ABSTRACT.
It is important to study the recent malaria incidence trends in urban areas resulting from rapid urbanization that can lead to changes in environmental conditions for malaria. This retrospective study assessed trends in malaria patients, their distribution according to parasite species, patient demographics, and weather data for the past 8 years at a malaria clinic in the National Institute of Malaria Research, New Delhi, India. We overlaid the effects of environmental factors such as rainfall, relative humidity, and temperature on malaria incidence. The malaria data were digitized for a period spanning 2012 to 2019, during which 36,892 patients with fever attended the clinic. Of these, 865 (2.3%) were diagnosed with malaria microscopically. Plasmodium vivax was predominant (96.2%), and very few patients were of Plasmodium falciparum (3.5%) or mixed infections (0.3%). The patients with malaria were within a 10-km radius of the clinic. Males (70.9%) were more commonly affected than females (29.1%). Of the total malaria patients, a majority (∼78%) belonged to the > 15-year age group. A total of 593 malaria patients (68.6%) received primaquine. These patients were most commonly diagnosed in April through October. Furthermore, there was a lag of 1 month between the rainfall peak and the malaria case peak. The peak in malaria cases corresponded to a mean temperature of 25 to 30°C and a relative humidity of 60% to 80%. This analysis will be useful for policymakers in evaluating current interventions and in accelerating malaria control further in urban areas of India.
INTRODUCTION
Malaria, a vector-borne disease, is a public health problem in developing countries, predominantly affecting the African and Asian regions.1 In South Asia, India has witnessed a decreasing trend in malaria in the past few decades.1 However, it still contributes significantly to morbidity and mortality in parts of India (https://nvbdcp.gov.in/), where ∼80% of reported malaria patients are from hilly, tribal, and difficult-to-access areas.3,4 In 2019, ∼340,000 cases of malaria were reported in the country.2 In India, malaria is commonly caused by either Plasmodium falciparum or Plasmodium vivax, and other species involved can be Plasmodium malariae, Plasmodium knowlesi, and Plasmodium ovale.5 In 2019, 63.1% of malaria cases were the result of P. falciparum and 36.9% were the result of P. vivax.2
It is important to study recent malaria trends in urban areas of India because the country is targeting elimination by 2030 and different timelines are set for each state based on the epidemiological situation and level of malaria transmission. Under the National Framework for Malaria Elimination, National Center for Vector Borne Disease Control (NCVBDC) guidelines, Delhi being a low malaria transmission area, falls under category 1 states/union territories, and the target was to eliminate malaria by 2022.2 In the National Institute of Malaria Research, a malaria clinic has been in operation since 2010 where walk-in febrile patients are tested for malaria by microscopy using thin and thick blood smears. After setting up the clinic, home visits and fever surveillance was done in surrounding areas so that the local population become aware of the clinic. The details of the patients with positive malaria infection are sent through e-mail to the district malaria officer. Furthermore, the patients testing positive for malaria are provided treatment per the national drug policy issued by the NCVBDC for malaria. We analyzed clinic data for 2012 to 2019. Our analysis will be useful for policymakers in accelerating further the elimination efforts to combat malaria in urban areas.
METHODS
Study area and design.
The study was conducted at the malaria clinic located in the National Institute of Malaria Research, Dwarka, New Delhi, in northern India. The target population comprised malaria-suspected participants attending the clinic from 2012 to 2019. The clinic was attended primarily by patients from nearby areas. Also, patients referred from nearby hospitals attended the clinic for confirmation of malaria diagnosis.
Data collection.
Malaria diagnosis was done as per WHO protocol using microscopic examination of peripheral blood smear slides. The variables included in the study were the date of the patient visit, method of diagnosis, species of parasite, age, gender, glucose-6-phosphate dehydrogenase (G6PD) assessment, and treatment received. The data were collected and entered by trained personnel, including a WHO level 1 certified malaria microscopist. Standard operating procedures for sample collection, smear preparation, and examination of slides were followed.6 At the study site, a blood smear was considered positive if one or more malaria parasites were seen, and negative if none were found in 200 fields. After blood examination, any individuals found positive for malaria were provided treatment according to national guidelines. Furthermore, on the day of positive diagnosis, G6PD testing was done using the dichlorophenol indophenol dye decolorization (semiquantitative) method.7 G6PD testing was not done in children younger than 5 years, nor was it done if patients were unwilling to undergo G6PD testing. Patients with a normal G6PD level were provided primaquine in a dose of 0.25 mg/kg in a follow-up visit on the third day. Primaquine was given as a single dose (0.75 mg/kg body weight) for P. falciparum, and a 14-day course (0.25 mg/kg/day) was prescribed for P. vivax infection. Patients were counseled regarding the importance of completing the course of primaquine. The medication was not changed for the patients who were taking medications from outside or those attending the clinic for malaria confirmation only.
