Summary
Here in we report the development of a Pt-V/CeO2 catalyst performing under mild conditions in amide hydrogenation. Ceria with different morphologies was employed as support in this study. We further developed a glycol-thermal technique that yields thermally stable quantum dot ceria, which can be applied as a support. A systematic investigation revealed the importance of proximity between the small crystalline hydrogenating sites (Pt) and oxophilic sites (V). The study showed that oxygen vacancies on the ceria surface oxidize both Pt and V, poisoning the hydrogenation reaction. In contrast, the absence of oxygen vacancies promoted the hydrogenating ability of Pt sites and also improved their ability to participate in the H2 spillover mechanism and in situ formation of oxophilic V3+. This study demonstrates how the engineering of the oxygen vacancies on the surface of the redox support can manipulate the nature of active sites toward specific reactions.
Subject areas: Catalysis, materials science, nanomaterials
Graphical abstract

Highlights
-
•
Preparations of ceria with different concentrations of oxygen vacancies
-
•
Preparation of quantum dot ceria using the glycol-thermal method
-
•
Oxygen vacancies on ceria facilitate the oxidation of loaded Pt and V
-
•
Low concentration of oxygen vacancies promotes the reduction of Pt and V
Catalysis; Materials science; Nanomaterials
Introduction
The reduction reaction is a commonly used transformation in organic chemistry and is extensively applied in laboratory and industrial processes.1 Considering the reduction of carbonyls within different functional groups, the carbonyl bonds within amides (carboxamides) are the hardest to reduce compared to other functional groups containing carbonyl groups.
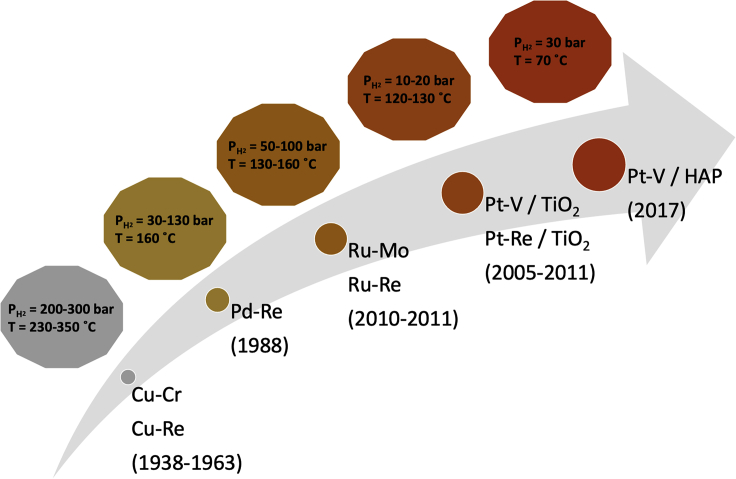
Therefore, high temperatures (230-350°C) and H2 pressures of 200-300 bar are required for their catalytic reduction using hydrogen.1,2 However, this reduction is important in the pharmaceutical, dye, and polymer industries.1,3,4 The difficulties associated with the reductive deoxygenation of amides are due to the tautomerization of this functional group between amide and imine structures. Therefore, both C-O and C-N bonds are partially unsaturated. Thus, it is of great importance to design a catalyst that directs the tautomerization reaction toward C=N formation (imine) and dehydration of the O-H group if one aims for selective reductive deoxygenation.1 Otherwise, a mixture of alcohols, products of C-N cleavage, and amines, products of C-O scission, will be obtained. As shown in Figure 1, most of the catalysts applied for amide hydrogenation are bimetallic catalysts composed of hydrogenating and oxophilic metals. Recent advancements in catalyst development for this application have helped overcome the thermodynamics for this challenging reaction.5,6,7,8 Work done by Mitsudome et al. using state of the art Pt-V/HAP (hydroxyapatite) drastically lowered both the temperature and pressure required for amide hydrogenation compared to what has been reported in the past.8 In their study, Pt sites were decorated with V. They showed that both Pt and V have small crystallite sizes and have proximity. Pt was found to assist in situ reduction of V5+ to V3+ with high oxophilicity.8
Figure 1.
The progressive path towards developing bimetallic heterogeneous catalysts for amide hydrogenation1
Pt is known as an efficient active metal for hydrogenation reactions.9,10 Furthermore, ceria as support for Pt is known to bring some advantages to the catalysts, such as better hydrogenation ability and a hydrogen spillover mechanism.11,12,13 The latter phenomenon allows the surface coverage with atomic/ionic H during the hydrogenation reaction, which enhances the hydrogenation rate and eases the in situ reduction of the second metal oxide (if present).14 Furthermore, it is known that ceria, depending on the exposed facet on the surface, the surface area, and the concentration of oxygen vacancies, can cause significant changes to the behavior of the active sites.15 Therefore, we decided to explore the influence of the support’s physiochemical properties in Pt-V/CeO2, where the ceria morphology is altered. We have discovered that ceria with different morphology16,17,18,19 contains different concentrations of oxygen vacancies that not only affect the oxidation states of both platinum and vanadium on the surfaces of these catalysts, but also control the performance of the hydrogen spillover mechanism during the hydrogenation of 4-acetylmorpholine. Our study provides direct evidence of the extraordinary ability of ceria (redox support) in controlling the properties of the loaded metals, which is very important for heterogeneous catalysts applied in hydrogenation or oxidative reactions.
Results and discussion
Active metal distribution and supports’ morphologies
To first establish the effect of preparation techniques on the properties of the final catalysts, the reference catalyst Pt-V/HAP was prepared via the reported method of Mitsodume et al.8 We obtained a catalyst where Pt and V showed close proximity as per the reference study (Figures S1E and S1F). In contrast, the application of H2PtCl6 as a Pt precursor and water as the reaction medium resulted in a catalyst with totally different properties (Figures S1C and S1D). The crystallite sizes of Pt were much larger, and the closeness of Pt and V was not apparent. Thus, the preparation technique using the organometallic precursors (as done in the study by Mitsodume et al.)8 and organic solvents as the media, appears to be a powerful technique in synthesizing bimetallic catalysts where the synergistic relationship between these two metals is essential. According to fundamental studies on the acac precursors used in this study, Pt(acac)2 and VO(acac)2 have similar structural geometries of square planar and square pyramidal, respectively.20,21 This allows them to adsorb on the same sites on the surface of the support, where they remain even after calcining and decomposing the ligands coordinated to these metals in their complex forms.20,21 Therefore, Pt and V remain in close proximity on the final catalyst surface. Furthermore, the use of a low boiling point organic solvent like acetone (in the impregnation technique) with minimal interaction with applied supports (due to the hydrophobic nature of acetone), allowed for synthesizing supported materials containing active sites with small crystallite sizes, because the solvent can be removed at room temperature and there is minimal effect of the solvent altering the surface properties of the support during catalyst preparation. The obtained crystallite sizes for Pt correspond well to the Pt size measured by Mitsudome et al. where Pt-V/HAP was studied. Therefore, we decided to use the acac method to load Pt and V on the support (including the reference catalyst) for this study. Ceria (as support) with different morphologies, was prepared using the hydro-thermal and glycol-thermal methods.22,23,24,25 In the naming applied for these catalysts, LS, NS, MM, NR, and NC refer to catalysts containing ceria with large sphere, nanosphere, mixed morphology, nanorods, and nanocube morphologies, respectively. The ceria with different morphologies is in the form of the fluorite phase with a face-centered cubic Bravais lattice (fcc).26 Different morphologies allow the exposure of different facets on their surfaces.
