Abstract
Agriculture is a backbone of global economy and most of the population relies on this sector for their livelihood. Chitosan as a biodegradable material thus can be explored for in various fields in its nano form to replace non-biodegradable and toxic compounds. The chitosan has appealing properties like biocompatibility, non-toxicity, biodegradability, and low allergenic, making it useful in several applications including in agriculture sector. Because of their unique properties, chitosan nanoparticles (ChNPs) are extensively applied as a bioagent in various biological and biomedical processes, including wastewater treatment, plant growth promoter, fungicidal agent, wound healing, and scaffold for tissue engineering.
Furthermore, the biocompatibility of chitosan nanoparticles (ChNPs) is reported to have other biological properties such as anti-cancerous, antifungal, antioxidant activities, even induces an immune response in the plant, and helps manage biotic and abiotic stresses. Chitosan can also find its application in wastewater treatment, hydrating agents in cosmetics, the food industry, paper, and the textile industry as adhesive, drug-delivering agent in medical as well as for bioimaging. Since chitosan has low toxicity, the nano-formulation of chitosan can be used for the controlled release of fertilizers, pesticides, and plant growth promoters in agriculture fields. The ChNPs applications in precision farming being a novel approach in recent developments. Here we have comprehensively reviewed the major points in this review are; the synthesis of ChNPs by biological resources, their modification and formulation for increasing its applicability, their modified types, and the different agricultural applications of ChNPs.
Keywords: Biological synthesis, Chitosan nanoparticles (ChNPs), Fertilizer, Fungicidal agent, Plant growth promoter
Biological synthesis; Chitosan nanoparticles (ChNPs); Fertilizer; Fungicidal agent; Plant growth promoter.
1. Introduction
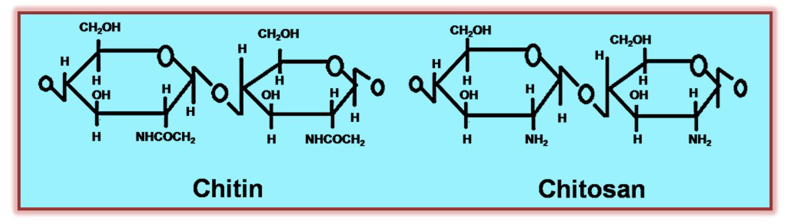
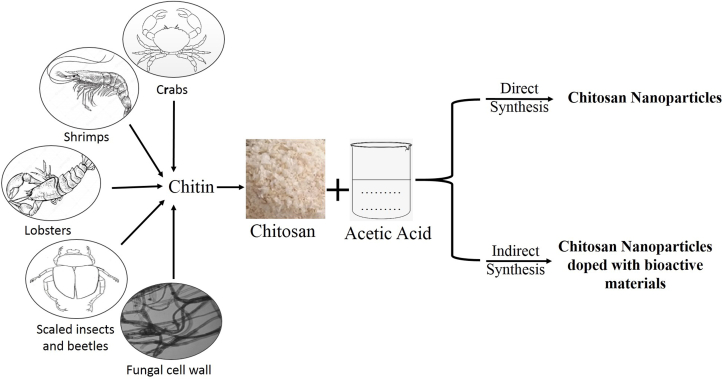
After cellulose, chitin is the major natural polymer in the world. The primary sources exploited are two marine crustaceans, viz. shrimp, crabs, lobster, and crawfish (in general 20–30% on a dry basis), and are sufficient to support a commercial chitin/chitosan industry [1]. Chitin, a cellulose-like polysaccharide, is a linear, poly-β-(1,4)-N-acetyl-D glucosamine [2]. Chitin occurs in nature as ordered crystalline microfibrils. It is found in three polymorphic forms: α chitin, β-chitin, and γ-chitin. Chitosan, also known as deacetylated chitin, is a naturally occurring polycationic polysaccharide derived from partial deacetylation of chitin (as shown in Figure 1). Estimates of the global annual production of shell wastes from crab, lobster, shrimp, krill, and clam/oyster, on a dry basis, are as much as 1.44 million metric tons.
Figure 1.
Structure of chitin and chitosan.
The degree of deacetylation, described by the molar fraction of deacetylated units or percentage of deacetylation, and the molecular weight of chitosan, were found to affect these properties. Due to its unique properties, chitosan is being extensively applied as a bioagent in various biological and biomedical processes like water treatment, wound-healing materials, as a drug carrier, and scaffold for tissue engineering. The cationic nature of chitosan is unique. The current world supply of chitinous wastes could support 50 to 100 million pounds [1]. Primary U.S. sources of crustaceans that are processed into chitin and chitosan are Dungeness crab (Cancer magister) and the Pacific shrimp (Pandalus borealis) [3]. Formerly, King crab (Paralithodes camtschaticus) was proposed as a chitin/chitosan resource; however, it is no longer available in sufficient quantity.
In agriculture sector, chitosan nanoparticles (ChNPs) by themselves can act as growth enhancers and potent antimicrobial agent against pathogenic fungi and bacteria [4]. Alternatively, they can also act as a nanocarriers for existing agrochemicals, hence are referred to as chitosan-based agronanochemicals [5, 6, 7]. In plant pathogen control and disease management, chitosan with or without the amalgamation of macronutrients could act as a substituted sustainable potent biocide agent against crop pathogens like bacteria, fungi and viruses [8]. Chitosan possesses a sustainable choice to be used as conventional fungicides against various diseases such as Fusarium wilt and head blight in chickpea and wheat, leaf blast in rice, stalk rot and leaf spot in maize as well as blast in finger millet [9]. Foliar application of oligo-chitosan and oligo-chitosan nano-silica demonstrated that soybean seed yield increased 10.5 and 17.0% for oligo-chitosan and oligo-chitosan nano-silica [10]. Systematic analysis of application of ChNPs (1–100 μg/mL) and chitosan showed adsorption of ChNPs on the surface of wheat seeds was higher than that of chitosan. Chitosan NPs application (5 μg/mL) induced the auxin-related gene expression [11]. The appealing properties of chitosan include biocompatibility, non-toxicity, biodegradability, and low allergenicity, making the chitosan valuable in several applications.
Chitosan is well known bio-stimulant used for promotion of growth and manage the stresses including pest and diseases [12]. Nanoparticles are more efficient with lesser molecular weights, have improved bioavailability, increased half-life and greater surface area to volume ratio. Therefore, present review is based on synthesis and characterization of ChNPs which holds the potent alternative for chemical pesticides and as bio-stimulant used in plant disease management. The latest researches on the application of ChNPs clearly indicate its benefit on plant productivity, plant protection against the attack of pathogens, and extension of the commercialization. Chitosan is a biodegradable material thus can be explored for various fields in its nano form to replace non-biodegradable and toxic compounds.
2. Definition, sources, and synthesis of chitosan nanoparticles (ChNPs)
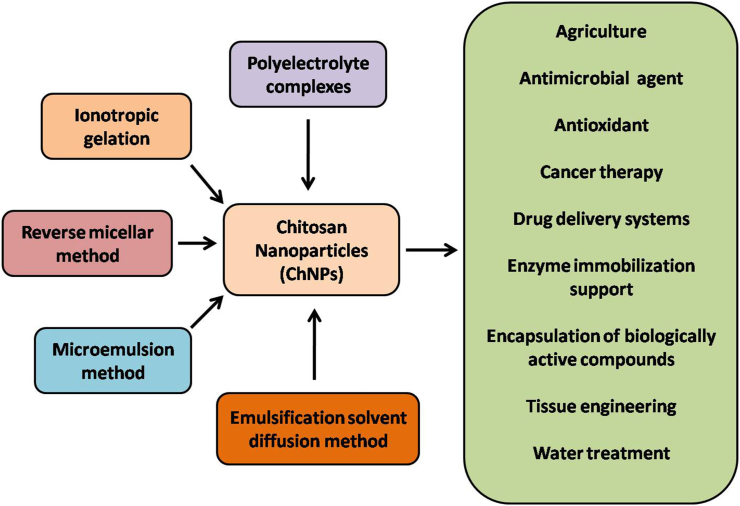
Recently, ChNPs has developed a lot of attention for a wide range of applications in the agricultural, biomedical, and pharmaceutical industries. Chitosan NPs are synthesized through numerous methods by “bottom-up” approaches such as polymerization or a reverse micelle medium or microemulsion methods, and top-down techniques like milling, high-pressure homogenization, and ultra-sonication are also applied [13]. Various methods of ChNPs synthesis and their applications are represented in Figure 2.
