for encephalitis (confer below) [27] and that could not be accounted for by another cause, such as toxic or metabolic factors;
-
•
encephalitis/meningitis defined as an altered mental status lasting ≥ 24 hours (encephalopathy) with one of the following criteria: white blood cell count in CSF ≥ 5/mm3, or detection of SARS-CoV-2 by RT-PCR in CSF, or presence of a compatible acute lesion on brain MRI.
As previously defined by the United States National Institutes of Health [28], the severity of the illness was classified as mild, moderate, severe, or critical. Patients classified as having encephalopathy or meningoencephalitis could additionally have developed a stroke episode or a movement disorder.
2.2. Standard plasma and CSF investigations to explore the infectious status of patients
A large panel of infections was systematically screened in plasma (serological tests for dengue virus, chikungunya virus, zika virus, human immunodeficiency virus, human T-lymphotropic virus, cytomegalovirus, Epstein Barr virus, leptospirosis, hepatitis B and C viruses) and in CSF (RT-PCR for varicella-zoster virus, herpes simplex virus, enterovirus, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella) to search for possible co-infections. Any acute co-infection was a criterion of exclusion.
CSF protein concentrations were analyzed with a Cobas®-Roche automated analyzer. Abnormal protein levels in CSF were considered if > 0.4 g/L. CSF/serum albumin ratios were analyzed and evaluated as abnormal when ≥ 0.0075. CSF white and red cell countings were performed using Kova Slides®. CSF immunoglobulin G (IgG) index was determined and considered increased when > 0.7. Isoelectric focusing was performed on CSF and serum samples using the Sebia Capillarys® system. Five patterns have been previously described [29]: type 1: no specific band in CSF and serum (normal); type 2: specific oligoclonal IgG bands in the CSF and no corresponding band in serum (intrathecal IgG synthesis); type 3: IgG oligoclonal bands in CSF and additional identical bands in the CSF and serum (intrathecal IgG synthesis); type 4: similar oligoclonal bands in the CSF and serum (systemic, no intrathecal IgG synthesis); type 5: monoclonal bands in CSF and serum (no IgG synthesis in CNS). CSF COVID-19 serology (IgG and IgM) was assessed using a Standard Q COVID-19 IgM/IgG Combo Test (SD Biosensor via Orgentec) and RT-PCR with a EurobioPlex SARS-CoV-2 Multiplex kit (Eurobio Scientific). The presence of onconeural antibodies was also analyzed in blood and CSF by immunohistochemistry and a cell-based assay (French reference center).
2.3. CSF neopterin as a marker of neuroinflammation
CSF neopterin was quantified by ultra performance liquid chromatography (UPLC) with fluorometric detection and Empower software for calculation and quantification (Waters®). The upper average reference value for neopterin was previously determined to be 5 nmol/L by Perret Liaudet et al. [30].
2.4. CSF biomarkers of neuronal injury and neurodegeneration
CSF collection, sampling, and storage were performed in a single laboratory using standard procedures prescribed in a consensus paper [31]. According to preanalytical recommendations, CSF samples were collected and aliquoted in polypropylene test tubes (Sarstedt, reference 62.610.201 and 62.558.201).
CSF neurofilament light chain (NfL) measurements were performed using an Nf-light® ELISA kit from Uman Diagnostics. Non-COVID patients with psychiatric illnesses (n = 20) – patients suffering from depressive syndrome associated with a cognitive complaint without progression during a two-year follow-up, and a normal CSF biomarker profile) [32] – were taken as a control group for NeuroCOVID patients in this assay. For detecting 14-3-3 protein, a Peggy Sue® automated Western blot system (Protein Simple, San Jose, CA, USA) was used. According to Fourier et al. [33], qualitative results interpreted were expressed as negative, positive, or intermediate.
Core Alzheimer's disease (AD) CSF biomarker assays (T-tau, P-tau 181, Aß1-42, and Aß1-40) were performed using a Lumipulse G600II automated analyzer (Fujirebio®). Typical cut-off values for parameters associated with AD risk were based on international criteria [34]. These values were determined locally and are as follows: T-tau > 400 ng/L, P-tau > 60 ng/L, Aß1-42 < 550 ng/L and/or Aß1-42/Aß1-40 ratio < 0.055.
2.5. Statistical analyses
All results are expressed in median and interquartile range (IQR). Non-parametric statistical analyses (Mann-Whitney, Spearman's rho correlation) were performed due to the small sample size. The significance level was defined as P < 0.05. Statistical analysis was performed using version 19.1 of the MedCalc Statistical Software (MedCalc Software bv, Ostend, Belgium) and the R Statistical Software (v4.1.1; R Core Team 2021).
2.6. Standard protocol approvals, registrations, and patient consent
The study was classified as an observational study according to French health regulations. The study was approved by the local ethics committee (number A17200704), and oral informed consent was obtained from all participants after providing them with written explanations. The study was performed according to the approved protocol.
The control group of non-COVID patients with psychiatric illnesses came from a study (NCT04001270) published and approved by the institutional review board of the Université Claude Bernard Lyon 1 and Hospices Civils de Lyon [32].
2.7. Data availability
Data will be made available by the corresponding author upon reasonable request. The data are not publicly available because they contain information that could compromise our patients’ privacy.
2.8. Literature summary
To facilitate the discussion through a global overview of CSF findings in patients with NeuroCOVID, we presented our results in a table together with a summary of data from previous studies (Table 1 ).
Table 1.
