Abstract
Introduction
As part of the Centers for Medicare and Medicaid Innovation Practice Transformation Network, an integrated healthcare system implemented a multimodal, population health-based hypertension clinical pathway program (HCPP) focused on hypertension management.
Aim
To determine whether the HCPP was associated with changes in hypertension control or process-of-care measures and whether associations varied for sites serving higher versus lower proportions of historically underserved patients.
Setting
An integrated academic health system encompassing 5 clinic networks and 85 primary and specialty care sites.
Program Description
The HCPP was implemented at some sites (adopters) but not others (non-adopters) and had four components: (1) stakeholder engagement; (2) clinical staff retraining; (3) electronic health record-based prompts; and (4) performance monitoring and feedback. Program goals were to encourage clinical teams to increase the frequency of follow up visits and adopt standardized approaches to blood pressure (BP) measurements and antihypertensive medication regimen advancement defined as adding or titrating existing medication.
Program Evaluation
This quasi-experimental study used 2017–2019 data from 63,497 patients with hypertension and multivariable difference-in-differences analyses to evaluate changes in outcomes at 19 adopter versus 39 non-adopter sites before and after HCPP implementation. Adoption was associated with 3.5 times differentially greater odds of a BP reassessment (OR 3.5, 95% CI 3.3–3.8), 11% differentially greater odds of BP control (BP<140/90 mmHg) (OR 1.11, 95% CI 1.07–1.15), and 12% differentially greater odds of having non-severely elevated BP (systolic BP < 155 mmHg) (OR 1.12, 95% CI 1.05–1.19). HCPP adoption was not associated with differential changes in 90-day follow-up BP measurement. Adoption was associated with 23% differentially greater odds of appropriate medication advancement (OR 1.23, 95% CI 1.04–1.46). A similar pattern was observed when limiting comparisons to sites caring for a higher proportion of historically underserved populations.
Discussion
A multimodal population health approach to transforming hypertension care was associated with improved BP outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11606-022-07522-4.
KEY WORDS: hypertension, population health
Introduction
Hypertension remains the leading cause of death and disability-adjusted life years worldwide.1 Hypertension accounts for up to 35% of cases of cardiovascular disease2 and 34% of new cases of end-stage renal disease3 in the USA. Hypertension control has improved in the USA4 but despite these gains, hypertension control rates remain suboptimal.5 Although some health systems have achieved hypertension control for most of their patients,6–8 control is achieved in fewer than half of all patients nationwide.9
In 2018, the University of Washington (UW) Medicine health system implemented a multimodal Hypertension Clinical Pathway Program (HCPP) to improve hypertension control in the primary care setting. Its goal was to improve blood pressure (BP) control by improving BP assessment processes and management. Concurrent with the HCPP, UW Medicine implemented a health equity initiative to reduce disparities in healthcare delivery.
The aims of this study were to 1) evaluate the association between HCPP adoption and hypertension control and 2) examine whether associations varied for sites based on their proportions of historically underserved patients.
Methods
Data Source and Study Population
This was a retrospective quasi-experimental study using data from January 1, 2017, to March 31, 2019, from UW Medicine, which included 5 clinic networks and 85 primary care and specialty care sites collectively serving approximately 200,000 patients during the study period.
The study population included adults (age 18 to 85 years) with a hypertension diagnosis, defined as having two or more visits in the prior 3 years or active on the problem list for the following International Classification of Diseases (ICD)-9 codes: 401.0, 401.9, 402.00, 402.10, 402.90, 403.00, 403.10, 403.90, 404.00, 404.10, 404.90; ICD-10: I11.9, I10, I12.9, I13.10. Consistent with the 2019 National Committee for Quality Assurance Healthcare Effectiveness Data and Information Set specifications, hypertension control meant BP < 140/90 mmHg and elevated BP meant BP > 140/90 mmHg.
HCPP
A multidisciplinary team composed of physicians with clinic management roles, nurses, and a pharmacist developed the HCPP for primary care sites. Its process improvement components were as follows: 1) BP re-measurement under standard conditions if the initial office BP was elevated; and 2) office BP follow up of patients with elevated BP within a month. The HCPP had three clinical guideline elements: 1) setting a patient-specific BP target; 2) using a standard diagnostic and management protocol including lifestyle recommendations (delivered by goal-setting and verbal and written patient education materials); and 3)initiating and titrating antihypertensive medication in a standardized way.2 The HCPP employed a multimodal approach with four key components: 1) stakeholder engagement; 2) clinical staff retraining; 3) electronic health record (EHR)-based prompts; and 4) performance monitoring and feedback.