Figure 2.
Methods of Chitosan Nanoparticles (ChNPs) synthesis and their applications in different fields.
The ionotropic gelation technique utilizes the electrostatic interaction between the amine group of chitosan and a negatively charged group of polyanion like tripolyphosphate. Chitosan can be dissolved in acetic acid, and NPs were formed spontaneously under mechanical stirring at room temperature. Changing the ratio of chitosan to the stabilizer can be modified the size and surface charge of particles [14]. Polyelectrolyte complex (PEC) formed by self-assembly of the cationic charged polymer and plasmid DNA due to fall in hydrophilicity because of charge neutralization between cationic polymer and DNA. The ChNPs can be synthesized spontaneously upon adding DNA into chitosan (in acetic acid) solution, under continuous stirring at room temperature. Polymer Grafting is a process of modifying polymer by attaching an active functional group. Examples are thiolation, esterification, and carboxylation, etc., these processes are used for active or passive targeting [15].
The pH-sensitive carboxymethyl chitosan (CMCS) NPs with fluorinated surface modification were prepared for efficient drug delivery. N-(3-Aminopropyl)-imidazole was pre-grafted onto CMCS to fabricate the pH-sensitive NPs, and then was surface-modified with perfluorobutyric anhydride to give the fluorinated NPs. The results suggested that the CMCS NPs had great potential to be efficient drug carriers for cancer chemotherapy [16].
2.1. Biologically synthesized chitosan nanoparticles (ChNPs)
Chitosan NPs can be synthesized by biological method with the help of different biomolecules. Sathiyabama and Parthasarathy [17] prepared ChNPs by adding anionic proteins isolated from Penicillium oxalicum culture to chitosan solutions. ChNPs with high antifungal activities are obtained through biological processes [17].
Anitha et al. [18] worked on nanoformulation of curcumin using dextran sulfate and chitosan. The result showed the preferential killing of cancer cells compared to normal cells by the curcumin-loaded NPs. Thus, the developed curcumin-loaded nanoformulation could be a promising candidate in cancer therapy. Figure 3 illustrated the graphical representation of biological synthesis and formulation of ChNPs.
Figure 3.
Biological synthesis and formulation of Chitosan Nanoparticles (ChNPs).
3. Role of biological chitosan NPs as a plant growth promoter
Modern agriculture has the primary concern regarding the production of food with good quality and sufficient quantity to meet the demand of population rise in the world, restraining environmental impacts. Hence, scientists started to think about nanotechnology in the field of agriculture [19]. Although various studies reported the use of chitosan in agriculture, the application of ChNPs has yet to be explored (Table 1). The oppositely charged polymers and the amine group of chitosan form several complexes that could be beneficial in the agriculture sector [20]. Different forms of chitosan are applied in the field for plant growth promotion in detail described in later sections.
Table 1.
Chitosan Nanoparticles (ChNPs) formulations and their effect on agriculture crops.
| Sr. no. | Type of Chitosan Nanoparticle formulation applied | Type of Application | Crop used | Effects on crop plants | Reference |
|---|---|---|---|---|---|
| 1 | Chitosan NPs | Seed treatment | Pennisetum glaucum | Leads to activation of early defense responses and elevation in nitric oxide accumulation against Downy mildew | [20, 26] |
| 2 | Chitosan-coated mesoporous silica NPs | Fruit surface treatment | Citrullus lanatus | Improved suppression during the fungal disease and altered expression of stress-related genes | [27] |
| 3 | ChNPs | Media enrichment | Capsicum annum | Increased plant heights, chlorophyll content, leaf numbers, leaf width as well as length | [28] |
| 4 | ChNPs | Foliar application | Camellia sinensis (L.) O. Kuntze | Induced the defense response in a nitric oxide dependent manner against Blister blight disease | [29, 30] |
| 5 | ChNPs + rhizobacteria (PS2 and PS10) | Stem Treatment | Zea mays | Improved seed germination, plant height, leaf area, internodes number and chlorophyll content in maize; enhanced dehydrogenase, alkaline phosphatase activity and fluorescein diacetate hydrolysis; increased stress tolerance mechanism | [31] |
| 6 | ChNPs | Foliar and soil application | Hordeum vulgare L. | Considerably augmented the leaf area and color, the number of grains per spike, the grain yield and the harvest index, reduced effect of drought stress | [21] |
| 7 | Chitosan-aloe vera gel coating | Post-Harvest Application | Mangifera indica L. | Coating mango fruit with chitosan will reduce the rate of rotting | [32] |
| 8 | Cu-ChNPs | Seed treatment and in vitro antifungal assay | Solanum lycopersicum Mill. | Significant growth promoting effect on germination of tomato seed, seedling length, fresh and dry weight; also in vitro antifungal activity against Alternaria solani and Fusarium oxysporum | [33] |
| 9 | Cu-ChNPs | Seed treatment | Z. mays | Higher percent germination, root and shoot length, root number, seedling length, fresh and dry weight and seedling vigor index; encouraged the activities of enzymes α-amylase and protease and increased the total protein content in germinating seeds | [34] |
| 10 | Cu-ChNPs | – | Z. mays | Defense against Curvularia leaf spot disease by inducing antioxidant and defense enzymes such as phenylalanine ammonia-lyses and polyphenol oxidase | [24, 25] |
| 11 | Chitosan–PVA and Cu NPs | Seed treatment | Solanum lycopersicum Mill | Increased tomato growth and chlorophylls ‘a’ and ‘b’, carotenoids, total chlorophylls, and superoxide dismutase content; activated synthesis of vitamin C and lycopene | [35] |
| 12 | ChNPs | Seed treatment | Triticum aestivum L. | Growth promoter, induces auxin-related gene expression, hastened indole-3-acetic acid (IAA) biosynthesis and transport, reduced IAA oxidase activity | [36] |
3.1. Free chitosan nanoparticles (ChNPs)
Chitosan is a non-hazardous, biocompatible, biodegradable, and natural biopolymer having a broad application. Chitosan on application to plant induces photosynthetic rate, stomatal closure, enhance antioxidant enzymes by signaling pathways of nitric oxide and hydrogen peroxide. It also stimulates the biosynthesis of amino acids, sugars, organic acids, and other metabolites, an essential component of stress tolerance and energy metabolism pathways [21].
Many researchers worked on ChNPs as plant growth promoters [20]. Chitosan enters the seeds through the imbibition and, as a result of seed/chitosan interaction, positively affects the seeds germination index, reduced germination and flowering time, increased plant growth, and biomass production [21]. Behboudi et al. [22] experimented with the effect of ChNPs under drought stress on Triticum aestivum L. seedlings. The wheat seeds were sown in soil after treatment with ChNPs. The ChNPs, at a concentration of 90 ppm, augmented relative water content, leaf area, photosynthesis rate, chlorophyll content, superoxide dismutase, and catalase activities, biomass, and yield compared to the control. Lastly, their outcome shows that using ChNPs at a concentration of 90 ppm could mitigate the unfavorable effects of drought on the wheat seedling growth under drought stress [22].
3.2. Conjugated chitosan nanoparticles (ChNPs)
Chitosan composed of randomly distributed β-(1–4)-linked D-glucosamine and N-acetyl-D-glucosamine (acetylated unit) as a linear hetero-polysaccharide. Chitosan is one of the few essential polysaccharides widely used in agriculture, biotechnology, food, chemical, medicine, feed, and environmental protection [9, 23, 24]. As a new drug delivery, conjugated NPs have been widely concerned by researchers in recent years. It can easily be conjugated with other moieties due to having an amine group.
In pot experiments, Cu-ChNPs demonstrated the growth-promoting effect in plant height, stem diameter, chlorophyll content, root length, and number. The defense response during NPs treatment showed higher antioxidant and defense enzymes [25].