Literature summary of NeuroCOVID studies with CSF analysis.
| NeuroCOVID studies | Delay (in days) between neuro. symptoms and LP med. [IQR] | Patients with neurological manifestations (n) | Neuroinflammation (CSF) |
Neuronal injury (CSF) |
Amyloid markers (CSF) |
Astrocyte reactivity (CSF GFAP) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCB (n) | Neopterin (n) | IgG index (n) | Albumin ratio (n) | Inflammatory cytokines (n) | NfL (ng/L) med. [IQR] | T-tau (ng/L) med. [IQR] | P-tau | 14-3-3 protein | Aß1-42 | Ratio Aß1-42/Aß1-40 | ||||
| Espindola et al. | NA | 58 | ↑ (3/38) |
NA | NA | NA | NA | 1694 [1091–3358]b | 318 [173–457]b | NA | NA | NA | NA | NA |
| Paterson et al. | NA | 34 21 CNS 10 PNS 3 others |
NA | NA | NA | NA | NA | ↑ CNS 1510 [857–14,800] vs. controls 872 [654–1200] |
→ CNS 585 [220–1788] vs. controls 289 [243–356] |
→a | NA | NA | ↓ (5/32) |
↓a CNS and PNS vs. controls |
| Garcia et al. | “early CSF collection group” n = 8 4 [1–6] “late CSF collection group” n = 10 20 [13–27] |
18 | → (5/18) |
NA | → (7/18) |
→ (7/18) |
↑ IL-6, TNFα, IL-10, IL-12p70 Stroke group (n = 7) vs. controls ↑ IL-10, IL-12p70 critical illness group (n = 8) vs. controls |
↑a 8657 [1400–18,333] |
NA | NA | NA | NA | NA | NA |
| Edén et al. (2020) | 11 [7–12] | 6 | → (6/6) |
↑ (6/6) |
→ (6/6) |
→ (6/6) |
NA | ↑ (2/6) 974 [669–1998] |
NA | NA | NA | NA | NA | NA |
| Virhammar et al. | NA | 19 | ↑ (1/18) |
NA | ↑ (4/17) |
↑ (1/19) |
↑a | ↑ (12/18) 1900 [773–3763] |
↑ (7/17) |
NA | NA | NA | NA | ↑ (3/18) |
| Alexopoulos et al. | NA | 8 | NA | NA | ↑ (1/8) |
↑ (3/8) |
NA | NA | NA | NA | Positive (4/8) |
NA | NA | NA |
| Ziff et al. | NA | 21 | NA | NA | NA | NA | ↑a TNFɑ, IL6, IL1β, IL8 vs. controls |
↑a vs. controls |
NA | NA | NA | ↓a vs. controls |
↓a vs. controls |
↓a vs. controls |
| Guasp et al. | NA | 60 | → (27/27) |
NA | NA | NA | ↑ MCP-1, G-CSF, IL18, IL6, IL8, MIG (n = 27) vs. controls |
↑ Encephalopathy (n = 16) 1543 [740–2083] vs. controls (n = 24) 764.5 [472.5–896.5] |
NA | NA | NA | NA | NA | NA |
| Edén et al. (2022) | NA | 23 | NA | ↑ vs. controls |
→ | → | ↑ TNFɑ, IL6, IL2 vs. controls |
↑a vs. controls |
NA | NA | NA | NA | NA | → vs. controls |
| Chaumont et al. | 5 [3–12] | 24 | ↑ (13/24) |
↑ (18/24) |
↑ (6/23) |
↑ (7/23) |
NA | ↑a 2905 [1428–7124] |
↑ (5/21) |
↑ (1/21) |
Intermediate (2/23) |
↓ (11/21) |
→ (20/20) |
NA |
CNS: patients with “central nervous system” injury; CSF: cerebrospinal fluid; GFAP: glial fibrillary acid protein; IgG: immunoglobulin G; IL: interleukin; IQR: interquartile range; LP: lumbar puncture; NA: not available; NfL: neurofilament light chain; OCB: oligoclonal bands; PNS: patients with “peripheral nervous system” injury; P-tau: phosphorylated-tau; TNF: tumor necrosis factor; T-tau: total-tau; vs.: versus; →: normal; ↑: increased; ↓: decreased.
The total number of patients is not available.
NeuroCOVID patients not compared to healthy controls.
3. Results
3.1. Clinical findings and management
We analyzed data from 24 NeuroCOVID patients. The median age was 62 years (IQR: 56–70), and males were more represented (62.5%) (Table 2 ). No patient had a previous medical history of neurological disease. Among these patients, encephalopathy was the main neurological syndrome (70.8%, n = 17) compared to meningoencephalitis (29.2%, n = 7). Eight of these patients (33%, six with encephalopathy and two with meningoencephalitis) also developed movement disorders, and five of them (21%, four with encephalopathy and one with meningoencephalitis) experienced a stroke. Disease severity in these patients was estimated to be either moderate (16.7%), severe (25%), or critical (58.3%). All patients hospitalized in ICU received mechanical ventilation (Table 2).
Table 2.