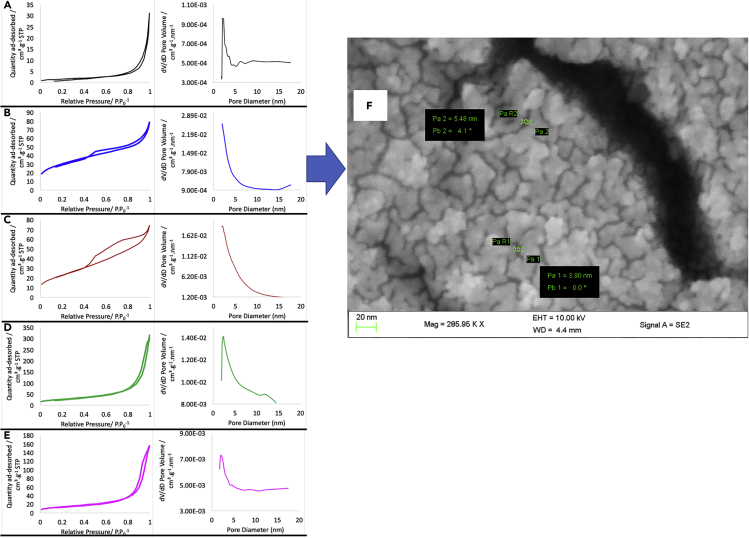
The final catalysts Pt-V/CeO2 (with different morphologies) showed different porous structures based on the obtained physisorption data (BET surface areas, pore sizes, and pore volumes are provided in Table S3). N2 adsorption-desorption isotherms of Pt-V/CeO2 (LS), Pt-V/CeO2 (NR), Pt-V/CeO2 (NC) consisted of hysteresis loops at high relative pressures indicating that the voids between the particles resulted in their porosity (Figure 2). In contrast, Pt-V/CeO2 (NS) and Pt-V/CeO2 (MM) showed type IV isotherms with H1 hysteresis loops with capillary condensation at lower P/P0. This indicates the presence of mesopores within the particles present in Pt-V/CeO2 (NS) and Pt-V/CeO2 (MM) as viewed using SEM (Figure 2).
Figure 2.
Porosity of the different Pt-V/CeO2 catalysts
N2 adsorption-desorption isotherms and pore size distribution of (A) Pt-V/CeO2 (LS), (B) Pt-V/CeO2 (NS), (C) Pt-V/CeO2 (MM), (D) Pt-V/CeO2 (NR) and (E) Pt-V/CeO2 (NC). (F) SEM image of mesopores over Pt-V/CeO2 (NS).
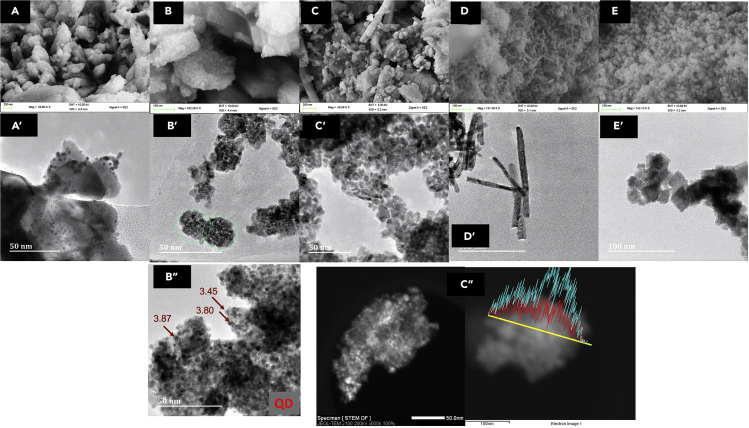
The HR-TEM and SEM images of these catalysts (Figure 3) showed that the targeted surface morphologies were obtained and remained intact during the material preparation. As evidenced by the EDX line scan of Pt-V/CeO2 (MM) shown in Figure 3, close proximity of Pt and V sites exists for these catalysts. Interestingly, the nanospheres within the Pt-V/CeO2 (NS) catalyst, prepared via a new modified glycol-thermal method,22 is composed of a grouping of several thermally stable unsupported quantum dot (QD) ceria units which are known to have extraordinary properties (particle size distribution is provided in Figure S2).27,28 Having nanospheres made of QD ceria resulted in a high surface area of 107 m2/g for this catalyst. To the best of our knowledge, obtaining unsupported QD ceria that is thermally stable (due to calcination at high temperature) has not been reported before. Applying this material as a support (carrier) will likely offer a new field for ceria chemistry in material sciences and heterogeneous catalysis.
Figure 3.
Electron microscope images of the different Pt-V/CeO2 catalysts
HR-TEM and SEM of images (A and A’) Pt-V/CeO2 (LS), (B and B’) Pt-V/CeO2 (NS) [the green circles show the formed nano-spheres, (C and C’) Pt-V/CeO2 (MM), (D and D’) Pt-V/CeO2 (NR) and (E and E’) Pt-V/CeO2 (NC). (B”) The small sizes of QD ceria were observed in Pt-V/CeO2 (NS) using HR-TEM. (C”) EDX-HR-TEM line scan of Pt-V/CeO2 (MM) highlights Pt and V’s proximity.
Catalytic deoxygenative hydrogenation of amide to amine
The catalysts were tested for the hydrogenation of 4-acetylmorpholine to n-ethyl morpholine as a model for the amide hydrogenation reaction. The results obtained for amide hydrogenation using heterogeneous catalysts in a high-pressure reactor depend highly on the reactor design.1 Therefore, we prepared the Pt-V/HAP catalyst via the procedure reported by Mitsudome et al.8 and tested it as a reference catalyst to establish a baseline for our study. In our reactor setup, we obtained a 45% yield to n-ethyl morpholine at 90°C and 30 bar of H2 pressure (reference values: yield = 70% at T = 70°C and p = 30 bar). The difference in reactor setup required us to operate at 90°C to obtain a comparable yield to that reported at 70°C over the same catalyst. Consequently, we chose 90°C for further testing. Furthermore, we tested the catalysts prepared using H2PtCl6 instead of Pt(acac)2 and observed no activity, indicating the critical role of the synthetic technique in obtaining a synergistic bimetallic catalyst.
Interestingly, Pt-V/CeO2 (NS) and Pt-V/CeO2 (MM) provided the highest yield to N-ethylmorpholine, 73%, and 79%, respectively (Table 1). These values are higher than the reference catalyst8 tested in our study. The monometallic catalysts did not show any activity signaling the importance of having bimetallic (bifunctional catalysts). Further optimization was done, and the results are provided in Table S1.
Table 1.
