Abstract
Gestational exposure to ambient fine particles (PM2.5) increases the risk of stillbirth, but the related disease burden is unknown, particularly in low- and middle-income countries (LMICs). We combine state-of-the-art estimates on stillbirths, and multiple exposure–response functions obtained from previous meta-analyses or derived by a self-matched case-control study in 54 LMICs. 13,870 stillbirths and 32,449 livebirths are extracted from 113 geocoded surveys from the Demographic and Health Surveys. Each stillbirth is compared to livebirth(s) of the same mother using a conditional logit regression. We find that 10-µg/m3 increase of PM2.5 is associated with an 11.0% (95% confidence interval [CI] 6.4, 15.7) increase in the risk of stillbirth, and the association is significantly enhanced by maternal age. Based on age-specific nonlinear PM2.5–stillbirth curves, we evaluate the PM2.5-related stillbirths in 137 countries. In 2015, of 2.09 (95% CI: 1.98, 2.20) million stillbirths, 0.83 (0.54, 1.08) million or 39.7% (26.1, 50.8) are attributable to PM2.5 exposure exceeding the reference level of 10 μg/m3. In LMICs, preventing pregnant women from being exposed to PM2.5 can improve maternal health.
Subject terms: Epidemiology, Health policy
Gestational exposure to ambient fine particles increases the risk of stillbirth, but the related disease burden is unknown, particularly in low- and middle-income countries (LMICs). Here, the authors combine state-of-the-art estimates on stillbirths, and multiple exposure–response functions to evaluate the burden in 137 countries.
Introduction
The United Nations (UN) calls the global burden of stillbirths a neglected tragedy1. The UN Inter-agency Group for Child Mortality Estimation (UN IGME) found that there were 2.0 million stillbirths (90% confidence interval [CI]: 1.9, 2.2) in 2019 globally, and recognized that progress in stillbirth prevention had slowed, particularly in low- and middle-income countries (LMICs), such as those in sub-Saharan Africa2. Most (98%) stillbirths are estimated to occur in LMICs3. However, this issue has received little attention. For instance, stillbirth was not included in the Millennium Development Goals4 or tracked by the Global Burden of Disease (GBD) study5, both of which counted livebirths only. Stillbirth is linked to disease burden such as bleeding or infection6, secondary infertility6,7, and psychological sequelae (grief, anxiety, posttraumatic stress) affecting the whole family8,9. In addition, the economic costs of stillbirth (including health-care costs and loss caused by the incapacity to work) affect individuals, healthcare systems, and society6. Therefore, stillbirth intervention could promote maternal health and sex equality.
Preventing stillbirth depends on a comprehensive understanding of the underlying risk factors. Recent studies have shown an epidemiological association between gestational exposure to fine particulate matter (PM2.5) and stillbirth10–14. Two meta-analyses of the association between stillbirth and PM2.5 relied on evidence found before 2020. Zhang et al.15 reported a pooled OR of 1.103 (95% CI 1.074, 1.131) per 10 μg/m3 increment in PM2.5 during pregnancy based on seven independent studies; Xie et al.11 reported a value of 1.15 (95% CI 1.07, 1.25) based on six of those seven studies. Due to the ubiquity of PM2.5 pollution, it may be a major contributor to the global burden of stillbirth.
How PM2.5 contributes to the global burden of stillbirth is unknown because of the following knowledge gaps. First, there is significant heterogeneity in PM2.5–stillbirth associations between studies11,13. A major reason for this is differential susceptibility according to demographic characteristics. For instance, the most mature exposure–response curves16, developed to assess the cardiovascular mortality risk for PM2.5, are stratified by age group. Similarly, maternal age can significantly modify the PM2.5–stillbirth association10,12. Therefore, a representative and age-specific exposure–response curve between PM2.5 and stillbirth is needed. Second, a few key inputs (e.g., the baseline risk of stillbirth and high-resolution spatial distribution of the population at risk) into the risk assessment of stillbirth became available only recently. The UN IGME developed the first state-of-the-art estimates of the total number of stillbirths for 195 countries2, and WorldPop generated gridded maps of pregnancies (including livebirths and stillbirths) with a spatial resolution of 1 × 1 km for 161 countries or regions17, covering Africa, Latin America and the Caribbean, which have high stillbirth rates.
We have developed a self-matched case-control method to evaluate the association between PM2.5 and stillbirths in Africa10 and South Asia14. The method has been recommended as a cost-effective approach to developing an exposure–response curve for PM2.5 and stillbirth18 from large-population data in LMICs. This study aims to present an assessment study to quantify the burden of PM2.5-related stillbirth, using exposure-response curves derived from our approach or other meta-analyses. Although establishing exposure-response curves was not within our major study aim, this study increased confidence on estimates by enlarging sample size, and specified the curves by age groups, compared to our previous analyses. Combining the curves with state-of-the-art estimates on the population at risk, PM2.5 concentration, and baseline risk, we evaluated the number of stillbirths attributable to PM2.5 exposure in 137 countries (Supplementary Table 1) from 2000 to 2019.
Results
Exposure–response curve
To establish the exposure–response curves for risk assessment, we analyzed 46,319 cases of gestation linked to 13,870 mothers from 1998 to 2016. The mean maternal age in the control group was 24.97 years with a standard deviation (SD) of 6.02 years, younger than the stillbirth group (mean 26.56 years; SD 7.02 years). The mean length of intervals between stillbirth and livebirth was 3.81 (SD = 2.45) years (Supplementary Table 2). Nightlight, as an indicator of developmental level, was 8.29 digit-number (DN) (SD = 16.14 DN) for the case group, higher than that for the control group (mean = 7.07 DN; SD = 14.99 DN; p value <2×10−16 for a paired test). This suggests that more stillbirths occur in more developed regions. The controls had a lower level of gestational exposure to PM2.5 (mean = 40.34 μg/m3, SD = 22.20 μg/m3), compared to stillbirth cases (mean = 40.96 μg/m3; SD = 23.04 μg/m3; p value <2×10−16 for a paired test). Country-specific distributions for the environmental variables are shown in Supplementary Fig. 1. The population characteristics for stillbirth and the secondary outcomes analyzed in this study are summarized in Table 1. The spatial distributions of the surveyed samples from the 54 LMICs are shown in Fig. 1a, along with the exposure level of PM2.5 in 2015.
Table 1.
