Abstract
Objective
The triglyceride and glucose (TyG) index is a useful marker of insulin resistance and is a predictor of several metabolic diseases. The aim of this study was to evaluate the association between the TyG index and all-cause or cardiovascular mortality using a large population-based cohort study database.
Methods
A total of 255,508 subjects in the Kangbuk Samsung Health Study cohort were enrolled. Cox proportional hazards models were used to analyze the risk of mortality.
Results
During a median 5.7-year follow-up, the cumulative all-cause and cardiovascular mortality was 0.47% and 0.07%. There was a nonlinear relationship between the TyG index and death, and moving from moderate to high, the TyG index levels were associated with an increase in the risk of death. The hazard ratio (HR) for all-cause and cardiovascular mortality of the TyG index was 1.21 [95% confidence interval (CI) 1.14–1.28] and 1.45 (95% CI 1.26–1.66) in the unadjusted model, respectively. After adjustment for covariates, the association between the TyG index and all-cause and cardiovascular mortality was attenuated. In the multivariable-adjusted model, the TyG index was associated with an elevated risk of all-cause mortality in women (HR 1.13, 95% CI 1.02–1.26) and a decreased risk in men (HR 0.92, 95% CI 0.85–0.99). The association between cardiovascular mortality and the TyG index was not statistically significant among either men or women in the multivariable-adjusted model.
Conclusions
The TyG index in a young, relatively healthy, population is associated with an elevated risk of all-cause and cardiovascular mortality. This association between the TyG index and all-cause mortality persists in women after multivariable adjustment.
KEY WORDS: glucose, homeostasis model assessment of insulin resistance (HOMA-IR), insulin resistance, mortality, triglycerides
INTRODUCTION
Insulin resistance is associated with several diseases1–4 and is a known health concern. However, the relationship between insulin resistance and mortality has been seldom studied5,6 and demonstrating this relationship is not straightforward. Several studies have shown that elevated insulin resistance is associated with an increased risk of cardiovascular mortality.7,8 However, few studies have evaluated the association between insulin resistance and overall mortality.9
Because the hyperinsulinemic-euglycemic clamp test is difficult to perform, the homeostasis model assessment of insulin resistance (HOMA-IR) has been widely used as an index of insulin resistance.10,11 HOMA-IR can be calculated by measuring fasting glucose and insulin levels; however, insulin measurement is expensive and is not included in routine health check-ups. The triglyceride and glucose (TyG) index has been recently reported to be a reliable marker of insulin resistance.12,13 Triglycerides and glucose measurements are included in many screening examinations, so the TyG index could be suitable for measuring insulin resistance in large population-based studies. Many studies have shown that the TyG index is a good predictor of many diseases associated with insulin resistance, such as hypertension, nonalcoholic fatty liver disease (NAFLD), and cardiovascular diseases.14–16 However, there were few large-scale, population-based studies that evaluated the association between the TyG index and mortality. The aim of this study was to evaluate the association between the TyG index and all-cause or cardiovascular mortality using a large population-based cohort study database.
METHODS
Study Design and Patients
We conducted this longitudinal cohort study using the Kangbuk Samsung Health Study dataset. This study includes 396,594 South Korean adults who underwent a comprehensive annual or biennial health examination at the clinics of the Kangbuk Samsung Hospital Total Healthcare Center in Seoul or Suwon, South Korea, from 2002 to 2012. More than 80% of participants were employees or spouses of employees of companies or local governmental organizations and the remaining participants were those voluntarily taking part in screening examinations. We excluded subjects who had prediabetes, diabetes, a history of taking anti-diabetic agents, agents for dyslipidemia, or missing information. Finally, a total of 255,508 subjects were enrolled and were followed up for a median of 5.7 years. The requirement for informed consent was waived because personal identifying information was not accessed. This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital according to the Declaration of Helsinki (2015-11-016-005).
Clinical and Laboratory Measurements
Information about lifestyle factors including cigarette smoking and daily alcohol consumption was obtained on the standardized self-questionnaire. Regular exercise was defined as the performance of vigorous exercise more than once a week. Subjects wore minimal clothing and no shoes during weight and height measurements. Blood pressure was measured by trained nurses using an automatic blood pressure monitor after the participants were seated for 10 min. Waist circumference was measured at the midpoint between the lower rib margin and the iliac crest. All blood samples were obtained after an overnight fast of at least eight hours, and plasma glucose, insulin, total cholesterol, triglyceride, high-density lipoprotein (HDL) cholesterol, apolipoprotein A-I, apolipoprotein B, aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), creatinine, and C-reactive protein (CRP) levels were measured. Serum glucose was measured by the hexokinase method using an autoanalyzer (ADIVA 1800; Siemens, Berlin, Germany) and serum insulin was measured using an immunoradiometric assay (Biosource, Nivelles, Belgium). The TyG index was calculated as Ln[fasting triglycerides (mg/dL) × fasting plasma glucose (mg/dL)/2] and HOMA-IR as [fasting insulin (μU/mL) × fasting plasma glucose (mg/dL)/405].
