Idiopathic nephrotic syndrome (INS) describes a group of rare glomerular diseases characterized by massive proteinuria and hypoalbuminemia in the absence of glomerular inflammatory lesions or immunoglobulin deposits. INS includes two main entities based on kidney biopsy findings: minimal-change nephrotic syndrome (MCNS) and focal segmental glomerulosclerosis (FSGS). The treatment of INS relies on steroids and/or immunosuppressive drugs (calcineurin inhibitors, cyclophosphamide, mycophenolate mofetil, and rituximab). Distinct clinical patterns, such as steroid sensitivity with frequent relapses (in 70–80% of cases) and primary or secondary steroid-resistant forms, can be observed. INS is considered a chronic medical condition that interferes with well-being and health-related quality of life in childhood as well as adulthood.
While genetic studies have revealed rare forms of inherited nephrotic syndrome, they failed to identify any genetic defect, except an association with some variants of class II MHC antigens, in INS with relapse [1]. Studies on genetic polymorphisms in the variable region of the T-cell receptor (TCR) β-chain have revealed selective recruitment of some β chain variable gene families in peripheral CD8+ T cells from nephrotic patients with frequent relapses [2].
The hypothesis that INS results from systemic dysregulation of the immune system is supported by clinical observations, such as its sensitivity to steroid therapy and immunosuppressive drugs, as well as the rapid occurrence of relapses upon antigen challenge (infection or vaccination), particularly in children.
Several disorders of T-cell subpopulations have been reported during relapses [3]. The frequency of Tregs is consistently reduced [4] and is restored after response to therapy [3]. The decrease in Treg cells during relapse is correlated with a significant decrease in the production of IL-2, which is crucial to the development and maintenance of Treg cells. Experimental evidence suggests that Treg cells contribute to controlling the humoral response by limiting the expansion of T follicular helper (TFH) and B cells. Depletion of Treg cells increases antibody class switching in B cells, while adoptive transfer of Treg cells suppresses the in vivo B-cell response. Interestingly, Treg cells prevent the expansion of autoreactive B cells and induce their apoptosis [5], which suggests that a decrease in Tregs may lead to escape of autoreactive, potentially class-switched memory B cells, which may contribute to the development of autoimmune diseases. Indeed, relapses are associated with more rapid reconstitution of class-switched memory B cells [6].
The finding that rituximab, a B-cell-depleting agent, maintains long-lasting INS remission indicates a potential role for B lymphocytes in the mechanisms of INS pathogenesis. In a multicenter, double-blind, randomized controlled trial of placebo vs. rituximab, we analyzed the modifications of T-cell subsets induced by B-cell depletion [3]. Our results suggest that rituximab does not interfere with the frequencies of CD4+ T cells, CD8+ T cells or CD45RO+ T cells, while relapses are associated with a decrease in the CD8+ T cell subset and an increase in the subset of CD4+CD45RO+CD30+ circulating memory T cells, which are involved in recall antibody responses. On the other hand, rituximab specifically reduces the frequency of TFH cells. Interestingly, recent studies showed expansion of TFH cells during relapses that was associated with a defect in immunoglobulin class switching, accounting for the increase in IgM production and low production of IgG affecting some subclasses, via unknown mechanisms [7, 8]. In another study, it was shown that T cells of patients who responded to rituximab exhibited a weaker response to PMA/ionomycin activation, characterized by lower percentages of CD3+CD4+CD154+, IFNγ+CD3+ and IL-2+CD3+ T cell subsets than in nonresponders, suggesting that hyporesponsiveness to T-cell stimulation could be used to identify patients likely to respond to rituximab [9].
The role of B cells in INS pathogenesis remains to be clarified. The frequency of B-cell subpopulations before and after rituximab therapy has been investigated in pediatric patients, and these investigations showed that memory B cell recovery is faster in patients with relapse than in those without relapse [6]. Rituximab is thought to eliminate germinal center B cells in human lymph nodes without affecting the TFH cell population [10]. This raises the possibility that the delayed reconstitution of class-switched memory B cells could result from qualitative alterations in TFH cells induced by B-cell depletion. Indeed, the expansion of class-switched memory B cells requires cognate TFH cells, since it is abrogated in the absence of the IL-21 receptor or CD40 ligand [11].
