Summary
A copper-mediated trifluoromethyltelluration of arylboronic acids with [Me4N][TeCF3] using air as an environmental friendly oxidant is presented. The reaction proceeded smoothly under mild conditions in the presence of Cu(OTf)2 and bipyridine to provide the corresponding trifluoromethyltellurated products in good yields. Vinylboronic acid and arylboronic acid pinacol ester were also suitable substrates in the conversion but the yields are low. This transformation featured simplicity, good functional group tolerance, and a wide range of substrates, allowing for a convenient access to various TeCF3-containing molecules, and represented the first Chan-Lam type trifluoromethyltelluration with the highly reactive [Me4N][TeCF3] salt.
Subject areas: Chemistry, Organic chemistry, Organic synthesis
Graphical abstract

Highlights
-
•
Mild reaction conditions and air as an environmental friendly oxidant
-
•
The first Chan-Lam cross-coupling with the elusive [Me4N][TeCF3] reagent
-
•
A useful method for the synthesis of various TeCF3-containing molecules
Chemistry; Organic chemistry; Organic synthesis
Introduction
Fluorine-containing functionalities are important design elements in the construction of pharmaceuticals, agrochemicals, and materials.1,2,3,4 To date, there have been more than 20% of pharmaceuticals and 30% of agrochemicals in the market containing at least one fluorine. Tellurium (Te) is a rare semi-metallic chalcogen and has unique semiconducting, photoconductive, thermoelectric, and acoustic-optic properties for applications in electronics, energy devices, sensors, imaging, and others.5,6,7,8 It can also be used as an antibacterial element with anti-leishmaniasis, anti-inflammatory, anti-atherosclerotic, and immunomodulatory properties.9,10,11 Given the well-recognized positive effects of fluorine on the physical, chemical, and biological properties of chemical entities, the TeCF3 group derived from the combination of trifluoromethyl moiety and tellurium would be an interesting and useful functionality in the fields of materials and life sciences.12,13 It was uncovered that the CF3 group strongly affects the polarization of tellurium center, reversing its properties from electronegative to relatively electropositive in RTeCF3,14 which possibly offers alternative binding modes in catalysis, chalcogen-bonding interaction, and bioactive systems.15,16 Although a variety of organotellurides have been utilized in synthesis, medicinal chemistry, and materials science,17,18,19 only few examples of CF3Te-containing compounds are harnessed because of the limited synthetic methods.
Although approaches for incorporation of OCF3, SCF3, and SeCF3 groups into organic molecules have been well documented in recent years,20,21,22,23,24,25,26,27,28 the synthesis of trifluoromethylated organotellurides has been rarely reported.29,30,31,32,33,34 The known trifluoromethyltelluration strategies include (1) the traditional trifluoromethylation of ditellurides with NaBH4 and CF3I or with TMSCF3 and KF, which was mainly employed by Umemoto and Togni in the development of Te-analogues of their famous electrophilic trifluoromethylation reagents,29,30 and (2) the nucleophilic substitution of several halides (e.g., acyl chlorides, benzyl bromide, methyl iodide, and methyl-4-bromobutyrate) with Me3SnTeCF3, KTeCF3 or [Me4N][TeCF3] as the TeCF3 reagent (Scheme 1).13,31,32,33,34 However, these approaches encountered obvious challenges, particularly if sensitive functionalities are present in the molecules, which led to very narrow substrate scope for the C-TeCF3 bond formation. Among the reported TeCF3 reagents, [Me4N][TeCF3] is more attractive as it is a thermally stable solid (decomposition at 185°C), easy to handle, and readily synthesized from Me3SiCF3, elemental tellurium, and [Me4N]F.31,32,33,34 Recently, Schoenebeck et al. disclosed a Pd-catalyzed trifluoromethyltelluration of aryl iodides with [Me4N][TeCF3], which represented the sole metal-catalytic direct introduction of TeCF3 group into organic scaffolds.14
Scheme 1.
Methods for C-TeCF3 bond formation
Encouraged by the previous transition-metal-catalyzed cross-coupling reactions of electrophilic or nucleophilic XCF3 (X = O, S, and Se) reagents with arylboronic acids,35,36,37,38,39,40,41,42,43,44,45 we set out to develop the first installation of TeCF3 functionality into these compounds. It has proved that the above-mentioned trifluoromethyl chalcogenylation exhibits different reaction profiles toward arylboronic acids in the construction of Ar-XCF3 (X = O, S, and Se) bonds, especially the Ar-OCF3 bonds, even though all X in XCF3 moieties belong to the chalcogen family.35,36,37,38,39,40,41,42,43,44,45 Given that organotellurides are much more easily oxidized by conventional oxidants in comparison with their sulfur and selenium analogues,34,46 the oxidative trifluoromethyltelluration with [Me4N][TeCF3] would be a challenging task as the overoxidation of [Me4N][TeCF3] and the products could be a potentially competing reaction, which might cause the formation of non-productive species. In this background, we employed copper salt and dry air as mild catalyst and oxidant to perform the cross-coupling of aryl boronic acid with the nucleophilic [Me4N][TeCF3] reagent.
Results and discussion
Optimization of reaction conditions
Pleasingly, when [1,1′-biphenyl]-4-ylboronic acid (1a) reacted with [Me4N][TeCF3] (2, 1.5 equiv) in acetone at room temperature under dry air (via balloon) for 4 h in the presence of Cu(OTf)2 (1 equiv) and 2,2′-bipyridine (bpy, 1 equiv), [1,1′-biphenyl]-4-yl(trifluoromethyl)tellane (3a) was formed in 83% yield (78% isolated yield) (Table 1, entry 1). The choice of copper salts had a central influence on the reaction. Taking CuOAc, CuI, Cu2O, CuSCN, CuOTf, Cu(MeCN)4BF4, Cu(MeCN)4PF6, CuO, Cu(OAc)2, Cu(acac)2, and CuSO4 instead of Cu(OTf)2 in the same conditions led to no formation of the desired product (3a) (Table 1, entry 2). The trifluoromethyltelluration with CuCl2 and CuBr2 gave very small amounts of 3a (4–6% yields) (Table 1, entries 3–4). Ligands could also affect the transformation. Replacement of 2,2′-bipyridine (bpy) with 4,4′-di-tert-butyl-2,2′-bipyridine (dtbpy), 4,4′-dimethyl-2,2′-bipyridine (dmbpy), 4,4′-dimethoxy-2,2′-bipyridine, N,N,N′,N′-tetramethylethylenediamine (TMEDA), and 1,10-phenanthroline (1,10-phen) under the standard conditions afforded 3a in lower yields (Table 1, entries 5–6 and Table S2 in the supplemental information). If phosphine ligands such as triphenylphosphine (PPh3) and tricyclohexylphosphine (PCy3)) were used in the same reactions, much poorer yields of 3a (4–21%) were obtained (Table 1, entries 7–8). Notably, when the trifluoromethyltelluration was performed in the absence of ligand under the standard conditions, 3a was formed in 53% yield (Table 1, entry 9). These results implicated that 2,2′-bipyridine was the optimal ligand and its presence could considerably improve the trifluoromethyltelluration. Furthermore, both acetone and DMF appeared to be the better solvents for the reaction as treatment of 1a and [Me4N][TeCF3] (1.5 equiv) with Cu(OTf)2/bpy (1 equiv) in other solvents (e.g., THF, toluene, DME, ethyl acetate, acetonitrile, and DMSO) under the same conditions afforded 3a in inferior yields (16–55%) (Table 1, entries 10–13 and Table S3 in supplemental information). Nonetheless, the use of undried acetone provided 3a in 39% yield (versus 83%), indicating that the residual water in solvent frustrated the trifluoromethyltelluration (Table 1, entry 14). Varying the reaction temperature from room temperature to 0°C or 50°C and increasing the equivalent of [Me4N][TeCF3] did not obviously change the yield of 3a (Tables S5 and S6 in supplemental information).
