Summary
Much of what we know about astrocyte form and function is derived from the study of gray matter protoplasmic astrocytes, whereas white matter fibrous astrocytes remain relatively unexplored. Here, we used the ribotag approach to isolate ribosome-associated mRNA and investigated the transcriptome of uninjured fibrous astrocytes from three regions: unmyelinated optic nerve head, myelinated optic nerve proper, and corpus callosum. Astrocytes from each region were transcriptionally distinct and we identified region-specific astrocyte genes and pathways. Energy metabolism, particularly oxidative phosphorylation and mitochondrial protein translation emerged as key differentiators of astrocyte populations. Optic nerve astrocytes expressed higher levels of neuroinflammatory pathways than corpus callosum astrocytes and we further identified CARTPT as a new marker of optic nerve head astrocytes. These previously uncharacterized transcriptional profiles of white matter astrocyte types reveal their functional diversity and a greater heterogeneity than previously appreciated.
Subject areas: Sensory neuroscience, cell biology, transcriptomics
Graphical abstract

Highlights
-
•
Transcriptional heterogeneity defines astrocytes across white matter tract regions
-
•
Pathways for energy metabolism are a key differentiator of astrocyte populations
-
•
Optic nerve astrocytes are enriched for neuroinflammatory pathways
-
•
Cartpt is a marker of optic nerve head astrocytes
Sensory neuroscience; Cell biology; Transcriptomics
Introduction
Astrocytes are the most abundant non-neuronal cell type within the CNS and have thousands of individual processes that extend out into the neuropil and interact with surrounding neurons, other glia, and blood vessels. They are involved in a wide variety of key CNS functions.1 From the late 19th century, morphological heterogeneity had been identified and astrocytes were classified into two main groups: protoplasmic astrocytes located in the gray matter and fibrous astrocytes in the white matter.2,3 Protoplasmic astrocytes are the best characterized and they exhibit substantial heterogeneity in form and function, both between and within brain regions. Many of our ideas of what an astrocyte is and what they do have come from studying these gray matter astrocytes.2,3,4 However, without understanding the extent of heterogeneity across the CNS, astrocytes continue to be discussed as a homogeneous population and the characteristics of astrocytes from one region are often inappropriately extrapolated to other disparate regions. In particular, very little is known about fibrous white matter astrocytes, a gap this study seeks to address by exploring the variability in transcriptional programs of astrocytes within the optic nerve head, optic nerve proper, and corpus callosum. We are particularly interested in the optic nerve because of its importance in optic neuropathies, and the nerve head region is a key site of pathophysiological changes in glaucoma, including biomechanical strain, neuroinflammation, and extracellular remodeling.
Astrocyte heterogeneity is now being increasingly addressed and there are many examples of this within the brain. Protoplasmic astrocytes of the hippocampus and striatum have equivalent somatic volumes, number of main processes, and total cell volume. However, striatal astrocytes occupy a larger territory and associate with more neuronal cell bodies, while hippocampal astrocytes exhibit more gap junctions and K+ currents, and associate with a larger number of excitatory synapses.5,6 Such morphological heterogeneity extends to astrocytes occupying the same brain region. Four morphological types have been identified in the cortical layers in differing proportions, even though they are all considered protoplasmic astrocytes.5,6
Significant insight into astrocyte heterogeneity and identity has been driven by experiments using transcriptome profiling. Such studies show considerable differences in the transcriptome of cortical astrocytes, cerebellar astrocytes, and cerebellar Bergmann glia.7 The cortex and hippocampus show a high degree of overlap between genes expressed, while other brain regions express unique mRNAs not expressed in astrocytes from any other brain regions studied.8 Transcriptional differences are also evident in astrocytes of the same region. Five transcriptionally different subpopulations have been identified across the different cortical layers, differing in many astrocyte genes including those involved in synapse formation, elimination, neurotransmission, and thus synaptogenic potential.6,9,10,11,12,13 Another study found seven astrocyte subtypes across the entire CNS,14 while another identified eight distinct types across nine major brain regions.15 Although astrocytes share many common genes that regulate essential processes, there is sufficient variation in gene expression at the inter- and intra-regional level to produce unique subtypes specialized to perform specific functions.
In the disease context, astrocyte heterogeneity may underpin the regional susceptibility to diseases. Studies of mouse astrocytes in the context of bacterial infection, stroke,16 and demyelination in the experimental allergic encephalomyelitis model17 suggest they adopt distinct transcriptional programs in response to different challenges. In amyotrophic lateral sclerosis, degeneration of spinal motor neurons occurs in regions where astrocyte subpopulations specifically downregulate Kir4.1 and Glt1.18,19,20 Similarly, in Huntington disease astrocytes from the striatum, where neuronal degeneration occurs, are shown to also preferentially downregulate Kir4.1. Astrocyte-specific overexpression in Kir4.1 increased striatal neuron survival and improved motor deficits.21 One possible reason for the regional difference in tumor susceptibility in the CNS is the location-dependent ability of astrocytes to proliferate, due to the differential expression of tumor suppressor genes.22 These findings demonstrate that astrocyte subtype and location are a factor in disease progression and neuronal survival.
The optic nerve is a white matter tract affected in many ocular diseases and consists largely of glial cells (astrocytes, microglia, oligodendrocytes, NG2 glial cells) and axons. It is anatomically separated into the proximal unmyelinated optic nerve head (ONH) and the distal myelinated optic nerve proper (ONP). Previous work suggested that ONH astrocytes are a unique population. Firstly, they have a distinct transversely oriented “baseball catchers’ mitt-like” morphology not seen in the more distal ONP, corpus callosum, and spinal cord.23 The ONH region contains only this one morphological type, unlike the ONP. Second, they show an immunocytochemical labeling profile that is very different from the ONP, with lower expression of aquaporin 4 (APQ4), and higher expression of the glial fibrillary acidic protein (GFAP), NES, VIM, LGALS3, MEGF10, and ABCA1.24,25,26 Third, they lie in a region of the optic nerve with no myelination and very few microglia and NG2 glial cells.23 Fourth, the ONH region possesses a vascular plexus with a complexity greater than that of the ONP.27,28 In glaucoma, astrocytes in this region upregulate phagocytic genes and function, compared to astrocytes further distal along the nerve.26 These differences suggest that ONH astrocytes have unique functional specializations that will be reflected in their transcriptional profile.
Here we used a ribotag approach to isolate ribosome-associated mRNA from astrocytes of different white matter regions, namely the ONH, ONP, and corpus callosum (CC), to determine whether they show distinct transcriptional programs. We found evidence of regional specialization of astrocytes, with notably key differences in metabolic profiles and neuroinflammatory pathways. Moreover, we identified CARTPT as a new marker of optic nerve head astrocytes.
Results
The ribotag approach enables the purification of astrocyte-specific ribosomal mRNA from the optic nerve head, optic nerve proper, and corpus callosum
To isolate astrocyte-specific ribosomal mRNA, we used the ribotag technique to genetically tag ribosomes in astrocytes.29,30,31 RiboTag mice were crossed to a constitutive GFAP-Cre line and experimental mice were homozygous for the RiboTag allele and carried the Cre transgene (Rpl22HA/HACre+, equal proportion of males and females used, aged 3–4 mths, see STAR Methods). Control mice were homozygous for the RiboTag allele but without the Cre transgene (Rpl22HA/HACre−). We then purified the astrocyte-specific ribosomal mRNA via immunoprecipitation using an antibody against hemagglutinin (HA). We have optimized the ribotag procedure for very small tissues such as the ONH and ONP (Figure 1A). Immunostaining against the HA tag demonstrated widespread expression of HA-tagged ribosomes throughout the ONH, ONP, and CC. To determine whether astrocytes were the major cell type expressing HA-tagged ribosomes, we colocalized HA with antibodies against cell-specific markers of astrocytes (GFAP, SOX9), microglia (IBA1), oligodendrocyte precursor cells (NG2), and oligodendrocytes (OLIG2; Figure 1B). We used two markers against astrocytes, one that labeled the nuclei (SOX9) and another that labeled the intermediate filaments (GFAP). For immunostaining with GFAP, transverse sections better show the extent of astrocyte morphology (Figure 1B). Notably, the HA labeling accompanying the GFAP stained section in Figure 1B showed that even ribosomal mRNA from distal processes of astrocytes could be detected and isolated with anti-HA. SOX9 is a specific marker of astrocyte nuclei in the optic nerve.25,32,33 We confirmed that HA immunostaining predominantly colocalized with SOX9 and GFAP, and not other markers (Figure 1B). We found a 93–98% overlap between HA and markers of astrocytes in the ONH, ONP, and CC (Figure 1C). Moreover, in the unmyelinated ONH and myelinated ONP every double-labeled SOX9/Hoescht-positive astrocyte colocalized with HA (n = 5 each region). There was no HA immunostaining in the control Rpl22HA/HACre− mice (Figure 1B).
Figure 1.
Characterization of the ribotag mouse and principal component analysis of samples
(A) The mouse eye imaged under a dissecting microscope showing areas of the optic nerve used for this study; the translucent unmyelinated ONH and the myelinated ONP. An equal length of ONP as ONH was used for experiments. Scale bar = 500 μm.
(B) Immunostaining for HA and cell-specific markers in the optic nerve and CC of Rpl22HA/HACre+ mice. For the optic nerve, all images are longitudinal sections of the nerve head region, except for the panels showing GFAP staining which are transverse sections. Transverse sections better show the extent of astrocyte morphology, particularly when GFAP is used. Notably, the HA labeling accompanying the GFAP staining shows that even ribosomal mRNA from distal processes of astrocytes can be detected and thus isolated with anti-HA (inset). The last panel is derived from control Rpl22HA/HACre- mice. Scale bar in top left panel = 50 μm and applies to all optic nerve panels except for the enlarged insets. Scale bar in the GFAP stained corpus callosum = 100 μm and applies to all corpus callosum panels except for the enlarged insets. Scale bar in the last panel = 50 μm.
(C) Quantification of cell-specific staining shows the majority of HA-tagged ribosomes were in astrocytes. SOX9 is a specific marker of astrocytes in the optic nerve only, and not for astrocytes in the CC, hence counts in the CC were performed on HA-positive cells that colocalized with GFAP and DAPI. n = 5 biological replicates for each region.
(D–F) qRT-PCR of the immunoprecipitate (IP) and input from ONH, ONP, and CC showing enrichment of astrocyte ribosomal mRNAs over other cell types. n = 4 per region. Mean ± SEM.
(G) Principal component analysis based on expression levels of the top 5000 most variable genes across all 17 samples.
(H) Heatmap with hierarchical clustering of the correlation in gene expression for all pairwise combinations of samples in the dataset.
(I) Number of DEGs for each independent pairwise comparison of tissue regions. Significance was adjusted p value < 0.05; no log2 fold change cutoff was applied.
To confirm the specific enrichment of astrocyte transcripts in the ribotag immunoprecipitated (IP) fraction compared to input (total mRNA from all cells), we evaluated the expression of cell type-specific genes using qRT-PCR. In all three tissue regions of Rpl22HA/HACre+ mice, when compared to the input, the IP fraction was strongly enriched in ribosomal mRNA for astrocyte markers and depleted in markers of microglia, oligodendrocytes, and neurofilaments (Figures 1D–1F). As expected, enrichment was not observed when control IgG was used in the immunoprecipitation step, nor when treating tissue derived from Rpl22HA/HACre− mice with the anti-HA antibody (Figure 1D).
