Abstract
This case report describes the first successful treatment of alopecia areata and atopic dermatitis with the Janus kinase 1 inhibitor upadacitinib in a paediatric patient. After minimal response to topical corticosteroids and spironolactone, improvements in hair growth on the scalp and body were noted after only 6 weeks of upadacitinib treatment.
Keywords: Alopecia areata, atopic dermatitis, Janus kinase inhibitors, hair diseases, skin diseases
Introduction
Alopecia areata (AA) is a genetically determined autoimmune disease that leads to hair loss. Alopecia onset is difficult to predict, with limited evidence on known triggers, and it has a significant impact on patients’ quality of life (QoL) due to its visible nature. It can wax and wane, and response to therapy varies by case. AA is a common subtype of alopecia and it leads to nonscarring hair loss. A recent North American study found a 0.11% prevalence rate of paediatric AA, with significantly higher incidence in Asian, Black and Hispanic children.1 AA of the scalp can present in diverse shapes and to various extent, from a single round patch to complete hair loss termed as alopecia totalis. One of the most common comorbidities of AA is atopic dermatitis (AD).2
AD is a chronic, intensely pruritic, and recurrent inflammatory disease. It is the most common inflammatory condition in children, with a lifetime prevalence estimated to be 10%–30%.3 AD is commonly associated with allergic rhinitis and asthma, referred to as the atopic triad. Similar to AA, AD also has a significant effect on QoL by impairing sleep, school and extracurricular activities as well being associated with psychosocial distress. AD treatments target both signs and symptoms of the disease. Systemic anti-inflammatory agents like cyclosporine and methotrexate are immunosuppressive and are generally restricted for those with severe, frequent flares who do not respond to topical corticosteroid treatment.4
In 2021, RINVOQ® (upadacitinib), an oral, once-daily selective Janus kinase (JAK) 1 inhibitor was approved in Canada for the treatment of adults and adolescents over 12 years of age with refractory moderate-to-severe AD.5 JAK inhibitors are a promising new treatment for AA, especially in those with chronic, severe types.6 Limited information is available on upadacitinib in paediatric AD cases with concurrent AA. We report the case of a 14-year-old adolescent with AA and AD treated successfully with upadacitinib.
Case report
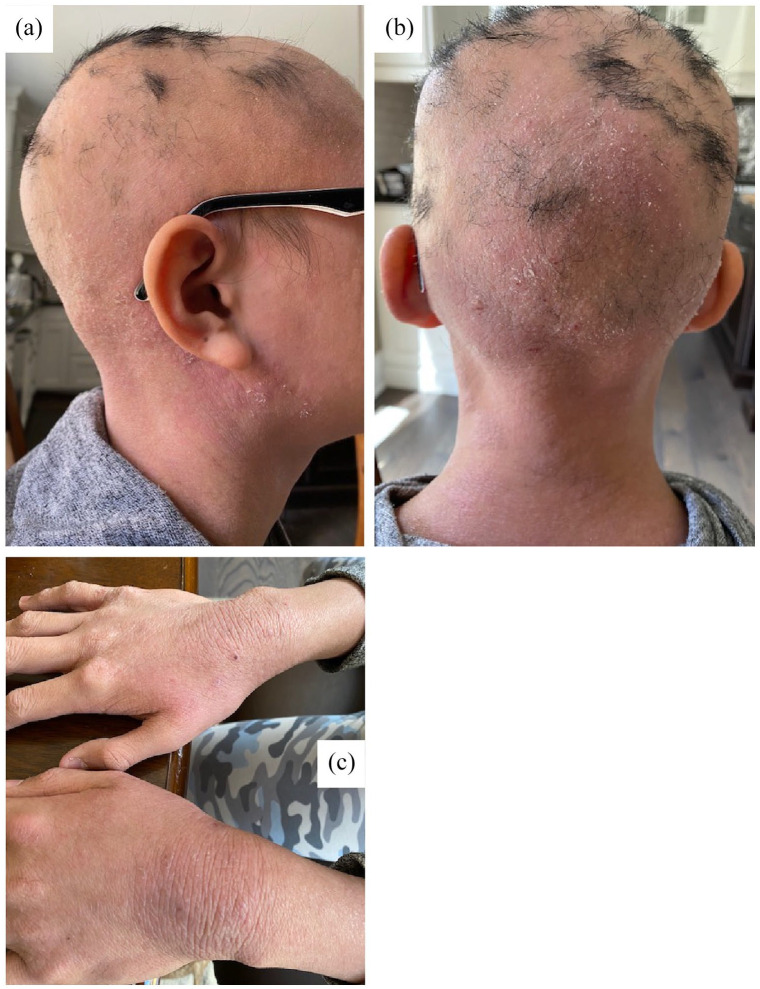
A 14-year-old male with Fitzpatrick phototype IV skin and a 13-year history of AA and a positive family history for alopecia presented to our dermatology clinic for evaluation of his hair loss. A full physical exam was deferred as the COVID-19 pandemic necessitated a virtual appointment, but photos demonstrated nonscarring alopecia totalis of the scalp with a few scattered tufts of hair and dry lichenified plaques on the bilateral dorsal wrists and neck (Figure 1). Photos also showed active and severe dermatitis on the scalp, face and hands with evidence of xerosis, erythema, scaling and lichenification. Subsequent physical exams in clinic revealed few additional findings, and dermoscopic evaluation showed exclamation point hairs, confirming the AA diagnosis.