Population characteristics for the dataset used to establish the exposure–response curves between PM2.5 and stillbirth or other similar outcomes
| Variable | Group | Outcomes and their matched controls | |||
|---|---|---|---|---|---|
| Stillbirth | Early stillbirth | Miscarriage | Pregnancy loss | ||
| Categorical variables | Number (percentage) | ||||
| Total | 46,319 (100%) | 32,665 (100%) | 279,147 (100%) | 358,131 (100%) | |
| Pregnancy loss | Control | 32,449 (70.1%) | 22,882 (70.1%) | 193,599 (69.4%) | 248,930 (69.5%) |
| Case | 13,870 (29.9%) | 9,783 (29.9%) | 85,548 (30.6%) | 109,201 (30.5%) | |
| Number of matched controls | 1 | 10,162 (21.9%) | 6,864 (21.0%) | 61,494 (22.0%) | 78,520 (21.9%) |
| 2 | 11,556 (24.9%) | 8,400 (25.7%) | 78,573 (28.1%) | 98,529 (27.5%) | |
| 3 | 8,988 (19.4%) | 6,924 (21.2%) | 58,152 (20.8%) | 74,064 (20.7%) | |
| 4 | 7,015 (15.1%) | 4,930 (15.1%) | 38,315 (13.7%) | 50,260 (14.0%) | |
| 5+ | 8,598 (18.6%) | 5,547 (17.0%) | 42,613 (15.3%) | 56,758 (15.8%) | |
| Maternal age (years) | <20 | 9,712 (21.0%) | 7,081 (21.7%) | 51,841 (18.6%) | 68,634 (19.2%) |
| 20–29 | 25,699 (55.5%) | 17,548 (53.7%) | 157,077 (56.3%) | 200,324 (55.9%) | |
| 30–34 | 6,638 (14.3%) | 4,667 (14.3%) | 41,342 (14.8%) | 52,647 (14.7%) | |
| > 34 | 4,270 (9.2%) | 3,369 (10.3%) | 28,887 (10.3%) | 36,526 (10.2%) | |
| Level of nightlight (NTL, Digit-Number) | Low (NTL ≤ 4) | 29,464 (63.6%) | 18,947 (58.0%) | 130,672 (46.8%) | 179,083 (50.0%) |
| Middle (4 < NTL ≤ 20.5) | 10,310 (22.3%) | 7,585 (23.2%) | 73,896(26.5%) | 91,791 (25.6%) | |
| High (NTL > 20.5) | 6,545 (14.1%) | 6,133 (18.8%) | 74,579 (26.7%) | 87,257 (24.4%) | |
| Parity | Nulliparous | 13,175(28.4%) | 8,634 (26.4%) | 75,080 (26.9%) | 96,889 (27.1%) |
| Multiparous | 33,144 (71.6%) | 24,031 (73.6%) | 204,067 (73.1%) | 261,242 (72.9%) | |
| Continuous variable | Mean (Standard deviation, interquartile range) | ||||
| Maternal age (years) | Total | 25.45 (6.38, 20.58 ~ 29.58) | 25.56 (6.62, 20.50 ~ 29.83) | 25.90 (6.43, 21.00 ~ 30.00) | 25.81 (6.44, 20.92 ~ 29.92) |
| Control | 24.97 (6.02, 20.42 ~ 28.83) | 24.92 (6.23, 20.17 ~ 29.00) | 25.01 (5.99, 20.50 ~ 28.83) | 25.00 (6.02, 20.42 ~ 28.83) | |
| Case | 26.56 (7.02, 21.08 ~ 31.50) | 27.05 (7.22, 21.50 ~ 32.00) | 27.90 (6.92, 22.58 ~ 32.58) | 27.66 (6.98, 22.33 ~ 32.42) | |
| Temperature (°C) | Total | 23.67 (4.68, 21.48 ~ 26.76) | 23.63 (5.06, 21.26 ~ 26.84) | 23.20 (6.24, 20.16 ~ 27.14) | 23.30 (5.96, 20.49 ~ 27.03) |
| Control | 23.74 (4.70, 21.54 ~ 26.81) | 23.69 (5.09, 21.32 ~ 26.87) | 23.22 (6.23, 20.24 ~ 27.11) | 23.33 (5.95, 20.57 ~ 27.03) | |
| Case | 23.53 (4.64, 21.37 ~ 26.60) | 23.50 (4.99, 21.12 ~ 26.77) | 23.14 (6.26, 19.94 ~ 27.20) | 23.22 (5.97, 20.31 ~ 27.05) | |
| Nightlight (Digit-Number) | Total | 7.44 (15.35, 0.00 ~ 6.40) | 9.70 (17.51, 0.00 ~ 9.00) | 13.69 (20.12, 0.00 ~ 19.00) | 12.52 (19.47, 0.00 ~ 16.00) |
| Control | 7.07 (14.99, 0.00 ~ 5.75) | 9.11 (17.01, 0.00 ~ 8.33) | 12.93 (19.67, 0.00 ~ 17.00) | 11.81 (19.01, 0.00 ~ 14.00) | |
| Case | 8.29 (16.14, 0.00 ~ 7.20) | 11.06 (18.54, 0.00 ~ 12.00) | 15.42 (21.01, 0.00 ~ 25.00) | 14.12 (20.40, 0.00 ~ 21.00) | |
| PM2.5 (µg/m3) | Total | 40.53 (22.46, 24.40 ~ 49.65) | 38.37 (24.53, 22.01 ~ 46.40) | 40.01 (29.54, 21.20 ~ 48.20) | 39.93 (28.30, 21.65 ~ 48.20) |
| Control | 40.34 (22.20, 24.41 ~ 49.38) | 38.07 (24.01, 22.07 ~ 46.22) | 39.57 (29.25, 21.00 ~ 47.70) | 39.53 (27.98, 21.53 ~ 47.78) | |
| Case | 40.96 (23.04, 24.35 ~ 50.22) | 39.07 (25.69, 21.87 ~ 46.89) | 41.01 (30.17, 21.57 ~ 49.27) | 40.83 (28.98, 21.92 ~ 49.17) | |
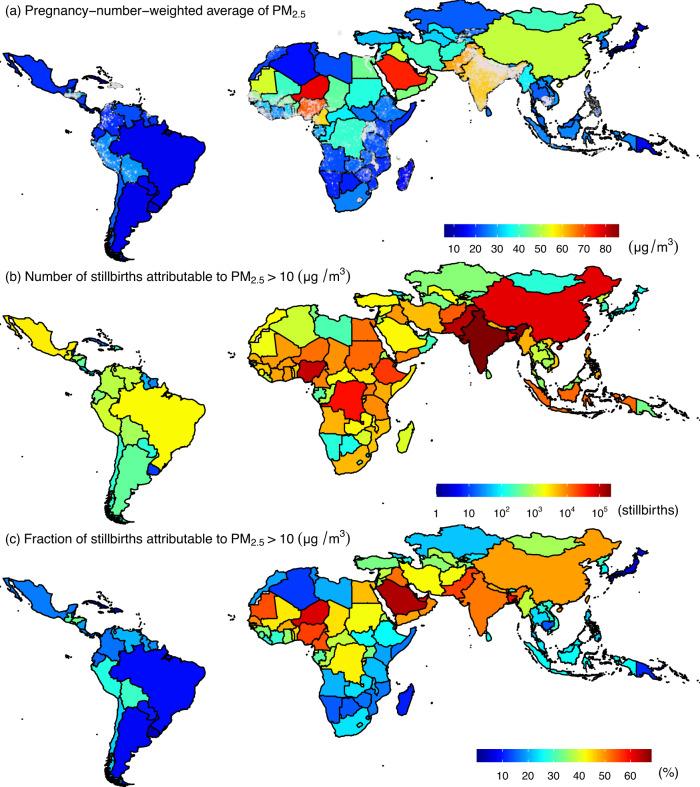
Fig. 1. Maps of stillbirths attributable to PM2.5 exposure in the 137 countries.
