Abstract
Listeria monocytogenes is a gram-positive, nonsporulating, food-borne pathogen of humans and animals that is able to invade many eukaryotic cells. Several listerial surface components have been reported to interact with eukaryotic cell receptors, but the complete mechanism by which the bacteria interact with all of these cell types remains largely unknown. In this work, we found that L. monocytogenes binds to human fibronectin, a 450,000-Da dimeric glycoprotein found in body fluids, on the surface of cells and in an insoluble component of the extracellular matrix. The binding of fibronectin to L. monocytogenes was found to be saturable and dependent on proteinaceous receptors. Five fibronectin-binding proteins of 55.3, 48.6, 46.7, 42.4, and 26.8 kDa were identified. The 55.3-kDa protein was proved to be present at the bacterial cell surface. The binding of L. monocytogenes to fibronectin adds to the number of molecules to which the bacterium is able to adhere and emphasizes the complexity of host-pathogen interactions.
Listeria monocytogenes, a gram-positive, nonsporulating, food-borne pathogen of humans and animals, is a facultative intracellular organism widely distributed in the environment. Human disease due to L. monocytogenes usually occurs in pregnant women, newborns, the elderly, and immunocompromised patients. Clinical manifestations range from mild flu-like symptoms and gastroenteritis to septicemia, central nervous system infections, and feto-maternal infections with abortion, premature labor, or birth of an infected child (5, 8, 12).
The pathogenicity of L. monocytogenes is due to its capacity to adhere to, invade, and multiply within a great variety of normally nonphagocytic cells (enterocytes, hepatocytes, fibroblasts, endothelial cells, and dendritic cells). In recent years, several listerial surface components have been reported to interact with these eukaryotic cells. Among the listerial molecules, the best characterized are the cell wall-anchored surface proteins internalin A (InlA) and internalin B (InlB). InlA is mainly responsible for adhesion to and invasion of enterocytes by binding to the host cell receptor E-cadherin, whereas InlB is mainly responsible for the uptake of the bacteria by hepatocytes and some epithelial or fibroblast cells by binding to an as yet unknown receptor. Binding of InlA and InlB to eukaryotic cells is followed by cytoskeletal rearrangement and internalization of the bacteria in a vacuole (2, 3, 18, 20). The proteins p60 and ActA were also reported to participate in the invasion of eukaryotic cells. The cell surface-associated protein p60 is a murein hydrolase whose activity is essential for bacterial septation. This protein is also implicated in the invasion of fibroblasts and hepatocytes (14, 16, 24, 30). ActA is a membrane-anchored protein involved in actin polymerization and in the intracellular bacterial motility leading to bacterial cell-to-cell spread. ActA was recently shown to bind the host cell heparan sulfate proteoglycans. This binding appears to play a role in the invasion of CHO epithelium-like cells (1). Apart from proteins, the bacterial surface polysaccharide α-d-galactose was also reported to interact with the human hepatocarcinoma cell line HepG2 and to play a role in the invasion of dendritic cells (4, 13). Although the cited molecules were proved to participate in the entry of L. monocytogenes into different mammalian cell types, mutants with deletions in adhesion protein genes or wild-type bacteria saturated with immunoglobulin (Ig) directed to these adhesion molecules were never completely impaired in their invasion capacity (1, 6, 7, 10, 13, 14, 20).
Due to the large pathogenic spectrum of L. monocytogenes (from mice to humans) and because of its capacity to invade many different cell types and tissues, it is very probable that some bacterial molecules are implicated in the recognition of components common to all of these numerous infected tissues. Fibronectin, collagen, laminin, proteoglycans, or other constituents of the extracellular matrix (ECM) are found in all eukaryotic cell tissues and are ubiquitously associated with the cell membrane. ECM components are thus ideal microbial adhesion targets that many intracellular and extracellular pathogens, including L. monocytogenes via its binding to heparan sulfate proteoglycans (1), have exploited for colonization of tissues and initiation of infection (9, 15, 19, 22, 23, 25, 28).