Table 1.
Cu-Mediated aerobic trifluoromethyltelluration of 1a with 2 under different conditions
| Entrya | Variation of the conditions | Yield (3a, %)b |
| 1 | none | 83 (78) |
| 2 | CuOAc, CuI, Cu2O, CuSCN, CuOTf, Cu(MeCN)4BF4, CuO, Cu(OAc)2, Cu(acac)2, and CuSO4 instead of Cu(OTf)2 | 0 |
| 3 | CuCl2 instead of Cu(OTf)2 | 6 |
| 4 | CuBr2 instead of Cu(OTf)2 | 4 |
| 5 | TMEDA instead of bpy | 48 |
| 6 | 1,10-phen instead of bpy | 62 |
| 7 | PPh3 instead of bpy | 21 |
| 8 | PCy3 instead of bpy | 4 |
| 9 | without ligand | 53 |
| 10 | DMF instead of acetone | 82 |
| 11 | THF instead of acetone | 55 |
| 12 | toluene instead of acetone | 51 |
| 13 | CH3CN instead of acetone | 16 |
| 14 | undried acetone | 39 |
| 15 | undried air instead of dry air | 78 |
| 16 | O2 instead of dry air | 74 |
| 17 | 8 h instead of 4 h in DMF | 60 |
| 18 | 1.5 equiv Cu(OTf)2/bpy was used | 74 |
| 19 | N2 instead of dry air, 24 h | 46 |
| 20 | 2.0 equiv Cu(OTf)2/bpy was used under N2, 24 h | 7 |
Reaction condition: 1a (0.2 mmol), [Me4N][TeCF3] (2, 0.3 mmol), [Cu] (0.2 mmol), bpy (0.2 mmol), acetone (2.5 mL), room temperature, 4 h, and dry air (via balloon).
The yields were determined by HPLC using pure [1,1′-biphenyl]-4-yl (trifluoromethyl)tellane (3a) as an external standard (tR = 10.10 min, λmax = 261 nm, water/methanol = 15/85 (v/v)). The isolated yield was depicted in the parenthesis.
Moreover, reaction of 1a, [Me4N][TeCF3] (1.5 equiv), and Cu(OTf)2/bpy (1 equiv) in acetone with undried air supplied 3a in a comparable yield (78 versus 83%) (Table 1, entry 15 versus entry 1). Using pure O2 instead of air in the same reaction provided 3a in a decreased yield (74 versus 83%) (Table 1, entry 16). It seemed that the stronger oxidant O2 (pure) slightly harmed the production of 3a. The standard reaction run for a longer time gave a lower yield of 3a as well (Table 1, entry 17). With prolonging the reaction time from 4 h to 12 h, the yield of 3a was gradually decreased (Table S4 in supplemental information). To our surprise, the equivalent of copper salt had a significant effect on the reaction (Table S7 in supplemental information). Reducing Cu(OTf)2 from 1 equiv to 0.2–0.8 equiv under the same conditions resulted in 0–16% yield of 3a. Increasing the equivalent of Cu(OTf)2 from 1 equiv to 1.5 equiv weakly prohibited the preparation of 3a (74% yield) (Table 1, entry 18). The lower yields obtained in the reactions using pure O2, longer reaction times, and excess Cu(OTf)2 might be caused by the decomposition or overoxidation of [Me4N][TeCF3] and the trifluoromethyltellurated product under these conditions. Furthermore, reaction of 1a with [Me4N][TeCF3] (1.5 equiv) and Cu(OTf)2/bpy (1 equiv) proceeded successfully under a N2 atmosphere, furnishing 3a in 46% yield after 24 h (Table 1, entry 19), which implied that the copper salt itself could be used as an oxidant in the trifluoromethyltelluration. Likewise, increasing the equivalents of Cu(OTf)2/bpy under the anaerobic conditions afforded 3a in much lower yields (Table 1, entry 20 and Table S8), which possibly supported again the negative impacts of excess copper salt on the reaction.
Substrate scope
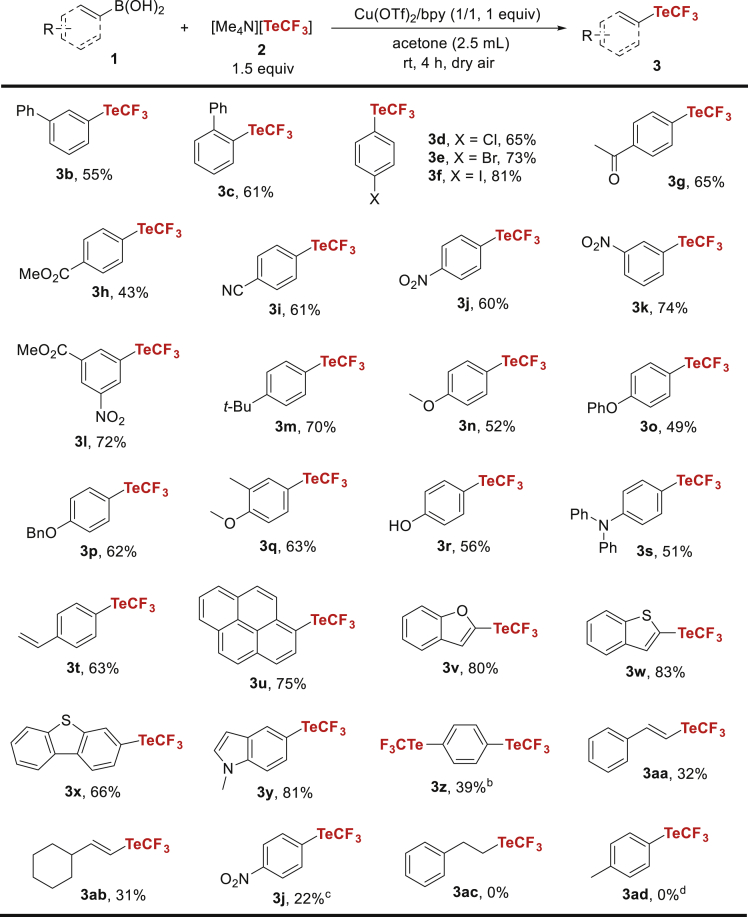
With establishment of the optimal reaction conditions (Table 1, entry 1), the substrate scope of this copper-mediated trifluoromethyltelluration was examined (Scheme 2). It was found that reactions of [1,1′-biphenyl]-3-ylboronic acid (1b) and [1,1′-biphenyl]-2-ylboronic acid (1c) with [Me4N][TeCF3] (1.5 equiv), Cu(OTf)2/bpy (1 equiv), and dry air in acetone at room temperature for 4 h afforded 3b in 55% yield and 3c in 61% yield. The chloro-, bromo-, and iodo-substituted arylboronic acids (1d-f) were well tolerated in the reaction, providing 3d-f in 65–81% yields. Boronic acids bearing strong electron-withdrawing groups such as keto (1g), ester (1h), cyano (1i) and nitro (1j-l) moieties on the phenyl rings were readily converted under the standard conditions to form the corresponding trifluoromethyltellurated products (3g-l) in 43–74% yields. Electron-rich arylboronic acids with tert-butyl, methoxy, phenoxy, benzyloxy, methyl/methoxy, hydroxyl, and diphenylamino substituents (1m-s) were also smoothly transformed in the reaction, furnishing the desired products (3m-s) in 49–70% yields. The position of substituents on the aryl rings of boronic acids had a little influence on the trifluoromethyltelluration (e.g., 3a versus 3b versus 3c and 3j versus 3k). The electronic features of the substituents showed no obvious laws of impacts on the transformation. In addition, (4-vinylphenyl)boronic acid (1t) and pyren-1-ylboronic acid (1u) reacted with [Me4N][TeCF3] in the presence of Cu(OTf)2/bpy and dry air to afford 3t in 63% yield and 3u in 75% yield, respectively. Heteroarylboronic acids such as benzofuran-2-ylboronic acid (1v), benzo[b]thiophen-2-ylboronic acid (1w), dibenzo[b,d]thiophen-3-ylboronic acid (1x), and (1-methyl-1H-indol-5-yl)boronic acid (1y) were also suitable substrates in the conversion, supplying 3v-y in 66–83% yields. 