PM2.5 exposure (a), number (b), and fraction (c) of stillbirths attributable to exposure in 2015 in the 137 countries. Gray dots in panel (a) are the surveyed locations for the samples from 54 low- and middle-income countries used to derive the exposure–response curves.
We examined the linear association between PM2.5 and stillbirth. We found a robust association between PM2.5 levels and stillbirths; the level of significance was not sensitive to adjustments of different covariates (Supplementary Fig. 2), and the association was not heterogenous between most subpopulation groups except between different maternal ages (Supplementary Fig. 3). According to the fully adjusted model, each 10 μg/m3 increment in PM2.5 was associated with a 11.0% (95% CI: 6.4, 15.7) increased risk of stillbirth. Regarding secondary outcomes, the association was estimated to be −1.4% (95% CI: −5.3, 2.7), 2.3% (95% CI: 1.5, 3.2) or 0.5% (95% CI: −0.4, 1.4) for early stillbirth, miscarriage, or pregnancy loss, respectively (Supplementary Fig. 2). Among all subtypes of pregnancy loss, stillbirth was most strongly associated with PM2.5 exposure. In addition, advanced maternal age significantly enhanced the PM2.5–stillbirth association (P = 0.032). For more details on subpopulation indicators and subpopulation-specific associations, please see Supplementary Table 2 and Supplementary Fig. 3.
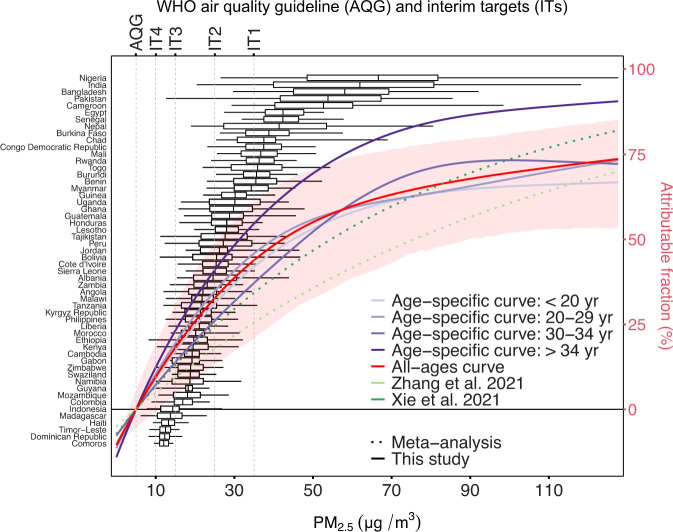
The linear models acted as the preliminary explorations for the development of nonlinear exposure–response curves. The findings suggested that the nonlinear curves should be stratified by maternal age. Using the fully adjusted model, we derived the nonlinear PM2.5–stillbirth associations with or without age-stratification (Fig. 2, Supplementary Fig. 4 and Supplementary Fig. 5). Generally speaking, the nonlinear models showed a sublinear curvature of the exposure–response relationships for both stillbirth and the secondary outcomes (Supplementary Fig. 4, and Supplementary Fig. 5). Consistent with the subpopulation-specific results estimated using linear models, the nonlinear curves generally showed a higher risk for mothers with more advanced maternal age, particularly those >34 years old. However, at different exposure levels, the significance of the modifying effect was different. At high exposure levels, the modification by age tended to be apparent (Fig. 2 and Supplementary Fig. 5). Therefore, a combination of the nonlinear curvature and the age’s modification could partially explain the heterogeneity between the country-specific linear associations between PM2.5 and stillbirth (Supplementary Fig. 6). In addition, uncertainties embedded in the pointwise estimates along the exposure–response curves were nonnegligible. For instance, according to the pointwise estimates, compared to the meta-analysis-based log-linear association reported by Xie et al.11, the sublinear curves showed higher risk at a low exposure level or lower risk at a high exposure level (Fig. 2). However, given the uncertainties, our exposure–response curves are comparable to those in prior meta-analyses (Fig. 2).
Fig. 2. Multiple exposure–response curves to link PM2.5 with stillbirth (right y-axis).
The all-ages curve and age-specific curves were derived from our self-matched case-control study of stillbirths from 54 countries (left y-axis). The corresponding PM2.5 exposures in those countries are shown as boxplots (The centre bars are medians; the box bounds and whiskers indicate for ranges from 25th to 75th and from 2.5th to 97.5th percentile, respectively.). The red ribbon is the pointwise 95% confidence interval for the all-ages curve.
Exposure assessment
Among the 137 countries, in 2015, the pregnancy-number-weighted average of PM2.5 was 43.24 μg/m3 for all populations at risk. Regarding age-specific subpopulations, the average of PM2.5 was 40.09, 44.8, 41.84, and 40.36 μg/m3 for pregnancies at maternal ages of <20, 20–29, 30–34, and >34 years. These results suggest that the exposure level among women at peak reproductive age (20–34 years) was higher than that in the adolescent pregnancy (<20 years old) and geriatric pregnancy (>34 years old) groups. Across locations where PM2.5 concentration was from 10 to 100 μg/m3, the proportion of pregnant women 20–29 years old was positively correlated with exposure level (Fig. 3). This correlation can in part explain the differences in age-specific PM2.5 averages. Population distributions by PM2.5 for different countries and regions are shown in Supplementary Fig. 7 and Supplementary Fig. 8, respectively.
Fig. 3. PM2.5 exposure distribution among the 137 countries.
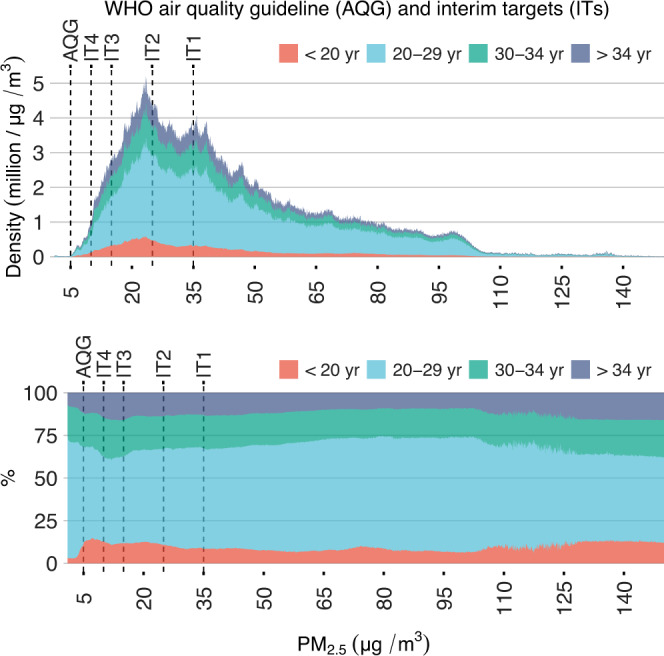
Distributions (top) and relative fractions (bottom) of age-specific populations at risk (i.e., pregnancies stratified by maternal age).