Transcriptomic analyses reveal different astrocyte profiles in the optic nerve and corpus callosum
Having validated that the IP samples were enriched for astrocyte ribosomal mRNA, we performed bulk RNA-seq on 6 ONH, 6 ONP, and 5 CC samples. Each ONH and each ONP sample comprised a pool of tissue dissected from 4 mice (2 females and 2 males). The ONH and ONP came from the same mouse. The CC samples did not require pooling. Principal component analysis (PCA) using the top 5000 genes with the greatest variance in expression level across all 17 samples showed that biological replicates clustered by tissue region. Samples from ONH and ONP are particularly distant from CC (Figures 1G and 1H). To determine genes specific to astrocytes from each tissue region, we performed three independent differential expression analyses for pairwise comparisons of astrocytes isolated from each of the three regions (Table S1). Analyses of ONH versus ONP astrocytes identified the smallest number of significantly differentially expressed genes (DEGs) (Figure 1I), confirming the greater similarity of astrocytes in these two regions compared to CC astrocytes, as was observed in the PCA analysis. Analyses of ONH vs CC and ONP vs CC each identified more than double the number of DEGs than the comparison of ONH vs ONP (Figure 1I). Thus, our data showed that astrocytes from the three regions have distinct transcriptional profiles.
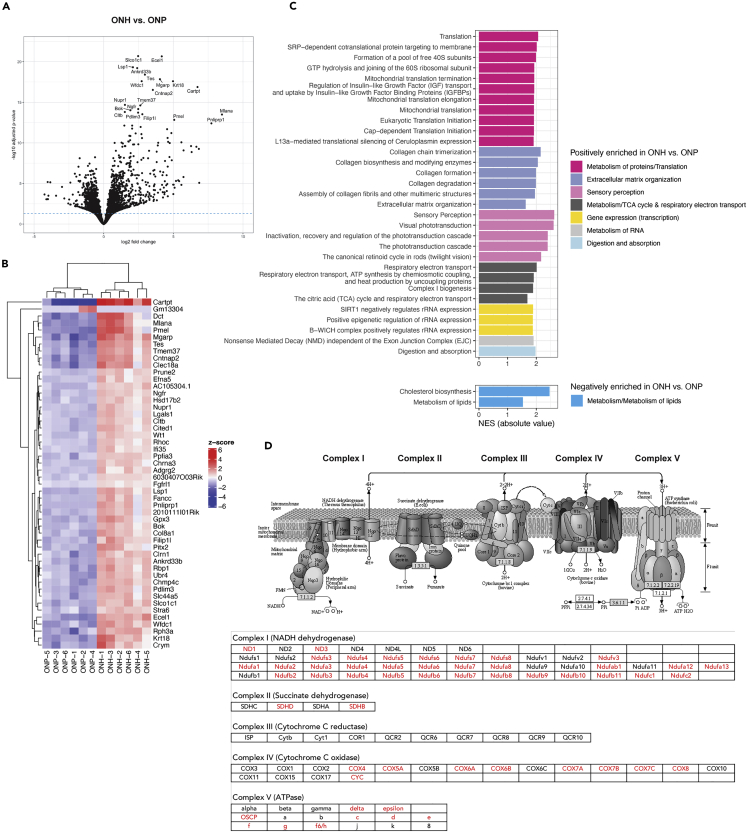
Optic nerve head astrocytes are enriched for mitochondrial protein translation, oxidative phosphorylation, antioxidant activity, and extracellular matrix dynamics
We next looked at overlapping DEGs between the independent pairwise comparisons to identify region-specific genes. We first focused on the ONH. We found a total of 2355 genes significantly different in comparisons of ONH vs CC and ONH vs ONP both. Of these, 1263 genes were enriched in both comparisons, suggesting specific enrichment of those genes in ONH astrocytes; and 975 genes were de-enriched in both comparisons, suggesting ONH-specific de-enrichment (Figure 2A and Table S2). The top 50 enriched and de-enriched genes in the ONH compared to the ONP and CC are shown in Figure 2B. Cartpt was one of the most highly enriched genes, making it a good candidate as a selective marker of ONH astrocytes (see below). Astrocytes of the ONH respond to mechanical stretch induced by changes in intraocular pressure,34 and accordingly, we found as one of the top genes the TRP channel Trpm1. The classical and alternate complement pathways are highly activated in ocular diseases such as age-related macular degeneration and glaucoma, and these pathways are common therapeutic targets.35,36 We found the enrichment of Complement Factor D (Cfd), an important component of the alternate pathway. Optic nerve head astrocytes also expressed high levels of Orosomucoids (Orm2, Orm3). These are a known mediator of astrocyte-microglial interaction and exert anti-inflammatory effects by modulating microglial activation and migration during inflammation.37,38 High levels of orosomucoids prevent the infiltration of microglia37,38 and so it is noteworthy that the ONH has very few microglia compared to the ONP region. Amongst the top enriched genes, we noted several related to pigment and melanin biosynthesis (Mlana, Tyrp1, Pmel, Dct), which is likely a result of residual retina and sclera remaining during the dissection of the ONH.
Figure 2.
Optic nerve head astrocyte-specific genes and pathways
(A) Overlap analysis showing the ONH astrocyte-specific differentially expressed enriched and de-enriched genes (adjusted p value < 0.05).
(B) Top 50 genes that were enriched and de-enriched in ONH astrocytes, based on rank from largest to lowest log2 fold change value (absolute value, log2 FC > 0 enriched, log2 FC < 0 de-enriched) derived from the comparison of ONH vs ONP.
(C) Over-representation analysis of the enriched and de-enriched genes using the Reactome database showing enriched pathways unique for ONH astrocytes. Listed are all pathways with adjusted p value < 0.05. Each pathway is color matched for the broader category it belongs to.
We next sought to understand the processes in which the ONH astrocyte-specific genes were involved. We ran two independent over-representation analyses (ORA) against the Reactome database, looking at enriched and de-enriched pathways (Figure 2C and Table S2). Among the most significant pathways distinguishing ONH astrocytes from others were extracellular matrix organization and the metabolism of proteins. Protein metabolism pathways encompassed a large number of genes encoding for eukaryotic translation initiation and elongation factors, as well as mitochondrial ribosomal proteins for translation, elongation, and termination (Table S2). Lipid metabolism was highly de-enriched, consistent with the fact that the ONH is unmyelinated. Several pathways related to visual phototransduction and sensory perception, which again was likely from residual retina/sclera remaining during the dissection of the ONH. Several of the genes in these pathways have multiple functions and were described early in the visual system (Rbp1, Rbp3; Table S2).
With the overlap analysis, we focused on genes differentially expressed in ONH versus ONP and CC both. To determine what distinguishes astrocytes in the two more closely related regions of the ONH and ONP, we re-examined the global results of our pairwise comparison of ONH vs ONP (Table S3). This confirmed Carpt as one of the top markers of ONH astrocytes (Figures 3A and 3B). Gene set enrichment analysis (GSEA) using ranked log2 fold change values for all genes assessed in this pairwise comparison identified several metabolic pathways specifically enriched in ONH astrocytes (Figure 3C). Beyond pathways related to mitochondrial protein translation, we found robust enrichment of oxidative phosphorylation pathways, including respiratory electron transport, ATP synthesis, complex I biogenesis, and the TCA cycle. Most genes encoded for complexes of the respiratory electron transport chain, in particular complex I (Figure 3D and Table S3), the complex producing the most reactive oxygen species (ROS).39 Subsequent GSEA against the molecular function subset of the gene ontology (GO) database further revealed enrichment of antioxidant activity, glutathione transferase activity and peroxidase activity (Table S3). We noticed Gsta1, a gene in the glutathione transferase activity pathway was also one of the top enriched genes specific to ONH astrocytes (Figure 2B). Thus, high oxidative phosphorylation in ONH astrocytes may require a concomitant increase in antioxidant activity to counter the higher amounts of ROS being produced. Astrocytes are known to be major contributors of glutathione, a potent antioxidant,40 and here we found that ONH astrocytes are particularly more so than other astrocytes. In all, ONH astrocytes were characterized by increased mitochondrial protein translation, oxidative phosphorylation, and antioxidant activity compared to other studied astrocytes. We validated the RNA-seq results with qRT-PCR of select genes and found a similar trend of differential expression (Figure S1).
Figure 3.
Pairwise comparison of optic nerve head and optic nerve proper astrocytes
(A) Volcano plot for all genes tested in ONH vs ONP astrocytes. The top 20 most significant genes are annotated. Dotted line indicates the p value cutoff for significance (0.05).
(B) Heatmap showing the variation in expression level across samples for the top 50 DEGs, based on rank from smallest to largest adjusted p value. The color code reflects the deviation from the mean expression across all samples for the given gene (Z score).
(C) Normalized enrichment scores (NES, in absolute value) of significant pathways identified in gene set enrichment analysis of ONH vs ONP astrocytes. Significance is adjusted p value < 0.05. Each pathway is color matched for the broader category it belongs to.
(D) Schematic of the oxidative phosphorylation pathway from KEGG and a table showing in red genes enriched in the metabolism/TCA cycle and respiratory electron transport pathway in panel (C). Many of the genes belonging to Complex I were enriched in our dataset.
Optic nerve proper astrocytes are enriched for the metabolism of lipids, cholesterol, steroids, and sphingolipids
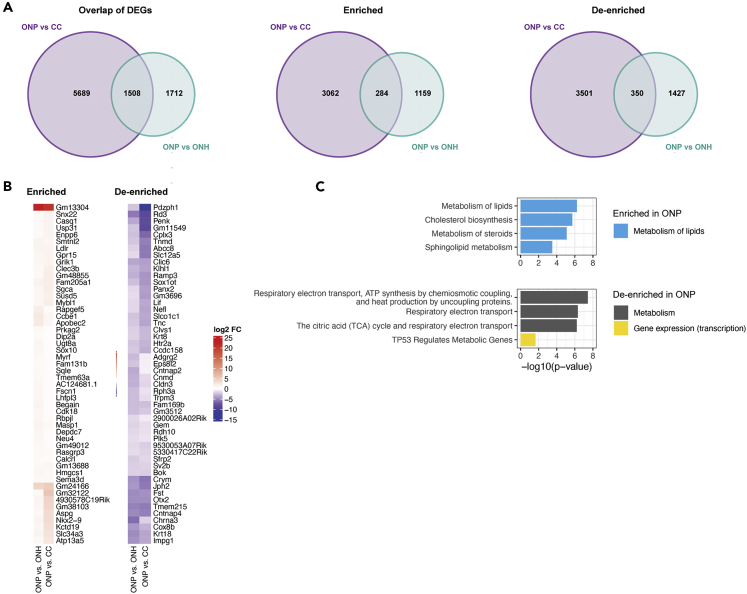
Overlap analysis of DEGs focusing on the ONP identified 1508 significant genes in common in the comparisons of ONP vs ONH and ONP vs CC both. Of these, 284 were enriched in both comparisons, suggesting specific enrichment of those genes in ONP astrocytes; and 350 genes were de-enriched in both comparisons, suggesting ONP-specific de-enrichment (Figure 4A and Table S4). This is a much smaller number of region-specific genes than ONH astrocytes, suggesting a more limited specialization of ONP astrocytes. Amongst the top enriched genes were several for cholesterol and lipid metabolism (Ldlr, Sqle, Hmgcs1; Figure 4B). Except for Gm13304, ONP-enriched genes also tended to show lower log2 fold change values (∼0.3–2) than those observed for top-enriched genes in ONH astrocytes (Table S2).
