Abstract
Chronic hepatitis B (CHB) infection remains a serious public health problem worldwide; however, the relationship between cholesterol levels and CHB remains unclear. We isolated peripheral blood mononuclear cells from healthy blood donors and CHB patients to analyze free cholesterol levels, lipid raft formation, and cholesterol metabolism-related pathways. Hepatitis B virus (HBV)-carrier mice were generated and used to confirm changes in cholesterol metabolism and cell-surface lipid raft formation in dendritic cells (DCs) in the context of CHB. Additionally, HBV-carrier mice were immunized with a recombinant HBV vaccine (rHBVvac) combined with lipophilic statins and evaluated for vaccine efficacy against HBV. Serum samples were analyzed for HBsAg, anti-HBs, and alanine aminotransferase levels, and liver samples were evaluated for HBV DNA and RNA and HBcAg. CHB reduced free cholesterol levels and suppressed lipid raft formation on DCs in patients with CHB and HBV-carrier mice, whereas administration of lipophilic statins promoted free cholesterol accumulation and restored lipid rafts on DCs accompanied by an enhanced antigen-presentation ability in vitro and in vivo. Cholesterol accumulation on DCs improved the rHBVvac-mediated elimination of serum HBV DNA and intrahepatic HBV DNA, HBV RNA, and HBcAg and promoted the rHBVvac-mediated generation and polyfunctionality of HBV-specific CD11ahi CD8αlo cells, induction of the development of memory responses against HBV reinfection, and seroconversion from HBsAg to anti-HBs. The results demonstrated the important role of cholesterol levels in DC dysfunction during CHB, suggesting that strategies to increase cholesterol accumulation on DCs might enhance therapeutic vaccine efficacy against HBV and support development toward clinical CHB treatment.
Keywords: Chronic hepatitis B, Dendritic cells, Cholesterol metabolism, Therapeutic vaccine
Subject terms: Hepatitis B, Immunization, Conventional dendritic cells
Introduction
Chronic hepatitis B (CHB) infection remains a serious public health problem worldwide. Patients with CHB have a higher risk of progressing to liver fibrosis, cirrhosis, and hepatocellular carcinoma (HCC) [1]. Despite numerous active drug and antiviral treatments, such as nucleoside/nucleotide-based therapy and interferon (IFN) therapy, the clinical management of CHB remains challenging owing to the failure to achieve full virologic suppression, a long treatment cycle, adverse side effects, and hepatitis B virus (HBV) recurrence during treatment [1, 2]. As a result, there remains an urgent need to develop novel strategies to clear HBV and achieve long-lasting immune control. Therefore, understanding the immunological mechanisms involved in CHB is important for developing potential therapeutic strategies.
CHB is difficult to cure mainly because of the immunosuppressive microenvironment formed in the liver of patients with CHB with a high proportion of regulatory T cells, myeloid-derived suppressor cells, and dysfunctional antigen-presenting cells (APCs) [3]. As professional APCs, dendritic cells (DCs) represent a link between innate and adaptive immune responses [4]. Previous studies have reported that DCs from CHB patients display impaired antigen-presenting and migratory capacities relative to those from healthy donors (HDs) [5]. Additionally, CHB can induce an incompetent DC phenotype characterized by downregulated costimulatory molecules and upregulated B7-H1 (programmed death-ligand 1; PD-L1) expression [6]. The functional impairment in DCs during CHB facilitates the exhaustion of HBV-specific T cells characterized by coexpression of inhibitory receptors, poor effector cytotoxic activity, altered transcription profiles, and metabolic impairment, leading to persistent infection and the progression of liver diseases in CHB patients [5, 7, 8]. However, the mechanisms involved in DC dysfunction induced by CHB have not been fully clarified.
Cholesterol is an indispensable lipid molecule that plays important roles in multiple biological processes, including the formation of lipid rafts, major histocompatibility complex (MHC) molecules, T-cell receptors (TCRs), B-cell receptors, and Toll-like receptors [9, 10]. The cholesterol level in immune cells reflects the dynamic balance among biosynthesis, uptake, efflux, and esterification. The key regulators of cholesterol biosynthesis include sterol regulatory element-binding protein 2 (SREBP2) and two rate-limiting enzymes [3-hydroxy-3-methylglutaryl coenzyme A reductase (HMGCR) and squalene epoxidase (SQLE)]. Low-density lipoprotein receptor (LDLR) is the dominant player responsible for cholesterol uptake from the circulation by peripheral immune cells. LDLR captures circulating LDL by endocytosis, after which LDL-carried cholesteryl esters gradually progress into free cholesterol through the endo-lysosomal system. Cholesterol efflux is mediated by ATP-binding cassette (ABC) transporters, including ABC subfamily A member 1 (ABCA1) and ABC subfamily G members 1/5/8, which contribute to the disposal of excess cholesterol from cells or excess cholesterol storage as cholesteryl esters [11, 12]. Esterification converts free cholesterol into neutral cholesteryl esters via acyl-CoA:cholesterol acyltransferase (ACAT; including ACAT1 and ACAT2) for either storage in lipid droplets or use as lipoprotein constituents to ensure cholesterol homeostasis [12]. A previous study showed that inhibiting cholesterol esterification reduced the accumulation of neutral lipid droplets by driving the translocation of esterified cholesterol to the plasma membrane [13, 14]. Free cholesterol in the plasma membrane is a critical structural component of lipid rafts, which can be identified by the presence of cholera toxin B subunit (CTxB). Lipid rafts act as cholesterol-enriched specialized microdomains and play crucial roles in membrane trafficking and signal transduction, as well as modulation of immune cell phenotypes and functions [12, 15, 16]. For example, enriched free cholesterol in the plasma membrane of CD8+ T cells enhances TCR clustering and the formation of immunological synapses accompanied by enhanced T-cell proliferation and functional effects [14]. Additionally, cholesterol accumulation promotes lipid raft formation and immune-signaling activation in natural killer (NK) cells, which in turn promote NK-cell effector functions against hepatoma cells [11]. In macrophages, cholesterol accumulation induces the activation of NOD-, LRR- and pyrin-domain-containing protein 3 (NLRP3) inflammasomes and the MyD88-mediated signaling pathway, which contributes to atherosclerosis progression [17, 18]. Moreover, cholesterol-enriched lipid rafts promote the accumulation of antigenic peptide–MHC class II complexes on APCs, which facilitates T-cell activation in vitro [19, 20]. Furthermore, hypercholesterolemia-induced cholesterol overload in CD11c+ DCs enhances the antigen presentation and T-cell priming abilities of these cells and promotes B-cell hyperplasia, thereby increasing the risk of autoimmune diseases [21–23]. These findings confirm the essential role of cholesterol in regulating immune cell activation; however, it remains unclear whether CHB affects cholesterol levels on DCs and subsequent dysfunction of DC-mediated anti-HBV immune responses.
In this study, we investigated the relationship between cholesterol levels and CHB. The results indicated that CHB reduced free cholesterol levels and lipid raft formation on DCs, thereby contributing to HBV persistence. The findings suggest that strategies to increase cholesterol accumulation on DCs might enhance therapeutic vaccine efficacy for clinical CHB treatment.
Materials and methods
Patient samples
Peripheral blood samples from patients with immune-tolerant CHB and HDs were collected at Shandong Provincial Hospital. This study was approved by the Institutional Review Board of Shandong Provincial Hospital (approval number: SWYX: NO. 2021-503) and conducted in accordance with the principles of the Declaration of Helsinki. Written informed consent was obtained from all study participants. All patients with immune-tolerant CHB conformed to the following inclusion criteria: aged between 18 and 65 years and HBV DNA > 2 × 104 IU/mL for hepatitis B e antigen (HBeAg)-positive patients without prior antiviral therapy. The exclusion criteria were liver disease of other etiologies [HCV infection, hepatitis D virus (HDV) infection, alcoholic liver disease, nonalcoholic fatty liver disease, primary biliary cholangitis, autoimmune hepatitis, hereditary metabolic liver disease, etc.], decompensated cirrhosis, HCC due to HBV or other etiologies, major systematic diseases, and other malignancies. The clinical characteristics of the patients with immune-tolerant CHB are shown in Supplementary Table 1.
Animals and reagents
Male C57BL/6J mice (5–6 weeks old) were obtained from Beijing HFK Bioscience Co., Ltd. (Beijing, China). B6.FVB-Tg (Tgax-DTR/EGFP) 57Lan/J mice (CD11c-DTR mice) were kindly gifted by Second Military University (Shanghai, China). All animal experiments were performed in accordance with the Guidelines for the Care and Use of Laboratory Animals and the Ethical Committee of Shandong University and using protocols approved by the Institutional Animal Care and Use Committee of Shandong University (approval number: 20023). A recombinant HBV vaccine (rHBVvac; Hansenula polymorpha) and hepatitis B surface antigen (HBsAg; H. polymorpha) were purchased from Dalian Hissen Bio-pharm. Co., Ltd. (Dalian, China). Recombinant hepatitis B e antigen (HBeAg) was purchased from Beijing Key-bio Biotech. Co., Ltd. (Beijing, China). Simvastatin and lovastatin were purchased from Sigma‒Aldrich (Shanghai, China). Alhydrogel® adjuvant 2% was purchased from InvivoGen (San Diego, CA, USA, Mainland). Recombinant adeno-associated virus (rAAV)8-HBV1.3 vectors containing 1.3-fold HBV genomes (genotype D, subtype ayw) were purchased from the Beijing FivePlus Molecular Medicine Institute (Beijing, China).
