Abstract
Objective
The study aims to systematically evaluate the risk factors of pulmonary infection in elderly patients with acute stroke.
Methods
PubMed, CENTRAL, CINAHL, Web of Science, Embase, and four Chinese databases (CNKI, SinoMed, VIP, and Wanfang databases) for studies involving risk factors of pulmonary infection in elderly patients with acute stroke were searched. Then, two researchers independently read the article titles and abstracts to screen the literature, extracted relevant research data, and evaluated the methodological quality. Finally, a meta-analysis was performed.
Results
In total, fifteen studies were included, with medium and high-grade quality. Meta-analysis results showed: age [OR = 1.70, 95% CI (1.27, 2.29), P = 0.0004], type of stroke [OR = 1.30, 95% CI (1.21, 1.40), P < 0.00001], conscious disturbance [OR = 2.27, 95% CI (1.44, 3.58), P = 0.0004], dysphagia [OR = 3.24, 95% CI (2.06, 5.12), P < 0.00001], diabetes mellitus [OR = 2.35, 95% CI (1.23, 4.48), P = 0.010], hypertension [OR = 2.05, 95% CI (1.83, 2.31), P < 0.0001], chronic obstructive pulmonary disease (COPD) [OR = 2.69, 95% CI (1.90, 3.81), P < 0.00001], hyperlipidemia [OR = 1.29, 95% CI (1.19, 1.39), P < 0.00001], invasive procedure [OR = 3.37, 95% CI (2.30, 4.94), P < 0.00001], hospital stays [OR = 1.41, 95% CI (1.22, 1.62), P < 0.00001], bedridden time [OR = 1.51, 95% CI (1.36, 1.68), P < 0.00001], and National Institute of Health Stroke Scale (NIHSS) score [OR = 1.67, 95% CI (1.02, 2.75), P = 0.04] were independent risk factors. Glasgow Coma Scale (GCS) score was not a risk factor. However, the relationship between atrial fibrillation, smoking history, and pulmonary infection in elderly patients with acute stroke needs further proof.
Conclusions
Age, type of stroke, conscious disturbance, dysphagia, diabetes mellitus, hypertension, COPD, hyperlipidemia, invasive procedure, hospital stays, bedridden time, and NIHSS score were risk factors for pulmonary infection in elderly patients with acute stroke.
Keywords: Elderly patients, Acute stroke, Pulmonary infection, Risk factors, Meta-analysis
Elderly patients; Acute stroke; Pulmonary infection; Risk factors; Meta-analysis.
1. Introduction
The population aged over 65 years is expected to reach 1500 million by 2050, compared to 524 million in 2010 [1]. Nearly fifteen million people suffer a stroke every year [2]. Acute stroke is a cerebrovascular circulatory disorder with sudden onset. It is a principal cause of disability and mortality around the world, leading to higher healthcare costs, with a global cost estimated to exceed United States dollar (USD) 721 billion (0.66% of the global gross domestic product) [3, 4]. Pulmonary infection is one of the most common and severe complications of acute stroke, with the complication rate being about 7–12% [5]. Elderly patients appear at higher risk of pulmonary infection due to low lung function and weakened body defense systems. When pulmonary infection occurs in elderly patients with acute stroke, it affects clinical outcomes, prolongs the length of hospitalization stay and costs, and aggravates the economic burden on families and society [6, 7]. Furthermore, pulmonary infection was a risk factor for poor prognosis (Odds Ratio, OR = 2.967) and death (OR = 5.493) among elderly patients with acute stroke [8]. As a result, identifying the risk factors and implementing early interventions based on the risk factors is critical for improving prognosis and quality of life and reducing the burden on their families and society. However, the research carried out in recent years had disparate results. For example, the study by Papagianni et al. did not approve of a relation between type 2 diabetes and pulmonary infection [9]. In contrast, others did [10]. Similarly, the study by Ishigami et al. agreed with an association between pulmonary infection and hypertension [11]. In contrast, others did not [12]. Therefore, we conducted a meta-analysis on the risk factors of pulmonary infection in elderly patients with acute stroke to provide the scientific basis for clinical prevention of pulmonary infection in elderly patients with acute stroke.
2. Methods
This review was reported according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) statement. The study protocol was registered under PROSPERO (Ref: CRD42022339906).
2.1. Search strategy
Comprehensive research on PubMed, Embase, CENTRAL, Web of Science, and the four Chinese databases, including National Knowledge Infrastructure (CNKI), Chinese Biomedical Literatures database (SinoMed), Wanfang Digital Periodicals (WanFang), and Chinese Science and Technology Periodicals (VIP) database were explored to identify the risk factors for pulmonary infection in elderly patients with acute stroke. The retrieval time was from the establishment of the database to May 1, 2022. The following search terms and logic were used: stroke (cerebrovascular accident OR cerebrovascular disorder OR cerebrovascular incident OR cerebral infarction OR brain ischemia OR cerebral hemorrhage OR brain hemorrhage OR CVA OR cerebrovascular apoplexy OR brain vascular accident OR cerebrovascular stroke OR Apoplexy OR cerebral stroke OR acute stroke OR intracranial embolism). In addition, they were combined with title/abstract words related to pulmonary infection (lung infection OR pneumonia), elderly (aged OR older OR elder OR geriatric OR elderly people OR old OR old people OR senior OR over 65), and risk factor (influencing factor OR relative factor OR predicted factor OR reason∗ OR correlated∗ OR predictor∗ OR influence∗ OR incident∗) through the Boolean operator AND. This search strategy was implemented in each of the databases mentioned above. Moreover, there were no restrictions on the date, country, publication status, or year of publication. However, the languages were confined to English and Chinese. Taking PubMed as an example, see Appendix 1 for the specific retrieval strategy.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: case-control studies or cohort studies; population aged 60 years or older, meet the diagnostic criteria of acute stroke, and confirmed by head computerized tomography (CT) or brain magnetic resonance imaging (MRI) [13] exposure factors include age, conscious disturbance, dysphagia, diabetes, chronic obstructive pulmonary disease (COPD), invasive procedure, hospital stays, bedridden time, etc.; the outcome measure was pulmonary infection after acute stroke. No limitation was exerted upon gender, race, or educational status. The exclusion criteria were as follows: studies that were not published in English or Chinese language; duplicate studies; no eligible data for extraction.
2.3. Data extraction
After the search was completed, the next step in data extraction was to screen the articles. Strictly following the inclusion and exclusion criteria, two reviewers (YQ WCX) retrieved and reviewed full-text articles after scrutinizing the titles and abstracts of all articles independently, then extracted data, discussed, and resolved any differences or negotiated with another researcher independently (WCX). After reading the full text, the step was marked as completed. The data extraction content included the first author, year of publication, the department of participants, country, population, sample size, and risk factors.