Figure 4.
Optic nerve proper astrocyte-specific genes and pathways
(A) Overlap analysis showing the ONP astrocyte-specific differentially expressed, enriched and de-enriched genes (adjusted p value < 0.05).
(B) Top 50 genes that were enriched and de-enriched in ONP astrocytes, based on rank from largest to lowest log2 fold change value (absolute value, log2 FC > 0 enriched, log2 FC < 0 de-enriched) derived from the comparison of ONP vs ONH.
(C) Over-representation analysis of the enriched and de-enriched genes using the Reactome database showing enriched pathways unique for ONP astrocytes. Listed are all pathways with adjusted p value < 0.05. Each pathway is color matched for the broader category it belongs to.
Accordingly,the most distinguishing characteristic of ONP astrocytes was the limited number and diversity of pathways specifically enriched or de-enriched (Figure 4C). Over-representation analysis on the ONP-enriched genes identified only three pathways, all related to the broad category of lipid metabolism, namely: cholesterol biosynthesis, metabolism of steroids, and sphingolipid metabolism (Figure 4C and Table S4). The enrichment of these pathways is not surprising as the ONP is myelinated. However, the fact that lipid metabolism is significantly more enriched than even in CC astrocytes, which also reside in a myelinated region, suggests further functional implications for this metabolic state in ONP. Pathways related to oxidative phosphorylation, including respiratory electron transport, ATP synthesis, and the TCA cycle, were de-enriched. The majority of genes in these pathways are encoded for complexes of the electron transport chain (cytochrome c oxidase subunits, ATP synthase subunits, electron transferring flavoproteins, NADH:ubiquinone oxidoreductase subnits, ubiquinol-cytochrome c reductase complexes) (Table S4). This is consistent with our observation of significant enrichment of these pathways in the ONH astrocytes.
Corpus callosum astrocytes are enriched for receptors, channels, transporters, and oxidative metabolism, and de-enriched for neuroinflammation
Finally, we focused on DEGs most specific to CC astrocytes. Overlap analysis identified 5566 genes significantly different in comparisons of CC vs ONH and CC vs ONP both. Of these, 2936 genes were enriched in both comparisons, suggesting specific enrichment of those genes in CC astrocytes; and 2603 genes were de-enriched in both comparisons, suggesting CC-specific de-enrichment (Figure 5A and Table S5). Unlike the optic nerve, amongst the top 50 differentially expressed genes were very few for metabolism/mitochondria (Figure 5B and Table S5). Transcription factors were prominently enriched, consistent with diverse transcriptional networks maintaining unique cell identities (Neurod2, Neurod6, Sp8, Dlx1, Dlx5, Emx1, Foxg1, Arx). Others were related to glutamate (Grin2c, Grm2) and dopamine (Drd1) receptors, a zinc transporter (Slc30a3), protein phosphatases (Ppp1r32, Ppp1r3g), extracellular matrix cell adhesion (Hapln1, Cdhr3), and thrombospondin 4 (Thbs4). Several de-enriched genes related to crystallins (Cryaa, Cryba1, Crybb2) and lysozymes (Lyz1, Lyz2), while many others encoded for neuroinflammation (Ccr1, Clec7a, Il2rg, Lilrb4a, Cd74).
Figure 5.
Corpus callosum astrocyte-specific genes and pathways
(A) Overlap analysis showing the CC astrocyte-specific differentially expressed, enriched and de-enriched genes (adjusted p value < 0.05).
(B) Top 50 genes that were enriched and de-enriched in CC astrocytes, based on rank from largest to lowest log2 fold change value (absolute value, log2 FC > 0 enriched, log2 FC < 0 de-enriched) derived from the comparison of CC vs ONP.
(C) Over-representation analysis of enriched and de-enriched genes using the Reactome database showing enriched pathways unique for CC astrocytes. Listed are all pathways with adjusted p value < 0.05, restricted to the top 46 most significant de-enriched pathways (see Table S5 for complete results). Each pathway is color matched for the broader category it belongs to.
(D–F) In red are key enzymes enriched in our dataset for the (D) glycogen synthesis and breakdown pathway, (E) glycolysis pathway, and (F) the TCA cycle including glutaminolysis.
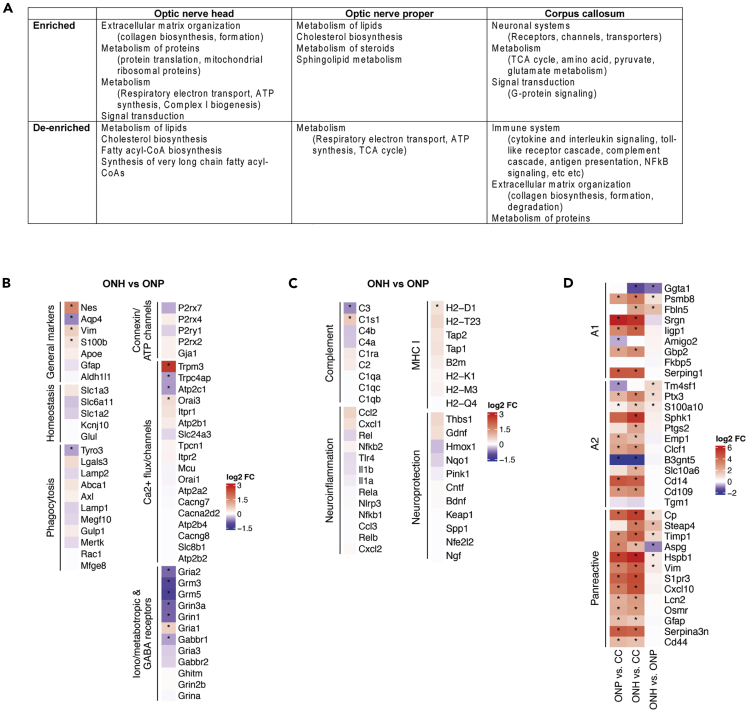
Over-representation analyses demonstrated that the majority of enriched pathways were related to neuronal systems, signal transduction (G-protein signaling), and metabolism (Figure 5C and Table S5). The CC does not contain neurons, but astrocytes also express genes that are more traditionally associated with neuronal cells, which explains the enrichment in neuronal system pathways we observed here. Genes found in pathways belonging to this category are encoded for general Ca2+ channels, Ca2+/calmodulin protein kinases, GABA/glycine receptors, K+ and Na+ channels, and solute carrier family transporters. As an important distinction, the metabolic pathways identified here were different from those in astrocytes of both optic nerve regions. Corpus callosum astrocytes showed enrichment of oxidative metabolic pathways, such as the TCA cycle, pyruvate, glycogen, glutamate, and glutamine metabolism (Figures 5C–5F), suggesting that CC astrocytes are energy demanding, but meet these demands differently. There was a significantly large number of de-enriched pathways (152 in total, adjusted p value < 0.05; Figure 5C and Table S5), including extracellular matrix organization, one of the top enriched pathways in the optic nerve. Surprisingly, almost half of the top 46 significantly de-enriched pathways were associated with neuroinflammation and immunity (Figure 5C). A de-enrichment of these pathways in CC astrocytes implies they were more highly expressed in optic nerve astrocytes, and both neuroinflammation and immunity have important roles in the optic nerve in glaucoma. In all, our data showed that CC astrocytes were transcriptionally very distinct from astrocytes in the optic nerve, even from those in the ONP, which is also considered a white matter tract, albeit in a different anatomical location. In particular, CC astrocytes were uniquely characterized as having the comparably lower expression of neuroinflammation and immune pathways. A summary of the pathways that characterize astrocytes from the different regions is shown in Figure 6A.
Figure 6.
Summary of region-specific astrocyte pathways and the expression of general astrocyte and neuroinflammation genes
(A) A summary of the region-specific astrocyte pathways identified in global, unbiased analyses.
(B) Log2 fold change values for canonical astrocyte genes, as determined in the differential expression analysis of ONH vs ONP samples. Stars indicate significant differences between the two groups (adjusted p value < 0.05).
(C) Log2 fold change values for neuroinflammation and neuroprotection-related genes, as determined in the differential expression analysis of ONH vs ONP samples. Stars indicate significant differences between the 2 groups (adjusted p value < 0.05).
(D) Log2 fold change values for reactivity-related genes, as derived from independent pairwise analyses of ONH vs ONP, ONH vs CC and ONP vs CC. Stars indicate significant differences between the 2 groups (adjusted p value < 0.05).
Expression of general astrocyte marker and homeostasis function genes
Having looked at genes and pathways distinguishing astrocytes in the three regions using a global, unbiased approach, we next turned our attention to genes of interest to astrocyte biology, including canonical astrocyte markers and genes involved in astrocyte homeostasis. In addition, previous immunohistochemical studies have highlighted features of ONH astrocytes that differentiate them from those in the ONP, such as higher levels of nestin (NES) and lower levels of aquaporin (AQP4), so we set out to determine whether these observations were reflected in our transcriptomic dataset. First, we looked at the cytoskeletal markers GFAP, vimentin (Vim) and Nes, the cytosolic markers S100b, Aldh1l1, Aldoc, the lipoprotein Apoe, and the water channel Aqp4 (Figure 6B). As expected, S100b, Vim, and Nes showed higher expression in the ONH. GFAP, Aldh1l1, Aldoc, and Apoe showed no differential expression. Although Aqp4 is a ubiquitous marker of astrocytes in the brain, it is not found in ONH astrocytes, and here we confirmed its low expression when compared to ONP astrocytes. Many of the genes related to general astrocyte functions were not differentially expressed between the two regions (Figure 6B). These included the glutamate uptake transporter Glt1 (Slc1a2) and glutamate aspartate transporter (GLAST; Slc1a3), the glutamate-metabolizing enzyme glutamine synthetase (Glul), the g-aminobutyric acid (GABA) transporter GAT3 (Slc6a11), and the major channel involved in potassium uptake Kir4.1 (Kcjn10). Genes related to connexins (Gja1), ATP receptors (P2rx2, P2rx4, P2rx7, P2ry1), and phagocytosis also showed no significant differential expression between ONH and ONP astrocytes. Many of the genes relating to Ca2+ flux were not differentially expressed; one exception was Trpm3. Trpm3 is the primary channel for store operated calcium entry in optic nerve astrocytes, which is essential for ATP-mediated Ca2+ signaling.41 The finding that it is more highly expressed in ONH versus ONP astrocytes suggests they have much higher Ca2+ dynamics and “glial excitability.” Many ionotropic and metabotropic glutamate and GABA receptor genes were de-enriched in the ONH (Figure 6B).
Optic nerve astrocytes are enriched for neuroinflammation pathways, with no difference between optic nerve head and optic nerve proper astrocytes
The ONH is densely populated with astrocytes and is an important site of neuroinflammation in glaucoma. Moreover, optic nerve astrocytes express very high levels of GFAP, much more than brain astrocytes, and therefore have been suggested to have a certain degree of neuroinflammation or reactivity at steady-state conditions. We wanted to determine if ONH astrocytes have higher expression of genes related to neuroinflammation and immunity under steady-state conditions, which could predispose the ONH environment to the development of neurodegeneration. We looked at the log2 fold change values of genes known from the literature to be involved in the pathogenesis of glaucoma,36,42,43,44 focusing on the comparison of ONH versus ONP (Figure 6C). We found that most were not differentially expressed, including the cytokines Il6, Il-1β, Il-1α, Tnfα, and Ifnγ, and other key players in pro-inflammatory pathways such as Tlr4, Nlrp3, Nfκβ, Cxcl2 (Mip2), Ccl2 (Mcp1), and MIP1α (Ccl3). Furthermore, most of the components of the classical complement pathway were also not differentially expressed apart from C3, which was de-enriched in the ONH compared to the ONP. C3 is a potential marker of astrocyte reactivity in the brain.45 Finally, genes related to MHC Class I and neuroprotection were also not differentially expressed (Figure 6C).
