Abstract
Bartonella species represent important causative agents of blood culture-negative infective endocarditis (IE). Their diagnosis represents a challenge for microbiologists and often relies on serological and molecular tools. However, even if the sensitivity of blood culture remains low, it should not be definitely ruled out. Indeed, we report the unusual case of a 22 year-old Guinean homeless man diagnosed with an IE due to Bartonella quintana. Unexpectedly, conventional blood cultures were positive after 13 days of incubation. Subculture was obtained on blood and chocolate agar, after 15 days of incubation in a 5 % CO2 atmosphere. Bacterial identification was obtained up to the species level using molecular tools (16S rRNA gene amplification and sequencing). A literature review of B. quintana blood culture-positive IE was conducted and revealed eighteen similar reported cases on a 25-year period. This case illustrates that, despite low sensitivity, Bartonella IE may be diagnosed thanks to prolonged blood culture.
Keywords: Bartonella quintana, Blood culture-positive, Infective endocarditis, Molecular tools
Introduction
Despite microbiological advances, blood culture-negative infective endocarditis (BCNIE) still represent around 5 % of all endocarditis cases [1], leading to diagnostic and therapeutic dilemma. Bartonella species, notably Bartonella henselae and Bartonella quintana, represent usual cause of BCNIE [1], [2]. Indeed, it is estimated that 3–4 % of all cases of IE can be due to Bartonella spp. [1]. B. quintana is transmitted by the human body louse and is known as the agent of trench fever. It is also responsible for infective endocarditis (IE) especially in people living in poor hygienic conditions [1], [3], [4].
B. quintana is a slow-growing bacterium and its microbiological culture requirements are responsible for a low yield of blood cultures [1]. Therefore, serology and molecular techniques are essential diagnostic tools.
Here we present an uncommon case of B. quintana IE with conventional positive blood cultures after 13 days of incubation in an automated system (BD Bactec™).
Case presentation
A 22 year-old Guinean homeless man was admitted to the emergency department for increased retrosternal chest pain and dyspnea NYHA II. Living in France for two years, he was followed for a symptomatic aortic insufficiency related to an aortic stenosis and a bicuspid aortic valve. The patient was afebrile, without deterioration of his general status or weight loss. No alcohol or tobacco intake was reported. A mild inflammatory reaction (C reactive protein = 20 mg/L (reference range 0–5 mg/L) and procalcitonin = 0.23 µg/L (reference range 0–0.06 µg/L)) was observed. HIV serology was negative.
Given the increase of the dyspnea, a transthoracic echocardiography was performed and revealed an increased aortic insufficiency as well as an oscillating mass strongly suspected to be a vegetative valvular endocarditis. At that time, the patient was meeting the modified Duke criteria of possible IE. Three sets (aerobic and anaerobic) of blood bottles were collected and the usual incubation protocol of 7 days has been extended to 14 days because of IE suspicion. A probabilistic treatment for IE without microbiological documentation combining amoxicillin (16 g per day), cefazolin (8 g per day) and doxycycline (200 mg per day) was initiated. No embolic complication or extracardiac localization was discovered during the extension medical check-up.
Blood cultures remained sterile during the first days of hospitalization. Therefore, screening for causes of BCNIE was performed including serological testing for Lyme disease, Mycoplasma pneumoniae, Bartonella spp., Coxiella burnetii, Brucella spp., Chlamydia psittaci, and a specific polymerase chain reaction (PCR) for Tropheryma whipplei.
Given the severe aortic insufficiency and the strong suspicion of IE, a surgical indication was retained and an aortic valve replacement by a bioprosthetic valve was performed.
The aortic valve was sent for conventional culture and molecular analysis targeting microorganisms responsible for BCNIE; 16s rRNA gene, specific T. whipplei and C. burnetii PCR were negative. However, PCR for Bartonella spp. returned positive while specific PCR targeting B. henselae was negative. Simultaneously, serological analysis for B. henselae and B. quintana were positive with high IgG titers respectively 1:2560 and 1:1280. Taken together, these results confirmed the suspected diagnosis of B. quintana IE. The histological examination concluded to a subacute endocarditis with lymphoplasmacytic infiltration. Therefore, the antibiotic treatment was switched to a combination of gentamicin (3 mg per kg per day) and doxycycline (200 mg per day), according to the ESC recommendations [5].