HBV mouse model
An HBV mouse model was generated through hydrodynamic injection of a volume of saline (equivalent to 10% body weight) containing 8 μg pAAV/HBV 1.2 plasmid (kindly provided by Pei-Jer Chen; National Taiwan University College of Medicine, Taipei, Taiwan) or intravenous injection of 1 × 1010 vector genome equivalent of rAAV8-HBV1.3, as previously described [24, 25]. At 6 weeks post-injection, serum HBsAg was detected, and mice with HBsAg levels >500 ng/mL (1000 ng/mL = 1.14 IU/mL) were defined as HBV-carrier mice.
Immunization protocol and HBV rechallenge
HBV-carrier mice (pAAV-HBV1.2) were divided into seven groups and immunized subcutaneously with phosphate-buffered saline (PBS), 2 μg rHBVvac (rHBVvac), 100 μg Alhydrogel 2% (alum), 100 μg simvastatin (Sim), 100 μg lovastatin (Lov), 2 μg rHBVvac combined with 100 μg simvastatin (Sim + rHBV), or 2 μg rHBVvac combined with 100 μg lovastatin (Lov + rHBV) weekly for 3 weeks (on Days 1, 8, and 15) (online Supplementary Fig. 1). Blood samples were obtained weekly from the lateral tail vein. For a memory response assay, immunized HBV-carrier mice were rechallenged with a hydrodynamic injection of 8 μg pAAV/HBV1.2 on Day 53 after treatment initiation. To confirm the anti-HBV effects of “Sim+rHBV” and “Lov+rHBV” in the rAAV8-HBV1.3 mouse model, mice were immunized subcutaneously with PBS, Sim+rHBV or Lov+rHBV weekly for 3 weeks (on Days 1, 8, and 15). To confirm the role of the HBV-specific T-cell response in anti-HBV effects, HBV-carrier mice were immunized subcutaneously with PBS or Sim+rHBV weekly for 3 weeks, and depleting antibodies against CD4 (BioXCell, clone: GK1.5) and CD8 (BioXCell, clone: 53–6.7) were administered by intraperitoneal injection (0.25 mg/mouse) on Days −1, 1, 6, and 13 between the HBV vaccination protocols (online Supplementary Fig. 1).
In vitro bone marrow-derived DC (BMDC) generation and associated functional assay
Murine BMDCs were generated as previously described [24, 26], and BMDCs were identified and analyzed to determine the percentage of CD11c+ cells by flow cytometry (>90%). To assess their antigen-presenting ability, BMDCs were loaded with 10 μg/mL fluorescein isothiocyanate-conjugated bovine serum albumin (FITC–BSA) and then incubated with Sim or Lov for 24 h or 36 h, after which the levels of FITC, CD86 costimulatory molecules, and MHC class II molecules on the BMDCs were evaluated using a BD FACSCelesta system (BD Biosciences, Franklin Lakes, NJ, USA). To assess the cholesterol and lipid raft levels on BMDCs in CHB, BMDCs were incubated with plasma from HDs or patients with CHB, treated with anti-HBs (Invitrogen) or left untreated, or treated with different doses of HBsAg and HBeAg for 36 h. The levels of cholesterol (Filipin III) and lipid rafts (CTxB) were then evaluated using a BD FACSCelesta system (BD Biosciences, Franklin Lakes, NJ, USA).
RNA-seq analysis
BMDCs were stimulated with 10 μg/mL Sim or Lov for 12 h, and total RNA was extracted by TRIzol reagent (Invitrogen, Carlsbad, CA, USA). Sequencing libraries were generated using a TruSeq RNA sample preparation kit (Illumina, San Diego, CA, USA) and sequenced on a HiSeq X platform (Illumina) by Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China).
Knockdown of LDLR by siRNA in vitro
PBMCs harvested from CHB patients were transfected with small-interfering RNA targeting LDLR (RiboBio, Guangzhou, China) for 24 h, followed by treatment with Sim (10 μg/mL) and cholesterol (10 μg/mL) for another 24 h. Then, the LDLR and cholesterol (Filipin III) levels on CD11c+ DCs were evaluated using flow cytometry.
Plasma biochemical analysis
Plasma was harvested from patients with CHB and HDs, and the levels of cholesterol (CHOL), high-density lipoprotein (HDL-C), low-density
lipoprotein (LDL-C) and triglycerides (TG) were measured using commercially available enzymatic kits (Medicalsystem, Ningbo, China) with a Beckman Coulter Olympus AU480 Chemistry Analyzer according to the manufacturer’s instructions.
Quantitative polymerase chain reaction (qPCR) analysis of cholesterol metabolism-related pathways in peripheral blood mononuclear cells (PBMCs) and BMDCs
Human PBMCs were isolated from the peripheral blood of healthy blood donors and patients with CHB using Ficoll–Paque PLUS (Solarbio, Beijing, China). Total RNA was isolated from human PBMCs and mouse BMDCs by TRIzol reagent (Invitrogen), and real-time PCR was performed on a LightCycler 96 system (Roche, Basel, Switzerland) using UltraSYBR reagent (CW Biotech, Beijing, China). The PCR primers are shown in Supplementary Table 2.
Confocal microscopy
Mouse BMDCs were treated with 10 μg/mL Sim or Lov for 24 h, followed by incubation on poly-L-lysine-coated coverslips, fixation with 4% formaldehyde, and staining with Filipin III (Cayman Chemical, Ann Arbor, MI, USA) at 37 °C for 1 h and FITC-conjugated CTxB (Sigma‒Aldrich) at 4 °C for 30 min in the dark. The cells were then washed with PBS and examined using a microscope (Carl Zeiss AG, Jena, Germany). Confocal images were acquired and analyzed under a Zeiss LSM 900 with Airyscan 2 software (Carl Zeiss, Germany).
Serum HBsAg, anti-HBs, and alanine aminotransferase (ALT) detection
Specific enzyme-linked immunosorbent assay (ELISA) kits for HBsAg (Autobio, Zhengzhou, China) and anti-HBs (Wantai Bio-pharm, Beijing, China) were used according to the manufacturer’s instructions. Serum ALT levels were measured using a commercially available kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Immunohistochemistry (IHC)
HBcAg in liver tissue was stained with an anti-HBcAg monoclonal antibody (dilution: 1:500; #GB058604, Gene Tech Co., Ltd., Shanghai, China) and then incubated with horseradish peroxidase-conjugated goat anti-mouse IgG (#ZB-2305; ZSGB-Bio Co., Ltd., Beijing, China).
HBV DNA and HBV RNA detection
Serum HBV DNA was measured by qPCR using an HBV DNA kit (Da An Gene, Guangzhou, China). Intrahepatic HBV genomic DNA was extracted using a gDNA kit (Tiangen Biotech, Beijing, China). Total RNA was extracted from livers from HBV-carrier mice using TRIzol reagent (Invitrogen), and RNA was reverse transcribed into cDNA using a cDNA synthesis kit (CW Biotech). Real-time PCR for HBV DNA and RNA was performed on a LightCycler 96 system (Roche) using UltraSYBR reagent (CW Biotech), as previously described [24, 26]. The PCR primers are shown in Supplementary Table 3.
Mononuclear cell isolation
Mononuclear cells (MNCs) were isolated from the liver, spleen, draining lymph node (dLN), and peripheral blood as previously described [24, 26]. Briefly, liver tissue was passed through a 200-μm nylon cell strainer, followed by centrifugation at 100 × g for 1 min to remove the hepatocytes. The single-cell suspension was then centrifuged at 400 × g for 10 min, and the residual cells were collected and layered over 40% Percoll (GE Healthcare, Uppsala, Sweden). Hepatic MNCs were obtained after centrifugation at 800 × g for 25 min, followed by red blood cell (RBC) lysis and washing with 1× PBS. The spleen was passed through a 200-μm nylon cell strainer, and splenic MNCs were obtained after RBC lysis and washing. The dLN was passed through a 200-μm nylon cell strainer, and LN MNCs were obtained after washing with 1× PBS. PBMCs were collected in heparinized tubes and obtained after RBC lysis and washing with 1× PBS.