1,4-Phenylenediboronic acid (1z) underwent the similar functionalization to form the doubly trifluoromethyltellurated product (3z) in 39% yield. The reaction showed good functional group tolerance and a broad range of substrates. Besides, the present trifluoromethyltelluration was applicable to vinylboronic acids and arylboronic acid pinacol ester. The standard reactions of (E)-styrylboronic acid (1aa) and (E)-(2-cyclohexylvinyl)boronic acid (1ab) with [Me4N][TeCF3] and Cu(OTf)2/bpy provided 3aa and 3ab (E-configuration) in 31–32% yields without observation of the Z-isomers. The similar treatment of [Me4N][TeCF3] and Cu(OTf)2/bpy with 4,4,5,5-tetramethyl-2-(4-nitrophenyl)-1,3,2-dioxaborolane (1ac) instead of (4-nitrophenyl)boronic acid (1j) in the presence of K3PO4 under dry air at 60°C for 24 h gave 3j in 22% yield. These data suggested that vinylboronic acid and arylboronic acid pinacol ester were less reactive substrates in the reaction. Efforts to improve the yields of these products were made but failed because of the easy degradation of [Me4N][TeCF3] under the reaction conditions. Unfortunately, when phenethylboronic acid (1ad) and potassium trifluoro(p-tolyl)borate (1ae) reacted with [Me4N][TeCF3] and Cu(OTf)2/bpy under the standard or modified conditions, no desired products (3ac and 3ad) were observed, suggesting that the Cu-mediated aerobic trifluoromethyltelluration was not amenable to alkyl boronic acid and trifluoro(aryl)borate.
Scheme 2.
Cu-Mediated aerobic trifluoromethyltelluration of diverse arylboronic acids (1) with 2.a
a Reaction conditions: 1 (0.2 mmol), [Me4N][TeCF3] (0.3 mmol), Cu(OTf)2 (0.2 mmol), bpy (0.2 mmol), acetone (2.5 mL), rt, dry air, and 4 h. Isolated yields.
b DMF was used as a solvent instead of acetone.
c 4,4,5,5-Tetramethyl-2-(4-nitrophenyl)-1,3,2-dioxaborolane (1ac) was used instead of (4-nitrophenyl)boronic acid (1j). The reaction was run at 60 °C under dry air for 24 h with K3PO4 (0.2 mmol) as an additive.
d Potassium trifluoro(p-tolyl)borate (1ae) was used as a substrate.
Mechanistic studies
To probe the possible reaction mechanism, several NMR experiments were carried out (see the supplemental information). 19F NMR analyses of the reaction mixtures of 1a, [Me4N][TeCF3] (1.5 equiv), and Cu(OTf)2/bpy (1 equiv) in acetone under dry air at room temperature for 10 min, 0.5 h, 1 h, and 2 h showed the gradual production of 3a with elongation of the reaction time (Figures S1–S4). The trifluoromethyltelluration proceeded fast as the reaction run for 10 min provided 3a in 45% yield (Figure S1). Of interest, if [Me4N][TeCF3] (1.5 equiv), Cu(OTf)2/bpy (1 equiv), and acetone were mixed without 1a under dry air or N2 atmosphere at room temperature for 4 h, [Me4N][TeCF3] was completely transformed and the complicated mixtures were obtained according to the 19F NMR spectroscopy (Figures S5 and S6). Addition of 1a (1 equiv) to a mixture of [Me4N][TeCF3] (1.5 equiv), Cu(OTf)2/bpy (1 equiv), and acetone that was already maintained at room temperature under dry air or N2 for 4 h gave 3a in 3–4% or 36% yield(s) after reacting for another 4 h (Table S9). These data revealed that the timely trifluoromethyltelluration of 1a with [Me4N][TeCF3] and Cu(OTf)2/bpy was preferable as the stepwise addition of 1a to a prior mixture of [Me4N][TeCF3] and Cu(OTf)2/bpy led to much poorer yields of 3a under the standard conditions (Table S9). Surprisingly, when [Me4N][TeCF3] was exposed to dry air without using copper salts and 1a in acetone at room temperature for 4 h, no useful CF3 species were found and [Me4N][TeCF3] fully decomposed (Figure S7). Moreover, no signals of CF3TeTeCF347,48 were observed in the 19F NMR spectra of all trials (Figures S1–S7). These results indicated that (1) [Me4N][TeCF3] decomposed very fast under air, (2) the copper salt might stabilize the ‒TeCF3 anion by forming the relatively more stable [CuTeCF3] species, and (3) CF3TeTeCF3 was not generated and might not be the intermediacy of the reaction.
Plausible reaction mechanism
On the basis of the above findings and previous studies,49,50 a plausible reaction mechanism was suggested in Scheme 3. Initially, ligand exchange of Cu(OTf)2/bpy with [Me4N][TeCF3] (2) and arylboronic acid (1) forms a rational LCuII(aryl)(TeCF3) intermediate (4), which is oxidized in the presence of air to afford a LCuIII(aryl)(TeCF3) species (5). Then, the LCuIII(aryl)(TeCF3) complex (5) undergoes reductive elimination to provide the trifluoromethyltellurated product (3). Alternatively, reductive elimination of 4 in the absence of air can also form the final product (3) but with lower efficiency (entry 19, Table 1 and supplemental information). Because Cu(I) salts did not trigger the trifluoromethyltelluration (entry 2, Table 1), oxidation of the in situ formed Cu(I) species by air to regenerate Cu(II) or Cu(III) intermediate for sustaining the catalytic cycle might be excluded in the reaction. This may also be supported by the observation that the reactions with catalytic amounts of Cu(OTf)2 gave very low yields of the desired product (Table S7).