In this work, we studied the capacity of L. monocytogenes to adhere to fibronectin, a 450,000-Da dimeric glycoprotein found in body fluids, on the surface of cells, and in an insoluble component of the ECM.
Five previously described (11) nonclonal clinical isolates of L. monocytogenes (strains 90/636 [sv1/2a, esterase type IB], 90/187 [sv4b, esterase type IIC], 90/207 [sv1/2b, esterase type IIC], and 91/463 [sv1/2c, esterase type IIF]) were tested for binding to human fibronectin. L. monocytogenes cells grown overnight in brain heart infusion (BHI) broth were centrifuged at 4°C (10,000 × g), washed three times in phosphate-buffered saline (PBS), and resuspended in PBS to an optical density at 600 nm of 0.5. Multiwell microtiter plates (Nunc-Immunoplate, Maxisorp F 96; Nunc, Roskilde, Denmark) were then coated with 100 μl of this bacterial suspension (or 100 μl of PBS as a blank) per well, air dried overnight at 37°C, washed with PBS, and saturated (300 μl of 2% [wt/vol] bovine serum albumin-PBS solution per well; incubation for 2 h at 37°C). After washings with PBST (PBS containing 0.05% [vol/vol] Tween 20), a human fibronectin (catalog no. 688851; Boehringer, Mannheim, Germany) solution in PBST containing 0.2% (wt/vol) bovine serum albumin (catalog no. A-3803; Sigma, St. Louis, Mo.) was added at 100 μl per well. After incubation (2 h at 37°C), washings with PBST, and the addition of peroxidase-labelled rabbit anti-human fibronectin Igs (Dako, Copenhagen, Denmark) (100 μl of a 1/4,000 dilution in PBST containing 0.2% [wt/vol] bovine serum albumin per well) and incubation for 1.5 h at 37°C, excess reagent was removed by successive washings with PBST and PBS, and peroxidase substrate was added (100 μl [per well] of a 0.1 M sodium acetate buffer [pH 5.5] containing 3,3′,5,5′-tetramethylbenzidine at 0.1 mg/ml and 0.01% [vol/vol] H2O2) and the mixture was incubated for 10 to 30 min at room temperature in the dark. The reaction was stopped by the addition of 100 μl of 2 M H2SO4 per well, and the A450 of each well was measured in an enzyme-linked immunosorbent assay (ELISA) plate reader (Titertek MS 212; ICN, Costa Mesa, Calif.).
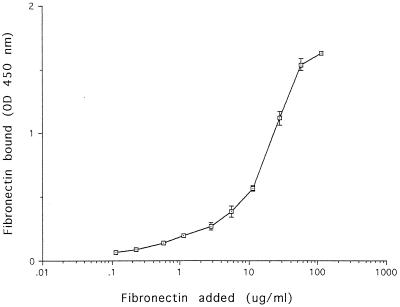
In Fig. 1, a typical result obtained with strain 90/636 is shown (similar results were obtained with the other four strains tested). Soluble fibronectin bound in a dose-related manner to the strains of L. monocytogenes tested and not to the bovine serum albumin used as a control. This suggested the presence of one or more saturable receptors for fibronectin on the cell surface. As similar results were obtained with all five of the strains tested, strain 90/636 was choosen for further experiments.
FIG. 1.
Binding of soluble fibronectin to L. monocytogenes. Intact L. monocytogenes 90/636 cells were immobilized on a microtiter plate and incubated with increasing concentrations of fibronectin. After washings, bacterial attachment of fibronectin was quantitated spectrophotometrically by an ELISA with peroxidase-labelled rabbit anti-human fibronectin Ig and tetramethylbenzidine as the substrate. Each square represents the average of three separate determinations after subtraction of the background value (0.082) obtained in the absence of added fibronectin. Blank values obtained when similar experiments were done in the absence of L. monocytogenes cells were lower than 0.06 optical density (OD) unit. Standard errors not comprised within squares are indicated by bars.