Stakeholder engagement included monthly sharing of data, best practices, and barrier identification with site leadership. Clinical staff retraining was conducted via webinars and face-to-face individual and small group interactions including competency validation. It included instruction in BP measurement, identification of patients whose BP was not controlled, and the application of the HCPP to specific patients. New EHR-based tools, including individual patient BP history, clinician action prompts, documentation templates with guidelines for lifestyle management and medication advancement and visual aids regarding application of the HCPP, were added in October 2018. A hypertension care dashboard was developed to allow clinicians to monitor their patients’ BP control and to provide feedback on BP control and HCPP process measures. Each site leader received monthly reports on its performance on key process and outcome measures. The HCPP was finalized in December 2017.
Study Periods and Groups
The HCPP was adopted in 19 primary care sites in two phases, with pre-HCPP and post-HCPP periods varying by phase. Phase 1 included 15 sites with a pre-HCPP period from January 2017 to December 2017, and a post-HCPP period from January 2018 to March 2019. Phase 2 included four sites and pre- and post-program periods of January 2017 to July 2018 and August 2018 to March 2019, respectively. Phase 2 sites served a higher proportion of patients from historically underserved groups, such as patients who were non-White, non-English speaking, or insured through Medicaid.
The post-HCPP period was divided into two sub-periods. First, there was an initial “post HCPP pilot” period during which adopter sites were trained in the HCPP and provided performance feedback (January to June 2018 for phase 1 adopter sites; August to October 2018 for phase 2 adopter sites). This was followed by a “post-HCPP maintenance” period without training but continued performance feedback (July 2018 to March 2019 for phase 1 adopter sites; November 2018 to March 2019 for phase 2 adopter sites). Over the study period, 39 sites that did not adopt the HCPP were defined as control, “non-adopter” sites.
Study Outcomes
We evaluated two process measures: 1) BP recheck—having BP re-measured on the same day after an elevated BP measurement; and 2) 90-day follow-up—having at least one BP measurement at the same site within 90 days of a confirmed elevated BP. We evaluated two outcome measures: 1) BP controlled (BP < 140/90 mmHg); and 2) non-severely elevated BP (systolic BP < 155 mmHg). This latter measure identified individuals with moderate to severe hypertension that was not associated with acute complications.
We included medication regimen advancement as an exploratory outcome, defined as a change or addition of drug class or an increase in daily dose within 90 days of an elevated BP among patients with medication information. Data on prescribed drug class, order duration, and daily dose was obtained from the EHR. In order to have medication data 6 months prior to and 6 months after the index date for uncontrolled BP, medication regimen advancement was examined only among patients from the phase 1 adopter and non-adopter sites.
Statistical Analysis
We examined the unadjusted temporal trends in each outcome of interest separately in patients from phase 1 adopter, phase 2 adopter, and non-adopter sites. In our main analysis, we then estimated the association between HCPP adoption and study outcomes using a difference-in-differences (DID) analytic approach.
We used multivariate logistic regression models to examine the parallel trend assumptions in outcomes during the pre-HCPP period, comparing phase 1 and phase 2 adopters separately with non-adopters. We estimated an interaction term between linear month and group status (phase 1 adopters vs. non-adopters; phase 2 adopters vs. non-adopters), allowing for a different intercept in sites that received the intervention while controlling for age, sex, race, English as preferred language, insurance, and comorbidities (Table 1). We used cluster-corrected standard errors to account for repeated visits per patient.
Table 1.