Scheme 3.
A plausible reaction mechanism for the Cu-mediated trifluoromethyltelluration
Conclusion
In conclusion, we have developed a convenient method for trifluoromethyltelluration of boronic acids with [Me4N][TeCF3] under air. The reaction proceeded smoothly at room temperature in the presence of Cu(OTf)2 to give the trifluoromethyltellurated products in good yields. Air was used as a cheap and environmental benign oxidant. Arylboronic acids bearing either electron-donating or -withdrawing groups as well as the sensitive functionalities on the aryl rings were readily converted to form the corresponding TeCF3 products. Vinylboronic acid and arylboronic acid pinacol ester were also suitable substrates in the trifluoromethyltelluration. The reaction showed advantages of simplicity, mild conditions, good functional group tolerance, and a wide range of substrates, which represents the first Cu-mediated Chan-Lam cross-coupling with the elusive [Me4N][TeCF3] reagent.
Limitations of the study
The choice of copper salt is very critical for the reaction. When other copper salt is used instead of Cu(OTf)2, none or extremely low yields of the product are obtained. In addition, this method is not suitable for trifluoromethyltelluration of alkylboronic acid and trifluoro(aryl)borate. [Me4N][TeCF3] is extremely sensitive to air.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| 4-Biphenylboronic acid | Meryer | CAS: 5122-94-1 |
| 3-Biphenylboronic acid | Meryer | CAS: 5122-95-2 |
| 2-Biphenylboronic acid | Bidepharm | CAS: 4688-76-0 |
| 4-Chlorophenylboronic acid | Bidepharm | CAS: 1679-18-1 |
| 4-Bromophenylboronic acid | Bidepharm | CAS: 5467-74-3 |
| 4-Iodophenylboronic acid | Bidepharm | CAS: 5122-99-6 |
| 4-Acetylphenylboronic acid | Bidepharm | CAS: 149104-90-5 |
| 4-Methoxycarbonylphenylboronic acid | Bidepharm | CAS: 99768-12-4 |
| 4-Cyanophenylboronic acid | Heowns | CAS: 126747-14-6 |
| 4-Nitrophenylboronic acid | Bidepharm | CAS: 24067-17-2 |
| 2-Nitrophenylboronic acid | Bidepharm | CAS: 5570-19-4 |
| 4-Methoxy-3-methylphenylboronic acid | Bidepharm | CAS: 175883-62-2 |
| 4-tert-Butylphenylboronic acid | Leyan | CAS: 123324-71-0 |
| 4-Methoxyphenylboronic acid | Bidepharm | CAS: 5720-07-0 |
| 4-Phenoxyphenylboronic acid | Macklin | CAS: 51067-38-0 |
| 4-Benzyloxybenzeneboronic acid | Leyan | CAS: 146631-00-7 |
| 4-Hydroxyphenylboronic acid | Macklin | CAS: 71597-85-8 |
| 4-(Diphenylamino)phenylboronic acid | Macklin | CAS: 201802-67-7 |
| 4-Vinylphenylboronic acid | Macklin | CAS: 2156-04-9 |
| 1-Pyreneboronic acid | Heowns | CAS: 164461-18-1 |
| 2-Benzofuranylboronic acid | Rhawn | CAS: 98437-24-2 |
| Benzothiophene-2-ylboronic acid | Macklin | CAS: 98437-23-1 |
| Dibenzothiophene-4-boronic acid | Meryer | CAS: 108847-20-7 |
| 1-Methylindole-5-boronic acid | Rhawn | CAS: 192182-55-1 |
| 1,4-Phenylenebisboronic acid | Bidepham | CAS: 4612-26-4 |
| (E)-Styreneboronic acid | Macklin | CAS: 6783-05-7 |
| (E)-(2-Cyclohexylvinyl)boronic acid | Macklin | CAS: 37490-33-8 |
| 1,4-Phenylenebisboronic acid | Bidepham | CAS: 4612-26-4 |
| Trifluoromethanesulfonicacidcopper(II) | Bidepharm | CAS: 34946-82-2 |
| 2,2′-Bipyridine | Bidepharm | CAS: 366-18-7 |
| Other | ||
| LC-100 II HPLC | WuFeng | https://www.wufengtech.com/about-wufeng/introduction |
| A360 Ultraviolet visible spectrophotometer | Aoyi Instrument | http://www.aoe-sh.com/aoesh-Products-13728467 |
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Cheng-Pan Zhang (cpzhang@whut.edu.cn, zhangchengpan1982@hotmail.com).
Materials availability
All materials generated in this study are available within the article and the supplemental information or from the lead contact upon reasonable request.
Method details
All reactions were carried out under a nitrogen atmosphere unless otherwise specified. The NMR spectra were recorded in CDCl3 or CD3CN on a 500 MHz (for 1H), 471 MHz (for 19F), or 126 MHz (for 13C) spectrometry. All chemical shifts were reported in ppm relative to TMS (0 ppm) for 1H NMR and to PhOCF3 (−58.0 ppm) for 19F NMR. The coupling constants were reported in Hertz (Hz). The following abbreviations were used to explain the multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet. The HPLC experiments were carried out on a Wufeng LC-100 II instrument (column: Shodex, C18, 5 μm, 4.6 × 250 mm), and the HPLC yields of the product were determined by using the corresponding pure compound as an external standard. MS experiments were performed on a TOF-Q ESI instrument. Melting points of the solid products were measured and uncorrected. Solvents were dried before use according to the literature.51 [Me4N][TeCF3] was synthesized according to the literature.33 Other reagents in the reactions were all purchased from the commercial sources and used without further purification.
Procedure for the synthesis of [Me4N][TeCF3] (2)
Under a nitrogen atmosphere, a Schlenk tube (500 mL) was charged with tellurium powder (8.83 g, 50 mmol, 1.0 equiv), anhydrous THF (300 mL), and TMSCF3 (8.89 g, 62.5 mmol, 1.25 equiv) with stirring and cooled to −60°C. Anhydrous [Me4N]F (4.65 g, 50 mmol, 1.0 equiv) was quickly added. After 1 hour at −60°C, the mixture was warmed to room temperature for 12 hours. The resulting mixture was filtered through a celite pad in a nitrogen-filled glovebox. The filtrate was discarded and the solid/celite was washed with MeCN (5 × 15 mL). The combined MeCN solutions were concentrated to roughly 30 mL and cooled to −30°C for 12 h. Then, Et2O (300 mL) was added. The precipitates were collected and dried under vacuum to give the product (2) as an off-white powder (5.67 g, 42%). 1H NMR (500 MHz, CD3CN) δ 3.14 (s, 12H). 19F NMR (471 MHz, CD3CN) δ 0.5 (s, 3F).