The oligo-chitosan was prepared through degradation of chitosan solution (4%) having 0.5% H2O2 by gamma Co-60 radiation and the silica NPs by calcinations of acid-treated rice husk at 700 °C for 2 h. These oligo-chitosan and oligo-chitosan-silica NPs were employed on soybean seed yield in the experimental field by foliar application. The exciting results indicated an increased soybean seed yield by 10.5 and 17.0% for oligo-chitosan and oligo-chitosan silica NPs, respectively, over the control [37].
Somayyeh and Masouleh [38] evaluated the effect of chitosan and magnetism in lily yearling bulblets to synthesize photosynthetic compounds. Carboxymethyl chitosan (CM) and Magnetic nanocomposite (MN) were used during the production of the yearling bulblet. The results indicated that MN highly affected the photosynthetic pigments and the amount of starch in lily bulbs. The highest amount of CM showed soluble carbohydrates and amylase [38].
3.3. Polymeric chitosan nanoparticles (ChNPs)
Polymeric ChNPs could be synthesised from synthetic as well as natural polymers. These NPs could be used due to their simplicity to modify its surface and stability. Biopolymeric NPs have additional advantages such as accessibility from marine (for e.g. chitin and chitosan) or agricultural (such as starch, cellulose and pectin) resources, biocompatibility, biodegradability, and non-toxicity. Chitosan NPs are biodegradable polymers hence these are mainly studied as delivery systems for slow and controlled release of active ingredients, stabilization of biomolecules such as proteins, genetics materials, and peptides [39, 40]. In another research study, Pereira et al. [41] developed the two systems of polymeric ChNPs with the alginate/chitosan and chitosan/tripolyphosphate NPs for the delivery of plant hormone gibberellic acid (GA). These systems showed efficient changes on both morphological and biochemical parameters, which resulting in increasing the leaf area and root length, and the chlorophylls level and carotenoids content in Phaseolus vulgaris (French beans) [41]. There are several systems have been developed that demonstrated the good potential by providing excellent stability and effectiveness of this plant hormone like GA in agriculture applications [42].
3.4. Encapsulated chitosan nanoparticles (ChNPs)
Encapsulation technique is essential for food processing, bioengineering industries, and agriculture fields. For the encapsulation of active food ingredients, immobilization of enzymes and as carriers of different molecules used in agriculture and fertilizers, chitosan has been widely employed in industries. In recent years, metal encapsulated chitosan-NMs have to pay more attention because of their dual activity as a plant protection agent and plant growth promoter [24].
The delivery of ChNPs loaded with nitrogen, phosphorus, and potassium (NPK) by foliar application on the wheat seedlings was investigated by Abdel-Aziz et al. [23]. Chitosan-NPK-NPs were quickly applied onto leaf surfaces and penetrated the stomata by gas uptake, evading direct interaction with soil systems. The results discovered that the NPs be taken up and transported via phloem tissue. When treated with nano chitosan-NPK fertilizer, wheat seedlings provoked a significant increase in wheat yield variables than respective control seedlings [23]. In another study, Choudhary et al. [25] synthesized Cu/Zn ChNPs and tested them against crop pathogenic fungal species like Culvularia lunata.
Furthermore, Cu/Zn ChNPs are involved in inducing enzymes amylase and protease related to the mobilization of food for seed germination [25]. To develop an effective nano delivery system, plant growth regulators may be encapsulated in the chitosan nanocarriers that slowly release the hormones with higher bioavailability. In another study, Pereira et al. [41] reported the growth-promoting effect of chitosan-gibberellic acid NPs in French beans that exhibited a 37% and 82% boosting in root development and leaf area, respectively, as compared to the free hormone gibberellic acid.
4. Importance of biologically synthesized chitosan NPs over other nanoparticles
Sathiyabama and Parthasarathy [17] biologically synthesized the ChNPs and evaluated their antifungal activity against some phytopathogenic fungi for e.g. Alternaria solani, F. oxysporum, and Pyricularia grisea. Whereas in another work, chitosan and ChNPs had been used to induce the biotic stress tolerance in tomato (Solanum lycopersicum) plant against bacteria such as gram positive and gram negative, fungi like Fusarium solani, and viruses like potato spindle tuber viroid (PSTV), bean/tomato bushy stunt virus (TBSV), and tobacco/TNV and also to initiate the immune response against them where ChNPs are used, which are sensed by plant PRR (pattern recognization receptor) which then induces the immune response in the plant [43].
Chitosan has an advantage over other NPs as they are very versatile and biocompatible, have low toxicity, and can be degraded easily, while other NPs can cause toxicity [44]. Chitosan NPs act as antimicrobial agents that show antibacterial, antifungal, antiviral, antioxidant activity, induce an immune response in the plant and helps in managing abiotic and biotic stresses. Since chitosan has low toxicity, nano-formulation of chitosan can be applied for the controlled-release of fertilizers, pesticides, and plant growth promoters in agriculture fields. Chitosan could also be employed for wastewater treatment, hydrating agents in cosmetics, in the food industry, in paper and textile industry as adhesive, as a drug-delivering agent in medical, and bioimaging [9].
The ChNPs and the chitosan-based agronanochemicals applied in agriculture could be organized by several methods, including emulsion cross-linking, precipitation, spray drying, ionic gelation, and sieving and reverse micellar processes [45]. The techniques mentioned are chemical and physical, which are having some demerits such as sieving method has been documented to fabricate NPs with irregular shape and size; however, the emulsion cross-linking process is relatively tedious and require cross-linking agents like alginate, formaldehyde, and glutaraldehyde, that may reason the impediments because of its incongruity with the active ingredients used as agrochemicals. The resultant particles size mostly depends upon the droplet size of the emulsion, which consecutively relies on the degree of cross-linking, surfactant type, the molecular weight of chitosan, and stirring speed [9]. The reverse micellar method is thermodynamically stable produced the uniformly distributed, small-sized chitosan nanoparticulate system. But this method needs a specialized surfactant solution like cetyl trimethyl ammonium bromide (CTAB), which is somewhat toxic and expensive; also, the process is quite laborious. Precipitation methods developed the ChNPs with no stability, irregular shape, and lower mechanical strength. The spray drying processes have been broadly employed to fabricate dry granules, pellet, and powder forms of chitosan. The techniques use the sequential addition of active ingredients and cross-linking agents to the chitosan solution dispersed in acetic acid. The precursor solution then underwent an evaporation method under hot air steam that ultimately forms the desired NPs.
The physical and chemical synthesis methods have several disadvantages, as described earlier, which leads to applying biological resources in the fabrication of ChNPs. The biologically synthesized ChNPs have several advantages: stability, regularity in shape and size, bioavailability, biocompatibility as the biologically active compounds taken part in the capping and the reducing process, non-toxic, and no added instrumentation or labor requirement, and so on. Therefore, the scientific community in recent years focuses on the green or biogenic synthesis of NPs using biological resources. In agriculture, nanoformulations are mainly aimed at enhancing the benefits of agrochemicals and chitosan though concurrently diminishing the undesirable results. Because of the amphiphilic nature, the encapsulation of chitosan may conquer the deprived solubility of several agrochemicals in water, provided that unusual use of inert chemicals in conventional agrochemicals, thus, tumbling their toxicity level [46]. Besides these, chitosan has provided excellent protection to the encapsulated agrochemicals because of their bioadhesive properties that enhance the stability and bioavailability in the plant. The efficacy of chitosan-based agronanochemicals, compared to conventional agrochemical to embark upon the actual tribulations faced by the agriculture industry, must be appraised by checking all the above parameters.
The biologically synthesized chitosan and chitosan-based agronanochemicals could be applied in different functions and play various roles as slow or controlled discharge formulations, plant growth enhancement, and biocidal or antimicrobial activity against plant pathogens and pests are described in detail in the sections below.