The catalytic hydrogenation of 4-acetylmorpholine to n-ethyl morpholine
| Catalyst | Temp./oC | Press./bar | Reaction time/h | Yield/% |
| Pt-V/HAPa | 70 | 30 | 1 | 70 |
| Pt-V/HAPb | 90 | 30 | 3 | 45 |
| Pt-V/CeO2 (LS) | 90 | 30 | 3 | 13 |
| Pt-V/CeO2 (NS) | 90 | 30 | 3 | 73 |
| Pt-V/CeO2 (MM) | 90 | 30 | 3 | 79 |
| Pt-V/CeO2 (NR) | 90 | 30 | 3 | 41 |
| Pt-V/CeO2 (NC) | 90 | 30 | 3 | 37 |
| Pt/CeO2 (MM) | 90 | 30 | 3 | 0 |
| V/CeO2 (MM) | 90 | 30 | 3 | 0 |
| CeO2 (MM) | 90 | 30 | 3 | 0 |
Catalyst loading: 0.1 g, Reaction scale = 0.5 mmol of amide.
HAP, Hydroxyapatite; LS, Large-sphere; NS, Nano-sphere; MM, Mixed morphology; NR, Nanorods; NC, Nanocubes.
The reported values are obtained from reference 8.8
These results are obtained using an in-house reactor over the reference catalyst.
Correlation between oxygen vacancies and ease of hydrogenation
Raman analysis was done (Figure 4A) to study the concentration of the oxygen vacancies (Ov) in the different catalysts. The peak at 590-595 cm−1 is due to the presence of Ov in the ceria lattice. The F2g peak that appears at 500 cm−1 is assigned to the fluorite structure of ceria (the symmetric bending of the oxygen ions surrounding the cerium ions).29 Ov/F2g can be used to establish the relative concentration of Ov in the structure of ceria-based catalysts. Based on Raman analysis the concentration of Ov in the ceria lattice of the studied catalysts followed the order of Pt-V/CeO2 (NR) > Pt-V/CeO2 (NC) > Pt-V/CeO2 (NS) > Pt-V/CeO2 (MM) > Pt-V/CeO2 (LS) (Table S2).
Figure 4.
Oxygen vacancy identification and reducibility of the Pt-V/CeO2 catalysts
(A) Raman spectra of the catalysts.
(B) H2-TPR analyses of the various catalysts in the temperature range of 50-300°C. The areas under the blue peaks are assigned to easy to reduce PtOx species (<100°C). The areas under the red peaks are assigned to hard to reduced PtOx species (>100°C).
H2-TPR analysis was done to gain insight into different types of PtOx in these catalysts (Figure 4 and Table S4). Based on XPS data, it was found that the majority of VOx species in these catalysts were V2O3 which was difficult to reduce, in agreement with Mitsudome et al.’s findings who used the same technique and precursor to load V over HAP.8 This phase of vanadia reduces at temperatures higher than 500°C. Also, ceria reduces at temperatures higher than 450°C. Since the focus of this H2-TPR study was to investigate the ease of reduction of loaded PtOx over ceria with different morphologies, the H2-TPR experiments were carried out in the temperature range of 50-300°C. The large differences between the acquired H2-TPR curves indicate the significant contribution of the support morphology to the performance of the catalysts by providing them with different concentrations of active sites, oxygen vacancies, and so forth. The exposed facets on the surface of the support appear to influence the metal-support interactions between PtOx species and ceria. It is known that bulk PtO and PtO2 with no metal support interactions (MSI) reduce at temperatures below room temperature. Therefore, these peaks are assigned to Pt2+ that interact with ceria in the form of Pt-O-Ce.30,31 Considering this fact, the differences noted in the reduction temperature of PtOx species in these catalysts are merely the result of MSI, thus it was found more applicable to present them as hard and easy to reduce PtOx species. Therefore, the catalysts composed of ceria with large spheres, nanocubes, and nanorods mainly contained hard to reduced PtOx species. In contrast, catalysts with mixed morphologies and nanospheres containing ceria of QD size showed a high concentration of easily reducing PtOx on their surfaces. In addition, the catalysts comprising the highest concentration of oxygen vacancies within their structures (Pt-V/CeO2 (NR) and Pt-V/CeO2 (NC)) were found to delay the reduction of PtOx species. However, the catalysts with a relatively low concentration of oxygen vacancies in their structures (Pt-V/CeO2 (MM) and Pt-V/CeO2 (NS)) facilitated the low-temperature reduction of PtOx species on the surfaces of these catalysts. Therefore, an inverse relationship between the ease of reduction of PtOx and both crystallite size of ceria (support) and the concentration of Ov vacancies in these Pt-V/CeO2 catalysts can be concluded.
Effect of oxygen vacancies on oxidation states of loaded metals on the surface
XPS analysis was done to study the surfaces of these catalysts. Six materials, namely CeO2 (MM), Pt-V/CeO2 (MM), Pt/CeO2 (MM), V/CeO2 (MM), CeO2 (NR), and Pt-V/CeO2 (NR) were subjected to XPS analysis. The Pt-V/CeO2 (MM) and Pt-V/CeO2 (NR) catalysts were chosen to represent the best and worst catalysts in the study to identify the natures of active sites for this hydrogenation reaction over them.
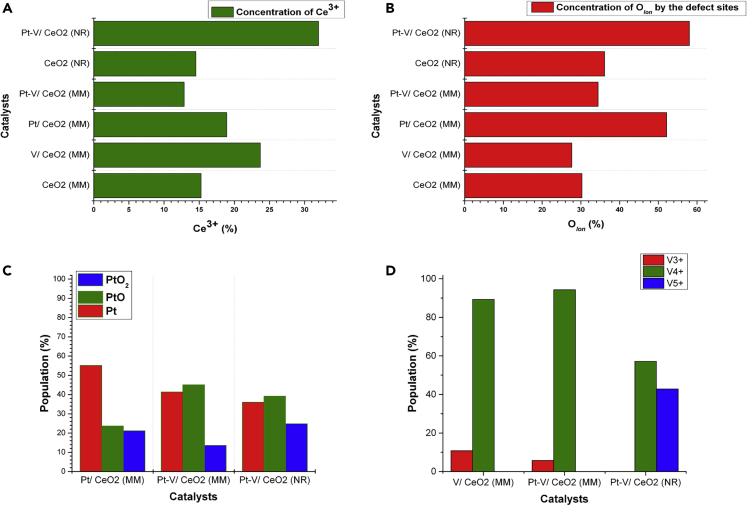
XP spectra of Ce 3d lines in the different catalysts were used to measure the concentration of Ov on each surface (Figure 5A). It was found that both CeO2 (MM) and CeO2 (NR) morphologies contain almost the same amounts of Ce3+ (correlates directly to the concentration of surface Ov) on their surfaces. The addition of either Pt or V (in monometallic form) increased the concentration of Ov on the surface of CeO2 (MM). However, this effect was suppressed when both Pt and V were loaded on the surface of CeO2 (MM). This data clearly shows that PtOx and VOx on the surface of CeO2 cause the reduction of ceria on the point of contact between the active metal (oxides) and the support with redox properties via MSI (metal support interaction). However, this phenomenon was not seen when close proximity of Pt and V was established, suggesting the electronic exchanges between two metals (oxides) minimizes their electronic requirements from the support. Thus, similar concentrations of oxygen vacancies on both CeO2 (MM) and Pt-V/CeO2 (MM) surfaces were noted. However, this discussion does not apply when the concentration of oxygen vacancies on CeO2 (NR) and Pt-V/CeO2 (NR) surfaces are compared. These results indicate that the measured concentration of oxygen vacancies in the final catalysts (Raman) does not necessarily come from the original support. The ceria with different exposed facets interacts differently with the redox-active sites (Pt and V in this case), which alters the concentration of oxygen vacancies detected in the original support.