General procedure for Cu-mediated trifluoromethyltelluration of aryl boronic acid (1) with [Me4N][TeCF3] (2)
In a nitrogen-filled glovebox, a sealed tube was charge with 1 (0.2 mmol), 2 (0.3 mmol), Cu(OTf)2 (0.2 mmol), bpy (0.2 mmol), and acetone (2.5 mL), and was taken out of the glovebox. Then, air dried by anhydrous CaCl2 was introduced into the tube via a balloon. The mixture was reacted at room temperature for 4 hours and the solvent was removed under reduced pressure. The residue was purified by flash column chromatography on silica gel using a mixture of petroleum ether (pentane) and ethyl acetate as eluents to give the desired product (3).
[1,1′-Biphenyl]-4-yl(trifluoromethyl)tellane (3a)14
54.6 mg, 78% yield. Petroleum ether as eluent for column chromatography. Yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.08 (d, J = 8.1 Hz, 2H), 7.62–7.57 (m, 4H), 7.48 (t, J = 7.6 Hz, 2H), 7.41 (t, J = 7.3 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −25.4 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 142.1, 140.9, 138.9, 127.9, 127.5, 127.0, 126.2, 107.4, 101.7 (q, J = 354.0 Hz).
[1,1′-Biphenyl]-3-yl(trifluoromethyl)tellane (3b)
38.2 mg, 55% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.25 (s, 1H), 7.99 (d, J = 7.5 Hz, 1H), 7.70 (d, J = 7.7 Hz, 1H), 7.60 (d, J = 7.2 Hz, 2H), 7.48 (t, J = 7.4 Hz, 2H), 7.45-7.39 (m, 2H). 19F NMR (471 MHz, CDCl3) δ −25.2 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 143.0, 140.1, 140.0, 139.9, 130.1, 129.0, 128.9, 127.9, 127.2, 110.3, 102.8 (q, J = 353.4 Hz). IR (KBr): 3058, 3031, 2963, 1584, 1557, 1501, 1471, 1449, 1395, 1261, 1083, 1042, 1017, 866, 797, 753, 724, 697 cm−1. HRMS-ESI (m/z) calcd. for [C13H10F3Te]+ ([M + H]+): 352.9792; found: 352.9792.
[1,1′-Biphenyl]-2-yl(trifluoromethyl)tellane (3c)
42.9 mg, 61% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CD3CN) δ 8.04 (d, J = 7.8 Hz, 1H), 7.44-7.37 (m, 2H), 7.33-7.32 (m, 3H), 7.24 (td, J = 7.7, 1.9 Hz, 1H), 7.16 (m, 2H). 19F NMR (471 MHz, CD3CN) δ −27.4 (s, 3F). 13C NMR (126 MHz, CD3CN) δ 149.3, 144.2, 140.6, 130.1, 129.7, 128.9, 128.8, 128.4, 128.1, 114.4, 104.2 (q, J = 353.7 Hz). IR (KBr): 3057, 2924, 1718, 1647, 1579, 1460, 1446, 1322, 1296, 1248, 1083, 1019, 1007, 950, 749, 723, 702 cm−1. HRMS-ESI (m/z) calcd. for [C13H10F3Te]+ ([M + H]+): 352.9792; found: 352.9791.
(4-Chlorophenyl)(trifluoromethyl)tellane (3d)14
39.8 mg, 65% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 8.3 Hz, 2H), 7.33 (d, J = 8.3 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ −25.5 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 142.8, 137.1, 130.3, 107.2, 102.8 (q, J = 353.4 Hz).
(4-Bromophenyl)(trifluoromethyl)tellane (3e)14
51.5 mg, 73% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.85 (d, J = 8.3 Hz, 2H), 7.49 (d, J = 8.4 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ −25.4 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 143.0, 133.2, 125.5, 107.9, 102.7 (q, J = 353.5 Hz).
(4-Iodophenyl)(trifluoromethyl)tellane (3f)
64.8 mg, 81% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.71-7.67 (m, 4H). 19F NMR (471 MHz, CDCl3) δ −25.3 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 142.9, 139.1, 108.9, 102.7 (q, J = 353.5 Hz), 97.6. IR (KBr): 2924, 1554, 1468, 1375, 1252, 1115, 1081, 1070, 1044, 998, 802, 724, 686 cm−1. HRMS-ESI (m/z) calcd. for [C7H4F3INaTe]+ ([M + Na]+): 424.8265; found: 424.8279.
1-(4-((Trifluoromethyl)tellanyl)phenyl)ethan-1-one (3g)14
40.8 mg, 65% yield. A mixture of petroleum ether/ethyl acetate = 20/1 (v/v) as eluents for column chromatography. Yellow solid. 1H NMR (500 MHz, CDCl3) δ 8.08 (d, J = 8.2 Hz, 2H), 7.90 (d, J = 8.3 Hz, 2H), 2.62 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −24.7 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 197.5, 141.0, 138.0, 129.3, 116.0, 103.0 (q, J = 353.9 Hz), 26.6.
Methyl 4-((trifluoromethyl)tellanyl)benzoate (3h)
28.8 mg, 43% yield. A mixture of petroleum ether/ethyl acetate = 30/1 (v/v) as eluents for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.06 (d, J = 8.1 Hz, 2H), 7.99 (d, J = 8.0 Hz, 2H), 3.94 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −24.7 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 166.4, 140.9, 131.6, 130.6, 115.7, 103.0 (q, J = 353.2 Hz), 52.4. IR (KBr): 3003, 2955, 2840, 1728, 1590, 1562, 1437, 1391, 1285, 1184, 1105, 1082, 1012, 965, 849, 824, 800, 755, 724, 689 cm−1. HRMS-ESI (m/z) calcd. for [C9H7F3NaO2Te]+ ([M + Na]+): 356.9353; found: 356.9358.
4-((Trifluoromethyl)tellanyl)benzonitrile (3i)
36.2 mg, 61% yield. A mixture of petroleum ether/ethyl acetate = 20/1 (v/v) as eluents for column chromatography. Yellow solid. M.p.: 90.7–91.6°C. 1H NMR (500 MHz, CDCl3) δ 8.09 (d, J = 8.0 Hz, 2H), 7.62 (d, J = 7.9 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ −24.4 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 141.2, 132.9, 117.9, 115.8, 114.0, 103.2 (q, J = 353.0 Hz). IR (KBr): 3057, 3030, 2962, 2920, 2228, 1932, 1589, 1580, 1480, 1402, 1386, 1305, 1261, 1088, 1012, 827, 721 cm−1. HRMS-ESI (m/z) calcd. for [C8H4F3NNaTe]+ ([M + Na]+): 323.9251; found: 323.9257.
(4-Nitrophenyl)(trifluoromethyl)tellane (3j)
38.2 mg, 60% yield. A mixture of petroleum ether/ethyl acetate = 15/1 (v/v) as eluents for column chromatography. Yellow solid. M.p.: 68.5–69.5°C. 1H NMR (500 MHz, CDCl3) δ 8.18-8.14 (m, 4H). 19F NMR (471 MHz, CDCl3) δ −24.2 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 149.0, 141.5, 124.4, 118.0, 103.3 (q, J = 355.4 Hz). IR (KBr): 3104, 2922, 2852, 1659, 1633, 1596, 1574, 1520, 1472, 1389, 1363, 1347, 1311, 1274, 1106, 1082, 1056, 1010, 852, 845, 737, 725, 706 cm−1. HRMS-ESI (m/z) calcd. for [C7H5F3NO2Te]+ ([M + H]+): 321.9330; found: 321.9345.