General characteristics of 24 COVID-19 patients with neurological manifestations.
| n = 24a | |
|---|---|
| Age (in years) | 62 [56–70] |
| Sex | |
| Female | 9 (37.5%) |
| Male | 15 (62.5%) |
| Comorbidities | |
| Hypertension | 13 (54.2%) |
| Diabetes mellitus | 10 (41.7%) |
| Obesity | 7 (29.2%) |
| Cancer | 4 (16.7%) |
| Chronic alcoholism | 2 (8.3%) |
| Chronic kidney disease | 0 (0.0%) |
| Chronic cardiac disease | 1 (4.2%) |
| Obstructive Sleep Apnea | 1 (4.2%) |
| NIH severity | |
| Mild | 0 (0.0%) |
| Moderate | 4 (16.7%) |
| Severe | 6 (25.0%) |
| Critical | 14 (58.3%) |
| Neurological syndromes | |
| Encephalopathy | 17 (70.8%) |
| Meningoencephalitis | 7 (29.2%) |
| Additional movement disorders | 8 (33.3%) |
| Additional stroke | 5 (20.8%) |
| Time between first infectious symptoms and neurological manifestation (in days) | 8 [1–17] |
| ICU hospitalization | 13 (54.2%) |
| Duration of ICU stay (in days) | 28 [17–33] |
| Mechanical ventilation | 13 (54.2%) |
| Duration (in days) | 22 [16–29] |
NIH: National Institutes of Health; ICU: intensive care unit.
Median [IQR]; n (%).
3.2. CSF immune reaction and neuroinflammation
All patients had a lumbar puncture (LP) a median five days (IQR 3–12) after the onset of neurological symptoms. CSF findings are shown in Table 3 . Except for CSF pleocytosis, protein levels, and albumin ratios, which were higher in patients with meningoencephalitis, no significant difference was observed in biomarkers of the immune response and neuroinflammation between the two clinical subgroups.
Table 3.
CSF findings in 24 COVID-19 patients with neurological manifestations.
| Total n = 24a |
Encephalopathy n = 17a |
Meningoencephalitis n = 7a |
P | |
|---|---|---|---|---|
| Time between first neurological symptoms and LP (in days) (n = 23) | 5 [3–12] | 5 [3–13] | 4 [2–4] | 0.121 |
| WCC (cell/μL) | 2 [0–5] | 1 [0–2] | 8 [6–28] | < 0.001 |
| Abnormal WCC (> 4/μL) | 7 (29%) | 0 (0%) | 7 (100%) | < 0.001 |
| Protein (g/L) | 0.42 [0.29–0.60] | 0.34 [0.22–0.44] | 0.62 [0.52–0.97] | 0.003 |
| Abnormal protein (> 0.4 g/L) | 12 (50%) | 5 (29%) | 7 (100%) | 0.005 |
| Isoelectric focusing of CSFb (n = 23) | 0.199 | |||
| Type 1 pattern | 10 (43%) | 6 (35%) | 4 (67%) | |
| Type 2 pattern | 2 (8.7%) | 1 (5.9%) | 1 (17%) | |
| Type 3 pattern | 11 (48%) | 10 (59%) | 1 (17%) | |
| IgG index (n = 23) | 0.62 [0.47–0.71] | 0.62 [0.49–0.65] | 0.60 [0.42–0.74] | 0.972 |
| Abnormal IgG index (> 0.7) | 6 (26%) | 3 (18%) | 3 (50%) | 0.279 |
| Albumin ratio (CSF/serum) (n = 23) | 0.006 [0.004–0.010] | 0.005 [0.004–0.007] | 0.012 [0.008–0.015] | 0.020 |
| Abnormal albumin ratio (≥ 0.0075) | 7 (30%) | 3 (18%) | 4 (67%) | 0.045 |
| Neopterin (nmol/l) (n = 24) | 9.1 [5.6–22.1] | 9.1 [6.6–22.9] | 6.4 [5.3–20.4] | 0.525 |
| Abnormal neopterin (> 5 nmol/L) | 18 (75%) | 13 (76%) | 5 (71%) | 1.000 |
| Positive anti-SARS-CoV-2 IgM (n = 15) | 2 (13%) | 0 (0%) | 2 (50%) | 0.057 |
| Positive anti-SARS-CoV-2 IgG (n = 17) | 6 (35%) | 4 (33%) | 2 (40%) | 1.000 |
| NfL (ng/L) (n = 19) | 2905 [1428–7124] | 3177 [2017–5846] | 1428 [918–7766] | 0.368 |
| Total-tau (ng/L) (n = 21) | 256 [199–394] | 265 [206–417] | 199 [170–294] | 0.407 |
| Abnormal T-tau (> 400) | 5 (24%) | 4 (25%) | 1 (20%) | 1.000 |
| Phosphorylated-tau 181 (ng/L) (n = 21) | 32 [20–37] | 33 [21–43] | 22 [20–32] | 0.363 |
| Abnormal P-tau (> 60) | 1 (4.8%) | 1 (6.2%) | 0 (0%) | 1.000 |
| Aß1-42 peptide (ng/L) (n = 21) | 536 [432–904] | 516 [423–838] | 808 [475–904] | 0.660 |
| Abnormal Aß1-42 (< 550) | 11 (52%) | 9 (56%) | 2 (40%) | 0.635 |
| Aß1-42/Aß1-40 ratio (n = 21) | 0.082 [0.060–0.096] | 0.082 [0.059–0.091] | 0.097 [0.073–0.101] | 0.130 |
| Abnormal Aß1-42/Aß1-40 (< 0.055) | 0 (0%) | 0 (0%) | 0 (0%) | |
| 14-3-3 protein (n = 23) | 0.526 | |||
| Intermediate | 2 (8.7%) | 1 (6.2%) | 1 (14%) | |
| Negative | 21 (91%) | 15 (94%) | 6 (86%) |
CSF: cerebrospinal fluid; IgG: immunoglobulin G; IgM: immunoglobulin M; NfL: neurofilament light chain; P-tau: phosphorylated-tau; T-tau: total-tau; WCC: white cell count. In bold, corresponds to significant results (p < 0.05).