Patient Characteristics at Non-Adopter (Control) and Phase 1 Adopter Sites in the Pre- and Post-HCPP Adoption Periods
| Factor | Pre-period (Jan 2017–Dec 2017) |
Post-period: pilot (Jan–Jun 2018) |
Post-period: maintenance (Jul 2018–Mar 2019) |
|||
|---|---|---|---|---|---|---|
| Non-adopters | Phase 1 adopters | Non-adopters | Phase 1 adopters | Non-adopters | Phase 1 adopters | |
| N | 10240 | 28413 | 7954 | 23510 | 8914 | 26484 |
| Age, mean (SDa) | 66.33 (14.68) | 60.49 (14.62) | 67.17 (14.63) | 61.43 (14.50) | 67.17 (14.50) | 61.50 (14.39) |
| Male | 5221 (51.0%) | 14628 (51.5%) | 4000 (50.3%) | 12024 (51.1%) | 4529 (50.8%) | 13701 (51.7%) |
| Racial groups | ||||||
| White non-Hispanic | 8028 (78.4%) | 20180 (71.0%) | 6210 (78.1%) | 16753 (71.3%) | 6856 (76.9%) | 18575 (70.1%) |
| African American or Black | 470 (4.6%) | 1726 (6.1%) | 359 (4.5%) | 1393 (5.9%) | 457 (5.1%) | 1575 (5.9%) |
| American Indian or Alaska Native | 81 (0.8%) | 237 (0.8%) | 55 (0.7%) | 196 (0.8%) | 68 (0.8%) | 219 (0.8%) |
| Asian | 989 (9.7%) | 2958 (10.4%) | 769 (9.7%) | 2394 (10.2%) | 875 (9.8%) | 2728 (10.3%) |
| Multiple races | 68 (0.7%) | 210 (0.7%) | 65 (0.8%) | 184 (0.8%) | 69 (0.8%) | 199 (0.8%) |
| Native Hawaiian or Other Pacific Islander | 50 (0.5%) | 301 (1.1%) | 51 (0.6%) | 254 (1.1%) | 59 (0.7%) | 261 (1.0%) |
| Unknown | 323 (3.2%) | 2234 (7.9%) | 245 (3.1%) | 1832 (7.8%) | 319 (3.6%) | 2404 (9.1%) |
| White Hispanic | 231 (2.3%) | 567 (2.0%) | 200 (2.5%) | 504 (2.1%) | 211 (2.4%) | 523 (2.0%) |
| English as preferred language | 9582 (93.7%) | 27002 (95.1%) | 7443 (93.6%) | 22309 (94.9%) | 8302 (93.2%) | 25170 (95.0%) |
| Primary Insurance | ||||||
| Commercial | 3129 (30.6%) | 13171 (46.4%) | 2317 (29.2%) | 10447 (44.5%) | 2700 (30.4%) | 12282 (46.5%) |
| Medicaid | 769 (7.5%) | 2357 (8.3%) | 592 (7.5%) | 2037 (8.7%) | 697 (7.8%) | 2210 (8.4%) |
| Medicare | 6099 (59.7%) | 12497 (44.1%) | 4867 (61.3%) | 10700 (45.6%) | 5283 (59.5%) | 11629 (44.0%) |
| Other | 22 (0.2%) | 27 (0.1%) | 13 (0.2%) | 17 (0.1%) | 11 (0.1%) | 17 (0.1%) |
| Self-Pay | 126 (1.2%) | 145 (0.5%) | 97 (1.2%) | 122 (0.5%) | 135 (1.5%) | 127 (0.5%) |
| Tricare | 7 (0.1%) | 39 (0.1%) | 11 (0.1%) | 43 (0.2%) | 7 (0.1%) | 42 (0.2%) |
| Worker's comp | 57 (0.6%) | 120 (0.4%) | 42 (0.5%) | 99 (0.4%) | 50 (0.6%) | 106 (0.4%) |
| Diabetes | 2784 (27.2%) | 7555 (26.6%) | 2213 (27.8%) | 6714 (28.6%) | 2466 (27.7%) | 7097 (26.8%) |
| Heart failure | 1016 (9.9%) | 1772 (6.2%) | 803 (10.1%) | 1571 (6.7%) | 885 (9.9%) | 1602 (6.0%) |
| Ischemic vascular disease or myocardial infarction | 3054 (29.8%) | 4839 (17.0%) | 2425 (30.5%) | 4288 (18.2%) | 2660 (29.8%) | 4484 (16.9%) |
| Cerebrovascular disease | 1567 (15.3%) | 2452 (8.6%) | 1226 (15.4%) | 2115 (9.0%) | 1321 (14.8%) | 2224 (8.4%) |
| Renal disease | 1796 (17.5%) | 2858 (10.1%) | 1469 (18.5%) | 2546 (10.8%) | 1559 (17.5%) | 2622 (9.9%) |
| End-stage renal disease | 232 (2.3%) | 326 (1.1%) | 197 (2.5%) | 283 (1.2%) | 202 (2.3%) | 282 (1.1%) |
aSD, Standard deviation
In our main analysis, we estimated multivariate logistic DID regression models for each outcome, adjusting for age, sex, race, English as preferred language, insurance, time in months, and comorbidities, and again cluster-corrected standard errors to account for repeated visits per patient. We adjusted for the social construct of race10,11 because of persistent disparities in healthcare access, hypertension prevalence, control, and outcomes in racial/ethnic minorities relative to non-Hispanic Whites. We controlled for English language12 because communication barriers and treatment biases may differentially impact access to care and hypertension control of patients with limited English proficiency. Our DID estimator was the interaction between study period indicator (pre- versus post-HCPP) and group status (phase 1 adopter vs. control). We repeated these analyses comparing phase 2 adopter versus non-adopter sites. As robustness checks, we applied DID models for phase 1 adopter sites that compared the pre-HCPP period to each post-HCPP subperiod separately.