Rainfall data were acquired from the Ministry of Earth Sciences, India Meteorological Department released under the National Data Sharing and Accessibility Policy. The humidity and temperature data were obtained from the National Aeronautics and Space Administration Langley Research Center Prediction of Worldwide Energy Resource Project funded through the NASA Earth Science/Applied Science Program.
Data analysis.
After entering the data in Microsoft Office Excel worksheets (version 16.65 Microsoft Corp., Redmond, WA), data analysis was done using the same software. Descriptive statistics for showing the trends according to year, gender, species, and season were used. Line graphs were used to depict the trend over the years. Seasons were categorized as summer (March, April, and May), monsoon (June, July, August, and September), and winter (October, November, December, January, and February).
RESULTS
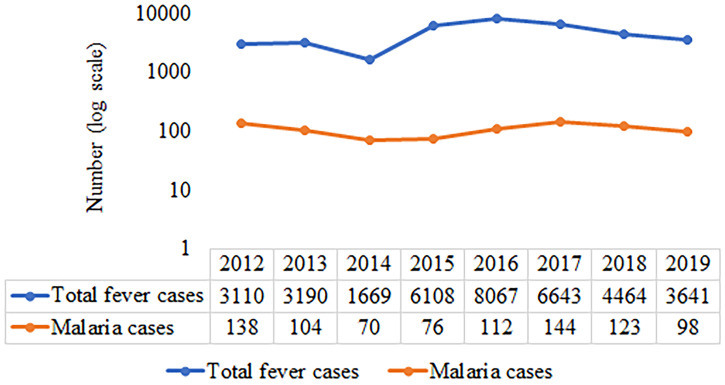
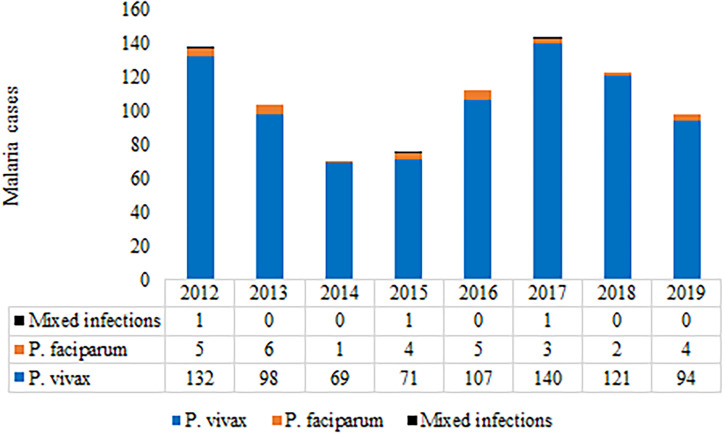
From 2012 to 2019, 36,892 patients with fever attended the clinic, of whom 865 (2.3%) were diagnosed microscopically as malaria positive, and all patients were reported from localities falling within the 10-km radius of the Institute and are thus Delhi patients. During the past decade, there was significant variation in the annual number of malaria patients reported, with 70 being the minimum in 2014 and 144 being the maximum reported in 2017 (Figure 1). Plasmodium vivax was predominant (n = 832), with very few cases (n = 30) reported of P. falciparum (Figure 2) and three cases of mixed infections, one each in 2012, 2015, and 2017. Of 865 patients with malaria, G6PD assessment was done in 808 patients (93.4%) using dichlorophenol indophenol, a semiquantitative method, and six male patients were found to be G6PD deficient (0.7%).
Figure 1.
Annual trend in total fever and malaria patients from 2012 to 2019. This figure appears in color at www.ajtmh.org.
Figure 2.
Species trends from 2012 to 2019. P. = Plasmodium. This figure appears in color at www.ajtmh.org.
Per the malaria clinic record review, males (70.9%) were found to be infected with malaria more commonly than females (29.1%) (Table 1). Of the total malaria patients, a majority (∼78%) belonged to the > 15-year age group. The prevalence was 6.7%, and 15.1% among those younger than 5 years and 5 to 15 years respectively.
Table 1.