Mortality Assessment
In Korea, all deaths are reported to the Korea National Statistical Office. Thus, we were able to evaluate deaths by matching subject identification numbers to death certificates from the National Statistical Office. The causes of death were coded according to the International Classification of Diseases, 10th revision. Cardiovascular death was defined as ICD-10 code I10, I11, I12, I20, I21, I24, I25, I27, I35, I40, I42, I46, I48, I49, I50, I51, I60, I61, I62, I63, I64, I67, I69, I70, I71, I72, or I85. All-cause and cardiovascular mortality was investigated.
Statistical Analyses
Data for categorical factors are reported as percentages, and continuous variables are presented as mean ± standard deviation. The follow-up time was computed from baseline until the occurrence of death. The proportional hazards assumption was examined using log plots of survival versus time. Cox proportional hazards models were used to determine the hazard ratio (HR) with the corresponding 95% confidence interval (CI) for mortality associated with the TyG index. The multivariable model was adjusted for age, sex, body mass index (BMI), systolic blood pressure, low-density lipoprotein (LDL) cholesterol, daily alcohol consumption, regular physical activity, and smoking status. Kaplan-Meier survival curves were constructed to compare survival rates according to the TyG index quartile. The TyG index quartile (Q) is as follows: Q1, < 7.96; Q2, 7.97–8.32; Q3, 8.32–8.70; Q4, ≥ 8.71. To account for nonlinear associations, we examined restricted cubic spline transformations for continuous predictors and used the likelihood ratio test to examine the linearity assumption. All statistical analyses were performed using R version 3.6.3 (Vienna, Austria; http://www.rproject.org). A P value < 0.05 was considered significant.
RESULTS
Baseline characteristics of the study population are presented in Table 1. The mean subject age was 37.63 years and BMI was 22.90 kg/m2. Among subjects, 25.46% were current smokers, 9.67% performed regular physical exercise, 11.15% had hypertension, and 8.26% had hypercholesterolemia. There were different baseline characteristics between men and women. Age, regular physical activity, HDL cholesterol, and apolipoprotein A-I were higher in women than in men, while current smoker, alcohol consumption, BMI, waist circumference, blood pressure (systolic and diastolic), hypertension, hypercholesterolemia, fasting plasma glucose, fasting serum insulin, total cholesterol, triglycerides, LDL cholesterol, non-HDL cholesterol, apolipoprotein B, AST, ALT, GGT, creatinine, CRP, the TyG index, and HOMA-IR were lower in women.
Table 1.
Baseline Characteristics of the Subjects
| Variables | Total | Men | Women | P-value |
|---|---|---|---|---|
| n | 255,508 | 134,455 | 121,053 | |
| Age, years | 37.63 ± 8.75 | 37.54 ± 8.52 | 37.73 ± 8.99 | < 0.001 |
| Body mass index, kg/m2 | 22.90 ± 3.01 | 23.99 ± 2.78 | 21.70 ± 2.80 | < 0.001 |
| Waist circumference, cm | 78.59 ± 9.07 | 83.54 ± 7.48 | 73.31 ± 7.50 | < 0.001 |
| Current smoking, % | 25.46 (65,041) | 44.38 (59,669) | 4.44 (5372) | < 0.001 |
| Daily alcohol consumption, g/day | 9.43 ± 15.92 | 14.73 ± 18.53 | 3.29 ± 8.88 | < 0.001 |