5. Other roles/functions of chitosan nanoparticles (ChNPs) in agriculture
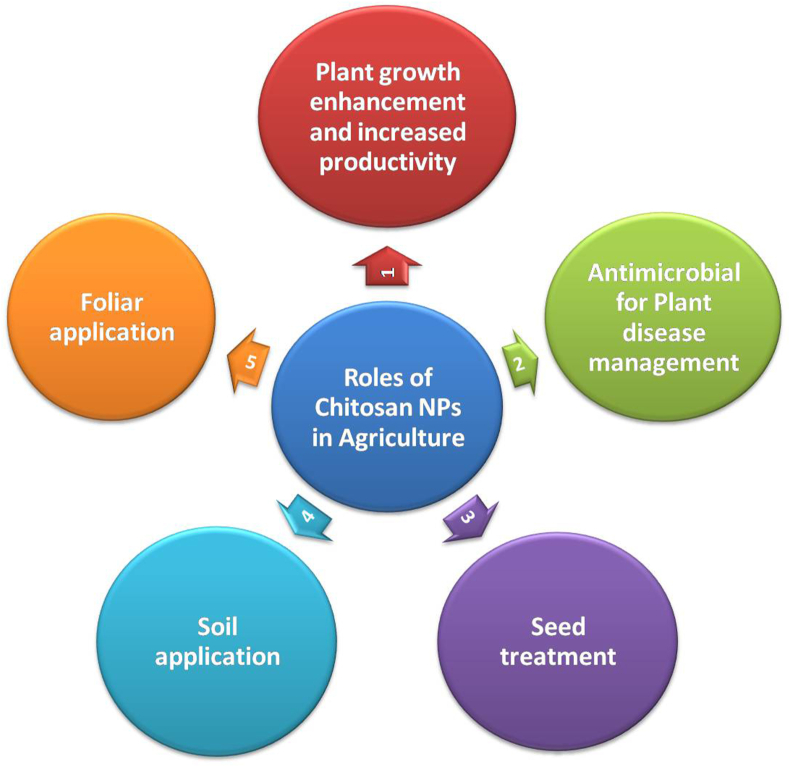
In the agriculture sector, ChNPs by themselves could act as an antimicrobial agent against the crop pathogenic microorganisms like fungi for eg. Pyricularia grisea, Alternaria solani, and Fusarium oxysporum and bacteria like gram positive and gram negative, and other insect pests like Aphis gossypii, Callosobruchus chinensis, and Callosobruchus maculatus and as a plant growth promoter [8, 9]. The formulations of ChNPs have the capability to enhance the plant defense mechanisms by obtaining the defense enzyme functions upon its application. Xing et al. [47] reported significant antifungal effect of oleoyl-chitosan nano-formulation against several pathogens. The comparative antimicrobial activity of ChNPs and bulk chitosan counterpart on A. solani, P. grisea, and F. oxysporum was investigated by Sathiyabama and Parthasarathy [48]. Treatment of seeds followed by foliar application with chitosan induces the resistance of tomato plants to Phytophtora infestans and A. solani [49]. Chitosan NPs application at anthesis (1000–5000 ppm, molecular mass 161–810 kDa, deacetylation degree 75–90%) is effective to control Fusarium head blight of wheat caused by Fusarium graminearum [50]. The anthracnose disease of cucumber caused by Colletotrichum spp. was efficiently controlled through foliar application of 0.05% and 0.1% chitosan [51]. Further the life cycle of the nano-fertilized wheat plants was shorter than normal-fertilized wheat plants (130 days compared with 170 days for yield production) [23]. Chitosan combined with waste silica may allow farmers to reduce the use of NKP fertilizers to improve corn production in Indonesia with environmental and economic advantages [52]. Maize seeds treated with Cu-ChNPs for 4 h (0.04–0.16%, Chitosan 50–190 kDa, deacetylation degree 80%), followed by spraying of plants every day for 35 days show enhanced plant height, stem diameter, root length and number, chlorophyll content, ear length and weight/plot, grain yield/plot and weight [26]. Besides this, they could also act as nanocarriers for some accessible agrochemicals that are generally referred to as chitosan-based agronanochemicals [7, 46]. The nanocarrier system facilitates the agriculturally active ingredients encapsulated by covalent or ionic inter or intramolecular bonds or entrapment in a chitosan polymeric matrix to develop an adequate formulation nano delivery system [7]. The recently reported studies on ChNPs have proven the various efficient ways of applying ChNPs in agriculture crops and fields as shown in Figure 4.
Figure 4.
Roles of chitosan nanoparticles (ChNPs) in agriculture.
As these ChNPs have shown their ability as a potent plant growth promoter [33], antimicrobial activity through various modes against pathogens of bacterial and fungal origin [53] and also induces an immune response in plants against viruses [43]. They may be employed directly to the soil as a soil applicant or a foliar applicant to show the systemic effect on the plant body [54]. The nanoformulations of chitosan are also applied for post-harvest uses as fruit nanocoatings to improve shelf life and prevent any damage due to microbes [47, 55]. In the above Table 1 the different types of ChNPs and their effect on the various crops has been presented.
5.1. Plant growth enhancement and increased productivity
In recent years, ChNPs and their nanoformulations have been extensively researched as a plant growth enhancer. The positively charged rich protonated chitosan demonstrates enhanced affinity towards the cell membranes, ensuing in increased reactivity in the plant system. In addition, chitosan has a nitrogen content of around 9–10% that serves as a macronutrient for the plant [45]. Alternatively, chitosan could be integrated with plant nutrients phosphorus (P), nitrogen (N), magnesium (Mg), potassium (K), sulfur (S), calcium (Ca), boron (B), iron (Fe), copper (Cu2+), manganese (Mn), zinc (Zn) and nickel (Ni). The application of ChNPs and micro/macronutrients nanocarrier in plant growth promotion in wheat, maize, French beans, and Robusta coffee, has been documented by several researchers [23, 24, 34, 56, 57, 58, 59, 60, 61, 62].
The nanoformulations of chitosan have been extensively applied as an unconventional method in seed treatment to promote the germination rate and enhance biomass accumulation. Furthermore, chitosan nanoformulations have been employed as a growth promoter by improving the nutrients uptake, chlorophyll content, and photosynthesis rate. For instance, chitosan oligomer with a high molecular weight and ChNPs of three variable average diameter sizes by High Resolution Transmission Electron Microscopy (HRTEM), i.e., small with 420 nm, medium with 750 nm, and 970 nm (large) size, when sprayed on the Robusta coffee seedlings leaves, demonstrated better nutrient uptake of Ca, K, N, Mg, P, etc., by ChNPs at all sizes, than that of chitosan oligomer. The nutrient uptake was affected; with the size was insignificant [56]. Alternatively, an impact of length of ChNPs could be observed on the content of chlorophyll and photosynthesis rate. The enhancement in the chlorophyll content and photosynthesis rate have been reported up to 61% and 29% for small, 81% and 59% for medium and 61% and 72% for large-sized NPs, respectively, by treating ChNPs. The treatment of ChNPs has also improved the vegetative growth of the seedlings compared to the chitosan oligomer treated and the untreated control seedlings [56]. In another study, Zayed et al. [60] demonstrated the abiotic stress (salinity stress) tolerance by Phaseolus vulgaris seedlings when supplementation of ChNPs was given to them.
Furthermore, chitosan-polymethacrylic acid-NPK NPs nanoformulation has been developed and applied for the wheat crop [23]. The efficacy of the nanoformulation was evaluated with the conventional bulk NPK fertilizer. The nanoformulation with 500 mg/mL of N, 60 mg/mL of P, and 400 mg/mL of K applied to wheat, evidenced for the plant height 41.29 cm, main spike weight 0.178 g, crop yield 6.95 g/plant, and harvesting index 26.94. At a similar quantity, the bulk NPK fertilizer evidenced for the same parameters were found the plant height 38.85 cm, main spike weight 0.136 g, crop yield 6.13 g/plant, and harvesting index 21.64, which showed the superior nanoformulation potential as plant growth and crop yield improvers of wheat [23]. The efficacy of copper sulfate (CuSO4), bulk chitosan, and Cu-ChNPs was investigated on the growth of maize seedlings by Saharan et al. [34] that showed the significant impact of the nanoformulations on the development of maize seedlings, α-amylase and protease activity, and total protein content. Depending upon the finding, it was assumed that the nanoformulations could facilitate penetration into seeds and, consequently, enhance seed metabolism; most probably, bulk chitosan may extend a film coating on the seed surface, which, therefore, prohibited their entrée to water as well as nutrients.