Astrocytes respond to all forms of injuries and disease through a process referred to as reactivity, and recently particular gene signatures have been identified as being associated with whether the reactive response is purportedly damaging (A1 phenotype), purportedly supportive (A2 phenotype), or pan-reactive.45,46 Although in this study we are assessing normal tissue, the high level of GFAP and vimentin in normal optic nerve astrocytes suggests they may already be somewhat reactive, and thus we performed this analysis. There are caveats in categorizing astrocytes in this manner (see discussion). Optic nerve head astrocytes showed enrichment of a few but not many of the A1, A2, and pan-reactive marker genes compared to ONP astrocytes (Figure 6D). However, compared to those in the CC, both ONH and ONP astrocytes showed significant enrichment in virtually all the genes, with no bias toward any one signature. This is consistent with the de-enrichment of many neuroinflammation pathways in CC astrocytes. Finally, we used GSEA to ask whether key astrocyte signaling pathways involved in reactivity and the injury response were enriched in ONH versus ONP astrocytes at steady-state. We restricted this follow-up GSEA to six pathways: nuclear factor kappa B (NFkB), calcineurin-nuclear factor activating of T-cells (CaN-NFAT), Mitogen-Activated protein kinase (MAPK), Janus Kinases/Signal Transducer and Activator of Transcription (JAK/STAT), Wnt/b-catenin, and sonic hedgehog, sourced from the GO, KEGG, and Reactome databases. Only one pathway, positive regulation of MAPK cascade from the GO database was significantly enriched (Figure S1). In all, we did not find strong evidence for a robust differential neuroinflammation activity between steady-state ONH and ONP astrocytes. However, in comparison with CC astrocytes (Figure 5C), both optic nerve astrocyte populations have higher expression of neuroinflammatory pathways.
CARTPT is a specific marker of optic nerve head astrocytes
Cocaine- and amphetamine-regulated transcript (Cartpt) was one of the most enriched genes in ONH astrocytes. It is a neuropeptide first found in the rat ventral striatum in response to intraperitoneal injections of cocaine and amphetamine and is involved in a wide range of physiological functions including stress, feeding behavior, autonomic regulation, fluid balance, metabolic processes, endocrine control, immune function and neuroprotection.47,48 In the retina, CARTPT is known to label ON-OFF direction-selective ganglion cells and a subpopulation of amacrine cells.49,50,51,52 Immunohistochemical staining with a CARTPT-specific antibody revealed bright labeling of cells within the unmyelinated ONH region and the myelination transition zone only (Figures 7A–7C), and no labeling in the more distal myelinated ONP. CARTPT labeling was perinuclear and colocalized with SOX9-positive astrocytes, but not with IBA1 (Figures 7D–7F). Cell counts determined that 82–91% of astrocytes in the unmyelinated ONH region were CARTPT-positive (Figure S1). In the retina, we observed sparse labeling of somas in the ganglion cell layer, presumably the ON-OFF direction-selective ganglion cells, and in the outer most layer of the inner plexiform layer. CARTPT did not colocalize with GFAP in retina (Figures 7G and 7H), nor was there any labeling in the corpus callosum (Figure 7L). We used duplex in-situ hybridization to confirm the colocalization of Cartpt mRNA with ONH astrocytes (Figures 7I and 7J), that in the retina there was no colocalization of Cartpt mRNA with GFAP (Figure 7K), and there was no Cartpt mRNA in the corpus callosum (Figures 7M and 7N).
Figure 7.
CARTPT is a marker of optic nerve head astrocytes
(A–C) Immunohistochemical labeling for CARTPT and myelin basic protein (MBP) demonstrated CARTPT primarily within the unmyelinated ONH region and the myelin transition zone, with no labeling in the distal myelinated ONP. Scale bars: A = 100 μm and applies to A–C; D = 50 μm and applies to D and F; G and H = 50 μm; I = 100 μm; K and M = 1 mm; L = 50 μm.
(D–F) CARTPT labeling was restricted to the perinuclear space of SOX9-positive astrocytes. There was no colocalization of CARTPT with IBA1.
(G and H) In the retina, CARTPT labeled a sparse population of somas within the ganglion cell layer (GCL) that did not colocalize with glial fibrillary acidic protein (GFAP) (arrows), and puncta in the outer most layer of the inner plexiform layer (IPL). Arrowheads in panel G indicate non-specific blood vessel staining.
(I–K) Duplex in-situ hybridization demonstrating the colocalization of Cartpt mRNA (red) specifically to ONH astrocytes (GFAP mRNA, turquoise). In the retina Cartpt mRNA did not colocalize with GFAP. Nuclei are stained with hematoxylin (purple).
(L–N) Immunohistochemistry and duplex in-situ hybridization showed there was no Cartpt expression in the corpus callosum.
Discussion
We performed RNA-seq on astrocytes from two optic nerve regions and the CC to investigate whether these represent transcriptionally distinct populations. Our results revealed significant heterogeneity both within the optic nerve and between it and the CC, highlighting greater diversity among white matter astrocytes than was previously appreciated. Although the ONH makes up only a small portion of the optic nerve (approximately 150–250 μm long in the mouse), our results indicate the presence of a unique population of astrocytes within this region, distinct from those of the myelinated ONP. This finding is consistent with our current view that the ONH represents a complex microenvironment with different glial support requirements from the adjacent myelinated region. Astrocytes of the CC were unlike those in the optic nerve altogether.
Due to the small size of the mouse optic nerve—particularly the unmyelinated head region, home to approximately 200 astrocytes—isolation of these cells for transcriptomic studies is challenging. Previous studies have investigated whole optic nerves, followed by immunohistochemistry to localize changes in expression to astrocytes,24,53,54,55 or employed less physiological isolation methods such as culturing astrocytes,56,57,58 which inevitably alters gene expression. FACS has rarely been used to isolate astrocytes of the optic nerve, although it has been well developed to isolate optic nerve microglia.59,60,61 In part, this may stem from limitations of the FACS methods, including the requirement for clean single-cell suspensions (challenging from a sponge-like densely myelinated tissue), cell loss (problematic with small cell populations), and the loss of RNA from distal processes (known to be physiologically relevant in astrocytes). These points may also complicate single-cell RNA-seq of the optic nerve, and to date, we are not aware of any prior studies using this method. Here, ribotag immunoprecipitation efficiently isolated astrocyte-specific ribosomal mRNA from small complex tissues in vivo. Ribosomal mRNAs more closely reflect the proteins being functionally used by the cell, or the active translational state of the cell, rather than the transcriptome irrespective of functional relevance.29,30 As well, the sequencing is weighted toward more frequently translated transcripts. This approach also isolates mRNA being translated in distal processes, a population of mRNAs often lost using other methods. For this study, we crossed Rpl22 mice with a Cre line driven by the astrocytic GFAP promoter, but other Cre lines could be used (e.g., Cx3Cr1-Cre to isolate microglia/macrophages).31
Our study identified three transcriptionally distinct populations of astrocytes. For the transcriptome derived from the ONH sample, we believe the profile is representative of one astrocyte population. Our previous morphological study showed that there was only one morphological type,23 and because the mouse ONH contains so few astrocytes it is unlikely we missed others. However, both the ONP and CC contain a variety of morphological types. Some have main processes oriented in parallel with the axonal fibers, some are oriented transversely, while others specifically in the optic nerve lie flat adjacent to the glia limitans.23 Therefore, the ONP and CC sample transcriptome profile likely represents multiple populations. While single-cell RNA-seq analysis would provide better resolution, the aforementioned difficulties present significant hurdles to that approach. We compared the heterogeneity found in our study, with brain studies of inter-regional astrocyte variation and found a similar difference; the number of DEGs that identified astrocytes in each of our studied white matter regions was similar to the number of DEGs that identified astrocytes in different gray matter brain regions.8,13
Energy metabolism emerged as a key differentiator of astrocyte populations. Astrocytes use both glycolytic and oxidative metabolism, with the latter producing more ATP per metabolite. As the main energy substrate, glucose can be metabolized via glycolysis to pyruvate, at which point it is either converted to lactate in the cytosol or enters the mitochondria for catabolism via the TCA cycle to either acetyl CoA or oxaloacetate (e.g., oxidative metabolism of glucose). Glucose can also be metabolized via the pentose phosphate pathway to generate sugars for nucleotides and reducing equivalents or stored as glycogen. Optic nerve head astrocytes were enriched for mitochondrial ribosomal protein translation and oxidative phosphorylation pathways, in comparison CC astrocytes were enriched in other key metabolic pathways: (1) they showed enrichment of glycogen metabolism and expressed high levels of all the key enzymes in the glycogen synthesis and breakdown pathways (Figure 5D), (2) they did not show enrichment of the glycolytic pathway. Many of the key glycolytic enzymes from glucose phosphorylation (glucose-6-phosphate) to pyruvate synthesis were not differentially expressed in CC versus ONH astrocytes (Figure 5E), (3) Ldhb, the key enzyme in converting pyruvate to lactate was highly enriched in CC astrocytes, (4) oxidative metabolism was highly enriched. In virtually all TCA cycle enzymatic steps, the expression was higher in CC versus ONH astrocytes (Figure 5F). (5) Lastly, there are multiple inputs into the TCA cycle, one of these important anaplerotic mechanisms is the activation of glutaminolysis, which converts glutamine to glutamate and subsequently to alpha-ketoglutarate (Figure 5F). Corpus callosum astrocytes exhibited enrichment of the key enzymes for this process (Figure 5F).