| Regular physical activity, % | 9.67 (24,714) | 6.08 (8172) | 13.67 (16,542) | < 0.001 |
| Hypertension, % | 11.15 (28,483) | 15.20 (20,440) | 6.64 (8043) | < 0.001 |
| Hypercholesterolemia, % | 8.26 (21,102) | 10.22 (13,735) | 6.09 (7367) | < 0.001 |
| Systolic blood pressure, mm Hg | 111.88 ± 13.65 | 116.05 ± 12.39 | 107.24 ± 13.49 | < 0.001 |
| Diastolic blood pressure, mm Hg | 72.10 ± 9.87 | 75.40 ± 9.24 | 68.42 ± 9.23 | < 0.001 |
| Fasting plasma glucose, mg/dL | 89.13 ± 6.30 | 89.97 ± 6.14 | 88.18 ± 6.35 | < 0.001 |
| Fasting serum insulin, μU/mL | 4.32 ± 3.20 | 4.37 ± 3.52 | 4.26 ± 2.81 | < 0.001 |
| Total cholesterol, mg/dL | 191.13 ± 33.67 | 196.15 ± 33.66 | 185.57 ± 32.79 | < 0.001 |
| Triglyceride, mg/dL | 112.55 ± 72.75 | 135.21 ± 82.39 | 87.37 ± 49.26 | < 0.001 |
| HDL cholesterol, mg/dL | 57.08 ± 13.24 | 52.67 ± 11.35 | 61.98 ± 13.48 | < 0.001 |
| LDL cholesterol, mg/dL | 111.07 ± 29.21 | 117.44 ± 29.10 | 104.01 ± 27.67 | < 0.001 |
| Non-HDL cholesterol, mg/dL | 134.05 ± 34.16 | 143.47 ± 33.67 | 123.59 ± 31.55 | < 0.001 |
| Apolipoprotein A-I, mg/dL | 141.86 ± 23.90 | 136.79 ± 22.15 | 146.86 ± 24.50 | < 0.001 |
| Apolipoprotein B, mg/dL | 87.88 ± 23.30 | 94.83 ± 22.86 | 81.06 ± 21.65 | < 0.001 |
| Aspartate aminotransferase, U/L | 23.46 ± 21.95 | 25.82 ± 28.37 | 20.84 ± 10.47 | < 0.001 |
| Alanine aminotransferase, U/L | 24.13 ± 26.35 | 30.14 ± 32.43 | 17.45 ± 14.58 | < 0.001 |
| Gamma-glutamyl transferase, U/L | 26.04 ± 31.01 | 36.20 ± 37.01 | 14.75 ± 16.30 | < 0.001 |
| Creatinine, mg/dL | 0.96 ± 0.20 | 1.09 ± 0.16 | 0.82 ± 0.14 | < 0.001 |
| C-reactive protein, mg/dL | 0.10 ± 0.27 | 0.12 ± 0.29 | 0.09 ± 0.24 | < 0.001 |
| The TyG index | 8.37 ± 0.54 | 8.57 ± 0.52 | 8.14 ± 0.46 | < 0.001 |
| HOMA-IR | 0.96 ± 0.74 | 0.98 ± 0.82 | 0.94 ± 0.64 | < 0.001 |
Data are expressed as mean ± standard deviation or a percentage (number)
P-value for the comparison between men and women
TyG index, triglyceride and glucose index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance
Over a median 5.7 years of follow-up, the cumulative all-cause and cardiovascular mortality was 0.47% (1207 deaths) and 0.07% (174 deaths), respectively (Tables 2 and 3). Incidence for all-cause death was 0.982 cases/1000 person-years in men and 0.558 cases/1000 person-years in women (Table 2). The unadjusted HRs for all-cause mortality of the TyG index, triglyceride, glucose, and HOMA-IR were 1.21 (95% CI 1.14–1.28), 1.13 (95% CI 1.08–1.18), 1.05 (95% CI 1.00–1.12), and 1.00 (95% CI 0.95–1.06), respectively. After adjustment for covariates, the association between the TyG index and all-cause mortality was attenuated. Triglyceride and glucose were not associated with all-cause mortality after adjusting for covariates, but HOMA-IR was still associated. After adjusting for age, the TyG index was associated with an elevated risk of all-cause mortality in women (HR 1.11, 95% CI 1.01–1.22), but not in men (HR 0.94, 95% CI 0.88–1.01). In the multivariable-adjusted model, the TyG index was associated with an elevated risk of all-cause mortality in women (HR 1.13, 95% CI 1.02–1.26) and a decreased risk in men (HR 0.92, 95% CI 0.85–0.99).
Table 2.