Median [IQR]; n (%).
Type 1: no specific band in CSF and serum (normal); type 2: specific oligoclonal IgG bands in the CSF and no corresponding band in serum (intrathecal IgG synthesis); type 3: IgG oligoclonal bands in CSF and additional identical bands in the CSF and serum (intrathecal IgG synthesis); type 4: similar oligoclonal bands in the CSF and serum (systemic, no intrathecal IgG synthesis); type 5: monoclonal bands in CSF and serum (no IgG synthesis in CNS).
Isoelectric focusing patterns 2 and 3, which indicate intrathecal IgG synthesis, were identified in two (8.7%) and 11 (47.8%) of the patients, respectively. Type 2 or 3 patterns were observed in 25% of patients with moderate forms, 50% of patients with severe forms, and 70% of those with critical forms, without a statistical relationship between disease severity and band pattern (P = 0.275). CSF neopterin was increased in 75% (n = 18) of patients (median 9.1 nmol/L, IQR 5.6–22.1). No correlation was found between CSF neopterin level and the delay of LP (rho = 0.25, P = 0.25), nor between CSF neopterin levels and duration of ICU stay (rho = 0.152, P = 0.62).
3.3. CSF biomarkers of neuronal injury and neurodegeneration
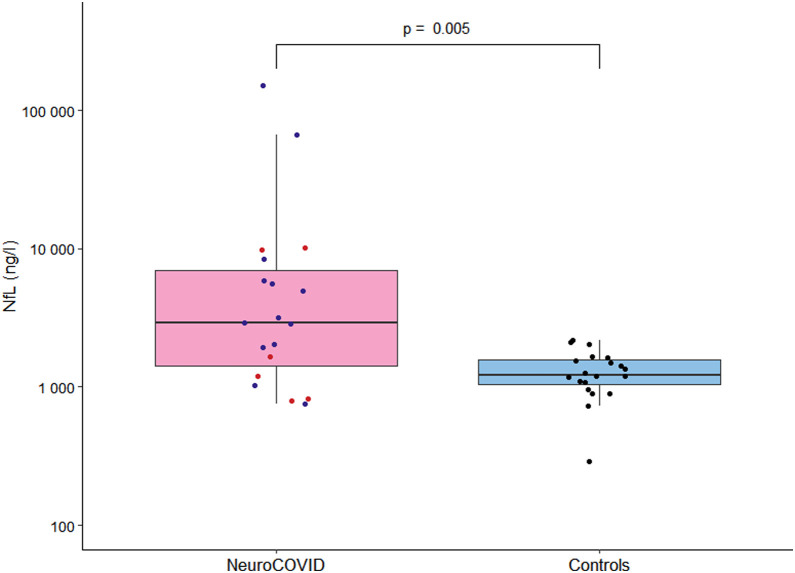
CSF NfL levels were significantly higher than in the control group of non-COVID-19 patients with psychiatric illnesses (2905 ng/l, IQR 1428–7124 versus 1222 ng/L, IQR 1049–1566) (Fig. 1 , Table 3). There was no correlation between CSF NfL level and age (rho = 0.337, P = 0.135), CSF NfL level and the delay of LP (rho = 0.09, P = 0.71), and CSF NfL level and duration of ICU stay (rho = -0.442, P = 0.17).
Fig. 1.
Level of CSF NfL in NeuroCOVID group and controls. Boxplots of cerebrospinal fluid (CSF) neurofilament light chain (NfL) in NeuroCOVID (n = 19, 13 encephalopathies in blue and six meningoencephalitis in red) and controls of non-COVID patients with psychiatric illnesses (n = 20, in black).
Total-tau protein levels were increased in 5/21 patients (24%, four with encephalopathy, one with meningoencephalitis). One patient with a concomitant elevation of T-tau (2577 ng/L) and P-tau (64 ng/L) also demonstrated high NfL levels (66,560 ng/L) and intermediate levels of 14-3-3 protein, while amyloid markers (Aß1-42 level and Aß1-42/Aß1-40 ratio) were not significantly modified. There was a positive correlation between T-tau and NfL CSF levels (rho = 0.510 and P = 0.036) in the whole cohort.
CSF 14-3-3 protein was negative in 21/23 patients and intermediate in 2/23 (one encephalopathy with additional stroke and one meningoencephalitis).
Aß1-42 peptide was lowered in 11/21 (52.4%) patients (median 536 ng/L, IQR 432–904). The Aß1-42/Aß1-40 amyloid ratio was, however, normal in the 21 patients analyzed (0.082, IQR 0.060–0.096). Among the 11 patients with low levels of Aß1-42, nine had encephalopathy, and two had meningoencephalitis. Three of the patients with reduced CSF Aß1-42 levels had a concomitant increase in CSF/serum albumin ratio. The median CSF NfL concentration was increased in these patients (2852 ng/L, IQR 1948–5626), but T-tau or P-tau levels remained normal. Overall, none of the patients had a typical CSF pattern evocative of Alzheimer's disease pathophysiology.
4. Discussion
We analyzed CSF biomarkers in a group of 24 well-characterized SARS-CoV-2 infected patients developing acute CNS injury. We found features consistent with active neuronal damage and immune reaction restricted to the CNS in most patients. Consistent with previous studies, our findings provide evidence that CNS immune activation occurs in NeuroCOVID patients together with neuronal injury and impaired amyloid processing. This, further sheds light on disease pathogenesis and mechanisms of neurological sequelae secondary to SARS-CoV-2 infection while raising concerns about the long-term impact of NeuroCOVID on brain function.