We also calculated the number of pregnancies exposed to PM2.5 above the WHO reference level. Of the pregnancies in the study domain, 99.96%, 98.87%, 93.51%, 73.16%, and 53.69% were beyond the WHO AQG (5 μg/m3), IT4 (10 μg/m3), IT3 (15 μg/m3), IT2 (25 μg/m3), and IT1 (35 μg/m3), respectively (Fig. 3). For the cleanest of the 137 countries, the Bahamas (pregnancy-number-weighted average of PM2.5 = 5.58 μg/m3), 82.90% of pregnancies were above the AQG. Therefore, the AQG might be too rigorous, and thus we selected IT4 as our major referent level for minimum risk (C0) in the assessment model (Eq. 4). For instance, in the dataset used to develop the exposure–response curves, no sample was exposed to PM2.5 < 5 μg/m3 (Fig. 2). Effects estimated based on the PM2.5–stillbirth curves for such low levels of exposure were extrapolations and thus unreliable. Country-level assessments of exposure are shown in Supplementary Table 3.
Risk assessment
According to UN IGME estimates2, globally, there were 2,131,914 stillbirths in 2015. Of them, 98.03% (2,089,918) were from the 137 countries in our assessment. In those countries, on average, 45.51% (95% CI: 29.24, 58.07), 39.66% (95% CI: 26.07, 50.85), 33.50% (95% CI: 22.77, 42.70), 22.05% (95% CI: 15.55, 27.71), and 13.45% (95% CI: 8.60, 17.68) of those stillbirths were attributable to gestational exposure to PM2.5 exceeding the WHO AQG, IT4, IT3, IT2, and IT1, respectively. The attributable fraction indicated a total number of 0.95 (95% CI: 0.61, 1.23) million, 0.83 (95% CI: 0.54, 1.08) million, 0.70 (95% CI: 0.48, 0.90) million, 0.46 (95% CI: 0.32, 0.58) million, and 0.28 (95% CI: 0.18, 0.37) million PM2.5-associated stillbirths in 2015, respectively. Considering the uncertainties, our results are not sensitive to use of different exposure–response curves. Referring to the main level of minimum risk (i.e., IT4), the number of stillbirths attributable to PM2.5 was estimated to be 0.81 (95% CI: 0.41, 1.12) million, 0.54 (95% CI: 0.38, 0.68) million, and 0.70 (95% CI: 0.30, 1.02) million according to the curve derived from the meta-analysis conducted by Xie et al.11, the meta-analysis by Zhang et al.15, or our all-ages model, respectively (Supplementary Fig. 9). Those estimates are comparable to the result from our main model: 0.83 (95% CI: 0.54, 1.08) (i.e., the age-specific curves).
The point-estimates from various PM2.5–stillbirth curves were affected by the referent exposure level of minimum risk used. When using a strict referent (e.g., AQG or IT4), point-estimates from the sublinear curves were larger than those from log-linear curves based on previous meta-analyses. When using a loose referent (e.g., IT1), among the four curves, the log-linear curve reported by Xie et al.11 produced the largest point-estimates (Supplementary Fig. 9). The difference could be explained by the estimated curvature of the exposure–response relationship. The sublinear curvature suggests that low-concentration exposure is more impactful than high-concentration exposure, whereas using a loose referent makes the risk assessment focus on the effect of high-concentration exposure.
Country-level estimates of the fraction and number of stillbirths attributable to PM2.5 are shown in Fig. 1 and Supplementary Table 4. Estimates from different exposure-response curves showed a similar spatial pattern (Supplementary Figure 10). The countries with the largest numbers of PM2.5-related stillbirths were India (217000 [95% CI: 151000, 279000]), Pakistan (110000 [95% CI: 77000, 142000]), Nigeria (93000 [95% CI: 51000, 137000]), China (64000 [95% CI: 42000, 81000]), and Bangladesh (49000 [95% CI: 35000, 61000]). The countries with the highest fraction of stillbirths attributable to PM2.5 were Qatar (71.16% [95% CI: 56.17, 80.88]), Saudi Arabia (68.38% [95% CI: 52.37, 79.06]), Kuwait (66.08% [95% CI: 48.76, 77.87]), Niger (65.68% [95% CI: 50.15, 76.77]), and the United Arab Emirates (64.63% [95% CI: 46.47, 76.45]). South Asia, sub-Saharan Africa, and the Arabian Desert were hotspots of PM2.5-related stillbirths, due to high exposure and baseline stillbirth rate.
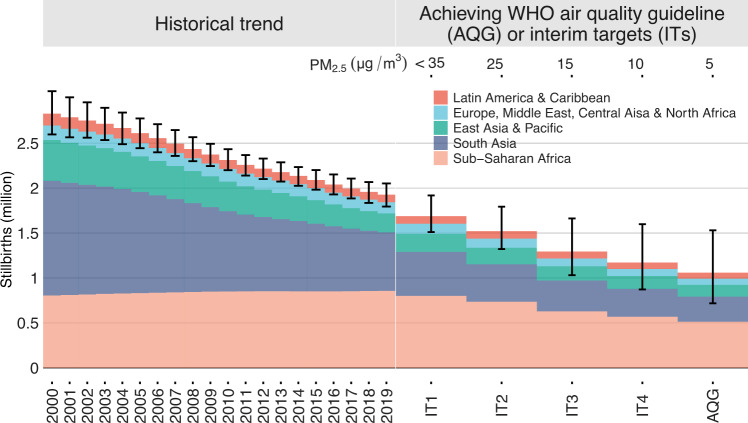
Within the study domain, the total number of stillbirths decreased at an annual rate of 1.95% (95% CI: 1.76, 2.15) from 2000 (2.83 [95% CI: 2.60, 3.08] million) to 2009 (2.37 [95% CI: 2.24, 2.49] million), and stably decreased by 2.05% (95% CI: 1.99, 2.12) from 2010 (2.31 [95% CI: 2.20, 2.43] million) to 2019 (1.93 [95% CI: 1.79, 2.05] million). By contrast, as shown in Supplementary Fig. 9, the number of stillbirths attributable to PM2.5 (>10 μg/m3) slowly decreased by 0.54% (95% CI: −0.03, 1.11) from 2000 (0.97 [95% CI: 0.59, 1.30] million) to 2009 (0.93 [95% CI: 0.61, 1.20] million), and the reduction rate increased to 2.84% (95% CI: 3.24, 2.43) from 2010 (0.90 [95% CI: 0.59, 1.15] million) to 2019 (0.71 [95% CI: 0.45, 0.92] million). These results suggest that the improved air quality in some of the 137 countries (e.g., China) might underlie the reduction in the global burden of stillbirths. Therefore, meeting the WHO air quality targets could prevent stillbirths. Achieving the IT1, IT2, IT3, IT4 or AQG target for long-term PM2.5 could reduce the number of stillbirths by 0.21 (95% CI: 0.13, 0.28) million, 0.37 (95% CI: 0.26, 0.47) million, 0.59 (95% CI: 0.39, 0.76) million, 0.71 (95% CI: 0.45, 0.92) million, or 0.83 (95% CI: 0.52, 1.08) million, respectively (Fig. 4). In terms of point-estimates, the health benefit from achieving AQG (0.83 million reduction) is comparable to the total number of stillbirths avoided by 20-year changes in all influencing factors (0.90 million reduction).