Figure 5.
XPS of the CeO2-based materials
(A) The concentration of Ce3+ (Ov) on the catalyst surface was obtained by analyzing the XPS of the Ce 3d lines.
(B) Concentration of ionic oxygen species in the different materials obtained from studying the XPS of O 1s lines.
(C) Quantifying different oxidation states of Pt on the catalysts’ surface by studying the XPS of Pt 4f.
(D) Quantifying different oxidation states of V on the catalysts’ surface by studying the XPS of V 2p.
Contributing factors in determining the ability of catalysts in amide hydrogenation
According to published articles, ceria with nanorod morphology has exposed (110) facets on its surface, whereas this facet is much less exposed in the catalyst with mixed morphology.32,33,34 The interaction between the exposed (110) facet on the surface of CeO2 (NR) and PtOx and VOx loaded over it (in Pt-V/CeO2 (NR)) resulted in different charge transfer effects to what was discussed for Pt-V/CeO2 (MM). In the case of Pt-V/CeO2 (NR), the proximity of Pt and V (oxides) does not switch the electron exchange between PtOx-CeO2 or VOx-CeO2 to PtOx-VOx modes. As a result, the bimetallic Pt-V/CeO2 (NR) showed to have a high surface oxygen vacancy content since both PtOx and VOx contributed toward ceria reduction to Ce3+ (as they did in the case of the monometallic CeO2 (MM) catalysts) (Figures 5A and 5B). These results support stronger MSI in Pt-V/CeO2 (NR) than Pt-V/CeO2 (MM). The concentration of surface Ov calculated using Ce3+ content in the XPS of the Ce 3d line was similar to the calculated concentration of ionized oxygen ions in the XPS of O 1s with slight variation, likely caused by the observation of O ions in all oxides (PtOx, VOx, and CeO2) instead of specifically for CeO2.
Studying the 4f orbital of PtOx in the Pt/CeO2 (MM) (Figure 5C) catalyst using XPS showed that more than 50% of Pt is in the metallic state, while approximately 20% each is present as PtO and PtO2. However, these ratios were different for Pt-V/CeO2 (MM). The co-addition of V resulted in lowering of the Pt° content (40%), an increase in PtO content (45%) and a decrease in PtO2 content (15%) when Pt/CeO2 (MM) and Pt-V/CeO2 (MM) were compared. Pt-V/CeO2 (NR) showed the highest concentration of PtO2 (25%) and the lowest Pt° content (36%). The PtO content in this catalyst was calculated to be (39%). Based on these XPS results, the addition of V into these catalysts oxidized the Pt species, and the change of support from mixed morphology to nanorods further catalyzed the oxidation of Pt species.
XPS analysis of V 2p orbitals (Figure 5D) in V/CeO2 (MM) and Pt-V/CeO2 (MM) showed V4+ as dominating species (89 and 94%, respectively). The remaining V species were V3+ which has high oxophilicity. The co-existence of Pt with V favored less formation of V3+ on the catalyst surface. Conversely, no V3+ was detected in Pt-V/CeO2 (NR), and high V4+ (58%) and V5+ (42%) contents on the surface of this catalyst were measured. This surprising result highlights the extraordinary capability of ceria with nanorods morphology and exposed (110) facets to oxidize the loaded metals (Pt and V) over it at the price of Ov formation on its surface. This discovery explains well why ceria with nanorod (or nanocube) morphology is popular for oxidative catalysis but not for hydrogenation.35 The formation of high in abundance V5+ with poor oxophilic properties and hard to reduce PtOx species on the surface of this catalyst explains the poor catalytic data obtained for this catalyst.
Both XPS results and H2-TPR data showed that the presence of oxygen vacancies on the surface of ceria results in the oxidation of the hydrogenating sites and makes their reduction more difficult. This correlation is better shown in Figure 6. An inverse relationship between the concentration of oxygen vacancies (calculated using Raman) and the specific reaction yield (yield/surface area) was noticed. Furthermore, ceria with a high oxygen vacancy content can oxidize the Pt and V loaded on it (VOx with oxophilic properties in this study). These two metals (Pt and V) thus consume the oxygen from the surface of the ceria to remain in the oxidized phase. Therefore, using ceria with a high oxygen vacancy concentration has an adverse effect on reduction reactions where the active sites are metals in the reduced state. In contrast, literature has shown that it provides a significant advantage to oxidative reactions (metal oxides as active sites).36,37,38 Ceria is well known to be a redox support and an oxygen reservoir.15 Therefore, the oxygen vacancies on its surface act differently from other materials because these defect sites become centers to promote the migration of lattice oxygen from the bulk of ceria to the surface.39 As a result, the surface of ceria is highly oxidized, and these oxidizing properties increase as the concentration of oxygen vacancies on the surface increases. The oxygen vacancies act as oxygen shuttles to the surface of the ceria, as discussed in part, in other literature. Now considering that the oxygen vacancies on the surface of ceria induce the movement of oxygen from the bulk to the surface of this support, it expresses why the lower concentration of oxygen vacancies eases the reduction of supported metal oxides. This is indeed a very ceria-specific case. The effect of oxidizing support (ceria) can only be discussed for catalysis that follows a heterogeneous mechanism with an interaction between the support and the active metals. Recyclability tests were performed to confirm whether this catalyst activates amide via heterogeneous or homogeneous pathways. Interestingly, the best catalyst (Pt-V/CeO2 (MM)) in this study could maintain its performance for at least three cycles. This further supports that this reaction proceeds via a heterogeneous mechanism since the discussed support’s effects in the formation of desired active sites cannot be argued if any metal ions dissolved in the solution were responsible for the achieved recyclability results. Besides this fact, there is no study showing that Pt in ionic forms (dissolved in the reaction media) can catalyze this challenging hydrogenation reaction.40
Figure 6.
Activity of the different catalysts in the hydrogenation of 4-acetylmorpholine and recyclability data
(A) Correlation between the specific yields (yield/surface area) and the concentration of oxygen vacancies (from Raman analysis) in different catalysts.
(B) Recyclability test of Pt-V/CeO2 (MM) for hydrogenation of 4-acetylmorpholine to n-ethyl morpholine.
Based on other reports15 and our work, we find the oxygen vacancies to be key to the unique chemistry that ceria offers to materials when used as a support. They strongly contribute to the oxidation states of the supported metals on them. Therefore, carefully engineering them is significant to their enhanced catalytic activity. Hence, ceria, with no oxygen vacancies can sometimes be a better support for hydrogenation reactions than carbon-based materials. On the contrary, it can be an excellent support for oxidation catalysis if it consists of a high concentration of oxygen vacancies on its surface.