Axons are reliant on astrocytes for energy. Mammalian white matter tracts are metabolically demanding due to the extensive energy requirements of axons,62,63 yet they possess a relatively restricted substrate supply due to a vascular network that is generally less dense than that of gray matter.64 Dense astrocyte populations in these regions are the main energy providers to axons via the astrocyte-neuron lactate transfer shuttle.65,66 Here, glucose taken up from capillaries by astrocyte endfeet is stored as glycogen in astrocytes, a function exclusive to astrocytes in the CNS, and then delivered to axons as lactate when needed via release through monocarboxylate transporters (MCT1 on astrocytes).67 In this role glycogen is stored as an energy buffer and only converted to lactate when ambient glucose alone is insufficient to meet the immediate energy demands. Lactate becomes the preferred energy source for fueling axons when glucose is very low or when there is increased neuronal activity (e.g., increased firing).68,69 This metabolic support is not solely a local function, as studies have demonstrated that astrocytes in both white70 and gray matter71 are capable of redistributing metabolic resources to stressed regions of tissue through gap junction (Cx43) coupled networks. However, significant variation exists in this provisioning even between white matter regions, with glial support networks in the CC showing greater involvement of oligodendrocytes72 than has been shown in the optic nerve.73 In this context it is interesting that while we observed distinct populations of astrocytes that neighbor each other and differentiate by how they metabolize energy (e.g., ONH vs ONP astrocytes), they are nonetheless linked in a single regional network.70 Given that CC astrocytes are enriched for glycogen synthesis, lactate production, and oxidative metabolism, and therefore presumably the astrocyte-neuron lactate shuttle, they may be able to better meet energy demands during high levels of neuronal activity or stress. In contrast, ONH astrocytes are enriched for oxidative phosphorylation, a highly oxygen-consuming process that also produces ROS, and therefore may increase its susceptibility to oxidative stress in injury/diseases. These results indicate that energy production and very likely usage are not uniform across white matter astrocytes and that the astrocyte-neuron lactate transfer shuttle could be more relied upon in certain CNS regions. The differential expression of metabolic pathways across regions may be an important factor for the discordant conclusions regarding the universality of the astrocyte-neuron lactate shuttle.74
Our finding that ONH astrocytes are enriched for oxidative phosphorylation is consistent with the view that the ONH environment is under unique stress and more metabolically demanding than the myelinated ONP and CC. Not only do ganglion cell axons have high signaling activity and thus metabolism, in this region they are unmyelinated. Moreover, ONH tissues and cells experience biomechanical strain from constant fluctuations in IOP and eye movements that rotate the globe independent of the optic nerve. Having astrocytes with a high capacity for oxidative phosphorylation is energetically advantageous; oxidative phosphorylation yields 36 molecules of net ATP per molecule of glucose whereas the glycolytic pathway yields 2 molecules of ATP.75 It is noteworthy also that the vascular network in the ONH region is denser and more complex than that of the myelinated ONP,28 most likely enabling greater provision of oxygen. The high energy demand of the ONH environment also suggests that it is particularly vulnerable to energy insufficiency. To counter the production of ROS by-products from high oxidative phosphorylation, ONH astrocytes have a higher level of antioxidant activity. Astrocytes have a primary role in providing antioxidant support to surrounding neurons through several systems: the glutathione system, thioredoxin/peroxiredoxin system, superoxidase dismutases, and catalase.75 They are the primary producers of and can store glutathione.40 Our GO analysis of molecular function found glutathione transferase and peroxidase activity to be high, both of which had overlapping gene sets that included: glutathione S-transferase (Gsta1, Gsta2, Gsta4, Gsta5), glutathione peroxidase (Gpx1, Gpx3, Gpx4, Gpx7, Gpx8), and peroxiredoxin (Prdx4, Prdx5). These genes play an important role in the enzymatic catabolism of H2O2. In fact, Gsta1 was one of the top most enriched genes specific to ONH astrocytes (Figure 2B). Interestingly, Prdx2, Prdx5, and Sod2 were amongst the top 50 most de-enriched genes unique to ONP astrocytes.
Our analysis demonstrated that optic nerve astrocytes were enriched for neuroinflammatory pathways in steady-state conditions, suggesting they may be prone to inflammation in optic neuropathies such as glaucoma. Astrocytes from both optic nerve regions also showed enrichment of most A1, A2, and pan-reactive gene signatures when compared to CC astrocytes. An important note here is that without complimentary functional tests we cannot conclude whether optic nerve astrocytes are more reactive per se or whether they exist in a different homeostatic state. Moreover, caution is needed in interpreting the results from this classification as there are several caveats: (1) we are studying normal tissue, whereas the classification was defined based on two acute injury models, and (2) the classification was based on the response of gray matter cortical astrocytes, and how this translates to white matter astrocytes is unknown. There are additional concerns with the classification and these have been well described in the recent astrocyte consensus statement.46 Notwithstanding these caveats, the classification provides a framework and we performed the analysis to allow comparisons with other studies that have used the nomenclature.
In conclusion, we have identified three transcriptionally distinct populations of white matter astrocytes, providing insight into their functional diversity. Metabolism played a key role in astrocyte identity, and it could be more important than previously appreciated in determining how the regional astrocytes respond to injuries involving the optic nerve. Finally, we identified CARTPT as a marker of optic nerve head astrocytes.
Limitations of the study
Due to inherent limitations with the mouse optic nerve—small tissue size, resistance to enzymatic dissociation, and low absolute numbers of ONH astrocytes—we have utilized tissue dissection and bulk RNA-seq approaches rather than a single-cell RNA-seq approach. While this made our study possible, it carries its own limitations. The most notable of these is a limited ability to detect astrocyte heterogeneity within a given tissue region. This is not an issue with ONH, as we are confident from existing morphological data that there is only a single population in this region (Sun et al., 2009). However, the myelinated optic nerve is a much larger anatomical region, with astrocytes of varying morphologies, and due to the resolution of our bulk RNA-seq method we are less able to evaluate the transcriptional heterogeneity of astrocytes within this region and whether multiple populations may be present.
Additionally, the myelin transition zone delineating these two regions forms an uneven demarcation rather than a smooth line. It is likely that our dissected ONHs contain small numbers of astrocytes from the myelinated region of the nerve, and that as a result, our transcriptional data may, in fact, understate the heterogeneity between these two regions.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit polyclonal anti-HA (1:400) | Phoenix Pharmaceuticals | Cat# H-003-61, RRID:AB_2922768 |
| Goat monoclonal Anti-hSOX9 antibody (1:100) | R&D Systems | Cat# AF3075, RRID:AB_2194160 |
| Chicken polyclonal Anti-GFAP antibody (1:2000) | Abcam | Cat# ab4674, RRID: AB_304558 |
| Rabbit polyclonal anti-Iba1 antibody (1:400) | FUJIFILM Wako Shibayagi | Cat# 019-19741, RRID:AB_839504 |
| Goat polyclonal anti-Olig2 antibody (1:100) | R&D Systems | Cat# AF2418, RRID:AB_2157554 |
| Rabbit polyclonal anti-NG2 antibody (1:400) | EMD Millipore | Cat# AB5320, RRID:AB_11213678 |
| Mouse monoclonal anti-hCART antibody (1:600) | R&D Systems | Cat# MAB163, RRID:AB_2275127 |
| Alexa Fluor 488-AffiniPure F(ab')2 Fragment Donkey Anti-Mouse IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 715-546-150, RRID:AB_2340849 |
| Alexa Fluor 594-AffiniPure Donkey Anti-Mouse IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 715-585-150, RRID:AB_2340854 |
| Alexa Fluor 594-AffiniPure F(ab')2 Fragment Donkey Anti-Chicken IgY (IgG) (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 703-586-155, RRID:AB_2340378 |
| Alexa Fluor 594-AffiniPure Donkey Anti-Goat IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 705-585-147, RRID:AB_2340433 |
| Alexa Fluor® 594 AffiniPure Donkey Anti-Rabbit IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 711-585-152, RRID:AB_2340621 |
| Alexa Fluor 647-AffiniPure Donkey Anti-Goat IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 705-605-147, RRID:AB_2340437 |
| Alexa Fluor 488-AffiniPure Donkey Anti-Rabbit IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 711-545-152, RRID:AB_2313584 |
| Alexa Fluor 488-AffiniPure Donkey Anti-Goat IgG (H+L) (1:800) | Jackson ImmunoResearch Labs | Cat# 705-545-147, RRID:AB_2336933 |
| Critical commercial assays | ||
| RNeasy Plus Micro Kit | Qiagen | Cat #74034 |
| Smart-Seq HT Ultra-low input RNA kit | Takara Biosciences | Cat #634437 |
| Deposited data | ||
| Raw and analyzed data | This paper | GEO: GSE211515 |
| Experimental models: Organisms/strains | ||
| Mouse: B6J.129(Cg)-Rpl22tm1.1Psam/SjJ | The Jackson Laboratory | JAX: 029977, RRID:IMSR_JAX:029977 |
| Mouse: B6.Cg-Tg (GFAP-Cre) 73.12Mvs/J | The Jackson Laboratory | JAX: 012886, RRID:IMSR_JAX:012886 |
| Oligonucleotides | ||
| RNAscope Probe - Mm-Gfap | Advanced Cell Diagnostics | Cat# 313211 |
| RNAscope Probe - Mm-Cartpt-C2 | Advanced Cell Diagnostics | Cat# 432001-C2 |
| RNAscope Positive Control Probe - Mm-Ppib | Advanced Cell Diagnostics | Cat# 313911 |
| RNAscope Probe - Mm-Polr2a-C2 | Advanced Cell Diagnostics | Cat# 312471-C2 |
| RNAscope Negative Control Probe - DapB | Advanced Cell Diagnostics | Cat# 310043 |
| Cartpt-F: CCCGAGCCCTGGACATCTA | Eurofins Genomics | Cocaine- And Amphetamine-Regulated Transcript Protein |
| Cartpt-R: GCTTCGATCTGCAACATAGCG | Eurofins Genomics | N/A |
| Alox8-F: CTGTCAGCATCGTGGGAACC | Eurofins Genomics | Arachidonate 8-Lipoxygenase |
| Alox8-R: GGAAGCGTCACCTCGAAGTC | Eurofins Genomics | N/A |
| Pth2r-F: GGCTGATTCTCAGTAGCTGTCT | Eurofins Genomics | Parathyroid Hormone 2 Receptor |
| Pth2r-R: GGGCCAACAAATGATCCCATC | Eurofins Genomics | N/A |
| Gsta1-F: CGCAGACCAGAGCCATTCTC | Eurofins Genomics | Glutathione S-transferase, Alpha 1 |
| Gsta1-R: TTGCCCAATCATTTCAGTCAGA | Eurofins Genomics | N/A |
| Mgarp-F: CATCAAAGCAAGTGAGACGTACAG | Eurofins Genomics | Mitochondria Localized Glutamic Acid Rich Protein |
| Mgarp-R: AGCAGTGTCTCCAGGCTCTGAA | Eurofins Genomics | N/A |
| Slco1c1-F: GATCCAGACCCTTGCGAACAT | Eurofins Genomics | Solute Carrier Organic Anion Transporter Family Member 1C1 |
| Slco1c1-R: GATATCCGACTGTAAAGGATGG | Eurofins Genomics | N/A |
| Bbox1-F: CTGACAAACGTGGTGAGATCA | Eurofins Genomics | Gamma-Butyrobetaine Hydroxylase 1 |
| Bbox1-R: CCACATTGTTGGCATCAATCTTG | Eurofins Genomics | N/A |
| Ttn-F: CTACGTGGTAGAAAAGCGAGAAA | Eurofins Genomics | Titin |
| Ttn-R: ACACCGTACTTGTTGACAGCC | Eurofins Genomics | N/A |
| GFAP-F: ACATCGAGATCGCCACCTACA | Eurofins Genomics | Glial Fibrillary Acidic Protein |
| GFAP-R: GATTTGGTGTCCAGGCTGGTT | Eurofins Genomics | N/A |
| S100B-F: TGGTTGCCCTCATTGATGTCT | Eurofins Genomics | S100 Calcium Binding Protein B |
| S100B-R: CCCATCCCCATCTTCGTCC | Eurofins Genomics | N/A |
| Gapdh-F: GGTTGTCTCCTGCGACTTCAA | Eurofins Genomics | Glyceraldehyde-3-Phosphate Dehydrogenase |
| Gapdh-R: CCTGTTGCTGTAGCCGTATTCAT | Eurofins Genomics | N/A |
| Aqp4-F: ATCATGGGAAACTGGGCAAAC | Eurofins Genomics | Aquaporin 4 |
| Aqp4-R: CTCCACATCAGGACAGAAGACATACT | Eurofins Genomics | N/A |
| Aldh1l1-F: TTTCAACTCGACACTCAACACTT | Eurofins Genomics | Aldehyde Dehydrogenase 1 Family Member L1 |
| Aldh1l1-R: GTCCCGCTTTGGTGACTAGG | Eurofins Genomics | N/A |
| Iba1-F: AAGGCCCAGCAGGAAGAGA | Eurofins Genomics | Ionized calcium binding adaptor molecule 1 |
| Iba1-R: GGAGATCCTCATCATTGCTGTA | Eurofins Genomics | N/A |
| NEFH-F: GAAACACCAAGTGGGAGATGG | Eurofins Genomics | Neurofilament Heavy Chain |
| NEFH-R: GAGCTTTCTGTAAGCGGCAAT | Eurofins Genomics | N/A |
| PTPRC-F: CAGAAACGCCTAAGCCTAGTTG | Eurofins Genomics | Protein Tyrosine Phosphatase Receptor Type C |
| PTPRC-R: AGGCAAGTAGGGACACTTCATAG | Eurofins Genomics | N/A |
| MBP-F: TCACAGCGATCCAAGTACCTG | Eurofins Genomics | Myelin Basic Protein |
| MBP-R: CCCCTGTCACGCTAAAGAA | Eurofins Genomics | N/A |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Daniel Sun (daniel_sun@meei.harvard.edu).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
All animals were handled in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research, and all procedures were approved by the Institutional Animal Care and Use Committee at Schepens Eye Research Institute. Mice aged 3–4 months were housed in a 12 h light/dark cycle and received food and water ad libitum. Two mouse strains were used in this study: (1) B6J.129(Cg)-Rpl22tm1.1Psam/SjJ (The Jackson Laboratory, Bar Harbor, ME, strain #029977), called RiboTag, and (2) B6.Cg-Tg (GFAP-Cre) 73.12Mvs/J (The Jackson Laboratory, Bar Harbor, ME, strain #012886). The ribotag approach is a strategy that efficiently isolates ribosome-associated mRNA transcripts from specific cell types from complex tissues in vivo.29,30 The RiboTag mouse has a modification in the 60S ribosomal protein gene, Rpl22, such that in the presence of Cre recombinase the floxed exon 4 of Rpl22 will be replaced with a hemagglutinin (HA)-tagged exon 4, effectively labeling the cells’ polyribosomes so that they can be isolated by immunoprecipitation.29,30 RiboTag mice can be crossed to a variety of Cre recombinase driver mouse lines, and in our case, we use a GFAP-Cre line in which a mouse GFAP promoter sequence directs expression of Cre recombinase in astrocytes. Both mice lines were on a C57Bl/6 background. We initially crossed GFAP-Cre recombinase expressing mice with hemizygous RiboTag mice to get offspring heterozygous for the RiboTag allele (Rpl22HA/+). These heterozygous RiboTag mice were then bred together to obtain experimental mice that were homozygous for the RiboTag allele with the Cre transgene (Rpl22HA/HA Cre+).