2.4. Quality appraisal
Quality assessment of case-control and cohort studies was evaluated by the Newcastle-Ottawa scale (NOS), which includes 8 items in 3 sections with 9 scores. The scores of 0–3, 4–6, and 7–9 were considered low, medium, and high-quality studies, respectively.
3. Statistical analysis
Stata 15.0 software was used for data analysis. The statistics were reported as odds ratios (ORs) and 95% confidence intervals (CIs). The heterogeneity of the included studies was judged by the Q test (test level is alpha = 0.1) and I2. If P ≤ 0.1 or I2 ≥ 50%, there was statistical heterogeneity between the studies, and the random effects model was applied. Instead, the fixed effects model was applied for meta-analysis. Meanwhile, meta-regression analysis, subgroup analysis, and one-by-one elimination of included studies of sensitivity analysis were carried out to explore the source of heterogeneity. We compared the pooled results from the fixed-effects model and the random-effects model to evaluate the stability of the results. Egger's test was used to detect publication bias.
4. Results
4.1. Study search and study characteristics
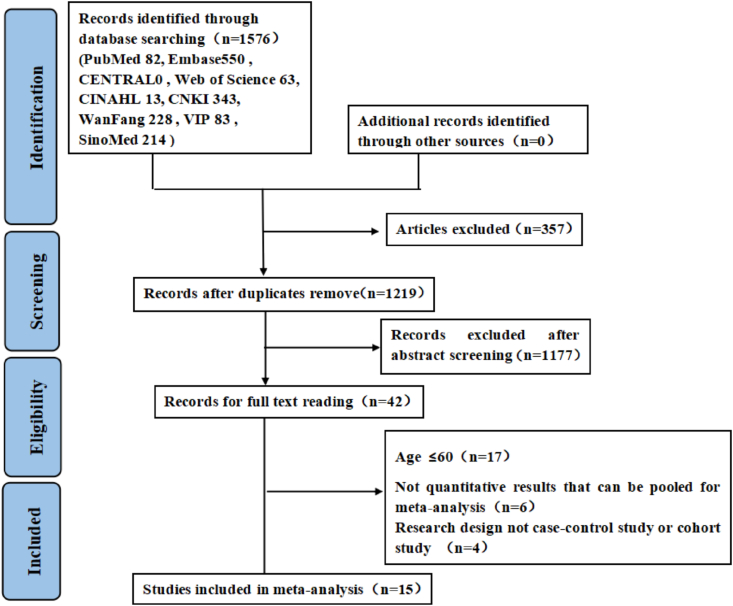
Search results and reasons for exclusion are shown in Figure 1. A total of 1,576 citations were identified from our search strategy. After removing duplicates, 1,219 studies remained, and 15 studies [10, 11, 12, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25] with 4,717 elderly patients with acute stroke met the inclusion criteria and were evaluated in the meta-analysis. Table 1 details the study's characteristics and the quality assessment results.
Figure 1.
Flowchart for selection of included studies.
Table 1.
Characteristics of included studies.
| References | Population | Department | Country | Infected group | Non-infected group | Study Design | Factors |
|---|---|---|---|---|---|---|---|
| Liu 2011 [12] | Acute ischemic stroke | Department of internal neurology | China | 34 | 97 | Prospective cohort study | 1、4 |
| Meng 2013 [14] | Acute stroke | Department of internal neurology | China | 24 | 136 | Retrospective case-control study | 2、4、5 |
| Gu 2015 [15] | Acute stroke | Department of internal neurology | China | 70 | 60 | Retrospective case-control study | 1、2、4、5、13、16 |
| Zhuang 2019 [16] | Acute ischemic stroke | Emergency department | China | 58 | 60 | Retrospective case-control study | 1、3、6、7、8、10 |
| Xie 2019 [17] | Acute stroke | Neurosurgery department | China | 84 | 80 | Prospective cohort study | 7、16、17 |
| Wang QL 2014 [18] | Acute ischemic stroke | Internal medicine | China | 36 | 140 | Retrospective case-control study | 1、2、5、8、9、16 |
| Wang KJ 2014 [19] | Acute ischemic stroke | Internal medicine | China | 68 | 215 | Retrospective case-control study | 1、2、4、8、12 |
| Shi 2015 [20] | Acute ischemic stroke | Department of internal neurology | China | 46 | 146 | Retrospective case-control study | 1、6、9、12 |
| Huang 2017 [21] | Acute stroke | Geriatrics | China | 30 | 160 | Retrospective case-control study | 2、4、5、7、8、9、13、14、15、16 |
| Cheng 2014 [22] | Acute stroke | Department of internal neurology | China | 52 | 94 | Retrospective case-control study | 1、4、16 |
| Chen DN 2021 [23] | Acute ischemic stroke | Department of internal neurology | China | 108 | 1005 | Retrospective case-control study | 1、4、8、13 |
| Sun 2016 [10] | Acute stroke | Department of internal neurology | China | 69 | 795 | Retrospective case-control study | 1、2、4、5、7、8、9、13、14、15、17 |
| Chen 2019 [24] | Acute ischemic stroke | NR | China | 254 | 504 | Retrospective case-control study | 1、4、6、8、9、16 |
| Ishigami 2012 [11] | Acute ischemic stroke | Geriatric Emergency Ward | Japan | 38 | 80 | Prospective cohort study | 15 |
| Feng 2014 [25] | Acute ischemic stroke | Department of internal neurology | China | 40 | 134 | Prospective cohort study | 8、10、15 |
Note: Acute stroke includes acute ischemic stroke and acute hemorrhagic stroke; 1 = Age, 2 = Chronic obstructive pulmonary disease (COPD), 3 = Atrial fibrillation, 4 = Conscious disturbance, 5 = Bedridden time, 6 = Smoking history, 7 = National Institute of Health Stroke Scale (NIHSS) score, 8 = Dysphagia, 9 = Diabetes mellitus, 10 = Glasgow Coma Scale (GCS) score, 11 = Comorbidity, 12 = Massive cerebral infarction, 13 = Hospital stays, 14 = Hyperlipemia, 15 = Hypertension, 16 = Invasive procedure, 17 = Type of stroke.
4.2. Quality assessment
The quality of all included studies ranged from 6 to 8, indicating medium or high quality (Tables 2 and 3).
Table 2.