Fig. 4. Numbers of total stillbirths and remaining stillbirths after achieving PM2.5 targets set by the World Health Organization (WHO) among the 137 countries from 2000 to 2019.
The effects of achieving the targets were estimated from the age-specific PM2.5–stillbirth curves. Error bars are the corresponding 95% confidence intervals estimated by Monte Carlo approach.
Discussion
We explored the association between PM2.5 and stillbirth in LMICs and performed the first global risk assessment on PM2.5-related stillbirths. Based on the lower boundary of the main result (i.e., referring to IT4 and using the age-specific PM2.5–stillbirth curves), at least a quarter of stillbirths are attributable to PM2.5 exposure during gestation.
An association between stillbirths and PM2.5 has been reported in North America19,20, Europe21, East Asia13,22,23, South Asia14, and Africa10. Recently, the association was confirmed by evidence on outcomes closely related to stillbirth, including spontaneous abortion23 and miscarriage19. For instance, using the same epidemiological design as this study (i.e., a case-crossover design) to analyze 3583 women in the Nurses’ Health Study II, Gaskins et al.19 reported an OR for spontaneous abortion per 2.0 μg/m3 increment in PM2.5 of 1.10 (1.04, 1.17). Due to the limited sample size, we have reported ORs for an outcome combining early stillbirth and stillbirth for each 10 μg/m3 increment total PM2.5 of 1.09 (95% CI: 1.05–1.14) in Africa10 and 1.07 (1.02–1.12) in South Asia14, using DHS data. In this study, which included samples from South America and Southeast Asia, and excluding early stillbirths, the OR was estimated to be 1.11 (95% CI 1.06, 1.16), consistent with previous findings.
Although potential biological mechanisms for the association between PM2.5 exposure and pregnancy loss are not clear yet, some pathways can explain it to some extent. First, fine ambient particles may directly cross the placental barrier, and trigger hypoxic or immune-mediated injuries, which can cause irreversible embryonic damages leading to stillbirth24. Second, PM2.5 exposure during pregnancy has been reported to increase the maternal methemoglobin level, which can induce fetal oxidative stresses and inhibit the oxygen transport25. Finally, placental abnormalities are more frequently found in stillbirths than in livebirths26, and have been considered as possible mechanisms to explain the fetal deaths. PM2.5 exposure has positively correlated with placental global DNA methylation27, and suboptimal placental perfusion induced by the increased blood viscosity28, both of which can lead to placental dysfunctions.
Our study adds evidence verifying the adverse effect of PM2.5 on stillbirth, an important but neglected public health issue. According to an UN IGME study2, although the average rate of stillbirth decreased from 2000 to 2019, the trend was slower than that of the mortality rate of children under 5 years old, an indicator of maternal health. This suggests that efforts to promote maternal health were unequal for different adverse outcomes, and interventions relevant to stillbirths are inadequate. In addition, no decrease in the stillbirth rate was found for 34 countries in sub-Saharan Africa, 16 countries in East Asia and the Pacific, and 15 countries in Latin America and the Caribbean, which reflects the geographic inequality in efforts to prevent stillbirths. Current efforts to prevent stillbirth focus on medical service improvements, such as providing ongoing intrapartum monitoring and timely intervention in cases of complications, increasing coverage of prenatal care, and strengthening emergency obstetric care and caesarean section. Furthermore, our findings show that exposure to an adverse environment during pregnancy can increase the risk of stillbirth. Compared to clinical risk factors, environmental ones are usually unseen, due to their lesser effects at the individual level. However, prolonged exposure renders them of public-health importance. Clean air policies, which have been enacted in some LMICs, such as China29–31, can prevent stillbirths. In addition, personal protections against air pollution, i.e., wearing masks, installing air purifiers, avoiding going outside when air pollution occurs, could also protect vulnerable pregnant women.
There are some limitations of this study to mention. Those related to the development of PM2.5–stillbirth curves, including those on the data quality (e.g., underreporting of stillbirth, misclassified outcomes and recall bias), exposure assessment (e.g., exposure misclassification introduced by residential mobility during pregnancy, and limited temporal resolution in DHS data on gestational length), and overlooked confounders (particularly, longitudinal risk factors on birth outcomes, e.g., antenatal clinic attendance32, gestational hypertension and diabetes) are discussed in the previous reports10,12,14 and are not repeated here. For instance, underreporting stillbirths could not be completely avoided, and the potential bias due to underreporting or misclassifying of stillbirth has been evaluated by a simulation analysis12, which might cause an underestimated PM2.5–stillbirth association. Even though we used the best-available estimates on the global burden, this issue could lead to underestimated numbers of PM2.5–related stillbirths, and the bias might be also varied between countries. The current study included all eligible controls and the unbalanced number of controls might introduce bias. However, our previous studies examined other alternative control selections (i.e., healthy control, nearby control, and nulliparous control) and found the bias had little influence on the estimated associations. Additionally, in this study, our epidemiological models had considered the heterogeneity between countries as the random effects. Some limitations as mentioned above (e.g., incomparable data quality between countries) are reasons underlying the heterogeneity. Therefore, modelling heterogeneity could help to control for potential bias. Here, we focus on the limitations of the risk assessments. First, most risk assessments, including ours, assume that exposure–response curves are generalizable. In other words, the PM2.5–stillbirth curves derived from a sampled population were assumed to predict the risk in the general population. To improve the generalizability of the exposure–response curves used in the main assessment model, we controlled for between-country heterogeneity in the epidemiological models (Eqs. 1–2), examined whether subpopulation-specific effects of PM2.5 were homogenous, developed age-specific assessments to address the potential heterogeneity embedded in the effect of PM2.5, and derived sublinear curves to model the variation in the marginal effect of PM2.5 between regions with different levels of long-term exposure. For LMICs, where stillbirths were prevalent, the above efforts made our PM2.5–stillbirth curves more representative than those based on meta-analyses of evidence from both LMICs and high-income countries. However, the generalizability was still questionable. For instance, in most risk assessments, the toxicity of PM2.5 is assumed to be homogenous, which may be invalid. Particularly, in the sub-Saharan Africa and Arabian Desert areas, hotspots of PM2.5-related stillbirths (Fig. 1), PM2.5 is rich in dust particles. Whether exposure to dust particles is associated with stillbirths is unknown and should be investigated. Therefore, risk-assessment results in those regions should be interpreted with caution. In future, data on PM2.5 components from multiple sources should be collected and ensembled for relevant exposure and risk assessment. Second, the stillbirth risk in this study is not independent of other health impacts from PM2.5, and thus our results should not be interpreted as an extra burden that adds to previous findings, such as preterm births attributable to PM2.5. Short gestation, an outcome included in GBD assessment of PM2.516, increases the probability of stillbirth, and thus can act as a mediating pathway for the PM2.5–stillbirth association. In our models, because we did not adjust for gestational duration, the exposure–response curves present the total effect of PM2.5, including the indirect effect mediated by short gestation and the direct effect on stillbirth. Finally, due to the limited data, our assessments were annual and at the country level, limiting the policy implications of the findings. Gestational PM2.5 exposure can vary seasonally, so that the risk of PM2.5-related stillbirth varies throughout the year. Planning conception for a particular time can reduce gestational PM2.5 exposure, but this requires further risk assessment studies with a fine temporal resolution. In conclusion, we developed age-specific exposure–response curves to assess the risk of stillbirth attributable to gestational PM2.5 exposure using individual-level data from 54 LMICs and applied the curves to evaluate the disease burden in 137 countries in which 98% of global stillbirths occur. We found that PM2.5 exposure contributed to 39.7% (95% CI: 26.1, 50.8) of stillbirths in the 137 countries. Meeting the WHO air quality targets could thus prevent a considerable number of stillbirths.