(3-Nitrophenyl)(trifluoromethyl)tellane (3k)
47.1 mg, 74% yield. A mixture of petroleum ether/ethyl acetate = 20/1 (v/v) as eluents for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.85 (s, 1H), 8.32 (m, 2H), 7.56 (t, J = 7.9 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −24.7 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 148.4, 146.9, 135.7, 130.6, 125.1, 110.2, 103.2 (q, J = 354.3 Hz). IR (KBr): 3091, 2925, 2861, 2208, 1743, 1598, 1526, 1464, 1417, 1347, 1298, 1274, 1111, 1079, 999, 935, 899, 864, 806, 729, 716, 670 cm−1. HRMS-ESI (m/z) calcd. for [C7H5F3NO2Te]+ ([M + H]+): 321.9330; found: 321.9326.
Methyl 3-nitro-5-((trifluoromethyl)tellanyl)benzoate (3l)
54.3 mg, 72% yield. A mixture of petroleum ether/ethyl acetate = 5/1 (v/v) as eluents for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 8.99 (s, 1H), 8.94 (s, 1H) 8.90 (s, 1H), 4.01 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −24.3 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 163.9, 148.4, 147.2, 139.2, 133.0, 126.1, 110.5, 103.3 (q, J = 354.3 Hz), 53.2. IR (KBr): 3085, 3007, 2957, 2872, 1732, 1605, 1536, 1447, 1411, 1348, 1279, 1195, 1115, 1079, 980, 902, 836, 774, 736, 725, 661 cm−1. HRMS-ESI (m/z) calcd. for [C9H6F3NNaO4Te]+ ([M + Na]+): 401.9204; found: 401.9216.
(4-(Tert-butyl)phenyl)(trifluoromethyl)tellane (3m)
45.7 mg, 69% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.93 (d, J = 8.1 Hz, 2H), 7.38 (d, J = 8.1 Hz, 2H), 1.34 (s, 9H). 19F NMR (471 MHz, CDCl3) δ −25.6 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 153.7, 141.3, 127.1, 106.3, 102.6 (q, J = 354.2 Hz), 34.9, 31.2. IR (KBr): 3076, 2965, 2928, 2869, 1586, 1487, 1463, 1396, 1380, 1365, 1267, 1203, 1113, 1083, 1007, 823, 724, 549 cm−1. HRMS-ESI (m/z) calcd. for [C11H13F3NaTe]+ ([M + Na]+): 354.9924; found: 354.9930.
(4-Methoxyphenyl)(trifluoromethyl)tellane (3n)14
31.8 mg 52% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 8.7 Hz, 2H), 6.87 (d, J = 8.6 Hz, 2H), 3.84 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −26.5 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 161.4, 143.6, 115.8, 102.6 (q, J = 354.2 Hz), 99.6, 55.3.
(4-Phenoxyphenyl)(trifluoromethyl)tellane (3o)
35.8 mg, 49% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.94 (d, J = 8.4 Hz, 2H), 7.40 (t, J = 7.9 Hz, 2H), 7.19 (t, J = 7.3 Hz, 1H), 7.08 (d, J = 8.0 Hz, 2H), 6.94 (d, J = 8.4 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ −26.1 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 159.8, 155.8, 143.6, 130.0, 124.4, 120.0, 119.5, 102.7 (q, J = 354.2 Hz), 102.0. IR (KBr): 3064, 2925, 1577, 1483, 1397, 1276, 1244, 1198, 1171, 1082, 1006, 867, 828, 794, 752, 723, 692 cm−1. HRMS-ESI (m/z) calcd. for [C13H10F3OTe]+ ([M + H]+): 368.9741; found: 368.9747.
(4-(Benzyloxy)phenyl)(trifluoromethyl)tellane (3p)
39.6 mg, 62% yield. A mixture of petroleum ether/ethyl acetate = 20/1 (v/v) as eluents for column chromatography. Yellow solid. M.p.: 47.1–48.7°C. 1H NMR (500 MHz, CDCl3) δ 7.92 (d, J = 8.7 Hz, 2H), 7.45-7.40 (m, 4H), 7.36 (t, J = 7.0 Hz, 1H), 6.86 (d, J = 8.8 Hz, 2H), 5.10 (s, 2H). 19F NMR (471 MHz, CDCl3) δ −26.4 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 160.6, 143.6, 136.3, 128.7, 128.2, 127.5, 116.6, 102.6 (q, J = 353.9 Hz), 99.9, 70.0. IR (KBr): 3093, 3064, 3033, 2950, 2921, 2890, 2856, 2525, 1890, 1581, 1563, 1487, 1471, 1454, 1383, 1285, 1247, 1176, 1076, 1006, 916, 858, 829, 806, 750, 720, 698 cm−1. HRMS-ESI (m/z) calcd. for [C14H11F3NaOTe]+ ([M + Na]+): 404.9717; found: 404.9720.
(4-Methoxy-3-methylphenyl)(trifluoromethyl)tellane (3q)
40.1 mg, 63% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.81 (d, J = 8.3 Hz, 1H), 7.77 (s, 1H), 6.78 (d, J = 8.3 Hz, 1H), 3.86 (s, 3H), 2.22 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −26.6 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 159.6, 143.9, 141.3, 128.8, 111.4, 102.5 (q, J = 354.3 Hz), 99.2, 55.3, 16.0. IR (KBr): 3006, 2962, 2926, 2843, 1586, 1572, 1489, 1465, 1441, 1387, 1301, 1281, 1253, 1179, 1141, 1083, 1031, 886, 808, 751, 723 cm−1. HRMS-ESI (m/z) calcd. for [C9H10F3OTe]+ ([M + H]+): 320.9741; found: 320.9746.
4-((Trifluoromethyl)tellanyl)phenol (3r)
32.5 mg, 56% yield. A mixture of petroleum ether/ethyl acetate = 5/1 (v/v) as eluents for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.88 (d, J = 8.4 Hz, 2H), 6.81 (d, J = 8.5 Hz, 2H), 5.20 (s, 1H). 19F NMR (471 MHz, CDCl3) δ −26.5 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 157.5, 143.9, 117.3, 102.6 (q, J = 353.2 Hz), 99.7. IR (KBr): 3379, 3056, 3007, 2925, 2853, 1649, 1593, 1579, 1489, 1427, 1361, 1262, 1246, 1176, 1081, 1007, 825, 723, 512 cm−1. HRMS-ESI (m/z) calcd. for [C7H6F3OTe]+ ([M + H]+): 292.9428; found: 292.9429.
N,N-Diphenyl-4-((trifluoromethyl)tellanyl)aniline (3s)
45.0 mg, 51% yield. Petroleum ether as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.80 (d, J = 8.3 Hz, 2H), 7.32 (t, J = 7.5 Hz, 4H), 7.16 (d, J = 7.6 Hz, 4H), 7.12 (t, J = 7.4 Hz, 2H), 6.97 (d, J = 8.4 Hz, 2H). 19F NMR (471 MHz, CDCl3) δ −26.3 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 149.9, 146.8, 142.8, 129.6, 125.6, 124.2, 122.6, 102.6 (q, J = 354.8 Hz), 99.9. IR (KBr): 3061, 3036, 2925, 2853, 1576, 1488, 1451, 1403, 1330, 1316, 1284, 1191, 1180, 1156, 1082, 1029, 1003, 921, 899, 819, 754, 722, 696 cm−1. HRMS-ESI (m/z) calcd. for [C19H15F3NTe]+ ([M + H]+): 444.0214; found: 444.0220.