Figure 1.
Clinical photographs of the patient at first appointment. Images were captured by the patient, which were reviewed. Photos of alopecia totalis of the scalp with a few scattered tufts of hair and erythematous dry skin. (a) Lateral view shows patches of hair in the parietal vertex. (b) Posterior view shows hair patches in the occipital vertex. (c) Picture of dorsal hands with severe and active dermatitis with xerosis, scaling and erythematous lichenification bilaterally.
Having initially developed alopecia as an infant, the patient described a waxing and waning course without full regrowth, initially affecting his scalp and later his eyebrows, sparing the eyelashes. A year prior, he was previously treated with intralesional corticosteroid injections with minimal improvement. He was also prescribed high potency topical corticosteroids and 0.1% protopic cream for the scalp. The patient also had concurrent eczema of the skin, later diagnosed as AD, and exhibited the atopic triad, with allergenic rhinitis and asthma. He intermittently applied topical corticosteroids during AD flare-ups. He had allergies to sesame seeds, grass, tree nuts and eggs but was otherwise fit and well and on no other medication. His family history was significant for androgenic alopecia in the father. He endorsed significant impacts on his QoL and self-esteem related to his alopecia.
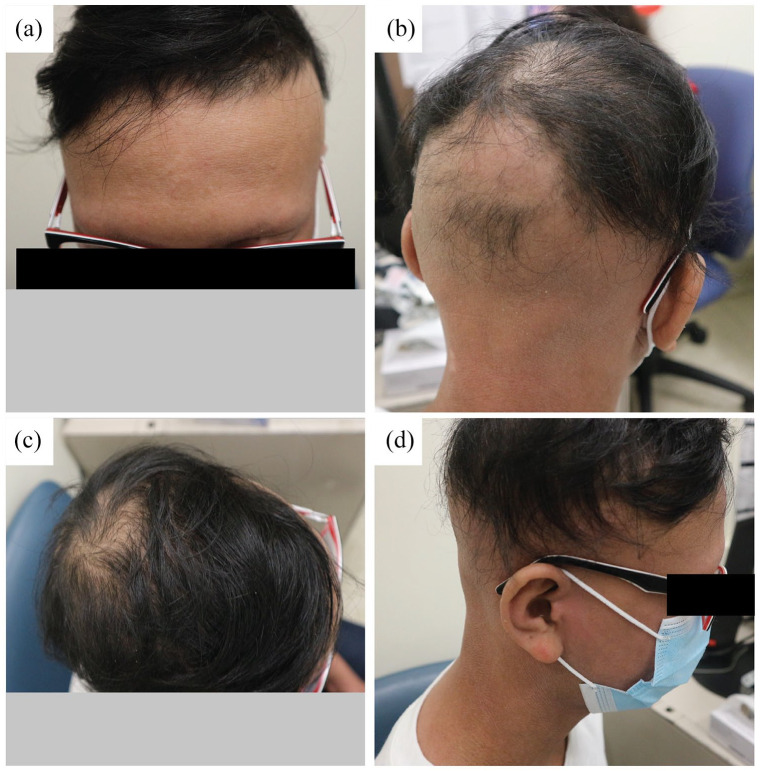
After the initial visit, early treatment strategies discussed included systemic immunosuppressive treatment with methotrexate. In addition, topical application of stronger potency steroids and supplementation with folic acid, vitamin D and iron were administered concurrently. Due to concerns by the caregiver about the adverse events associated with methotrexate, administration was delayed for 16 months. In the meantime, the alopecia worsened with no improvement in hair growth. Five months after initial assessment, both the AA and AD worsened with frequent flare-ups, and cyclosporine 5 mg/kg/day was initiated for AD management. Treatment was well tolerated, with new hair growth in the frontal scalp and noticeable improvements in AD after just a month (Figure 2), as well as further improvements in the eyebrows after 5 months.