Analysis of intracellular cytokines
For analysis of intracellular interleukin (IL)-2, IFN-γ, and tumor necrosis factor (TNF)-α, 2 × 106 hepatic MNCs were stimulated with 30 ng/mL phorbol 12-myristate 13-acetate (PMA; Beyotime, Shanghai, China) and 1 μg/mL ionomycin (Beyotime) in the presence of IL-2 (100 U/mL; Changchun Institute of Biological Products Co., Ltd., Changchun, China) and brefeldin A (BFA, 5 μg/mL; BioLegend, San Diego, CA, USA) for 4 h at 37 °C. Data were collected using a BD FACSymphony A3 or BD FACSCelesta system (BD Biosciences) and analyzed with FlowJo software (FlowJo, LLC, Ashland, OR, USA). The antibodies are shown in Supplementary Table 4.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software (v6.0; GraphPad Software, La Jolla, CA, USA). An unpaired Student’s t-test was applied for comparisons between two groups, and differences among multiple groups were analyzed by one-way analysis of variance. Differences achieving p < 0.05 were considered statistically significant. All further materials and methods can be found in the online Supplementary Materials and Methods.
Results
CHB decreases cholesterol levels and impairs lipid raft formation on DCs
To clarify whether CHB affects cholesterol metabolism in DCs and lipid raft formation on DCs, we isolated PBMCs from healthy donors and patients with immune-tolerant CHB. Figure 1A shows significantly reduced levels of free cholesterol (Filipin III), lipid rafts (CTxB), and LDLR on DCs among PBMCs isolated from immune-tolerant CHB patients relative to those from HDs (online Supplementary Fig. 2A). Additionally, we found significant positive correlations between the levels of free cholesterol and those of lipid rafts or LDLR on DCs (Fig. 1B); however, CHB did not affect the free cholesterol levels on CD4+ or CD8+ T cells or NK cells (Fig. 1C). To confirm the effect of CHB on cholesterol metabolism in immune cells, we detected the expression of genes associated with cholesterol metabolism in PBMCs. Compared with HDs, patients with CHB exhibited clearly decreased mRNA levels of genes associated with cholesterol biosynthesis, such as HMGCR, 3-hydroxy-3-methylglutaryl-CoA synthase 1 (HMGCS1), and SQLE, but did not show effects on SREBF2 or the cholesterol-efflux gene ABCA1 (Fig. 1D); these changes were accompanied by increased plasma LCAT1 levels (Fig. 1E).
Fig. 1.
CHB decreases cholesterol levels and impairs lipid raft formation on DCs. A PBMCs were harvested from patients with immune-tolerant CHB and healthy donors (HDs) for flow cytometric analysis of cholesterol (Filipin III), lipid raft (CTxB), and LDLR levels on CD11c+ DCs. B Correlation analysis between cholesterol (Filipin III) and lipid raft (CTxB) levels or LDLR levels on CD11c+ DCs among PBMCs. C Cholesterol (Filipin III) levels on CD4+ and CD8+ T cells and NK cells. D Analysis of the mRNA levels of genes associated with cholesterol metabolism in PBMCs by qPCR with normalization against GAPDH levels. E Plasma was harvested from CHB patients and healthy controls, and LCAT1 levels were analyzed by ELISA. Data represent the mean ± SEM (n ≥ 9). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control. GAPDH glyceraldehyde 3-phosphate dehydrogenase, SEM standard error of the mean
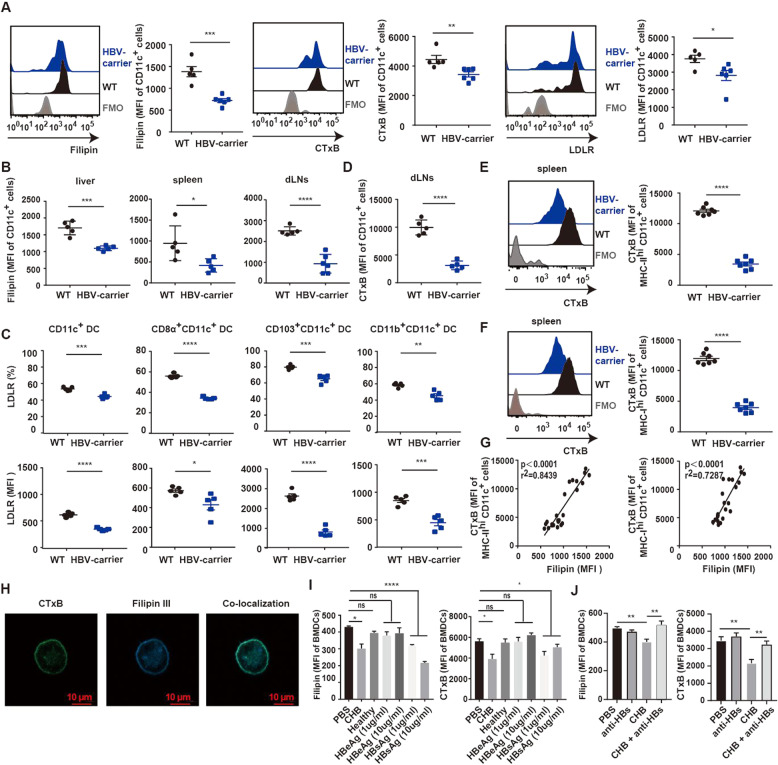
We then used the HBV-carrier mouse model to confirm these findings. Consistently, we found that HBV-carrier mice showed decreased cholesterol levels on DCs from PBMCs compared to DCs from WT mice, accompanied by significantly downregulated levels of lipid rafts and LDLR (Fig. 2A, online Supplementary Fig. 2B). The magnitude of the reduction in the cholesterol level on DCs was more significant than that for the levels on T cells and NK cells (online Supplementary Fig. 3). A similar phenomenon was shown for DCs from the liver, spleen, and dLNs, and the same results were observed for type 1 conventional DC (cDC1) subsets (Fig. 2B–F, online Supplementary Fig. 2C). Additionally, we found a positive correlation between cholesterol and lipid raft levels on DCs (Fig. 2G), with confocal microscopy revealing the colocalization of lipid rafts with membrane cholesterol in BMDCs (Fig. 2H). To elucidate the HBV components contributing to this phenomenon, we incubated mouse BMDCs with plasma from patients with CHB or HDs or different doses of HBsAg and HBeAg. The results showed that plasma derived from patients with CHB reduced cholesterol levels and lipid raft formation, with HBsAg exerting similar inhibitory effects, whereas neither healthy plasma nor HBeAg treatment resulted in these changes (Fig. 2I). Additionally, HBsAg depletion recovered the cholesterol and lipid raft levels in BMDCs treated with CHB plasma (Fig. 2J), indicating that HBsAg might be the major factor responsible for this effect. These results suggested that CHB decreased cholesterol levels on DCs, resulting in impaired lipid raft formation.
Fig. 2.
CHB decreases cholesterol levels and lipid raft formation on DCs in HBV-carrier mice. A–F HBV-carrier mice were screened 6 weeks after hydrodynamic injection of pAAV/HBV 1.2. A PBMCs from HBV-carrier mice and wild-type mice were harvested for flow cytometric analysis of cholesterol (Filipin III), lipid raft (CTxB), and LDLR levels on CD11c+ DCs. B MNCs from the liver, spleen and dLNs were harvested from HBV-carrier mice, and cholesterol (Filipin III) levels on CD11c+ cells were analyzed by flow cytometry. C Flow cytometric analysis of LDLR expression on CD11c+ DCs, CD8α+ CD11c+ DCs (cDC1s), CD103+ CD11c+ DCs (cDC1s), and CD11b+ CD11c+ DCs (cDC2s) in the spleen. D Flow cytometric analysis of lipid raft (CTxB) levels on CD11c+ cells from the dLNs. Flow cytometric analysis of lipid raft (CTxB) levels on E mature MHC class IIhi CD11c+ DCs and F MHC class Ihi CD11c+ DCs from the spleen. G Correlation analysis between cholesterol (Filipin III) and lipid raft (CTxB) levels on MHC class IIhi CD11c+ cells (left) and MHC class Ihi CD11c+ cells (right). H Following the generation of BMDCs, staining images were captured to visualize the colocalization of cholesterol (Filipin III, blue) and lipid rafts (CTxB, green) on BMDCs by confocal microscopy (magnification, 63×; scale bar, 10 µm). I BMDCs were incubated with plasma from CHB patients or healthy donors or with different doses of HBsAg and HBeAg for 36 h, followed by flow cytometric analysis of cholesterol (Filipin III, left) and lipid raft (CTxB, right) levels. J BMDCs were incubated with plasma from patients with CHB or with anti-HBs for 36 h, followed by flow cytometric analysis of cholesterol (Filipin III, left) and lipid raft (CTxB, right) levels. Data represent the mean ± SEM (n ≥ 5). *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control. SEM standard error of the mean
Enhanced cholesterol accumulation and lipid raft formation induced by lipophilic statins improves DC maturation
To confirm the roles of cholesterol metabolism in DC maturation and function, we administered lipophilic statins, including Sim and Lov, based on their previously demonstrated abilities to increase cholesterol synthesis and LDLR activity and reduce levels of cholesterol esters on hepatocytes [27, 28], neurons [29], and NK cells [30], suggesting an ability to elevate free cholesterol levels. RNA-seq analysis indicated that Sim or Lov administration upregulated the expression of key genes associated with cholesterol synthesis, transport, and absorption, whereas the expression of genes related to cholesterol efflux and esterification was downregulated in BMDCs (Fig. 3A). Consistently, qPCR confirmed that Sim or Lov treatment clearly increased the expression of cholesterol biosynthesis-related genes, such as SREBF2, HMGCS1, HMGCR, and SQLE, but suppressed the expression of the cholesterol efflux-related gene ABCA1, as well as that of inducible degrader of LDLR (Fig. 3B), which was accompanied by increased LDLR mRNA and protein levels (Fig. 3C, D). Moreover, compared with control BMDCs, BMDCs treated with Sim or Lov showed elevated cholesterol levels (Fig. 3E) and lipid raft formation (Fig. 3F), which could be further enhanced by the addition of exogenous cholesterol (Fig. 3G, H). Furthermore, we identified a positive correlation between cholesterol levels and lipid raft formation on BMDCs (Fig. 3I).