A large proportion of the SARS-CoV-2 patients developing CNS abnormalities demonstrated CNS immune activation. In particular, agarose gel isoelectric focusing allowed us to show that CSF oligoclonal bands of IgG were present in 57% of our patients. By contrast, in previous studies, intrathecal IgG synthesis was inconstantly reported and observed in only 2 to 8% of patients [18], [20], [35]. In the present study, the median delay between the onset of neurological symptoms and LP was much shorter (median of five days) compared to 14 days [21] and 11 days [19] in the two studies in which intrathecal IgG synthesis was not detected (Table 1), suggesting that the delay between neurological manifestations and LP might account for such differences.
CSF neopterin is a well-established immune activation marker with elevated concentrations seen in many inflammatory states, including infections, autoimmune disorders, and primary CNS lymphoma [36], [37], [38]. Our study found that CSF neopterin was increased in 75% of all NeuroCOVID cases. Our finding agrees with the data reported by Eden et al. [19], in which 6/6 NeuroCOVID patients (four with encephalopathy and two with altered mental status) exhibited high levels of CSF neopterin. Under inflammatory conditions, neopterin in the brain is produced by microglia and astrocytes in response to stimulation by interferon gamma [39]. An elevation of CSF neopterin was reported when brain neuroinflammation results from viral infections, especially in herpes virus encephalitis, enterovirus meningoencephalitis [40], and in HIV-1-associated neurocognitive disorders [36]. Overall, biomarkers of focal immune reaction and neuroinflammation could provide valuable tools for diagnosing NeuroCOVID, for example, to distinguish between stroke due to SARS-CoV-2 infection versus non-inflammatory/infectious etiologies.
A substantial proportion of our patients had abnormal CSF levels of biomarkers that reflect neuronal injury, including NfL, T-tau, and to a lesser extent, protein 14-3-3. NfL is a cytoskeletal protein mainly expressed in large myelinated axons [41]. The positive correlation that normally exists between elevated CSF NfL and age in healthy individuals [42] was absent in NeuroCOVID patients suggesting that ongoing neuronal insults may cover up the age effect. Accordingly, this correlation is also absent in inflammatory, neurodegenerative, traumatic, and cerebrovascular diseases [43], [44], [45] where NfL is thought to be passively released into the CSF. CSF T-tau is also a well-studied biomarker that can be taken as a tool not only for prediction but also for diagnosing AD [46]. Of twenty-one patients, five had elevated levels of CSF T-tau. Among them, one patient with encephalopathy associated with stroke demonstrated high T-tau levels (2577 ng/L, i.e. five times higher than the cut-off value) and an intermediate level of protein 14-3-3, suggesting more extensive neuronal damage in this individual. Changes in CSF biomarkers of neuronal injury have been reported during various CNS infections. In a large retrospective study of 281 patients with CNS infections [40], T-tau and protein 14-3-3 were reported abnormally high in the CSF of patients developing herpes simplex virus (HSV) encephalitis. High levels of T-tau were also observed in patients with HSV encephalitis undergoing LP within seven days after the onset of symptoms suggesting that a sharp increase in T-tau occurs in the first days following HSV infection [40]. In NeuroCOVID patients with CNS injury, previous studies reported increased levels of NfL [18], [19], [21], [22], whereas T-Tau was found inconstantly increased [18], [22] (Table 1). Elevated CSF 14-3-3 protein was reported in four of eight critical COVID-19 patients with encephalopathy in the study from [23] (Table 1). Overall, our results confirm that neuronal damage is significant during the acute phase of NeuroCOVID and suggest that CSF NfL is the most reliable biomarker of neuronal injury in this context.
In our group of patients, 52.4% (nine with encephalopathy, two with encephalitis) developed changes in CSF amyloid biomarkers, but none presented abnormalities suggestive of Alzheimer's disease amyloidosis. Aß1-42 levels were significantly reduced in these patients but with no concomitant reduction of Aß1-42/Aß1-40 ratios as expected in amyloidosis [47]. Besides, no concomitant increase of T-tau and P-tau was observed in these particular patients. Changes in CSF amyloid biomarkers were also previously reported in two other studies describing NeuroCOVID patients with CNS and peripheral nervous system (PNS) damages. Paterson et al. [22] reported a decrease in the Aß1-42/Aß1-40 ratio but normal T-tau or P-tau levels in three patients with Guillain-Barre syndrome and two with encephalopathy (Table 1). CSF amyloid Aß1-40, Aß1-42, and Aß1-42/Aß1-40 ratio, as well as soluble amyloid precursor protein metabolites (sAPPα and sAPPß), were significantly reduced in another study describing 21 COVID-19 patients with PNS and CNS injuries [24]. Overall, these results suggest that SARS-CoV-2 infection may possibly induce a down-regulation of amyloid precursor protein processing, possibly resulting in a global reduction in ß-amyloid peptide production [24] that is not found in the pathophysiology of AD. This is consistent with previous reports on neuroinflammatory conditions and CNS infections where decreases in both Aß1-40 and Aß1-42 were reported [40], [48], [49]. One may also assume that the clearance and elimination of amyloid metabolites might be enhanced in patients with higher CSF/serum albumin ratios traducing increased BBB permeability. Note, however, that CSF/serum albumin ratios were elevated in only one-third of patients with reduced CSF Aß1-42. From a more general point of view, the amyloid precursor protein being considered as an innate antiviral defense factor [50], [51], [52], alterations in its metabolism are not totally unexpected in NeuroCOVID patients.
