Abstract
Attractive targeted sugar baits (ATSBs) are a potential vector control tool that exploits the sugar-feeding behaviour of mosquitoes. We evaluated the sugar-feeding behaviour of Anopheles mosquitoes as part of baseline studies for cluster randomised controlled trials of ATSBs. Mosquitoes were collected indoors and outdoors from two villages in western Kenya using prokopack aspirations, malaise tent traps and ultraviolet (UV) light traps. Individual mosquitoes were subjected to the cold anthrone test to assess the presence of sugar. Overall, 15.7% of collected mosquitoes had fed on natural sugar sources. By species and sex, the proportion sugar-fed was 41.3% and 27.7% in male and female Anopheles funestus, 27.2% and 12.8% in male and female An. arabiensis, and 9.7% and 8.3% in male and female An. coustani, respectively. Sugar-feeding was higher in unfed than blood-fed mosquitoes and higher in male than gravid mosquitoes. Anopheles mosquitoes obtained sugar meals from natural sources during all physiological stages, whether they rest indoors or outdoors. These findings offer a potential avenue to exploit for the control of mosquitoes, particularly with the advent of ATSBs, which have been shown to reduce mosquito densities in other regions.
Subject terms: Entomology, Malaria
Introduction
The scale-up of malaria control since 2000 has resulted in substantial reductions in the burden of malaria globally. Modelling studies suggest that much of the reduction was due to vector control efforts, particularly long-lasting insecticidal nets (LLINs) and indoor residual spraying (IRS)1. However, despite these gains, malaria remains endemic in sub-Saharan Africa and is one of the leading causes of morbidity and mortality2. Furthermore, recent reports indicate that the decline in malaria burden has stalled or reversed in some areas3. There is a need for additional control tools to be integrated with the existing approaches2,4.
Attractive targeted sugar baits (ATSBs) are a potential new vector control tool that exploits the sugar feeding behaviour of mosquitoes. While female mosquitoes require blood for egg maturation, both males and females require sugar to meet energy needs. Mosquitoes obtain sugar from various sources, including floral and extra-floral nectaries, fruits and honeydew5–8. In West Africa, it has been demonstrated that sugar baits comprised of juice from local fruits are attractive to mosquitoes9,10 which may be exploited to target mosquitoes responsible for transmitting vector-borne diseases in humans or domestic animals. The efficacy of sugar baits spiked with an oral toxin was first demonstrated in field trials in Israel against An. claviger in underground cisterns11 and against An. sergentii and An. caspius in a desert oasis12. Subsequently, a small-scale trial in Mali where vegetation was sprayed with a sugar solution plus boric acid resulted in an estimated 90% reduction in older mosquitoes13. More recently, a larger trial using a prototype of a commercial bait station also resulted in substantial reductions14, suggesting that ATSBs could be a promising new malaria control tool.
However, ATSBs have only been evaluated in areas characterised by an arid climate with limited vegetation, where ATSBs may face little competition from natural sugar sources. In more tropical climates, mosquitoes are thought to have access to a wider variety of natural sugar sources, which may limit feeding on ATSBs and/or require alternative placement strategies. To better understand the potential for ATSBs in areas with a wider variety of natural sugar sources, we characterised the sugar feeding behaviour of the primary Anopheles vectors of malaria as part of baseline studies for population-based controlled trials of ATSBs in western Kenya where malaria transmission is high and perennial15.
Methodology
Study sites
This study was conducted in Mabinju (-0.1849075, 34.3727141) and Abidha (-0.1552102, 34.4014668) villages in Rarieda sub-County, Siaya County, western Kenya. The area is part of the KEMRI-CDC Health and Demographic Surveillance System (HDSS)16 where a population of 262,215 as of 2019 has been continuously monitored for in- and out-migration and births and deaths and where regular surveys of malaria prevalence have been conducted for over a decade17. Mabinju and Abidha were selected as historically, high numbers of Anopheles mosquitoes have been observed in these villages18,19.
Most people in the study area are of the Luo ethnic group and earn their living through subsistence farming and fishing. The primary vectors in this area are An. arabiensis, An. gambiae sensu stricto and An. funestus. Malaria transmission is highest following the long rains which occur from April to June with a smaller peak following the short rains in October/November. However, rainfall and malaria transmission may occur throughout the year. The study area was the site of a large-scale trial of insecticide-treated nets (ITNs) and now receives LLINs through periodic mass campaigns and routine distribution to pregnant women and children through antenatal clinics and child welfare clinics18,20–22. In the 2020 Malaria Indicator Survey, 77.9% of households in the Lake-Endemic region of western Kenya owned at least one net23. In a study in this area in 2015, LLIN usage across all ages was 87.0% and 91.2% in children under 5 years. Despite high coverage of LLINs malaria prevalence by microscopy was 36.1% across all ages and 39.0% among children under 5 years15.
Mosquito sampling
Mosquito sampling was done in and around 10 randomly selected dwellings (structures) in each village for 2 weeks each month during the cool dry season between July and September 2020 using three trapping methods. Ultraviolet (UV) light traps (Model 512, John W. Hock Company, Gainesville, Florida, USA) were used to collect mosquitoes in three positions in or outdoors near each selected dwelling (structure): (1) Indoors (B), (2) 10 m outdoors from the structure (outdoors near to structure; UVLT-C) and (3) immediately outside the compound (outdoors-outside compound; UVLT-D) at a distance of 15 to 20 m from the structure with an indoor trap. Indoors, the traps were set 1.5 m above the ground at the foot of an occupied bednet. Indoor traps were set at 17:00 and collected at 07:00 the following morning to avoid inconveniencing the household members.