The development of an efficient nano delivery system of hormones could be possible by encapsulating the plant growth regulators into chitosan nanocarriers for slow release and with greater bioavailability. The plant growth regulators are nothing but the plant hormones, like auxins, cytokinins, gibberellins, ethylene, and abscisic acid, which are chemicals responsible for plant cell development and growth. Gibberellic acid-ChNPs demonstrated an increase of root development by 37% and in leaf area by 82% in French beans than free gibberellic acid [59]. Pereira et al. [59] highlighted the beneficial effects of the nanoparticulate systems by reporting the formation of the more lateral roots in the Phaseolus vulgaris seedlings supplemented with the γ-polyglutamic acid-gibberellic acid-ChNPs compared to the free hormones. The seeds of chickpea, when undergone the treatment of thiamine-ChNPs, showed a more significant germination percentage (90%) compared to the combination of Thiamine-chitosan (84%) and water control (75%) [62]. The seedlings treated with a nanoparticulate system demonstrated a 10-fold increase in auxin levels and more defense enzymes than the untreated control seedlings.
5.2. Antimicrobial for crop pathogens and pests to manage diseases in plants
In plant-pathogen control and disease management, chitosan alone or in combination with macronutrients could substitute effective, sustainable biocide agents against crop pathogens like bacteria, fungi, and viruses. Chitosan alone or in integration with other active agents demonstrated promising perspective as a sustainable choice to the conventional fungicide application against Fusarium wilt and head blight disease in chickpea and wheat, blast leaf of rice, stalk rot after flowering and leaf spot in maize, blast disease of finger millet, and other [9]. The formulations of ChNPs integrated by polyacrylic acid provide the tremendous potential to manage the attack of some common pests such as cotton aphids (Aphis gossypii) and beetles during soybean cultivation [8]. Many studies have discovered the ChNPs formulation to enhance the plant defense mechanisms by obtaining the defense enzyme functions. Additionally, Xing et al. [47] demonstrated the in-vitro antifungal activity of oleoyl-chitosan nanoformulation against some crop pathogenic fungi like Alternaria tenuissima, Botryosphaeria dothidea, Fusarium culmorum, Gibberella zeae, Nigrospora oryzae, and Nigrospora spaerica. From which A. tenuissima, N. oryzae, N. spaerica, and B. dothidea showed significant antifungal effects categorized as chitosan-sensitive fungi, while G. zeae and F. culmorum could be classified as chitosan-resistance fungi [47]. Chitosan (CS)-g-poly (acrylic acid) (PAA) NPs was found to be sensitive to the fungi like Aspergillus flavus (75%), F. oxysporum (30%), Aspergillus terreus (40%), Fusarium solani (41%), Alternaria tenuis (40%), and Sclerotium rolfsii (36%) [8]. Furthermore, the in-vitro spore germination and mycelial growth of Alternaria alternata (90%), Rhizoctonia solani (60%), and Macrophomina phaseolina (63%) was efficiently inhibited during Cu-ChNPs treatment [63]. The comparative antimicrobial activity of ChNPs and bulk chitosan counterparts on A. solani, P. grisea, and F. oxysporum was investigated by Sathiyabama and Parthasarathy [17]. The ChNPs exhibited a more significant percentage of mycelia growth inhibition than that of bulk chitosan. It has been accounted that the smaller size, higher porosity, and more significant zeta potential of ChNPs make it highly stable, which ultimately affects the tested fungal pathogens.
The bulk chitosan (BCS), ChNPs, and ChNPs supplemented by ethanolic blueberry extract (ChNPs-EBE) showed inhibitory effect on A. alternata; in this case, the trend was found to be ChNPs-EBE (83.3%), ChNPs (83.1%) > BCS (6%) only [64]. Their inhibitory effect on Colletotrichum gloeosporioides showed the trend as ChNPs-methanol extract (79.6%) > ChNPs (57%) > BCS (9.4%). In another study, Kheiri et al. [50] used chitosan with three variable molecular weights (MW), i.e., the lowest 161 kDa MW, medium 300 kDa, and highest 810 kDa, the fabrication of the nanoparticulate system. The formed NPs demonstrate lower zeta potential and a larger mean size with the MW increase and, therefore, lowered the in-vitro antifungal activity on Fusarium graminearum. The ChNPs of lower MW demonstrates 2-fold greater antifungal activity than NPs of medium and higher MW chitosan. This is because the smaller size facilitates the easy cell penetration and greater charge makes the ChNPs of lower MW more stable.
Furthermore, the formation of chitosan-agrochemical NPs, the chitosan nano delivery system was loaded with agrochemicals as the active agent that offer the proscribed release properties with higher efficiency and potency, as the active constituent could arrive at the target cell or parts of the plant more efficiently within a definite time [65]. The essential parameters considered in the design and development of chitosan-agrochemicals NPs involves active agent’s loading, encapsulation competence, discharge profile, particle size, and shape. Several studies reported and considered all these mentioned parameters during the design and development of these nanoformulations. Ye et al. [66] developed an herbicide (diuron) nanocarrier system as a photosynthetic inhibitor using cross-linking 2-nitro benzyl and carboxymethyl chitosan with the mean HRTEM diameter size of 140 nm to control the weed growth. A mechanism of photo-controlled release developed these nanoformulations. In another study, Kumar et al. [67] developed an intelligent formulation of alginate-chitosan nanocapsules with the size 30–40 nm diameter by HRTEM for the proscribed release of acetamiprid. In the present system, the proscribed release properties were accomplished at three different pHs, where a 50% of release of insecticide was observed at pH 10 after 24 h, and after 24 h at pH 7 and 4, compared to merely about 6 h for the conventional insecticide release at all pHs. Maluin and Hussein [9] reviewed the applications of chitosan-based agronanochemicals as a sustainable choice for the protection of crops, in which the authors have discussed the variable chitosan-based agronanochemicals as a controlled release formulation, as a plant growth enhancer, and as a biocide against the crop pathogens and pests.
5.3. Seed treatment
Seed treatment is a better and advanced approach as compared to soil amendment. It is a targeted and controlled delivery approach of active plant ingredients that reduces their overuse and decomposition in soil. A most recent approach is based upon maintaining the integrity of seed coat and simultaneously reducing water solubility using biodegradable hybrid coats of chitosan for seeds [68]. The strategy of chitosan application in seed treatment is considered as the primary artificial defense activation in plants against the different infectious agents. Differential characteristic features of chitosan at various molecular weights make it an excellent seed treating agent. The biopolymer with a high molecular weight of chitosan could be applied as a covering film around the seeds to protect the infection by pathogens [69]. Chitosan seed coating can also be used as a deliverance system for different products used in plant protection, fertilizers, and plant growth-promoting micronutrients [70]. Chitosan is used as a film for seed, which helps deliver fertilizers, micronutrients, and plant protection products such as essential oils and others. It helps elicit systemic confrontation in the plants [20].
5.4. Soil application
Although chemical-based fertilizers and pesticides have high and immediate impacts on crop yield, they also negatively affect the environment and consumers. Less than 0.1% of agrochemicals are delivered to plant systems, and the rest are washed off into the atmosphere [71]. Chitosan NPs are studied for their utilization in agriculture as a soil applicant to manage various fungal and bacterial diseases, as a nanofertilizer, and as an efficient delivery system for agrochemicals. The agrochemicals encapsulation in chitosan nanoformulations can offer a controlled release system for agrochemicals. This has helped to comparatively evaluate the effect of chitosan-based agronanochemicals and their counterparts on soil microbial populations. There is no significant effect of ChNPs on the soil enzyme activity and microbial population compared to the chemical fertilizers [9, 72]. Maruyama et al. [73] have shown an improved effect on microbial population after applying chitosan-alginate-herbicide NPs to soil [54, 73].