Conclusion
Successful recyclable catalysts were developed for the hydrogenation of 4-acetylmorpholine to n-ethyl morpholine. The crystallite sizes of Pt and V sites and their proximity were found to be the key to achieving any activity over these Pt-V/CeO2 catalysts. In experiments toward preparing ceria with different morphologies, we discovered a new procedure to prepare unsupported thermally stable quantum dot ceria that can be employed as a support in heterogeneous catalysis. Furthermore, we found that oxygen vacancies are responsible for the oxidation of the active sites, which is undesired for the hydrogenation reaction but beneficial for the oxidation reaction. Ceria with nanorod morphology interacts strongly with Pt and V, thus forming a high concentration of oxygen vacancies on its surface to strengthen the bonding and oxidizing ability. In contrast, ceria with mixed morphology does not catalyze the oxygen vacancy formation and, thus, maximizes the synergistic interactions between Pt and V, with minimal interactions with the support. Therefore Pt-V/CeO2 with mixed morphology outperformed the other catalysts during the hydrogenation of the amide to the amine.
Limitations of the study
This catalytic reaction generates water (in situ), which poisons the catalytic sites. Therefore, molecular sieves should be included in the catalytic testing. Furthermore, the generality of the catalytic hydrogenation with other amides has not been investigated.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | CAS |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Water | Double distilled | Produced using distillation |
| Cerium(III) nitrate hexahydrate (99%) | Sigma-Aldrich | 10294-41-4 |
| Ethylene glycol (ReagentPlus, ≥99%) | Sigma-Aldrich | 107-21-1 |
| Propionic anhydride (≥99%) | Sigma-Aldrich | 123-62-6 |
| Ce(NH4)2(NO3)6 (ACS reagent, ≥98.5%) | Sigma-Aldrich | 16774-21-3 |
| NaOH (reagent grade, ≥98%, pellets (anhydrous)) | Sigma-Aldrich | 1310-73-2 |
| Pt(acac)2 (97%) | Sigma-Aldrich | 15170-57-7 |
| VO(acac)2 (98%) | Sigma-Aldrich | 3153-26-2 |
| Acetone (ACS reagent, ≥99.5%) | Sigma-Aldrich | 67-64-1 |
| Molecular sieves, 4 Å (powder, 325 mesh particle size) | Sigma-Aldrich | 70955-01-0 |
| 1,2-Dimethoxyethane (anhydrous, 99.5%) | Sigma-Aldrich | 110-71-4 |
| 4-acetylmorpholine (99%) | Sigma-Aldrich | 1696-20-4 |
| H2 gas (Instrumental grade 5.0.0) | Afrox | 1333-74-0 |
| Autoclave reactor for material preparation | Paar instrument | (Series 4560 Mini Reactors, 100–600 mL) |
| Autoclave reactor for catalytic testing | Parker Autoclave engineers | Mini reactor-50 mL |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Holger B. Friedrich (friedric@ukzn.ac.za).
Materials availability
This study did not generate new unique materials. The protocols below allow the synthesis of special morphologies.
Method details
General information
The elemental compositions of the fresh and used catalysts were determined using a Perkin Elmer Optima 5300 DV inductively coupled plasma-optical emission spectrometer (ICP-OES). The nitrogen physisorption analyses were done in a Micromeritics Tri-Star II. The samples were degassed (Micromeritics FlowPep 060 instrument) under nitrogen flow at 200°C overnight before the physisorption analysis. Raman spectra of the fresh catalysts were obtained using a Renishaw inVia Raman microscope at λ = 514 nm, 50% laser power, and 10 s of exposure time. The catalyst morphology was viewed using a Zeiss Ultra plus Scanning Electron Microscope (SEM). The bulk structures of the catalysts were viewed using a Jeol JEM-2100, while a High Resolution-Transmission Electron Microscope (HR-TEM [Jeol JEM-2100]) was used (in STEM mode) to determine the elemental distribution (mapping). H2-TPR (temperature-programmed reduction) was carried out in a Micromeritics 2920 Autochem II analyzer equipped with a TCD detector. In this analysis, 30 mL/min of 5% H2/Ar flowed, while the temperature was changed in the range of 80°C–300°C (heating rate = 10 degrees/min). X-ray photoelectron spectra (XPS) were recorded with a Thermo Scientifc Multilab 2000 spectrometer equipped with the Al K radiation source (1486.6 eV). All the binding energies were referenced to the C(1s) peak (284.8 eV). (Additional experimental data are included in the supplemental information associated to this work.)
To synthesize the support (ceria with different morphologies), an autoclave reactor equipped with a base residence vessel (volume = 400 mL) with the ability to hold self-generated pressure (up to 20 bar) at a maximum temperature of 200°C and stirred at a rate of 1000 rpm was used. An oven and tube furnace are needed to dry and calcine the catalysts. A small autoclave reactor was used to carry out the hydrogenation reaction operating in a temperature range of 50-200°C with a reaction chamber with a volume of 20 mL.
Preparation of Pt-V/ceria with different morphologies
Synthesis of ceria with nanosphere morphology. Timing: [Two days]
-
1.
6.5 g of Ce(NO3)3.6H2O was dissolved in 200 mL of ethylene glycol and charged in the autoclave reactor.
-
2.
4.3 mL of propionic anhydride and 6.5 mL of double distilled water were then added to the solution described above.
-
3.
The mixture was made in the autoclave reactor with a PTFE (Polytetrafluoroethylene) liner with the volume of 450 mL.
-
4.
The reactor was pressurized (20 bar) using nitrogen at room temperature to do a leak check for an hour.
-
5.
Then, the pressure was released and the reactor was sealed (with no gas pressure this time) and heated to 180°C with rotation rate of 1000 rpm. The reaction time (24 h) was started when the targeted temperature was reached.
-
6.
High temperature (180°C) in the sealed system created in situ pressure of 2-5 bar.
-
7.
After the reaction completion, the reactor was cooled down to room temperature and the mixture was centrifuged. The recovered solid was washed three times with water.
-
8.
The resultant paste was left in the oven overnight at a temperature of 100°C (drying step).
-
9.
The obtained solid after the drying step was crushed using a mortar and pestle for 10 min.
-
10.
The obtained powder was calcined in the tube furnace. The powder was heated (heating rate = 2.2 degrees/min) to 400°C under air flow and kept isothermally at 400°C for another three hours.
-
11.
The final powder (ceria) was well mixed and referred to as CeO2 with nanospheres morphology (CeO2-NS)
Synthesis of ceria with mixed morphology. Timing: [Two days]
-
1.
32.84 g of (NH4)2Ce(NO3)6 was dissolved in 250 mL of ethylene glycol and charged in the autoclave reactor.
-
2.
17 mL of propionic anhydride and 26 mL of double distilled water were then added to the solution described above.
-
3.
The mixture was made in the autoclave reactor with a PTFE liner with the volume of 450 mL.
-
4.
The reactor was pressurized (20 bar) using nitrogen at room temperature to do a leak check for an hour.
-
5.
Then, the pressure was released and the reactor was sealed (with no gas pressure this time) and heated to 180°C with rotation rate of 1000 rpm. The reaction time (24 h) was started when the targeted temperature was reached.
-
6.
High temperature (180°C) in the sealed system created in situ pressure of 2-5 bar.
-
7.