Methods
Study population
Valid records of pregnancy outcomes were obtained from the Demographic and Health Surveys (DHS) program from 1998 to 2016. The DHS program is an ongoing series of standardized, nationally representative surveys conducted in more than 90 countries every 5 years. In each wave, samples are obtained via a multi-stage stratified cluster sampling approach to guarantee representativeness. Women aged 15–49 years are selected for in-depth surveys on a broad range of health issues, including maternal and child health, nutrition, fertility, and reproductive health. Details of the sampling frame, survey questionnaire, and data are available after completing a simple registration process followed by a request for access on the DHS website (https://www.dhsprogram.com/). Global positioning system (GPS) devices were used to collect geospatial information for each primary sampling unit (e.g., urban wards and rural villages) in most survey waves. From 113 geocoded surveys of 54 LMICs (Fig. 1a), we extracted variables related to reproductive history and individual-level demographic features (Table 1) regarding the analyzed mothers, as described previously10,14. For each mother, to minimize recall bias, we incorporated only the most recent case of pregnancy loss and all available controls in the study period. Additional details on the dataset and data preparation can be found on the DHS website and in previous publications10,14.
The major health outcome for this study was stillbirth, uniformly defined as babies born with no sign of life at ≥28 weeks of gestation for all the studied countries. Because the DHS reports gestation in months, here, all early terminated pregnancies with a gestational length ≥ 7 months were defined as stillbirths. For sensitivity analysis, we collected similar outcomes, including early stillbirth (gestational length 5–6 months), miscarriage (gestation < 5 months), and pregnancy loss (i.e., any of stillbirth, early stillbirth, or miscarriage).
Procedures and consent from all participants for DHS surveys have been reviewed and approved by ICF Institutional Review Board (IRB), and an IRB in the host country. This study is based on the publicly available DHS data, and thus no further ethic approval is required.
Environmental variables
In previous studies, we assessed monthly exposure to PM2.5 according to a combination of multiple datasets. Recently, a new study33 generated state-of-the-art monthly PM2.5 concentrations from 1998 to 2019 with a fine spatial resolution of 0.01° × 0.01° (~1 km × 1 km), by fusing chemical transport models, satellite remote sensing measurements, and geographic variables. Accordingly, we updated the exposure assessment by applying the new product, which was in good agreement with the ground-surface observations (R2 = 0.84).
To control for the effects of climate on stillbirth, we obtained monthly temperatures with a spatial resolution of ~50 km from the Modern-Era Retrospective analysis for Research and Applications (MERRA-2) from 1998 to 2016. We also used a satellite nightlight as an indicator of economic growth. Nightlight is correlated with several socioeconomic factors, such as population density and the regional product value, and has been used to explore the effect of developmental level on human health34. We obtained annual nightlight data with a raw spatial resolution of 1 × 1 km from a harmonized product from 1998 to 201635.
Epidemiological method to derive age-specific exposure–response curves
We performed a self-matched case-control study of the association between pregnancy loss and environmental exposures. The approach is documented in previous studies10,12, and is briefly summarized here. The design is similar to (but subtly different from) a case-crossover study36 or sibling-matched study37, and has been used to link air pollution and adverse outcomes, including stillbirth10,14, preterm birth36, and low birthweight37. We examined how PM2.5 exposure varied across multiple gestations of the same mother with different outcomes. Therefore, we focused on mothers who reported at least one stillbirth and created a fixed effect (θ) according to the mothers’ IDs. We used logit regression of the fixed effect (also known as a conditional logit regression) for epidemiological analysis. The model is as follows:
| 1 |
where the subscripts i and t denote the mother ID and time index of a specific gestation, pi,t denotes the probability of stillbirth, xi,t is the target exposure variable, β is the coefficient of the estimated effect, zi,t γ controls for the potential confounders, and θi is a nuisance parameter to control for the fixed effect. The fixed effect denotes all temporally invariant factors that affect stillbirth and includes variables such as genetics and geographic conditions (e.g., climate region). The linear effect was evaluated as the odds ratio (OR = eβ×10) of stillbirth per 10 µg/m3 increase in PM2.5. Because the spatial confounders are controlled for by the fixed effect, the covariates (zi,t) focus on longitudinal effects, including the nonlinear effect of maternal age, which is modeled as a spline with three degrees of freedom (DF), parity, the nonlinear effect of temperature as modeled by a 3-DF spline, seasonality as a 4-DF spline of month, and satellite nightlight. The covariates also included two random effect terms including the country-specific trends and country-specific slope of PM2.5 to control for heterogeneity.
The target exposure variable (xi,t) denotes the concentration of PM2.5 during a hazard time-window (HTM) for stillbirths. The controls and cases were matched for the HTM length. For each mother, the HTM started from the month of conception and its duration was determined by the gestational month of stillbirth. Therefore, we used HTMs of the same length for all samples (i.e., one case and one or multiple controls) affiliated with a mother. Therefore, the exposure indicator is termed the gestation-adjusted PM2.5, referring to a previous study38, and we have applied it in previous analyses12,37,39.
To estimate the age-specific nonlinear exposure–response curves, Eq. (1) was modified as follows:
| 2 |
where f denotes a set of thin-plate spline functions. To develop age-specific curves, regression coefficients for the spline terms were estimated by strata of four maternal-age groups (i.e., <20 years, 20–29 years, 30–34 years, and >34 years). We also generated an exposure–response curve for all ages from an alternative model, which estimated the coefficients for spline terms without age stratification. The exposure-response functions estimated by Eq. (2) or obtained from previous meta-analyses are documented in the Supplementary Data 1.