The Risk of All-Cause Mortality According to Various Indices
| The TyG index | Triglycerides | Glucose | HOMA-IR | |
|---|---|---|---|---|
| All-cause mortality | ||||
| Events, n | 1207 | |||
| Person-years | 1,523,253.747 | |||
| Incidence, per 1000 person-years | 0.792 | |||
| Unadjusted | 1.21 (1.14–1.28) | 1.13 (1.08–1.18) | 1.05 (1.00–1.12) | 1.00 (0.95–1.06) |
| Age- and sex-adjusted | 0.97 (0.91–1.03) | 1.00 (0.95–1.05) | 0.95 (0.90–1.00) | 1.03 (1.01–1.05) |
| Multivariable-adjusted* | 0.97 (0.90–1.03) | 0.99 (0.93–1.04) | 0.95 (0.89–1.00) | 1.03 (1.01–1.04) |
| Men | ||||
| Events, n | 827 | |||
| Person-years | 841,776.731 | |||
| Incidence, per 1000 person-years | 0.982 | |||
| Unadjusted | 0.96 (0.89–1.03) | 0.97 (0.91–1.04) | 0.96 (0.90–1.02) | 0.92 (0.84–1.00) |
| Age-adjusted | 0.94 (0.88–1.01) | 0.97 (0.90–1.04) | 0.91 (0.85–0.97) | 1.03 (1.00–1.06) |
| Multivariable-adjusted* | 0.92 (0.85–0.99) | 0.94 (0.87–1.02) | 0.91 (0.85–0.97) | 1.03 (1.01–1.05) |
| Women | ||||
| Events, n | 380 | |||
| Person-years | 681,477.016 | |||
| Incidence, per 1000 person-years | 0.558 | |||
| Unadjusted | 1.49 (1.37–1.63) | 1.24 (1.19–1.29) | 1.20 (1.08–1.33) | 1.10 (1.03–1.16) |
| Age-adjusted | 1.11 (1.01–1.22) | 1.10 (1.03–1.17) | 1.06 (0.96–1.18) | 1.11 (1.05–1.18) |
| Multivariable-adjusted* | 1.13 (1.02–1.26) | 1.09 (1.02–1.16) | 1.07 (0.96–1.20) | 1.13 (1.07–1.20) |
*Adjusted for age, sex, body mass index, systolic blood pressure, LDL cholesterol, daily alcohol consumption, regular physical activity, current smoking
TyG index, triglyceride and glucose index; HOMA-IR, homeostatic model assessment of insulin resistance
Table 3.
The Risk of Cardiovascular Mortality According to Various Indices
| The TyG index | Triglycerides | Glucose | HOMA-IR | |
|---|---|---|---|---|
| Cardiovascular mortality | ||||
| Events, n | 174 | |||
| Person-years | 1,523,253.747 | |||
| Incidence, per 1000 person-years | 0.114 | |||
| Unadjusted | 1.45 (1.26–1.66) | 1.23 (1.15–1.33) | 1.11 (0.96–1.29) | 0.97 (0.82–1.15) |
| Age- and sex-adjusted | 1.17 (1.01–1.37) | 1.15 (1.04–1.27) | 0.98 (0.85–1.14) | 1.03 (0.98–1.08) |
| Multivariable-adjusted* | 1.04 (0.88–1.25) | 1.10 (0.97–1.24) | 0.96 (0.82–1.12) | 1.02 (0.96–1.08) |
| Men | ||||
| Events, n | 125 | |||
| Person-years | 841,776.731 | |||
| Incidence, per 1000 person-years | 0.148 | |||
| Unadjusted | 1.18 (0.99–1.40) | 1.14 (1.00–1.29) | 0.95 (0.80–1.12) | 1.01 (0.91–1.13) |
| Age-adjusted | 1.16 (0.98–1.39) | 1.14 (1.00–1.31) | 0.91 (0.77–1.07) | 1.04 (1.00–1.08) |
| Multivariable-adjusted* | 1.03 (0.84–1.26) | 1.07 (0.90–1.26) | 0.89 (0.75–1.05) | 1.02 (0.97–1.07) |
| Women | ||||
| Events, n | 49 | |||
| Person-years | 681,477.016 | |||
| Incidence, per 1000 person-years | 0.072 | |||
| Unadjusted | 1.70 (1.34–2.15) | 1.29 (1.19–1.39) | 1.49 (1.09–2.04) | 0.75 (0.52–1.09) |
| Age-adjusted | 1.12 (0.85–1.47) | 1.16 (1.01–1.33) | 1.24 (0.91–1.69) | 0.81 (0.58–1.14) |
| Multivariable-adjusted* | 1.04 (0.77–1.39) | 1.12 (0.96–1.29) | 1.27 (0.92–1.76) | 0.84 (0.60–1.19) |
*Adjusted for age, sex, body mass index, systolic blood pressure, LDL cholesterol, daily alcohol consumption, regular physical activity, current smoking
TyG index, triglyceride and glucose index; HOMA-IR, homeostatic model assessment of insulin resistance
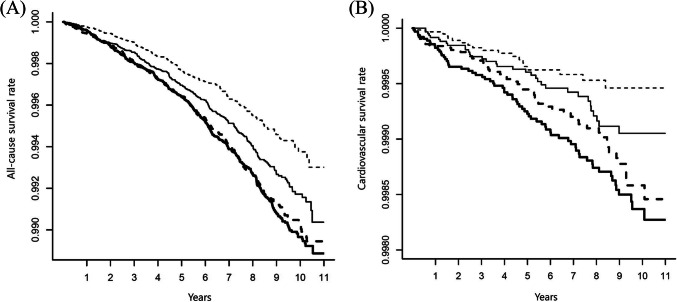
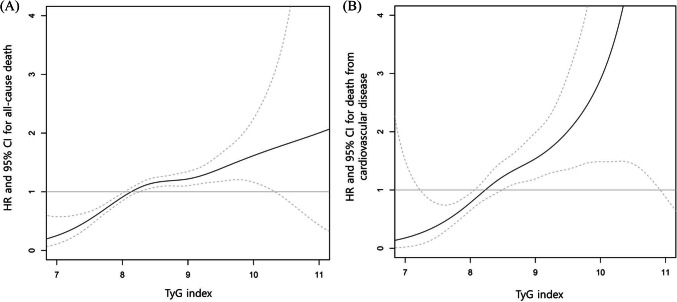