Risk of bias assessment results of case-control studies.
| Included studies | Study population selection |
Comparability |
Exposure |
NOS score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | ||
| Meng 2013 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Gu 2015 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Zhuang 2019 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Wang QL 2014 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 6 |
| Wang KJ 2014 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Shi 015 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Huang 2017 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 0 | 7 |
| Cheng 2014 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Chen DN 2021 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 7 |
| Sun 2016 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Chen 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
1: Is the case definition adequate? 2: Representativeness of the cases; 3: Selection of Controls; 4: Definition of Controls; 5A: Study controls for _______________ (Select the most important factor); 5B: Study controls for any additional factor (This criteria could be modified to indicate specific control for a second important factor); 6: Ascertainment of exposure; 7: Same method of ascertainment for cases and controls; 8: Non-Response rate.
Table 3.
Risk of bias assessment results of cohort studies.
| Included studies | Study population selection |
Comparability |
Exposure |
NOS score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5A | 5B | 6 | 7 | 8 | ||
| Liu 2011 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 8 |
| Xie 2019 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 6 |
| Ishigami 2012 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| Feng 2014 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 8 |
1: Representativeness of the exposed cohort; 2: Selection of the non-exposed cohort; 3: Ascertainment of exposure; 4: Demonstration that outcome of interest was not present at start of study; 5A: study controls for _____________ (select the most important factor); 5B: study controls for any additional factor (This criterion could be modified to indicate specific control for a second important factor); 6: Assessment of outcome; 7: Was follow-up long enough for outcomes to occur; 8: Adequacy of follow up of cohorts.
4.3. Meta-analysis of the risk factors
4.3.1. Age
In total, eight of fifteen included studies on patient age's impact on pulmonary infection. Because there was significant heterogeneity, a random-effects model was used for the meta-analysis. The results showed that age is a risk factor [OR = 1.70, 95% CI (1.27, 2.29), P = 0.0004]. Sensitivity analysis did not change the heterogeneity significantly. Forest plots are shown in the Appendix.
4.3.2. Type of stroke
Two studies reported the relationship between the type of stroke and pulmonary infection, and there was no statistical heterogeneity between studies (P = 0.64, I2 = 0%). The fixed-effects model was used for analysis. The results showed that the type of stroke is a risk factor [OR = 1.30, 95% CI (1.21, 1.40), P < 0.00001]. Forest plots are shown in the Appendix.
4.3.3. Conscious disturbance
Seven studies reported the relationship between conscious disturbance and pulmonary infection, and there was obvious heterogeneity among studies (P < 0.0001, I2 = 91%). Therefore, the random-effects model was used for analysis. The results showed that conscious disturbance is a risk factor for pulmonary infection in elderly patients with acute stroke [OR = 2.27, 95% CI (1.44, 3.58), P = 0.0004]. Sensitivity analysis was performed to explore the heterogeneity. The sensitivity analysis results showed that the research of Wang QL et al. [18] was the primary source of heterogeneity. After excluding the study of Wang et al., there was no heterogeneity among the studies (P = 0.14, I2 = 40%). Forest plots are shown in the Appendix.
4.3.4. Dysphagia
Eight studies reported the relationship between dysphagia and pulmonary infection. There was heterogeneity among the studies (P < 0.0001, I2 = 86%). Therefore, a random-effects model was used for analysis. The results showed that dysphagia is a risk factor for pulmonary infection in elderly patients with acute stroke [OR = 3.24, 95% CI (2.06, 5.12), P < 0.00001]. Sensitivity analysis was performed to explore the heterogeneity. The sensitivity analysis results showed that the studies of Wang QL et al. [18] and Chen DN et al. [23] were the primary sources of heterogeneity. After excluding them, there was no heterogeneity between studies (P = 0.50, I2 = 0%). Forest plots are shown in the Appendix.
4.3.5. Diabetes
Five studies reported the relationship between diabetes mellitus and pulmonary infection, and there was obvious heterogeneity among the studies (P < 0.0001, I2 = 93%) Therefore, a random-effects model was used for analysis. The results showed that diabetes mellitus is a risk factor [OR = 2.35, 95% CI (1.23, 4.48), P = 0.010]. After excluding the study by Chen et al.and Shi et al. the heterogeneity was found to reduce from 93% to 75%. Forest plots are shown in the Appendix.
4.3.6. Hypertension
Three studies were included, and there was no heterogeneity among the studies (P = 0.97, I2 = 0%). The fixed-effects model was used for analysis. The results showed that hypertension is the risk factor [OR = 2.05, 95% CI (1.83, 2.31), P < 0.00001]. Forest plots are shown in the Appendix.
4.3.7. COPD
Six studies were included, and there was obvious heterogeneity among studies (P < 0.0001, I2 = 90%). The random effect model was used for analysis. The results showed that hypertension was the risk factor for pulmonary infection in elderly patients with acute stroke [OR = 2.69, 95% CI (1.90, 3.81), P < 0.00001]. Sensitivity analysis was performed to explore the heterogeneity. The sensitivity analysis results showed that the studies of Wang QL et al. and Gu Q et al. were the primary source of heterogeneity. After excluding them, there was no heterogeneity between studies (P = 0.21, I2 = 34%). Forest plots are shown in the Appendix.
4.3.8. Atrial fibrillation
Two studies were included, and there was no heterogeneity between studies (P = 0.16, I2 = 48%), so the fixed-effects model was used for analysis. The results showed that atrial fibrillation is the risk factor [OR = 2.06, 95% CI (1.07, 3.98), P = 0.03]. Forest plots are shown in the Appendix.
4.3.9. Hyperlipemia
Two studies were included, and there was no heterogeneity among the studies (P = 0.99, I2 = 0%). The fixed-effect model was used for analysis. The results showed that hyperlipemia is the risk factor [OR = 1.29, 95% CI (1.19, 1.39), P < 0.00001]. Forest plots are shown in the Appendix.
4.3.10. Smoking history
Four studies were included, and there was heterogeneity between them (P < 0.0001, I2 = 93%). The random-effects model was used for analysis. The results showed that smoking history is not associated with pulmonary infection [OR = 1.84, 95% CI (0.96, 3.53), P = 0.07]. Sensitivity analysis was conducted due to high heterogeneity. The studies by Chen et al., and Gu et al. might be a source of heterogeneity because the heterogeneity was reduced after excluding the study, with I2 decreasing from 93% to 0%. Forest plots are shown in the Appendix.
