Abstract
Four-dimensional (4D) printing is a promising technology that provides solutions for compelling needs in various fields. Most of the reported 4D printed systems are based on the temporal shape transformation of printed subjects. Induction of temporal heterogenicity in functions in addition to shape may extend the scope of 4D printing. Herein, we report a 4D printing approach using plant protein (zein) gel inspired by the amyloid fibrils formation mechanism. The printing of zein gel in a specialized layered-Carbopol supporting bath with different water concentrations in an ethanol-water mixture modulates hydrophobic and hydrogen bonding that causes temporal changes in functions. The part of the construct printed in a supporting bath with higher water content exhibits higher drug loading, faster drug release and degradation than those printed in the supporting bath with lower water content. Tri-segment conduit and butterfly-shaped construct with two asymmetrical wings are printed using this system to evaluate biomedical function as nerve conduit and drug delivery system. 4D printed conduits are also effective as a drug-eluting urethral stent in the porcine model. Overall, this study extends the concept of 4D printing beyond shape transformation and presents an approach of fabricating specialized baths for 4D printing that can also be extended to other materials to obtain 4D printed medical devices with translational potential.
Keywords: Zein gels, Water-driven 4D printing, Protein self-assembly, Tunable degradation, Controlled drug release
Graphical abstract
Highlights
-
•
Expanding scope of 4D printing beyond shape morphing.
-
•
Preparation of printable amyloid-inspired zein gel.
-
•
Preparation of specialized layered supporting bath with gradient water concentration to tune molecular interactions of zein.
-
•
Temporal control over drug loading, drug release rate, porosity, and degradation rate of printed constructs.
1. Introduction
Four-dimensional (4D) printing is an emerging area in additive manufacturing; this technology helps in developing demanding products in electronics, biomedical sciences, robotics, and others, that cannot be produced using conventional approaches. In 4D printing, time is integrated into the fourth dimension with three-dimensional (3D) printing [1,2]. Briefly, transformation of shapes, properties, and functions of 3D printed structures over time, when exposed to stimuli, is termed 4D printing [[3], [4], [5]]. “Smart” materials can transform over time in response to external stimuli and aid 4D printing [6,7]. In this regard, stimuli-responsive polymers that can respond to pH, light, temperature, water, magnetic field or chemicals are commonly employed materials in 4D printing [8,9]. Several comprehensive reviews on this topic have been published [1,10,11]. In the medical sciences, tissue engineering and drug delivery are the major areas in which 4D printing can be applied to achieve desired outcomes [1,4]. Despite advancement in the field, this can only be considered the early beginning of expectations [12]. There are a few reports on the use of 4D printing in the biomedical field. Han et al. used 4D-printed microneedles to enhance tissue adhesion. These microneedles have curved backward-facing barbs that can deform to interlock with the tissue when inserted into the skin [13]. Malocchi et al. used 4D-printed PVA-based structures for prolonged gastric retention [14]. Zhang et al. employed poly(glycerol dodecanoate) acrylate with low transition temperature to prepare 4D printed shape-adaptive vascular conduits [15]. Material selection is crucial from the translational medicine aspect of 4D printing because of biocompatibility and degradation concerns [12]. Moreover, a single material with a low cost, free availability and simplicity of 4D printing is preferable. Only a limited number of biocompatible materials that can be applied for 4D printing are available [12].
The application of sustainable materials for biomedical or pharmaceutical applications is required to mitigate the use of synthetic plastics. Maize is one of the most cultivated cereal crops [16]. Using cereal proteins obtained in bioethanol production (such as zein) is an attractive approach [17]. α-zein has shown significant potential for use in drug delivery systems and tissue engineering. In our previous reports, we prepared ivermectin tablets [18], floating tablets [19,20], heparin-loaded microspheres and electrospun films for cardiovascular applications [21], microcarriers for large-scale cell production [22], scaffolds for bone tissue engineering [23], and sciatic nerve injury repair [24]. Our group submitted a patent application for the 3D printing application of zein gel for the first time [25]. Recently, Tavares-Negrete et al. reported zein gel as 3D printing ink that demonstrated slow gelation (21 d) and low yield stress (<30 Pa), and the printed structures exhibited cell adhesion and proliferation properties [26]. Zein can self-assemble in water because of its hydrophobic nature. Therefore, we hypothesized that controlling this water-responsive self-assembly can be exploited to tune the functions of printed constructs.
Degradation rate, drug loading or drug release rate and porosity are essential properties in biomaterials and their control is desirable to achieve optimum results. For instance, controlling the degradation rate is vital for scaffolds and should ideally be matched with the tissue regeneration rate [27]. Nerve conduits should support nerve growth from the proximal to the distal stump without collapse [28]. Therefore, it requires specific designing of conduits with tunable degradation of proximal or distal parts. In another scenario, the degradation of ureteral stents from the distal to the proximal end is desirable to avoid urinal blockage [29]. There is also a demand for scaffolds with gradient properties (like porosity and degradation) [30,31]. Moreover, concentration gradient is also be useful in the biomedical or pharmaceutical field [32]. For instance, gradient concentration of growth factor plays an important role in determining cell fate; therefore, developing scaffolds with a concentration gradient of bioactive molecules is helpful in modulating cell-material interactions [33,34]. The control over the properties of the scaffold using a simple method is desirable and will help to design more suitable scaffolds.
In this study, zein gel was applied in additive manufacturing to produce scaffolds with controlled properties. The gelation mechanism of zein solution was investigated and cryo-TEM was used for tracing the transformation process from zein microspheres in solution to fibril structure in gel. The related change in protein secondary structure was characterized by FTIR spectroscopy. Shear-thinning, yield stress and elastic recovery of gels were measured to assess their printability. A customized supporting/sacrificial bath was prepared using Carbopol with different water and ethanol concentrations to induce heterogenicity in printed constructs with time (fourth dimension). The structural heterogenicity in printed constructs was proposed to induce the transformation of functions such as degradation rate, drug loading, and drug release. Doxorubicin and ciprofloxacin-loaded zein gels were printed in the supporting bath as proof-of-concept. The degradation rates of nerve conduits composed of three 4D-printed segments were evaluated for heterogenicity in rat nerve injury models. Finally, in vivo efficacy of the printed ciprofloxacin-loaded conduits as ureteral stents was assessed in pigs.
2. Materials and methods
2.1. Materials
Zein was procured from WAKO Pure Chemical Industries Ltd., Japan. Protease XIV from Streptomyces griseus (3.5 U/mg) was purchased from Sigma-Aldrich, USA, and Ciprofloxacin HCl (CPFX) was purchased form Shanghai Macklin Biochemical Co., Ltd., China. Doxorubicin hydrochloride (DOX) was obtained from Aladdin, China. Carbopol 980 was purchased from Lubrizol Co., USA.
2.2. Preparation and characterization of zein gels
Zein gel was prepared by dissolving zein (20 and 30% w/v) in 80% v/v ethanol in water. The solutions were stirred using magnetic stirring at 1500 rpm till gelation. The gelation was confirmed by inverting the glass vial. Structural characterization of gel was performed using cryo-TEM, ThT-binding assay, and FTIR spectroscopy. Rheological characteristics were also analyzed. Details of methods can be found in supporting information (Section 1.1).
2.3. Printability assessment
First, the printability of zein gels was assessed using a 3D bioprinter (3D Discovery, RegenHU, Switzerland), and the optimal parameters for zein gels were determined. Gels were loaded into plastic syringes and extruded via a 0.5 mm (21 G) diameter printing needle. An air-pressure extrusion (pneumatic extrusion) method was used. The pressure and speed of the printing head were optimized by filament continuity and diameter. A 3 cm filament was extruded on the glass side to evaluate the continuity and diameter of the filament. The pressure was adjusted depending on zein concentrations (80–100 kPa for 20% zein, 120–150 kPa for 30% zein), and printing speed was set at 14 mm/s for 20% and 8–10 mm/s for 30%, zein-containing gel. The printing was performed at 22 ± 2 °C.