Distribution of malaria positivity by age and gender
| Age group, years | Male | Female | Total, n | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| < 5 | 32 | 57.1 | 26 | 42.9 | 58 |
| 5–15 | 75 | 57.3 | 56 | 42.7 | 131 |
| > 15 | 506 | 74.8 | 170 | 25.2 | 676 |
Overall, primaquine was given to 593 patients with malaria (68.6%): a 14-day course to 574 patients (68.9%) with P. vivax infection and to two patients (67%) with mixed infection; a single dose was given to 17 patients (56.7%) with P. falciparum infection. Primaquine was not prescribed to 38 patients (4.4%) because 14 were pregnant, 17 were anemic, 6 were G6PD deficient, and 1 woman was lactating. A total of 234 patients (27.0%) did not return for follow-up visits for primaquine despite contacting them by phone. For patients with P. vivax infection (n = 832), chloroquine was prescribed to the majority of them (n = 809), two were given artemisinin-based combination therapy, nine were referred to the hospital, and information on treatment was not available for 13 patients. For patients with P. falciparum infection (n = 30), artemisinin-based combination therapy was prescribed to the majority of them (24 patients were given artesunate–sulfadoxine–pyrimethamine, and three patients were provided with artemether–lumefantrine), and information on treatment was not available for 3 patients.
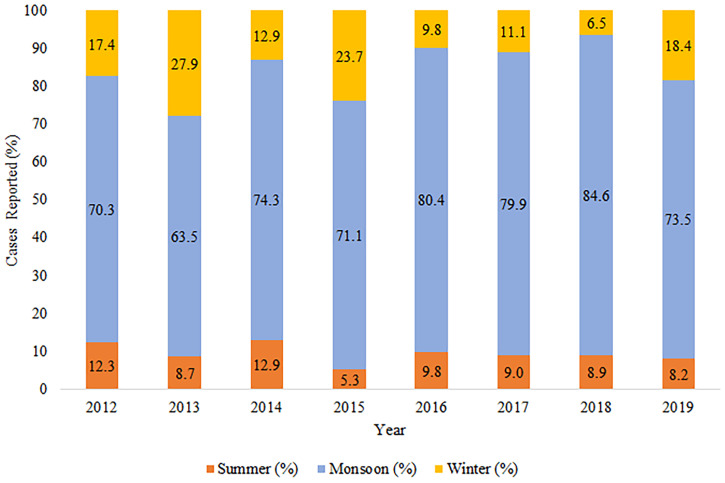
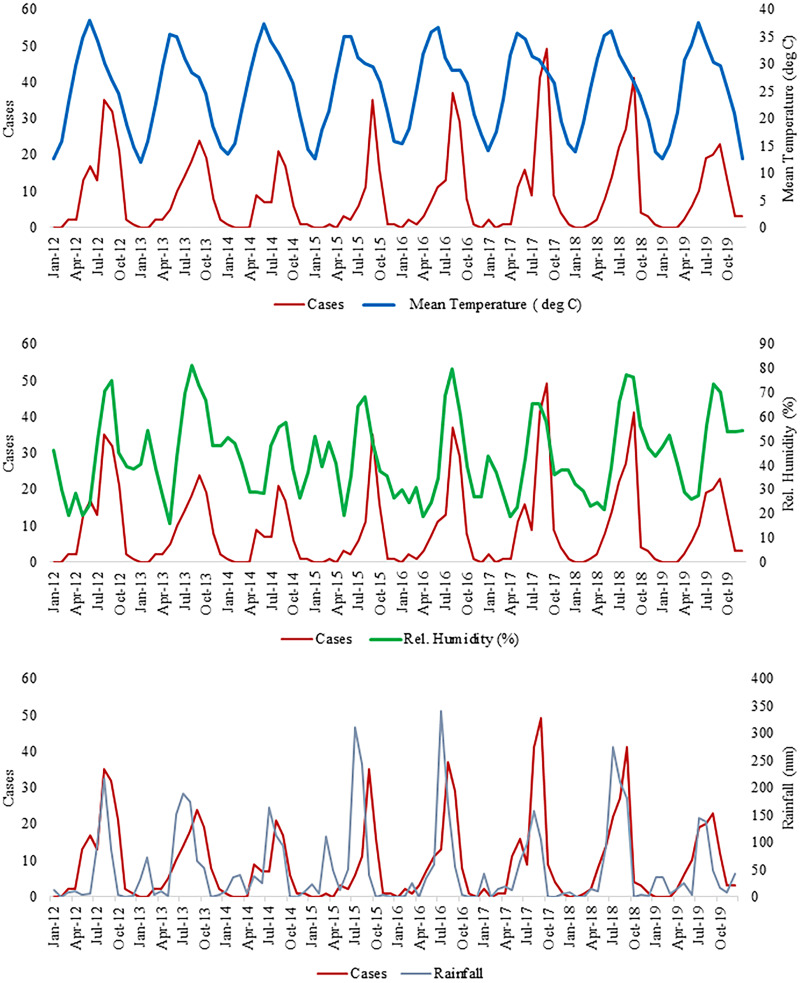
During the study period, there was a significant seasonal trend in the occurrence of malaria, and the maximum number of cases was reported during the monsoon season (Figures 3 and 4). However, malaria cases were reported throughout the year and in other seasons as well. Figure 4 depicts the relation between rainfall and malaria cases. The peak in malaria cases followed the peak in rainfall, with a lag of almost 1 month. The variation in the occurrence of malaria corresponded to changes in relative humidity, and the peak in malaria cases corresponded to a mean temperature range of 25 to 30°C (Figure 4).