4.3.11. Invasive procedure
Seven studies were included, and there was heterogeneity among studies (P < 0.0001, I2 = 85%). Therefore, the random-effects model was used for analysis. The results showed that invasive procedure is a risk factor [OR = 3.37, 95% CI (2.30, 4.94), P < 0.00001]. Sensitivity analysis was applied to explore the source of heterogeneity, after excluding the study by Wang QL et al., and Xie et al., the heterogeneity reduced from 85% to 79%. Forest plots are shown in the Appendix.
4.3.12. Hospital stays
Six studies were included, and there was obvious heterogeneity among them (P < 0.0001, I2 = 90%). Therefore, the random-effect model was used for analysis. The results showed that hospital stays are a risk factor for pulmonary infection in elderly acute stroke [OR = 1.41, 95% CI (1.22, 1.62), P < 0.00001]. Sensitivity analysis was performed to explore the heterogeneity. The results of sensitivity analysis showed that the study of Xie LN et al. and Chen DN et al. were the main sources of heterogeneity. After exclusion, there was no heterogeneity between studies (P = 0.99, I2 = 0%). Forest plots are shown in the Appendix.
4.3.13. Bedridden time
Three studies were included, and there was no heterogeneity among the studies (P = 0.98, I2 = 0%). Therefore, a fixed effect model was used for analysis. The results showed that bedridden time is a risk factor [OR = 1.51, 95% CI (1.36, 1.68), P < 0.00001]. Forest plots are shown in the Appendix.
4.3.14. NIHSS score
Three studies were included, and there was obvious heterogeneity among them (P < 0.0001, I2 = 97%). Therefore, the random-effect model was used for analysis. The results showed that the NIHSS score is associated with pulmonary infection in elderly patients with acute stroke [OR = 1.67, 95% CI (1.02, 2.75), P = 0.04]. Sensitivity analysis was performed to explore the heterogeneity. The results showed that the study of Xie LN's study was a major source of heterogeneity. After exclusion, there was no heterogeneity between the studies (P = 1.00, I2 = 0%). Forest plots are shown in the Appendix.
4.3.15. GCS score
Two studies were included, and there was obvious heterogeneity between them (P < 0.0001, I2 = 96%). Therefore, the random-effect model was used for analysis. The results showed that the GCS score is not a risk factor [OR = 2.73, 95% CI (0.26, 28.82), P = 0.40]. Forest plots are shown in the Appendix.
4.4. Meta-regression analyses
Meta-regression was used to analyze factors with high statistical heterogeneity. Age, conscious disturbance, dysphagia, diabetes, COPD, smoking history, invasive procedure, hospital stays, and NIHSS score were used as dependent variables. In contrast, study design, publication year, sample size, literature quality score, and department as covariates. However, the results suggested that none of the above potential factors is the major source of heterogeneity (P > 0.05) (Table 4).
Table 4.
Meta regression analyses results.
| Covariate | Β | 95% CI | P |
|---|---|---|---|
| Age | |||
| Study design | 1.747 | −0.902–4.395 | 0.127 |
| Publication year | −0.272 | −0.909–0.365 | 0.268 |
| Sample size | −0.000 | −0.003–1.259 | 0.705 |
| Literature quality score | −1.652 | −4.562–1.259 | 0.169 |
| Department | 0.072 | −1.611–1.755 | 0.920 |
| Conscious disturbance | |||
| Study design | −1.278 | −8.078–5.523 | 0.504 |
| Publication year | −0.039 | −1.398–1.321 | 0.914 |
| Sample size | 0.001 | −0.009–0.011 | 0.758 |
| Literature quality score | 0.260 | −1.563–2.081 | 0.602 |
| Department | 0.398 | −0.832–1.629 | 0.443 |
| Dysphagia | |||
| Study design | 0.364 | −2.417–3.146 | 0.705 |
| Publication year | −0.073 | −0.566–0.420 | 0.670 |
| Sample size | 0.001 | −0.004–0.006 | 0.670 |
| Literature quality score | −0.521 | −1.762–0.719 | 0.273 |
| Department | 0.246 | −1.117–1.608 | 0.675 |
| Diabetes | |||
| Publication year | −0.349 | −4.216–3.518 | 0.456 |
| Sample size | −0.001 | −0.036–0.0341 | 0.818 |
| Literature quality score | 0.182 | −19.049–19.413 | 0.924 |
| Department | −0.198 | −1.964–1.568 | 0.745 |
| COPD | |||
| Publication year | −0.232 | −1.253–0.790 | 0.432 |
| Sample size | 0.001 | −0.002–0.004 | 0.465 |
| Literature quality score | −0.626 | −2.812–1.560 | 0.343 |
| Department | 0.197 | −0.942–1.337 | 0.656 |
| Smoking history | |||
| Publication year | −0.001 | −1.036–1.035 | 0.998 |
| Sample size | −0.002 | −0.007–0.004 | 0.387 |
| Literature quality score | −1.078 | −4.023–1.867 | 0.256 |
| Department | −0.002 | −4.144–4.140 | 0.998 |
| Invasive procedure | |||
| Study design | −1.185 | −12.728–10.359 | 0.702 |
| Publication year | 0.015 | −0.868–0.898 | 0.949 |
| Sample size | 3.10e − 07 | −0.004–0.004 | 1.000 |
| Literature quality score | −0.038 | −2.035–1.960 | 0.943 |
| Department | 0.207 | −1.042–1.456 | 0.687 |
| Hospital stays | |||
| Study design | −1.851 | −23.041–19.338 | 0.467 |
| Publication year | 0.010 | −1.453–1.472 | 0.947 |
| Sample size | −0.000 | −0.008–0.008 | 0.831 |
| Literature quality score | 0.160 | −2.443–2.763 | 0.578 |
| Department | 0.193 | −0.210–0.560 | 0.254 |
| NIHSS score | |||
| Study design | 0.639 | −3.134–4.412 | 0.277 |
| Publication year | −0.243 | −1.714–1.228 | 0.283 |
| Sample size | 0.001 | −0.010–0.011 | 0.590 |
| Literature quality score | 0.373 | −1.980–2.724 | 0.294 |
| Department | −0.412 | −7.996–7.172 | 0.615 |
4.5. Subgroup analyses
Subgroup analysis was performed to address the clinical heterogeneity. According to the meta-analysis results, a subgroup analysis was performed on the risk factors of age, conscious disturbance, dysphagia, diabetes, COPD, smoking history, invasive procedure, and hospital stays. The results showed that the literature quality score might be the source of conscious disturbance and dysphagia heterogeneity. However, there was no evident heterogeneity for other factors. Regarding combined effect results, except for the study design and department in the age subgroup analysis, the department in the diabetes group, the rest were consistent with the overall results. Table 5 shows the results of the subgroup analysis.
Table 5.