2.4. 4D printing of zein gel in the external supporting bath
A scheme for preparing the supporting bath and ink is given in Fig. S1. In 0.01 N sodium hydroxide containing different water concentrations (30–100% v/v in ethanol), 0.25% (w/v) Carbopol 980 was suspended to prepare the supporting bath. The porogen l-menthol (50% of zein) was added to zein gel (with 20% w/v zein) for 4D printing. Before stirring, 2% w/w (of zein) ciprofloxacin HCl (CPFX) or 0.00825% w/w (of zein) doxorubicin hydrochloride (DOX) was added to the zein solution to prepare drug-loaded gels. Different edible pigments (green and blue) were also used in the Carbopol bath to differentiate layers with different water concentrations to evaluate the mixing of different supporting baths. A 22 G needle (0.44 mm needle diameter) was used for printing. Conduits, butterfly, and flower-shaped structures were printed in situ and allowed to remain in the supporting bath for transformation with time, followed by washing to remove Carbopol. The structures were dried in an oven at 37 °C. Printed structures were evaluated for morphological analysis, in vitro drug release, in vitro degradation, antibacterial and cytotoxicity (detailed methods can be found in supporting information, Section 1.2.)
2.5. Evaluation of degradation of 4D printed conduits as nerve conduit in the rat model
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) in Shanghai Jiao Tong University (No. A2016036). Male Sprague Dawley (SD) rats (200–250 g) were bought from Charles River Laboratory Animal Co., LTD (Zhejiang, China). The implantation experiment of nerve conduits was performed according to our previous method [35]. Briefly, the sciatic nerve of the right hip was separated and exposed, and a nerve segment of 15 mm was cut. Then, the 17 mm conduits were sutured to the proximal and distal stumps of the sciatic nerve. Autologous nerve transplantation was used as a control. All animals were housed and fed routinely after suturing the wound.
After implantation of 3 or 10.5 months, the conduits were separated and fixed in 4% paraformaldehyde solution. Then, they were dehydrated, hyalinized and embedded in paraffin. The sample slices (5 μm) were prepared and stained by an H&E staining kit (Beyotime, Shanghai, China) according to the manufacturer's protocol. All slices were scanned by a high-resolution slide scanning system (Panoramic MIDI, 3DHISTECH Ltd., Budapest, Hungary) and observed by K-Viewer 1.5.5.2.
2.6. Evaluation of in vivo efficiency of 4D printed stents in the swine model
Two types of stents (length 3.5 cm, average diameter 2.05 ± 0.08 mm) were prepared in Carbopol supporting medium containing 75% (CB75) or 40% water (CB40) using CPFX-loaded zein gel. After washing and drying, the stents were placed in a barium sulfate suspension (200 mg/mL) to incorporate contrast for X-ray imaging. The polyurethane stents (6.5F, 2 cm) were immersed in an E. coli suspension (1 × 108 CFU/mL) for 1 h and dried in a biosafety cabinet as a control.
All animal experiments were conducted at Shanghai Jiagan Biotechnology Co., Ltd (Shanghai, China) following the animal welfare requirements of ISO 10993–2:2006 and were approved by the ethical committee of the company vide approval no. JGLL20200921. The experimental subjects were three Yorkshire pigs (male, average weight 46.0 ± 3.0 kg). For stent implantation, the ureter was cut longitudinally to a length of 1 cm. A polyurethane stent with E. coli was first placed in the upper section of each ureter, and subsequently, CPFX-loaded zein stents were implanted in the lower ureter. In one ureter, a zein stent printed in Carbopol supporting bath with 75% water was implanted. In the other ureter, a zein stent printed in the Carbopol supporting bath with 40% water was implanted to compare its antibacterial efficacy. Finally, the ureter and abdominal incision were sutured using 5–0 PDS absorbable sutures. An intravenous pyelogram (IVP) was performed after injecting 40 mL of iohexol (35 mg/100 mL) intravenously via a marginal ear vein to evaluate urine drainage efficacy through the stent days after stent implantation. The pigs were euthanized four days after the implantation, and their kidneys, ureters, bladders, and stents were harvested aseptically. The collected stents were cut into pieces. Parts 2 mm in length from each conduit's upper, middle, and lower parts were immersed in 2 mL PBS, followed by sonication for 15 min in an ultrasonic bath to detach the attached bacteria. The agar plate method was used for the bacterial count in PBS to evaluate the antibacterial efficacy of the 4D-printed stents.
3. Results and discussion
3.1. Preparation and structural characterization of zein gel
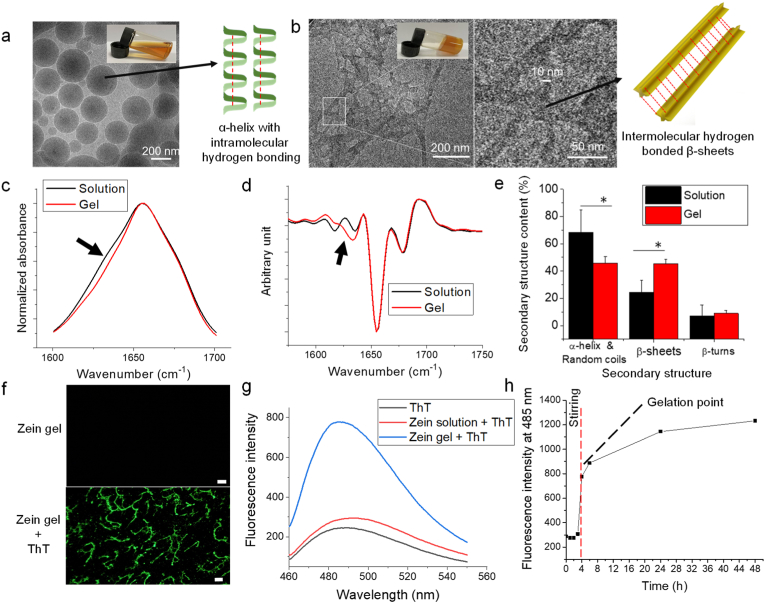
Structural characterization was done using Cryo-TEM, FTIR and ThT-binding assay. Zein formed spherical self-assemblies after dissolution in hydroalcoholic solvent (Fig. 1a), while in zein gel, a fibrous network was observed with a minimum diameter of 10 nm (Fig. 1b). FTIR analysis showed a small shoulder at 1625-1635 cm−1 indicates the formation of β-sheets during gelation (Fig. 1c). Furthermore, a shift in β-sheets content was observed from the secondary-derivative spectrum indicating a new β-band (Fig. 1d). Amide I peak resolving also showed a reduction in alpha content and random coils (1640-1658 cm−1) while an increase in β-sheets content (1620-1640 cm−1) (Fig. 1e and Fig. S2). The new β band in zein gel is due to the attribution of stronger hydrogen bonds in the intermolecular β-sheet structure [36]. A positive ThT-binding with zein gel showed that the gelation of zein solution is amyloid-inspired (Fig. 1f–h). Overall, the gelation mechanism of zein solution is inspired by amyloid fibrils and hydrogen bonding drives fibrils to form a network for gel. Amyloid fibrillation is previously known for pathological conditions. Recently, it is established that fibril formation is a generic property of protein and was considered a promising material owing to its unique properties; however, there are limited studies that were reported on plant-based proteins which can act as sustainable sources [37].
Fig. 1.