Outdoor traps were suspended from unoccupied buildings and trees approximately 1.5 m above the ground and sheltered from the rain. The traps were placed away from shelters where animals were kept and no attractant other than the UV lights were deployed with the traps. The collection cups from these outdoor light traps were collected hourly from 17:00 to 07:00. Mosquitoes were also collected using a single standard 6 m malaise tent trap (Model 3012, John W. Hock Company, Gainesville, Florida, USA) set approximately 50 m from the nearest structure in an open field within Mabinju village. The malaise trap collections were done in tandem with the outdoor UV light traps. Resting collections were done in the mornings from 07:00 to 11:00 using prokopack (Model 1419, John W. Hock Company, Gainsville, Florida, USA) aspirators indoors and outdoors. Outdoor aspirations were done in and around clay pots and other water storage containers outside the structure. Collections were done by moving the prokopack aspirators under the eaves and within dark corners around the structure and vegetation where mosquitoes were likely to rest. Mosquito collections indoors were done to determine the natural sugar-feeding rate of host-seeking and resting mosquitoes for UVLT and prokopack aspiration respectively.
Collections done outdoors using UVLT near and outside the compound were to determine the sugar-feeding rate of mosquitoes closer to the dwelling. Malaise tent trap collections were to determine the sugar-feeding rate of mosquitoes free flying in the wild.
Mosquito processing
For processing, mosquitoes were transported to the field laboratory in Rarieda sub-County. Transportation from the collection sites to the field laboratory took approximately 30 min and lab processing took another 30 min. Upon arrival at the lab, live mosquitoes were knocked down either in chloroform or by freezing at − 20 °C. Anopheles mosquitoes were identified to species level by trained technician using dissecting microscopes and Anopheles taxonomic keys24; the sex and abdominal status of females (blood fed, non-blood fed, or gravid) were also recorded. The alcohol precipitation method and conventional polymerase chain reaction were used to speciate a subset of Anopheles gambiae sl to sibling species25,26.
Individual Anopheles mosquitoes were subjected to the cold anthrone test27 to assess the presence of sugar. The anthrone reagent was prepared as follows: 500 ml of 72% sulphuric acid was made by adding acid to distilled water, after which 1 g of anthrone powder was added to the solution. The mixture turned to a yellow solution after mixing. Individual mosquitoes were placed in a 96-well round-bottomed ELISA plate. The mosquitoes were then crushed singly using pestles, and 200 µl of the cold anthrone reagent was added. The plates were incubated for 45 min at room temperature, after which the results were scored based on the intensity of the green–blue colouration: yellow (no change in colour), light green, green and dark green. Yellow was interpreted as mosquitoes that were not sugar-fed, while light green, green and dark green indicated increasing levels of sugar feeding (scored as 0, 1, 2 and 3).
All Anopheles mosquitoes were included in anthrone testing independent of their abdominal status and sex. However, only An. gambiae, An. funestus and An. coustani were included for further analysis as inadequate numbers of the other species were available. For all analyses, gravid and half-gravid mosquitoes were combined into a single category referred to hereafter as “gravid”.
Data analysis
Data was recorded in a Microsoft Excel spreadsheet with the date, collection method, location, collection hour, species, sex, abdominal status and anthrone result. The anthrone result was also converted to a binary outcome (any level of sugar feeding versus no sugar feeding). Logistic regression was used to compare feeding rates between species, collection method, abdominal status, and time of collection. All models were adjusted for repeated measures on mosquitoes from the same collection (location, structure, and date) using generalised estimating equations (GEE). All statistical analyses were carried out using SAS version 9.4 and R statistical package version 4.0.3.
Ethical considerations
The study was reviewed and approved by the Kenya Medical Research Institute Scientific and Ethics Review Unit (SERU 3613) and by the Institutional Review Board of the Liverpool School of Tropical Medicine Research Ethics Committee (18-015). The study was also approved through a reliance agreement between the IRB of the US Centers for Disease Control and Prevention and KEMRI SERU (CDC IRB 7112). Permission to conduct the study was also obtained from the respective leaders and elders of each of the two villages. Informed consent was obtained verbally from inhabitants of the structures where mosquito collections were conducted in English, Kiswahili or Dholuo.
Results
Anopheles species variation
A total of 18,386 Anopheles mosquitoes were collected from the two villages over the 3-month collection period, with 2 weeks of sampling each month. Eight different Anopheles species were identified though most were An. coustani (n = 11,623; 63%), An. funestus (n = 5049; 27%) or An. gambiae s.l. (n = 1572; 9%). By PCR, most An. gambiae s.l. were An. arabiensis (n = 275; 96%), the rest being An. gambiae s.s. Other species of Anopheles mosquitoes that were collected only outdoors included An. maculipalpis (N = 4), An. pharoensis (N = 114), An. rufipes (N = 10), An. squamosus (N = 11) and An. parensis (N = 3). The total number of mosquitoes tested by species, abdominal status and collection method/location are presented in Table 1.
Table 1.