Subgroup analyses.
| Subgroup | Number of studies | Results of Heterogeneity |
Effect model | Meta-analysis |
||
|---|---|---|---|---|---|---|
| (I2, %) | P | OR (95% CI) | P | |||
| Age | ||||||
| Cohort studies | 2 | 95.0 | p < 0.0001 | Random | 2.84 [0.43, 18.62] | p = 0.28 |
| Case-control studies | 6 | 91.0 | p < 0.0001 | Random | 1.71 [1.11, 2.65] | p = 0.02 |
| Department of internal neurology | 5 | 90.0 | p < 0.0001 | Random | 1.62 [1.25, 2.11] | p = 0.0003 |
| Another department | 3 | 95.0 | p < 0.0001 | Random | 2.52 [0.36, 17.52] | p = 0.35 |
| Conscious disturbance | ||||||
| Cohort studies | 2 | 0 | p = 0.46 | Fixed | 5.17 [1.82, 14.69] | p = 0.002 |
| Case-control studies | 5 | 93.0 | p < 0.0001 | Random | 1.99 [1.25, 3.18] | p = 0.0004 |
| Department of internal neurology | 4 | 62.0 | p = 0.05 | Random | 2.04 [1.64, 2.52] | p < 0.00001 |
| Another department | 3 | 90.0 | p < 0.0001 | Random | 1.06 [1.04, 1.08] | p < 0.00001 |
| NOS≥7 | 5 | 50.0 | p = 0.09 | Fixed | 2.09 [1.74, 2.50] | p < 0.00001 |
| NOS<7 | 2 | 50.0 | p = 0.15 | Fixed | 1.06 [1.04, 1.08] | p < 0.00001 |
| Dysphagia | ||||||
| Cohort studies | 2 | 0 | p = 0.73 | Fixed | 1.75 [1.17, 2.62] | p = 0.006 |
| Case-control studies | 6 | 87.0 | p < 0.0001 | Random | 3.59 [3.08, 4.18] | p < 0.00001 |
| Department of internal neurology | 3 | 86.0 | p = 0.001 | Random | 3.13 [1.66, 5.90] | p = 0.0004 |
| Another department | 5 | 85.0 | p < 0.0001 | Random | 3.28 [1.59, 6.76] | p = 0.01 |
| NOS≥7 | 6 | 68.0 | p = 0.009 | Random | 2.53 [2.13, 2.99] | p < 0.00001 |
| NOS<7 | 2 | 0 | p = 0.56 | Fixed | 6.52 [4.96, 8.57] | p < 0.00001 |
| Diabetes | ||||||
| Department of internal neurology | 2 | 93.0 | p = 0.0002 | Random | 3.14 [1.25, 7.85] | p = 0.01 |
| Another department | 3 | 93.0 | p < 0.0001 | Random | 1.93 [0.77, 4.83] | p = 0.16 |
| COPD | ||||||
| Department of internal neurology | 3 | 84.0 | p = 0.002 | Random | 2.11 [1.50, 2.97] | p < 0.00001 |
| Another department | 3 | 92.0 | p < 0.0001 | Random | 3.52 [1.55, 8.01] | p = 0.003 |
| Smoking history | ||||||
| Department of internal neurology | 2 | 91.0 | p = 0.007 | Random | 2.07 [0.90, 4.72] | p = 0.08 |
| Another department | 2 | 97.0 | p < 0.0001 | Random | 1.65 [0.32, 8.56] | p = 0.55 |
| Invasive procedure | ||||||
| Department of internal neurology | 3 | 85.0 | p = 0.001 | Random | 2.82 [1.57, 5.06] | p = 0.0005 |
| Another department | 4 | 86.0 | p < 0.0001 | Random | 3.90 [2.27, 6.72] | p < 0.00001 |
| NOS≥7 | 5 | 85.0 | p < 0.0001 | Random | 2.89 [1.88, 4.45] | p < 0.00001 |
| NOS<7 | 2 | 79.0 | p = 0.003 | Random | 5.26 [2.23, 12.40] | p = 0.0001 |
| Hospital stays | ||||||
| Department of internal neurology | 3 | 80.0 | p = 0.007 | Random | 1.29 [1.11, 1.51] | p = 0.0009 |
| Another department | 3 | 87.0 | p = 0.0004 | Random | 1.66 [1.25, 2.21] | p = 0.0005 |
4.6. Sensitivity analysis and publication bias
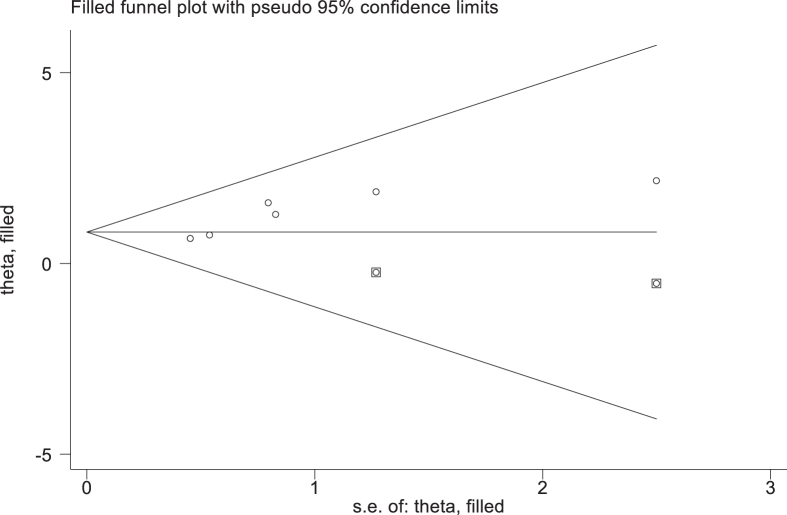
The stability of the combined results was analyzed by converting the fixed-effects model and the random-effects model. The results showed that except for the two factors of atrial fibrillation, smoking history, the other risk factors did not change much, suggesting that the results were stable and reliable. In addition, Egger's test was performed on the risk factors according to the number of included literature, and the results showed that only invasive procedure had publication bias. The bias was corrected by trim-and-fill method, as shown in Figure 2 and Table 6.
Figure 2.
Invasive operation.
Table 6.