All-cause (Fig. 1A) and cardiovascular (Fig. 1B) survival rates decreased with increasing the TyG index Q1 to Q4 (P < 0.001, respectively). However, there was a nonlinear relationship between the TyG index and risk for all-cause and cardiovascular mortality (Fig. 2). Moving from moderate to high, the TyG index levels were associated with an increased risk. The TyG index level from which the risk increases appeared to be 8.23 in all-cause mortality and 8.14 in cardiovascular mortality. In addition, the TyG index showed a significant correlation with HOMA-IR (r = 0.26, 95% CI 0.25–0.26, P < 0.001). The sensitivity and specificity of the TyG index for all-cause mortality were 71.33% and 42.92% (cutoff value 8.23), and cardiovascular mortality were 84.48% and 36.16% (cutoff value 8.14), respectively. On the other hand, the sensitivity and specificity of HOMA-IR for all-cause mortality were 46.73% and 41.07% (cutoff value 0.70), and cardiovascular mortality were 47.13% and 39.33% (cutoff value 0.68), respectively.
Figure 1.
All-cause (A) and cardiovascular (B) survival rates according to the triglyceride and glucose (TyG) index quartile in the unadjusted model (P < 0.001, respectively). Q1, broken line; Q2, solid line; Q3 bold broken line; Q4, bold solid line.
Figure 2.
Restricted cubic spline curve of the risk for all-cause (A) and cardiovascular mortality (B) according to the triglyceride and glucose (TyG) index in the unadjusted model. Dotted lines represent 95% confidence interval (CI). HR, hazard ratio.
After 1,523,253.747 person-years of follow-up, there were 174 cases of cardiovascular death (0.114 cases/1,000 person-years) (Table 3). The unadjusted HRs for cardiovascular mortality of the TyG index, triglyceride, glucose, and HOMA-IR were 1.45 (95% CI 1.26–1.66), 1.23 (95% CI 1.15–1.33), 1.11 (95% CI 0.96–1.29), and 0.97 (95% CI 0.82–1.15), respectively. In the age- and sex-adjusted model, HR for cardiovascular mortality of the TyG index was 1.17 (95% CI 1.01-1.37). However, after multivariable adjustment, the association between cardiovascular mortality and the TyG index was weakened and was not statistically significant among either men or women. Triglyceride, glucose, and HOMA-IR did not show a significant association with cardiovascular mortality after adjustment for covariates, except triglyceride in the age- and sex-adjusted model. In the unadjusted model, the TyG index was associated with an elevated risk of cardiovascular mortality in women (HR 1.70, 95% CI 1.34–2.15), but not in men (HR 1.18, 95% CI 0.99–1.40). The association between cardiovascular mortality and the TyG index tended to be high, but it was not statistically significant after age adjustment or multivariable adjustment in both men and women.
DISCUSSION
In this study, the all-cause and cardiovascular mortality rate increased with increasing the TyG index quartile despite the relatively low mortality because the study population is young (mean age 37.63 years) and healthy. There was a nonlinear relationship between the TyG index and death, and moving from moderate to high, the TyG index levels were associated with an increase in the risk of death. The risk for all-cause and cardiovascular mortality of the TyG index was elevated in the unadjusted model, but it was attenuated in the multivariable-adjusted model. In the multivariable-adjusted model, the TyG index was associated with an elevated risk of all-cause mortality only in women. The association between cardiovascular mortality and the TyG index was not statistically significant among either men or women in the multivariable-adjusted model.