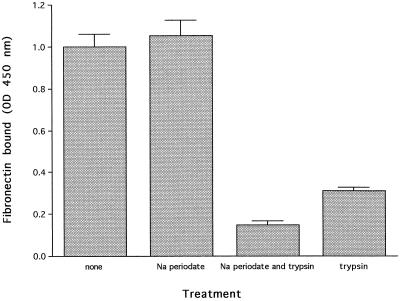
To investigate the chemical nature of the receptor(s), proteins or polysaccharides at the bacterial surface were removed by trypsin and mild periodate oxidation, respectively. Microtiter plates coated with L. monocytogenes (as described above) were then either incubated with PBS as a control (200 μl per well); with a solution containing trypsin (catalog no. T-8003; Sigma) at 10 mg/ml (wt/vol), 10 mM CaCl2, and 20 mM Tris · Cl (pH 7.4) (200 μl per well; incubation for 2.5 h at 37°C); or successively with 10 mM Na m-periodate in sodium acetate buffer (pH 4.5) (200 μl per well; incubation for 1 h at room temperature in the dark) and 50 mM sodium borohydride in PBS (200 μl per well; incubation for 30 min at room temperature) as described by Woodward et al. (29). After washings, some of the wells treated with Na m-periodate and sodium borohydride were incubated with the solution containing trypsin at 10 mg/ml, 10 mM CaCl2, and 20 mM Tris · Cl (pH 7.4) (200 μl per well; incubation for 2.5 h at 37°C). Plates were then washed with PBS and saturated (300 μl of 2% [wt/vol] bovine serum albumin-PBS solution per well; incubation for 2 h at 37°C). Binding of human fibronectin (fibronectin at 25 μg/ml [wt/vol] in PBST containing 0.2% [wt/vol] bovine serum albumin [100 μl per well]) was then assayed by ELISA as described above. Fibronectin binding to treated and untreated cells was then compared. Figure 2 shows that the binding of fibronectin to trypsin-treated cells was strongly reduced, indicating that the receptor(s) is in great part proteinaceous. Treatment of the cells with Na periodate did not affect the binding of fibronectin, but polysaccharides at the cell surface seem to protect some of the fibronectin-binding protein(s) as bacterial incubation with Na periodate before trypsinization further reduced fibronectin binding to L. monocytogenes cells.
FIG. 2.
Effect of trypsin hydrolysis and periodate oxidation of L. monocytogenes cells on the binding of soluble human fibronectin. Intact L. monocytogenes 90/636 cells immobilized on a microtiter plate were treated with PBS, Na periodate, trypsin, or both Na periodate and trypsin. After washings, the bacteria were incubated with fibronectin (25 μg/ml). The attachment of fibronectin was quantitated spectrophotometrically by an ELISA with peroxidase-labelled rabbit anti-human fibronectin and tetramethylbenzidine as the substrate. Binding data are presented as the means ± the standard deviations for six determinations. OD, optical density.
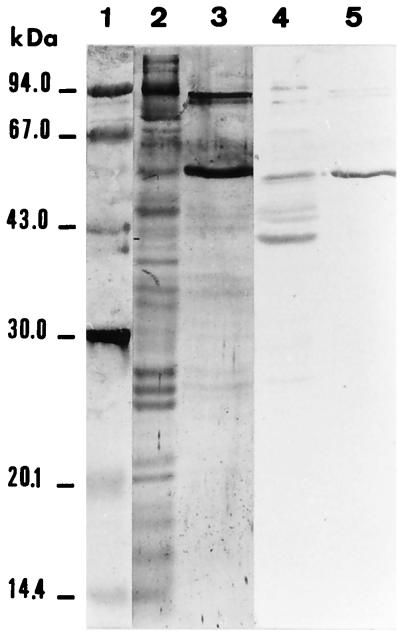
L. monocytogenes proteins that bind fibronectin were next identified by ligand affinity. To that end, bacteria grown overnight in 10 ml of BHI broth at 37°C were washed with PBS, resuspended in 1 ml of PBS containing 10 mM phenylmethylsulfonyl fluoride and 1 mM EDTA, and finally lysed by sonication (Sonics and Materials, Inc., Danbury, Conn.) with ice bath cooling. The sonicate was then fractionated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (4 and 12% polyacrylamide for stacking and separating gels, respectively) under denaturing conditions. Electrophoresed components were then transferred from the polyacrylamide gel to polyvinylidene difluoride (PVDF) membranes (Immobilon-P; Millipore, Bedford, Mass.) by the use of a Transblot