Method details
Preparation of reagents
Homogenization buffer (HB)
To prepare the homogenization buffer, 2.5 mL of 1 M Tris (pH 7.4; ThermoFisher Scientific, #17926), 2.5 mL of 2 M KCl (RNase free; Fisher Scientific, #AM9640G), 600 μL of 1 M MgCl2 (RNase free; Fisher Scientific, #AM9530G) and 500 μL of NP-40 (ThermoFisher Scientific, #28324) were added to a 50 mL Falcon tube. The volume was made up to 50 mL with DNAse/RNAse free water and vortexed until all components were dissolved. On the day of the experiment, homogenization buffer supplemented (HB-S) was prepared by adding 5 μL of 1 M DTT (VWR, #97061-340), 50 μL of protease inhibitor (VWR, #97063-970), 25 μL of RNasin (VWR, #PAN2615), 100 μL of Cycloheximide (5 mg/mL; Fisher Scientific, #AC357420050), and 50 μL of Heparin (100 mg/mL; Fisher Scientific, #BP252420) in a 15 mL Falcon tube. The volume was made up to 5 mL with HB buffer. The HB-S solution was prepared fresh just prior to homogenization.
High salt buffer (HSB)
High salt buffer was prepared by adding 2.5 mL of 1 M Tris (pH 7.4; ThermoFisher Scientific, #17926), 7.5 mL of 2 M KCl (RNase free; Fisher Scientific, #AM9640G), 600 μL of 1 M MgCl2 (RNase free; Fisher Scientific, #AM9530G), and 500 μL of NP-40 (ThermoFisher Scientific, #28324) in a 50 mL Falcon tube. The final volume was brought to 50 mL with DNase/RNase free water and vortexed. The high salt buffer supplemented (HSB-S) was prepared before the washing step by adding 40 μL of cycloheximide (5 mg/mL; Fisher Scientific, #AC357420050), 10 μL of protease inhibitor (VWR, #97063-970), and 5 μL of RNasin (VWR, #PAN2615), in a 2 mL Eppendorf tube and the volume made up to 2 mL with HSB. This solution was prepared fresh before the washing step.
Preparation of tissue and ribotag immunoprecipitation
Th following procedures have been optimized from29,30 for very small tissues. Mice were euthanized with carbon dioxide followed by cervical dislocation. The skull and the brain were removed, and each eye together with the optic nerve carefully dissected out without imposing any mechanical stress or damage to the tissue. The ONH, ONP and CC were micro-dissected on ice in chilled PBS. We considered the translucent unmyelinated portion of the optic nerve as the ONH (Figure 1A). In order to obtain sufficient RNA, 4 ONHs and 4 ONP (2 males and 2 females) were pooled for n = 1 each. Each of the ONH and ONP came from the same mouse and an equal length of ONP as ONH was used. We used the most proximal portion of the ONP and the remainder of the optic nerve was not sequenced. Pooling was not required with the larger CC and here equal numbers of male and female mice were used. Once dissected, tissues were placed in 150 μL of HB-S buffer, centrifuged for 2 mins at 1000 rpm at 4°C, then homogenized using a PYREX Glass Pestle in the microcentrifuge tubes on ice. The lysate was further centrifuged for 10 mins at 10,000 rpm at 4°C, then the supernatant collected. 10 μL of the supernatant was mixed with 350 μL of RLT lysis buffer (RNeasy Micro Kit, Qiagen) and kept as Input, the remaining supernatant was used for immunoprecipitation (IP).
HA-tagged ribosomes were captured by adding 2.5 μL of anti-HA antibody (BioLegend, #MMS-101R) to the IP supernatant and incubated for 4 hours at 4°C on a mini-tube rotator with gentle mixing. This antibody-supernatant is then added to 55 μL of pre-washed Pierce Protein A/G magnetic beads and incubated overnight at 4°C on a mini-tube rotator. The following day, the Eppendorf tubes were placed in a Dynamag-2 magnetic rack on ice and washed twice with HSB-S buffer. The non-precipitated flow-through from each immunoprecipitated sample was collected and RNA was purified for enrichment analysis using a RNeasy Micro Kit (Qiagen). The CC samples were immunoprecipitated with 200 μL of magnetic beads and bead equilibration carried out with 800 μL of HB-S buffer, with all other steps being similar.
RNA-seq library generation and sequencing
RNA was extracted using the RNeasy Plus Micro Kit (Qiagen) and the quantity and quality were measured on an Agilent 2100 Bioanalyzer. Typical yields and RIN scores are shown in Figure S1. Typically, 3.95–37.4 ng of RNA was used to make libraries; to avoid batch effects, library preparation and sequencing were done at the same time for all samples. mRNA was extracted with oligo-dT beads, capturing poly(A) tails, and cDNA libraries were made with the Smart-Seq HT Ultra-low input RNA kit (Takara Biosciences). The cDNA samples were normalized and sequenced on an Illumina HiSeq 4000 with paired-end 2x150 bp reads at 20–30 million reads per sample. Library preparation and sequencing were performed by Genewiz (Chelmsford, MA).
RNA-seq mapping, analysis, and statistics
Sequencing data was processed for alignment to the mouse genome, quality control and transcript quantification using the bcbio-nextgen bulk RNA-seq pipeline (version 1.2.8-1c563f1). More specifically, reads were aligned to NCBI build mm10 of the mouse genome (Mus musculus) using the STAR aligner (version 2.6.1d). Additional transcript-level information was sourced from Ensembl release 94. Sequencing data quality control was performed using FastQC (version 0.11.8), Qualimap (version 2.2.2d) and Samtools (version 1.9), and summarized with MultiQC (version 1.9). Transcripts were quantified through a quasi-alignment approach using Salmon (version 0.14.2). Transcript-level counts were then aggregated at the gene level and imported into R using tximport. Downstream analyses were performed in R (version 4.1.0). Independent, pairwise differential expression analyses contrasting gene expression between 2 interest groups were performed with DESeq2 (version 1.34.0). For subsequent functional analyses and graphical representations, DESeq2-derived log2 fold change values were corrected using the ashr shrinkage estimator. Over representation analyses (ORA) and geneset enrichment analyses (GSEA) were run using clusterProfiler (version 4.2.2) and ReactomePA (version 1.38.0). Significance for differential expression was defined as an adjusted p-value < 0.05, using Benjamini-Hochberg’s procedure for multiple comparisons adjustment. No cutoff on log2 fold change was used to define significant genes.
Immunohistochemistry
Optic nerve, retina and brain sections were incubated with blocking solution (10% donkey serum, 0.5% Triton-X, 1% BSA) for one hour, then incubated with primary antibodies also for one hour at room temperature. Tissues were then washed in PBS (pH 7.4; 3 × 5 mins), and the secondary antibody applied for one hour at room temperature. Following incubation, the tissues were washed in PBS (pH 7.4; 3 × 5 mins) and cover-slipped with Prolong Diamond Antifade Mountant (Thermo Fisher Scientific). Whole retinas were incubated in the primary antibodies for three days at 4°C and the secondary for two days. Slides were imaged on a Leica TCS SP8 confocal microscope. All fluorescent images were maximum intensity projections. The brightness and contrast of the final images were adjusted using Adobe Photoshop 2022; no other digital image processing was performed.
In-situ hybridization
In-situ hybridizations were carried out according to the manufacturer’s instructions using the RNAscope duplex detection chromogenic kit (Advanced Cell Diagnostics, #322500). Optic nerve and retina were fixed overnight at 4°C in 4% paraformaldehyde and cryoprotected in 30% sucrose in 1x PBS. In-situ hybridization was performed on 15-μm thick tissue sections and hybridized with mouse GFAP (#313211) and CARTPT (#432001-C2). To assess probe specificity, two types of controls were used: (1) mouse PPIB (peptidylprolyl isomerase B, #313911) and mouse Polr2a (polymerase II polypeptide A, #312471-C2) as positive controls, and (2) bacterial DapB (dihydrodipicolinate reductase, #310043) as a negative control. Tissue sections were counterstained with 50% Mayer’s hematoxylin, mounted using Vecta Shield mounting medium (Vector Laboratories, #H-1500), and bright-field images were obtained with a Nikon Eclipse 800 microscope interfaced with Olympus DP controller software.
Quantitative RT-PCR
RNA was extracted using the RNeasy Plus Micro Kit (Qiagen) and the quantity and quality were measured on an Agilent 2100 Bioanalyzer. Synthesis of cDNA was performed using the Smart-Seq HT kit (Takara Biosciences) following the manufacturer’s protocol, and quantified using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific). Enrichment and de-enrichment of cell specific gene transcripts in IP RNA relative to Input RNA, as well as validation of RNA sequencing data were confirmed by qRT-PCR (Applied Biosystems StepOnePlus Real-time PCR system) and using the SYBR Green PCR Master Mix (Applied Biosystems, ThermoFisher Scientific, Waltham, MA). Primers used targeted astrocytes, microglia, oligodendrocytes and neurofilament. Melting curves were examined to verify amplification specificity. Each gene and glyceraldehyde phosphate dehydrogenase (GAPDH) were interrogated in triplicates. All technical replicates had cycle thresholds (CT) that differed from each other by less than 1.0 CT and a CT standard deviation <0.3. All gene expression levels were normalized to GAPDH using the ddCT method.