Remission following B-cell depletion suggests that glomerular disease could be induced by some B lymphocyte subsets, such as autoreactive B cells, but some arguments support a more intricate mechanism: (i) immunofluorescence studies on renal biopsies consistently show the absence of Ig deposits in INS relapse, and (ii) remission can be maintained despite complete reconstitution of the peripheral B-cell compartment, while relapses can occur in the presence of sustained B-cell depletion [12]. In addition to their roles in antibody-mediated mechanisms, B cells are also involved in antigen presentation, T-cell activation/regulation and the production of cytokines and growth factors. Thus, B cells may facilitate disease activity by sustaining pathogenic T-cell responses through antibody-independent mechanisms. Therefore, although rituximab may be considered an innovative therapeutic agent for frequently relapsing, steroid-dependent INS, the mechanisms by which it interferes with T-cell dysregulation remain unclear.
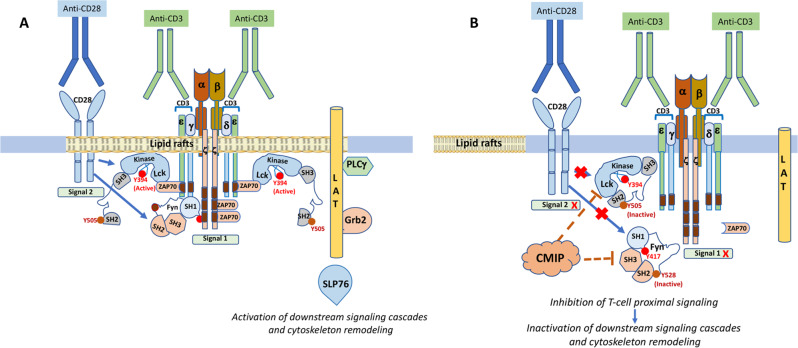
CMIP (CMaf-inducing protein) is a poorly characterized gene that was initially identified in T cells of patients with INS [13]. In normal lymphoid tissue, CMIP is selectively expressed in lymphoid follicles, mainly at the interface of B-cell and T-cell zones [14], while in peripheral T cells, CMIP is scarcely detected under resting conditions [13]; this pattern suggests that under physiological conditions, CMIP acts primarily in secondary lymphoid organs. On the other hand, CMIP was found to be highly expressed by a subset of yet-uncharacterized T cells. Selective expression of CMIP in peripheral T cells by targeted transgenesis in mice results in T-cell dysfunction. CMIP interferes with the early events in T-cell signaling by inhibiting lipid raft clustering and the activation of Src kinases (Fyn and Lck) and ZAP-70 (Fig. 1). Transgenic T cells exhibit a lower proliferative capacity and are less prone to produce cytokines, notably IL2, after stimulation [13]. Moreover, CMIP binds to the P85 subunit of PI3 kinase and prevents its dissociation from the p110 catalytic subunit, which results in inhibition of PI3 kinase activation and downstream signaling molecules, notably Akt. It is interesting to note that Akt activation is inhibited in vitro and in vivo in T cells and podocytes overexpressing CMIP, as well as in INS ([15] and manuscript in preparation). These observations may account for the lower reactivity of T lymphocytes to mitogens and the decrease in delayed hypersensitivity reported in patients with INS. However, a clear identification of CMIP function in the immune system is mandatory for a better understanding of its role in INS pathogenesis.
Fig. 1.
CMIP inhibits proximal T-cell signaling. A Costimulation by anti-CD3/CD28 antibodies mimics T-cell activation induced by ligation of the TCR to a peptide bound to a major histocompatibility complex (MHC) class II-antigen. In both cases, signal 1 (dependent on TCR ligation) and signal 2 (CD28 costimulation) trigger rapid Src kinase-mediated phosphorylation of the immunoreceptor tyrosine-based activation motif (ITAM), a conserved domain of signal-transducing chains of the TCR complex. Phosphorylated ITAMs serve as binding sites for the ZAP-70 kinase, which is activated by phosphorylation by the Src kinase Lck, resulting in stability of the ITAM-ZAP-70 interaction. The clustering of active TCRs and the recruitment of Lck/Fyn and ZAP-70 occur in lipid rafts (LRs), which are plasma membrane microdomains that are enriched in cholesterol and glycosphingolipids and serve as signaling platforms. Activation of ZAP-70 induces phosphorylation of the transmembrane adapter molecule LAT (linker for activation of T cells) and leukocyte phosphoprotein of 76 kDa (SLP-76) at multiple tyrosine residues, contributing to the assembly of a signaling complex in LRs through protein‒protein or protein–lipid interactions, which in turn leads to cytoskeletal reorganization, the formation of immunological synapses and efficient signal transmission from LRs to downstream signaling cascades, ultimately resulting in the activation of transcription factors such as NF-kB, NFAT, and AP-1. B Overexpression of CMIP inhibits the activation of Src kinases and ZAP-70, suggesting that CMIP interferes with proximal T-cell signaling and prevents the clustering of lipid rafts, reorganization of the cytoskeleton and activation of downstream signaling cascades
Competing interests
The authors declare no competing interests.