unit (217 Multiphor 2; LKB, Bromma, Sweden). The transfer buffer contained 20% (vol/vol) methanol, 0.039 M glycine, and 0.048 M Tris (pH 8.8), and runs were made at 0.8 mA/cm2 of membrane for 2 h. Transblotted PVDF membranes processed as described by the producer were first incubated for 30 min with PBS containing 3% (wt/vol) bovine serum albumin (room temperature) and then for 2 h (at 37°C) with human fibronectin (an 80-μg/ml solution in PBST containing 0.2% [wt/vol] bovine serum albumin). After repeated washings with PBST, membranes were incubated for 1.5 h with peroxidase-labelled rabbit anti-human fibronectin Igs (Dako) (1/1,000 dilution in PBST containing 0.2% [wt/vol] bovine serum albumin). After successive washings with PBST and PBS, a color reaction was developed by addition of α-chloronaphthol (Bio-Rad, Richmond, Calif.) to a final concentration of 2.8 mM in the presence of 0.015% (vol/vol) hydrogen peroxide. Fibronectin bound mainly to two proteins of 55.3 and 42.4 kDa but also reacted slightly less with proteins of 48.6, 46.7, and 26.8 kDa (Fig. 3, lane 4). No reaction occurred when the membrane was incubated with the antifibronectin Ig in the absence of fibronectin (results not shown). When similar experiments were done with cell wall extracts of the bacteria prepared as described by Tabouret et al. (27), a 55.3-kDa protein was found to react strongly with fibronectin, indicating that at least one of the identified fibronectin-binding proteins is expressed at the cell surface of bacteria grown in BHI at 37°C. As conformational fibronectin-binding sites should be destroyed by the denaturing procedure used, it is conceivable that more fibronectin-binding proteins exist.
FIG. 3.
Identification of L. monocytogenes fibronectin-binding proteins. L. monocytogenes 90/636 sonicate (15 μg of protein in lane 2 and 50 μg in lane 4) and cell surface extract (10 μg in lanes 3 and 5) were fractionated by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis and transblotted to PVDF membranes, which were either stained with colloidal gold (lanes 2 and 3) or incubated with human fibronectin (lanes 4 and 5). Membrane-bound fibronectin was revealed by peroxidase-labelled rabbit anti-human fibronectin and α-chloronaphthol as the substrate. Molecular size markers (lane 1) were stained with colloidal gold.
In conclusion, we report here that L. monocytogenes is able to specifically bind to fibronectin, a glycoprotein component of body fluids, of the surface of cells, and of the ECM. Fibronectin has been reported to play a prominent role in multiple cellular processes, including cell-cell and cell-ECM adhesion, cell migration, differentiation, platelet function, wound healing, and interaction with the cytoskeleton. All of these functions are related to the capacity of fibronectin to interact with a great variety of other molecules, e.g., collagen, heparin, fibrin, DNA, proteoglycans, integrins, and others. Moreover, by developing the ability to bind fibronectin, several prokaryotic and eukaryotic pathogenic microorganisms have exploited the adhesion properties of fibronectin to mediate adherence to and colonization and invasion of host cells and tissues. All of these processes involve the binding of specific surface receptors to discrete domains of the fibronectin molecule (9, 15, 19, 22, 23, 25, 28).