As an exploratory analysis, we examined the association between HCPP adoption and 90-day medication regimen advancement using a pre-post analytic design. For the phase 1 adopter and non-adopter sites, we defined a “pre” cohort of patients diagnosed with hypertension as of July 1, 2017, that had at least one elevated BP from July 1, 2017, to Dec 31, 2017. The “post” cohort were patients with hypertension as of July 1, 2018, and had at least one elevated BP from July 1, 2018, to Dec 31, 2018. For each patient in the pre- or post-cohort, we defined an index date as the date of first elevated BP within the respective study periods and included all medication data 6 months prior to and 6 months after the index date to estimate medication regimen advancement. We used a multivariate logistic regression model with 90-day medication regimen advancement as the dependent variable, adjusting for age, sex, race, English as preferred language, insurance, and comorbidities. Our estimator consisted of the interaction between pre- vs. post period indicator and group status (phase 1 adopter vs. non-adopter sites), the coefficient of which reflects the magnitude of association between the HCPP and the outcome.
We reported results as means with standard deviations or frequencies with percentages. For two-group comparisons, chi-square tests were used for categorical variables and t-tests were used for continuous variables. Statistical tests were two-sided with statistical significance set to 0.05. All analyses were performed in STATA (version 14, StataCorp LP, College Station, TX, USA). The University of Washington institutional review board deemed this study to be “minimal risk” (IRB # STUDY00007194).
Results
Our sample included 63,497 patients with hypertension from 58 sites. Compared to non-adopter sites, phase 1 adopters served patients who were younger, more frequently non-White, preferred a language that was not English for communication, and had commercial insurance (Table 1). They had fewer comorbidities, such as heart failure, ischemic vascular diseases, metastatic cancer, cerebrovascular, or renal disease. These patterns were consistent across study periods.
Compared to non-adopter sites, phase 2 adopters served patients who were younger, more often male and more often non-White, preferred non-English for communication, had Medicaid as primary insurance, and had more comorbidities (eTable 1 in the Appendix). These differences in patient characteristics were consistent across study periods.
Across study periods, phase 2 adopter sites served patients who were more often male, non-White, preferred a language other than English for communication, had Medicaid insurance, and had more comorbidities, as compared to patients served at phase 1 adopter sites (eTable 1 in the Appendix).
Table 2 shows unadjusted outcomes before and after HCPP adoption among patients from the phase 1 adopter, phase 2 adopter, and non-adopter sites. Among phase 1 adopter sites, BP recheck rate increased from a mean of 25% in the pre-adoption period to 51% in the post-period. BP control increased from 60 to 66%, and non-severely elevated BP increased from 84 to 86%. Similar magnitude of increases occurred among phase 2 adopter sites after HCPP adoption.
Table 2.