Fig. 3.
Cholesterol accumulation and lipid raft formation following administration of lipophilic statins promote DC maturation and antigen presentation. A–C BMDCs were treated with 10 μg/mL Sim or Lov for 12 h. A The heatmap shows genes related to cholesterol metabolism and demonstrates the significant variation in expression following Sim or Lov treatment relative to control treatment determined by RNA-seq analysis. B, C Analysis of the mRNA levels of genes associated with cholesterol metabolism in Sim- and Lov-treated BMDCs versus control cells by qPCR with normalization against GAPDH expression. BMDCs were treated with different doses of Sim or Lov for 24 h, and D flow cytometry was performed to analyze LDLR expression on the BMDCs. Flow cytometry and confocal microscopy were performed to analyze E cholesterol (Filipin III) and F lipid raft (CTxB) levels on BMDCs. BMDCs were stimulated with different doses of Sim or Lov with the addition of exogenous cholesterol (10 μg/mL) for 24 h, followed by flow cytometric analysis of G cholesterol (Filipin III) and H lipid raft (CTxB) levels on the BMDCs. I Correlation analysis of cholesterol (Filipin III) and lipid raft (CTxB) levels on BMDCs. J BMDCs were treated with 10 μg/mL Sim or Lov for 12 h. The heatmap showed genes related to DC and T-cell activation and migration and demonstrates the significant variation between treated BMDCs and control cells determined by RNA-seq analysis. K BMDCs were loaded with 10 μg/mL FITC–BSA protein and incubated with different doses of Sim or Lov for 24 h or 36 h, followed by flow cytometric analysis to detect phagocytosis of the labeled proteins in the BMDCs. BMDCs were incubated with different doses of Sim or Lov for 24 h or 36 h, followed by flow cytometric analysis of L MHC class II, M CD86, N CD83, and O CD80 expression on BMDCs. Data represent the mean ± SEM (n ≥ 5). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control. GAPDH glyceraldehyde 3-phosphate dehydrogenase, SEM standard error of the mean
Further analysis of the transcriptome of Sim- and Lov-treated BMDCs showed that among genes related to the activation and migration of DCs and T cells, immune system processes and response to stimulus were functionally enriched in these BMDCs relative to control BMDCs (Fig. 3J and online Supplementary Fig. 4A–C). These findings suggested that Sim or Lov might augment DC activation and function. Indeed, we found that Sim or Lov treatment enhanced the DC-mediated phagocytosis of FITC–BSA-labeled proteins (Fig. 3K) accompanied by elevated expression of MHC class II molecules, CD86, CD80, and CD83 on BMDCs (Fig. 3L–O and online Supplementary Fig. 4D). These results demonstrated that DC maturation and antigen presentation might be improved by lipophilic statin-induced cholesterol accumulation and lipid raft formation.
Cholesterol accumulation on DCs efficiently eliminates HBV in pAAV-HBV1.2 mice
DC maturation and antigen presentation determine the adaptive immune response to a pathogen during vaccination. Therefore, we determined whether lipophilic statins could enhance the adaptive immune response in HBV-carrier mice. We immunized HBV-carrier mice with rHBVvac combined with Sim (Sim + rHBV), rHBVvac combined with Lov (Lov + rHBV), Sim alone, Lov alone, or rHBVvac alone and then evaluated the HBV clearance efficiency. Compared with control mice, Sim+rHBV-vaccinated mice had significantly decreased serum HBsAg levels, with these levels being nearly undetectable on Day 28 after treatment initiation, and the therapeutic effects could be maintained for at least 52 days (Fig. 4A). Furthermore, we found low levels of serum HBV DNA (Fig. 4B) and intrahepatic HBcAg (Fig. 4C), HBV DNA, and HBV RNA, as well as low levels of HBV covalently closed circular DNA (cccDNA), after treatment (Fig. 4D). Interestingly, 50% (3/6) of Sim+rHBV-vaccinated mice achieved seroconversion from HBsAg to anti-HBs (Fig. 4E) but showed no significant mononuclear cell infiltration or liver damage during the treatment cycle (online Supplementary Fig. 5). Consistently, we found the same phenomena in Lov+rHBV-vaccinated mice (Fig. 4A–E, Supplemental Fig. 5), and the anti-HBs seroconversion rate was approximately 28.57% (2/7). Notably, Sim+rHBV-triggered HBV clearance was significantly attenuated in HBV-carrier CD11c–DTR mice treated with diphtheria toxin (Fig. 4F), confirming the role of DCs in Sim+rHBV- and Lov+rHBV-induced anti-HBV effects. However, monotherapy with rHBVvac, alum adjuvant, Sim or Lov alone was not sufficient to eliminate HBV in HBV-carrier mice (Fig. 4A, B, D, E).
Fig. 4.
Cholesterol accumulation on DCs eliminates HBV in pAAV-HBV1.2 mice. HBV-carrier mice were screened 6 weeks after hydrodynamic injection of pAAV/HBV 1.2, followed by subcutaneous immunization with rHBVvac (2 μg), Alhydrogel 2% (100 μg), Sim (100 μg), Lov (100 μg), Sim + rHBV or Lov+rHBV three times with 1-week intervals; control mice were administered PBS. A The relative levels of serum HBsAg in mice were detected by CLIA, B serum HBV DNA copy number was measured on Day 28 after treatment, and C HBcAg expression in hepatocytes on Day 28 post-immunization was determined by IHC staining (magnification, 200×; scale bar, 100 µm). D qPCR analysis of intrahepatic HBV intermediate product 3.5-kb RNA, HBV total RNA, HBV cccDNA, and HBV DNA was performed with normalization against GAPDH expression on Day 28 after treatment. The data are shown on a log10 scale. E Serum anti-HBs levels on Day 28 after treatment. F HBV-carrier-CD11c-DTR mice were intraperitoneally injected with diphtheria toxin (4 ng/g body weight) 24 h before treatment, followed by subcutaneous immunization with Sim+rHBV three times with 1-week intervals; control mice were administered PBS. Relative levels of serum HBsAg were detected by CLIA. Immunized mice were rechallenged with 8 μg pAAV/HBV 1.2 on Day 53 after treatment initiation, and G serum HBsAg and H anti-HBs levels were evaluated by CLIA and ELISA, respectively. I Intrahepatic HBV intermediate product 3.5-kb RNA, HBV total RNA, HBV cccDNA, and HBV DNA were analyzed by qPCR with normalization against GAPDH expression on Day 7 after rechallenge, and the data are shown on a log10 scale. Data represent the mean ± SEM (n ≥ 3). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. controls. GAPDH glyceraldehyde 3-phosphate dehydrogenase, SEM standard error of the mean
The major challenge in clinical CHB therapy is the high incidence of recurrence after therapy discontinuation. To identify whether vaccination can induce memory responses to protect against HBV reinfection, we rechallenged Sim+rHBV- and Lov+rHBV-vaccinated mice with HBV on Day 53 after treatment initiation. Compared with control mice, vaccinated mice had nearly undetectable serum HBsAg levels (Fig. 4G), and 75% of the mice generated high levels of protective anti-HBs (Fig. 4H). Additionally, we did not detect intrahepatic HBV cccDNA, HBV DNA, or HBV RNA in Sim+rHBV- and Lov+rHBV-vaccinated mice (Fig. 4I). These results suggested that the cholesterol accumulation initiated by lipophilic statins contributed to DC-triggered anti-HBV immune responses.
