Abstract
Aortobronchial fistula often requires emergency surgery as it causes rapidly progressing respiratory failure. Some patients present with recurrent hemoptysis episodes. We report the case of a patient who was saved by elective surgery after aortobronchial fistula following aortic pseudoaneurysm rupture into the lung. An 84-year-old man, who had undergone mechanical Bentall replacement of the ascending aorta 20 years ago, complained of persistent fever. Computed tomography indicated a pseudoaneurysm at the distal anastomosis of the ascending aorta, and an urgent surgery was planned. During hospitalization, his aneurysm ruptured, suddenly penetrating the right lung and triggering acute respiratory failure and unconsciousness before the planned urgent operation. Mechanical ventilation was immediately provided; his respiratory status remarkably improved through intensive care and medical therapy. After nutritional status recovery, partial arch replacement was performed as an elective operation without any intervention for the injured lung. He did not exhibit any respiratory or neurological complications postoperatively.
Keywords: Cardiovascular, surgery, infectious diseases, aortobronchial fistula, arch replacement, rupture into the lung, pseudoaneurysm
Introduction
Aortobronchial fistula (ABF) following aortic pseudoaneurysm rupture into the lung can be managed by emergency operation because it causes rapidly progressing respiratory failure. Occasionally, the salvage treatment has to be given up owing to co-morbidity or severe patient frailty. However, there is limited knowledge regarding the life-saving possibility of elective surgery after long-term medical treatment. Here, we report a case of ABF after Bentall procedure, successfully treated with elective surgery after 8-week medical therapy.
Case
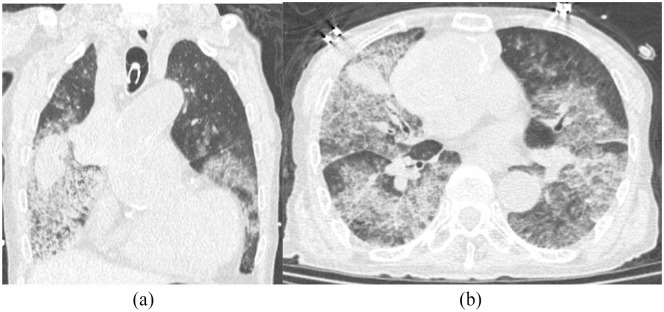
An 84-year-old man had undergone mechanical Bentall procedure and ascending aorta replacement at another hospital 20 years ago. One year prior, he began experiencing pain in multiple joints. Fever continued for 4 months, and he was admitted to our hospital for diagnosis. Computed tomography (CT) revealed a pseudoaneurysm (diameter: 87 mm × 50 mm) at the distal anastomosis immediately adjacent to the right upper lung (Figure 1), which increased rapidly by 20 mm over the previous 2 years. An elective surgery was planned. Thereafter, further examinations were performed. Blood culture, synovial fluid culture, fluorodeoxyglucose-positron emission tomography/CT, and magnetic resonance imaging were performed and did not indicate any infection. Then, the patient was consulted to a specialist. Polyarthritis was diagnosed, and steroid therapy was initiated. During the treatment, the patient suffered from pneumonia in the right lung, which was improved with Ceftriaxione administration (2 g/day for 1 week). After 2 weeks, he suddenly developed desaturation with hemoptysis, indicating intrapulmonary perforation (Figures 2 and 3). CT showed that the blood flow in the perforated right upper lobe of the lung had reached the left lung via the bronchi, with bilateral consolidation. The severe respiratory failure was managed using a respirator.
Figure 1.
Pseudoaneurysm (87 mm × 50 mm) at the distal anastomosis site: (a) axial view and (b) coronal view.
Figure 2.
Blood in the lung parenchyma (pink; white arrow) communicated with the pseudoaneurysm (green; block arrow): (a) frontal view and (b) right side view.
Figure 3.
Pulmonary window setting: (a) coronal view and (b) axial view.