(Color online) Preparation and structural characterization of zein gel. Cryo-TEM micrographs of zein self-assemblies (a) in solution and (b) in gel which appeared as fibril network with the thinnest fibril of 10 nm diameter. c) FTIR absorption normalized spectra of zein solution and gel. d) Secondary derivative spectra of zein solution and gel (mean spectra, n = 4, normalized spectra in amide I range, black arrows indicate a change in IR features whiles gelation). e) Quantification of the secondary structure of zein in solution and gel form (n = 4, independent samples). f) Fluorescence microscopic images of zein gel with and without ThT. g) Zein gel after staining with ThT (scale bar-25 μm). c) The fluorescence spectrum of zein solution and gel after adding ThT. h) Dynamic increase in fluorescence intensity at 485 nm with ThT-binding. *p < 0.05.
3.2. Rheological properties and printability of zein gel
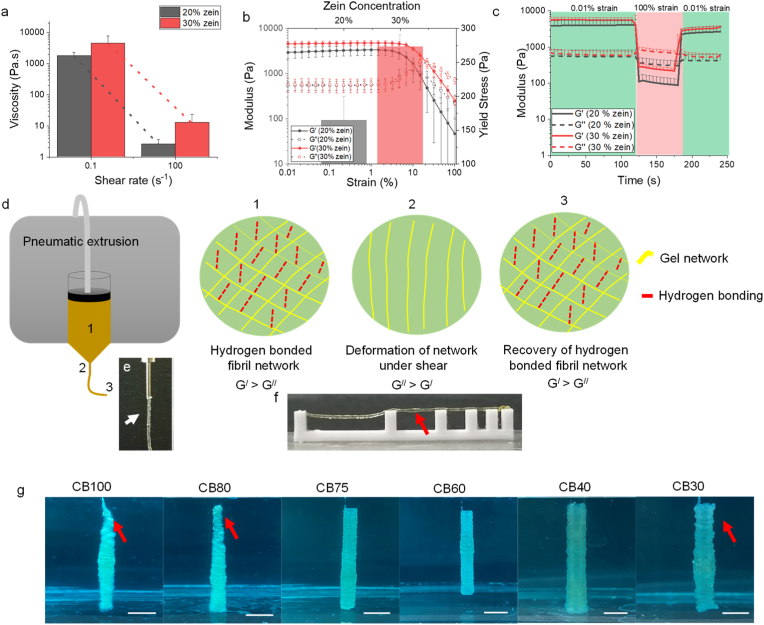
The rheological properties of the gels were evaluated to establish a theoretical estimation of printability. Shear-thinning, yield stress and rapid structural recovery (elastic recovery) are essential for direct ink writing (DIW) printing inks [38,39]. The gels exhibited shear-thinning behavior with low viscosity at a higher shear rate (Fig. 2a). The strain/stress sweep curves showed that the zein gels had a viscoelastic nature, with a higher elastic modulus value than the viscous modulus at low strain (Fig. 2b). Yield stress is considered a critical characteristic of 3D printing inks because it can ensure the stability of the printed structure [40]. The stress tolerated by a gel before deformation is called yield stress. The gel with 20% w/v zein had 164.4 ± 35.2 Pa yield stress and the gel with 30% zein had 272.8 ± 17.4 Pa (Fig. 2b). Values of yield stresses were found to be superior to previously reported zein gel [26]. The difference in the preparation method was a possible reason for the superior rheological properties. In the present work, the generation of amyloid fibrils under shear stress caused the more robust network formation. The structural recovery capability was also observed for all zein gels after passing through 100% strain, which enabled the stability of the printed structure (Fig. 2c). Overall, zein gels exhibit rheological properties suitable for direct ink writing. Upon extrusion, shear-thinning briefly enables them to flow continuously under shear stress greater than the flow point. As zein gel was driven by hydrogen bonds; therefore, the shear-thinning may be caused by the deformation of hydrogen bonds because hydrogen bonds are susceptible to break under extrusion shear and can self-assemble after extrusion [41] (Fig. 2d). This phenomenon resulted in a continuous filament-like gel extrusion (Fig. 2e). Yield stress and structural recovery further support the filament from collapsing under gravity (Fig. 2f) [42].
Fig. 2.
(Color online) Printability assessment of zein gel. (a) Viscosity vs shear rate curves of zein gels with different concentrations of zein. (b) Strain sweep curves of gels with different concentrations of zein. (c) Structural recovery capability of zein gels after passing through high shear (100% strain) for 60 s. (d) Scheme illustrating mechanism of zein gel extrusion. (e) Image showing filament of zein gel during extrusion indicated by the white arrow. (f) Image showing elastic recovery of the gel after extrusion, which enables the gel to support against collapse as indicated by the red arrow. (g) Images of printed conduits in supporting medium with different concentration of water which showed poor printing structural stability in water concentration ≥80% and <40%. CB100, CB80, CB75, CB60, CB40, CB30 shows Carbopol supporting baths with 100%, 80%, 75%, 60%, 40% and 30% water (scale bar‒5 mm).
Zein has hydrophobic nature and zein gels can self-assemble in an aqueous medium by hydrophobic interactions [43,44]. A supporting bath comprising Carbopol with different water concentrations in ethanol was used to induce water-responsive self-assembly. The printing process is illustrated in Video S1. Needle blockage due to the hydrophobic assembly of zein at a higher water concentration (≥80%) was the possible reason for poor printing. In comparison, at a lower concentration of water (<40%), free dispersion of gel in the supporting bath because of lower self-assembly caused non-uniform printing. The supporting bath's water concentrations (40–75%) were suitable for incessant printing zein gel (Fig. 2g).
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2022.11.009.
The following is the supplementary data related to this article:
3.3. 4D printing of zein gel in supporting bath
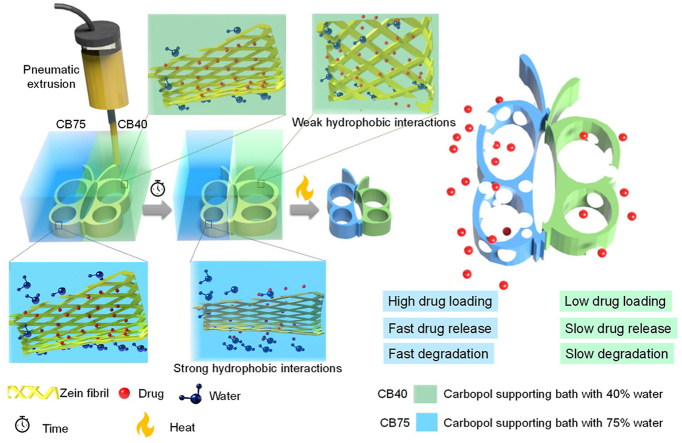
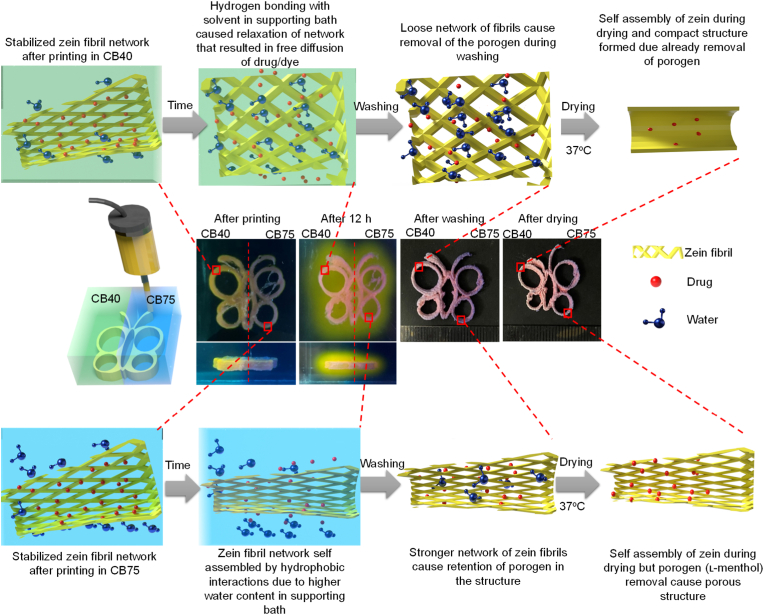
Hydrogen bonding exists among amyloid-like zein fibrils in zein gel and upon water immersion, hydrophobic interactions prevailed, leading to the solidification of the gel. Therefore, it was proposed that varying water concentration in the supporting baths can modulate hydrogen bonding and hydrophobic self-assembly in the printed structures. As a proof-of-concept, conduits were printed in Carbopol supporting bath with 40% (CB40), 60% (CB60), and 75% (CB75) v/v in ethanol and allowed to stay in the supporting bath for 12 h. There was no change in the size of CB60 was observed for 12 h; that might be due to balanced hydrogen and hydrophobic interactions between zein gel and the supporting bath. Therefore, the structure printed in CB60 was selected as a control to compare size changes in constructs printed in other supporting baths (CB75 and CB40). After 12 h in Carbopol bath, the printed structure in CB75 reduced in size (−14.3 ± 5.2% diameter, −17.6 ± 5.7% thickness, −5.5 ± 3.3%) due to hydrophobic attractions between zein molecules (Figs. S3a and b). In contrast, the construct printed in CB40 showed an increase in size (28.5 ± 8.1% diameter, 18.3 ± 4.5% thickness, 9.72 ± 1.8%) by forming hydrogen bonding with the supporting bath solvent due to low water concentration (Figs. S3a and b).
