Abstract
VlsE, the variable surface antigen of Borrelia burgdorferi, consists of two invariable domains at the amino and carboxyl termini and one central variable domain. The latter contains six invariable regions, IR1 to IR6, and six variable regions. In the present study, the antigenicity of all of the invariable regions in B. burgdorferi-infected monkeys, humans, and mice was assessed by peptide-based enzyme-linked immunosorbent assays. Only one invariable region, IR6, was antigenic in all animals of the three host species. IR2 and IR4 were also antigenic in mice.
Antigenic variation is an effective strategy developed by pathogenic microorganisms to evade the host immune system. Variable antigens such as the variant surface glycoprotein of African trypanosomes (4, 5), pilin of the bacterium Neisseria gonorrhoeae (11), the variable major protein (Vmp) of the spirochete Borrelia hermsii (13, 18, 20), and the variable surface antigen (Vmp-like sequence, Expressed; VlsE) of Borrelia burgdorferi (21) contain both invariable and variable domains. Antigenic variation affects only the variable domain. Even within this domain, short invariable regions (IRs) may be present. Both the invariable domains and short regions are important in maintaining the functional structure of the molecule (10, 14, 17). The variable domains are highly immunogenic and serve as the major target of the host immune response (4, 6). Invariable portions of the variant surface glycoprotein, pilin, and Vmp antigens have not been found to be antigenic during natural infections, although antibodies directed to these conserved sequences may be produced by immunization (1, 3, 9, 19).
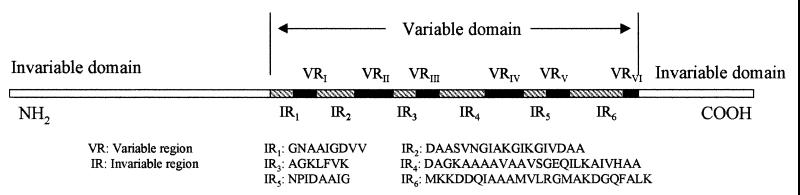
VlsE is a surface lipoprotein with a predicted molecular mass of 34 kDa in the B31 strain of B. burgdorferi sensu stricto (21). The two invariable domains at the amino and carboxyl termini together encompass approximately half of this molecule's length (21) (Fig. 1). The variable domain at the center contains six variable regions, VRI to VRVI, and six IRs, IR1, to IR6 (21) (Fig. 1). The six IRs remain unchanged during antigenic variation (21) and are conserved among strains and genospecies of B. burgdorferi sensu lato (12). This indicates that the IRs are critical for the physiologic function of VlsE and may thus be targets of immune intervention. An analysis of their antigenicity is therefore apposite. We had already examined the antigenicity of IR6, the most conserved IR, and found it to be immunodominant in humans and monkeys (12). In this study, we investigated the antigenicity of the remaining IRs, IR1 to IR5, in experimentally infected and immunized monkeys and mice and in humans with Lyme disease.
FIG. 1.
Diagrammatic illustration of the VlsE structure. VlsE consists of two invariable domains at the amino and carboxyl termini and one variable domain at the center. The variable domain contains six variable regions, VRI to VRVI, and six IRs, IR1 to IR6. The sequences of the IRs were obtained from one cloned variable domain of VlsE expressed by strain IP90 of B. garinii (12).
To determine the antigenicity of the IRs of VlsE, peptides were prepared by the fluorenylmethoxycarbonyl synthesis protocol (2) based on the sequences listed in Fig. 1. The peptide was covalently linked to biotin by the N-succinimidyl maleimide carboxylate method. The maleimide reagents were from Molecular Probes (Eugene, Oreg.), and the protocol suggested by the manufacturer was followed.