However, owing to the respiratory condition’s severity as depicted by the low PaO2/FiO2 ratio (70), increased age, presence of frailty, invasive surgery including lung resection, and the high risk of surgery, we did not recommend emergency surgical treatment. Instead, we followed a conservative medical approach, including mechanical ventilation, blood transfusion, antibiotics infusion, and facilitating coagulation promotion. The patient received a vitamin K antagonist (VKA) for mechanical valve as the prothrombin time international normalized ratio was 2.54; however, the value was immediately reversed and normalized by VKA and fresh frozen plasma. According to the sputum culture results, piperacillin/tazobactam 4.5 g × 3 times/day and micafungin (MCFG) 150 mg/day were administered from the day of hemoptysis for 2 and 4 weeks, respectively. Thereafter, hemoptysis was not observed. The PaO2/FiO2 ratio was improved with recovering of consolidation on chest radiograph and pulmonary edema on CT, resulting in successful weaning from the ventilator; extubation was performed 2 weeks after the hemoptysis was resolved.
After confirming the improvement of respiratory function and recovery of activities of daily living (ADLs), we concluded that the patient could withstand surgery; therefore, elective surgery was planned.
The chest was opened without any injury, and core cooling was initiated with an additional venous cannula into the right atrium. The pseudoaneurysm protruded to the left and right side of the prosthetic graft and was firmly adhered to the right pulmonary artery, superior vena cava, and the right upper lobe of the lung. Partial arch replacement, including the brachiocephalic artery under deep hypothermic circulatory arrest, was performed. Cardiac arrest was obtained with antegrade cardioplegia infusion. For the pseudoaneurysm on the distal anastomosis, the intima of the aortic posterior wall was perforated halfway around (Figure 4(a)) and reinforced by a felt strip to the inner and outer sides using the inclusion technique. The aneurysmal sac communicated to this punched-out intimal defect. While strong adhesion around the right pulmonary artery was observed, the aneurysm sac, all thrombi, and the tissue around distal anastomosis of old prosthetic graft were completely removed. Regarding fistula detection, the apparent fistula was not recognized by the surrounding hematoma, and because of tissue fragility, radical debridement was not performed (Figure 4(b)). Interestingly, the fistula is usually small and could be covered with hematoma.
Figure 4.
(a) Perforated intima of the aortic posterior wall. (b) Fistula covered by the surrounding hematoma.
Then, partial arch replacement with brachiocephalic artery reconstruction (Triplex advance; Terumo Corp., Tokyo, Japan) was performed (Figure 5). There were concerns regarding pulmonary hemorrhage from the injured lung after withdrawal from the cardiopulmonary bypass; however, bleeding was not observed. The ventilator was withdrawn on the same day and the patient did not exhibit any neurological complications. MCFG was resumed after surgery for 2 weeks of infusion and 2 weeks of oral administration. No evidence of fever or further infection was found during hospitalization.
Figure 5.

Postoperative three-dimensional computed tomography.
Despite the patient’s severe frailty owing to the preoperative conservative treatment, his conditions improved, he gradually began walking with mobility aids, and was transferred to a different hospital for rehabilitation on day 76. At 3 months postoperatively, he had a gastrointestinal perforation due to ileus and transferred to another hospital.
Discussion
Most ABF cases reportedly occur after prosthetic vascular graft replacement in the thoracic aorta;1 they are strongly associated with pseudoaneurysms.2 Picichè et al.3 reported a pseudoaneurysm similar to true aneurysms that grows at a faster rate and progressively enlarges, causing airways compression. This causes a local inflammatory response and pressure necrosis, further increasing the inflammatory process. Stable adhesions may also occur.4 In addition, the chronic pulsation of the pseudoaneurysm causes lung damages. Moreover, when the wall tension of the pseudoaneurysm increases significantly, it ruptures into the lungs.5 The degree of hemoptysis may depend on the fistula size. The fistula is usually small and is easily blocked by clots. Although it can remain closed for weeks or months, when the clots resolve or dislocate, a new bleeding may occur.3
CT, bronchoscopy, and transesophageal echocardiography are common modalities to diagnose ABF.6 Although bronchoscopy is the most sensitive and specific method for confirming the bleeding source, it may be harmful by dislodging clots overlying the fistula tract.7 ABF is often deadly without treatment and surgical or endovascular treatment8,9 should be performed without delays to prevent clot dislocation and hemoptysis. Despite the reported relation between ABF and endovascular repair, further issues remain, including ischemic necrosis of the bronchial wall,8 chronic endoleak into the adjacent lung leading to erosion, stent graft penetration through the aortic wall into the lung,10 and recurrence of ABF and infection.9 As a salvaging operation, zone zero thoracic endovascular aortic repair could be considered; however, open surgical repair was selected here because the false aneurysm infection could not be denied.