Bi-layered Carbopol supporting baths (CB75/CB40) were also prepared to induce the structural heterogenicity in a single printed construct (Fig. S4). No mixing of colors was observed with time that showed stability or restrictive movement of solvent molecules among different Carbopol gels. The bi-layered Carbopol can print structures with gradient gaps between printed layers, which is difficult to achieve using conventional 3D printing methods (Fig. S5 and Video S2) [45]. Interestingly, the printed structure's shape was transformed differently during drying; the construct printed in CB75 exhibited a small shape change, while the construct printed in CB40 shrank during drying (Fig. 3c, d and S7). Overall, the size change from the designed structure to the dried final printed structure for CB75, CB60 and CB40 is given in Fig. S3e. Dye-loaded zein gel was printed to evaluate the influence of structural heterogenicity on dye/drug loading and release. In the case of dye (rhodamine)-loaded zein gel, higher dye release was observed in the CB40 during curing (12 h) (Fig. S6 and Video S3) than those printed in CB75. For overall structural transformation, a mechanism was proposed with an example of a dye-loaded butterfly-shaped construct in bi-layered Carbopol supporting bath (CB75/CB40), as shown in Fig. 3. In CB75, zein tended to self-assemble quickly, and hydrophobic attractions prevailed, leading to contraction of construct/filament. In CB40, hydrogen bonds between the zein molecules and the solvent of the supporting medium prevailed due to low water concentration, causing an increase in construct/filament, forming a loose network. This behavior is illustrated in Video S4. This phenomenon can also be explained in the recent study reported by Nandakumar et al. regarding self-assembly of amphiphilic polypeptide in the presence of a water/ethanol mixture [46]. Increased ethanol concentration in water/ethanol mixture induced stronger hydrogen bonding among water molecules and reduced its effect on zein, causing lower hydrophobic self-assembly as shown by CB40. Concerning dye/drug release in the Carbopol bath, encapsulation of dye/drug between hydrophobic assembled segments of zein led to the slow release in CB75. Conversely, in CB40, a loose extruded filament structure was formed owing to hydrogen bond dominance, causing relatively free diffusion of dye/drug in the supporting medium (Fig. 3). This time-dependent phenomenon resulted in different drug loadings and sizes dependent on the supporting medium water content. Another feature of the printed structures was the size transformation during drying. Structures printed in CB40, with larger size after printing and washing than structures printed in CB75, shrank after drying. The removal of porogen (like drug) in CB40 and during washing due to loosen structure may lead to compact structure while retention of porogen in CB75 due to self-assembled structure may cause porous structure during heat drying by sublimation.
Fig. 3.
(Color online) Mechanism of structural changes during printing of zein in Carbopol bath with different water concentrations (CB75 and CB40). Hydrophobic interactions between zein molecules in response to the external supporting medium were responsible for the change in size and dye/drug release behavior from the printed gel. CB75 and CB40 shows Carbopol supporting bath with 75% and 40% water, respectively.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.bioactmat.2022.11.009.
The following are the supplementary data related to this article:
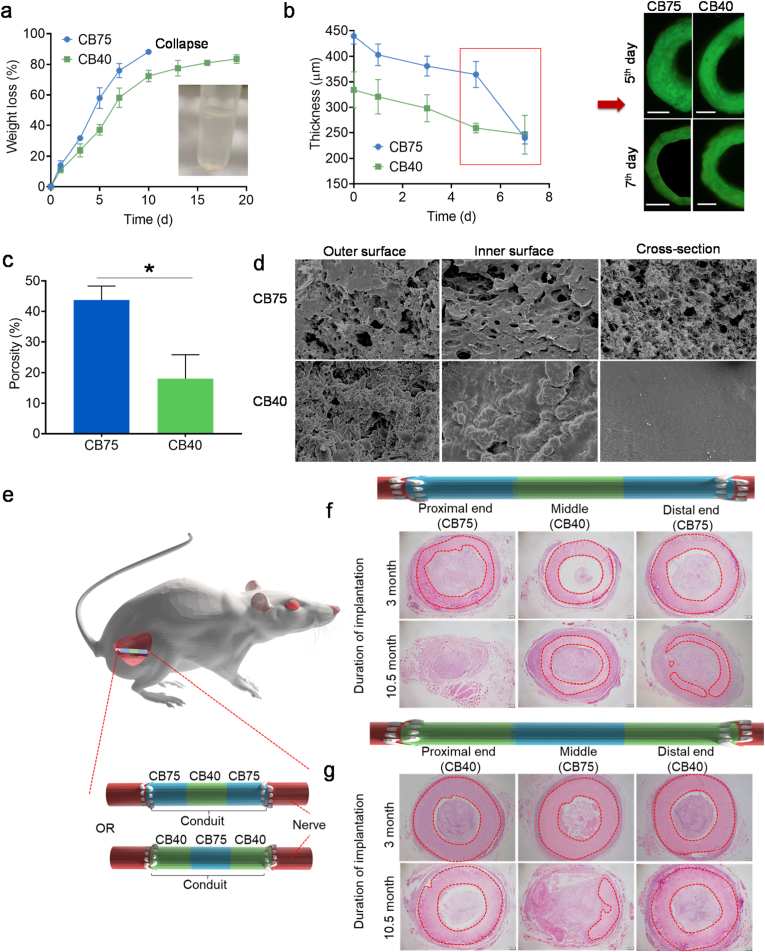
The degradation rates of the printed constructs after drying (conduit-shaped) were evaluated by calculating percentage weight loss in the degradation medium with protease XIV. The water concentration in the Carbopol bath was critical for determining the degradation rate. The degradation rate was significantly decreased with a decrease in the water concentration from 75% to 40% in the supporting bath, as shown in Fig. 4a. Conduits printed in CB75 were degraded 88.2 ± 1.17% in 10 days and the loss the integrity of the remaining structure (collapse) as shown in the inset of Fig. 4a. The wall thickness of conduits during degradation was also reduced (Fig. 4b). Conduits printed in CB40 had a stable wall thickness owing to low degradation rate than those printed in CB75 and CB60, in which thickness reduced significantly from 5 to 7th days (Fig. 4b). Conduits in Carbopol supporting medium with tri-layered water concentrations (CB75-CB40-CB75 or CB40-CB75-CB40) were also 4D printed successfully and were evaluated for degradation in vitro firstly. Conduits printed in the CB75-CB40-CB75 bath demonstrated a stable structure in the degradation medium due to robust core. In contrast, conduits printed in CB40-CB75-CB40 bath collapsed in seven days owing to the fast degradation of the middle section (Fig. S7). Conduits printed in CB75 had higher porosity than those printed in CB40 (Fig. 4c). Higher porosity facilitated the penetration of the medium that caused faster degradation, while conduits with lower porosity (CB40) had restricted water penetration. The pore size distribution and volume depend on the supporting medium, which can be adjusted based on the intended use of the scaffold (Fig. 4d and Fig. S8). The degradation of tri-segment conduits was also evaluated in vivo as nerve conduit in rat sciatic injury model (15 mm) (Fig. 4e). CB75-CB40-CB75 conduit showed faster terminal degradation due to presence of high porous CB75 section, while middle section was degraded slowly (CB40) as indicated in H&E stained samples (Fig. 4f). The proximal part was degraded faster than distal part of conduit that was due to faster nerve regeneration at proximal section. Contrarily, in CB40-CB75-CB40 conduits, the middle section (CB75) was degraded faster than the terminal position (CB40) (Fig. 4g). The in vivo degradation evaluation validated the potential translational application of 4D printed conduits with the potential to control the degradation rate. This strategy provides an approach to control the degradation rate of different sections of a single printed scaffold by just adjusting the composition of the supporting bath.