Insulin resistance produces proinflammatory cytokines, promotes atherosclerosis, and has been associated with several diseases including type 2 diabetes, hypertension, dyslipidemia, cardiovascular disease, NAFLD, and malignancy.17–24 It seems reasonable that insulin resistance is likely associated with elevated mortality, but few studies have evaluated this hypothesis. The current gold standard method for measuring insulin resistance is the hyperinsulinemic-euglycemic clamp test, but this is not easy to perform for large populations because it is time-consuming and invasive. Therefore, HOMA-IR, which is relatively simple to estimate, is widely used as a surrogate marker of insulin resistance. Several studies reported that HOMA-IR was associated with a higher risk of cardiovascular mortality.25–27 However, the association between HOMA-IR and all-cause mortality has not been evaluated much, and results are conflicting.27–29 A recent meta-analysis of seven studies involving 26,976 nondiabetic adults found that the relative risk of overall and cardiovascular mortality between the highest and lowest categories of HOMA-IR was 1.34 (95% CI 1.11–1.62, P = 0.002) and 2.11 (95% CI 1.01–4.41, P = 0.048), respectively.30 However, to calculate HOMA-IR, fasting insulin which is not routinely measured in health check-ups is needed, so it may be limited to the use of HOMA-IR as a universal marker especially in developing countries. In this study, the TyG index as a simple and easy-to-calculate marker was associated with an elevated risk of all-cause and cardiovascular mortality. These results also support that insulin resistance would be associated with mortality.
The TyG index is regarded as a reliable insulin resistance marker due to its high correlation with the hyperinsulinemic-euglycemic clamp test.12,13 As an alternative insulin resistance marker, the TyG index could be useful for identifying insulin resistance in healthy, prediabetic, diabetic, and adolescent subjects.12,31 Many studies have shown that the TyG index might be effective for predicting patients at risk for hypertension, diabetes, atherosclerosis, NAFLD, and cardiovascular events.14–16,32,33 In Korean adults, the TyG index is an independent predictor of coronary artery calcification progression, which itself is a risk factor for cardiovascular disease.34 The TyG index cutoff value for insulin resistance has not been firmly defined, but it ranges from 8.0 to 8.8 across several studies.14–16,34,35 In this study, the TyG index level from which the risk for death increases appeared to be 8.23 in all-cause mortality and 8.14 in death from cardiovascular disease, which is similar to previous studies.
However, there were few studies to evaluate the relationship between mortality and the TyG index. This study represented that the TyG index might be a reliable mortality indicator and was comparable to HOMA-IR. In addition, triglyceride and glucose alone were not associated with all-cause and cardiovascular mortality. It is not clear why the TyG index shows a more strong association with mortality than triglyceride and glucose alone, but a possible explanation might be that both lipotoxicity and glucotoxicity play an important role in insulin resistance pathogenesis.36,37 Unlike many insulin resistance indicators that only include glycemic parameters, the TyG index measures both lipotoxicity and glucotoxicity through the measurement of triglycerides and glucose. Further studies are needed to identify the underlying mechanisms.
The association between elevated risk of mortality and the TyG index was different between men and women in this study. It is not easy to explain clearly these differences; one possible explanation was that men had shown an unhealthy lifestyle. For example, men were more likely to be smokers and drinkers, did less regular physical activity, and had more hypertension and hypercholesterolemia compared to women. Because those characteristics were strong risk factors for mortality, the association between the TyG index and mortality might not have been significant in men after adjustment for covariates. Even the TyG index was inversely associated with all-cause mortality in men after the multivariable-adjusted model. The attenuated association between the TyG index and mortality after adjustment might be that conventional risk factors were more influential, not that the TyG index was not associated with mortality. Another possible explanation is that it could be due to differences in energy metabolism, adiposity, and hormone function.38 Due to these differences, the clinical impact of the TyG index on death may appear to be stronger in women than in men.
The TyG index has several strengths for use in both clinical and research settings. Other components of metabolic syndrome (obesity, hypertension, and diabetes) are associated with cardiovascular disease and death. However, we also wanted to evaluate risks in populations without those three established diseases. The TyG index may be a useful marker in a broader general population as insulin resistance has been associated with cardiovascular disease and death in non-diabetic and non-obese patients.30,33 Compared to insulin measurement methods that are expensive and not standardized in most developing countries, triglycerides and glucose can be easily measured almost anywhere in the world. Furthermore, triglycerides and glucose are usually included in health check-ups, while insulin is not. Using the TyG index, insulin resistance can be measured for subjects in low-income countries, and associations between the TyG index and various diseases can be evaluated for large population-based studies.
Our study and findings have several limitations. First, the all-cause and cardiovascular mortality was relatively low because the participants were young, healthy, and regularly received comprehensive health screenings. Further studies with longer follow-up intervals are needed to clarify the association between the TyG index and mortality. Second, insulin resistance of the subjects might not be high compared with that of Western populations because most Koreans are not obese (mean 23 kg/m2). Third, the follow-up duration (mean 5.7 years) of this study might not have been long enough to investigate mortality. Fourth, as results could vary across ethnic populations, the finding might not be generalizable to other populations. Finally, we did not have information on subject medications, lifestyles, habitual food intakes, mental health, sleeping status, or other diseases, so we could not determine which factors might influence the association between the TyG index and mortality. Nevertheless, this study is valuable because it has evaluated the association between the TyG index and mortality using a large population-based cohort study database.