5.5. Foliar application
Numerous synthetic chemical fertilizers and pesticides prevent crops from attacking pests and diseases and provide nutrients such as nitrogen, phosphates, and minerals to increase agriculture productivity. But the use of synthetic fertilizers, pesticides cause damage to the quality and fertility of the soil. Moreover, the applied biomolecules are fully absorbed by the plants and the large quantity of these biomolecules' runoff into water bodies or leach in soil due to rain and irrigation. Nanotechnology has been demonstrated to help minimize the loss and enhanced the nutrient uptake by the plant. Due to its small size, this nanoformulation can reach deep into the soil [74]. These nutrients, growth hormones, and fertilizers are encapsulated in NPs and spray in the soil. The nanoformulation acts as a delivering agent and prevents the nutrient from coming in contact with soil microbes. They slowly release their content in the soil, which plants effectively take up. Due to its exceptional advantageous property, scientists employ chitosan in the agriculture field [75, 76].
Foliar application of ChNPs is used to increase the growth and production in the plant. Chitosan NPs get easily absorbed by leaves, penetrate the plant through stomata, travel down into the plant through the phloem, and provide nutrient to a different part [77]. Van et al. [78] worked on the biophysical characteristics of ChNPs and the greenhouse growth study of Robusta coffee. The ChNPs increased the nutrient uptake of nitrogen by 9.8–27.4%, phosphorous by 17.3–30.4%, and that potassium by 30–45%, and it also impacts coffee seeding growth. Abdel-Aziz et al. [77] demonstrated the foliar application of ChNPs-NPK fertilizer to improve wheat yield, developed on the two different soils. The foliar application increased the output of the wheat plant, and it also reduced the life cycle of the crop. Whereas in another work where they studied the foliar application of ChNPs-NPK fertilizer affected the chemical composition of wheat grains found a change in the composition of wheat grain with increased element content such as potassium and phosphorous while a decrease in nitrogen and protein content also accumulation of carbohydrates [77].
6. Conclusions and future perspectives
It can be concluded from the available literature that the ChNPs could be a versatile, biodegradable, and biocompatible, low toxicity, and easy degrading alternative to presently available agrochemicals. In combination with the other metallic and metal oxide NPs, they show a considerable range of activities. Chitosan NPs can be applied in various fields based on their properties such as antibacterial, antifungal, antiviral, antioxidant activity, inducing an immune response in the plant, and helps in managing biotic, and abiotic stress so they could be implicated in wastewater treatment, hydrating agents in cosmetics, food industries, the paper, and textile industries as adhesive, drug-delivering agent in medical, and bioimaging. But the thorough studies on product development and method optimization are required before commercial production and in vivo use. Various methods are used for the application of ChNPs in agriculture crops and fields. Chitosan NPs could be applied on crops by methods like dust and foliar sprays, soil application, and seeds treatment. Similarly, ChNPs explored in the ever-growing field of agriculture and sustainable agricultural practices. Chitosan NPs can be a potential substitute for toxic and non-degradable compounds with biocatalytic activity. As ChNPs are discovered to have the ability to encapsulate the various agro-supplements, they could be employed for the controlled deliberations of fertilizers, pesticides, and plant growth promoters in agriculture fields. The research community working in the area of crop protection and improvement will be the targeted beneficiaries. The ChNPs will be a cost-effective alternative for the use of toxic chemicals in the field of agriculture. Hence, green synthesised ChNPs would be a boon for the agriculture sector.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This work was supported by the Ministry of Science and Higher Education of the Russian Federation [No. 220-5234-7520].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest's statement
The authors declare no conflict of interest.
Additional information
No additional information provided.
Footnotes
This article is a part of the “Crop management using nanotechnology” Special issue.
References
- 1.Faqir Y., Ma J., Chai Y. Chitosan in modern agriculture production. Plant Soil Environ. 2021;67(12):679–699. [Google Scholar]
- 2.Vani R., Stanley S.A. Studies on the extraction of chitin and chitosan from different aquatic organisms. Adv. Biotech. 2013;12(12):12–15. [Google Scholar]
- 3.Papineau A.M., Hoover D.G., Knorr D., Farkas D.F. Antimicrobial effect of water-soluble chitosans with high hydrostatic pressure. Food Biotechnol. 1991;5(1):45–57. [Google Scholar]
- 4.Kong M., Chen X.G., Xing K., Park H.J. Antimicrobial properties of chitosan and mode of action: a state of the art review. Int. J. Food Microbiol. 2010;144(1):51–63. doi: 10.1016/j.ijfoodmicro.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 5.Campos E.V., Oliveira J.L., da Silva C.M., Pascoli M., Pasquoto T., Lima R., Abhilash P.C., Fernandes F.L. Polymeric and solid lipid nanoparticles for sustained release of carbendazim and tebuconazole in agricultural applications. Sci. Rep. 2015;5(1):1–4. doi: 10.1038/srep13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernández-Téllez C.N., Rodríguez-Córdova F.J., Rosas-Burgos E.C., Cortez-Rocha M.O., Burgos-Hernández A., Lizardi-Mendoza J., Torres-Arreola W., Martínez-Higuera A., Plascencia-Jatomea M. Activity of chitosan–lysozyme nanoparticles on the growth, membrane integrity, and β-1, 3-glucanase production by Aspergillus parasiticus. 3 Biotech. 2016;7(5):1–3. doi: 10.1007/s13205-017-0913-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashyap P.L., Xiang X., Heiden P. Chitosan nanoparticle-based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015;77:36–51. doi: 10.1016/j.ijbiomac.2015.02.039. [DOI] [PubMed] [Google Scholar]
- 8.Sahab A.F., Waly A.I., Sabbour M.M., Nawar L.S. Synthesis, antifungal and insecticidal potential of Chitosan (CS)-g-poly (acrylic acid) (PAA) nanoparticles against some seed borne fungi and insects of soybean. Int. J. ChemTech Res. 2015;8(2):589–598. [Google Scholar]
- 9.Maluin F.N., Hussein M.Z. Chitosan-based agronanochemicals as a sustainable alternative in crop protection. Molecules. 2020;25(7):1611. doi: 10.3390/molecules25071611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van P.D., Du B.D., Van T.H., Hien N.Q. Preparation and foliar application of oligochitosan-nanosilica on the enhancement of soybean seed yield. Int. J. Environ. Agric. Biotechnol. 2017;2(1) [Google Scholar]
- 11.Li R., He J., Xie H., Wang W., Bose S.K., Sun Y., Hu J., Yin H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.) Int. J. Biol. Macromol. 2019;126:91–100. doi: 10.1016/j.ijbiomac.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 12.Muley A.B., Shingote P.R., Patil A.P., Dalvi S.G., Suprasanna P. Gamma radiation degradation of chitosan for application in growth promotion and induction of stress tolerance in potato (Solanum tuberosum L.) Carbohydr. Polym. 2019;210:289–301. doi: 10.1016/j.carbpol.2019.01.056. [DOI] [PubMed] [Google Scholar]
- 13.Rizeq B.R., Younes N.N., Rasool K., Nasrallah G.K. Synthesis, bioapplications, and toxicity evaluation of chitosan-based nanoparticles. Int. J. Mol. Sci. 2018;16:101–112. doi: 10.3390/ijms20225776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini S.F., Rezaei M., Zandi M., Farahmandghavi F. Fabrication of bio-nanocomposite films based on fish gelatin reinforced with chitosan nanoparticles. Food Hydrocolloids. 2015;44:172–182. [Google Scholar]