The number of An. gambiae, An. funestus and An. coustani collected using different trapping methods and tested for sugar by cold anthrone test.
| Species | Collection method | Male | Non-blood fed | Blood fed | Gravid* |
|---|---|---|---|---|---|
| An. funestus | Aspiration indoor | 1039 | 342 | 452 | 460 |
| Aspiration outdoor | 122 | 60 | 24 | 17 | |
| Malaise | 11 | 12 | 0 | 0 | |
| UVLT indoor | 615 | 1258 | 19 | 58 | |
| UVLT outdoor (C) | 30 | 279 | 5 | 5 | |
| UVLT outdoor (D) | 39 | 193 | 2 | 7 | |
| An. gambiae | Aspiration indoor | 34 | 18 | 34 | 28 |
| Aspiration outdoor | 28 | 22 | 19 | 4 | |
| Malaise | 8 | 22 | 0 | 0 | |
| UVLT indoor | 61 | 191 | 16 | 8 | |
| UVLT outdoor (C) | 64 | 537 | 5 | 8 | |
| UVLT outdoor (D) | 59 | 393 | 1 | 12 | |
| An. coustani | Aspiration indoor | 0 | 5 | 0 | 0 |
| Aspiration outdoor | 2 | 11 | 6 | 1 | |
| Malaise | 10 | 101 | 6 | 3 | |
| UVLT indoor | 28 | 464 | 75 | 2 | |
| UVLT outdoor (C) | 489 | 3716 | 451 | 25 | |
| UVLT outdoor (D) | 958 | 4895 | 359 | 16 |
*Includes both gravid and half gravid.
Sugar feeding
The proportion and intensity of sugar feeding by species
Overall, 15.7% of all the mosquitoes caught using the different trapping methods had fed on natural sugar sources. Anopheles funestus males and females had the highest rate of sugar feeding with 41.3% and 27.7%, respectively. For An. gambiae s.l., 27.2% of males and 12.8% of females had fed on sugar, while this was 9.7% and 8.3% for male and female An. coustani. These interspecific trends were similar when sugar feeding was classified based on the intensity of the anthrone result (Table 2). Sugar feeding rates were significantly higher in An. funestus compared to both An. gambiae s.l. and An. coustani for both males and females while sugar feeding rates in An. gambiae s.l. were significantly higher than those of An. coustani (p < 0.05 for all comparisons; Supplementary Tables 1a & 1b).
Table 2.
The intensity of sugar feeding by species. Zero is interpreted as unfed mosquitoes while increasing intensities of sugar feeding are indicated as 1, 2 and 3.
| Species | Anthrone result | Males | Females | ||
|---|---|---|---|---|---|
| Number | Percent (95% CI) | Number | Percent (95% CI) | ||
| An. funestus | 0 | 1090 | 58.7 (51.4–66) | 2310 | 72.3 (69.7–75) |
| 1 | 364 | 19.6 (15.5–23.7) | 380 | 11.9 (10.4–13.4) | |
| 2 | 234 | 12.6 (10.1–15.1) | 243 | 7.6 (6.5–8.7) | |
| 3 | 168 | 9.1 (6.9–11.3) | 260 | 8.1 (6.8–9.5) | |
| Any sugar | 766 | 41.3 (34.0–48.6) | 883 | 27.7 (25.0–30.3) | |
| An. gambiae | 0 | 185 | 72.8 (64.3–81.3) | 1149 | 87.2 (84.3–90) |
| 1 | 23 | 9.1 (5.1–13) | 105 | 8.0 (5.5–10.4) | |
| 2 | 20 | 7.9 (4.1–11.7) | 32 | 2.4 (1.6–3.2) | |
| 3 | 26 | 10.2 (5.4–15.1) | 32 | 2.4 (1.5–3.4) | |
| Any sugar | 69 | 27.2 (18.7–35.7) | 169 | 12.8 (10.0–15.7) | |
| An. coustani | 0 | 1343 | 90.3 (88.5–92.1) | 9296 | 91.7 (90.8–92.6) |
| 1 | 88 | 5.9 (4.7–7.2) | 609 | 6.0 (5.3–6.7) | |
| 2 | 27 | 1.8 (1.1–2.6) | 171 | 1.7 (1.4–2) | |
| 3 | 29 | 2.0 (1.2–2.7) | 60 | 0.6 (0.4–0.7) | |
| Any sugar | 144 | 9.7 (7.9–11.5) | 840 | 8.3 (7.4–9.2) | |
Sugar feeding by sex and abdominal status
Higher rates of sugar feeding were observed in males compared to females and in non-blood fed females compared to blood-fed mosquitoes across all the species. Sugar feeding rates among gravid females varied by species, with rates in An. funestus similar to those of blood-fed females and rates in An. coustani were similar to non-blood fed mosquitoes. Sugar feeding rates among gravid An. gambiae s.l. were intermediate between blood-fed and non-blood fed females (Table 3). In An. funestus, higher sugar feeding rates were observed in males and non-blood fed females compared to blood-fed and gravid mosquitoes (p < 0.001 for all comparisons). Sugar feeding rates were not significantly different between An. funestus males and non-blood fed females (p = 0.105) or between blood-fed and gravid females (p = 0.596) (Supplementary Tables 2a & 2b). Sugar feeding among male An. gambiae was significantly higher than blood-fed, non-blood fed or gravid females of the same species (p < 0.01 for all comparisons). Non-blood fed An. gambiae s.l. were significantly more likely to have sugar compared to blood-fed An. gambiae s.l. (p = 0.038). There was no significant difference in sugar feeding rates between gravid An. gambiae s.l. and either blood-fed (p = 0.184) or non-blood fed An. gambiae s.l. (p = 0.523) (Supplementary Tables 3a & 3b). Sugar-feeding rates were higher among male than female Anopheles coustani (p < 0.001), and higher among non-blood-fed females than blood-fed females (p < 0.001). There were no other statistically significant pairwise comparisons for An. coustani (Supplementary Tables 4a & 4b).
Table 3.