In conclusion, the TyG index in a young, relatively healthy, population is associated with an elevated risk of all-cause and cardiovascular mortality. This association between the TyG index and all-cause mortality persists in women after multivariable adjustment. Although there was a nonlinear relationship between the TyG index and death, moving from moderate to high, the TyG index levels were associated with an increase in the risk of death. Considering that the TyG index might have the advantage of convenience and cost-effectiveness, it will be easy to find and manage the patients with high TyG index in the general population.
Author Contribution
Kyung-Soo Kim: Conceptualization, Formal analysis, Interpreted the data, Writing — original draft. Sangmo Hong: Interpreted the data, revised the manuscript for intellectual content. You-Cheol Hwang: Interpreted the data, revised the manuscript for intellectual content. Hong-Yup Ahn: Formal analysis, interpreted. Cheol-Young Park: Conceptualization, Formal analysis, interpreted the data, writing — review and editing.
Declarations
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tenenbaum A, Adler Y, Boyko V, et al. Insulin resistance is associated with increased risk of major cardiovascular events in patients with preexisting coronary artery disease. Am Heart J. 2007;153:559–65. doi: 10.1016/j.ahj.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Lee SH, Han K, Kim HS, Cho JH, Yoon KH, Kim MK. Predicting the development of myocardial infarction in middle-aged adults with type 2 diabetes: a risk model generated from a nationwide population-based cohort study in Korea. Endocrinol Metab (Seoul) 2020;35:636–46. doi: 10.3803/EnM.2020.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bu SY. Genetically mediated lipid metabolism and risk of insulin resistance: insights from mendelian randomization studies. J Lipid Atheroscler. 2019;8:132–43. doi: 10.12997/jla.2019.8.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huh JH, Ahn SG, Kim YI, et al. Impact of Longitudinal changes in metabolic syndrome status over 2 years on 10-year incident diabetes mellitus. Diabetes Metab J. 2019;43:530–8. doi: 10.4093/dmj.2018.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perseghin G, Calori G, Lattuada G, et al. Insulin resistance/hyperinsulinemia and cancer mortality: the Cremona study at the 15th year of follow-up. Acta Diabetol. 2012;49:421–8. doi: 10.1007/s00592-011-0361-2. [DOI] [PubMed] [Google Scholar]
- 6.Pan K, Nelson RA, Wactawski-Wende J, et al. Insulin resistance and cancer-specific and all-cause mortality in postmenopausal women: the women’s health initiative. J Natl Cancer Inst. 2020;112:170–8. doi: 10.1093/jnci/djz069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DECODE Insulin Study Group Plasma insulin and cardiovascular mortality in non-diabetic European men and women: a meta-analysis of data from eleven prospective studies. Diabetologia. 2004;47:1245–56. doi: 10.1007/s00125-004-1433-4. [DOI] [PubMed] [Google Scholar]
- 8.Gast KB, Tjeerdema N, Stijnen T, Smit JW, Dekkers OM. Insulin resistance and risk of incident cardiovascular events in adults without diabetes: meta-analysis. PLoS One. 2012;7:e52036. doi: 10.1371/journal.pone.0052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang O, Matsushita K, Coresh J, et al. Mortality implications of prediabetes and diabetes in older adults. Diabetes Care. 2020;43:382–8. doi: 10.2337/dc19-1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–95. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 11.Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: Past, present and future. Crit Rev Clin Lab Sci. 2015;52:180–90. doi: 10.3109/10408363.2015.1023429. [DOI] [PubMed] [Google Scholar]
- 12.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 13.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–51. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 14.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16:175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang S, Du T, Zhang J, et al. The triglyceride and glucose index (TyG) is an effective biomarker to identify nonalcoholic fatty liver disease. Lipids Health Dis. 2017;16:15. doi: 10.1186/s12944-017-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 17.Bornfeldt KE, Tabas I. Insulin resistance, hyperglycemia, and atherosclerosis. Cell Metab. 2011;14:575–85. doi: 10.1016/j.cmet.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moon J, Kim JY, Yoo S, Koh G. Fasting and postprandial hyperglycemia: their predictors and contributions to overall hyperglycemia in korean patients with type 2 diabetes. Endocrinol Metab (Seoul) 2020;35:290–7. doi: 10.3803/EnM.2020.35.2.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee I, Lee HH, Cho Y, et al. Association between serum bilirubin and the progression of carotid atherosclerosis in type 2 diabetes. J Lipid Atheroscler. 