References
- 1.Lane BM, Cason R, Esezobor CI, Gbadegesin RA. Genetics of childhood steroid sensitive nephrotic syndrome: an update. Front Pediatr. 2019;7:8. doi: 10.3389/fped.2019.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank C, Herrmann M, Fernandez S, Dirnecker D, Boswald M, Kolowos W, et al. Dominant T cells in idiopathic nephrotic syndrome of childhood. Kidney Int. 2000;57:510–7. doi: 10.1046/j.1523-1755.2000.00870.x. [DOI] [PubMed] [Google Scholar]
- 3.Boumediene A, Vachin P, Sendeyo K, Oniszczuk J, Zhang SY, Henique C, et al. NEPHRUTIX: a randomized, double-blind, placebo vs Rituximab-controlled trial assessing T-cell subset changes in Minimal Change Nephrotic Syndrome. J Autoimmun. 2018;88:91–102. doi: 10.1016/j.jaut.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, et al. Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol. 2011;139:314–20. doi: 10.1016/j.clim.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 5.Zhao DM, Thornton AM, DiPaolo RJ, Shevach EM. Activated CD4+CD25+ T cells selectively kill B lymphocytes. Blood. 2006;107:3925–32. doi: 10.1182/blood-2005-11-4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Rava L, et al. B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol. 2016;27:1811–22. doi: 10.1681/ASN.2015050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kemper MJ, Altrogge H, Ganschow R, Muller-Wiefel DE. Serum levels of immunoglobulins and IgG subclasses in steroid sensitive nephrotic syndrome. Pediatr Nephrol. 2002;17:413–7. doi: 10.1007/s00467-001-0817-7. [DOI] [PubMed] [Google Scholar]
- 8.Yang X, Tang X, Li T, Man C, Yang X, Wang M, et al. Circulating follicular T helper cells are possibly associated with low levels of serum immunoglobulin G due to impaired immunoglobulin class-switch recombination of B cells in children with primary nephrotic syndrome. Mol Immunol. 2019;114:162–70. doi: 10.1016/j.molimm.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Chan CY, Liu ID, Resontoc LP, Ng KH, Chan YH, Lau PY, et al. T lymphocyte activation markers as predictors of responsiveness to rituximab among patients with FSGS. Clin J Am Soc Nephrol. 2016;11:1360–8. doi: 10.2215/CJN.11941115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallin EF, Jolly EC, Suchanek O, Bradley JA, Espeli M, Jayne DR, et al. Human T-follicular helper and T-follicular regulatory cell maintenance is independent of germinal centers. Blood. 2014;124:2666–74. doi: 10.1182/blood-2014-07-585976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zabel F, Fettelschoss A, Vogel M, Johansen P, Kundig TM, Bachmann MF. Distinct T helper cell dependence of memory B-cell proliferation versus plasma cell differentiation. Immunology. 2017;150:329–42. doi: 10.1111/imm.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rood IM, Huussen J, Wetzels JF, Deegens JK. Rituximab can induce remission of nephrotic syndrome in the absence of peripheral B-cells. Nephrology. 2015;20:667–8. doi: 10.1111/nep.12492. [DOI] [PubMed] [Google Scholar]
- 13.Oniszczuk J, Sendeyo K, Chhuon C, Savas B, Cogne E, Vachin P, et al. CMIP is a negative regulator of T cell signaling. Cell Mol Immunol. 2020;17:1026–41. doi: 10.1038/s41423-019-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Audard V, Zhang SY, Copie-Bergman C, Rucker-Martin C, Ory V, Candelier M, et al. Occurrence of minimal change nephrotic syndrome in classical Hodgkin lymphoma is closely related to the induction of c-mip in Hodgkin-Reed Sternberg cells and podocytes. Blood. 2010;115:3756–62. doi: 10.1182/blood-2009-11-251132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang SY, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, et al. c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal. 2010;3:ra39. doi: 10.1126/scisignal.2000678. [DOI] [PMC free article] [PubMed] [Google Scholar]