Cholesterol accumulation rescues lipid raft formation and reverses CHB-induced DC dysfunction
We then evaluated whether cholesterol levels and lipid raft formation on DCs were restored in Sim+rHBV- and Lov+rHBV-vaccinated mice. cDCs include two functionally distinct lineages: CD8α+ and CD103+ cDC1s and CD11b+ cDC2s [31, 32]. cDC1s are functionally specialized for antigen cross-presentation and are particularly important in the induction of CD8+ T-cell responses against pathogens, whereas cDC2s are mainly involved in eliciting T helper (Th)17- and Th2-mediated immune responses. We found that the CHB infection-induced decreases in cholesterol levels on intrahepatic (Fig. 5A) and splenic (Fig. 5B) DCs and cDC1s were reversed by Sim or Lov treatment, with the levels becoming similar to those in the wild-type control. Additionally, Sim+rHBV and Lov+rHBV vaccination both significantly upregulated LDLR activity (Fig. 5C) and lipid raft formation on DCs (Fig. 5D), accompanied by reductions in PD-L1 levels and elevations in MHC class I levels (Fig. 5E, F). Additionally, we observed similar phenomena for DCs and cDC1s in the dLNs (online Supplementary Fig. 6A–C), with the absolute numbers of CD11c+ DCs and cDC1s increased in the dLNs following Sim+rHBV or Lov+rHBV vaccination relative to control treatment (online Supplementary Fig. 6D).
Fig. 5.
Cholesterol accumulation rescues lipid raft formation and reverses CHB-induced DC dysfunction. MNCs from the liver and spleen were harvested from HBV-carrier mice on Day 28 after treatment, and cholesterol (Filipin III) levels on CD11c+ DCs, CD8α+ CD11c+ DCs (cDC1s), and CD103+ CD11c+ DCs (cDC1s) in the A liver and B spleen were analyzed by flow cytometry. C Flow cytometric analysis of LDLR expression on CD11c+ DCs, CD8α+ CD11c+ DCs (cDC1s), and CD103+ CD11c+ DCs (cDC1s) in the spleen. D Flow cytometric analysis of lipid raft (CTxB) levels on CD11c+ cells and PD-L1 and MHC class I molecules on E CD8α+ CD11c+ DCs (cDC1s) and F CD103+ CD11c+ DCs (cDC1s) in the spleen. G PBMCs harvested from CHB patients were treated with Sim (10 μg/mL) with (10 μg/mL) or without cholesterol for 24 h, followed by flow cytometric analysis of cholesterol (Filipin III) and lipid raft (CTxB) levels on CD11c+ DCs. PBMCs harvested from patients with CHB were transfected with small-interfering RNA targeting LDLR for 24 h (H), followed by treatment with Sim (10 μg/mL) and cholesterol (10 μg/mL) for another 24 h and I evaluation of cholesterol (Filipin III) levels on CD11c+ DCs by flow cytometry. Data represent the mean ± SEM (n ≥ 5). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control. SEM standard error of the mean
Given the importance of cDC2s in the production of anti-HBs during vaccination, we also detected changes in cDC2s. Interestingly, we found that Sim+rHBV and Lov+rHBV vaccination both increased the levels of cholesterol and LDLR on cDC2s (online Supplementary Fig. 7A–C), with further analysis also confirming increases in MHC class II molecules and a reduction in PD-L1 levels on cDC2s in the spleen and dLNs, which are vital in enhancing the humoral response (online Supplementary Fig. 7D, E). Furthermore, Sim or Lov alone was able to restore cholesterol levels in the context of reduced PD-L1 levels and increased MHC class I and II molecule expression (online Supplementary Fig. 8A–D). However, Sim or Lov alone showed no significant effect on the absolute numbers of DCs (online Supplementary Fig. 8E). Moreover, we detected significant elevations in cholesterol levels and lipid raft formation on CD11c+ DCs among PBMCs from patients with CHB following Sim treatment, which could be further enhanced by exogenous addition of cholesterol (Fig. 5G). However, we noted that among PBMCs from patients with CHB, LDLR knockdown (Fig. 5H) alleviated the Sim-induced increases in cholesterol on CD11c+ DCs mediated through exogenous cholesterol uptake pathways (Fig. 5I). These results showed that cholesterol accumulation on DCs could restore lipid raft formation and enhance DC function in HBV-carrier mice.
Cholesterol accumulation on DCs induces the generation and polyfunctionality of HBV-specific CD8+ T cells
We then determined whether cholesterol accumulation on DCs could trigger a T-cell response against HBV. Following Sim+rHBV or Lov+rHBV vaccination, we detected significant increases in the proportion and absolute number of HBV-specific CD11ahi CD8αlo cells in the liver (Fig. 6A and online Supplementary Fig. 9). Our previous studies showed that effector killer cell lectin-like receptor G1 (KLRG1)+ CD8+ T cells exhibited strong cytolytic function and anti-HBV effects and were dominant in HBV clearance and the memory response protecting against HBV reinfection [24]. Interestingly, in the present study, we observed that Sim+rHBV and Lov+rHBV vaccination both promoted the generation of KLRG1+ CD8+ T cells (Fig. 6B) and that the frequency of intrahepatic HBV-specific CD11ahi CD8αlo cells simultaneously expressing three immune checkpoint molecules [lymphocyte activating 3 (LAG3)+ programmed cell death protein-1 (PD-1)+ T cell immunoglobulin and mucin domain-containing protein-3 (TIM-3)+] was decreased in Sim+rHBV- and Lov+rHBV-vaccinated mice (Fig. 6C). Furthermore, Sim±rHBV and Lov±rHBV vaccination both induced a higher frequency of polyfunctional CD8± T cells simultaneously expressing two cytokines (IFN-γ+ IL-2+) than control treatment (Fig. 6D); this was accompanied by increased expression of CD107a (Fig. 6E, F). Moreover, Sim+rHBV-induced anti-HBV effects were significantly suppressed in the presence of an anti-CD4 antibody and completely eliminated in the presence of an anti-CD8 antibody (Fig. 6G). However, rHBVvac, Sim or Lov alone could not induce a significant HBV-specific CD8+ T-cell response (online Supplementary Fig. 10).
Fig. 6.
Cholesterol accumulation on DCs promotes the polyfunctionality of HBV-specific CD8+ T cells. MNCs from the liver and spleen were harvested from HBV-carrier mice on Day 28 after treatment. A The frequency and absolute numbers of CD11ahi CD8αlo cells in hepatic CD8+ T cells. B The expression of KLRG1 among HBV-specific CD11ahi CD8αlo cells in the spleen. C The ability of hepatic HBV-specific CD11ahi CD8αlo cells to express one (1+), two (2+) or three (3+) types of coinhibitory receptors (LAG3, TIM-3, and PD-1) was analyzed by flow cytometry. Each pie chart represents the proportion of each coinhibitory receptor population. D Hepatic MNCs (2 × 106) were stimulated with PMA/ionomycin in vitro for 4 h in the presence of BFA (5 μg/mL). The ability of HBV-specific CD11ahi CD8αlo cells to produce one (1+), two (2+), or three (3+) types of cytokines (IFN-γ, TNF-α, IL-2) was analyzed by flow cytometry. Each pie chart represents the proportion of each cytokine population. Expression of CD107a among HBV-specific CD11ahi CD8αlo cells from E liver or F spleen. G HBV-carrier mice were screened 6 weeks after hydrodynamic injection of pAAV/HBV 1.2, followed by subcutaneous immunization with Sim+rHBV three times with 1-week intervals; control mice were administered PBS. CD4+ T cells and CD8+ T cells were depleted with anti-CD4 and anti-CD8 antibodies, respectively, on Days -1, 1, 6, and 13 between the HBV vaccination protocols. The relative levels of serum HBsAg in mice were detected by CLIA. Data represent the mean ± SEM (n ≥ 5). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control mice. SEM standard error of the mean
A previous study reported that the rAAV8-HBV1.3 mouse model shows more efficient and homogeneous HBV transduction than models initiated by hydrodynamic injection [33]. Therefore, we validated the influence of cholesterol on DCs using the rAAV8-HBV1.3 mouse model. We found that Sim+rHBV- and Lov+rHBV-vaccinated mice demonstrated significantly decreased serum HBsAg levels relative to control mice (Fig. 7A), along with restored cholesterol levels and lipid raft formation on DCs (Fig. 7B, C). Additionally, Sim+rHBV and Lov+rHBV vaccination both promoted the generation and polyfunctionality of HBV-specific CD8+ T cells (Fig. 7D–G) in the rAAV8-HBV1.3 mouse model. These data suggested that cholesterol accumulation on DCs promoted the rHBVvac-induced generation and polyfunctionality of HBV-specific CD11ahi CD8αlo cells.
Fig. 7.