(Trifluoromethyl)(4-vinylphenyl)tellane (3t)
37.6 mg 63% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.96 (d, J = 7.9 Hz, 2H), 7.38 (d, J = 7.9 Hz, 2H), 6.73 (dd, J = 17.6, 10.9 Hz, 1H), 5.83 (d, J = 17.5 Hz, 1H) 5.35 (d, J = 10.8 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −25.6 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 141.7, 139.4, 135.9, 127.6, 116.0, 108.8, 102.7 (q, J = 354.2 Hz). IR (KBr): 3066, 3011, 2925, 2853, 1705, 1629, 1586, 1552, 1486, 1464, 1419, 1391, 1256, 1185, 1082, 1026, 1009, 988, 917, 831, 723 cm−1. HRMS-ESI (m/z) calcd. for [C9H7F3NaTe]+ ([M + Na]+): 324.9455; found: 324.9455.
Pyren-1-yl(trifluoromethyl)tellane (3u)
59.9 mg, 75% yield. Petroleum ether as eluent for column chromatography. Yellow solid. M.p.: 101.8–102.9°C. 1H NMR (500 MHz, CDCl3) δ 8.73 (dd, J = 7.7, 3.1 Hz, 1H), 8.53 (dd, J = 9.1, 3.3 Hz, 1H), 8.20 (t, J = 7.1 Hz, 2H), 8.12-8.07 (m, 2H), 8.03 (t, J = 7.3 Hz, 1H), 8.00-7.96 (m, 2H). 19F NMR (471 MHz, CDCl3) δ −25.1 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 141.6, 135.8, 133.6, 132.1, 130.9, 130.7, 129.2, 129.2, 127.0, 126.4, 126.2, 125.5, 124.7, 124.1, 110.3, 103.0 (q, J = 355.7 Hz), 100.0. IR (KBr): 3041, 2150, 1921, 1801, 1636, 1599, 1585, 1454, 1426, 1414, 1379, 1298, 1242, 1205, 1179, 1153, 1143, 1124, 1085, 1008, 967, 902, 843, 818, 757, 719, 708 cm−1. HRMS-ESI (m/z) calcd. for [C17H10F3Te]+ ([M + H]+): 400.9792; found: 400.9804.
2-((Trifluoromethyl)tellanyl)benzofuran (3v)
50.5 mg, 80% yield. Petroleum ether as eluent for column chromatography. Yellow solid. M.p.: 57.4–58.8°C. 1H NMR (500 MHz, CDCl3) δ 7.64 (d, J = 7.7 Hz, 1H), 7.59 (d, J = 8.2 Hz, 1H), 7.46 (s, 1H), 7.38 (t, J = 7.3 Hz, 1H), 7.29 (t, J = 7.3 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −24.9 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 159.6, 128.4, 126.8, 126.2, 123.3, 123.3, 121.3, 111.6, 103.9 (q, J = 355.7 Hz). IR (KBr): 3141, 2922, 2851, 1518, 1471, 1435, 1301, 1249, 1163, 1145, 1123, 1082, 1028, 1007, 910, 879, 860, 822, 780, 754, 741, 723, 604 cm−1. HRMS-ESI (m/z) calcd. for [C9H5F3NaOTe]+ ([M + Na]+): 338.9252; found: 338.9250.
2-((Trifluoromethyl)tellanyl)benzo[b]thiophene (3w)
54.8 mg, 83% yield. Petroleum ether as eluent for column chromatography. Yellow solid. M.p.: 58.9–60.5°C. 1H NMR (500 MHz, CDCl3) δ 7.91-7.87 (m, 3H), 7.42-7.40 (m, 2H). 19F NMR (471 MHz, CDCl3) δ −26.1 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 147.3, 141.8, 140.6, 125.8, 124.8, 124.0, 121.6, 103.3 (q, J = 355.7 Hz), 99.6. IR (KBr): 3056, 3019, 2924, 2852, 2190, 1958, 1925, 1812, 1703, 1676, 1624, 1590, 1483, 1453, 1406, 1320, 1298, 1246, 1178, 1158, 1111, 1086, 946, 922, 872, 830, 757, 723, 707, 556 cm−1. HRMS-ESI (m/z) calcd. for [C9H5F3NaSTe]+ ([M + Na]+): 354.9019; found: 354.9023.
3-((Trifluoromethyl)tellanyl)dibenzo[b,d]thiophene (3x)
50.2 mg, 66% yield. Petroleum ether as eluent for column chromatography. Yellow solid. M.p.: 127.4–129.2°C. 1H NMR (500 MHz, CDCl3) δ 8.25 (d, J = 7.9 Hz, 1H), 8.12-8.08 (m, 2H), 7.88 (d, J = 7.2 Hz, 1H), 7.52-7.48 (m, 2H) 7.40 (t, J = 7.6 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −24.2 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 151.7, 141.2, 138.8, 136.6, 135.1, 127.3, 125.5, 124.7, 123.9, 122.7, 122.4, 103.7 (q, J = 355.7 Hz), 103.4. IR (KBr): 3054, 2923, 1915, 1799, 1649, 1579, 1522, 1446, 1435, 1379, 1316, 1303, 1248, 1104, 1085, 1024, 942, 864, 794, 753, 721 cm−1. HRMS-ESI (m/z) calcd. for [C13H7F3NaSTe]+ ([M + Na]+): 404.9175; found: 404.9175.
1-Methyl-5-((trifluoromethyl)tellanyl)-1H-indole (3y)
52.8 mg, 81% yield. A mixture of petroleum ether/ethyl acetate = 15/1 (v/v) as eluents for column chromatography. Yellow solid. M.p.: 89.3–90.4°C. 1H NMR (500 MHz, CDCl3) δ 8.34 (s, 1H), 7.85 (d, J = 8.4 Hz, 1H), 7.30 (d, J = 8.4 Hz, 1H), 7.09 (d, J = 3.1 Hz, 1H), 6.54 (d, J = 3.0 Hz, 1H), 3.81 (s, 3H). 19F NMR (471 MHz, CDCl3) δ −26.7 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 137.1, 135.8, 134.5, 130.2, 129.9, 110.8, 102.5 (q, J = 355.7 Hz), 101.4, 98.8, 32.9. IR (KBr): 2924, 2854, 2820, 1727, 1614, 1596, 1508, 1471, 1420, 1355, 1326, 1278, 1243, 1094, 882, 795, 761, 724, 600 cm−1. HRMS-ESI (m/z) calcd. for [C10H9F3NTe]+ ([M + H]+): 329.9744; found: 329.9755.