A peptide-based enzyme-linked immunosorbent assay (ELISA) protocol was used. Ninety-six-well ELISA plates were coated with 100 μl of 4-μg/ml streptavidin (Pierce Chemical Company, Rockford, Ill.) per well in coating buffer (0.1 M carbonate buffer, pH 9.2) and incubated at 4°C overnight. The remaining steps were conducted in a rotatory shaker at room temperature. After two 3-min washes with 200 μl of phosphate-buffered saline–Tween 20 (PBS/T, phosphate-buffered saline containing 0.1% Tween 20, pH 7.4) per well at 200 rpm, 200 μl of 5-μg/ml biotinylated peptide dissolved in blocking solution (PBS/T supplemented with 5% nonfat dry milk) was applied to each well. The plate was shaken at 150 rpm for 2 h. After three washes with PBS/T, 50 μl of serum (mouse, monkey, or human) diluted 1:200 with blocking solution was added to each well. The plate was incubated at 150 rpm for 1 h and then washed three times with PBS/T. Each well then received 100 μl of 0.2-μg/ml goat anti-monkey immunoglobulin G (IgG) (γ chain specific [Kirkegaard & Perry Laboratories, Gaithersburg, Md.]), 0.5-μg/ml anti-mouse IgG (heavy and light chain specific [Sigma Chemical Co., St. Louis, Mo.]), or 0.1-μg/ml anti-human IgG (heavy and light chain specific [Pierce]), each conjugated to horseradish peroxidase and dissolved in blocking solution. The plate was incubated for 1 h while being shaken. After four washes with PBS/T, each for 3 to 6 min, the antigen-antibody reaction was probed by using the TMB Microwell peroxidase substrate system (Kirkegaard & Perry), and color was allowed to develop for 10 min. The enzyme reaction was stopped by addition of 100 μl of 1 M H3PO4. Optical density (OD) was measured at 450 nm.
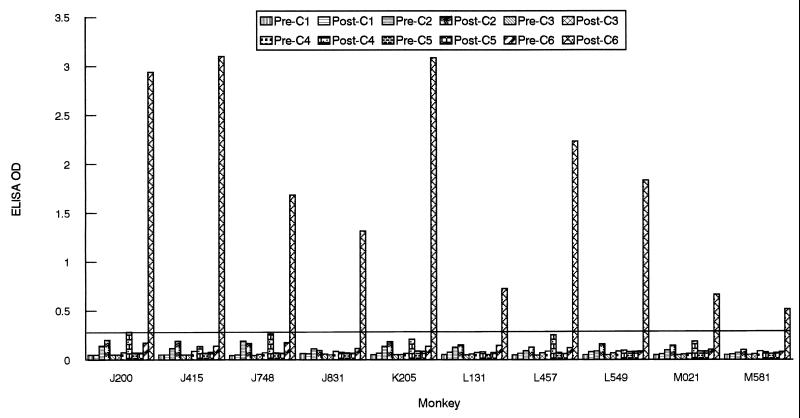
To assess the antigenicity of the IRs in monkeys, serum specimens from 10 rhesus monkeys (2 to 4 years old; Macaca mulatta) that had been infected by the bite of Ixodes scapularis nymphal ticks were used. The ticks were themselves infected with either of the B. burgdorferi sensu stricto strains JD1 (15) and B31 (16). Serum samples obtained at 4 to 6 weeks postinfection were used to examine the antibody response by the peptide-based ELISA. Except for a strong response to IR6, no significant antibody responses to the five remaining IRs, IR1 to IR5, were detected (Fig. 2). Serum samples obtained from some of the monkeys after 3 years of infection also were tested. No detectable responses to IR1 to IR5 were observed, whereas the anti-IR6 response persisted (data not shown).
FIG. 2.
Antigenicity of IRs of VlsE in infected monkeys. Serum samples were collected from monkeys at 0 (“Pre-”) and 4 to 6 (“Post-”) weeks postinoculation. Animals were infected by tick inoculation either with the JD1 strain of B. burgdorferi (animals J200, J415, J748, J831, K205, and L131) or with the B31 strain (L457, L549, M021, and M581). Antibody levels were assessed by peptide-based ELISAs. The cutoff value (0.270) was based on the mean OD plus 3 SDs of 10 monkey preimmune samples when six peptides were separately used as an ELISA antigen.