Cholesterol accumulation on DCs triggers an anti-HBV immune response in rAAV8-HBV1.3 mice. HBV-carrier mice were screened 6 weeks after intravenous injection of rAAV8-HBV1.3 vectors, followed by subcutaneous immunization with Sim+rHBV or Lov+rHBV three times with 1-week intervals; control mice were administered PBS. A Serum HBsAg levels detected by CLIA after treatment. MNCs were harvested from the liver, spleen, and dLNs of HBV-carrier mice on Day 21 after treatment, and B cholesterol (Filipin III) and C lipid raft (CTxB) levels on CD11c+ DCs from the liver and dLNs were analyzed by flow cytometry. D The frequency of CD11ahi CD8αlo cells among hepatic CD8+ T cells. The ability of HBV-specific CD11ahi CD8αlo cells from the E liver or F spleen to express one (1+), two (2+) or three (3+) types of coinhibitory receptors (LAG3, TIM-3, and PD-1) was analyzed by flow cytometry. Each pie chart represents the proportion of each coinhibitory receptor population. G Hepatic MNCs (2 × 106) were stimulated with PMA/ionomycin in vitro for 4 h in the presence of BFA (5 μg/mL). The ability of HBV-specific CD11ahi CD8αlo cells to produce one (1+), two (2+), or three (3+) types of cytokines (IFN-γ, TNF-α, IL-2) was analyzed by flow cytometry. Each pie chart represents the proportion of each cytokine population. Data represent the mean ± SEM (n ≥ 6). *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001 vs. control mice. CLIA chemiluminescent immunoassay, SEM standard error of the mean
Discussion
Cholesterol plays important structural and metabolic roles in cellular and systemic functions. A disturbed cholesterol balance is the main cause of cardiovascular disease, neurodegenerative diseases, autoimmune diseases, and cancers, as well as other diseases [12, 21, 22, 34]. Excessive cholesterol accumulation on tumor-infiltrating CD8+ T cells induces signaling related to the endoplasmic reticulum stress–X-box-binding protein 1 pathway, which in turn promotes immune checkpoint molecule expression and functional exhaustion of CD8+ T cells [35]. Another study showed that accelerating cholesterol uptake on LDLR-overexpressing NK cells elevated plasma membrane cholesterol levels and augmented NK-cell cytotoxicity and the antihepatoma response [11]. Additionally, cholesterol accumulation on DCs and macrophages improves antigen presentation, inflammatory cytokine secretion, and NLRP3 inflammasome activation, which in turn accelerates atherosclerosis and autoimmune diseases [21, 22, 36]. These findings suggest the variability in the cholesterol metabolism status in different immune cells and its involvement in disease progression; however, the role of cholesterol metabolism in CHB remains unknown. In the present study, we found that CHB was associated with reduced free cholesterol levels on DCs and impaired lipid raft formation. Further analysis showed no significant differences in CHOL, HDL-C, LDL-C, or TG levels between patients with CHB and healthy controls (online Supplementary Fig. 11). However, we found that the application of HBsAg or serum from patients with CHB was able to suppress free cholesterol levels and lipid raft formation on DCs but that depletion of HBsAg limited the CHB plasma-mediated effects on BMDCs, indicating the ability of HBsAg to decrease cholesterol biosynthesis and/or LDLR-mediated endocytosis in CHB-infected DCs. This might represent a new mechanism involved in CHB-induced immune tolerance.
Lipophilic statins (also known as mevalonate pathway inhibitors), such as Sim and Lov, exert regulatory effects on monocytes, DCs, and CD4+ T cells. Sim enhances a lipopolysaccharide-induced proinflammatory response in macrophages by regulating the c-Fos, c-Jun, and SREBP-cleavage-activating protein–SREBP2 complex [37, 38]. Additionally, Sim treatment induces the activation and proliferation of CD4+ T cells accompanied by increases in IL-6 secretion, which extends survival following lethal sepsis [39]. Notably, Sim downregulates PD-1 and 2B4 (CD244) transcription to restore exhausted CD8+ T cells [35]. Xia et al. demonstrated that lipophilic statins could be used as potent vaccine adjuvants to improve the immune responses to several antigens, such as human papillomavirus type 16 E7 and hemagglutinin A1 [40]. Moreover, statin administration inhibits cholesterol esterification and elevates LDLR expression on hepatocytes, thereby increasing lipoprotein levels [27, 29]. Importantly, long-term statin use is associated with a low risk of HCC development in patients with CHB [41, 42] and results in a sustained virologic response accompanied by high LDL expression [43], possibly due to DC activation following statin treatment. Interestingly, in the present study, we found a positive correlation between cholesterol and lipid raft levels on DCs from patients with CHB or mice, and combination of lipophilic statins with rHBVvac increased cholesterol accumulation and lipid raft formation on DCs in HBV-carrier mice, which in turn enhanced the antigen phagocytosis, antigen presentation, and activating activities of DCs in vivo and in vitro. The administration of lipophilic statins in the context of rHBVvac-triggered HBV clearance was significantly attenuated by the depletion of DCs, indicating that the anti-HBV effects depended on DCs that functionally recovered with lipophilic statin treatment. Therefore, we speculated that lipophilic statins promoted anti-HBV effects mainly by elevating cholesterol accumulation and lipid raft formation on DCs, not by interrupting the chain of virus infection and transmission to other cells, as previously described [44–46]. Additionally, we confirmed that Sim treatment restored free cholesterol levels on DCs from patients with CHB, which might explain the low risk of HCC development in patients with CHB following long-term statin use. Based on these findings, we employed HBV-carrier mice to investigate the role of cholesterol in DC-triggered anti-HBV immune responses in the context of treatment with Sim or Lov.
Importantly, we confirmed that Sim+rHBV- and Lov+rHBV-mediated HBV clearance were dependent on DC activation. There are two major types of cDCs: cDC1s and cDC2s [31, 32]. cDC1s expressing CD8α or CD103 are superior in antigen cross-presentation and activate CD8+ T cells against pathogens. We found that Sim or Lov treatment combined with rHBVvac restored cholesterol levels and lipid raft formation and induced cDC1 maturation and differentiation in the liver, spleen, and dLNs, accompanied by higher levels of MHC class I molecules and reductions in PD-L1 levels. Importantly, Sim or Lov administration enhanced the rHBVvac-induced generation and polyfunctionality of HBV-specific CD8+ T cells, thereby disrupting immune tolerance and augmenting HBV clearance in the pAAV-HBV1.2 and rAAV8-HBV1.3 mouse models. Additionally, the Sim+rHBV-induced anti-HBV effects were mainly dependent on antigen-specific T cells, especially CD8+ T cells. Furthermore, Sim+rHBV and Lov+rHBV treatment prevented the recurrence of HBV infection, which is currently a major challenge in clinical CHB therapy.
Elevated CD11a expression and downregulated CD8α expression on CD8+ T cells are widely used to track antigen-primed CD8+ T cells during pathogen infection or vaccination [24, 47]. HBV-specific CD8+ T-cell functions predict the efficacy of therapeutic vaccines against HBV; however, in CHB, HBV-specific T cells are generally exhausted and express multiple coinhibitory receptors, such as cytotoxic T lymphocyte-associated protein-4 (CTLA-4), PD-1, LAG3, and TIM-3, and exhibit impaired cytotoxic activity [48, 49]. Blockade of PD-1 and CTLA-4 can restore exhausted CD8+ T cells and improve their proliferation and cytokine secretion, thereby benefiting clinical CHB treatment [50, 51]. In the present study, we observed significant increases in HBV-specific CD11ahi CD8αlo cells following Sim+rHBV or Lov+rHBV vaccination, which were accompanied by downregulated expression of immune checkpoint molecules and increased generation of polyfunctional CD8+ T cells with elevated production of the proinflammatory cytokines IL-2, IFN-γ, and TNF-α and expression of the degranulation marker CD107a. Moreover, Sim+rHBV and Lov+rHBV vaccination both promoted the generation of KLRG1+ CD8+ T cells, which are dominant in effector and memory responses against HBV [24]. Previous studies have reported that increases in free cholesterol enhance lipid raft formation on CD8+ T cells and TCR signaling in these cells, accompanied by enhanced expansion and functionality [13, 14]. Similarly, in the present study, we found that Sim+rHBV and Lov+rHBV treatment both rescued free cholesterol levels on HBV-specific CD11ahi CD8αlo cells in HBV-carrier mice (online Supplementary Fig. 12). Given the elimination of HBV induced by Sim+rHBV or Lov+rHBV in the absence of elevated ALT levels, the therapeutic effects of these vaccination regimens might be mediated by noncytolytic CD8+ T-cell effects [24, 52].
cDC2s expressing CD11b favor the polarization of CD4+ T cells toward Th2 responses that contribute to the production of anti-HBs. We found that Sim+rHBV and Lov+rHBV treatment both ameliorated the CHB infection-induced decrease in cholesterol levels and activation of cDC2s and facilitated seroconversion to anti-HBs against HBV. Sim+rHBV-mediated HBV clearance was partly dependent on HBV-specific CD4+ T-cell responses in our study. A previous study showed that treatment with rHBVvac alone did not efficiently enhance DC activation [53] or induce the immunological effects induced by Sim+rHBV or Lov+rHBV. Notably, Sim or Lov alone restored cholesterol levels and affected the expression of PD-L1 and MHC class I and II molecules with a mature phenotype; however, Sim or Lov alone showed no significant effect on the absolute numbers of DCs and HBV-specific CD11ahi CD8αlo cells or on HBV clearance in HBV-carrier mice because of the absence of HBsAg in vivo. Bile acids play important roles in cholesterol homeostasis through the classical pathway and the acidic pathway and could also affect the function of DCs related to the development of autoimmune uveitis [54, 55]. Whether bile acids can affect the function of DCs and anti-HBV effects in CHB needs to be further confirmed.