Frequency of sugar feeding by species, sex and abdominal status.
| Status | An. gambiae | An. funestus | An. coustani | |||
|---|---|---|---|---|---|---|
| Number | Percent (95% CI) | Number | Percent`(95% CI) | Number | Percent (95% CI) | |
| Nonblood-fed | 1183 | 13.4 (10.4–16.5) | 2144 | 30.4 (27.2–33.5) | 9192 | 8.6 (7.6–9.6) |
| Blood-Fed | 75 | 5.3 (0.6–10.1) | 502 | 21.9 (17.9–26) | 897 | 4.9 (3.3–6.5) |
| Gravid | 60 | 10 (2.8–17.2) | 547 | 22.3 (17.6–27) | 47 | 8.5 (0.9–16.1) |
| Male | 254 | 27.2 (18.7–35.7) | 1856 | 41.3 (34.0–48.6) | 1487 | 9.7 (7.9–11.5) |
Sugar feeding rates by collection method and location
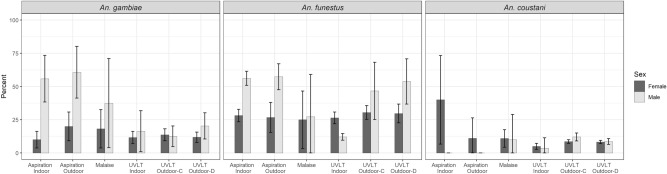
Sugar feeding rates by species, sex and collection method are provided in Fig. 1. Sugar feeding rates among female An. funestus or female An. gambiae s.l. were not significantly different based on method and/or collection location (P > 0.05 for all comparisons; Supplementary Tables 5a & 5b and 7a &7b). For male An. funestus, sugar feeding rates were highest among those collected by aspiration, indoors or outdoors, and UVLT, both close to and more distant from the structure. The lowest sugar feeding rates were observed among mosquitoes collected by UVLT indoors. In pairwise comparisons, sugar feeding rates were significantly lower among male An. funestus collected by UVLT indoors compared to those collected by aspiration indoors, aspiration outdoors, UVLT-C (close to the structure) or UVLT-D (distant from structure) (p < 0.001 for all comparisons; Supplementary Tables 6a & 6b). Male An. gambiae s.l. collected by aspiration indoors or outdoors were significantly more likely to be sugar-fed compared to those captured by UVLT indoors (p < 0.05 for both comparisons), UVLT-C (p < 0.001 for both comparisons), and UVLT-D (p < 0.003 for both comparisons). No other pairwise comparisons of sugar feeding by collection method were statistically significant among An. gambiae males.
Figure 1.
Sugar feeding rates of An. gambiae, An. funestus and An. coustani by collection method, location, species, and sex. Error bars represent the 95% confidence limits.
For An. coustani females, the highest rates of sugar feeding were observed among those collected by indoor aspiration (40.0%), although only 5 mosquitoes were available for testing. Despite the low numbers, sugar feeding rates were significantly higher in An. coustani collected by indoor aspiration compared to malaise traps, UVLT indoor, UVLT-C, and UVLT-D (p < 0.02 for all comparisons; Supplementary Tables 9a & 9b). Low numbers of An. coustani males were available for testing, and only six pairwise comparisons were possible. None were statistically significant.
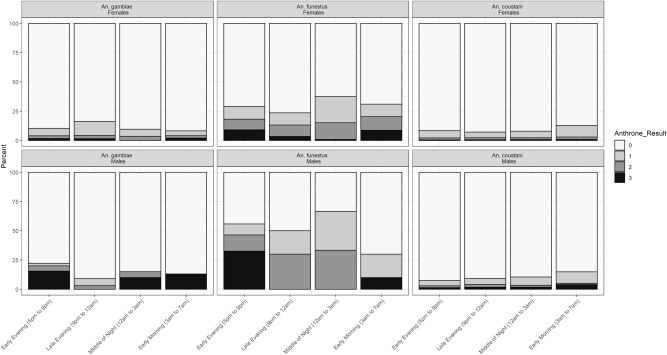
Sugar feeding by time of collection
Due to high variation in mosquito numbers from 1 h to the next, outdoor hourly data were grouped into categories of 3 or 4 h: early evening (5–9 pm), late evening (9 pm to 12 am), middle of the night (12–3 am), or early morning (3–7 am) (Fig. 2). For An. funestus and An. gambiae s.l., there was little evidence of differences in the proportion of sugar-fed mosquitoes by time. The only statistically significant pairwise comparison was for An. funestus females collected in the middle of the night, (12–3 am) which were significantly more likely to be sugar-fed than An. funestus females collected in the late evening (9 pm to 12 am) (p = 0.022). No other pairwise comparisons for An. funestus or An. gambiae were statistically significant (Supplementary tables 11a, 11b, 12a, 12b, 13a, 13b, 14a & 14b).
Figure 2.
Sugar feeding intensity by the time. Anthrone results (0 = no sugar, 1, 2 and 3 = increasing levels of sugar feeding).
Female An. coustani collected between 3 and 7 am were significantly more likely to be sugar-fed than those collected in the early evening (5–9 pm), late evening (9 pm to 12 am), and the middle of the night (12–3 am) (P < 0.01 for all comparisons; Supplementary Tables 15a & 15b). For An. coustani males, the sugar-fed proportion was also highest in the early morning. The differences were statistically significant when compared to feeding rates among An. coustani males collected in the early evening (5–9 pm; p = 0.010) or in the late evening (9 pm to 12am; p = 0.036) (Supplementary Tables 16a & 16b).