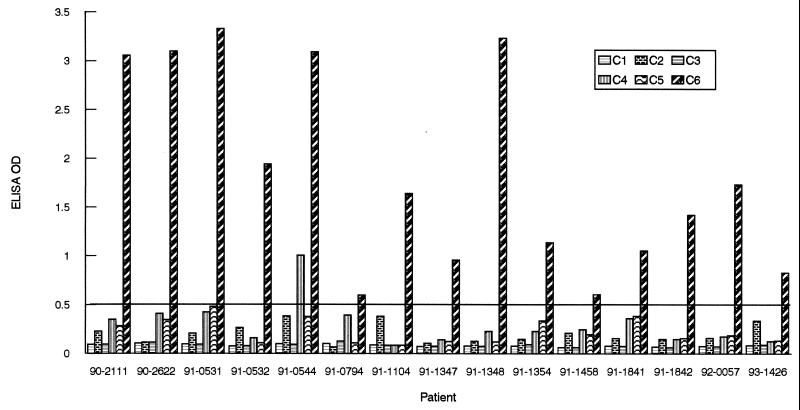
For humans, the antigenicity of the IRs was examined with the aid of 15 sera randomly selected from a panel of 41 serum samples provided by the Centers for Disease Control and Prevention (CDC). All the samples were collected from Lyme disease patients who had signs and symptoms that satisfied the CDC clinical case definition (8). The baseline represented the mean OD value plus 3 standard deviations (SDs) of 97 human sera collected from a local hospital in Louisiana, where Lyme disease is not endemic. Regardless of the peptides used as ELISA antigens, calibrated baselines were similar, approximately 0.5. Only one sample (91-0544) showed a significant response to IR4. As with the monkey serum samples, IR6 was the only IR that was ostensibly antigenic (Fig. 3).
FIG. 3.
Antigenicity of IRs of VlsE in humans. Fifteen samples were randomly selected from a CDC panel of 41 Lyme disease sera. Antibody levels were assessed by peptide-based ELISAs. The cutoff value (0.500) was based on the mean OD plus 3 SDs of human sera collected from hospitalized patients in an area where Lyme disease is not endemic.
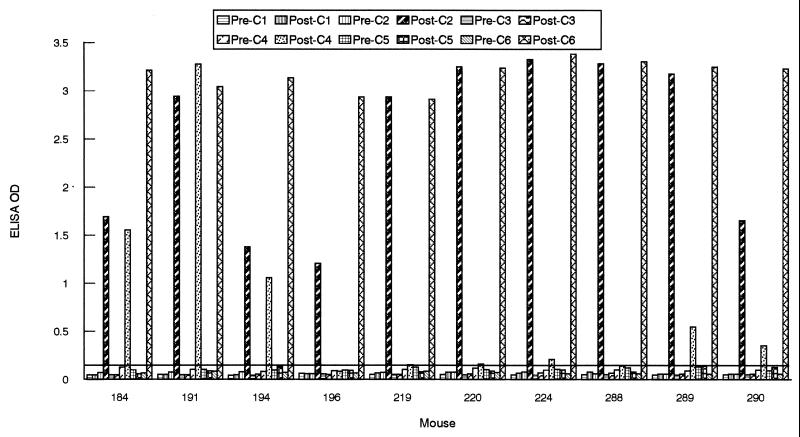
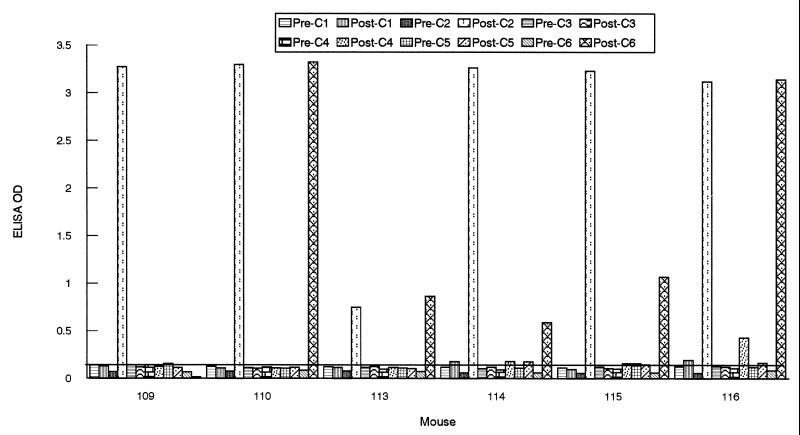
A remarkably different response was observed when the humoral response was investigated with mice. Ten animals (6- to 8-week-old C3H/HeN mice [Jackson Laboratories, Bar Harbor, Maine]) were infected with B. burgdorferi sensu stricto Sh-2-82 (low passage; a gift from Denee Thomas, University of Texas Health Science Center, San Antonio) by subcutaneous needle inoculation with 108 spirochetes administered in 1 ml of BSK-H medium (Sigma Chemical Co.) or by the bite of B31-infected I. scapularis nymphal ticks. In addition to IR6, a strong antibody response to IR2 was detected in all 10 infected mice, and five animals (184, 191, 194, 289, and 290) also responded, in some cases vigorously, to IR4 (Fig. 4).
FIG. 4.