Fig. 4.
(Color online) Evaluation of degradation of conduits printed in Carbopol bath. (a) Degradation of conduits printed in CB75 and CB40. (b) Wall-thickness reduction of conduits during degradation, which showed that conduits printed in CB40 have stable thickness of wall with due to low degradation rate, while those printed in CB75 and CB40 had significant reduction in thickness especially from 5 to 7th day of degradation as shown in fluorescence images (scale bar – 500 μm). CB40 (green) and CB75 (lblue) shows Carbopol supporting baths with 40 and 75% water, respectively. (e) Schematic illustration of animal experiment for evaluation of degradation of printed conduits as nerve conduit. H&E images of (e) CB75-CB40-CB75 and (f) CB40-CB75-CB40 conduits after 3 and 10.5 months of implantation in rats (red area indicate implanted conduit). *p < 0.05, results of the printed structures in CB75 and CB40 were compared using Student's t-test.
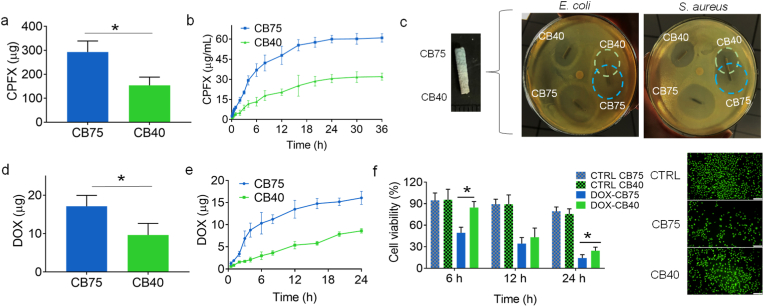
To provide proof-of-concept of structural heterogenicity application, Ciprofloxacin HCl (CPFX)-loaded conduits and Doxorubicin (DOX)-loaded butterfly-shaped constructs were printed in bi-layered Carbopol supporting bath (CB75/CB40). CPFX is a commonly used antibiotic and DOX is used as an anti-tumor agent. CPFX loading and drug release rates were higher in the conduits printed in CB75 than those printed in CB40 (Fig. 5a and b). Owing to higher drug loading and drug release in conduits printed in CB75, these conduits showed a larger zone of inhibition against both E. coli and S. aureus than those conduits prepared in CB40 (Fig. 5c). Antimicrobial activity of scaffolds in the fabrication of scaffolds to prevent post-implantation infection and treatment of infections [47,48]. Butterfly-shaped constructs loaded with DOX were also printed in bi-layered Carbopol supporting bath with half printing in CB75 and CB40. The wings of butterfly also became asymmetric due to the difference in water concentration (Fig. 3). Like CPFX, DOX loading and release higher from structures printed in CB75 were observed at a higher rate than printed in CB40 (Fig. 5d and e). The structures were dissected into two portions to evaluate the cytotoxicity of the DOX-loaded butterflies (Fig. S9). Higher cytotoxicity against BEL7402 cells was found inside the butterfly printed in CB75 (Fig. 5f). Zein has been applied for the preparation of scaffolds for tissue engineering and doxorubicin-loaded scaffold can be applied as bone implant to provide local delivery of drug to treat bone tumor [49,50]. In this regard, biphasic controlled release may be helpful in achieving initial rapid release and long-term sustained release. Differential drug release depending upon water concentration of the supporting bath can be explained by the porosity. The higher porosity of constructs printed in CB75 caused faster release by easy penetration of the dissolution medium.
Fig. 5.
(Color online) 4D printing of drug-loaded zein gel in Carbopol supporting system with 75% (CB75) and 40% (CB40) water with time-dependent drug loading and release. (a) Ciprofloxacin HCl (CPFX) loading in printed conduits in CB75 and CB40 (n = 3). (b) CPFX release over time in PBS from printed conduits (n = 3). (c) Antibacterial efficacy of CPFX-loaded conduits against E. coli and S. aureus. (d) Doxorubicin HCl (DOX) loading in the printed butterfly (after drying) in CB75 and CB40 (n = 3). (e) DOX release over time in PBS from the printed structure (n = 3). (f) Cell viability with time when incubated in DOX-loaded butterfly (n = 3) and fluorescence microscope images to show living cells stained by FDA, when incubated in DOX-loaded butterfly portion, which were printed in CB75 or CB40. *p < 0.05, results of the printed structures in CB75 and CB40 were compared using Student's t-test.
4D printing is commonly employed for shape transformations over time under external stimuli. Ali et al. utilized external ionic concentration and electrical stimulus to induce reversible contactless bending of polyelectrolyte hydrogels [51]. Moreover, crosslinking gradients in gel-based printing inks have been reported to induce transformation in time dimension. Ding et al. reported oxidized and methacrylate alginate (OMA)-based micro-flake hydrogel for 4D bioprinting. The cross-linking gradient in the hydrogel later controls the shape morphing of the printed construct [52]. Furthermore, gradient crosslinking was also used to develop silk microneedle patches for multi-drug delivery at different rates [53]. The present study's gradient hydrophobic assembly was exploited to induce heterogenicity of drug loading, drug release rates, and degradation over time with a simpler approach of designing a tailor-made supporting bath that was validated by printing different structures. Water-responsive ability is a promising zein gel feature that can be further evaluated for other applications.