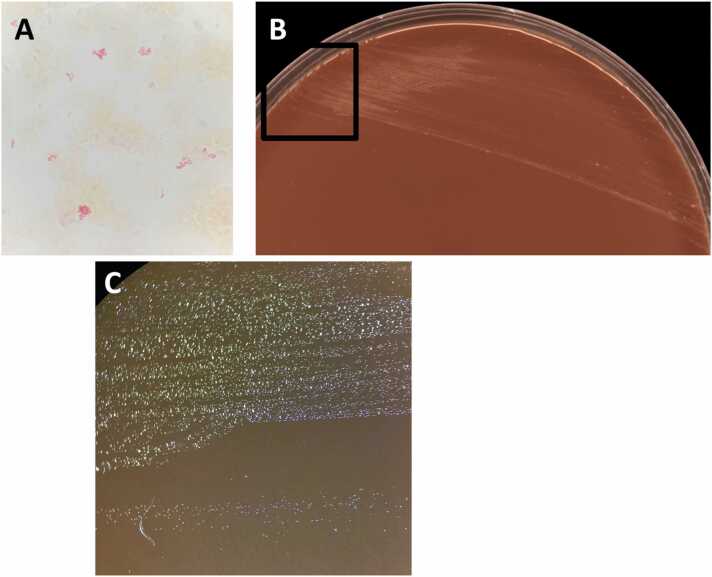
Surprisingly, two aerobic blood bottle cultures out of three were detected positive after 13 days of incubation while anaerobic blood bottle cultures remained sterile. Gram staining showed rod-shaped Gram-negative, poorly stained and pleomorphic bacilli (Fig. 1A). Given the context and previous results, a DNA extraction and a PCR assay selectively targeting Bartonella spp. and B. henselae (Realcycler® universal, Progenie molecular, Spain) were performed directly from the positive blood bottle. Bartonella spp PCR assay came back positive whereas specific PCR assay targeting B. henselae returned negative. Subcultures were performed on horse blood and chocolate agar plates (bioMérieux, Marcy-l′Étoile, France) and incubated at 35 °C in a 5 % CO2 atmosphere. After 15 days of incubation, tiny and shiny colonies were observed on both horse blood and chocolate agar plates (Figs. 1B and 1C). Catalase and oxidase reactions were negative. Vitek MS mass spectrometry identification was unsuccessful but neither B. quintana nor B. henselae was included in the current V3.2 version of the VitekMS database. After DNA extraction, 16S rRNA gene amplification and sequencing were performed with universal primers 27f and 1492r yield as previously described, yielding a 1246 bp-fragment [6]. The obtained fragment matched that of B. quintana with 99.7 % similarity according to BIBI database and 100 % according to BLAST analysis. Despite optimized microbiological culture, including aerobic and anaerobic blood bottles and systematic subculture on horse blood and chocolate agar plates, the valve culture remained sterile.
Fig. 1.
Gram staining of a positive blood bottle culture (Total magnification × 1000) (A); Bartonella quintana culture grown on chocolate agar plate after 15 days of culture (B) and a zoom of the black rectangular area (Total magnification × 15) (C).
After surgery and adjusted antibiotic treatment, the patient remained afebrile, and his recovery was promptly favorable. Follow-up blood culture during seven days after the treatment start was negative, and no clinical relapse was observed. As recommended, after an initial bitherapy with doxycycline and gentamicin, doxycycline (200 mg per day) was prescribed for five weeks after the surgical management [5]. After a one-month follow-up period and a favorable recovery, the patient was discharged from the hospital. Outcome remains favorable a year after the initial IE episode, with no evidence for relapse.
Discussion
B. quintana is a facultative intracellular, fastidious aerobic Gram-negative bacterium responsible for trench fever [1]. Humans represent the only known reservoir of this zoonotic bacteria transmitted by human body louse [1]. The first case of B. quintana IE was reported in 1993 in the USA [7], and since around a hundred cases have been described (79 cases were reported worldwide (excluding United Stated), and 13 cases in North America [8], [9]).
Major risk factors of B. quintana IE are low socioeconomic status, homelessness, HIV infection, and drug or alcohol abuse. A preponderance of the male gender is also observed [1], [3], [4], [10], [11], [12]. Most of the patients suffering from B. quintana IE did not have previously known valvular diseases, unlike our patient [1]. The aortic valve appeared to be the most frequently affected [9], [11], [12], [13], [14], [15], [16], as in the present case.
Laboratory diagnosis of Bartonella IE remains difficult because cultures are often negative. Serological and molecular analysis performed on blood or valvular tissue samples are thus frequently necessary to establish the diagnosis.