2020;9:195–204. doi: 10.12997/jla.2020.9.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim KS, Lee BW, Kim YJ, Lee DH, Cha BS, Park CY. Nonalcoholic fatty liver disease and diabetes: part ii: treatment. Diabetes Metab J. 2019;43:127–43. doi: 10.4093/dmj.2019.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhee EJ, Kwon H, Park SE, et al. Associations among obesity degree, glycemic status, and risk of heart failure in 9,720,220 Korean adults. Diabetes Metab J. 2020;44:592–601. doi: 10.4093/dmj.2019.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marwitz SE, Gaines MV, Brady SM, et al. Cross-sectional and longitudinal examination of insulin sensitivity and secretion across puberty among non-hispanic black and white children. Endocrinol Metab (Seoul). 2020;35:847–57. doi: 10.3803/EnM.2020.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo JE, Shim JS, Lee H, Kim HC. Association between the thigh muscle and insulin resistance according to body mass index in middle-aged Korean adults. Diabetes Metab J. 2020;44:446–57. doi: 10.4093/dmj.2019.0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jung I, Kwon H, Park SE, et al. Increased risk of cardiovascular disease and mortality in patients with diabetes and coexisting depression: a nationwide population-based cohort study. Diabetes Metab J. 2021;45:379–89. doi: 10.4093/dmj.2020.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hedblad B, Nilsson P, Engström G, Berglund G, Janzon L. Insulin resistance in non-diabetic subjects is associated with increased incidence of myocardial infarction and death. Diabet Med. 2002;19:470–5. doi: 10.1046/j.1464-5491.2002.00719.x. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Ryu J, Ahn SY, et al. Association of insulin resistance with lower glomerular filtration rate and all-cause mortality in the Korean elderly population: a community-based prospective cohort study. Tohoku J Exp Med. 2013;231:271–9. doi: 10.1620/tjem.231.271. [DOI] [PubMed] [Google Scholar]
- 27.Ausk KJ, Boyko EJ, Ioannou GN. Insulin resistance predicts mortality in nondiabetic individuals in the U.S. Diabetes Care. 2010;33:1179–85. doi: 10.2337/dc09-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh P, Preiss D, Lloyd SM, et al. Contrasting associations of insulin resistance with diabetes, cardiovascular disease and all-cause mortality in the elderly: PROSPER long-term follow-up. Diabetologia. 2014;57:2513–20. doi: 10.1007/s00125-014-3383-9. [DOI] [PubMed] [Google Scholar]
- 29.Kim KS, Lee YM, Lee IK, Kim DJ, Jacobs DR, Jr, Lee DH. Paradoxical associations of insulin resistance with total and cardiovascular mortality in humans. J Gerontol A Biol Sci Med Sci. 2015;70:847–53. doi: 10.1093/gerona/glu194. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Li J, Zheng S, Luo Q, Zhou C, Wang C. Fasting insulin, insulin resistance, and risk of cardiovascular or all-cause mortality in non-diabetic adults: a meta-analysis. Biosci Rep. 2017;37(5). [DOI] [PMC free article] [PubMed]
- 31.Kang B, Yang Y, Lee EY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes (Lond) 2017;41:789–92. doi: 10.1038/ijo.2017.14. [DOI] [PubMed] [Google Scholar]
- 32.Lee SH, Kwon HS, Park YM, et al. Predicting the development of diabetes using the product of triglycerides and glucose: the Chungju Metabolic Disease Cohort (CMC) study. PLoS One. 2014;9:e90430. doi: 10.1371/journal.pone.0090430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambrinoudaki I, Kazani MV, Armeni E, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27:716–24. doi: 10.1016/j.hlc.2017.05.142. [DOI] [PubMed] [Google Scholar]
- 34.Park K, Ahn CW, Lee SB, et al. Elevated TyG index predicts progression of coronary artery calcification. Diabetes Care. 2019;42:1569–73. doi: 10.2337/dc18-1920. [DOI] [PubMed] [Google Scholar]
- 35.Unger G, Benozzi SF, Perruzza F, Pennacchiotti GL. Triglycerides and glucose index: a useful indicator of insulin resistance. Endocrinol Nutr. 2014;61:533–40. doi: 10.1016/j.endonu.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 36.Vasques AC, Novaes FS, de Oliveira MS, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 37.Lee SB, Kim MK, Kang S, et al. Triglyceride glucose index is superior to the homeostasis model assessment of insulin resistance for predicting nonalcoholic fatty liver disease in Korean adults. Endocrinol Metab (Seoul) 2019;34:179–86. doi: 10.3803/EnM.2019.34.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hwang YC, Ahn HY, Park SW, Park CY. Nonalcoholic fatty liver disease associates with increased overall mortality and death from cancer, cardiovascular disease, and liver disease in women but not men. Clin Gastroenterol Hepatol. 2018;16:1131–7. doi: 10.1016/j.cgh.2017.11.026. [DOI] [PubMed] [Google Scholar]