In this case, as the clots did not resolve or dislocate, new bleeding did not occur and the patient was successfully managed through surgery. Hemostasis was achieved with appropriate blood pressure management, clotting factor supplementation, and positive-pressure respiration in the intensive care unit. Surgery risks for lung perforation are high in extremely frail patients or those in critical conditions before surgery. Thus, instead of emergency surgery, elective surgery could be an option after the patient’s condition improves.
Conclusion
We encountered a rare case of anastomotic pseudoaneurysm rupture after thoracic aortic surgery accompanied by severe respiratory failure due to ABF. Cases with respiratory failure due to ABF may be treated with elective surgery. In high-risk cases where emergency surgery cannot be performed, if stabilization of the patient’s respiratory condition can be achieved instead of immediately resorting to best supportive care, elective surgery can save lives.
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Footnotes
Author contributions: AM: conceptualization and writing of the original draft. YM: reviewing, editing, and supervision. KY: data curation. TM and YK: data curation. All authors have read and approved the final manuscript.
Availability of data and materials: Data sharing is not applicable to this article as no data sets were generated or analyzed during this study.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval: Our institution does not require ethical approval for reporting this case.
Informed consent: Written informed consent was obtained from the patient for his anonymized information to be published in this article.
ORCID iD: Atsuyuki Mitsuishi  https://orcid.org/0000-0001-5851-2736
https://orcid.org/0000-0001-5851-2736
References
- 1. Favre JP, Gournier JP, Adham M, et al. Aortobronchial fistula: report of three cases and review of the literature. Surgery 1994; 115: 264–270. [PubMed] [Google Scholar]
- 2. Kazerooni EA, Williams DM, Abrams GD, et al. Aortobronchial fistula 13 years following repair of aortic transection. Chest 1994; 106: 1590–1594. [DOI] [PubMed] [Google Scholar]
- 3. Picichè M, De Paulis R, Fabbri A, et al. Postoperative aortic fistulas into the airways: etiology, pathogenesis, presentation, diagnosis, and management, presentation. Ann Thorac Surg 2003; 75: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 4. Sullivan KL, Steiner RM, Smullens SN, et al. Pseudoaneurysm of the ascending aorta following cardiac surgery. Chest 1988; 93: 138–143. [DOI] [PubMed] [Google Scholar]
- 5. Picichè M, De Paulis R, Chiariello L. Unusual origin and fistulization of an aortic pseudoaneurysm: “off-pump” surgical repair. Ann Thorac Surg 1999; 68: 1406–1407. [DOI] [PubMed] [Google Scholar]
- 6. Kpodonu J, Rodriguez-Lopez JA, Ramaiah VG, et al. Endoluminal graft therapy for the treatment of an aorto-bronchial fistula: mid-term follow-up. J Card Surg 2008; 23: 530–532. [DOI] [PubMed] [Google Scholar]
- 7. Piciche `M, De Paulis R, Fabbri A, et al. Postoperative aortic fistulas into the airways: etiology, pathogenesis, presentation, diagnosis, and management. Ann Thorac Surg 2003; 75: 1998–2006. [DOI] [PubMed] [Google Scholar]
- 8. Hu H, Spadaccio C, Zhu J, et al. Management of aortobronchial fistula: experience of 14 cases. J Card Surg 2021; 36: 156–161. [DOI] [PubMed] [Google Scholar]
- 9. Canaud L, Ozdemir BA, Bahia S, et al. Thoracic endovascular aortic repair for aortobronchial fistula. Ann Thorac Surg 2013; 96: 1117–1121. [DOI] [PubMed] [Google Scholar]
- 10. Girdauskas E, Falk V, Kuntze T, et al. Secondary surgical procedures after endovascular stent grafting of the thoracic aorta: successful approaches to a challenging clinical problem. J Thorac Cardiovasc Surg 2008; 136: 1289–1294. [DOI] [PubMed] [Google Scholar]