Beyond acute infection, there is still a challenge to distinguish neurological sequelae of SARS-CoV-2 infection from early neurodegenerative processes [53]. Post-infectious immune response generated by anti-neuronal autoantibodies [54] or persistent viral replication in tissue reservoirs such as the olfactory mucosa [2] could mediate residual and chronic neuroinflammation. Therefore, longitudinal clinical, biological, and neuropathological studies are needed to better understand long-term consequences of these processes.
Our study has some limitations, such as the small sample size and the lack of a prospective control group of COVID-19 patients without neurological symptoms. Also, the variable delay between the onset of neurological symptoms and LP could be a possible confounding factor. Nevertheless, one of the strengths of our study was that participants were well-characterized and formed a relatively homogeneous group. Another strength is the harmonization of sample collection and handling, assuring robust and comparable results between patients.
Overall, our study showed that CSF biomarkers of neuroinflammation and neuronal injury are elevated in acute NeuroCOVID patients. We speculate that neuroinflammation, demonstrated by elevation of CSF neopterin and intrathecal synthesis of IgG as well as BBB disruption, could trigger neuronal damage and compromise amyloid precursor peptide processing. Our results confirm the idea that anti-inflammatory drugs are essential at an early phase of the disease [55]. While their effects appear to improve outcomes in the acute phase of the disease [56], the impact on residual neurological disability is still poorly understood and requires better understanding.
Author's contributions
Hugo Chaumont: conceptualization, investigation, project administration, validation, writing – original draft preparation; Flora Kaczorowski: conceptualization, data curation, formal analysis, investigation, resources, writing – original draft preparation; Aurore San-Galli: investigation, resources, writing – original draft preparation; Patrick Pierre Michel: validation, writing – review and editing; Benoit Tressières: data curation, formal analysis, methodology, software, validation; Emmanuel Roze: validation, writing – review and editing; Isabelle Quadrio: validation, writing – review and editing; Annie Lannuzel: project administration, supervision, validation, writing – review and editing.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R., et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 2.de Melo G.D., Lazarini F., Levallois S., Hautefort C., Michel V., Larrous F., et al. COVID-19 – related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med. 2021;13:eabf8396. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Francistiová L., Klepe A., Curley G., Gulya K., Dinnyés A., Filkor K. Cellular and molecular effects of SARS-CoV-2 linking lung infection to the brain. Front Immunol. 2021;12:730088. doi: 10.3389/fimmu.2021.730088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song E., Bartley C.M., Chow R.D., Ngo T.T., Jiang R., Zamecnik C.R., et al. Divergent and self-reactive immune responses in the CNS of COVID-19 patients with neurological symptoms. Cell Rep Med. 2021;2:100288. doi: 10.1016/j.xcrm.2021.100288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohammadi S., Moosaie F., Aarabi M.H. Understanding the immunologic characteristics of neurologic manifestations of SARS-CoV-2 and potential immunological mechanisms. Mol Neurobiol. 2020;57:5263–5275. doi: 10.1007/s12035-020-02094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaumont H., Meppiel E., Roze E., Tressières B., de Broucker T., Lannuzel A. Long-term outcomes after NeuroCOVID: a 6-month follow-up study on 60 patients. Rev Neurol. 2022;178:137–143. doi: 10.1016/j.neurol.2021.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frontera J.A., Yang D., Lewis A., Patel P., Medicherla C., Arena V., et al. A prospective study of long-term outcomes among hospitalized COVID-19 patients with and without neurological complications. J Neurol Sci. 2021;426:117486. doi: 10.1016/j.jns.2021.117486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krey L., Huber M.K., Höglinger G.U., Wegner F. Can SARS-CoV-2 infection lead to neurodegeneration and Parkinson's disease? Brain Sci. 2021;11:1654. doi: 10.3390/brainsci11121654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bouali-Benazzouz R., Benazzouz A. Covid-19 infection and Parkinsonism: Is there a link? Mov Disord. 2021;36:1737–1743. doi: 10.1002/mds.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritchie K., Chan D., Watermeyer T. The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun. 2020;2:fcaa069. doi: 10.1093/braincomms/fcaa069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sencio V., Machelart A., Robil C., Benech N., Hoffmann E., Galbert C., et al. Alteration of the gut microbiota following SARS-CoV-2 infection correlates with disease severity in hamsters. Gut Microbes. 2022;14:2018900. doi: 10.1080/19490976.2021.2018900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venegas-Borsellino C., Sankararaman S., Roche K., Burns J.B., Landis R.M. Impact of COVID-19 on the intestinal microbiome. Curr Nutr Rep. 2021;10:300–306. doi: 10.1007/s13668-021-00375-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun M., Ma K., Wen J., Wang G., Zhang C., Li Q., et al. A review of the brain-gut-microbiome axis and the potential role of microbiota in Alzheimer's disease. JAD. 2020;73:849–865. doi: 10.3233/JAD-190872. [DOI] [PubMed] [Google Scholar]