Results of sensitivity analysis and publication bias.
| Risk factor | Effect model OR [95% CI] |
Stability | Egger's test |
||
|---|---|---|---|---|---|
| Fixed model | Random model | T value | p value | ||
| Age | 1.23 [1.16, 1.30] | 1.56 [1.16, 2.11] | Stable | 1.26 | 0.277 |
| Type of stroke | 1.30 [1.21, 1.40] | 1.30 [1.21, 1.40] | Stable | — | — |
| Conscious disturbance | 1.06 [1.05, 1.09] | 2.27 [1.44, 3.58] | Stable | -1.14 | 0.305 |
| Dysphagia | 3.28 [2.84, 3.78] | 3.24 [2.06, 5.12] | Stable | 0.36 | 0.731 |
| Diabetes mellitus | 2.08 [1.76, 2.46] | 2.35 [1.23, 4.48] | Stable | 0.73 | 0.519 |
| Hypertension | 2.05 [1.83, 2.31] | 2.05 [1.83, 2.31] | Stable | — | — |
| COPD | 2.04 [1.86, 2.24] | 2.69 [1.90, 3.81] | Stable | 2.72 | 0.053 |
| Atrial fibrillation | 2.06 [1.07, 3.98] | 2.34 [0.84, 6.47] | Unstable | — | — |
| Hyperlipemia | 1.29 [1.19, 1.39] | 1.29 [1.19, 1.39] | Stable | — | — |
| Smoking history | 1.47 [1.27, 1.70] | 1.84 [0.96, 3.53] | Unstable | 0.53 | 0.651 |
| Invasive procedure | 2.81 [2.44, 3.23] | 3.37 [2.30, 4.94] | Stable | 3.98 | 0.011 |
| Hospital stays | 1.30 [1.25, 1.35] | 1.41 [1.22, 1.62] | Stable | 1.31 | 0.260 |
| Bedridden time | 1.51 [1.36, 1.68] | 1.51 [1.36, 1.68] | Stable | — | — |
| NIHSS score | 1.24 [1.16, 1.32] | 1.67 [1.02, 2.75] | Stable | — | — |
| GCS score | 1.38 [0.96, 1.97] | 2.73 [0.26, 28.82] | Stable | 0.96 | 0.515 |
5. Discussion
5.1. Findings from the meta-analysis
A total of 15 studies were included, stipulating the inclusion and exclusion criteria of the study subjects, consistent with the criteria of our study. The quality of included studies was evaluated by NOS, indicating that the overall quality of included studies was high. Three of fifteen included studies [11, 15, 25] controlled the influence of underlying diseases and other important confounding factors on the results. Five studies [11, 15, 16, 21, 23] did not specify the basis for the selection of the control group, which may be limited by the study site and personnel. Seven studies [16, 17, 18, 19, 20, 21, 25] did not describe whether the non-response rate was the same in the case and control groups. The results of transforming different effect models showed that the combined total effect size of the meta-analysis was stable, and Egger's test indicated that the results were reliable. Pulmonary infection is the most common complication of acute stroke, which is one of the main reasons for the deterioration of disease and death of elderly patients with acute stroke. As a result, it is critical to help clinicians accurately identify acute stroke risk factors in elderly patients. The meta-analysis in our study showed that age, type of stroke, conscious disturbance, dysphagia, diabetes, hypertension, COPD, hyperlipidemia, invasive procedure, length of hospital stays, length of bedridden time, and NIHSS score were the most important risk factors for pulmonary infection except smoking history and GCS score.
5.1.1. General factor
A previous study has shown a significant association between age and pulmonary infection, consistent with our results [26]. A study suggested a 1.2-fold increase in risk per 10-year increase in age [27]. Nervous center dysfunction in patients with acute stroke, and pulmonary edema in the early stage were possible reasons for the increased risk of pulmonary infection. Therefore, for elderly patients with acute stroke, medical staff should formulate nursing programs and take timely and effective treatment measures to prevent pulmonary infection.
5.1.2. Disease factor
Our study did not specify which type of stroke was more likely to develop lung infections. In contrast, the study by Wu XL et al. [28] reported that a hemorrhagic stroke was more common than an ischemic stroke in pulmonary infection [29], as patients with hemorrhagic stroke may have an acute onset with the occurrence of earlier and faster pulmonary congestion, conscious disturbance, dysphagia, and weakened cough reflex, increasing the risk of aspiration. Moreover, the incidence of pulmonary infection in acute stroke patients with conscious disturbance was 1.7-fold that of those without conscious disturbance [30], mainly due to aspiration. Together with our results, these data suggest intensive oral care for patients should be provided to prevent pulmonary infection.
The risk of pulmonary infection in stroke patients with dysphagia was about three-fold that of patients without dysphagia [31], consistent with our results. Systematic screening and evaluation of dysphagia could significantly reduce the risk of pulmonary infection and improve the prognosis of patients [32]. Research reported that the time of screening and evaluation of dysphagia is associated with the risk of pulmonary infection [33]. Delaying the screening and evaluation of dysphagia may significantly increase the risk of pulmonary infection [34]. Therefore, it is recommended that all stroke patients undergo dysphagia screening and evaluation within 4 h of admission [35]. The NIHSS score is an indicator for evaluating the severity of a stroke, and our study also found that the NIHSS score is a risk factor, which is consistent with previous reports [36]. Therefore, assessing the NIHSS scale in time is significant for evaluating patient prognosis.
Patients with chronic disease may suffer from pulmonary infection easily. For example, for patients with diabetes, the hyperglycemic environment in the body is conducive to the growth and reproduction of bacteria, thereby aggravating the pulmonary microcirculation disorder and increasing the possibility of pulmonary infection [37]. In patients with hypertension, pulmonary congestion and changes in sympathetic activity caused by elevated blood pressure may lead to pulmonary infection [11]. In COPD patients, decreased airway clearance, aggravated airway inflammation, and decreased lung compliance may lead to pulmonary infection. Therefore, medical staff should strictly control blood glucose and blood pressure, actively carry out pulmonary rehabilitation education, and encourage patients to perform pulmonary rehabilitation training such as pursed-lip breathing to improve lung function.
5.1.3. Therapeutic factor
The risk of lung infection in patients who underwent invasive procedures was 12.8-fold that of patients who did not [8], which was consistent with our study. Strictly mastering the indications for invasive procedures and stopping invasive procedures on time can reduce the risk of pulmonary infection in patients [14]. Therefore, when performing invasive procedures, medical staff should strictly follow the principles of the aseptic technique and avoid unnecessary invasive procedures.
The length of hospital stays and bedridden time were risk factors, which was consistent with the findings of Wang WL [38]. The odds of developing a pulmonary infection within three-day hospital stays were 50% lower than the odds of a one-week hospital stay [39], and the risk of pulmonary infection in ischemic stroke gradually increased with the length of hospital stays [40]. A previous study indicated that bedridden acute stroke patients were at risk of developing pulmonary infection, which increases the risk of poor prognosis and death [8]. In addition, bedridden time was related to pulmonary infection, which may be due to the weakened ability of patients to expectorate sputum spontaneously, leading to the formation of hypostatic pneumonia. To prevent pulmonary infection in elderly patients with acute stroke who have been hospitalized and bedridden for a long time, interventions such as helping patients get out of bed, rehabilitation exercise, and so on should be implemented as soon as possible.