Antigenicity of IRs of VlsE in infected mice. Serum samples were collected from mice at 0 (“Pre-”) and 4 to 6 (“Post-”) weeks postinoculation. Animals were infected either with the B31 strain of B. burgdorferi (184, 191, 194, and 196) by tick inoculation or with the Sh-2-82 strain (219, 220, 224, 288, 289, and 290) by needle inoculation. Antibody levels were assessed by peptide-based ELISAs. The cutoff value (0.140) was based on the mean OD plus 3 SDs of 10 mouse preimmune samples when six peptides were separately used as an ELISA antigen.
The sequences of the six IRs are conserved among the strains and genospecies of B. burgdorferi sensu lato (12). However, a single amino acid substitution may diminish or destroy the reactivity of an epitope. The sequences of the peptides used in this study were derived from a cassette segment of the IP90 strain of Borrelia garinii (a detailed comparison of the IR sequences of IP90, B31, and 297 strains is available in reference 12). It is possible that the peptide-based ELISA failed to detect the antibody response because the sequences of the IR probes used in the ELISA were not sufficiently conserved compared to those of the B31, Sh-2-82, or JD1 strain used for infection. However, the fact that all of the infected mice responded to IR2 and that five mice also responded to IR4 indicates that these two IRs were also antigenically conserved.
To further investigate the antigenicity of the six IRs, we also immunized mice with a recombinant protein, P7-1, described previously (12), which contained the six IRs IR1 to IR6 with the same sequences listed in Fig. 1. Six mice were given three injections each, at biweekly intervals, of 10 μg of P7-1 emulsified with RiBi adjuvant (RiBi ImmunoChem Research, Inc., Hamilton, Mont.). Two weeks after the last injection, the antibody titer was determined by the peptide ELISA. The results presented in Fig. 5 are in agreement with our observations in the mouse infection experiment (Fig. 4), indicating that IR1, IR3, and IR5 are not antigenic even after immunization under the influence of adjuvant. In addition, one rhesus monkey was immunized with a mixture of P7-1 and three additional recombinant proteins, each of which contained at least one cassette segment of VlsE cloned from the IP90 strain. Antibody responses to the six IRs reproduced the observations obtained in the monkey infection experiment (data not shown).
FIG. 5.