- 14.Vavougios G.D. SARS-CoV-2 dysregulation of PTBP1 and YWHAE/Z gene expression: a primer of neurodegeneration. Med Hypotheses. 2020;144:110212. doi: 10.1016/j.mehy.2020.110212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindlau A., Widmann C.N., Putensen C., Jessen F., Semmler A., Heneka M.T. Predictors of hippocampal atrophy in critically ill patients. Eur J Neurol. 2015;22:410–415. doi: 10.1111/ene.12443. [DOI] [PubMed] [Google Scholar]
- 16.Iwashyna T.J., Ely E.W., Smith D.M., Langa K.M. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304:1787. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widmann C.N., Heneka M.T. Long-term cerebral consequences of sepsis. Lancet Neurol. 2014;13:630–636. doi: 10.1016/S1474-4422(14)70017-1. [DOI] [PubMed] [Google Scholar]
- 18.Virhammar J., Nääs A., Fällmar D., Cunningham J.L., Klang A., Ashton N.J., et al. Biomarkers for central nervous system injury in cerebrospinal fluid are elevated in COVID-19 and associated with neurological symptoms and disease severity. Eur J Neurol. 2021;28:3324–3331. doi: 10.1111/ene.14703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edén A., Kanberg N., Gostner J., Fuchs D., Hagberg L., Andersson L.-M., et al. CSF biomarkers in patients with COVID-19 and neurological symptoms: a case series. Neurology. 2021;96:e294–e300. doi: 10.1212/WNL.0000000000010977. [10.1212/WNL.0000000000010977] [DOI] [PubMed] [Google Scholar]
- 20.Espíndola O.M., Brandão C.O., Gomes Y.C.P., Siqueira M., Soares C.N., Lima M.A.S.D., et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int J Infect Dis. 2021;102:155–162. doi: 10.1016/j.ijid.2020.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia M.A., Barreras P.V., Lewis A., Pinilla G., Sokoll L.J., Kickler T., et al. Cerebrospinal fluid in COVID-19 neurological complications: neuroaxonal damage, anti-SARS-Cov2 antibodies but no evidence of cytokine storm. J Neurol Sci. 2021;427:117517. doi: 10.1016/j.jns.2021.117517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paterson R.W., Benjamin L.A., Mehta P.R., Brown R.L., Athauda D., Ashton N.J., et al. Serum and cerebrospinal fluid biomarker profiles in acute SARS-CoV-2-associated neurological syndromes. Brain Commun. 2021;3:fcab099. doi: 10.1093/braincomms/fcab099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexopoulos H., Magira E., Bitzogli K., Kafasi N., Vlachoyiannopoulos P., Tzioufas A., et al. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7:e893. doi: 10.1212/NXI.0000000000000893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziff O.J., Ashton N.J., Mehta P.R., Brown R., Athauda D., Heaney J., et al. Amyloid processing in COVID-19-associated neurological syndromes. J Neurochem. 2022;161(2) doi: 10.1111/jnc.15585. [jnc.15585] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guasp M., Muñoz-Sánchez G., Martínez-Hernández E., Santana D., Carbayo Á., Naranjo L., et al. CSF biomarkers in COVID-19 associated encephalopathy and encephalitis predict long-term outcome. Front Immunol. 2022;13:866153. doi: 10.3389/fimmu.2022.866153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edén A., Grahn A., Bremell D., Aghvanyan A., Bathala P., Fuchs D., et al. Viral antigen and inflammatory biomarkers in cerebrospinal fluid in patients with COVID-19 infection and neurologic symptoms compared with control participants without infection or neurologic symptoms. JAMA Netw Open. 2022;5:e2213253. doi: 10.1001/jamanetworkopen.2022.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meppiel E., Peiffer-Smadja N., Maury A., Bekri I., Delorme C., Desestret V., et al. Neurologic manifestations associated with COVID-19: a multicentre registry. Clin Microbiol Infect. 2020;27(3) doi: 10.1016/j.cmi.2020.11.005. [S1198743X20306984] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [insert date]. n.d. [PubMed]
- 29.Deisenhammer F., Bartos A., Egg R., Gilhus N.E., Giovannoni G., Rauer S., et al. Guidelines on routine cerebrospinal fluid analysis. Report from an EFNS task force. Eur J Neurol. 2006;13:913–922. doi: 10.1111/j.1468-1331.2006.01493.x. [DOI] [PubMed] [Google Scholar]
- 30.Perret-Liaudet A., Boibieux A., Gabet J., Biron F., Haond P., Peyramond D., et al. VIII International Conference on AIDS/III STD World Congress; Amsterdam, The Netherlands: 1992. CSF neopterin in neurological disease in HIV-1 infection. PoB 3497. [Google Scholar]
- 31.Vanderstichele H., Bibl M., Engelborghs S., Le Bastard N., Lewczuk P., Molinuevo J.L., et al. Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's biomarkers standardization initiative. Alzheimers Dement. 2012;8:65–73. doi: 10.1016/j.jalz.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Lardeux P., Fourier A., Peter E., Dorey A., Muñiz-Castrillo S., Vogrig A., et al. Core cerebrospinal fluid biomarker profile in anti-LGI1 encephalitis. J Neurol. 2022;269:377–388. doi: 10.1007/s00415-021-10642-2. [DOI] [PubMed] [Google Scholar]
- 33.Fourier A., Dorey A., Perret-Liaudet A., Quadrio I. Detection of CSF 14-3-3 protein in sporadic Creutzfeldt-Jakob disease patients using a new automated capillary western assay. Mol Neurobiol. 2018;55:3537–3545. doi: 10.1007/s12035-017-0607-2. [DOI] [PubMed] [Google Scholar]