After the reaction completion, the reactor was cooled down to room temperature and the mixture was centrifuged. The recovered solid was washed three times with water.
-
8.
The resultant paste was left in the oven overnight at a temperature of 100°C (drying step).
-
9.
The obtained solid after the drying step was crushed using a mortar and pestle for 10 min.
-
10.
The obtained powder was calcined in the tube furnace. The powder was heated (heating rate = 2.2°/min) to 400°C under air flow and kept isothermally at 400°C for another 3 h.
-
11.
The final powder (ceria) was well mixed and referred to as CeO2 with mixed morphology (CeO2-MM)
Synthesis of ceria with nanorod and nanocube morphologies.34 timing: [Two days in each case]
-
1.
3.74 g of Ce(NO3)3.6H2O and 38.4 g were dissolved in 20 and 140 mL of double distilled water to prepare CeO2 with nanorod (CeO2-NR) and nanocube (CeO2-NC) morphologies, respectively. Both solutions were mixed vigorously for 30 min to ensure total solubilization of the added chemicals
-
2.
The mixture was set up in an autoclave reactor with a PTFE liner with a volume of 450 mL.
-
3.
The reactor was pressurized (20 bar) using nitrogen at room temperature to do a leak check for an hour.
-
4.
Then, the pressure was released and the reactor was sealed (with no gas pressure this time) and heated to 100°C and 180°C to obtain CeO2-NR and CeO2-NC, respectively with rotation rate of 1000 rpm. The reaction time (24 h) was started when the targeted temperature was reached.
-
5.
After the reaction completion, the reactor was cooled down to room temperature and the mixture was centrifuged. The recovered solid was washed three times with ethanol and three times with water. The pHs of filtrates were monitored using a pH meter to make sure of the complete removal of the base (NaOH) from the catalyst (a pH of seven was obtained).
-
6.
The resultant paste was left in the oven overnight at a temperature of 100°C (drying step).
-
7.
The obtained solids after the drying step were crushed using a mortar and pestle for 10 min.
-
8.
The obtained powder was calcined in a tube furnace. The powder was heated (heating rate = 2.2°/min) to 400°C under air flow and kept isothermally at 400°C for another 3 h.
The final powders (ceria) were well mixed and referred to as CeO2-NR and CeO2-NC, respectively.
Loading of Pt and V over ceria with different morphologies using the acac method.8 timing: [one day]
-
1.
0.4 mmol of Pt(acac)2 and 0.4 mmol of VO(acac)2 were added to 90 mL of acetone.
-
2.
The resultant mixture was stirred for 30 min until both complexes were fully dissolved.
-
3.
Thereafter, ceria (with different morphologies) was added to the solution.
-
4.
The formed mixture was left stirring for 4 h.
-
5.
The acetone was removed using a rotary evaporator set at 50°C and a rotation rate of 150 rpm.
-
6.
The obtained solid was dried (while in the round bottom flask [RBF]) in an oven set at 110°C overnight.
-
7.
The formed solid was removed from the RBF, and then crushed and mixed for 10 min using a mortar and pestle.
-
8.
The obtained fine powder was calcined in the tube furnace under airflow. The calcination method consisted of a temperature ramp from room temperature to 300°C at a rate of 1.5°/min and isothermal thermal treatment at 300°C for 3 h.
-
9.
After the calcination step, the obtained powder was crushed and mixed (using mortar and pestle) for an additional 10 min.
Catalytic testing (hydrogenation of amide to amine)
Timing: 3–10 h
This part of the protocol describes the method that was adopted (with slight modifications) from the work published by Mitsudome et al.8 In this protocol 4-acetylmorpholine was reduced to 4-ethylmorpholine under hydrogen pressure over Pt-V/CeO2 catalysts with different morphologies.
-
1.Charging the reactor with reagents and solvents
-
a.0.1 g of molecular sieves 4Å (0.1 g) were loaded in a 25 mL stainless -steel autoclave
-
b.Then, 0.1 g of catalyst was loaded in the reactor.
-
c.After addition of the solids, 5 mL of solvent (1,2-dimethoxyethane [DME]) was added into the reactor.
-
d.n-octane was added (17.8 μL) as an internal standard.
-
e.Finally. 4-acetylmorpholine (64.6μL) was added to the reactor.
-
a.
-
2.After addition of all the reaction components, the reactor was sealed under 30 bar hydrogen pressure. A leak check was done to ensure that the reactor was fully sealed and held the pressure.
-
a.After confirming the absence of any leakage in the reactor, the reactor was repressurized to the desired pressure (20–40 bar), and the reactor heater was switched on and the desired temperature was set.
-
b.The reaction time started after the set temperature was reached.
-
a.
-
3.
After the reaction completion, the reaction mixture was transferred to a centrifuge tube to separate the solid from the solution.
-
4.0.2 μL of the filtrate was injected to a GC equipped with a FID detector. The GC was calibrated for analyses of starting materials as well as any possible products.
-
a.Using the added internal standard (n-octane) and GC calibration data, reaction conversion was determined. No additional peaks to the expected chemicals (DME, 4-acetylmorpholine, n-octane, 4-ethylmorpholine) were observed in the obtained GC chromatographs.
-
a.
General procedure for recyclability tests
The hydrogenation reaction was done as described above. After the reaction, Pt-V/CeO2 was removed by centrifuging and the yield was determined by GC analysis. The catalyst was washed 3 times with 1,2-dimethoxyethane and calcined at 300°C for 3 h in air for recyclability.
Expected outcomes
The described protocol yields Pt-V/CeO2 with different morphologies. The use of the acac method to prepare these materials resulted in close proximity of Pt and V in these catalysts. Indeed proximity is the key to success of these catalysts and is an independent parameter from the morphology of the supports. The morphologies of the supports affect the concentration of oxygen vacancies on catalyst surfaces, which ultimately impact the degree of oxidation of the Pt sites over the catalysts. Therefore, it is concluded that a lower concentration of oxygen vacancies favors this hydrogenation reaction since it results in Pt and V with lower oxidation states.
Limitations
The catalytic testing suffers from limitations, which should be taken into the account. The presence of water (introduced initially or generated in situ) poisons this catalysis and can result in obtaining irreproducible results. In addition, only an amide molecule was investigated in this study. In terms of catalyst preparation, there are some challenges as well. The reaction yield to synthesise ceria with nanosphere morphology is low and hard to scale up. The length of the nanorod in CeO2-NR had an inverse relationship with the stirring rate of the autoclave reactor. The use of acetone, Pt(acac)2, and VO(acac)2 during catalyst preparation is absolutely critical to the structures of these catalysts and Pt-V proximity.
Troubleshooting
As mentioned above, the presence of water in the reaction media results in poor catalytic performance, which is often irreproducible. To avoid facing this issue, we realized the following actions are effective.
-
1.
All the solvents, catalysts and reagents should be stored in a desiccator at all times.
-
2.
The molecular sieves (4 Α°) should be activated by heating at a high temperature before being charged into the reactor. Water is generated, as a side product, due to the decarboxylation of the amide group in this reaction and must be removed as it is formed to allow the reaction to go to completion.
-
3.