Quantification and statistical analysis
Statistical details of experiments can be found in the figure legends. Statistical analysis of the RNA-seq data is described above. Immunohistochemistry and duplex in-situ hybridizations were performed on n = 5 biological replicates.
Acknowledgments
This work and members of the Sun lab (A.G.M. and P.F.C.) were supported by NIH-NEI R01 EY029268-01, a Research to Prevent Blindness Grant, and a NIH Core Grant for Vision Research P30EY003790. Work by the Harvard Chan Bioinformatics Core was conducted with support from Harvard Catalyst | The Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL 1TR002541) and financial contributions from Harvard University and affiliated centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated centers, or the National Institutes of Health.
Author contributions
A.G.M. and D.S. conceived the experiments. A.G.M. performed most of the experiments with P.F.C. helping with the immunohistochemistry. A.M.J. analyzed the RNA-seq data. D.S. wrote the article with input from the other authors.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research.
Published: December 22, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105568.
Supplemental information
Differentially expressed genes in independent pairwise comparisons of ONP vs CC, ONH vs CC, and ONH vs ONP (no criteria applied).
ONH-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 2. Details of the analysis and criteria used are indicated in the heading of each tab.
Gene set enrichment analysis on pairwise comparison of ONH versus ONP astrocytes, and analysis against the molecular function subset of the gene ontology (GO) database, related to Figure 3. Details of the analysis and criteria used are indicated in the heading of each tab.
ONP-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 4. Details of the analysis and criteria used are indicated in the heading of each tab.
CC-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 5. Details of the analysis and criteria used are indicated in the heading of each tab.
Data and code availability
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact on request. Raw data have been deposited at GEO and publicly available at the date of publication. Accession numbers are listed in the key resources table.
-
•
This article does not report original code.
References
- 1.Sofroniew M.V., Vinters H.V. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pestana F., Edwards-Faret G., Belgard T.G., Martirosyan A., Holt M.G. No longer underappreciated: the emerging concept of astrocyte heterogeneity in neuroscience. Brain Sci. 2020;10:168. doi: 10.3390/brainsci10030168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khakh B.S., Deneen B. The emerging nature of astrocyte diversity. Annu. Rev. Neurosci. 2019;42:187–207. doi: 10.1146/annurev-neuro-070918-050443. [DOI] [PubMed] [Google Scholar]
- 4.Westergard T., Rothstein J.D. Astrocyte diversity: current insights and future directions. Neurochem. Res. 2020;45:1298–1305. doi: 10.1007/s11064-020-02959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chai H., Diaz-Castro B., Shigetomi E., Monte E., Octeau J.C., Yu X., Cohn W., Rajendran P.S., Vondriska T.M., Whitelegge J.P., et al. Neural circuit-specialized astrocytes: transcriptomic, proteomic, morphological, and functional evidence. Neuron. 2017;95:531–549.e9. doi: 10.1016/j.neuron.2017.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanjakornsiripan D., Pior B.-J., Kawaguchi D., Furutachi S., Tahara T., Katsuyama Y., Suzuki Y., Fukazawa Y., Gotoh Y. Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat. Commun. 2018;9:1623. doi: 10.1038/s41467-018-03940-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle J.P., Dougherty J.D., Heiman M., Schmidt E.F., Stevens T.R., Ma G., Bupp S., Shrestha P., Shah R.D., Doughty M.L., et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2009;139:1022. doi: 10.1016/j.cell.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morel L., Chiang M.S.R., Higashimori H., Shoneye T., Iyer L.K., Yelick J., Tai A., Yang Y. Molecular and functional properties of regional astrocytes in the adult brain. J. Neurosci. 2017;37:8706–8717. doi: 10.1523/jneurosci.3956-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bayraktar O.A., Bartels T., Holmqvist S., Kleshchevnikov V., Martirosyan A., Polioudakis D., Ben Haim L., Young A.M.H., Batiuk M.Y., Prakash K., et al. Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat. Neurosci. 2020;23:500–509. doi: 10.1038/s41593-020-0602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller S.J., Philips T., Kim N., Dastgheyb R., Chen Z., Hsieh Y.-C., Daigle J.G., Datta M., Chew J., Vidensky S., et al. Molecularly defined cortical astroglia subpopulation modulates neurons via secretion of Norrin. Nat. Neurosci. 2019;22:741–752. doi: 10.1038/s41593-019-0366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morel L., Men Y., Chiang M.S.R., Tian Y., Jin S., Yelick J., Higashimori H., Yang Y. Intracortical astrocyte subpopulations defined by astrocyte reporter Mice in the adult brain. Glia. 2019;67:171–181. doi: 10.1002/glia.23545. [DOI] [PubMed] [Google Scholar]
- 12.John Lin C.C., Yu K., Hatcher A., Huang T.-W., Lee H.K., Carlson J., Weston M.C., Chen F., Zhang Y., Zhu W., et al. Identification of diverse astrocyte populations and their malignant analogs. Nat. Neurosci. 2017;20:396–405. doi: 10.1038/nn.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batiuk M.Y., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C., Koeppen J., Viana J.F., Oliveira J.F., Voet T., et al. Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 2020;11:1220. doi: 10.1038/s41467-019-14198-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeisel A., Hochgerner H., Lönnerberg P., Johnsson A., Memic F., van der Zwan J., Häring M., Braun E., Borm L.E., La Manno G., et al. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saunders A., Macosko E.Z., Wysoker A., Goldman M., Krienen F.M., de Rivera H., Bien E., Baum M., Bortolin L., Wang S., et al. Molecular diversity and specializations among the cells of the adult mouse brain. Cell. 2018;174:1015–1030.e16. doi: 10.1016/j.cell.2018.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zamanian J.L., Xu L., Foo L.C., Nouri N., Zhou L., Giffard R.G., Barres B.A. Genomic analysis of reactive astrogliosis. J. Neurosci. 2012;32:6391–6410. doi: 10.1523/jneurosci.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Itoh N., Itoh Y., Tassoni A., Ren E., Kaito M., Ohno A., Ao Y., Farkhondeh V., Johnsonbaugh H., Burda J., et al. Cell-specific and region-specific transcriptomics in the multiple sclerosis model: focus on astrocytes. Proc. Natl. Acad. Sci. USA. 2018;115:E302–E309. doi: 10.1073/pnas.1716032115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y., Gozen O., Watkins A., Lorenzini I., Lepore A., Gao Y., Vidensky S., Brennan J., Poulsen D., Won Park J., et al. Presynaptic regulation of astroglial excitatory neurotransmitter transporter GLT1. Neuron. 2009;61:880–894. doi: 10.1016/j.neuron.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenblum L.T., Trotti D. EAAT2 and the molecular signature of amyotrophic lateral sclerosis. Adv. Neurobiol. 2017;16:117–136. doi: 10.1007/978-3-319-55769-4_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaiser M., Maletzki I., Hülsmann S., Holtmann B., Schulz-Schaeffer W., Kirchhoff F., Bähr M., Neusch C. Progressive loss of a glial potassium channel (KCNJ10) in the spinal cord of the SOD1 (G93A) transgenic mouse model of amyotrophic lateral sclerosis. J. Neurochem. 2006;99:900–912. doi: 10.1111/j.1471-4159.2006.04131.x. [DOI] [PubMed] [Google Scholar]
- 21.Tong X., Ao Y., Faas G.C., Nwaobi S.E., Xu J., Haustein M.D., Anderson M.A., Mody I., Olsen M.L., Sofroniew M.V., et al. Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington’s disease model mice. Nat. Neurosci. 2014;17:694–703. doi: 10.1038/nn.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeh T.H., Lee D.Y., Gianino S.M., Gutmann D.H. Microarray analyses reveal regional astrocyte heterogeneity with implications for neurofibromatosis type 1 (NF1)-regulated glial proliferation. Glia. 2009;57:1239–1249. doi: 10.1002/glia.20845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun D., Lye-Barthel M., Masland R.H., Jakobs T.C. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 2009;516:1–19. doi: 10.1002/cne.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qu J., Giovanni S.D., Jakobs T.C. The time course of gene expression during reactive gliosis in the optic nerve. PLoS One. 2013;8:e67094. doi: 10.1371/journal.pone.0067094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernstein S.L., Guo Y., Kerr C., Fawcett R.J., Stern J.H., Temple S., Mehrabian Z. The optic nerve lamina region is a neural progenitor cell niche. Proc. Natl. Acad. Sci. USA. 2020;117:19287–19298. doi: 10.1073/pnas.2001858117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen J.V., Soto I., Kim K.-Y., Bushong E.A., Oglesby E., Valiente-Soriano F.J., Yang Z., Davis C.-H.O., Bedont J.L., Son J.L., et al. Myelination transition zone astrocytes are constitutively phagocytic and have synuclein dependent reactivity in glaucoma. Proc. Natl. Acad. Sci. USA. 2011;108:1176–1181. doi: 10.1073/pnas.1013965108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrison J.C., Johnson E.C., Cepurna W.O., Funk R.H. Microvasculature of the rat optic nerve head. Invest. Ophthalmol. Vis. Sci. 1999;40:1702–1709. [PubMed] [Google Scholar]
- 28.May C.A., Lütjen-Drecoll E. Morphology of the murine optic nerve. Invest. Ophthalmol. Vis. Sci. 2002;43:2206–2212. [PubMed] [Google Scholar]
- 29.Sanz E., Yang L., Su T., Morris D.R., McKnight G.S., Amieux P.S. Cell-type-specific isolation of ribosome-associated mRNA from complex tissues. Proc. Natl. Acad. Sci. USA. 2009;106:13939–13944. doi: 10.1073/pnas.0907143106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanz E., Bean J.C., Carey D.P., Quintana A., McKnight G.S. RiboTag: ribosomal tagging strategy to analyze cell-type-specific mRNA expression in vivo. Curr. Protoc. Neurosci. 2019;88:e77. doi: 10.1002/cpns.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haimon Z., Volaski A., Orthgiess J., Boura-Halfon S., Varol D., Shemer A., Yona S., Zuckerman B., David E., Chappell-Maor L., et al. Re-evaluating microglia expression profiles using RiboTag and cell isolation strategies. Nat. Immunol. 2018;19:636–644. doi: 10.1038/s41590-018-0110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lozano D.C., Choe T.E., Cepurna W.O., Morrison J.C., Johnson E.C. Early optic nerve head glial proliferation and jak-stat pathway activation in chronic experimental glaucoma. Invest. Ophthalmol. Vis. Sci. 2019;60:921–932. doi: 10.1167/iovs.18-25700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelley K.W., Inoue H., Molofsky A.V., Oldham M.C. Variation among intact tissue samples reveals the core transcriptional features of human CNS cell classes. Nat. Neurosci. 2018;21:1171–1184. doi: 10.1038/s41593-018-0216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi H.J., Sun D., Jakobs T.C. Astrocytes in the optic nerve head express putative mechanosensitive channels. Mol. Vis. 2015;21:749–766. [PMC free article] [PubMed] [Google Scholar]