Unadjusted Outcomes in Patients Before and After the HCPP Adoption at Phase 1 Adopter, Phase 2 Adopter, and Non-Adopter Sites
| Phase 1 adopter vs. non-adopter | Phase 2 adopter vs. non-adopter | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Phase 1 adopter | Non-adopter | Phase 2 adopter | Non-adopter | |||||||
| Pre period | Post period | Pre period | Post Period | Pre period | Post Period | Pre period | Post Period | |||
| Entire period | Pilot | Main-tenance | ||||||||
| Process measures | ||||||||||
| BP rechecka | 24.9% | 51.1% | 39.4% | 59.5% | 16.0% | 17.7% | 11.6% | 34.8% | 15.9% | 19.5% |
| 90-day follow-upb | 68.2% | 65.4% | 70.1% | 61.8% | 67% | 63% | 80% | 72% | 67% | 59% |
| Clinical outcomes | ||||||||||
| BP control | 59.9% | 65.6% | 64.0% | 66.8% | 65.8% | 68.9% | 60.3% | 65.2% | 66.8% | 69.2% |
| Non-severely elevated BP | 84.3% | 86.3% | 85.7% | 86.8% | 89.9% | 90.4% | 83.6% | 85.4% | 90.1% | 90.4% |
| Exploratory outcome | ||||||||||
| Medication advancementc | 28.6% | 32.5% | 22.8% | 23.0% | ||||||
aBP recheck, same-day blood pressure (BP) recheck among those with an elevated BP measurement
bAt least one BP measurement in the same site within 90 days of a confirmed elevated BP; the post-period ends in December 2018 for this outcome to allow for a 90-day follow-up period.
cOnly evaluated among the subset of patients with medication data within a year before and after the adoption of the HCPP
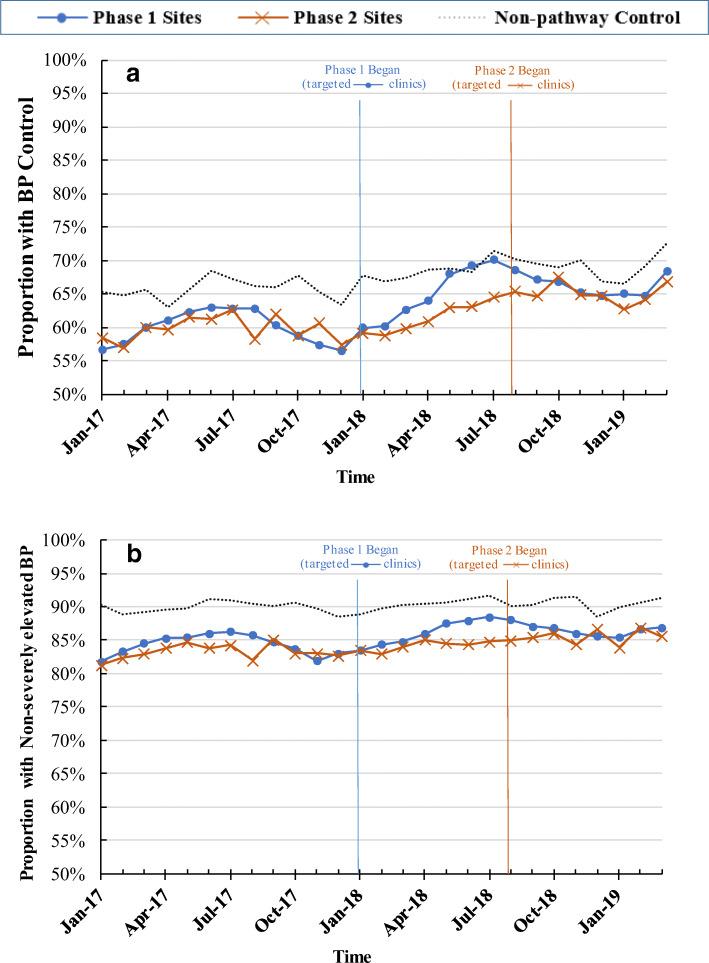
In adjusted analyses, changes were smaller among the non-adopter sites: BP recheck rate increased from 16 to 18%, BP control from 66 to 69%, and non-severely elevated BP remained at approximately 90%. The proportion with 90-day follow up BP measurement decreased over time in all three groups. Figures 1 and 2 show the trends in process and outcome measures by study group. There was no evidence of divergent trends in outcomes in the pre-HCPP period by study group (eTable 1 in the Appendix).