Even if serological sensitivity is high in IE cases, Bartonella spp. serology suffers from a lack of specificity, leading to a risk of false positive results. Indeed, cross-reactivity has been reported with Chlamydophila pneumoniae [17] or Coxiella burnetii [18], both causative agents of IE. A cross-reactivity between B. henselae and B. quintana, is also frequently reported, as observed in the reported case, making diagnosis up to the species level difficult [1]. Therefore, molecular tools remain the cornerstone of Bartonella spp. IE diagnosis allowing identification to the species level. 16S rRNA gene PCR and sequencing or specific Bartonella spp. PCR may be performed using different molecular targets (internal transcribed spacer (ITS), RNA polymerase β-subunit-encoding gene (rpoB), and citrate synthase gene (gltA)) [17]. Sensitivity is higher when performed from valvular tissues (72 %) compared to whole blood or serum (58 %) [1].
If positive, culture from blood or infected tissues asserts to designate an active infection. However, the sensitivity of culture remains very low: 20 % for blood culture and 30 % for valve culture [1]. Indeed, the growth of this bacterium on agar bases requires addition of different supplements like hemoglobin, erythrocytes, or hemin as well as extended incubation times. Blood or chocolate agar plates should be incubated under a CO2-enriched atmosphere (5 %) for at least 14 days and microbiologists should regularly and carefully look for tiny colonies [1]. If liquid medium supports the growth of Bartonella species, their detection from automated blood cultures system remains difficult partly because they produce little CO2. Surprisingly, Shepard and colleagues reported a high rate of positive blood culture (76.9 %) in a cohort of B. quintana IE. Unfortunately, they gave no information on the way bacterial culture were performed [19].
In culture-positive cases, identification of bacterial colonies represents another challenge. Indeed, MALDI-TOF mass spectrometry is rarely contributive because B. quintana spectrum is not included in the database. Thus, molecular methods are necessary.
Eighteen cases of B. quintana IE with positive blood culture were identified in PubMed database. The shortest time of blood cultures positivity was 10–12 days [15] and the average was 20 days. Cases with positive blood cultures remain uncommon and additional methods are suggested to improve culture sensitivity like the use of cell culture [4] or the use of acridine orange before the realization of subculture [1]. Lysis centrifugation was also described to encourage the growth of B. quintana in culture [20].
Conclusion
BCNIE diagnosis, including Bartonella species IE, still represents a challenge for microbiologists and often relies on serological and molecular tools. Even if the sensitivity of blood culture remains low, the reported case highlighted that B. quintana IE may be diagnosed thanks to prolonged blood culture, prolonged subculture under a 5 % CO2 atmosphere and accurate observation of blood agar/chocolate plates.
CRediT authorship contribution statement
Writing – original draft: Manon Robert and Anne-Gaëlle Leroy; Writing – review & editing: all; Clinical record reviewing/ data collection: Manon Robert; Technical support: Gaëlle Croizier; Supervision: Anne-Gaëlle Leroy and Stéphane Corvec.
Ethical approval
According to French and Europeans legislations, the use of anonymous data does not need the approval of the ethics committee.
Consent
According to French and Europeans legislations, patient’s consent was waived to use anonymous data. Patients of our hospital are systematically informed that their data may be used anonymously for research purpose.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
All authors report no conflict of interest relevant to this article.
Acknowledgments
The authors acknowledge the technical team for their help, especially Jihane El Khobzi and Gaëlle Croizier, as well as cardiology and infectious diseases teams.