3.4. Efficacy of printed conduits as a ureteral stent in a porcine model
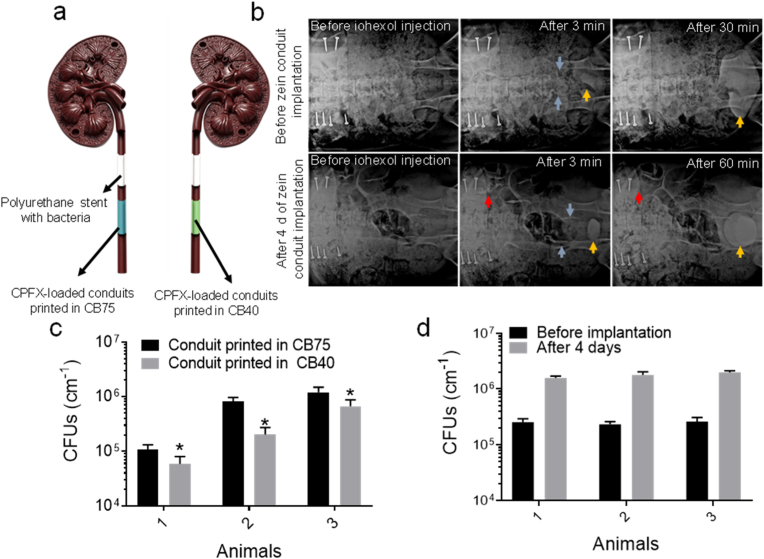
In a porcine model, 4D printed zein conduits were evaluated as drug-eluting, biodegradable ureteral stents. Ureteral stents are commonly used to maintain ureteral luminal patency. Hydronephrosis and infection in upper urinary tract malformations include stenosis of the ureteropelvic junction, giant ureteral, vesicoureteral reflux, and ureteral cyst [54], which require placement of a ureteral stent for 1–2 months after surgery, are commonly observed in patients. Ureteral stents are also necessary to drain urine in hydronephrosis for a long time because of the retroperitoneal metastasis of abdominal tumors [55]. However, long-term implantation can form a biofilm, aggravating ureteral stenosis, and re-implantation of stents might be challenging [56,57]. Biofilms can sustainably release bacteria and toxins, which are difficult to eliminate by drugs [58,59]. Therefore, the prevention of biofilm formation on ureteral stents is crucial. Therefore, the antibacterial function of biodegradable stents is required for controlled drug elution. In this study, a rational experiment was designed to evaluate urine drainage and antibacterial efficacy of the CPFX-loaded conduits printed in a CB75 or CB40 to validate differential drug release (Fig. 6a). Before the operation, renal pelvises, calyces, and ureters were observed 3 min after intravenous injection of iohexol, indicating that the secretion and excretion functions worked well. The contrast agent in the renal pelvises and ureters disappeared 30 min after injection (Fig. 6b). Four days after surgery, renal pelvises and ureters appeared 3 min after injection. The renal pelvises and ureters did not dilate, indicating good secretion and excretion. The contrast agent was passed through the stent implantation sites. Sixty minutes after the injection, the contrast agent in the renal pelvises and ureters disappeared (Fig. 6b). Although the time required to excrete the contrast agent was slightly longer than that before surgery, the kidney secretion and excretion were regained, mainly due to adequate drainage through the zein stents. Furthermore, the CFUs count test showed that stents printed in CB75 had significantly higher CFUs than those printed in CB40 (Fig. 6c). The number of CFUs in these stents indicates tunable drug release in conduits in situ printed in the supporting bath with different ethanol concentrations, validating the 4D printing design. The number of CFUs counted in polyurethane stents significantly increased four days after implantation, but no significant difference was observed among the different animals (Fig. 6d). Overall, zein stents showed in vivo urine drainage efficacy and antibacterial activity, which can be exploited in the future to prepare 4D printed drug-eluting biodegradable ureteral stents. It is important to mention that the number of CFUs in the upper section of the zein conduits was higher than that in the lower section four days after implantation. Therefore, separate stents printed in CB75 and CB40 were used instead of a single 75%–40% stent to avoid false results. Another feature of 4D printed zein ureteral stents is controllable degradation, which can be used to prepare stents with a controlled degradation rate.
Fig. 6.
(Color online) Efficiency of printed conduits as ureteral stent in the porcine model. (a) Implantation sites of stents. (b) Radiographs before and after four days of implantation of conduits as ureteral stent (blue arrows). Iohexol was injected to visualize urine drainage through stents and after 3 and 30 min of iohexol injection, radiographs show urine accumulation in urinary bladder (yellow arrow) and clearance of renal pelvises and calyces (red arrow). After the stent implantation, clearance of iohexol was prolonged to 60 min. (c) Number of CFUs per cm of zein conduits printed in supporting bath CB75 and CB40 after four days of implantation in pigs as ureteral stent. (d) Number of CFUs per cm of polyurethane stent before and after four days of implantation. *p < 0.05, results of conduits printed in CB75 and CB40 were compared using Student's t-test.
4. Conclusion
In conclusion, hydrogen-bonded amyloid-like fibrils were found to be responsible for the formation of zein gel under shear stress. Zein gel has water-induced self-assembly property that was exploited for 4D printing using a tailor-made supporting bath. Hydrophobic attraction caused differential self-assembly in the supporting bath with different water concentrations. This modulation of hydrophobic self-assembly controlled the properties of the printed structures with great importance, especially for biomedical purposes, and provides tunable drug loading, drug release, and degradation rate. The animal study further validated the significant translational potential of such 4D-printed structures. This principle may be further extended to other materials for 4D printing.
Data availability
The authors declare that all relevant data of this study are available from the corresponding authors.
CRediT authorship contribution statement
Yubei Zhang: Methodology, Validation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Ali Raza: Methodology, Validation, Formal analysis, Investigation, Visualization, Writing – original draft, Writing – review & editing. Ya-Qi Xue: Investigation. Ganggang Yang: Investigation. Uzma Hayat: Investigation. Jingwen Yu: Investigation. Chang Liu: Investigation. Hua-Jie Wang: Investigation. Jin-Ye Wang: Conceptualization, Supervision, Project administration, Resources, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the National Key R&D Program of China (2019YFE0101200), the Science and Technology Commission Shanghai Municipality, China (13JC1403400, 18490740200), the Foreign Young Talent Program from the Ministry of Science and Technology, China (QN2022134003L), and the Plan of Jiaxing Innovation and Elites Leading, China.