Figure 1.
Process measures over time in the phase 1 adopter, phase 2 adopter, and non-adopter sites. a Proportion with BP recheck; b proportion with 90-day follow-up. Vertical lines indicate timing of HCPP introduction in each phase.
Figure 2.
Clinical outcomes over time in the phase 1 adopter, phase 2 adopter, and non-adopter sites. a Proportion with BP control; b proportion with non-severely elevated BP (systolic BP < 155 mm Hg). Vertical lines indicate timing of HCPP introduction in each phase.
In adjusted analyses among phase 1 adopter sites, HCPP adoption was associated with a 3.5-fold differentially greater odds of BP recheck among patients with an elevated BP (OR 3.5; 95% CI 3.30–3.76), an 11% differentially greater odds of BP control (OR 1.11; 95% CI 1.07–1.15) and 12% differentially greater odds of having non-severely elevated BP (OR 1.12; 95% CI 1.05–1.19) (Table 3). There was no significant association between HCPP adoption and differential changes in 90-day follow-up among patients with elevated BP (OR 1.06; 95% 1.00–1.13).
Table 3.
Adjusted odds ratios of outcomes in the phase 1 adopter sites after the HCPP was adopted, accounting for secular trend of non-adopter sites (difference-in-difference analysis)
| Entire post-period (Jan 2018–Mar 2019) | Post-period “pilot” (Jan–Jun 2018) | Post-period “maintenance” (Jul 2018–Mar 2019) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Na | aORb (95% CI) | p value | N | aOR (95% CI) | p value | N | aOR (95% CI) | p value | |
| Process measures | |||||||||
| BP recheckc | 29,994 | 3.52 (3.3–3.76) | <0.001 | 18,529 | 2.43 (2.23–2.63) | <0.001 | 22,648 | 4.7 (4.36–5.06) | <0.001 |
| 90-day follow-upd | 33,997 | 1.06 (1–1.13) | 0.07 | 20,750 | 1.06 (0.98–1.14) | 0.1 | 24,482 | 1.06 (0.98–1.15) | 0.1 |
| Clinical outcomes: | |||||||||
| BP control | 40,688 | 1.11 (1.07–1.15) | <0.001 | 31,464 | 1.08 (1.03–1.13) | <0.001 | 35,398 | 1.14 (1.08–1.19) | <0.001 |
| Non-severely elevated BP | 40,688 | 1.12 (1.05–1.19) | <0.001 | 31,464 | 1.09 (1.01–1.17) | 0.03 | 35,398 | 1.14 (1.06–1.23) | <0.001 |
aNumber of patients
baOR, adjusted odds ratios, determined from coefficient of interaction term (pathway status × post-period) with 3 post HCPP periods evaluated relative to pre-period: post-HCPP pilot period (6 months after HCPP adoption), maintenance period (7–15 months after HCPP adoption), and pilot and maintenance period combined (entire period)
cBP recheck, same-day blood pressure (BP) recheck among those with an elevated BP measurement
dAt least one BP measurement in the same site within 90 days of a confirmed elevated BP; the post-period ends in December 2018 for this outcome to allow for a 90-day follow-up period
*Only evaluated among the subset of patients with medication data within a year before and after the adoption of the HCPP
There were stronger associations between HCPP adoption and BP recheck in the post-HCPP maintenance period (OR 4.70; 95% CI 4.36–5.06) than in the post-HCPP pilot period (OR 2.43; 95% CI 2.23–2.63) (Table 3). OR of BP control was slightly higher in the post-HCPP maintenance period (OR 1.14, 95% CI 1.08–1.19) than in the post-HCPP pilot period (OR 1.08; 95% CI 1.03–1.13). Associations were also stronger between HCPP adoption and non-severely elevated BP in the post-HCPP maintenance period (OR 1.14; 95% CI 1.06–1.23) than in the post-HCPP pilot period (OR 1.09; 95% CI 1.01–1.17).