Figure 3.
Seasonal distribution of malaria from 2012 to 2019. This figure appears in color at www.ajtmh.org.
Figure 4.
Annual trend in mean temperature, relative humidity, and rainfall with number of malaria patients. Rel. = relative. This figure appears in color at www.ajtmh.org.
DISCUSSION
Over an 8-year study period, we were able to assess the epidemiological trends in the occurrence of malaria, and their distribution according to parasite species, patient demographics, and weather data. Overall, 36,892 blood slides were examined, and 865 malaria cases were reported, with a slide positivity rate of 2.34%. Despite the fluctuating trend in malaria cases, ∼70 to 144 patients with patients were reported annually in this study. In 2014, the number of patients with malaria was the least, and this finding is reported in another study from Delhi as well.8 From 2015 to 2019, there was a significant number of patients with dengue in Delhi, reflected in the increased number of patients reporting fever.2 Plasmodium vivax accounted for the majority of the patients (> 95%). In India, P. vivax is mainly responsible for urban malaria, and the percentage of cases attributed to P. vivax has increased in urban areas.9 The increase in malaria cases in urban areas is mainly attributed to uncontrolled urbanization, new construction sites, and a migratory population.10,11 Thus, it is important to target interventions for controlling the rise in P. vivax cases as this is also associated with severe malaria.12–14 Easier availability of rapid diagnostic test kits through pharmacies could aid in the early diagnosis of malaria and the timely initiation of treatment, thus preventing complications.15 However, it is prudent to confirm such patients with conventional microscopy to avoid false-positive results. Furthermore, different surveillance mechanisms per the transmission dynamics of malaria that cover high-risk populations such as migrants could accelerate malaria elimination.16 Involvement of private health-care providers and use of real-time digital platforms, fever tracking apps, and digital dashboards may assist in the early recognition of an increase in patients.16–18
In our study, males were found to be affected by malaria more commonly than females, corroborating findings from previous studies. The reason could be relatively more involvement of males in outdoor activities, thus making them exposed to frequent mosquito bites and thus infection.19–25 However, the real pattern in the community could be different from the pattern observed in the clinic, because the clinic population is not necessarily representative of the Delhi population. In terms of age distribution, patients who were 15 to 44 years old were found to be commonly affected by malaria. Similar findings were reported in earlier studies as well.20,22–24,26,27
Overall, primaquine was given to 593 patients with malaria (68.6%) after G6PD testing. The reasons for low primaquine use were pregnancy, G6PD deficiency, patients unwilling to undergo G6PD testing, and patients not returning for follow-up visits for primaquine. The low use of primaquine by patients with P. vivax malaria increases the chances of relapse resulting from the persistence of hypnozoites.28 Thus, tafenoquine administered as a single dose could be considered an option in similar settings for clearing the parasite reservoir.29 In P. falciparum malaria, primaquine act as gametocytocidal and thus blocks the transmission of the parasite to mosquitoes. Thus, from a malaria elimination standpoint, it is vital to administer primaquine for its effects on hypnozoites in P. vivax, and gametocytes in P. falciparum. For this, minimizing the number of visits to the health-care facility for G6PD testing and initiating primaquine may increase its use.