- 34.Molinuevo J.L., Blennow K., Dubois B., Engelborghs S., Lewczuk P., Perret-Liaudet A., et al. The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's biomarkers standardization initiative. Alzheimers Dement. 2014;10:808–817. doi: 10.1016/j.jalz.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 35.Lewis A., Frontera J., Placantonakis D.G., Lighter J., Galetta S., Balcer L., et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J Neurol Sci. 2021;421:117316. doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagberg L., Cinque P., Gisslen M., Brew B.J., Spudich S., Bestetti A., et al. Cerebrospinal fluid neopterin: an informative biomarker of central nervous system immune activation in HIV-1 infection. AIDS Res Ther. 2010;7:15. doi: 10.1186/1742-6405-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Viaccoz A., Ducray F., Tholance Y., Barcelos G.K., Thomas-Maisonneuve L., Ghesquières H., et al. CSF neopterin level as a diagnostic marker in primary central nervous system lymphoma. Neuro Oncol. 2015;17:1497–1503. doi: 10.1093/neuonc/nov092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furukawa Y., Nishi K., Kondo T., Tanabe K., Mizuno Y. Significance of CSF total neopterin and biopterin in inflammatory neurological diseases. J Neurol Sci. 1992;111:65–72. doi: 10.1016/0022-510X(92)90113-Y. [DOI] [PubMed] [Google Scholar]
- 39.Ghisoni K., Martins R., de P., Barbeito L., Latini A. Neopterin as a potential cytoprotective brain molecule. J Psychiatr Res. 2015;71:134–139. doi: 10.1016/j.jpsychires.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 40.Di Stefano A., Alcantarini C., Atzori C., Lipani F., Imperiale D., Burdino E., et al. Cerebrospinal fluid biomarkers in patients with central nervous system infections: a retrospective study. CNS Spectr. 2020;25:402–408. doi: 10.1017/S1092852919000981. [DOI] [PubMed] [Google Scholar]
- 41.Gafson A.R., Barthélemy N.R., Bomont P., Carare R.O., Durham H.D., Julien J.-P., et al. Neurofilaments: neurobiological foundations for biomarker applications. Brain. 2020;143:1975–1998. doi: 10.1093/brain/awaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vågberg M., Norgren N., Dring A., Lindqvist T., Birgander R., Zetterberg H., et al. Levels and age dependency of neurofilament light and glial fibrillary acidic protein in healthy individuals and their relation to the brain parenchymal fraction. PLoS ONE. 2015;10:e0135886. doi: 10.1371/journal.pone.0135886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khalil M., Enzinger C., Langkammer C., Ropele S., Mader A., Trentini A., et al. CSF neurofilament and N-acetylaspartate related brain changes in clinically isolated syndrome. Mult Scler. 2013;19:436–442. doi: 10.1177/1352458512458010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khalil M., Teunissen C.E., Otto M., Piehl F., Sormani M.P., Gattringer T., et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 45.Kuhle J., Plattner K., Bestwick J.P., Lindberg R.L., Ramagopalan S.V., Norgren N., et al. A comparative study of CSF neurofilament light and heavy chain protein in MS. Mult Scler. 2013;19:1597–1603. doi: 10.1177/1352458513482374. [DOI] [PubMed] [Google Scholar]
- 46.Blennow K., de Leon M.J., Zetterberg H. Alzheimer's disease. Lancet. 2006;368:387–403. doi: 10.1016/S0140-6736(06)69113-7. [DOI] [PubMed] [Google Scholar]
- 47.Biscetti L., Salvadori N., Farotti L., Cataldi S., Eusebi P., Paciotti S., et al. The added value of Aβ42/Aβ40 in the CSF signature for routine diagnostics of Alzheimer's disease. Clinica Chimica Acta. 2019;494:71–73. doi: 10.1016/j.cca.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 48.Zetterberg H., Blennow K. Fluid biomarkers for mild traumatic brain injury and related conditions. Nat Rev Neurol. 2016;12:563–574. doi: 10.1038/nrneurol.2016.127. [DOI] [PubMed] [Google Scholar]
- 49.Krut J.J., Zetterberg H., Blennow K., Cinque P., Hagberg L., Price R.W., et al. Cerebrospinal fluid Alzheimer's biomarker profiles in CNS infections. J Neurol. 2013;260:620–626. doi: 10.1007/s00415-012-6688-y. [DOI] [PubMed] [Google Scholar]
- 50.Chai Q., Jovasevic V., Malikov V., Sabo Y., Morham S., Walsh D., et al. HIV-1 counteracts an innate restriction by amyloid precursor protein resulting in neurodegeneration. Nat Commun. 2017;8:1522. doi: 10.1038/s41467-017-01795-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gisslén M., Krut J., Andreasson U., Blennow K., Cinque P., Brew B.J., et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63. doi: 10.1186/1471-2377-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eimer W.A., Vijaya Kumar D.K., Navalpur Shanmugam N.K., Rodriguez A.S., Mitchell T., Washicosky K.J., et al. Alzheimer's disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron. 2018;99 doi: 10.1016/j.neuron.2018.06.030. [56-63.e3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spudich S., Nath A. Nervous system consequences of COVID-19. Science. 2022;375:267–269. doi: 10.1126/science.abm2052. [DOI] [PubMed] [Google Scholar]
- 54.Franke C., Ferse C., Kreye J., Reincke S.M., Sanchez-Sendin E., Rocco A., et al. High frequency of cerebrospinal fluid autoantibodies in COVID-19 patients with neurological symptoms. Brain Behav Immun. 2021;93:415–419. doi: 10.1016/j.bbi.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaumont H., San-Galli A., Martino F., Couratier C., Joguet G., Carles M., et al. Mixed central and peripheral nervous system disorders in severe SARS-CoV-2 infection. J Neurol. 2020;267:3121–3127. doi: 10.1007/s00415-020-09986-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Merad M., Blish C.A., Sallusto F., Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375:1122–1127. doi: 10.1126/science.abm8108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available by the corresponding author upon reasonable request. The data are not publicly available because they contain information that could compromise our patients’ privacy.