6. Strengths and limitations
As far as we know, this study is the first meta-analysis to analyze the risk factors for pulmonary infection in elderly patients with acute stroke. Moreover, a comprehensive search strategy was conducted in nine electronic databases to limit the possibility of missing any research, and the quality of included studies is high or medium. In addition, the study protocol was registered under PROSPERO for better research integrity and transparency. Finally, meta-aggression, subgroup and sensitivity analyses were conducted to explore the possible reasons for heterogeneity. However, there are some limitations to this study. First, the included studies were conducted mainly in China, which leaves some possibilities for bias. Second, it is impossible to analyze the risk factors in different age groups due to the limited data and reports in the literature. We sought to conducting relevant original studies in the future. Third, we only identified the risk factors. In the future, a prediction model should be established to help clinicians make decisions and consult [41].
7. Conclusion
To sum up, this study showed that age, type of stroke, conscious disturbance, dysphagia, diabetes, hypertension, COPD, hyperlipidemia, invasive procedure, length of hospital stays, bedridden time, and NIHSS score were the main factors in connection with the incidence of pulmonary infection in elderly patients with acute stroke. However, the relationship between atrial fibrillation, smoking history, and pulmonary infection needs further proof. Medical staff should strengthen the assessment and management of high-risk groups of elderly acute stroke patients and make targeted interventions to reduce the risk of pulmonary infection and promote early recovery.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Prof Jing Gao was supported by Sichuan Mental Health Education Research Center Project [XLJKJY2203A].
Data availability statement
Data will be made available on request.
Declaration of interest’s statement
The authors declare no conflict of interest.
Additional information
Supplementary content related to this article has been published online at [URL].
Contributor Information
Jing Gao, Email: gaojing@cdutcm.edu.cn.
Chaoming Hou, Email: 19941012@cdutcm.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.World Health Organization World Report on Ageing and Health. https://www.who.int/ageing/events/world-report-2015-launch/en/
- 2.Kim J., Thayabaranathan T., Donnan G.A., Howard G., Howard V.J., Rothwell P., et al. Global stroke statistics 2019. Int. J. Stroke. 2020;15(8):819–838. doi: 10.1177/1747493020909545. [DOI] [PubMed] [Google Scholar]
- 3.Lavados P.M., Hennis A.J., Fernandes J.G., et al. Stroke epidemiology, prevention, and management strategies at a regional level: Latin America and the Caribbean. Lancet Neurol. 2007;6(4):362–372. doi: 10.1016/S1474-4422(07)70003-0. [DOI] [PubMed] [Google Scholar]
- 4.Feigin V.L., Brainin M., Norrving B., Martins S., Sacco R.L., Hacke W., et al. World stroke organization (WSO): global stroke fact sheet 2022. Int. J. Stroke. 2022;17(1):18–29. doi: 10.1177/17474930211065917. [DOI] [PubMed] [Google Scholar]
- 5.Urra X., Laredo C., Zhao Y., Amaro S., Rudilosso S., Renu A., et al. Neuroanatomical correlates of stroke-associated infection and stroke-induced immunodepression. Brain Behav. Immun. 2017;60:142–150. doi: 10.1016/j.bbi.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Hannawi Y., Hannawi B., Rao C.P., Suarez J.I., Bershad E.M. Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc. Dis. 2013;35(5):430–443. doi: 10.1159/000350199. [DOI] [PubMed] [Google Scholar]
- 7.Mcculloch L., Smith C.J., Mccoll B.W. Adrenergic-mediated loss of splenic marginal zone B cells contributes to infection susceptibility after stroke. Nat. Commun. 2017;8 doi: 10.1038/ncomms15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan M., Li Q., Zhang R., Zhang W., Zou N., Qin X.Y., et al. Risk factors for and impact of poststroke pneumonia in patients with acute ischemic stroke. Medicine. 2021;100(12) doi: 10.1097/MD.0000000000025213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papagianni M., Tziomalos K., Kostaki S. Pneumonia increases mortality risk in patients admitted with acute ischemic stroke independently of stroke severity. Europ. Stroke J. 2018;3(1):340. [Google Scholar]
- 10.Sun M.J., Chen L.D., Kou X.L., Tang W.G., Yu X.L. Risk factors and prevention strategies of the elderly acute cerebral apoplexy patients with pulmonary infections. Chin. J. Nosocomio. 2016;26(7):1517–1519. [Google Scholar]
- 11.Ishigami K., Okuro M., Koizumi Y., Satoh K., Iritani O., Yano H., et al. Association of severe hypertension with pneumonia in elderly patients with acute ischemic stroke. Hypertens. Res. 2012;35(6):648–653. doi: 10.1038/hr.2012.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J.J., Zhang J., He M.L. Study on correlation between risk factors and prognosis of pulmonary infection in elderly patients with acute cerebral infarction. Chin. J. Geriatr Heart Brain Vessel Dis. 2011;13(9):824–826. [Google Scholar]
- 13.Luo A.L., Zhang H.M., Cheng M.F., Luo J.J., Shi Y., Hu X.L. Meta-analysis of therapeutic effect of enteral nutrition on acute stroke combined with dysphagia patients. Chin. J. Gerontol. 2020;40(21):4502–4510. [Google Scholar]
- 14.Meng X.B., Wang Z.R., Wang Y.Z., Hu X.Y. Risk factors of pulmonary infections in elderly patients with acute brain stroke. Chin. J. Nosocomio. 2013;23(19):4637–4639. [Google Scholar]
- 15.Gu Q., Shen M.H., Qian M.J., Sun R. Risk factors for pulmonary infections in elderly patients with acute stroke and prevention measures. Chin. J. Nosocomio. 2015;25(10):2265–2275. [Google Scholar]