Discussion
This study assessed the natural sugar feeding behaviour of Anopheles malaria vectors in western Kenya to identify potential aspects that could be exploited in the design and deployment of ATSBs, and the potential for natural sugar sources to compete against ATSBs. Sugar feeding was observed across all the main malaria vectors, in both males and females in different physiological states. Furthermore, sugar-fed mosquitoes were detected by several different trapping methods deployed indoors and outdoors. However, there was variation in sugar feeding among the three species tested, between the males and females, and between females in different physiological states. There were also differences in the proportion of sugar-fed mosquitoes by trapping methods, though this varied by species. Anopheles funestus had the highest levels of sugar feeding, while An. coustani had the lowest level of sugar feeding. The proportion of sugar-fed An. gambiae was intermediate. Sugar feeding by males was consistently higher than females within each species. Lower levels of sugar feeding were observed in blood-fed females compared to non-blood females, while levels of sugar feeding among gravid females varied between the different species. Male mosquitoes collected using the aspiration method had higher sugar feeding rates than those from the UV light trap collections. Overall sugar feeding did not vary substantially throughout the night, but the highest intensity of sugar feeding based on the scoring of the cold anthrone test was observed in early evenings and late mornings although it was not possible to determine how long mosquitoes remained positive for sugar in the current experiment.
There have been limited studies of sugar feeding by natural populations of African malaria vectors. In a previous study in two sites in western Kenya, overall sugar feeding rates among An. gambiae s.l. and An. funestus were 14.4% for host-seeking females and 6.3% for indoor resting females, with no significant differences between the sites or the species28. The lack of difference observed between species contrasts with the current study where An. funestus was more frequently found sugar-fed compared to An. gambiae s.l. Furthermore, the previous study found higher sugar feeding rates among host-seeking females than indoor resting females. The reason for these differences is not clear, although a shift in species composition among An. gambiae s.l. from predominantly An. gambiae s.s. to predominantly An. arabiensis may have contributed to some of the differences observed. In a study in Mali 44.9% of female and 45.1% of male An. coluzzii had fed on natural sugar sources based on the cold anthrone test14. After attractive sugar bait stations (ASBs) were introduced, sugar feeding on natural sources declined. The proportion that had fed on natural sources was lower than the proportion that had fed on ASBs suggesting that in that setting, natural sugar sources did not interfere with feeding on the artificial bait stations14.
There were clear differences in sugar feeding by species in the current study, with the highest overall feeding rates among An. funestus and lowest rates among An. coustani for both males and females. Compared to other species tested in previous studies, sugar feeding rates among the three Anopheles species in the current study were relatively low. Among host-seeking An. freeborni in California, 23% were sugar-fed while 36% of non-blood fed, resting females collected in the evening and 55% of non-blood-fed, resting females collected in the morning were sugar-fed. In stark contrast to the current study, 94% of gravid An. freeborni were sugar-fed29. Feeding rates among Culex quinquefaciatus in Texas, as measured by the hot anthrone test, were 60% for males and 72.3% for females30, while sugar feeding rates among male and female Aedes albopictus in New York were 49.6% and 41.8%, respectively31. Variable sugar feeding rates have been observed in Aedes aegypti, with high rates observed in Texas30 and Mali32, while others report little to no sugar feeding by adult female Ae. aegypti33–36. As noted above, approximately 45% of male and female An. coluzzii were sugar fed by the cold anthrone test in Mali14.
Based on the low feeding rates observed in some field studies33,37 and laboratory studies34,38,39, Ae. aegypti were thought to derive most of their energetic needs from frequent blood feeding. Similarly, laboratory studies suggested that blood-feeding alone is adequate to meet the nutritional and energetic needs of An. gambiae s.s.40. The relatively low sugar feeding rates observed in the current study, particularly among blood-fed females, suggest wild An. gambiae s.l. and An. funestus are also able to meet their energetic needs largely through blood-feeding. These two species, along with Ae. aegypti, share similar behavioural traits that may lead to reduced sugar feeding and increased reliance on blood. These species tend to be associated with the domestic and peri-domestic environment and preferentially feed on humans. Aedes aegypti has been shown to have increased fitness when fed on human blood alone compared to human blood plus sugar or mouse blood, with or without access to sugar34. The difference was attributed to low isoleucine levels in human blood, as supplementing blood meals with this amino acid resulted in lower energetic reserves and lower egg output. It is possible that An. gambiae s.s. and An. funestus, which feed preferentially on humans, have evolved similar mechanisms to utilise blood meals, although this has not been investigated. However, observations from the current study do not entirely support this hypothesis as the highest prevalence of sugar feeding was in An. funestus which has a strong preference for humans, particularly compared to An. coustani which feeds on humans at much lower frequencies than An. funestus or An. gambiae s.l. Furthermore, by molecular identification, An. gambiae s.l. were mostly An. arabiensis which has emerged as the predominant species in the An. gambiae complex in this region18. In contrast to An. funestus or An. gambiae s.s., An. arabiensis tends to be much more opportunistic in its feeding behaviours and frequently feeds on cattle, as evidenced by blood meal identification studies41,42 and the generally lower sporozoite rates compared to An. funestus19,43,44.