There was a significant seasonal trend in the occurrence of malaria across the years. The peak in malaria cases was observed primarily in July through October, which corresponds to and follows the peak in rainfall in the study area. This is in agreement with the seasonal pattern observed in studies for P. vivax in Indian settings, resulting from the favorable environment for mosquito breeding, as reported in earlier studies.10,30 Notably, there were reports of malaria cases (∼30% of total malaria cases) in the post-monsoon and pre-monsoon seasons. However, the numbers were low when compared with cases occurring during the monsoon season, pointing to factors other than rainfall. This might be attributed to the commonly occurring phenomenon of relapses for P. vivax malaria.
Climatic factors, including temperature and humidity, played a significant role in explaining the occurrence of malaria cases, and similar findings have been reported.10 The climate affects mosquito longevity, biting rate, and host-locating ability, and thus is important in disease transmission.31 Humidity of ∼55% to 80% is most favorable for malaria transmission, and in the our study it was 60% to 80% during peak transmission.30,32 A temperature of 25 to 30°C is most favorable for the mosquito life cycle, and it corresponds to the peak in the number of malaria cases.33–35 The 1-month lag in rainfall peak and peak in malaria cases was corroborated by other studies,30,33,36 and the peak is a result of an increase in the breeding sites for mosquitoes and an increase in mosquito survival because of the humidity in the environment after rainfall.37,38
Our study had certain limitations. Because it was based on malaria clinic data, the magnitude of the problem in the community can only be presumed and may not reflect the real pattern in the community. Another limitation is the unavailability of information regarding employment of the patients attending the clinic. Thus, we could not comment on disease transmission based on job profiles or working patterns of the patients.
In conclusion, malaria remains a public health problem in India, especially so in urban areas, and P. vivax is the dominant parasite reported in the study area. Males were affected by malaria more commonly than females. The use of primaquine was low among patients with malaria. The peak in malaria cases corresponded to a humidity of 60% to 80%, and followed the peak in rainfall, with a lag of almost 1 month. Thus, a targeted and collaborative approach is critical in achieving the national goal of malaria elimination by 2030.
ACKNOWLEDGMENTS
We appreciate the sincere efforts by the National Institute of Malaria Research staff for the diagnosis of malaria patients and maintaining clinical records. We also thank the editor for the feedback provided. The American Society of Tropical Medicine and Hygiene (ASTMH) assisted with publication expenses.
REFERENCES
- 1. World Health Organization , 2021. World Malaria Report 2021. Available at: https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2021. Accessed May 4, 2022.
- 2.Singh N, Singh OP, Sharma VP, 1996. Dynamics of malaria transmission in forested and deforested region of Mandla district, Central India, Madhya Pradesh. J Am Mosq Cont Assoc 12: 225–234. [PubMed] [Google Scholar]
- 3. Sharma RK, Thakor HG, Saha KB, Sonal GS, Dhariwal AC, Singh N, 2015. Malaria situation in India with special reference to tribal areas. Indian J Med Res 141: 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ranjha R, Sharma A, 2021. Forest malaria: the prevailing obstacle for malaria control and elimination in India. BMJ Glob Health 6: e005391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaturvedi R, Deora N, Bhandari D, Parvez S, Sinha A, Sharma A, 2020. Trends of neglected Plasmodium species infection in humans over the past century in India. One Health 11: 100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization , 2016. Malaria Microscopy Quality Assurance Manual: version 2. Geneva, Switzerland: WHO. [Google Scholar]
- 7. Benstein R, 1962. DPIP decolourisation test for G6PD deficiency. Nature 194: 192.13868283 [Google Scholar]
- 8. Kumar G, Singh RK, 2016. Situation analysis of malaria in Delhi. Int J Mosq Res 3: 36–39. [Google Scholar]
- 9. Anvikar AR. et al. , 2016. Epidemiology of Plasmodium vivax malaria in India. Am J Trop Med Hyg 95: 108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santos-Vega M, Bouma MJ, Kohli V, Pascual M, 2016. Population density, climate variables and poverty synergistically structure spatial risk in urban malaria in India. PLoS Negl Trop Dis 10: e0005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kerr-Wilson CO, Mackay DF, Smith GC, Pell JP, 2012. Meta-analysis of the association between preterm delivery and intelligence. J Public Health (Oxf) 34: 209–216. [DOI] [PubMed] [Google Scholar]