The role of fibronectin-binding activity in the pathogenesis of listerial infection remains to be elucidated. A first hypothesis would be that the coating of bacteria with fibronectin leads to attachment to host cells by a two-step mechanism. The first step would be mediated by fibronectin and/or other ECM components and would be relatively nonspecific in that it would occur with a great variety of host cell types and molecules. This initial adherence would facilitate the interaction with a second receptor (e.g., InlA, InlB, p60, etc.), resulting in high-affinity, host cell-specific attachment and leading to internalization of the bacteria by previously described mechanisms. On the other hand, it is still possible that fibronectin, by a bridging mechanism with β1-integrin via the RGD sequence of fibronectin, allows L. monocytogenes to use this eukaryotic receptor for entry (at a low level) into certain host cells. In this connection, certain pathogens, such as Mycobacterium leprae, M. bovis BCG, and Streptococcus pyogenes, have already been reported to use fibronectin to invade eukaryotic cells (17, 21, 26). A last hypothesis would be that the coating of the bacterium with fibronectin may mask L. monocytogenes and prevent its recognition by the host immune system and would thus represent another mechanism developed by the bacteria to evade the host defense system.
The binding of L. monocytogenes to fibronectin, described in this paper, adds to the number of molecules to which the bacterium is able to adhere and emphasizes that, as in other bacterial pathogens, adherence to host tissues is a very complex and multifactorial mechanism.
Acknowledgments
We are grateful to M. Braibant for reading the manuscript.
REFERENCES
- 1.Alvarez-Dominguez C, Vazquez-Boland J A, Carrasco-Marin E, Lopez-Mato P, Leyva-Cobian F. Host cell heparan sulfate proteoglycans mediate attachment and entry of Listeria monocytogenes, and the listerial surface protein ActA is involved in heparan sulfate receptor recognition. Infect Immun. 1997;65:78–88. doi: 10.1128/iai.65.1.78-88.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braun L, Ohayon H, Cossart P. The InlB protein of Listeria monocytogenesis sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 3.Cossart P, Lecuit M. Interactions of Listeria monocytogeneswith mammalian cells during entry and actin-based movement: bacterial factors, cellular ligands and signaling. EMBO J. 1998;17:3797–3806. doi: 10.1093/emboj/17.14.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowart R, Lashmet J, McIntosh M, Adams T. Adherence of a virulent strain of Listeria monocytogenesto the surface of a hepatocarcinoma cell line via lectine-substrate interaction. Arch Microbiol. 1990;153:282–286. doi: 10.1007/BF00249083. [DOI] [PubMed] [Google Scholar]
- 5.Dalton C B, Austin C C, Sobel J, Hayes P S, Bibb W F, Graves L M, Swaminathan B, Proctor M E, Griffin P M. An outbreak of gastroenteritis and fever due to Listeria monocytogenesin milk. N Engl J Med. 1997;336:100–105. doi: 10.1056/NEJM199701093360204. [DOI] [PubMed] [Google Scholar]
- 6.Dramsi S, Biswas I, Maguin E, Braun L, Mastroeni P, Cossart P. Entry of Listeria monocytogenesinto hepatocytes requires expression of InlB, a surface protein of the internalin multigene family. Mol Microbiol. 1995;16:251–261. doi: 10.1111/j.1365-2958.1995.tb02297.x. [DOI] [PubMed] [Google Scholar]
- 7.Drevets D A, Sawyer R T, Potter T A, Campbell P A. Listeria monocytogenesinfects human endothelial cells by two distinct mechanisms. Infect Immun. 1995;63:4268–4276. doi: 10.1128/iai.63.11.4268-4276.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farber J M, Peterkin P I. Listeria monocytogenes, a food-borne pathogen. Microbiol Rev. 1991;55:476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foster T J, Hook M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 10.Gaillard J L, Jaubert F, Berche P. The inlAB locus mediates the entry of Listeria monocytogenesinto hepatocytes in vivo. J Exp Med. 1996;183:359–369. doi: 10.1084/jem.183.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilot P, Genicot A, André P. Serotyping and esterase typing for analysis of Listeria monocytogenespopulations recovered from foodstuffs and from human patients with listeriosis in Belgium. J Clin Microbiol. 1996;34:1007–1010. doi: 10.1128/jcm.34.4.1007-1010.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilot P, Hermans C, Yde M, Gigi J, Janssens M, Genicot A, André P, Wauters G. Sporadic case of listeriosis associated with the consumption of a Listeria monocytogenes-contaminated ‘Camembert’ cheese. J Infect. 1997;35:195–197. doi: 10.1016/s0163-4453(97)91974-5. [DOI] [PubMed] [Google Scholar]