Sugar feeding is an important aspect of mosquito behaviour that is poorly understood. Differences in rates of sugar feeding by collection method, time of collection and mosquito physiological state may provide insights into this largely unstudied aspect of mosquito biology. Resting male An. gambiae s.l., collected by aspiration, were more likely to be sugar-fed than those that were actively flying and captured in UV light traps. Higher rates of sugar feeding in resting males and females have been observed in Ae. aegypti and Cx. quinquefaciatus30, although sample sizes were relatively small and the only statistically significant difference was for Cx. quinquefaciatus females. Differences in the prevalence of sugar feeding based on the method of collection have also been observed for Culex tarsalis45. In An. freeborni, male mosquitoes were thought to feed after swarming in the evening and then seek out resting sites while sugars are converted to glycogen46. Similar patterns were seen among female An. freeborni29. The higher levels of sugar feeding among resting An. gambiae s.l. and An. funestus males suggest similar patterns of sugar feeding followed by resting. However, there was little evidence of temporal variation in sugar feeding among either males or female An. gambiae s.l. or An. funestus. The lack of variation by time may be partly due to the slow digestion of sugars. However, little is known about rates of digestion of sugar meals in An. gambiae or An. funestus, particularly among natural populations in western Kenya and interpretation of the proportion sugar fed by time of night should be interpreted with caution. In other species, sugar meals have been observed to be digested within 24 h31,47 while at least one study reported detection of sugar in Ae. aegypti up to 4 days after ingestion. Slow digestion may have obscured temporal patterns of overall sugar feeding, but the intensity of the cold anthrone results may be indicative of sugar feeding patterns. High intensity results were primarily observed from outdoor collections in the early evening (5–9 pm) for An. funestus males with another peak in high intensity anthrone results from collections in the early morning (3–7 am). A similar though less distinct pattern was observed for An. funestus females suggesting sugar feeding in this species occurs before commencing nocturnal activities and again before searching out resting sites during the day.
ATSBs have been proposed as a supplementary vector control intervention for malaria control in sub-Saharan Africa based on studies demonstrating their efficacy against An. gambiae s.l. mosquitoes in Mali14,48. Modelling studies indicate that the minimum ATSB daily excess mortality (measured by daily ATSB feeding rates) in Mali, western Kenya and western Zambia, to achieve a 30%49 reduction in malaria incidence compared to areas without ATSBs, are approximately 2.5%, (unpublished data). Although many mosquitoes would likely continue to feed on natural sources, the present study may provide estimates of the maximum potential of ATSBs in western Kenya. Overall feeding rates by An. funestus females were 27.7%, suggesting ATSBs could be targeted against this species. Feeding rates were lower among An. gambiae s.l. However, An. gambiae s.l. are predominantly An. arabiensis, which frequently feeds on non-human hosts and is a less efficient vector than An. funestus in western Kenya18. However, this study does not indicate how wild mosquitoes in western Kenya will respond to the deployment of ATSBs. In Mali, natural sugar feeding declined after the introduction of ASBs suggesting that artificial sugar sources could compete with natural sources. Furthermore, An. gambiae and Ae. aegypti increase blood feeding when sugar is withheld50,51. If the converse is true, ITNs and ATSBs may be complementary vector control tools as ITNs reduce blood feeding by mosquitoes which may in turn increase the frequency of sugar feeding to meet their energetic needs.
There were some limitations to this study. First, it was conducted over 3 months and therefore did not account for seasonal variation in sugar feeding behaviours described in other species30. Furthermore, the rates of sugar meal digestion are unknown in An. gambiae or An. funestus. Different rates of digestion of the sugar meals could affect comparisons in sugar feeding rates among these mosquitoes. Finally, the method used to assess sugar feeding was the cold anthrone test which provides information on the presence or absence of sugar but is not quantitative and relies on subjective assessment of colour intensity for a relative amount of sugar. A more detailed analysis of quantities of sugar and possibly glycogen, which is produced as sugar is digested, would provide more detailed insights into the sugar feeding behaviours of mosquitoes in western Kenya.
Conclusions
This study indicates that Anopheles mosquitoes of both sexes obtain sugar meals from natural sources during all physiological stages related to blood-feeding and egg maturation, whether they rest indoors or outdoors. These findings offer a potential area to exploit for the control of mosquitoes, particularly with the potential advent of ATSBs for the control of malaria in Africa.
Supplementary Information
Acknowledgements
We are grateful to the KEMRI Entomology field team for all the hard work on long, cold and wet nights. We thank Dr Angela Harris of IVCC and Prof. Martin Donnelly of LSTM for their technical input in the write-up of this manuscript.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centres for Disease Control and Prevention. This manuscript is published with the permission of the Director-General of the Kenya Medical Research Institute.
Abbreviations
- LLINs
Long lasting insecticidal nets
- IRS
Indoor residual spraying
- SSA
Sub Saharan Africa
- ATSB
Attractive targeted sugar baits
- ASB
Attractive sugar bait
- KEMRI
Kenya Medical Research Institute
- CDC
Centers for Disease Control and Prevention
- HDSS
Health and demographic surveillance system
- ITN
Insecticide treated bed net
- SERU
Scientific and ethics review unit
- UVLT-C
Ultraviolet light trap, close to a house or structure
- UVLT-D
Ultraviolet light trap, distant from a house or structure
- CDC-LT
CDC light trap
Author contributions
S.O., J.K.: Study design, sample collection, sample processing, data collection, data analysis, drafting the manuscript. S.A., B.P., N.Y., M.O.: Sample collection, sample processing. V.M., B.A., D.M., J.E., A.M.S., F.t.K.: Study design, data analysis. J.E.G. and E.O.: Conceptualisation of the study, study design, sample collection, data analysis, drafting of the manuscript. All authors read and approved this manuscript.
Funding
The Innovative Vector Control Consortium (IVCC), UK, which is funded by grants from the Bill and Melinda Gates Foundation and other funding partners.
Data availability
All data generated or analysed during this study are in this published article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-25004-9.
References
- 1.Bhatt S, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization World malaria report 2020: 20 years of global progress and challenges. (2020).
- 3.WHO . World Malaria Report 2021. Geneva: World Health Organization; 2021. [Google Scholar]
- 4.Organization, w. H. High burden to high impact: a targeted malaria response (2018).