Exposure and risk assessment
The population-weighted mean of PM2.5 concentration or attributable fraction is used to present the level of exposure or relevant risk, respectively, in traditional assessment studies. For stillbirths, the population at risk was pregnant women, so the number of pregnancies, instead of the total population, should be used as the weight. Therefore, we obtained gridded maps of total pregnancy number (Ps) with a spatial resolution of 1 km × 1 km, in 2015, for 161 countries or regions17. To further quantify the age-specific populations at risk (Ps, k), in each pixel (s), we further divided the total number of pregnancies (Ps) using the following equation:
| 3 |
where s, k, and i denote the indexes for spatial pixel, age group, and country, respectively; s ∈ i is pixel–country relationship (i.e., the sth pixel is within the ith country); Ws,k is the gridded female population at a reproductive age (10–54 years); and Ri, k is the age-specific fertility rate for all pixels in ith country. Ws,k was obtained from WorldPop products on the sex/age-specific populations across the 1 km × 1 km grid in 201540; Ri, k was obtained from the estimates generated by the GBD study41. Ps,k was first derived according to 5-year age groups, and then the results were aggregated into four groups (i.e., <20 years, 20–29 years, 30–34 years, and >34 years) to be matched with age-specific exposure–response curves. Finally, the age-specific populations at risk (Ps, k) were used as the weights for exposure and risk assessment. Because gridded maps of pregnancies were only available in 2015, we assumed that the weights were constant, and selected the assessments in 2015 as the main results.
The country-level exposure and risk were assessed for 2000 to 2019, using the following equations:
| 4 |
where s, k, i, and y denote the indexes for spatial pixel, age group, country, and year, respectively; Cs,y is the gridded annual average of PM2.5 concentrations; C0 is a referent exposure level for minimum risk; Exposurei,y is the average exposure level of PM2.5 and Exposurei,y,k is an age-specific average; fk is an age-specific exposure–response curve, estimated from Eq. (2); AFs,k,y is the attributable fraction for a pixel and AFi,y is its country-level weighted mean; Ni,y represents the total number of stillbirths for the ith country and yth year; and ANi,y is the annual and country-level number of stillbirths attributable to PM2.5 exposure. C0 was selected from the air quality guideline (AQG) or interim targets (IT) on long-term concentration of PM2.5, launched by the World Health Organization (WHO) in 202142. Due to the computational burden, the empirical confidence intervals (CI) for AFi,y and ANi,y only considered the uncertainties embedded in the exposure–response curves and stillbirth baseline (Ni,y). We used a Monte Carlo approach to simulate the corresponding CIs. In sensitivity analyses, we replaced the age-specific exposure–response curves by (1) the all-ages curve estimated in this study, (2) the log-linear curve from the meta-analysis by Zhang et al.15 or (3) a log-linear curve from Xie et al.11.
All analyses were run in R software. Statistical inference for the regression models was performed using the R package survival.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by National Natural Science Foundation of China (42175182, T.X.), PKU-Baidu Fund (2020BD031, T.X.), Energy Foundation (G-2208-34045, G-2107-33169, and R-2109-33379, T.Z.), and CAMS Innovation Fund for Medical Sciences (2017-I2M-1-004, T.G.).
Author contributions
Study design: T.X.; Draft of the manuscript: T.X. & M.T.; Data preparation: T.X., Jia.L., He.L., R.W., Ho.L. & Y.L.; Data analysis: T.X. & M.T.; Result interpretation: T.G., Jiw.L., & P.L.; Conception of the study: T.X. & T.Z.; All co-authors revised the manuscript together.
Peer review
Peer review information
Nature Communications thanks Benn Sartorius and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
The datasets used in this study are publicly available. The Demographic and Health Survey (DHS) dataset, PM2.5 dataset, MERRA-2, stillbirth number, and population datasets of pregnancies and age-specific females are available from https://www.dhsprogram.com/, https://sites.wustl.edu/acag/datasets/surface-pm2-5/, https://disc.gsfc.nasa.gov/datasets?project=MERRA-2, http://childmortality.org/, and https://www.worldpop.org/, respectively.
Code availability
The R codes for the epidemiological analyses are documented in the Supplementary Data 2. The R codes and relevant data to reproduce the figures are also within Supplementary Data 2.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tao Xue, Mingkun Tong.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-022-34250-4.
References
- 1.Hug L. et al. A neglected tragedy the global burden of stillbirths: report of the UN inter-agency group for child mortality estimation, 2020. United Nations Children’s Fund (2020).
- 2.Hug L, et al. Global, regional, and national estimates and trends in stillbirths from 2000 to 2019: a systematic assessment. Lancet. 2021;398:772–785. doi: 10.1016/S0140-6736(21)01112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lawn JE, et al. Stillbirths: rates, risk factors, and acceleration towards 2030. Lancet. 2016;387:587–603. doi: 10.1016/S0140-6736(15)00837-5. [DOI] [PubMed] [Google Scholar]
- 4.You D, Hug L, Ejdemyr S. Global, regional, and national levels and trends in under-5 mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Inter-agency Group for Child Mortality Estimation (vol 386, pg 2275, 2015) Lancet. 2015;386:2256–2256. doi: 10.1016/S0140-6736(15)00120-8. [DOI] [PubMed] [Google Scholar]
- 5.India State-Level Disease Burden Initiative Child Mortality Collaborators et al. Subnational mapping of under-5 and neonatal mortality trends in India: the Global Burden of Disease Study 2000-17. Lancet. 2020;395:1640–1658. doi: 10.1016/S0140-6736(20)30471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quenby S, et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658–1667. doi: 10.1016/S0140-6736(21)00682-6. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson MD. Management of recurrent early pregnancy loss. J. Reprod. Med. 2006;51:303–310. [PubMed] [Google Scholar]
- 8.Swanson KM, Chen HT, Graham JC, Wojnar DM, Petras A. Resolution of depression and grief during the first year after miscarriage: a randomized controlled clinical trial of couples-focused interventions. J. Women’s Health (Larchmt.) 2009;18:1245–1257. doi: 10.1089/jwh.2008.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grauerholz KR, Berry SN, Capuano RM, Early JM. Uncovering Prolonged Grief Reactions Subsequent to a Reproductive Loss: Implications for the Primary Care Provider. Front Psychol. 2021;12:673050. doi: 10.3389/fpsyg.2021.673050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue T, Zhu T, Geng G, Zhang Q. Association between pregnancy loss and ambient PM2.5 using survey data in Africa: a longitudinal case-control study, 1998-2016. Lancet Planet Health. 2019;3:e219–ee225. doi: 10.1016/S2542-5196(19)30047-6. [DOI] [PubMed] [Google Scholar]
- 11.Xie G, et al. Maternal exposure to PM2.5 was linked to elevated risk of stillbirth. Chemosphere. 2021;283:131169. doi: 10.1016/j.chemosphere.2021.131169. [DOI] [PubMed] [Google Scholar]
- 12.Xue T, et al. Estimation of pregnancy losses attributable to exposure to ambient fine particles in south Asia: an epidemiological case-control study. Lancet Planet Health. 2021;5:e15–e24. doi: 10.1016/S2542-5196(20)30268-0. [DOI] [PubMed] [Google Scholar]
- 13.Liang Z, et al. Maternal PM2.5 exposure associated with stillbirth: A large birth cohort study in seven Chinese cities. Int J. Hyg. Environ. Health. 2021;236:113795. doi: 10.1016/j.ijheh.2021.113795. [DOI] [PubMed] [Google Scholar]
- 14.Xue T, et al. Open fire exposure increases the risk of pregnancy loss in South Asia. Nat. Commun. 2021;12:3205. doi: 10.1038/s41467-021-23529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H. et al. Ambient air pollution and stillbirth: An updated systematic review and meta-analysis of epidemiological studies. Environ. Pollut.278, 116752 (2021). [DOI] [PubMed]
- 16.Murray CJL, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James WHM, et al. Gridded birth and pregnancy datasets for Africa, Latin America and the Caribbean. Sci. Data. 2018;5:180090. doi: 10.1038/sdata.2018.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ha S, Mendola P. Are we ready to establish a causal relationship between air pollution and pregnancy loss? Lancet Planet. Health. 2019;3:e198–e199. doi: 10.1016/S2542-5196(19)30073-7. [DOI] [PubMed] [Google Scholar]
- 19.Gaskins AJ, et al. Air pollution exposure and risk of spontaneous abortion in the Nurses’ Health Study II. Hum. Reprod. 2019;34:1809–1817. doi: 10.1093/humrep/dez111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tong D. et al. Targeted emission reductions from global super-polluting power plant units. Nat. Sustain.1, 59–68 (2018).