In adjusted analyses among phase 2 adopter sites, HCPP adoption was associated with a 12% greater odds of BP control (OR 1.12; 95% CI 1.04–1.20) and 11% greater in odds of having non-severely elevated BP (OR 1.11; 95% CI 1.02–1.22) (eTable 1 in the Appendix). HCPP adoption was also associated with 3.56 times differentially greater odds of BP recheck (OR 3.56; 95% CI 3.21–3.96) but not with differential change in 90-day follow up (OR 1.02; 95% CI 0.92–1.14).
In an exploratory analysis among the subset of patients with an elevated BP and medication data (n=16,504), HCPP adoption was associated with a 23% differentially higher odds of a medication regimen advancement in the post-period (OR 1.23; 95% CI 1.04–1.46).
Discussion
We found associations between an HCPP emphasizing standard BP measurement, follow-up, and medication protocols and several BP-related measures, including BP measurement, medication regimen advancement, and hypertension control. Within 6 months of HCPP adoption, phase 1 sites demonstrated differentially greater BP recheck, hypertension control, and rate of non-severely elevated BP among hypertensive patients, compared to non-adopters. Associations were stronger for BP recheck during a subsequent post-HCPP maintenance period. We found no significant association between HCPP and the rate of follow-up BP measurement at 90 days. We observed similar findings in the phase 2 sites despite differences in patient characteristics and comorbidities, demonstrating the effectiveness of the HCPP among sites that served more individuals from historically underserved populations.
The improvement in hypertension control could be partially due to more accurate BP measurement and improvement in lifestyle management. The increase in medication regimen advancement associated with our HCPP likely contributed to improved BP control.
We did not observe an association between the HCPP and 90-day follow-up BP measurement. This measure may take longer to impact: potential barriers to a follow-up BP measurement may include patient factors and site capacity and logistics. Patients often declined follow-up care at the recommended interval. During our study period, we did not capture BP follow-up carried out via phone calls or virtual care and home-based BP measurements. Thus, our 90-day BP follow-up rate may underestimate clinical and site intentions to schedule follow-up, as well as the actual follow-up rate.
Observed changes in BP recheck may be related to our approach to intervention design, which involved principles of team-based care as recommended strategies to improve hypertension care.2,13 Anecdotally, we observed that changes were greater for sites where medical assistants took greater ownership of the process improvement measures—evidence that a team-based approach may have engaged more team members in HCPP changes. Our findings were consistent with experiences from other health systems.7
We demonstrated the feasibility of the HCPP among sites that served a higher proportion of patients who were historically underserved (individuals who identified as non-White, preferred to use non-English for medical communications, or insured through Medicaid). We found similar associations between the HCPP with the main study outcomes in these sites. This is likely attributable to the collaborative nature and flexibility of the HCPP at the site level. Given the persistent disparities in hypertension control and its associated health outcomes across racial and socio-economic groups, future studies should examine the potential disparate impacts of the HCPP in sub-populations and identify strategies to improve equity in hypertension management.
Clinician feedback indicated that the ability to immediately see historical BP data via an EHR-based tool and to rapidly access the HCPP documents was more important than visual cues such as best practice alerts in promoting pathway concordant care. However, our study demonstrated that devoting time to provider engagement and training was necessary to achieve positive results. Merely creating additional EHR tools did not lead to improved BP control within the population.
Our study has some limitations. First, adoption statuses were not randomly assigned but rather determined by the sites’ choice. Therefore, unobserved differences between adopters and non-adopters may underlie between-group differences in baseline BP control. However, we used analytical methods (difference-in-difference) that accounted for these baseline differences. Second, although the HCPP was designed and implemented in a standardized way, variations in timing and thoroughness of adoption by sites did occur. Third, we could not determine relative contributions from specific components of the multi-modal HCPP to changes in study outcomes. For instance, our intervention improved BP measurement accuracy, but its contribution to better BP control is not clear. BP recheck has been found in other studies to be positively associated with BP control.14 Further research is needed to determine the most impactful elements of the HCPP. Fourth, because all clinic sites were part of a single health care system, contamination from an adopter site to a non-adopter site may have occurred, perhaps blunting any difference that otherwise would have occurred. There were overall trends toward increased BP control in all groups and yet despite that, we observed differentially greater increases in intervention group. Fifth, BP is a continuous measure and dichotomizing involved some arbitrariness. However, we chose widely used thresholds to enable consistency and comparison to other studies. Last, our hypertension definition excluded individuals with undiagnosed or uncoded hypertension, and those with fewer than two visits in the prior 3 years. It is possible that underserved populations may be disproportionately affected. Future study should examine the impact of applying a different hypertension definition.