- 5.Impoinvil DE, et al. Feeding and survival of the malaria vector Anopheles gambiae on plants growing in Kenya. Med. Vet. Entomol. 2004;18:108–115. doi: 10.1111/j.0269-283X.2004.00484.x. [DOI] [PubMed] [Google Scholar]
- 6.Foster WA. Mosquito sugar feeding and reproductive energetics. Annu. Rev. Entomol. 1995;40:443–474. doi: 10.1146/annurev.en.40.010195.002303. [DOI] [PubMed] [Google Scholar]
- 7.Van Handel E. Rapid determination of glycogen and sugars in mosquitoes. J. Am. Mosq. Control Assoc. 1985;1:299–301. [PubMed] [Google Scholar]
- 8.Yuval B. The other habit: sugar feeding by mosquitoes. Bull. Soc. Vector Ecol. 1992;17:7. [Google Scholar]
- 9.Stewart ZP, et al. Indoor application of attractive toxic sugar bait (ATSB) in combination with mosquito nets for control of pyrethroid-resistant mosquitoes. PLoS One. 2013;8:e84168. doi: 10.1371/journal.pone.0084168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller GC, et al. Field experiments of Anopheles gambiae attraction to local fruits/seedpods and flowering plants in Mali to optimize strategies for malaria vector control in Africa using attractive toxic sugar bait methods. Malar J. 2010;9:262. doi: 10.1186/1475-2875-9-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muller GC, Schlein Y. Efficacy of toxic sugar baits against adult cistern-dwelling Anopheles claviger. Trans. R. Soc. Trop. Med. Hyg. 2008;102:480–484. doi: 10.1016/j.trstmh.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Muller GC, Kravchenko VD, Schlein Y. Decline of Anopheles sergentii and Aedes caspius populations following presentation of attractive toxic (spinosad) sugar bait stations in an oasis. J. Am. Mosq. Control Assoc. 2008;24:147–149. doi: 10.2987/8756-971X(2008)24[147:DOASAA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Muller GC, et al. Successful field trial of attractive toxic sugar bait (ATSB) plant-spraying methods against malaria vectors in the Anopheles gambiae complex in Mali West Africa. Malar J. 2010;9:210. doi: 10.1186/1475-2875-9-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Traore MM, et al. Large-scale field trial of attractive toxic sugar baits (ATSB) for the control of malaria vector mosquitoes in Mali West Africa. Malar J. 2020;19:72. doi: 10.1186/s12936-020-3132-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuels AM, et al. Impact of community-based mass testing and treatment on malaria infection prevalence in a high-transmission area of western Kenya: a cluster randomized controlled trial. Clin. Infect. Dis. 2021;72:1927–1935. doi: 10.1093/cid/ciaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Odhiambo FO, et al. Profile: the KEMRI/CDC health and demographic surveillance system-western Kenya. Int. J. Epidemiol. 2012;41:977–987. doi: 10.1093/ije/dys108. [DOI] [PubMed] [Google Scholar]
- 17.Were V, et al. Trends in malaria prevalence and health related socioeconomic inequality in rural western Kenya: results from repeated household malaria cross-sectional surveys from 2006 to 2013. BMJ Open. 2019;9:e033883. doi: 10.1136/bmjopen-2019-033883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayoh MN, et al. Anopheles gambiae: historical population decline associated with regional distribution of insecticide-treated bed nets in western Nyanza Province Kenya. Malar J. 2010;9:62. doi: 10.1186/1475-2875-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCann RS, et al. Reemergence of Anopheles funestus as a vector of Plasmodium falciparum in western Kenya after long-term implementation of insecticide-treated bed nets. Am. J. Trop. Med. Hyg. 2014;90:597–604. doi: 10.4269/ajtmh.13-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Phillips-Howard PA, et al. Efficacy of permethrin-treated bed nets in the prevention of mortality in young children in an area of high perennial malaria transmission in western Kenya. Am. J. Trop. Med. Hyg. 2003;68:23–29. doi: 10.4269/ajtmh.2003.68.23. [DOI] [PubMed] [Google Scholar]
- 21.Hightower A, et al. Bed net ownership in Kenya: the impact of 3.4 million free bed nets. Malar J. 2010;9:183. doi: 10.1186/1475-2875-9-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WHO . Global Technical Strategy for Malaria 2016–2030. Geneva: World Health Organization; 2015. [Google Scholar]
- 23.Division of National Malaria Programme (DNMP) [Kenya] and ICF. 2021. Kenya Malaria Indicator Survey 2020: Key Indicators. Nairobi, Kenya and Rockville, Maryland, USA: DNMP and ICF. (2021).
- 24.Coetzee M. Key to the females of afrotropical Anopheles mosquitoes (Diptera: Culicidae) Malar J. 2020;19:70. doi: 10.1186/s12936-020-3144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins FH, et al. A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am. J. Trop. Med. Hyg. 1987;37:37–41. doi: 10.4269/ajtmh.1987.37.37. [DOI] [PubMed] [Google Scholar]
- 26.Scott JA, Brogdon WG, Collins FH. Identification of single specimens of the Anopheles gambiae complex by the polymerase chain reaction. Am. J. Trop. Med. Hyg. 1993;49:520–529. doi: 10.4269/ajtmh.1993.49.520. [DOI] [PubMed] [Google Scholar]
- 27.Van Handel, E. Detection of nectar in mosquitoes. Mosquito News (1972).