Antibody response to IRs of VlsE in immunized mice. Serum samples were collected from mice before immunization (“Pre-”) and at 2 weeks after the last injection (“Post-”) with P7-1 (12) and RiBi adjuvant. Antibody levels were assessed by peptide-based ELISAs. The cutoff value (0.140) was based on the mean OD plus 3 SDs of six mouse preimmune samples when six peptides were separately used as an ELISA antigen.
Among molecules that undergo antigenic variation, VlsE is unusual in that more than 75% of its primary structure is invariable. Such a prominent invariable portion of a variable surface antigen must elude potentially deleterious antibody responses. Several mechanisms are possible to this end: IRs or invariable domains may be (i) conformationally cryptic; (ii) exposed on the surface of the molecule but not on that of the spirochete; and (iii) nonantigenic, either because of an intrinsic lack of antigenicity or because other regions of the molecule are immunodominant. We have already demonstrated that IR6, the immunodominant IR of VlsE, is exposed on the molecule's surface but is not accessible to antibody on the surface of the spirochete (12). Why this is so remains to be investigated. VlsE is a bacterial lipoprotein (21). Mature bacterial lipoproteins are usually hydrophilic (VlsE is no exception) and are anchored to the membrane only by their cysteine-bound amino-terminal lipid moieties. It is therefore unlikely that IR inaccessibility to antibody is due to seclusion of these regions in the spirochetal membrane. Rather, inaccessibility may be the consequence of steric hindrance by other, closely packed, perhaps abundant, membrane proteins. This type of hindrance has already been demonstrated in the case of P66, an integral membrane protein of B. burgdorferi whose accessibility to antibody and proteases is hindered by lipoproteins (7). Here we have provided evidence that indicates that IR1, IR3, and IR5 might elude being targeted by antibody because they are poorly, or not at all, antigenic. This lack of antigenicity, perhaps due in part to the limited length of these regions, persisted across animal species and regardless of whether stimulation of an antibody response was attempted by infection or by immunization. Hence, it is possible that these invariable residues need not be occult but are exposed on the spirochetal surface without endangering the survival of the organism.
Acknowledgments
This work was supported by grants AI35027 and RR00164 from the National Institutes of Health and by a grant from SmithKline Beecham Biologicals.
REFERENCES
- 1.Barbet A F, McGuire T C. Crossreacting determinants in variant-specific surface antigens of African trypanosomes. Proc Natl Acad Sci USA. 1978;75:1989–1993. doi: 10.1073/pnas.75.4.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barony G, Merrifield R B. The peptides: analysis, synthesis, and biology. New York, N.Y: Academic Press, Inc.; 1980. pp. 3–285. [Google Scholar]
- 3.Barstad P A, Coligan J E, Raum M G, Barbour A G. Variable major proteins of Borrelia hermsii. Epitope mapping and partial sequence analysis of CNBr peptides. J Exp Med. 1985;161:1302–1314. doi: 10.1084/jem.161.6.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borst P, Cross G A M. Molecular basis for trypanosome antigenic variation. Cell. 1982;29:291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- 5.Borst P, Bitter W, Blundell P A, Chaves I, Cross G A M, Gerrits H, van Leeuwen F, McCulloch R, Taylor M, Rudenko G. Control of Vsg gene expression sites in Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:67–76. doi: 10.1016/s0166-6851(97)00184-9. [DOI] [PubMed] [Google Scholar]