Figure 2.
Clinical photographs of the patient after 1 month of treatment with cyclosporine. New hair growth in the frontal scalp. Confluent patches of smooth noon scarring alopecia in irregular areas of the occiput, vertex, parietal and temporal scalp. (a) Frontal, (b) posterior, (c) superior and (d) lateral view.
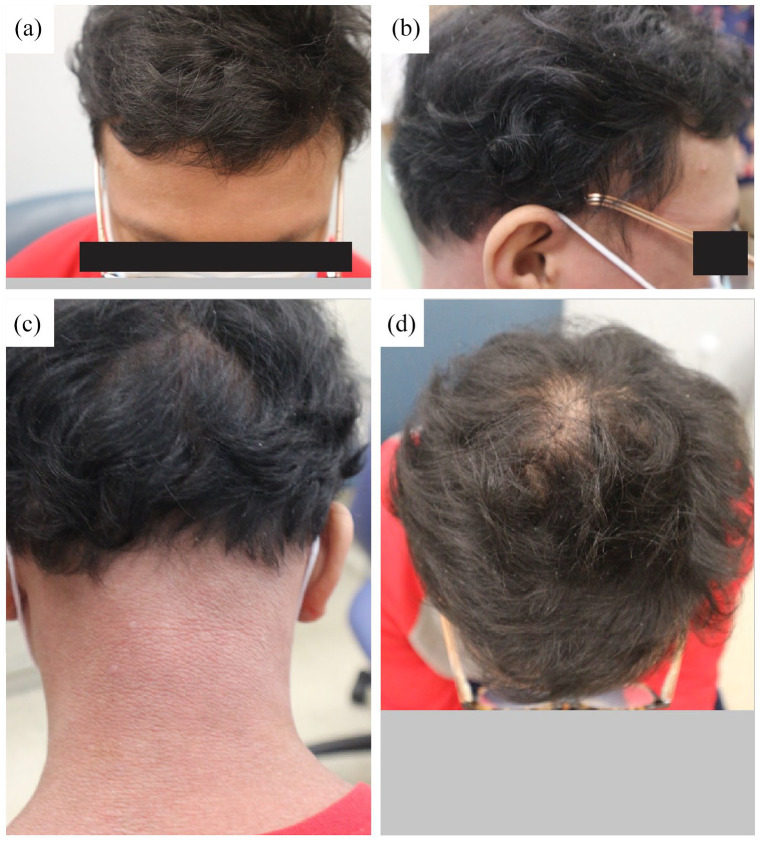
Unfortunately, after 7 months of cyclosporine, the patient reported new hair loss on the scalp and eyebrows in conjunction with a dermatitis flare-up. Low-dose systemic minoxidil (1.25 mg daily) was added to his regimen, but improvement in hair growth was minimal. Cyclosporin was discontinued and the family agreed to proceed with methotrexate, which was replaced with upadacitinib a month later. Improvements in both AA and AD were noted only 6 weeks after initiating upadacitinib, and by 5 months, the whole scalp was covered in hair (Figure 3). The patient continued to describe itching episodes, but his eczema also showed marked improvement.
Figure 3.
Clinical photographs of the patient after 5 months of treatment with upadacitinib. Significant hair growth in the entire scalp. (a) Frontal, (b) lateral, (c) posterior and (d) superior view of the scalp.
Discussion
Evidence on the dual efficacy of upadacitinib is limited.7,8 Here, we report for the first time a paediatric case with marked improvements in AD and AA after treatment with a recently approved JAK inhibitor, upadacitinib. One theory relates to the common downstream signalling cascade controlled by JAK inhibitors. Two of the four isoforms of the JAK family, namely, JAK1 and JAK3, interact with the intracellular domains of type I/II cytokine receptors.9
JAK phosphorylates activated cytokine receptors, allowing them to bind the transcription factor STAT, which upregulates the expression of various immune modulators.10 JAK1 activity promotes a number of interleukin signals that associate with the pathophysiology of AD and AA: specifically, interleukin (IL)-4, which promotes Th2 differentiation and associates with AA pathophysiology; interferon (IFN)-G and IL-15, which promote innate immunity via natural killer cell (NKC) differentiation and associate with AD; and IL6, which promotes innate immunity via phagocytosis and associates with AD.11–13 Thus, upadacitinib mitigates AD and AA through interleukin inhibition via JAK1/STAT suppression.