- 16.Zhuang J.Y., Wei W., Zhou L.L., Zhan K.D. Relevant factors of stroke-associated pneumonia in elderly patients with acute ischemic stroke. J. Guangxi Med. Univ. 2019;44(11):1339–1343. [Google Scholar]
- 17.Xie L.N., Zhu L.J. Analysis on risk factors for pulmonary infection in elderly patients with acute stroke. Chongqing Medicine. 2019;48(19):3325–3329. [Google Scholar]
- 18.Wang Q.L., Li X.Q., Wang D.H. Analysis of the influencing factors of pulmonary infection during hospitalization in elderly patients with acute cerebral infarction. J. Med. Inform. 2014;(8):195. [Google Scholar]
- 19.Wang K.J. Analysis of risk factors for pulmonary infection in elderly patients with acute cerebral infarction. J Chin. Pract. Diagn. Ther. 2014;28(9):935–936. [Google Scholar]
- 20.ShiY Liu T., Zhang X.M., Du A.P., Zeng Y.F. Analysis of risk factors for pneumonia after acute cerebral infarction in the elderly. Chin. Manip. Rehabilit. Medicin. 2015;6(9):65–66. [Google Scholar]
- 21.Huang B., Huang H.Y. Risk factors and preventive strategies of pulmonary infection in elderly patients with acute stroke. Clin. Res. Pract. 2017;2(23):26–27. [Google Scholar]
- 22.Cheng W., Li Y., Pan C.L. Analysis of risk factors for pulmonary infections in elderly patients with acute cerebral stroke. Chin. J. Nosocomiol. 2014;24(15):3734–3738. [Google Scholar]
- 23.Cheng D.N., Wu W.T., Xiao X.H., Xiao X.Y., Zeng M., Cui M.L., et al. The risk factors of pulmonary infection in elderly patients with acute ischemic stroke. Guangzhou Med. J. 2021;52(3):28–31. [Google Scholar]
- 24.Chen J., Gao Y.Q. Analysis of etiology, risk factors and prognosis of pulmonary infection in elderly patients with acute cerebral infarction. Int. J. Clin. Exp. Med. 2019;12(7):9231–9237. [Google Scholar]
- 25.Feng Y., Xu C.S., Yuan L.P. The association of blood pressure levels in acute stage with stroke-associated pneumonia in elderly patients with ischemic stroke. Chin. J. Geriatrics. 2014;33(9):995–997. [Google Scholar]
- 26.Xu H.S. Influencing factors of acute stroke complicated with pulmonary infection in the elderly. Chin. J. Gerontology. 2013;33(6):1413–1414. [Google Scholar]
- 27.Kwon H.M., Jeong S.W., Lee S.H., Yoon B.W. The pneumonia score: a simple grading scale for prediction of pneumonia after acute stroke. Am. J. Infect. Control. 2006;34(2):64–68. doi: 10.1016/j.ajic.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 28.WXL Risk factors for pulmonary infection and value of combined diagnosis of BNP, TNF-α, Lp(a) in elderly stroke. Chin J Nosocomiol. 2020;30(23):3612–3616. [Google Scholar]
- 29.Almeida S.R.M., Bahia M.M., Lima F.O., Paschoal I.A., Cardoso T.A.M.O., Li L.M. Predictors of pneumonia in acute stroke inpatients in emergency unit. Arq Neuropsiquiatr. 2015;73(5):415–419. doi: 10.1590/0004-282X20150046. [DOI] [PubMed] [Google Scholar]
- 30.Etiology L.H. Of pulmonary infection in elderly patients with stroke and risk factors. Chin. J. Nosocomiol. 2017;27(1):88–91. [Google Scholar]
- 31.Martino R., Foley N., Bhogal S., Diamant N., Speechley M., Teasell R. Dysphagia after stroke: incidence, diagnosis, and pulmonary complications. Stroke. 2005;36(12):2756–2763. doi: 10.1161/01.STR.0000190056.76543.eb. [DOI] [PubMed] [Google Scholar]
- 32.Hinchey J.A., Shephard T., Furie K., Smith D., Wang D., Tonn S., et al. Formal dysphagia screening protocols prevent pneumonia. Stroke. 2005;36(9):1972–1976. doi: 10.1161/01.STR.0000177529.86868.8d. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang M., Boaden E., Arima H., Lavados P.M., Billot L., Hackett M.L., et al. Dysphagia screening and risks of pneumonia and adverse outcomes after acute stroke: an international multicenter study. Int. J. Stroke. 2020;15(2):206–215. doi: 10.1177/1747493019858778. [DOI] [PubMed] [Google Scholar]
- 34.Bray B.D., Smith C.J., Cloud G.C., Enderby B., James M., Paley L., et al. The association between delays in screening for and assessing dysphagia after acute stroke, and the risk of stroke-associated pneumonia. J. Neurol. Neurosurg. Psychiatry. 2017;88(1):25–30. doi: 10.1136/jnnp-2016-313356. [DOI] [PubMed] [Google Scholar]
- 35.Chen X., Hou X.H., Cui X., Li G.Q., Gao Y.F., Yang L.J. Evidence summary: recognition of dysphagia in acute stroke patients. J. Nurs. Sci. 2019;34(14):97–100. [Google Scholar]
- 36.Almeida S.R., Bahia M.M., Lima F.O., Paschoal I.A., Cardoso T.A.M., Li L.M. Predictors of pneumonia in acute stroke in patients in an emergency unit. Arq Neuropsiquiatr. 2015;73(5):415–419. doi: 10.1590/0004-282X20150046. [DOI] [PubMed] [Google Scholar]
- 37.Liang Z.R., Li K.Y., Zhang Y.J., Liang W.W., Yao H., Cai H.R., et al. Pathogens and influencing factors of severe pneumonia in patients with ischemic stroke. Chin. J. Nosocomiol. 2019;29(11):1656–1659. [Google Scholar]
- 38.Wang W.L., Duan H.P., Liu B.Y., Li T., Wang Y.M. Pathogenic bacteria characteristics and risk factors analysis of acute cerebral stroke patients with pulmonary infection. Chin. J. Nosocomiol. 2017;27(11):2465–2468. [Google Scholar]
- 39.Patel U.K., Kodumuri N., Dave M., Lekshminarayanan A., Khan N., Kavi T., et al. Stroke-associated pneumonia: a retrospective study of risk factors and outcomes. Neurol. 2020;25(3):39–48. doi: 10.1097/NRL.0000000000000269. [DOI] [PubMed] [Google Scholar]
- 40.Hu Y., Yu C., Guo Y., Bian Z., Han Y., Yang L., et al. Pneumonia hospitalizations and the subsequent risk of incident ischaemic cardiovascular disease in Chinese adults. Int. J. Epidemiol. 2021;50(5):1698–1707. doi: 10.1093/ije/dyab039. [DOI] [PubMed] [Google Scholar]
- 41.Zheng L., Wen L., Lei W., Ning Z. Vol. 100. 2021. Added value of systemic inflammation markers in predicting pulmonary infection in stroke patients: a retrospective study by machine learning analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.