Conclusions
Our evaluation showed that, in an integrated healthcare system with widely dispersed primary care sites, a multimodal population health–based pathway program was associated with increased BP recheck rate, improved hypertension control, and appropriate antihypertensive medication regimen advancement. It had similar effects among sites that served a higher proportion of historically underserved patients. Further study is needed to understand the durability of the effects, program scalability, and the costs and feasibility of replicating it in different settings.
Supplementary Information
(DOCX 216 kb)
Author Contribution
None
Funding
The project described was supported by Funding Opportunity Number CMS-331-44-501 from the U.S. Department of Health & Human Services, Centers for Medicare & Medicaid Services. This funding was part of the Transforming Clinical Practice Initiative, authorized under and Social Security Act 1115(A) and in support of the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) to strengthen the quality of patient care and spend health care dollars more wisely.
Declarations
Conflict of Interest
The authors declare no competing interests.
Disclaimer
The contents provided are solely the responsibility of the authors and do not necessarily represent the official views of the U.S. Department of Health & Human Services or any of its agencies.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Forouzanfar MH, Liu P, Roth GA, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317(2):165–182. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high BP in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 3.Saran R, Li Y, Robinson B, et al. US Renal Data System 2014 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2015;66 Svii:S1–305. doi: 10.1053/j.ajkd.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010;303(20):2043–2050. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 5.Muntner P, Hardy ST, Fine LJ, et al. Trends in blood pressure control among US adults with hypertension, 1999-2000 to 2017-2018. JAMA. 2020;324(12):1190–1200. doi: 10.1001/jama.2020.14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fletcher RD, Amdur RL, Kolodner R, et al. Blood pressure control among US veterans: A large multiyear analysis of blood pressure data from the Veterans Administration Health Data Repository. Circulation. 2012;125:2462–2468. doi: 10.1161/CIRCULATIONAHA.111.029983. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe MG, Young JD. The Kaiser Permanente northern California story: Improving hypertension control from 44% to 90% in 13 years (2000 to 2013) J Clin Hypertens. 2016;18(4):260–261. doi: 10.1111/jch.12803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson GG, Pu J, Magid DJ, et al. Effect of the Million Hearts Cardiovascular Disease Risk Reduction Model on initiating and intensifying medications: A prespecified secondary analysis of a randomized clinical trial. JAMA Cardiol. 2021 Sep 1;6(9):1050-1059. 10.1001/jamacardio.2021.1565. [DOI] [PMC free article] [PubMed]
- 9.Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon-Moran D. Hypertension prevalence and control among adults: United States, 2015–2016. National Center Health Statistics Data Brief 2017 Oct;(289):1-8. [PubMed]
- 10.Deere BP, Ferdinand KC. Hypertension and race/ethnicity. Curr Opin Cardiol. 2020;35(4):342–350. doi: 10.1097/HCO.0000000000000742. [DOI] [PubMed] [Google Scholar]
- 11.Egan BM, Li J, Sutherland SE, Rakotz MK, Wozniak GD. Hypertension control in the United States 2009 to 2018: Factors underlying falling control rates during 2015 to 2018 across age- and race-ethnicity groups. Hypertension. 2021;78:578–587. 10.1161/HYPERTENSIONAHA.120.16418. [DOI] [PubMed]
- 12.Kim EJ, Kim T, Paasche-Orlow MK, Rose AJ, Hanchate AD. Disparities in hypertension associated with limited English proficiency. J Gen Intern Med. 2017;32(6):632–639. doi: 10.1007/s11606-017-3999-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention . Hypertension Control Change Package. 2. Atlanta, GA: Centers for Disease Control and Prevention, U.S. Department of Health and Human Services; 2020. [Google Scholar]
- 14.Einstadter D, Bolen SD, Misak JE, Bar-Shain DS, Cebul RD. Association of repeated measurements with blood pressure control in primary care. JAMA Intern Med. 2018;178(6):858–860. doi: 10.1001/jamainternmed.2018.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 216 kb)