- 35.Park D.H., Connor K.M., Lambris J.D. The challenges and promise of complement therapeutics for ocular diseases. Front. Immunol. 2019;10:1007. doi: 10.3389/fimmu.2019.01007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tezel G. Molecular regulation of neuroinflammation in glaucoma: current knowledge and the ongoing search for new treatment targets. Prog. Retin. Eye Res. 2022;87:100998. doi: 10.1016/j.preteyeres.2021.100998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jo M., Kim J.-H., Song G.J., Seo M., Hwang E.M., Suk K. Astrocytic orosomucoid-2 modulates microglial activation and neuroinflammation. J. Neurosci. 2017;37:2878–2894. doi: 10.1523/jneurosci.2534-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jha M.K., Jo M., Kim J.-H., Suk K. Microglia-astrocyte crosstalk: an intimate molecular conversation. Neuroscientist. 2019;25:227–240. doi: 10.1177/1073858418783959. [DOI] [PubMed] [Google Scholar]
- 39.Zhao R.-Z., Jiang S., Zhang L., Yu Z.-B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dringen R., Brandmann M., Hohnholt M.C., Blumrich E.-M. Glutathione-dependent detoxification processes in astrocytes. Neurochem. Res. 2015;40:2570–2582. doi: 10.1007/s11064-014-1481-1. [DOI] [PubMed] [Google Scholar]
- 41.Papanikolaou M., Lewis A., Butt A.M. Store-operated calcium entry is essential for glial calcium signalling in CNS white matter. Brain Struct. Funct. 2017;222:2993–3005. doi: 10.1007/s00429-017-1380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Williams P.A., Marsh-Armstrong N., Howell G.R., Lasker/IRRF Initiative on Astrocytes and Glaucomatous Neurodegeneration Participants Neuroinflammation in glaucoma: a new opportunity. Exp. Eye Res. 2017;157:20–27. doi: 10.1016/j.exer.2017.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soto I., Howell G.R. The complex role of neuroinflammation in glaucoma. Cold Spring Harb. Perspect. Med. 2014;4:a017269. doi: 10.1101/cshperspect.a017269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nickells R.W., Howell G.R., Soto I., John S.W.M. Under pressure: cellular and molecular responses during glaucoma, a common neurodegeneration with axonopathy. Annu. Rev. Neurosci. 2012;35:153–179. doi: 10.1146/annurev.neuro.051508.135728. [DOI] [PubMed] [Google Scholar]
- 45.Liddelow S.A., Guttenplan K.A., Clarke L.E., Bennett F.C., Bohlen C.J., Schirmer L., Bennett M.L., Münch A.E., Chung W.-S., Peterson T.C., et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Escartin C., Galea E., Lakatos A., O’Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhäuser C., Volterra A., Carmignoto G., Agarwal A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh A., de Araujo A.M., Krieger J.-P., Vergara M., Ip C.K., de Lartigue G. Demystifying functional role of cocaine- and amphetamine-related transcript (CART) peptide in control of energy homeostasis: a twenty-five year expedition. Peptides. 2021;140:170534. doi: 10.1016/j.peptides.2021.170534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Y., Zhang W., Klaus J., Young J., Koerner I., Sheldahl L.C., Hurn P.D., Martínez-Murillo F., Alkayed N.J. Role of cocaine- and amphetamine-regulated transcript in estradiol-mediated neuroprotection. Proc. Natl. Acad. Sci. USA. 2006;103:14489–14494. doi: 10.1073/pnas.0602932103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kay J.N., De la Huerta I., Kim I.-J., Zhang Y., Yamagata M., Chu M.W., Meister M., Sanes J.R. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J. Neurosci. 2011;31:7753–7762. doi: 10.1523/jneurosci.0907-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daigle T.L., Madisen L., Hage T.A., Valley M.T., Knoblich U., Larsen R.S., Takeno M.M., Huang L., Gu H., Larsen R., et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell. 2018;174:465–480.e22. doi: 10.1016/j.cell.2018.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martersteck E.M., Hirokawa K.E., Evarts M., Bernard A., Duan X., Li Y., Ng L., Oh S.W., Ouellette B., Royall J.J., et al. Diverse central projection patterns of retinal ganglion cells. Cell Rep. 2017;18:2058–2072. doi: 10.1016/j.celrep.2017.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tsai N.Y., Wang F., Toma K., Yin C., Takatoh J., Pai E.L., Wu K., Matcham A.C., Yin L., Dang E.J., et al. Trans-Seq maps a selective mammalian retinotectal synapse instructed by Nephronectin. Nat. Neurosci. 2022;25:659–674. doi: 10.1038/s41593-022-01068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson E.C., Jia L., Cepurna W.O., Doser T.A., Morrison J.C. Global changes in optic nerve head gene expression after exposure to elevated intraocular pressure in a rat glaucoma model. Invest. Ophthalmol. Vis. Sci. 2007;48:3161–3177. doi: 10.1167/iovs.06-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Johnson E.C., Doser T.A., Cepurna W.O., Dyck J.A., Jia L., Guo Y., Lambert W.S., Morrison J.C. Cell proliferation and interleukin-6-type cytokine signaling are implicated by gene expression responses in early optic nerve head injury in rat glaucoma. Invest. Ophthalmol. Vis. Sci. 2011;52:504–518. doi: 10.1167/iovs.10-5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Z., Quigley H.A., Pease M.E., Yang Y., Qian J., Valenta D., Zack D.J. Changes in gene expression in experimental glaucoma and optic nerve transection: the equilibrium between protective and detrimental mechanisms. Invest. Ophthalmol. Vis. Sci. 2007;48:5539–5548. doi: 10.1167/iovs.07-0542. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez M.R., Agapova O.A., Yang P., Salvador-Silva M., Ricard C.S., Aoi S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia. 2002;38:45–64. doi: 10.1002/glia.10051. [DOI] [PubMed] [Google Scholar]
- 57.Albalawi F., Lu W., Beckel J.M., Lim J.C., McCaughey S.A., Mitchell C.H. The P2X7 receptor primes IL-1β and the NLRP3 inflammasome in astrocytes exposed to mechanical strain. Front. Cell. Neurosci. 2017;11:227. doi: 10.3389/fncel.2017.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mandal A., Shahidullah M., Delamere N.A., Terán M.A. Elevated hydrostatic pressure activates sodium/hydrogen exchanger-1 in rat optic nerve head astrocytes. Am. J. Physiol. Cell Physiol. 2009;297:C111–C120. doi: 10.1152/ajpcell.00539.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tribble J.R., Harder J.M., Williams P.A., John S.W.M. Ocular hypertension suppresses homeostatic gene expression in optic nerve head microglia of DBA/2 J mice. Mol. Brain. 2020;13:81. doi: 10.1186/s13041-020-00603-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williams P.A., Braine C.E., Kizhatil K., Foxworth N.E., Tolman N.G., Harder J.M., Scott R.A., Sousa G.L., Panitch A., Howell G.R., et al. Inhibition of monocyte-like cell extravasation protects from neurodegeneration in DBA/2J glaucoma. Mol. Neurodegener. 2019;14:6. doi: 10.1186/s13024-018-0303-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howell G.R., Macalinao D.G., Sousa G.L., Walden M., Soto I., Kneeland S.C., Barbay J.M., King B.L., Marchant J.K., Hibbs M., et al. Molecular clustering identifies complement and endothelin induction as early events in a mouse model of glaucoma. J. Clin. Invest. 2011;121:1429–1444. doi: 10.1172/jci44646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ransom B.R., Orkand R.K. Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci. 1996;19:352–358. doi: 10.1016/0166-2236(96)10045-x. [DOI] [PubMed] [Google Scholar]
- 63.Harris J.J., Attwell D. The energetics of CNS white matter. J. Neurosci. 2012;32:356–371. doi: 10.1523/jneurosci.3430-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moody D.M., Bell M.A., Challa V.R. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR. Am. J. Neuroradiol. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 65.Wender R., Brown A.M., Fern R., Swanson R.A., Farrell K., Ransom B.R. Astrocytic glycogen influences axon function and survival during glucose deprivation in central white matter. J. Neurosci. 2000;20:6804–6810. doi: 10.1523/JNEUROSCI.20-18-06804.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pellerin L., Pellegri G., Bittar P.G., Charnay Y., Bouras C., Martin J.-L., Stella N., Magistretti P.J. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev. Neurosci. 1998;20:291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 67.Dringen R., Gebhardt R., Hamprecht B. Glycogen in astrocytes: possible function as lactate supply for neighboring cells. Brain Res. 1993;623:208–214. doi: 10.1016/0006-8993(93)91429-v. [DOI] [PubMed] [Google Scholar]
- 68.Tekkök S.B., Brown A.M., Westenbroek R., Pellerin L., Ransom B.R. Transfer of glycogen-derived lactate from astrocytes to axons via specific monocarboxylate transporters supports mouse optic nerve activity. J. Neurosci. Res. 2005;81:644–652. doi: 10.1002/jnr.20573. [DOI] [PubMed] [Google Scholar]
- 69.Brown A.M., Tekkök S.B., Ransom B.R. Glycogen regulation and functional role in mouse white matter. J. Physiol. 2003;549:501–512. doi: 10.1113/jphysiol.2003.042416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cooper M.L., Pasini S., Lambert W.S., D’Alessandro K.B., Yao V., Risner M.L., Calkins D.J. Redistribution of metabolic resources through astrocyte networks mitigates neurodegenerative stress. Proc. Natl. Acad. Sci. USA. 2020;117:18810–18821. doi: 10.1073/pnas.2009425117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rouach N., Koulakoff A., Abudara V., Willecke K., Giaume C. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 72.Meyer N., Richter N., Fan Z., Siemonsmeier G., Pivneva T., Jordan P., Steinhäuser C., Semtner M., Nolte C., Kettenmann H. Oligodendrocytes in the mouse corpus callosum maintain axonal function by delivery of glucose. Cell Rep. 2018;22:2383–2394. doi: 10.1016/j.celrep.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 73.Brown A.M., Rich L.R., Ransom B.R. Metabolism of glycogen in brain white matter. Adv. Neurobiol. 2019;23:187–207. doi: 10.1007/978-3-030-27480-1_7. [DOI] [PubMed] [Google Scholar]
- 74.Yellen G. Fueling thought: management of glycolysis and oxidative phosphorylation in neuronal metabolism. J. Cell Biol. 2018;217:2235–2246. doi: 10.1083/jcb.201803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baxter P.S., Hardingham G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016;100:147–152. doi: 10.1016/j.freeradbiomed.2016.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Differentially expressed genes in independent pairwise comparisons of ONP vs CC, ONH vs CC, and ONH vs ONP (no criteria applied).
ONH-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 2. Details of the analysis and criteria used are indicated in the heading of each tab.
Gene set enrichment analysis on pairwise comparison of ONH versus ONP astrocytes, and analysis against the molecular function subset of the gene ontology (GO) database, related to Figure 3. Details of the analysis and criteria used are indicated in the heading of each tab.
ONP-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 4. Details of the analysis and criteria used are indicated in the heading of each tab.
CC-specific differentially expressed, enriched, and de-enriched genes and pathways, related to Figure 5. Details of the analysis and criteria used are indicated in the heading of each tab.
Data Availability Statement
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact on request. Raw data have been deposited at GEO and publicly available at the date of publication. Accession numbers are listed in the key resources table.
-
•
This article does not report original code.