To avoid facing the challenges associated with the preparation of CeO2-NS, the concentration of cerium precursor in the reaction medium should be minimal. Therefore, a larger reactor might be needed to prepare more of this material in a single run. The rotation rate must be constant to obtain CeO2-NR with a fixed length of rods.
Acknowledgments
We thank Novomer Inc. (USA), the National Research Foundation (South Africa, grant 111660), and the University of KwaZulu-Natal for financial support. We also thank the Electron Microscopy Unit at the University of KwaZulu-Natal (Westville Campus).
Author contributions
M.D.F. and H.B.F. designed the project. M.D.F. synthesized, characterized, and tested the material under the supervision of H.B.F. M.D.F. and H.B.F. co-wrote the article. A.S.M. co-supervised the project. C.M. assisted with ICP analysis and discussion.
Declaration of interests
The authors declare no conflicts of interest.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: December 22, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105560.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request
References
- 1.Smith A.M., Whyman R. Review of methods for the catalytic hydrogenation of carboxamides. Chem. Rev. 2014;114:5477–5510. doi: 10.1021/cr400609m. [DOI] [PubMed] [Google Scholar]
- 2.Stein M., Breit B. Catalytic hydrogenation of amides to amines under mild conditions. Angew. Chem. Int. Ed. 2013;52:2231–2234. doi: 10.1002/anie.201207803. [DOI] [PubMed] [Google Scholar]
- 3.Smith Alan A., Dani P., Higginson Paul D., Pettman Alan J. 2005. Method For Catalytic Reduction Of Amides. WO patent WO 2005/066112 A1, patent application NL 2004000018 W, 2004/01/09, and granted 2005/07/21. [Google Scholar]
- 4.Kadyrov R. 2018. Catalytic Hydrogenation for Producing Amines from Carboxylic Acid Amides, Carboxylic Acid Diamides, Di-, Tri-, or Polypeptides, or Peptide Amides. US Patent US 9878975 B2, Patent Application US 201415034120 A, 2014/10/16, and granted 2018/01/30. [Google Scholar]
- 5.Artus Suarez L., Culakova Z., Balcells D., Bernskoetter W.H., Eisenstein O., Goldberg K.I., Hazari N., Tilset M., Nova A. The key role of the hemiaminal intermediate in the iron-catalyzed deaminative hydrogenation of amides. ACS Catal. 2018;8:8751–8762. doi: 10.1021/acscatal.8b02184. [DOI] [Google Scholar]
- 6.Kar S., Rauch M., Kumar A., Leitus G., Ben-David Y., Milstein D. Selective room-temperature hydrogenation of amides to amines and alcohols catalyzed by a ruthenium pincer complex and mechanistic insight. ACS Catal. 2020;10:5511–5515. doi: 10.1021/acscatal.0c01406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sitte N.A., Bursch M., Grimme S., Paradies J. Frustrated lewis pair catalyzed hydrogenation of amides: halides as active lewis base in the metal-free hydrogen activation. J. Am. Chem. Soc. 2019;141:159–162. doi: 10.1021/jacs.8b12997. [DOI] [PubMed] [Google Scholar]
- 8.Mitsudome T., Miyagawa K., Maeno Z., Mizugaki T., Jitsukawa K., Yamasaki J., Kitagawa Y., Kaneda K. Mild hydrogenation of amides to amines over a platinum-vanadium bimetallic catalyst. Angew. Chem. Int. Ed. 2017;56:9381–9385. doi: 10.1002/anie.201704199. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L., Zhou M., Wang A., Zhang T. Selective hydrogenation over supported metal catalysts: from nanoparticles to single atoms. Chem. Rev. 2020;120:683–733. doi: 10.1021/acs.chemrev.9b00230. [DOI] [PubMed] [Google Scholar]
- 10.Yu W., Porosoff M.D., Chen J.G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. Chem. Rev. 2012;112:5780–5817. doi: 10.1021/cr300096b. [DOI] [PubMed] [Google Scholar]
- 11.Dutta G., Waghmare U.V., Baidya T., Hegde M.S. Hydrogen spillover on CeO2/Pt: enhanced storage of active hydrogen. Chem. Mater. 2007;19:6430–6436. doi: 10.1021/cm071330m. [DOI] [Google Scholar]
- 12.Ahmed F., Alam M.K., Muira R., Suzuki A., Tsuboi H., Hatakeyama N., Endou A., Takaba H., Kubo M., Miyamoto A. Adsorption and dissociation of molecular hydrogen on Pt/CeO2 catalyst in the hydrogen spillover process: a quantum chemical molecular dynamics study. Appl. Surf. Sci. 2010;256:7643–7652. doi: 10.1016/j.apsusc.2010.06.021. [DOI] [Google Scholar]
- 13.Ma Y., Chi B., Liu W., Cao L., Lin Y., Zhang X., Ye X., Wei S., Lu J. Tailoring of the proximity of platinum single atoms on CeO2 using phosphorus boosts the hydrogenation activity. ACS Catal. 2019;9:8404–8412. doi: 10.1021/acscatal.9b01536. [DOI] [Google Scholar]
- 14.Choi M., Yook S., Kim H. Hydrogen spillover in encapsulated metal catalysts: new opportunities for designing advanced hydroprocessing catalysts. ChemCatChem. 2015;7:1048–1057. doi: 10.1002/cctc.201500032. [DOI] [Google Scholar]
- 15.Montini T., Melchionna M., Monai M., Fornasiero P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016;116:5987–6041. doi: 10.1021/acs.chemrev.5b00603. [DOI] [PubMed] [Google Scholar]
- 16.Kourtelesis M., Moraes T.S., Mattos L.V., Niakolas D.K., Noronha F.B., Verykios X. The effects of support morphology on the performance of Pt/CeO2 catalysts for the low temperature steam reforming of ethanol. Appl. Catal. B. 2021;284:119757. doi: 10.1016/j.apcatb.2020.119757. [DOI] [Google Scholar]
- 17.Wang Z., Huang Z., Brosnahan J.T., Zhang S., Guo Y., Guo Y., Wang L., Wang Y., Zhan W. Ru/CeO2 catalyst with optimized CeO2 support morphology and surface facets for propane combustion. Environ. Sci. Technol. 2019;53:5349–5358. doi: 10.1021/acs.est.9b01929. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto N., Mori K., Asahara K., Shibata S., Jida H., Kuwahara Y., Yamashita H. How the morphology of NiOx-decorated CeO2 nanostructures affects catalytic properties in CO2 methanation. Langmuir. 2021;37:5376–5384. doi: 10.1021/acs.langmuir.1c00546. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X., Li Y., Zhang L., Shen L., Xiao Y., Zhang Y., Au C., Jiang L. Insight into the effect of morphology on catalytic performance of porous CeO2 nanocrystals for H2S selective oxidation. Appl. Catal. B. 2019;252:98–110. doi: 10.1016/j.apcatb.2019.04.014. [DOI] [Google Scholar]