References
- 1.Okaro U., Addisu A., Casanas B., Anderson B. Bartonella species, an emerging cause of blood-culture-negative endocarditis. Clin Microbiol Rev. 2017;30:709–746. doi: 10.1128/CMR.00013-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edouard S., Nabet C., Lepidi H., Fournier P.-E., Raoult D. Bartonella, a common cause of endocarditis: a report on 106 cases and review. J Clin Microbiol. 2015;53:824–829. doi: 10.1128/JCM.02827-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brouqui P., Lascola B., Roux V., Raoult D. Chronic Bartonella quintana bacteremia in homeless patients. N Engl J Med. 1999;340:184–189. doi: 10.1056/NEJM199901213400303. [DOI] [PubMed] [Google Scholar]
- 4.Drancourt M., Mainardi J.L., Brouqui P., Vandenesch F., Carta A., Lehnert F., et al. Bartonella (Rochalimaea) quintana endocarditis in three homeless men. N Engl J Med. 1995;332:419–423. doi: 10.1056/NEJM199502163320702. [DOI] [PubMed] [Google Scholar]
- 5.Habib G., Lancellotti P., Antunes M.J., Bongiorni M.G., Casalta J.-P., Del Zotti F., et al. 2015 ESC Guidelines for the management of infective endocarditis: the Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC) Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36:3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 6.Aubin G.G., Bémer P., Guillouzouic A., Crémet L., Touchais S., Fraquet N., et al. First report of a hip prosthetic and joint infection caused by Lactococcus garvieae in a woman fishmonger▿. J Clin Microbiol. 2011;49:2074–2076. doi: 10.1128/JCM.00065-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spach D.H., Callis K.P., Paauw D.S., Houze Y.B., Schoenknecht F.D., Welch D.F., et al. Endocarditis caused by Rochalimaea quintana in a patient infected with human immunodeficiency virus. J Clin Microbiol. 1993;31:692–694. doi: 10.1128/jcm.31.3.692-694.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lam J.C., Fonseca K., Pabbaraju K., Meatherall B.L. Case report: Bartonella quintana endocarditis outside of the Europe–African gradient: comprehensive review of cases within North America. Am J Trop Med Hyg. 2019;100:1125–1129. doi: 10.4269/ajtmh.18-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohammadian M., Butt S. Endocarditis caused by Bartonella Quintana, a rare case in the United States. IDCases. 2019:17. doi: 10.1016/j.idcr.2019.e00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foucault C., Barrau K., Brouqui P., Raoult D. Bartonella quintana bacteremia among homeless people. Clin Infect Dis. 2002;35:684–689. doi: 10.1086/342065. [DOI] [PubMed] [Google Scholar]
- 11.Guyot A., Bakhai A., Fry N., Merritt J., Malnick H., Harrison T. Culture-positive Bartonella quintana endocarditis. Eur J Clin Microbiol Infect Dis. 1999;18:145–147. doi: 10.1007/s100960050244. [DOI] [PubMed] [Google Scholar]
- 12.Spach D.H., Kanter A.S., Daniels N.A., Nowowiejski D.J., Larson A.M., Schmidt R.A., et al. Bartonella (Rochalimaea) species as a cause of apparent “culture-negative” endocarditis. Clin Infect Dis. 1995;20:1044–1047. doi: 10.1093/clinids/20.4.1044. [DOI] [PubMed] [Google Scholar]
- 13.Christiansen S., Fehske W., Autschbach R. Aortic valve endocarditis with Bartonella quintana – a rare entity. Herz. 2005;30:761–763. doi: 10.1007/s00059-005-2652-2. [DOI] [PubMed] [Google Scholar]
- 14.Montcriol A., Benard F., Fenollar F., Ribeiri A., Bonnet M., Collart F., et al. Fatal myocarditis-associated Bartonella quintana endocarditis: a case report. J Med Case Rep. 2009;3:7325. doi: 10.4076/1752-1947-3-7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sondermeijer H.P., Claas E.C.J., Orendi J.M., Tamsma J.T. Bartonella quintana prosthetic valve endocarditis detected by blood culture incubation beyond 10 days. Eur J Intern Med. 2006;17:441–443. doi: 10.1016/j.ejim.2006.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Yoda M., Hata M., Sezai A., Unosawa S., Furukawa N., Minami K. First report of Bartonella Quintana endocarditis in Japan. Circ J. 2008;72:1022–1024. doi: 10.1253/circj.72.1022. [DOI] [PubMed] [Google Scholar]
- 17.Ghidey F.Y., Igbinosa O., Mills K., Lai L., Woods C., Ruiz M.E., et al. Case series of Bartonella quintana blood culture-negative endocarditis in Washington, DC. JMM Case Rep. 2016:3. doi: 10.1099/jmmcr.0.005049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rahimian J., Raoult D., Tang Y.-W., Hanna B.A. Bartonella quintana endocarditis with positive serology for Coxiella burnetii. J Infect. 2006;53:e151–e153. doi: 10.1016/j.jinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Shepard Z., Barahona L.V., Montalbano G., Rowan S.E., Franco-Paredes C., Madinger N. Bartonella quintana infection in people experiencing homelessness in the Denver metropolitan area. J Infect Dis. 2022:jiac238. doi: 10.1093/infdis/jiac238. [DOI] [PubMed] [Google Scholar]
- 20.Maurin M., Raoult D. Bartonella (Rochalimaea) quintana infections. Clin Microbiol Rev. 1996;9:273–292. doi: 10.1128/CMR.9.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]