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.11.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Gao B., Yang Q., Zhao X., Jin G., Ma Y., Xu F. 4D bioprinting for biomedical applications. Trends Biotechnol. 2016;34:746–756. doi: 10.1016/j.tibtech.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ryan K.R., Down M.P., Banks C.E. Future of additive manufacturing: overview of 4D and 3D printed smart and advanced materials and their applications. Chem. Eng. J. 2021;403 doi: 10.1016/j.cej.2020.126162. [DOI] [Google Scholar]
- 3.Chen X., Han S., Wu W., Wu Z., Yuan Y., Wu J., Liu C. Harnessing 4D printing bioscaffolds for advanced orthopedics. Small. 2022;18 doi: 10.1002/smll.202106824. [DOI] [PubMed] [Google Scholar]
- 4.Hann S.Y., Cui H., Nowicki M., Zhang L.G. 4D printing soft robotics for biomedical applications. Addit. Manuf. 2020 doi: 10.1016/j.addma.2020.101567. [DOI] [Google Scholar]
- 5.Demoly F., Dunn M.L., Wood K.L., Qi H.J., André J.-C. The status, barriers, challenges, and future in design for 4D printing. Mater. Des. 2021;212 doi: 10.1016/j.matdes.2021.110193. [DOI] [Google Scholar]
- 6.Falahati M., Ahmadvand P., Safaee S., Chang Y.C., Lyu Z., Chen R., Li L., Lin Y. Smart polymers and nanocomposites for 3D and 4D printing. Mater. Today. 2020;40:215–245. doi: 10.1016/j.mattod.2020.06.001. [DOI] [Google Scholar]
- 7.Champeau M., Heinze D.A., Viana T.N., de Souza E.R., Chinellato A.C., Titotto S. 4D printing of hydrogels: a review. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201910606. [DOI] [Google Scholar]
- 8.Makvandi P., Maleki A., Shabani M., Hutton A.R.J., Kirkby M., Jamaledin R., Fang T., He J., Lee J., Mazzolai B., Donnelly R.F., Tay F.R., Chen G., Mattoli V. Bioinspired microneedle patches: biomimetic designs, fabrication, and biomedical applications. Matter. 2022;5:390–429. doi: 10.1016/j.matt.2021.11.021. [DOI] [Google Scholar]
- 9.Kuang X., Roach D.J., Wu J., Hamel C.M., Ding Z., Wang T., Dunn M.L., Qi H.J. Advances in 4D printing: materials and applications. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201805290. [DOI] [Google Scholar]
- 10.Spiegel C.A., Hippler M., Münchinger A., Bastmeyer M., Barner-Kowollik C., Wegener M., Blasco E. 4D printing at the microscale. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201907615. [DOI] [Google Scholar]
- 11.Rastogi P., Kandasubramanian B. Breakthrough in the printing tactics for stimuli-responsive materials: 4D printing. Chem. Eng. J. 2019;366:264–304. doi: 10.1016/j.cej.2019.02.085. [DOI] [Google Scholar]
- 12.Agarwal T., Hann S.Y., Chiesa I., Cui H., Celikkin N., Micalizzi S., Barbetta A., Costantini M., Esworthy T., Zhang L.G., De Maria C., Maiti T.K. 4D printing in biomedical applications: emerging trends and technologies. J. Mater. Chem. B. 2021;9:7608–7632. doi: 10.1039/D1TB01335A. [DOI] [PubMed] [Google Scholar]
- 13.Han D., Morde R.S., Mariani S., La Mattina A.A., Vignali E., Yang C., Barillaro G., Lee H. 4D printing of a bioinspired microneedle array with backward-facing barbs for enhanced tissue adhesion. Adv. Funct. Mater. 2020;30 doi: 10.1002/adfm.201909197. [DOI] [Google Scholar]
- 14.Melocchi A., Uboldi M., Inverardi N., Briatico-Vangosa F., Baldi F., Pandini S., Scalet G., Auricchio F., Cerea M., Foppoli A., Maroni A., Zema L., Gazzaniga A. Expandable drug delivery system for gastric retention based on shape memory polymers: development via 4D printing and extrusion. Int. J. Pharm. 2019;571 doi: 10.1016/j.ijpharm.2019.118700. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C., Cai D., Liao P., Su J.-W., Deng H., Vardhanabhuti B., Ulery B.D., Chen S.-Y., Lin J. 4D Printing of shape-memory polymeric scaffolds for adaptive biomedical implantation. Acta Biomater. 2021;122:101–110. doi: 10.1016/j.actbio.2020.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grote U., Fasse A., Nguyen T.T., Erenstein O. Food security and the dynamics of wheat and maize value chains in africa and asia. Front. Sustain. Food Syst. 2021;4 https://www.frontiersin.org/article/10.3389/fsufs.2020.617009 [Google Scholar]
- 17.Tortorella S., Maturi M., Vetri Buratti V., Vozzolo G., Locatelli E., Sambri L., Comes Franchini M. Zein as a versatile biopolymer: different shapes for different biomedical applications. RSC Adv. 2021;11:39004–39026. doi: 10.1039/D1RA07424E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X., Sun Q., Wang H., Zhang L., Wang J.Y. Microspheres of corn protein, zein, for an ivermectin drug delivery system. Biomaterials. 2005;26:109–115. doi: 10.1016/j.biomaterials.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 19.Raza A., Hayat U., Wang H.-J., Wang J.-Y. Preparation and evaluation of captopril loaded gastro-retentive zein based porous floating tablets. Int. J. Pharm. 2020;579 doi: 10.1016/j.ijpharm.2020.119185. [DOI] [PubMed] [Google Scholar]
- 20.Raza A., Shen N., Li J., Chen Y., Wang J.Y. Formulation of zein based compression coated floating tablets for enhanced gastric retention and tunable drug release. Eur. J. Pharmaceut. Sci. 2019 doi: 10.1016/j.ejps.2019.01.025. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.J., Lin Z.X., Liu X.M., Sheng S.Y., Wang J.Y. Heparin-loaded zein microsphere film and hemocompatibility. J. Contr. Release. 2005;105:120–131. doi: 10.1016/j.jconrel.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 22.Li W., Han Y., Yang H., Wang G., Lan R., Wang J.Y. Preparation of microcarriers based on zein and their application in cell culture. Mater. Sci. Eng. C. 2016;58:863–869. doi: 10.1016/j.msec.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 23.Tu J., Wang H., Li H., Dai K., Wang J., Zhang X. Biomaterials; 2009. The in Vivo Bone Formation by Mesenchymal Stem Cells in Zein Scaffolds. [DOI] [PubMed] [Google Scholar]
- 24.Wang G.W., Yang H., Wu W.F., Zhang P., Wang J.Y. Design and optimization of a biodegradable porous zein conduit using microtubes as a guide for rat sciatic nerve defect repair. Biomaterials. 2017;131:145–159. doi: 10.1016/j.biomaterials.2017.03.038. [DOI] [PubMed] [Google Scholar]
- 25.Wang J.-Y., Wang H.-J. 2019. A Bioink for 3D Printing, the Preparation Method and Usage. PCT/CN2019/070746. [Google Scholar]
- 26.Tavares-Negrete J.A., Aceves-Colin A.E., Rivera-Flores D.C., Díaz-Armas G.G., Mertgen A.-S., Trinidad-Calderón P.A., Olmos-Cordero J.M., Gómez-López E.G., Pérez-Carrillo E., Escobedo-Avellaneda Z.J., Tamayol A., Alvarez M.M., Trujillo-de Santiago G. Three-dimensional printing using a maize protein: zein-based inks in biomedical applications. ACS Biomater. Sci. Eng. 2021;7:3964–3979. doi: 10.1021/acsbiomaterials.1c00544. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H., Zhou L., Zhang W. Control of scaffold degradation in tissue engineering: a review. Tissue Eng. B Rev. 2014;20:492–502. doi: 10.1089/ten.teb.2013.0452. [DOI] [PubMed] [Google Scholar]
- 28.Muheremu A., Ao Q. Past, present, and future of nerve conduits in the treatment of peripheral nerve injury. BioMed Res. Int. 2015;2015 doi: 10.1155/2015/237507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hadaschik B.A., Paterson R.F., Fazli L., Clinkscales K.W., Shalaby S.W., Chew B.H. Investigation of a novel degradable ureteral stent in a porcine model. J. Urol. 2008;180:1161–1166. doi: 10.1016/j.juro.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 30.Zhang N., Wang Y., Zhang J., Guo J., He J. Controlled domain gels with a biomimetic gradient environment for osteochondral tissue regeneration. Acta Biomater. 2021;135:304–317. doi: 10.1016/j.actbio.2021.08.029. [DOI] [PubMed] [Google Scholar]