- 28.Beier JC. Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera:Culicidae) in western Kenya. J. Med. Entomol. 1996;33:613–618. doi: 10.1093/jmedent/33.4.613. [DOI] [PubMed] [Google Scholar]
- 29.Holliday-Hanson ML, Yuval B, Washino RK. Energetics and sugar-feeding of field-collected anopheline females. J. Vector Ecol. 1997;22:83–89. [PubMed] [Google Scholar]
- 30.Olson MF, et al. Sugar feeding patterns for Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) mosquitoes in South Texas. J. Med. Entomol. 2020;57:1111–1119. doi: 10.1093/jme/tjaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fikrig K, et al. Sugar feeding patterns of New York Aedes albopictus mosquitoes are affected by saturation deficit, flowers, and host seeking. PLoS Negl. Trop. Dis. 2020;14:e0008244. doi: 10.1371/journal.pntd.0008244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sissoko F, et al. Frequent sugar feeding behavior by Aedes aegypti in Bamako, Mali makes them ideal candidates for control with attractive toxic sugar baits (ATSB) PLoS One. 2019;14:e0214170. doi: 10.1371/journal.pone.0214170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edman JD, Strickman D, Kittayapong P, Scott TW. Female Aedes aegypti (Diptera: Culicidae) in Thailand rarely feed on sugar. J. Med. Entomol. 1992;29:1035–1038. doi: 10.1093/jmedent/29.6.1035. [DOI] [PubMed] [Google Scholar]
- 34.Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J. Med. Entomol. 2001;38:411–422. doi: 10.1603/0022-2585-38.3.411. [DOI] [PubMed] [Google Scholar]
- 35.Spencer CY, Pendergast THT, Harrington LC. Fructose variation in the dengue vector, Aedes aegypti, during high and low transmission seasons in the Mae Sot region of Thailand. J. Am. Mosq. Control Assoc. 2005;21:177–181. doi: 10.2987/8756-971X(2005)21[177:FVITDV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 36.Scott TW, Takken W. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 2012;28:114–121. doi: 10.1016/j.pt.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Nayar JK, Sauerman DM., Jr The effects of nutrition on survival and fecundity in Florida mosquitoes. Part 3. Utilization of blood and sugar for fecundity. J. Med. Entomol. 1975;12:220–225. doi: 10.1093/jmedent/12.2.220. [DOI] [PubMed] [Google Scholar]
- 38.Gary RE, Jr, Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2001;38:22–28. doi: 10.1603/0022-2585-38.1.22. [DOI] [PubMed] [Google Scholar]
- 39.RE, G. J. Biology of the Malaria Vector Anopheles gambiae: Behavioral and Reproductive Components of Sugar Feeding. The Ohio State University (2005).
- 40.Straif SC, Beier JC. Effects of sugar availability on the blood-feeding behavior of Anopheles gambiae (Diptera:Culicidae) J. Med. Entomol. 1996;33:608–612. doi: 10.1093/jmedent/33.4.608. [DOI] [PubMed] [Google Scholar]
- 41.Massebo F, Balkew M, Gebre-Michael T, Lindtjorn B. Blood meal origins and insecticide susceptibility of Anopheles arabiensis from Chano in South-West Ethiopia. Parasit. Vectors. 2013;6:44. doi: 10.1186/1756-3305-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Githeko AK, Service MW, Mbogo CM, Atieli FK, Juma FO. Origin of blood meals in indoor and outdoor resting malaria vectors in western Kenya. Acta Trop. 1994;58:307–316. doi: 10.1016/0001-706X(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 43.Machani MG, et al. Phenotypic, genotypic and biochemical changes during pyrethroid resistance selection in Anopheles gambiae mosquitoes. Sci. Rep. 2020;10:19063. doi: 10.1038/s41598-020-75865-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayagaya VS, et al. The impact of livestock on the abundance, resting behaviour and sporozoite rate of malaria vectors in southern Tanzania. Malar J. 2015;14:17. doi: 10.1186/s12936-014-0536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reisen WK, Meyer RP, Milby MM. Patterns of fructose feeding by Culex tarsalis (Diptera: Culicidae) J. Med. Entomol. 1986;23:366–373. doi: 10.1093/jmedent/23.4.366. [DOI] [PubMed] [Google Scholar]
- 46.Yuval B, Fritz G. Multiple mating in female mosquitoes—evidence from a field population of Anopheles freeborni (Diptera: Culicidae) Bull. Entomol. Res. 1994;84:137–139. doi: 10.1017/S0007485300032326. [DOI] [Google Scholar]
- 47.Andersson IH, Jaenson TG. Nectar feeding by mosquitoes in Sweden, with special reference to Culex pipiens and Cx torrentium. Med. Vet. Entomol. 1987;1:59–64. doi: 10.1111/j.1365-2915.1987.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 48.Beier JC, Muller GC, Gu W, Arheart KL, Schlein Y. Attractive toxic sugar bait (ATSB) methods decimate populations of Anopheles malaria vectors in arid environments regardless of the local availability of favoured sugar-source blossoms. Malar J. 2012;11:31. doi: 10.1186/1475-2875-11-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fraser KJ, et al. Estimating the potential impact of attractive targeted sugar baits (ATSBs) as a new vector control tool for Plasmodium falciparum malaria. Malar J. 2021;20:151. doi: 10.1186/s12936-021-03684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foster WA, Eischen FA. Frequency of blood-feeding in relation to sugar availability in Aedes aegypti and Anopheles quadrimaculatus (Diptera: Culicidae) Ann. Entomol. Soc. Am. 1987;80(2):103–108. doi: 10.1093/aesa/80.2.103. [DOI] [Google Scholar]
- 51.Braks MA, Juliano SA, Lounibos LP. Superior reproductive success on human blood without sugar is not limited to highly anthropophilic mosquito species. Med. Vet. Entomol. 2006;20:53–59. doi: 10.1111/j.1365-2915.2006.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are in this published article.