- 12. Naing C, Whittaker MA, Nyunt Wai V, Mak JW, 2014. Is Plasmodium vivax malaria a severe malaria? A systematic review and meta-analysis. PLoS Negl Trop Dis 8: e3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Anvikar AR. et al. , 2020. Clinical and epidemiological characterization of severe Plasmodium vivax malaria in Gujarat, India. Virulence 11: 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kojom Foko LP, Arya A, Sharma A, Singh V, 2021. Epidemiology and clinical outcomes of severe Plasmodium vivax malaria in India. J Infect 82: 231–246. [DOI] [PubMed] [Google Scholar]
- 15. Rahi M, Sharma A, 2021. Free market availability of rapid diagnostics will empower communities to control malaria in India. Am J Trop Med Hyg 105: 281–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rahi M, Das P, Sharma A, 2021. Malaria elimination in India requires additional surveillance mechanisms. J Public Health (Oxf) 44: 227–231. [DOI] [PubMed] [Google Scholar]
- 17. Pal Bhowmick I. et al. , 2021. Validation of a mobile health technology platform (FeverTracker) for malaria surveillance in India: development and usability study. JMIR Form Res 5: e28951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahi M, Sharma A, 2022. Active engagement of private healthcare providers is needed to propel malaria elimination in India. Am J Trop Med Hyg 106: 1585–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Addisu A, Tegegne Y, Mihiret Y, Setegn A, Zeleke AJA, 2020. 7-Year trend of malaria at primary health facilities in northwest Ethiopia. J Parasitol Res 2020: 4204987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alemu A, Muluye D, Mihret M, Adugna M, Gebeyaw M, 2012. Ten year trend analysis of malaria prevalence in Kola Diba, North Gondar, northwest Ethiopia. Parasit Vectors 5: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Derbie A, Alemu M, 2017. Five years malaria trend analysis in Woreta Health Center, northwest Ethiopia. Ethiop J Health Sci 27: 465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dabaro D, Birhanu Z, Yewhalaw D, 2020. Analysis of trends of malaria from 2010 to 2017 in Boricha District, southern Ethiopia. Malar J 19: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Feleke DG, Gebretsadik D, Gebreweld A, 2018. Analysis of the trend of malaria prevalence in Ataye, North Shoa, Ethiopia between 2013 and 2017. Malar J 17: 323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gebretsadik D, Feleke DG, Fiseha M, 2018. Eight-year trend analysis of malaria prevalence in Kombolcha, South Wollo, North-Central Ethiopia: a retrospective study. Parasit Vectors 11: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Berhe B, Mardu F, Legese H, Negash H, 2019. Seasonal distribution and seven year trend of malaria in North West Tigrai: 2012–2018, Ethiopia. Trop Dis Travel Med Vaccines 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Alkadir S, Gelana T, Gebresilassie A, 2020. A five year trend analysis of malaria prevalence in Guba District, Benishangul-Gumuz regional state, western Ethiopia: a retrospective study. Trop Dis Travel Med Vaccines 6: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baharia RK, Yadav CP, Sharma A, 2021. Four decades of epidemiological data reveal trajectories towards malaria elimination in Kheda District (Gujarat), western part of India. BMJ Glob Health 6: e005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kevin Baird J, 2013. Malaria caused by Plasmodium vivax: recurrent, difficult to treat, disabling, and threatening to life—averting the infectious bite preempts these hazards. Pathog Glob Health 107: 475–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ahmad SS, Rahi M, Sharma A, 2021. Relapses of Plasmodium vivax malaria threaten disease elimination: time to deploy tafenoquine in India? BMJ Glob Health 6: e004558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kumar V. et al. , 2014. Forecasting malaria cases using climatic factors in Delhi, India: a time series analysis. Malar Res Treat 2014: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Manh BH. et al. , 2011. Social and environmental determinants of malaria in space and time in Viet Nam. Int J Parasitol 41: 109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Smith DL, Dushoff J, McKenzie FE, 2004. The risk of a mosquito-borne infection in a heterogeneous environment. PLoS Biol 2: e368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kumar P, Vatsa R, Sarthi PP, Kumar M, Gangare V, 2020. Modeling an association between malaria patients and climate variables for Keonjhar District of Odisha, India: a Bayesian approach. J Parasit Dis 44: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB, 2010. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci 107: 15135–15139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reiter P, 2001. Climate change and mosquito-borne disease. Environ Health Perspect 109 (Suppl 1): 141–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Loevinsohn ME, 1994. Climatic warming and increased malaria incidence in Rwanda. Lancet 343: 714–718. [DOI] [PubMed] [Google Scholar]
- 37. Briët OJ, Vounatsou P, Gunawardena DM, Galappaththy GN, Amerasinghe PH, 2008. Temporal correlation between malaria and rainfall in Sri Lanka. Malar J 7: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauderdale JM. et al. , 2014. Towards seasonal forecasting of malaria in India. Malar J 13: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]