- 21.Smith RB, et al. Impacts of air pollution and noise on risk of preterm birth and stillbirth in London. Environ. Int. 2020;134:105290. doi: 10.1016/j.envint.2019.105290. [DOI] [PubMed] [Google Scholar]
- 22.Kim JH, Choi YY, Yoo SI, Kang DR. Association between ambient air pollution and high-risk pregnancy: A 2015-2018 national population-based cohort study in Korea. Environ. Res. 2021;197:110965. doi: 10.1016/j.envres.2021.110965. [DOI] [PubMed] [Google Scholar]
- 23.Xue T. et al. Change in the number of PM2.5-attributed deaths in China from 2000 to 2010: Comparison between estimations from census-based epidemiology and pre-established exposure-response functions. Environ. Int.129, 430–437 (2019). [DOI] [PubMed]
- 24.Faiz AS, et al. Ambient air pollution and the risk of stillbirth. Am. J. Epidemiol. 2012;176:308–316. doi: 10.1093/aje/kws029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Green R, Sarovar V, Malig B, Basu R. Association of stillbirth with ambient air pollution in a California cohort study. Am. J. Epidemiol. 2015;181:874–882. doi: 10.1093/aje/kwu460. [DOI] [PubMed] [Google Scholar]
- 26.Ananthan A, Nanavati R, Sathe P, Balasubramanian H. Placental Findings in Singleton Stillbirths: A Case-control Study. J. Trop. Pediatr. 2019;65:21–28. doi: 10.1093/tropej/fmy006. [DOI] [PubMed] [Google Scholar]
- 27.Maghbooli Z, et al. Air pollution during pregnancy and placental adaptation in the levels of global DNA methylation. PLoS One. 2018;13:e0199772. doi: 10.1371/journal.pone.0199772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peters A, Doring A, Wichmann HE, Koenig W. Increased plasma viscosity during an air pollution episode: a link to mortality? Lancet. 1997;349:1582–1587. doi: 10.1016/S0140-6736(97)01211-7. [DOI] [PubMed] [Google Scholar]
- 29.Xue T, et al. Rapid improvement of PM2.5 pollution and associated health benefits in China during 2013–2017. Sci. China Earth Sci. 2019;62:1847–1856. doi: 10.1007/s11430-018-9348-2. [DOI] [Google Scholar]
- 30.Xue T. et al. New WHO Global Air Quality Guidelines Help Prevent Premature Deaths in China. Natl. Sci. Rev.9, (2022). [DOI] [PMC free article] [PubMed]
- 31.Xiao Q, et al. Tracking PM2.5 and O3 Pollution and the Related Health Burden in China 2013–2020. Environ. Sci. Technol. 2021;56:6922–6932. doi: 10.1021/acs.est.1c04548. [DOI] [PubMed] [Google Scholar]
- 32.Andegiorgish AK, Elhoumed M, Qi Q, Zhu Z, Zeng L. Determinants of antenatal care use in nine sub-Saharan African countries: a statistical analysis of cross-sectional data from Demographic and Health Surveys. BMJ Open. 2022;12:e051675. doi: 10.1136/bmjopen-2021-051675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Donkelaar A, et al. Monthly global estimates of fine particulate matter and their uncertainty. Environ. Sci. Technol. 2021;55:15287–15300. doi: 10.1021/acs.est.1c05309. [DOI] [PubMed] [Google Scholar]
- 34.Lane KJ, et al. Associations between Greenness, Impervious Surface Area, and Nighttime Lights on Biomarkers of Vascular Aging in Chennai, India. Environ. Health Perspect. 2017;125:087003. doi: 10.1289/EHP541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li X., Zhou Y., Zhao M., Zhao X. A harmonized global nighttime light dataset 1992–2018. Sci. Data7, 1–9 (2020). [DOI] [PMC free article] [PubMed]
- 36.Pereira G, Bell ML, Lee HJ, Koutrakis P, Belanger K. Sources of fine particulate matter and risk of preterm birth in Connecticut, 2000–2006: a longitudinal study. Environ. health Perspect. 2014;122:1117–1122. doi: 10.1289/ehp.1307741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li J, et al. Exposure to landscape fire smoke reduced birthweight in low- and middle-income countries: findings from a siblings-matched case-control study. Elife. 2021;10:e69298. doi: 10.7554/eLife.69298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebisu K, Malig B, Hasheminassab S, Sioutas C, Basu R. Cause-specific stillbirth and exposure to chemical constituents and sources of fine particulate matter. Environ. Res. 2018;160:358–364. doi: 10.1016/j.envres.2017.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Guan T, et al. Acute and chronic effects of ambient fine particulate matter on preterm births in Beijing, China: a time-series model. Sci. Total Environ. 2019;650:1671–1677. doi: 10.1016/j.scitotenv.2018.09.279. [DOI] [PubMed] [Google Scholar]
- 40.Pezzulo C, et al. Sub-national mapping of population pyramids and dependency ratios in Africa and Asia. Sci. Data. 2017;4:170089. doi: 10.1038/sdata.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, et al. Global age-sex-specific fertility, mortality, healthy life expectancy (HALE), and population estimates in 204 countries and territories, 1950–2019: a comprehensive demographic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1160–1203. doi: 10.1016/S0140-6736(20)30977-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide: executive summary. (2021). [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
The datasets used in this study are publicly available. The Demographic and Health Survey (DHS) dataset, PM2.5 dataset, MERRA-2, stillbirth number, and population datasets of pregnancies and age-specific females are available from https://www.dhsprogram.com/, https://sites.wustl.edu/acag/datasets/surface-pm2-5/, https://disc.gsfc.nasa.gov/datasets?project=MERRA-2, http://childmortality.org/, and https://www.worldpop.org/, respectively.
The R codes for the epidemiological analyses are documented in the Supplementary Data 2. The R codes and relevant data to reproduce the figures are also within Supplementary Data 2.