- 13.Guzman C A, Rohde M, Chakraborty T, Domann E, Hudel M, Wehland J, Timmis K N. Interaction of Listeria monocytogeneswith mouse dendritic cells. Infect Immun. 1995;63:3665–3673. doi: 10.1128/iai.63.9.3665-3673.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hess J, Gentschev I, Szalay G, Ladel C, Bubert A, Goebel W, Kaufmann S H E. Listeria monocytogenes p60 supports host cell invasion by and in vivo survival of attenuated Salmonella typhimurium. Infect Immun. 1995;63:2047–2053. doi: 10.1128/iai.63.5.2047-2053.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreis T, Vale D, editors. Guidebook to the extracellular matrix and adhesion proteins. Oxford, England: Oxford University Press; 1993. [Google Scholar]
- 16.Kuhn M, Goebel W. Identification of an extracellular protein of Listeria monocytogenespossibly involved in intracellular uptake by mammalian cells. Infect Immun. 1989;57:55–61. doi: 10.1128/iai.57.1.55-61.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuroda K, Brown E J, Telle W B, Russell D G, Ratliff T L. Characterization of the internalization of bacillus Calmette-Guerin by human bladder tumor cells. J Clin Investig. 1993;91:69–76. doi: 10.1172/JCI116202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lingnau A, Domann E, Hudel M, Bock M, Nichterlein T, Wehland J, Chakraborty T. Expression of the Listeria monocytogenes EGD inlA and inlBgenes, whose products mediate bacterial entry into tissue culture cell lines, by PrfA-dependent and -independent mechanisms. Infect Immun. 1995;63:3896–3903. doi: 10.1128/iai.63.10.3896-3903.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ljungh A, Moran A P, Wadstrom T. Interactions of bacterial adhesins with extracellular matrix and plasma proteins: pathogenic implications and therapeutic possibilities. FEMS Immunol Med Microbiol. 1996;16:117–126. doi: 10.1111/j.1574-695X.1996.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 20.Mengaud J, Ohayon H, Gounon P, Mege R M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenesinto epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 21.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patti J M, Allen B L, McGavin M J, Hook M. MSCRAMM-mediated adherence of microorganisms to host tissues. Annu Rev Microbiol. 1994;48:585–617. doi: 10.1146/annurev.mi.48.100194.003101. [DOI] [PubMed] [Google Scholar]
- 23.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruhland G J, Hellwig M, Wanner G, Fiedler F. Cell-surface location of Listeria-specific protein p60—detection of Listeriacells by indirect immunofluorescence. J Gen Microbiol. 1993;139:609–616. doi: 10.1099/00221287-139-3-609. [DOI] [PubMed] [Google Scholar]
- 25.Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- 26.Schorey J S, Li Q, McCourt D W, Bong-Mastek M, Clark-Curtiss J E, Ratliff T L, Brown E J. A Mycobacterium lepraegene encoding a fibronectin binding protein is used for efficient invasion of epithelial cells and Schwann cells. Infect Immun. 1995;63:2652–2657. doi: 10.1128/iai.63.7.2652-2657.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tabouret M, de Rycke J, Dubray G. Analysis of surface proteins of Listeriain relation to species, serovar and pathogenicity. J Gen Microbiol. 1992;138:743–753. doi: 10.1099/00221287-138-4-743. [DOI] [PubMed] [Google Scholar]
- 28.Westerlund B, Korhonen T K. Bacterial proteins binding to the mammalian extracellular matrix. Mol Microbiol. 1993;9:687–694. doi: 10.1111/j.1365-2958.1993.tb01729.x. [DOI] [PubMed] [Google Scholar]
- 29.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 30.Wuenscher M D, Köhler S, Bubert A, Gerike U, Goebel W. The iap gene of Listeria monocytogenesis essential for cell viability, and its gene product, p60, has bacteriolytic activity. J Bacteriol. 1993;175:3491–3501. doi: 10.1128/jb.175.11.3491-3501.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]