- 31.X. Niu, N. Li, Z. Du, X. Li, Integrated gradient tissue-engineered osteochondral scaffolds: challenges, current efforts and future perspectives, Bioact. Mater. 20 (2023) 574–597. https://doi.org/https://doi.org/10.1016/j.bioactmat.2022.06.011. [DOI] [PMC free article] [PubMed]
- 32.Hua D., Xiong R., Braeckmans K., Scheid B., Huang C., Sauvage F., De Smedt S.C. Concentration gradients in material sciences: methods to design and biomedical applications. Adv. Funct. Mater. 2021;31 doi: 10.1002/adfm.202009005. [DOI] [Google Scholar]
- 33.Peret B.J., Murphy W.L. Controllable soluble protein concentration gradients in hydrogel networks. Adv. Funct. Mater. 2008;18:3410–3417. doi: 10.1002/adfm.200800218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gurdon J.B., Bourillot P.-Y. Morphogen gradient interpretation. Nature. 2001;413:797–803. doi: 10.1038/35101500. [DOI] [PubMed] [Google Scholar]
- 35.Yu J., Lin Y., Wang G., Song J., Hayat U., Liu C., Raza A., Huang X., Lin H., Wang J.-Y. Zein-induced immune response and modulation by size, pore structure and drug-loading: application for sciatic nerve regeneration. Acta Biomater. 2022;140:289–301. doi: 10.1016/j.actbio.2021.11.035. [DOI] [PubMed] [Google Scholar]
- 36.Shivu B., Seshadri S., Li J., Oberg K.A., Uversky V.N., Fink A.L. Distinct β-sheet structure in protein aggregates determined by ATR-FTIR spectroscopy. Biochemistry. 2013;52:5176–5183. doi: 10.1021/bi400625v. [DOI] [PubMed] [Google Scholar]
- 37.Cao Y., Mezzenga R. Food protein amyloid fibrils: origin, structure, formation, characterization, applications and health implications. Adv. Colloid Interface Sci. 2019;269:334–356. doi: 10.1016/j.cis.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Ji H., Zhao J., Chen J., Shimai S., Zhang J., Liu Y., Liu D., Wang S. A novel experimental approach to quantitatively evaluate the printability of inks in 3D printing using two criteria. Addit. Manuf. 2022 doi: 10.1016/j.addma.2022.102846. [DOI] [Google Scholar]
- 39.Schwab A., Levato R., D'Este M., Piluso S., Eglin D., Malda J. Printability and shape fidelity of bioinks in 3D bioprinting. Chem. Rev. 2020;120:11028–11055. doi: 10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen Z., Zhao D., Liu B., Nian G., Li X., Yin J., Qu S., Yang W. 3D printing of multifunctional hydrogels. Adv. Funct. Mater. 2019;29 doi: 10.1002/adfm.201900971. [DOI] [Google Scholar]
- 41.Sun H., Kim Y., Kim Y.C., Park I.K., Suhr J., Byun D., Choi H.R., Kuk K., Baek O.H., Jung Y.K., Choi H.J., Kim K.J., Nam J.D. Self-standing and shape-memorable UV-curing epoxy polymers for three-dimensional (3D) continuous-filament printing. J. Mater. Chem. C. 2018;6:2996–3003. doi: 10.1039/C7TC04873D. [DOI] [Google Scholar]
- 42.Ribeiro A., Blokzijl M.M., Levato R., Visser C.W., Castilho M., Hennink W.E., Vermonden T., Malda J. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication. 2018;10 doi: 10.1088/1758-5090/aa90e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun C., Xiong Z., Chang Y., Li S., Zhang Y., Fang Y. Zein molecules in aqueous acetic acid solution: self-assembling behaviors and formation mechanism of gluten-free doughs. Innovat. Food Sci. Emerg. Technol. 2022;80 doi: 10.1016/j.ifset.2022.103092. [DOI] [Google Scholar]
- 44.Raza A., Hayat U., Zhang X., Wang J.-Y. Self-assembled zein organogels as in situ forming implant drug delivery system and 3D printing ink. Int. J. Pharm. 2022;627 doi: 10.1016/j.ijpharm.2022.122206. [DOI] [PubMed] [Google Scholar]
- 45.Chen D., Liu Q., Han Z., Zhang J., Song H., Wang K., Song Z., Wen S., Zhou Y., Yan C., Shi Y. 4D printing strain self-sensing and temperature self-sensing integrated sensor–actuator with bioinspired gradient gaps. Adv. Sci. 2020;7 doi: 10.1002/advs.202000584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nandakumar A., Ito Y., Ueda M. Solvent effects on the self-assembly of an amphiphilic polypeptide incorporating α-helical hydrophobic blocks. J. Am. Chem. Soc. 2020;142:20994–21003. doi: 10.1021/jacs.0c03425. [DOI] [PubMed] [Google Scholar]
- 47.Hayashi K., Shimabukuro M., Ishikawa K. Antibacterial honeycomb scaffolds for achieving infection prevention and bone regeneration. ACS Appl. Mater. Interfaces. 2022;14:3762–3772. doi: 10.1021/acsami.1c20204. [DOI] [PubMed] [Google Scholar]
- 48.Heidari G., Hassanpour M., Nejaddehbashi F., Sarfjoo M.R., Yousefiasl S., Sharifi E., Bigham A., Agarwal T., Borzacchiello A., Lagreca E., Di Natale C., Nikfarjam N., Vasseghian Y. Biosynthesized nanomaterials with antioxidant and antimicrobial properties. Mater. Chem. Horizons. 2022;1:35–48. doi: 10.22128/mch.2022.553.1005. [DOI] [Google Scholar]
- 49.Wu F., Wei J., Liu C., O'Neill B., Ngothai Y. Fabrication and properties of porous scaffold of zein/PCL biocomposite for bone tissue engineering. Compos. B Eng. 2012;43:2192–2197. doi: 10.1016/j.compositesb.2012.02.040. [DOI] [Google Scholar]
- 50.Chen M., Le D.Q.S., Hein S., Li P., V Nygaard J., Kassem M., Kjems J., Besenbacher F., Bünger C. Fabrication and characterization of a rapid prototyped tissue engineering scaffold with embedded multicomponent matrix for controlled drug release. Int. J. Nanomed. 2012;7:4285–4297. doi: 10.2147/IJN.S33083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zolfagharian A., Kaynak A., Khoo S.Y., Kouzani A.Z. Polyelectrolyte soft actuators: 3D printed chitosan and cast gelatin, 3D print. Addit. Manuf. 2018;5:138–150. doi: 10.1089/3dp.2017.0054. [DOI] [Google Scholar]
- 52.Ding A., Jeon O., Cleveland D., Gasvoda K.L., Wells D., Lee S.J., Alsberg E. Jammed micro-flake hydrogel for four-dimensional living cell bioprinting. Adv. Mater. 2022;34 doi: 10.1002/adma.202109394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z., Yang Z., Jiang J., Shi Z., Mao Y., Qin N., Tao T.H. Silk microneedle patch capable of on-demand multidrug delivery to the brain for glioblastoma treatment. Adv. Mater. 2022;34 doi: 10.1002/adma.202106606. [DOI] [PubMed] [Google Scholar]
- 54.Rodriguez M.M. Congenital anomalies of the kidney and the urinary tract (CAKUT), fetal pediatr. For. Pathol. 2014;33:293–320. doi: 10.3109/15513815.2014.959678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elsamra S.E., Leavitt D.A., Motato H.A., Friedlander J.I., Siev M., Keheila M., Hoenig D.M., Smith A.D., Okeke Z. Stenting for malignant ureteral obstruction: tandem, metal or metal-mesh stents. Int. J. Urol. 2015;22:629–636. doi: 10.1111/iju.12795. [DOI] [PubMed] [Google Scholar]
- 56.Kehinde E.O., Rotimi V.O., Al-Awadi K.A., Abdul-Halim H., Boland F., A-H A., P A. Factors predisposing to urinary tract infection after J ureteral stent insertion. J. Urol. 2002;167:1334–1337. doi: 10.1016/S0022-5347(05)65294-9. [DOI] [PubMed] [Google Scholar]
- 57.Arciola C.R., Campoccia D., Speziale P., Montanaro L., Costerton J.W. Biofilm formation in Staphylococcus implant infections. A review of molecular mechanisms and implications for biofilm-resistant materials. Biomaterials. 2012;33:5967–5982. doi: 10.1016/j.biomaterials.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 58.Lange D., Bidnur S., Hoag N., Chew B.H. Ureteral stent-associated complications-where we are and where we are going. Nat. Rev. Urol. 2015 doi: 10.1038/nrurol.2014.340. [DOI] [PubMed] [Google Scholar]
- 59.Arciola C.R., Campoccia D., Montanaro L. Implant infections: adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018;16:397–409. doi: 10.1038/s41579-018-0019-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that all relevant data of this study are available from the corresponding authors.