- 6.Borst P. Molecular genetics of antigenic variation. Immunol Today. 1991;12:A29–A33. doi: 10.1016/S0167-5699(05)80009-X. [DOI] [PubMed] [Google Scholar]
- 7.Bunikis J, Barbour A G. Access of antibody or trypsin to an integral outer membrane protein (P66) of Borrelia burgdorferiis hindered by Osp lipoproteins. Infect Immun. 1999;67:2874–2883. doi: 10.1128/iai.67.6.2874-2883.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. Case definition for infectious conditions under public health surveillance. Morbid Mortal Weekly Rep. 1997;46:20–21. [Google Scholar]
- 9.Cross G A M. Crossreacting determinants in the C-terminal region of trypanosome variant surface antigens. Nature. 1979;277:310–312. doi: 10.1038/277310a0. [DOI] [PubMed] [Google Scholar]
- 10.Forest K T, Bernstein S L, Getzoff E D, So M, Tribbick G, Geysen H M, Deal C D, Tainer J A. Assembly and antigenicity of the Neisseria gonorrhoeaepilus mapped with antibodies. Infect Immun. 1996;64:644–652. doi: 10.1128/iai.64.2.644-652.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagblom P, Segal E, Billyard E, So M. Intragenic recombination leads to pilus antigenic variation in Neisseria gonorrhoeae. Nature. 1985;315:156–168. doi: 10.1038/315156a0. [DOI] [PubMed] [Google Scholar]
- 12.Liang, F. T., A. L. Alvarez, Y. Gu, J. M. Nowling, R. Ramamoorthy, and M. T. Philipp. An immunodominant conserved region within the variable domain of VlsE, the variable surface antigen of Borrelia burgdorferi. J. Immunol., in press. [PubMed]
- 13.Meier J T, Simon M I, Barbour A G. Antigenic variation is associated with DNA rearrangements in a relapsing fever Borrelia. Cell. 1984;41:403–409. doi: 10.1016/s0092-8674(85)80013-1. [DOI] [PubMed] [Google Scholar]
- 14.Pays E, Nolan D P. Expression and function of surface proteins in Trypanosoma brucei. Mol Biochem Parasitol. 1998;91:3–36. doi: 10.1016/s0166-6851(97)00183-7. [DOI] [PubMed] [Google Scholar]
- 15.Philipp M T, Aydintug M K, Bohm R P, Jr, Cogswell F B, Dennis V A, Lanners H N, Lowrie R C, Jr, Roberts E D, Conway M D, Karaçorlu M, Peyman G A, Gubler D J, Johnson B J B, Piesman J, Gu Y. Early and early disseminated phases of Lyme disease in the rhesus monkey: a model for infection in humans. Infect Immun. 1993;61:3047–3059. doi: 10.1128/iai.61.7.3047-3059.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Philipp M T, Lobet Y, Bohm R P, Jr, Roberts E D, Dennis V A, Gu Y, Lowrie R C, Jr, Desmons P, Duray P H, England J, Hauser P, Piesman J, Xu K. The outer surface protein A (OspA) vaccine against Lyme disease: efficacy in the rhesus monkey. Vaccine. 1997;15:1872–1884. doi: 10.1016/s0264-410x(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 17.Reinitz D M, Aizenstein B D, Mansfield J M. Variable and conserved structural elements of the trypanosome variant surface glycoprotein. Mol Biochem Parasitol. 1992;51:119–132. doi: 10.1016/0166-6851(92)90207-z. [DOI] [PubMed] [Google Scholar]
- 18.Restrepo B I, Kitten T, Carter C J, Infante D, Barbour A G. Subtelomeric expression regions of Borrelia hermsiilinear plasmids are highly polymorphic. Mol Microbiol. 1992;6:3299–3311. doi: 10.1111/j.1365-2958.1992.tb02198.x. [DOI] [PubMed] [Google Scholar]
- 19.Rothbard J B, Fernandez R, Schoolnik G K. Strain-specific and common epitopes of gonococcal pili. J Exp Med. 1984;160:208–221. doi: 10.1084/jem.160.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoenner H G, Dodd T, Larsen C. Antigenic variation of Borrelia hermsii. J Exp Med. 1982;156:1297–1311. doi: 10.1084/jem.156.5.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang J R, Hardham J M, Barbour A G, Norris S J. Antigenic variation in Lyme disease borreliae by promiscuous recombination of VMP-like sequence cassettes. Cell. 1997;89:275–285. doi: 10.1016/s0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]