In summary, we determined that CHB, especially the presence of HBsAg, reduced free cholesterol levels and suppressed lipid raft formation on DCs (Fig. 8). Interestingly, treatment with lipophilic statins promoted cholesterol accumulation and restored lipid raft formation on DCs, possibly due to the combined effects of changes in cellular cholesterol synthesis, esterification, and absorption. Significantly, cholesterol accumulation on DCs enhanced antigen presentation and triggered efficient anti-HBV immune responses accompanied by signs of development of memory responses that were protective against HBV rechallenge. Identifying novel strategies to promote DC functions to restore exhausted HBV-specific CD8+ T cells and improve their effector function could efficiently eliminate HBV and achieve a functional cure [24, 52, 56]. Given the importance of DCs in initiating and maintaining HBV-specific T-cell responses, these findings suggest that maintaining high levels of free cholesterol on DCs might promote lipid raft formation and thereby enhance antigen presentation and T-cell priming. In the present study, low-dose statins were administered subcutaneously for a short period, which could avoid the risk of the side effects previously reported in patients given long-term statin treatment [57–60]. These results highlight an important role for cholesterol in DC dysfunction during CHB and suggest a novel strategy for increasing therapeutic vaccine efficacy against HBV.
Fig. 8.

Schematic illustration of cholesterol accumulation on dendritic cells reversing chronic hepatitis B virus infection-induced dysfunction. CHB reduced the free cholesterol levels and suppressed lipid raft formation on DCs in patients with CHB and in HBV-carrier mice. Strategies to increase cholesterol accumulation on DCs could promote antigen presentation, thereby enhancing therapeutic vaccine efficacy and rescuing exhausted HBV-specific CD8+ T cells
Supplementary information
Acknowledgements
This work was supported by the National Key Research and Development Programme (2021YFC2300603), the National Postdoctoral Programme for Innovative Talents (No. BX20190192), the China Postdoctoral Science Foundation (No. 2020M672064), and the National Science Foundation for Young Scientists of China (No. 82001687). We thank the Pharmaceutical Biology Sharing Platform of Shandong University and the Translational Medicine Core Facility of Shandong University for consultation and instrument availability supporting this work.
Author contributions
JZ, ZGT, and HJZ conceived and designed the experiments; HJZ, YTY, YCW, LHZ, and ALY performed the experiments; HJZ, YFH, ZYP, ZXW, JRY, and QJH analyzed the data; JZ and HJZ wrote the manuscript, and all authors critically read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at 10.1038/s41423-022-00939-1.
References
- 1.Tang LSY, Covert E, Wilson E, Kottilil S. Chronic Hepatitis B infection: A review. JAMA. 2018;319:1802–13. doi: 10.1001/jama.2018.3795. [DOI] [PubMed] [Google Scholar]
- 2.Gill US, Bertoletti A. Clinical trial design for immune-based Therapy of Hepatitis B virus. Semin Liver Dis. 2017;37:85–94. doi: 10.1055/s-0037-1600522. [DOI] [PubMed] [Google Scholar]
- 3.Li TY, Yang Y, Zhou G, Tu ZK. Immune suppression in chronic hepatitis B infection associated liver disease: A review. World J Gastroenterol. 2019;25:3527–37. doi: 10.3748/wjg.v25.i27.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Novak N, Koch S, Allam JP, Bieber T. Dendritic cells: bridging innate and adaptive immunity in atopic dermatitis. J Allergy Clin Immunol. 2010;125:50–59. doi: 10.1016/j.jaci.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 5.Yonejima A, Mizukoshi E, Tamai T, Nakagawa H, Kitahara M, Yamashita T, et al. Characteristics of impaired dendritic cell function in patients with Hepatitis B virus infection. Hepatology. 2019;70:25–39. doi: 10.1002/hep.30637. [DOI] [PubMed] [Google Scholar]
- 6.Li M, Zhou ZH, Sun XH, Zhang X, Zhu XJ, Jin SG, et al. Hepatitis B core antigen upregulates B7-H1 on dendritic cells by activating the AKT/ERK/P38 pathway: a possible mechanism of hepatitis B virus persistence. Lab Invest. 2016;96:1156–64. doi: 10.1038/labinvest.2016.96. [DOI] [PubMed] [Google Scholar]
- 7.Fisicaro P, Barili V, Rossi M, Montali I, Vecchi A, Acerbi G, et al. Pathogenetic mechanisms of T cell dysfunction in chronic HBV infection and related therapeutic approaches. Front Immunol. 2020;11:849. doi: 10.3389/fimmu.2020.00849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Man K, Gabriel SS, Liao Y, Gloury R, Preston S, Henstridge DC, et al. Transcription Factor IRF4 promotes CD8(+) T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity. 2017;47:1129–41. doi: 10.1016/j.immuni.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Montixi C, Langlet C, Bernard AM, Thimonier J, Dubois C, Wurbel MA, et al. Engagement of T cell receptor triggers its recruitment to low-density detergent-insoluble membrane domains. EMBO J. 1998;17:5334–48. doi: 10.1093/emboj/17.18.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hiltbold EM, Poloso NJ, Roche PA. MHC class II-peptide complexes and APC lipid rafts accumulate at the immunological synapse. J Immunol. 2003;170:1329–38. doi: 10.4049/jimmunol.170.3.1329. [DOI] [PubMed] [Google Scholar]
- 11.Qin WH, Yang ZS, Li M, Chen Y, Zhao XF, Qin YY, et al. High serum levels of cholesterol increase antitumor functions of nature killer cells and reduce growth of liver tumors in mice. Gastroenterology. 2020;158:1713–27. doi: 10.1053/j.gastro.2020.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21:225–45. doi: 10.1038/s41580-019-0190-7. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt NM, Wing PAC, Diniz MO, Pallett LJ, Swadling L, Harris JM, et al. Targeting human Acyl-CoA:cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat Commun. 2021;12:2814. doi: 10.1038/s41467-021-22967-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–5. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23. doi: 10.1084/jem.20151570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norata GD, Caligiuri G, Chavakis T, Matarese G, Netea MG, Nicoletti A, et al. The cellular and molecular basis of translational immunometabolism. Immunity. 2015;43:421–34. doi: 10.1016/j.immuni.2015.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–61. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bjorkbacka H, Kunjathoor VV, Moore KJ, Koehn S, Ordija CM, Lee MA, et al. Reduced atherosclerosis in MyD88-null mice links elevated serum cholesterol levels to activation of innate immunity signaling pathways. Nat Med. 2004;10:416–21. doi: 10.1038/nm1008. [DOI] [PubMed] [Google Scholar]
- 19.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 20.Eren E, Yates J, Cwynarski K, Preston S, Dong R, Germain C, et al. Location of major histocompatibility complex class II molecules in rafts on dendritic cells enhances the efficiency of T-cell activation and proliferation. Scand J Immunol. 2006;63:7–16. doi: 10.1111/j.1365-3083.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito A, Hong C, Oka K, Salazar JV, Diehl C, Witztum JL, et al. Cholesterol accumulation in CD11c(+) immune cells is a causal and targetable factor in autoimmune disease. Immunity. 2016;45:1311–26. doi: 10.1016/j.immuni.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 2017;25:1294–304. doi: 10.1016/j.cmet.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonacina F, Coe D, Wang G, Longhi MP, Baragetti A, Moregola A, et al. Myeloid apolipoprotein E controls dendritic cell antigen presentation and T cell activation. Nat Commun. 2018;9:3083. doi: 10.1038/s41467-018-05322-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao HJ, Han QJ, Wang G, Lin A, Xu DQ, Wang YQ, et al. Poly I:C-based rHBVvac therapeutic vaccine eliminates HBV via generation of HBV-specific CD8(+) effector memory T cells. Gut. 2019;68:2032–43. doi: 10.1136/gutjnl-2017-315588. [DOI] [PubMed] [Google Scholar]