- 15.Bari N.K., Fazil M., Hassan M.Q., Haider M.R., Gaba B., Narang J.K., Baboota S., Ali J. Brain delivery of buspirone hydrochloride chitosan nanoparticles for the treatment of general anxiety disorder. Int. J. Biol. Macromol. 2015;81:49–59. doi: 10.1016/j.ijbiomac.2015.07.041. [DOI] [PubMed] [Google Scholar]
- 16.Xu C., Zeng Y., Zheng X., Wang R.T. Surface-fluorinated and pH-sensitive carboxymethyl chitosan nanoparticles to overcome biological barriers for improved drug delivery in vivo. Carbohydr. Polym. 2019;208:59–69. doi: 10.1016/j.carbpol.2018.12.063. [DOI] [PubMed] [Google Scholar]
- 17.Sathiyabama M., Parthasarathy R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016;151:321–325. doi: 10.1016/j.carbpol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 18.Anitha A., Deepagan V.G., Divya V.V., Menon R.D., Nair S.V., Jayakumar R. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohydr. Polym. 2011;84(3):1158–1164. [Google Scholar]
- 19.Mukhopadhyay S.S. Nanotechnology in agriculture: prospects and constraints. Nanotechnol. Sci. Appl. 2014;7:63–71. doi: 10.2147/NSA.S39409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orzali L., Corsi B., Forni C., Riccioni L. In: Biological Activities and Application of marine Polysaccharides. Shalaby E., editor. InTech; 2017. Chitosan in agriculture: a new challenge for managing plant diseases; pp. 17–36. [Google Scholar]
- 21.Behboudi F., Sarvestani T.Z., Kassaee M.Z., Sanavi S.A.M.M., Sorooshzadeh A., Ahmadi S.B. Evaluation of chitosan nanoparticles effects on yield and yield components of barley (Hordeum vulgare L.) under late season drought stress. J. Water Environ. Nanotechnol. 2018;3:22–39. [Google Scholar]
- 22.Behboudi F., Tahmasebi-Sarvestani Z., Kassaee M.Z., Modarres-Sanavy S.A.M., Sorooshzadeh A., Mokhtassi-Bidgoli A. Evaluation of chitosan nanoparticles effects with two application methods on wheat under drought stress. J. Plant Nutr. 2019;42(13):1439. [Google Scholar]
- 23.Abdel-Aziz H.M., Hasaneen M.N., Omer A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Spanish J. Agric. Res. 2016;14 [Google Scholar]
- 24.Choudhary R.C., Kumaraswamy R.V., Kumari S., Pal A., Raliya R., Biswas P., Saharan V. In: Nanotechnology. Prasad R., Kumar M., Kumar V., editors. Springer; Singapore: 2017. Synthesis, characterization, and application of chitosan nanomaterials loaded with zinc and copper for plant growth and protection; pp. 227–247. [Google Scholar]
- 25.Choudhary R.C., Kumaraswamy R.V., Kumari S., Sharma S.S., Pal A., Raliya R., Biswas P., Saharan V. Cu-chitosan nanoparticle boost defence responses and plant growth in maize (Zea mays L.) Sci. Rep. 2017;7(9754):1–11. doi: 10.1038/s41598-017-08571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siddaiah C.N., Prasanth K.V.H., Satyanarayana N.R., et al. Chitosan nanoparticles having higher degree of acetylation induce resistance against pearl millet downy mildew through nitric oxide generation. Sci. Rep. 2018;8:2485. doi: 10.1038/s41598-017-19016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buchman J.T., Elmer W.H., Ma C., Landy K.M., White J.C., Haynes C.L. Chitosan-coated mesoporous silica nanoparticle treatment of Citrullus lanatus (watermelon): enhanced fungal disease suppression and modulated expression of stress-related genes. ACS Sustain. Chem. Eng. 2019;7:19649–19659. [Google Scholar]
- 28.Chookhongkha N., Sopondilok T., Photchanachai S. Effect of chitosan and chitosan nanoparticles on fungal growth and chilli seed quality. Acta Hortic. 2013;973:231–237. [Google Scholar]
- 29.Chandra S., Chakraborty N., Dasgupta A., Sarkar J., Panda K., Acharya K. Chitosan nanoparticles: a positive modulator of innate immune responses in plants. Sci. Rep. 2015;5 doi: 10.1038/srep15195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chandra S., Chakraborty N., Panda K., Acharya K. Chitosan-induced immunity in Camellia sinensis (L.) O. Kuntze against blister blight disease is mediated by nitric-oxide. Plant Physiol. Biochem. 2017;115:298–307. doi: 10.1016/j.plaphy.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 31.Khati P., Chaudhary P., Gangola S., Bhatt P., Sharma A. Nanochitosan supports growth of Zea mays and also maintains soil health following growth. 3 Biotech. 2017;7(1):81. doi: 10.1007/s13205-017-0668-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shah S., Hashmi M.S. Chitosan-aloe vera gel coating delays postharvest decay of mango fruit. Hortic. Environ. Biotechnol. 2020;61:279–289. [Google Scholar]
- 33.Saharan V., Sharma G., Yadav M., Choudhary M.K., Sharma S., Pal A., Raliya R., Biswas P. Synthesis and in vitro antifungal efficacy of Cu-chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015;75:346–353. doi: 10.1016/j.ijbiomac.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 34.Saharan V., Kumaraswamy R., Choudhary R.C., Kumari S., Pal A., Raliya R., Biswas P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016;64:6148–6155. doi: 10.1021/acs.jafc.6b02239. [DOI] [PubMed] [Google Scholar]
- 35.Hernández-Hernández H., González-Morales S., Benavides-Mendoza A., Ortega-Ortiz H., Cadenas-Pliego G., Juárez-Maldonado A. Effects of Chitosan-PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules. 2018;23(1):178. doi: 10.3390/molecules23010178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li R., He J., Xie H., Wang W., Bose S.K., Sun Y., Hu J., Yin H. Effects of chitosan nanoparticles on seed germination and seedling growth of wheat (Triticum aestivum L.) Int. J. Biol. Macromol. 2019;126:91–100. doi: 10.1016/j.ijbiomac.2018.12.118. [DOI] [PubMed] [Google Scholar]
- 37.Phu D.V., Du B.D., Tuan L.N.A., Tam H.V., Hien N.Q. Preparation and foliar application of Oligochitosan-Nanosilica on the enhancement of soybean seed yield. Int. J. Environ. Agric. Biotechnol. 2017;2(1):421–428. [Google Scholar]
- 38.Somayyeh S., Masouleh S. Increased assimilates in lily yearling bulblets by fertilizer supplement of magnetic nano-composite. Ornam. Horticul. 2019;25(3):247–254. [Google Scholar]
- 39.Ghormade V., Deshpande M.V., Paknikar K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011;29(6):792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Vodyashkin A.A., Kezimana P., Vetcher A.A., Stanishevskiy Y.M. Biopolymeric nanoparticles–multifunctional materials of the future. Polymers. 2022;14(11):2287. doi: 10.3390/polym14112287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira A.E.S., Silva P.M., Oliveira J.L., Oliveira H.C., Fraceto L.F. Chitosan nanoparticles as carrier systems for the plant growth hormone gibberellic acid. Colloids Surf. B Biointerfaces. 2017;150:141–152. doi: 10.1016/j.colsurfb.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Divya K., Jisha M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018;16:101–112. [Google Scholar]
- 43.Iriti M., Varoni E.M. Chitosan-induced antiviral activity and innate immunity in plants. Environ. Sci. Pollut. Res. 2015;22(4):2935–2944. doi: 10.1007/s11356-014-3571-7. [DOI] [PubMed] [Google Scholar]
- 44.Karlsson H.L., Toprak M.S., Fadeel B. Toxicity of metal and metal oxide nanoparticles. Handb. Toxicol. Met. 2015:75–112. [Google Scholar]
- 45.Agarwal M., Nagar D., Srivastava N., Agarwal M. Chitosan nanoparticles based drug delivery: an update. Int. J. Adv. Multidiscip. Res. 2015;2:1–13. [Google Scholar]
- 46.Campos E.V.R., de Oliveira J.L., Fraceto L.F., Singh B. Polysaccharides as safer release systems for agrochemicals. Agron. Sustain. Dev. 2015;35:47–66. [Google Scholar]