Upadacitinib was approved for AD indication after a large body of pre-clinical evidence was collected, and positive clinical trials were completed.5 Although upadacitinib has not been approved for AA indication, pre-clinical evidence and the theoretical rationale discussed above support its potential in treating this autoimmune disease. Herein, we outlined the patient outcome of an off-label indication for upadacitinib in treating AA. Alopecia and AD resolution was largely achieved by 6 weeks, and further improvements were noted by 5 months. Because JAK1 targets a multitude of interleukin pathways, including upstream promoters of Th2, NKC and macrophage activity, it is very possible that its inhibition can independently mitigate the pathophysiology associated with AA and AD. Conversely, it is also possible that upadacitinib’s inhibition of the JAK1/STAT pathway specifically treats the symptoms of AD, and that this improvement of AD symptoms indirectly leads to the resolution of AA. This second hypothesis is arguably supported by the comorbidity observed between AA and AD, with 17% of AA patients also developing AD.2
Clinical trials have shown promising results of using JAK1 inhibitors for the treatment of AD and theoretically on AA as they share pathogenetic aspects. In agreement with these data, our experience suggests that upadacitinib may be a therapeutic option not only for AD but also for patients with concurrent AA which has failed conventional therapy. Further investigations into JAK inhibitors are required to determine the long-term safety efficacy in AD and to elucidate the pathogenesis of AA.
Acknowledgments
The authors would like to acknowledge all Pediatric Dermatology staff at the Hospital for Sick Children who assisted in the care of this patient.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: C.S. has participated in advisory boards for Leo, Miravo, Pfizer, Sanofi; has received honoraria from AbbVie, Leo, Novartis, Pfizer, Sanofi and UCB and is an investigator for a trial with Arcutis.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Patient consent: The patients in this article have given written informed consent to publication of their case details.
ORCID iD: Adrienn N Bourkas  https://orcid.org/0000-0002-0664-8161
https://orcid.org/0000-0002-0664-8161
References
- 1. McKenzie PL, Maltenfort M, Bruckner AL, et al. Evaluation of the prevalence and incidence of pediatric alopecia areata using electronic health record data. JAMA Dermatol 2022; 158(5): 547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Conic RZ, Tamashunas NL, Damiani G, et al. Comorbidities in pediatric alopecia areata. J Eur Acad Dermatol Venereol 2020; 34(12): 2898–2901. [DOI] [PubMed] [Google Scholar]
- 3. Asher MI, Montefort S, Björkstén B, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006; 368(9537): 733–743. [DOI] [PubMed] [Google Scholar]
- 4. Maliyar K, Sibbald C, Pope E, et al. Diagnosis and management of atopic dermatitis: a review. Adv Skin Wound Care 2018; 31(12): 538–550. [DOI] [PubMed] [Google Scholar]
- 5. Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet 2021; 397(10290): 2151–2168. [DOI] [PubMed] [Google Scholar]
- 6. Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol 2019; 33(5): 850–856. [DOI] [PubMed] [Google Scholar]
- 7. Gambardella A, Licata G, Calabrese G, et al. Dual efficacy of upadacitinib in 2 patients with concomitant severe atopic dermatitis and alopecia areata. Dermatitis 2021; 32(1s): e85–e86. [DOI] [PubMed] [Google Scholar]
- 8. Cantelli M, Martora F, Patruno C, et al. Upadacitinib improved alopecia areata in a patient with atopic dermatitis: a case report. Dermatol Ther 2022; 35(4): e15346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwartz DM, Kanno Y, Villarino A, et al. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017; 16(12): 843–862. [DOI] [PubMed] [Google Scholar]
- 10. Hu X, Li J, Fu M, et al. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther 2021; 6(1): 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dillon KL. A comprehensive literature review of JAK inhibitors in treatment of alopecia areata. Clin Cosmet Investig Dermatol 2021; 14: 691–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howell MD, Kuo FI, Smith PA. Targeting the Janus kinase family in autoimmune skin diseases. Front Immunol 2019; 10: 2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Damsky W, King BA. JAK inhibitors in dermatology: the promise of a new drug class. J Am Acad Dermatol 2017; 76(4): 736–744. [DOI] [PMC free article] [PubMed] [Google Scholar]