- 25.Bian Y, Zhang Z, Sun Z, Zhao J, Zhu D, Wang Y, et al. Vaccines targeting preS1 domain overcome immune tolerance in hepatitis B virus carrier mice. Hepatology. 2017;66:1067–82. doi: 10.1002/hep.29239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H, Wang H, Hu Y, Xu D, Yin C, Han Q, et al. Chitosan nanovaccines as efficient carrier adjuvant system for IL-12 with enhanced protection against HBV. Int J Nanomed. 2021;16:4913–28. doi: 10.2147/IJN.S317113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsimikas S, Gordts P, Nora C, Yeang C, Witztum JL. Statin therapy increases lipoprotein(a) levels. Eur Heart J. 2020;41:2275–84. doi: 10.1093/eurheartj/ehz310. [DOI] [PubMed] [Google Scholar]
- 28.Schonewille M, de Boer JF, Mele L, Wolters H, Bloks VW, Wolters JC, et al. Statins increase hepatic cholesterol synthesis and stimulate fecal cholesterol elimination in mice. J Lipid Res. 2016;57:1455–64. doi: 10.1194/jlr.M067488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Kant R, Langness VF, Herrera CM, Williams DA, Fong LK, Leestemaker Y, et al. Cholesterol metabolism is a druggable axis that independently regulates tau and amyloid-beta in iPSC-derived Alzheimer’s disease neurons. Cell Stem Cell. 2019;24:363–75. doi: 10.1016/j.stem.2018.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gong Y, Klein Wolterink RGJ, Janssen I, Groot AJ, Bos GMJ, Germeraad WTV. Rosuvastatin enhances VSV-G lentiviral transduction of NK Cells via upregulation of the low-density lipoprotein receptor. Mol Ther Methods Clin Dev. 2020;17:634–46. doi: 10.1016/j.omtm.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlitzer A, Sivakamasundari V, Chen J, Sumatoh HR, Schreuder J, Lum J, et al. Identification of cDC1- and cDC2-committed DC progenitors reveals early lineage priming at the common DC progenitor stage in the bone marrow. Nat Immunol. 2015;16:718–28. doi: 10.1038/ni.3200. [DOI] [PubMed] [Google Scholar]
- 32.Noubade R, Majri-Morrison S, Tarbell KV. Beyond cDC1: Emerging roles of DC crosstalk in cancer immunity. Front Immunol. 2019;10:1014. doi: 10.3389/fimmu.2019.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du Y, Broering R, Li X, Zhang X, Liu J, Yang D, et al. In vivo mouse models for hepatitis b virus infection and their application. Front Immunol. 2021;12:766534. doi: 10.3389/fimmu.2021.766534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai L, Zou L, Meng L, Qiang G, Yan M, Zhang Z. Cholesterol metabolism in neurodegenerative diseases: molecular mechanisms and therapeutic targets. Mol Neurobiol. 2021;58:2183–201. doi: 10.1007/s12035-020-02232-6. [DOI] [PubMed] [Google Scholar]
- 35.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–56 e145. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–16. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsumoto M, Einhaus D, Gold ES, Aderem A. Simvastatin augments lipopolysaccharide-induced proinflammatory responses in macrophages by differential regulation of the c-Fos and c-Jun transcription factors. J Immunol. 2004;172:7377–84. doi: 10.4049/jimmunol.172.12.7377. [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Chi Z, Jiang D, Xu T, Yu W, Wang Z, et al. Cholesterol homeostatic regulator SCAP-SREBP2 integrates NLRP3 Inflammasome activation and cholesterol biosynthetic signaling in macrophages. Immunity. 2018;49:842–56. doi: 10.1016/j.immuni.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 39.Braga Filho JAF, Abreu AG, Rios CEP, Trovao LO, Silva DLF, Cysne DN, et al. Prophylactic treatment with simvastatin modulates the immune response and increases animal survival following lethal sepsis infection. Front Immunol. 2018;9:2137. doi: 10.3389/fimmu.2018.02137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Y, Xie Y, Yu Z, Xiao H, Jiang G, Zhou X, et al. The mevalonate pathway is a druggable target for vaccine adjuvant discovery. Cell. 2018;175:1059–73. doi: 10.1016/j.cell.2018.08.070. [DOI] [PubMed] [Google Scholar]
- 41.Chang FM, Wang YP, Lang HC, Tsai CF, Hou MC, Lee FY, et al. Statins decrease the risk of decompensation in hepatitis B virus- and hepatitis C virus-related cirrhosis: A population-based study. Hepatology. 2017;66:896–907. doi: 10.1002/hep.29172. [DOI] [PubMed] [Google Scholar]
- 42.Goh MJ, Sinn DH, Kim S, Woo SY, Cho H, Kang W, et al. Statin use and the risk of hepatocellular carcinoma in patients with chronic Hepatitis B. Hepatology. 2020;71:2023–32. doi: 10.1002/hep.30973. [DOI] [PubMed] [Google Scholar]
- 43.Rao GA, Pandya PK. Statin therapy improves sustained virologic response among diabetic patients with chronic hepatitis C. Gastroenterology. 2011;140:144–52. doi: 10.1053/j.gastro.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 44.Gorabi AM, Kiaie N, Bianconi V, Jamialahmadi T, Al-Rasadi K, Johnston TP, et al. Antiviral effects of statins. Prog Lipid Res. 2020;79:101054. doi: 10.1016/j.plipres.2020.101054. [DOI] [PubMed] [Google Scholar]
- 45.Shrivastava-Ranjan P, Flint M, Bergeron É, McElroy AK, Chatterjee P, Albariño CG, et al. Statins suppress Ebola virus infectivity by interfering with glycoprotein processing. mBio. 2018;9:e00660–18.. doi: 10.1128/mBio.00660-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Latif RG, Mohammed S, Elgendy IY. Statin therapy and SAR-COV-2: an available and potential therapy? Eur Heart J Cardiovasc Pharmacother. 2020;6:333–4. doi: 10.1093/ehjcvp/pvaa050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai D, Pham NL, Harty JT, Badovinac VP. Tracking the total CD8 T cell response to infection reveals substantial discordance in magnitude and kinetics between inbred and outbred hosts. J Immunol. 2009;183:7672–81. doi: 10.4049/jimmunol.0902874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shih C, Chou SF, Yang CC, Huang JY, Choijilsuren G, Jhou RS. Control and eradication strategies of Hepatitis B virus. Trends Microbiol. 2016;24:739–49. doi: 10.1016/j.tim.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 49.Wherry EJ. T cell exhaustion. Nat Immunol. 2011;12:492–9. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 50.Cox MA, Nechanitzky R, Mak TW. Check point inhibitors as therapies for infectious diseases. Curr Opin Immunol. 2017;48:61–7. doi: 10.1016/j.coi.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 51.Miller BC, Sen DR, Al Abosy R, Bi K, Virkud YV, LaFleur MW, et al. Subsets of exhausted CD8(+) T cells differentially mediate tumor control and respond to checkpoint blockade. Nat Immunol. 2019;20:326–36. doi: 10.1038/s41590-019-0312-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X, Dong A, Xiao J, Zhou X, Mi H, Xu H, et al. Overcoming HBV immune tolerance to eliminate HBsAg-positive hepatocytes via pre-administration of GM-CSF as a novel adjuvant for a hepatitis B vaccine in HBV transgenic mice. Cell Mol Immunol. 2016;13:850–61. doi: 10.1038/cmi.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao H, Han Q, Yang A, Wang Y, Wang G, Lin A, et al. CpG-C ODN M362 as an immunoadjuvant for HBV therapeutic vaccine reverses the systemic tolerance against HBV. Int J Biol Sci. 2022;18:154–65. doi: 10.7150/ijbs.62424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahlström A, Sayin SI, Marschall HU, Bäckhed F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016;24:41–50. doi: 10.1016/j.cmet.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Hu J, Wang C, Huang X, Yi S, Pan S, Zhang Y, et al. Gut microbiota-mediated secondary bile acids regulate dendritic cells to attenuate autoimmune uveitis through TGR5 signaling. Cell Rep. 2021;36:109726. doi: 10.1016/j.celrep.2021.109726. [DOI] [PubMed] [Google Scholar]
- 56.Meng Z, Chen Y, Lu M. Advances in targeting the innate and adaptive immune systems to cure chronic Hepatitis B Virus infection. Front Immunol. 2019;10:3127. doi: 10.3389/fimmu.2019.03127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thompson PD. What to believe and do about statin-associated adverse effects. JAMA. 2016;316:1969–70. doi: 10.1001/jama.2016.16557. [DOI] [PubMed] [Google Scholar]
- 58.Gurevich VS, Shovman O, Slutzky L, Meroni PL, Shoenfeld Y. Statins and autoimmune diseases. Autoimmun Rev. 2005;4:123–9. doi: 10.1016/j.autrev.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 59.Noël B. Autoimmune disease and other potential side-effects of statins. Lancet. 2004;363:2000. doi: 10.1016/S0140-6736(04)16423-4. [DOI] [PubMed] [Google Scholar]
- 60.Moosmann B, Behl C. Selenoprotein synthesis and side-effects of statins. Lancet. 2004;363:892–4. doi: 10.1016/S0140-6736(04)15739-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.