- 47.Xing K., Shen X., Zhu X., Ju X., Miao X., Tian J., Feng Z., Peng X., Jiang J., Qin S. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016;82:830–836. doi: 10.1016/j.ijbiomac.2015.09.074. [DOI] [PubMed] [Google Scholar]
- 48.Sathiyabama M., Parthasarathy R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2020;151:321–325. doi: 10.1016/j.carbpol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 49.Kiprushkina E.I., Shestopalova I.A., Pekhotina A.M., Kuprina E.E., Nikitina O.V. Protective-stimulating properties of chitosan in the vegetation and storing tomatoes. Progress Chem. Appl. Chitin Deriv. 2017;22:77–81. [Google Scholar]
- 50.Kheiri A., Jorf S.M., Malihipour A., Saremi H., Nikkhah M. Synthesis and characterization of chitosan nanoparticles and their effect on Fusarium head blight and oxidative activity in wheat. Int. J. Biol. Macromol. 2017;102:526–538. doi: 10.1016/j.ijbiomac.2017.04.034. [DOI] [PubMed] [Google Scholar]
- 51.Dodgson J.L., Dodgson W. Comparison of effects of chitin and chitosan for control of Colletotrichum sp. on cucumbers. J. Pure Appl. Microbiol. 2017;11(1):87–94. [Google Scholar]
- 52.Gumilar T.A., Prihastanti E., Haryanti S., Subagio A., Ngadiwiyana A. Utilization of waste silica and chitosan as fertilizer nanochisil to improve corn production in Indonesia. Adv. Sci. Lett. 2017;23:2447–2449. [Google Scholar]
- 53.Divya K., Vijayan S., George T.K., et al. Antimicrobial properties of chitosan nanoparticles: mode of action and factors affecting activity. Fibers Polym. 2017;18:221–230. [Google Scholar]
- 54.Bandara S., Du H., Carson L., Bradford D., Kommalapati R. Agricultural and biomedical applications of chitosan-based nanomaterials. Nanomaterials(Basel) 2020;10(10):1903. doi: 10.3390/nano10101903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Duan C., Meng X., Meng J., et al. Chitosan as a preservative for fruits and vegetables: a review on chemistry and antimicrobial properties. J. Bioresourc. Bioprod. 2019;4(1):11–21. [Google Scholar]
- 56.Minh H.D., Anh D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013;2:289–294. [Google Scholar]
- 57.Hasaneen M.N.A.-G., Abdel-Aziz H.M.M., Omer A.M. Effect of foliar application of engineered nanomaterials: carbon nanotubes NPK and chitosan nanoparticles NPK fertilizer on the growth of French bean plant. Biochem. Biotechnol. Res. 2016;4:68–76. [Google Scholar]
- 58.Deshpande P., Dapkekar A., Oak M.D., Paknikar K.M., Rajwade J.M. Zinc complexed chitosan/TPP nanoparticles: a promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017;165:394–401. doi: 10.1016/j.carbpol.2017.02.061. [DOI] [PubMed] [Google Scholar]
- 59.Pereira A.E.S., Sandoval-Herrera I., Zavala-Betancourt S., Oliveira H., Ledezma-Pérez A., Romero J., Fraceto L.F. γ-Polyglutamic acid/chitosan nanoparticles for the plant growth regulator gibberellic acid: characterization and evaluation of biological activity. Carbohydr. Polym. 2017;157:1862–1873. doi: 10.1016/j.carbpol.2016.11.073. [DOI] [PubMed] [Google Scholar]
- 60.Zayed M., Elkafafi S., Zedan A.M., Dawoud S.F. Effect of nano chitosan on growth, physiological and biochemical parameters of Phaseolus vulgaris under salt stress. J. Plant Prod. 2017;8:577–585. [Google Scholar]
- 61.Khalifa N.S., Hasaneen M.N. The effect of chitosan-PMAA-NPK nanofertilizer on Pisum sativum plants. 3 Biotech. 2018;8:193. doi: 10.1007/s13205-018-1221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muthukrishnan S., Murugan I., Selvaraj M. Chitosan nanoparticles loaded with thiamine stimulate growth and enhances protection against wilt disease in Chickpea. Carbohydr. Polym. 2019;212:169–177. doi: 10.1016/j.carbpol.2019.02.037. [DOI] [PubMed] [Google Scholar]
- 63.Ahuja I., Kissen R., Bones A.M. Phytoalexins in defense against pathogens. Trends Plant Sci. 2012;17:73–90. doi: 10.1016/j.tplants.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 64.Barrera-Necha L.L., Correa-Pacheco Z.N., Bautista-Baños S., Hernández-López M., Jiménez J.E.M., Mejía A.F.M. Synthesis and characterization of chitosan nanoparticles loaded botanical extracts with antifungal activity on Colletotrichum gloeosporioides and Alternaria species. Adv. Microbiol. 2018;8:286. [Google Scholar]
- 65.Duhan J.S., Kumar R., Kumar N., Kaur P., Nehra K., Duhan S. Nanotechnology: the new perspective in precision agriculture. Biotechnol Rep. 2017;1:11–23. doi: 10.1016/j.btre.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ye Z., Guo J., Wu D., Tan M., Xiong X., Yin Y., He G. Photo-responsive shell cross-linked micelles based on carboxymethyl chitosan and their application in controlled release of pesticide. Carbohydr. Polym. 2015;132:520–528. doi: 10.1016/j.carbpol.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 67.Kumar S., Chauhan N., Gopal M., Kumar R., Dilbaghi N. Development and evaluation of alginate-chitosan nanocapsules for controlled release of acetamiprid. Int. J. Biol. Macromol. 2015;81:631–637. doi: 10.1016/j.ijbiomac.2015.08.062. [DOI] [PubMed] [Google Scholar]
- 68.Pirzada T., de Farias B.V., Mathew R., Guenther R.H., Byrd M.V., Sit T.L., Pal L., Opperman C.H., Khan S.A. Recent advances in biodegradable matrices for active ingredient release in crop protection: towards attaining sustainability in Agriculture. Curr. Opin. Colloid Interface Sci. 2020;48:121–136. doi: 10.1016/j.cocis.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J.L., Zhao Y. Effect of molecular weight, acid, and plasticizer on the physicochemical and antibacterial properties of β-chitosan based films. J. Food Sci. 2012;77(5):E127–E136. doi: 10.1111/j.1750-3841.2012.02686.x. [DOI] [PubMed] [Google Scholar]
- 70.Malerba M., Cerana R. Chitosan effects on plant systems. Int. J. Mol. Sci. 2016;17(7):996. doi: 10.3390/ijms17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Camara M.C., Campos E.V.R., Monteiro R.A., Pereira A.E.S., de Freitas Proença P.L., Fraceto L.F. Development of stimuli-responsive nano-based pesticides: emerging opportunities for agriculture. J. Nanobiotechnol. 2019;17:100. doi: 10.1186/s12951-019-0533-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Namasivayam K.R.S., Aruna A., Gokila Evaluation of silver nanoparticles-chitosan encapsulated synthetic herbicide paraquat (AgNp-CS-PQ) preparation for the controlled release and improved herbicidal activity against Eichhornia crassipes. Res J Biotechnol. 2014;9:19–27. [Google Scholar]
- 73.Maruyama C.R., Guilger M., Pascoli M., Bileshy-José N., Abhilash P., Fraceto L.F., De Lima R. Nanoparticles based on chitosan as carriers for the combined herbicides imazapic and imazapyr. Sci. Rep. 2016;6 doi: 10.1038/srep19768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bautista-Díaz J., Cruz-Alvarez O., Hernández-Rodríguez O.A., Sánchez-Chávez E., Jacobo-Cuellar J.L., Preciado-Rangel P., Ávila-Quezada G.D., Ojeda-Barrios D. Zinc sulphate or zinc nanoparticle applications to leaves of green beans. Folia Hortic. 2021;33(2):365–375. [Google Scholar]
- 75.Shalaby T.A., Bayoumi Y., Abdalla N., Taha H., Alshaal T., Shehata S., Amer M., Domokos-Szabolcsy É., El-Ramady H. Nanoparticles, soils, plants and sustainable agriculture. Sustain. Agric. Rev. 2016:283–312. [Google Scholar]
- 76.Jatav G.K., De N. Application of nano-technology in soil-plant system. Asian J. Soil Sci. 2013;8(1):176–184. Corpus ID: 139340340. [Google Scholar]
- 77.Abdel-Aziz H.M., Hasaneen M.N., Omer A.M. Effect of foliar application of nano chitosan NPK fertilizer on the chemical composition of wheat grains. Egypt. J. Bot. 2018;58:87–95. [Google Scholar]
- 78.Van N.S., Minh H.D., Anh D.N. Study on chitosan nanoparticles on biophysical characteristics and growth of Robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013;2(4):289–294. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.