- 20.van Veen J.A.R., Jonkers G., Hesselink W.H. Interaction of transition-metal acetylacetonates with γ-Al2O3 surfaces. J. Chem. Soc. Faraday Trans. 1989;85:389–413. doi: 10.1039/F19898500389. [DOI] [Google Scholar]
- 21.Baltes M., Collart O., Van Der Voort P., Vansant E.F. Synthesis of supported transition metal oxide catalysts by the designed deposition of acetylacetonate complexes. Langmuir. 1999;15:5841–5845. doi: 10.1021/la981362b. [DOI] [Google Scholar]
- 22.Farahani M.D., Fadlalla M.I., Ezekiel I.P., Osman N.S.E., Moyo T., Claeys M., Friedrich H.B. Nb2O5 as a radical modulator during oxidative dehydrogenation and as a Lewis acid promoter in CO2 assisted dehydrogenation of octane over confined 2D engineered NiO–Nb2O5–Al2O3. Catal. Sci. Tech. 2021;11:5321–5334. doi: 10.1039/D1CY00550B. [DOI] [Google Scholar]
- 23.Liu Z., Li J., Buettner M., Ranganathan R.V., Uddi M., Wang R. Metal–support interactions in CeO2- and SiO2-supported cobalt catalysts: effect of support morphology, reducibility, and interfacial configuration. ACS Appl. Mater. Interfaces. 2019;11:17035–17049. doi: 10.1021/acsami.9b02455. [DOI] [PubMed] [Google Scholar]
- 24.Negreiros F.R., Fabris S. Role of cluster morphology in the dynamics and reactivity of subnanometer Pt clusters supported on ceria surfaces. J. Phys. Chem. C. 2014;118:21014–21020. doi: 10.1021/jp506404z. [DOI] [Google Scholar]
- 25.Gao Y., Wang W., Chang S., Huang W. Morphology effect of CeO2 support in the preparation, metal–support interaction, and catalytic performance of Pt/CeO2 catalysts. ChemCatChem. 2013;5:3610–3620. doi: 10.1002/cctc.201300709. [DOI] [Google Scholar]
- 26.Fadzil N.A.M., Rahim M.H.A., Maniam G.P. Brief review of ceria and modified ceria: synthesis and application. Mater. Res. Express. 2018;5:085019. [Google Scholar]
- 27.Sreekanth T.V.M., Nagajyothi P.C., Dillip G.R., Lee Y.R. Determination of band Alignment in the synergistic catalyst of electronic structure-modified graphitic carbon nitride-integrated ceria quantum-dot heterojunctions for rapid degradation of organic pollutants. J. Phys. Chem. C. 2017;121:25229–25242. doi: 10.1021/acs.jpcc.7b08568. [DOI] [Google Scholar]
- 28.Renuka N.K., Harsha N., Divya T. Supercharged ceria quantum dots with exceptionally high oxygen buffer action. RSC Adv. 2015;5:38837–38841. doi: 10.1039/C5RA01161B. [DOI] [Google Scholar]
- 29.Mkhwanazi T.P.O., Farahani M.D., Mahomed A.S., Singh S., Friedrich H.B. Engineering of catalytic sites of Pdx-Ce1-xO2-δ for dehydrogenation, oxygen insertion and reverse water gas shift reactions during methane combustion. Appl. Catal. B. 2020;275:119118. doi: 10.1016/j.apcatb.2020.119118. [DOI] [Google Scholar]
- 30.Jan A., Shin J., Ahn J., Yang S., Yoon K.J., Son J.-W., Kim H., Lee J.-H., Ji H.-I. Promotion of Pt/CeO2 catalyst by hydrogen treatment for low-temperature CO oxidation. RSC Adv. 2019;9:27002–27012. doi: 10.1039/C9RA05965B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanabe T., Nagai Y., Hirabayashi T., Takagi N., Dohmae K., Takahashi N., Matsumoto S.i., Shinjoh H., Kondo J.N., Schouten J.C., Brongersma H.H. Low temperature CO pulse adsorption for the determination of Pt particle size in a Pt/cerium-based oxide catalyst. Appl. Catal. A. 2009;370:108–113. doi: 10.1016/j.apcata.2009.09.030. [DOI] [Google Scholar]
- 32.Lin L., Yao S., Liu Z., Zhang F., Li N., Vovchok D., Martínez-Arias A., Castañeda R., Lin J., Senanayake S.D., et al. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C. 2018;122:12934–12943. doi: 10.1021/acs.jpcc.8b03596. [DOI] [Google Scholar]
- 33.Si R., Flytzani-Stephanopoulos M. Shape and crystal-plane effects of nanoscale ceria on the activity of Au-CeO2 catalysts for the water–gas shift reaction. Angew. Chem. Int. Ed. 2008;47:2884–2887. doi: 10.1002/anie.200705828. [DOI] [PubMed] [Google Scholar]
- 34.Mai H.-X., Sun L.-D., Zhang Y.-W., Si R., Feng W., Zhang H.-P., Liu H.-C., Yan C.-H. Shape-selective synthesis and oxygen storage behavior of ceria nanopolyhedra, nanorods, and nanocubes. J. Phys. Chem. B. 2005;109:24380–24385. doi: 10.1021/jp055584b. [DOI] [PubMed] [Google Scholar]
- 35.Vilé G., Colussi S., Krumeich F., Trovarelli A., Pérez-Ramírez J. Opposite face sensitivity of CeO2 in hydrogenation and oxidation catalysis. Angew. Chem. Int. Ed. 2014;53:12069–12072. doi: 10.1002/anie.201406637. [DOI] [PubMed] [Google Scholar]
- 36.Ganduglia-Pirovano M.V., Popa C., Sauer J., Abbott H., Uhl A., Baron M., Stacchiola D., Bondarchuk O., Shaikhutdinov S., Freund H.-J. Role of ceria in oxidative dehydrogenation on supported vanadia catalysts. J. Am. Chem. Soc. 2010;132:2345–2349. doi: 10.1021/ja910574h. [DOI] [PubMed] [Google Scholar]
- 37.Yang F., Bao X., Li P., Wang X., Cheng G., Chen S., Luo W. Boosting hydrogen oxidation activity of Ni in alkaline media through oxygen-vacancy-rich CeO2/Ni heterostructures. Angew. Chem. Int. Ed. 2019;58:14179–14183. doi: 10.1002/anie.201908194. [DOI] [PubMed] [Google Scholar]
- 38.Yang C., Yu X., Heißler S., Weidler P.G., Nefedov A., Wang Y., Wöll C., Kropp T., Paier J., Sauer J. O2 activation on ceria catalysts—the importance of substrate crystallographic orientation. Angew. Chem. Int. Ed. 2017;56:16399–16404. doi: 10.1002/anie.201709199. [DOI] [PubMed] [Google Scholar]
- 39.Chen D., He D., Lu J., Zhong L., Liu F., Liu J., Yu J., Wan G., He S., Luo Y. Investigation of the role of surface lattice oxygen and bulk lattice oxygen migration of cerium-based oxygen carriers: XPS and designed H2-TPR characterization. Appl. Catal. B. 2017;218:249–259. doi: 10.1016/j.apcatb.2017.06.053. [DOI] [Google Scholar]
- 40.Cabrero-Antonino J.R., Adam R., Papa V., Beller M. Homogeneous and heterogeneous catalytic reduction of amides and related compounds using molecular hydrogen. Nat. Commun. 2020;11:3893. doi: 10.1038/s41467-020-17588-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request