1,4-Bis((trifluoromethyl)tellanyl)benzene (3z)
36.6 mg, 39% yield. Petroleum ether as eluent for column chromatography. Yellow solid. M.p.: 54.4–55.6°C. 1H NMR (500 MHz, CDCl3) δ 7.95 (s, 4H). 19F NMR (471 MHz, CDCl3) δ −24.8 (s, 6F). 13C NMR (126 MHz, CDCl3) δ 142.1, 112.4, 103.0 (q, J = 354.1 Hz). IR (KBr): 2924, 2853, 1469, 1375, 1254, 1121, 1084, 1001, 809, 721 cm−1. HRMS-ESI (m/z) calcd. for [C8H5F6Te2]+ ([M + H]+): 474.8414; found: 474.8429.
(E)-Styryl(trifluoromethyl)tellane (3aa)
19.2 mg, 32% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 7.65 (d, J = 16.4 Hz, 1H), 7.45-7.37 (m, 5H), 7.33 (t, J = 6.6 Hz, 1H). 19F NMR (471 MHz, CDCl3) δ −25.0 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 151.0, 137.2, 129.2, 128.8, 126.8, 102.1 (q, J = 353.4 Hz), 94.6. IR (KBr): 3060, 3031, 2926, 1703, 1599, 1568, 1495, 1447, 1327, 1303, 1283, 1211, 1174, 1107, 1082, 964, 799, 731, 688 cm−1. HRMS-ESI (m/z) calcd. for [C9H8F3Te]+ ([M + H]+): 302.9635; found: 302.9631.
(E)-(2-cyclohexylvinyl)(trifluoromethyl)tellane (3ab)
18.9 mg, 31% yield. Pentane as eluent for column chromatography. Yellow oil. 1H NMR (500 MHz, CDCl3) δ 6.85 (d, J = 16.0 Hz, 1H), 6.61 (dd, J = 15.9, 6.8 Hz, 1H), 2.19 (m, 1H), 1.80-1.73 (m, 4H), 1.69-1.65 (m, 2H), 1.22-1.12 (m, 4H). 19F NMR (471 MHz, CDCl3) δ −25.7 (s, 3F). 13C NMR (126 MHz, CDCl3) δ 161.4, 101.7 (q, J = 353.7 Hz), 91.7, 44.9, 32.0, 25.9, 25.7. IR (KBr): 2954, 2925, 2854, 1661, 1630, 1598, 1463, 1377, 1299, 1260, 1115, 1085, 1030, 968, 802, 724, 659 cm−1. HRMS-ESI (m/z) calcd. for [C9H13F3NaTe]+ ([M + Na]+): 330.9924; found: 330.9922.
Acknowledgments
We thank Wuhan University of Technology, the “Hundred Talent” Program of Hubei Province (China), and the Natural Science Foundation of Shandong Province (China) (ZR2021MB138) for financial support.
Author contributions
C-P.Z. and J-Y.D. designed the project. J-Y.D., H-N.W. and Y-Q.X. performed the experiments and analyzed the data. C-P.Z. supervised the project and wrote the manuscript. All the authors discussed the results and commented on the manuscript.
Declaration of interests
The authors declare no competing interest.
Published: December 22, 2022
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2022.105566.
Supplemental information
Data and code availability
-
•
Original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Szabó K., Selander N. WILEY-VCH GmbH; 2021. Organofluorine Chemistry: Synthesis, Modeling, and Applications. [Google Scholar]
- 2.Meanwell N.A. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J. Med. Chem. 2018;61:5822–5880. doi: 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa Y., Tokunaga E., Kobayashi O., Hirai K., Shibata N. Current contributions of organofluorine compounds to the agrochemical industry. iScience. 2020;23:101467. doi: 10.1016/j.isci.2020.101467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kusoglu A., Weber A.Z. New Insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017;117:987–1104. doi: 10.1021/acs.chemrev.6b00159. [DOI] [PubMed] [Google Scholar]
- 5.He Z., Yang Y., Liu J.-W., Yu S.-H. Emerging tellurium nanostructures: controllable synthesis and their applications. Chem. Soc. Rev. 2017;46:2732–2753. doi: 10.1039/C7CS00013H. [DOI] [PubMed] [Google Scholar]
- 6.Hoffmann J.E., King M.G., Carapella S.C., Oldfield J.E., Putnam R.D. Kirk-Othmer Encyclopedia of Chemical Technology. Wiley-VCH Verlag GmbH & Co. KGaA; 2011. Tellurium and tellurium compounds. [Google Scholar]
- 7.Wu W., Qiu G., Wang Y., Wang R., Ye P. Tellurene: its physical properties, scalable nanomanufacturing, and device applications. Chem. Soc. Rev. 2018;47:7203–7212. doi: 10.1039/C8CS00598B. [DOI] [PubMed] [Google Scholar]
- 8.Hoover G.C., Seferos D.S. Photoactivity and optical applications of organic materials containing selenium and tellurium. Chem. Sci. 2019;10:9182–9188. doi: 10.1039/C9SC04279B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zare B., Nami M., Shahverdi A.-R. Tracing tellurium and its nanostructures inbiology. Biol. Trace Elem. Res. 2017;180:171–181. doi: 10.1007/s12011-017-1006-2. [DOI] [PubMed] [Google Scholar]
- 10.Turner R.J., Borghese R., Zannoni D. Microbial processing of tellurium as a tool in biotechnology. Biotechnol. Adv. 2012;30:954–963. doi: 10.1016/j.biotechadv.2011.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Vávrová S., Struhárňanská E., Turňa J., Stuchlík S. Tellurium: a rare element with influence on prokaryotic and eukaryotic biological systems. Int. J. Mol. Sci. 2021;22:5924. doi: 10.3390/ijms22115924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kirij N.V., Yagupolskii Y.L., Maggiarosa N., Tyrra W., Naumann D. Tris(trifluoromethyl)tellurate(II), [Te(CF3)3]−, the dimeric bis(trifluoromethyl)chlorotellurate(II), [(CF3)2TeCl]22−, and bis(trifluoromethyl)iodate(I), [I(CF3)2]−: the first hypervalent alkyl [10-Te-3] and [12-Te-4] species and a novel [10-I-2] compound. J. Fluor. Chem. 2001;112:213–218. doi: 10.1016/S0022-1139(01)00524-3. [DOI] [Google Scholar]
- 13.Park H., Edgar L.J., Lumba M.A., Willis L.M., Nitz M. Organotellurium scaffolds for mass cytometry reagent development. Org. Biomol. Chem. 2015;13:7027–7033. doi: 10.1039/C5OB00593K. [DOI] [PubMed] [Google Scholar]
- 14.Sperger T., Guven S., Schoenebeck F. Chemoselective Pd-catalyzed C-TeCF3 coupling of aryl iodides. Angew. Chem. Int. Ed. Engl. 2018;57:16903–16906. doi: 10.1002/anie.201810950. [DOI] [PubMed] [Google Scholar]
- 15.Scilabra P., Terraneo G., Resnati G. The chalcogen bond in crystalline solids: a world parallel to halogen bond. Acc. Chem. Res. 2019;52:1313–1324. doi: 10.1021/acs.accounts.9b00037. [DOI] [PubMed] [Google Scholar]
- 16.Tiekink E.R. Te-N secondary-bonding interactions in tellurium crystals: supramolecular aggregation patterns and a comparison with their lighter congeners. Coord. Chem. Rev. 2022;457:214397. doi: 10.1016/j.ccr.2021.214397. [DOI] [Google Scholar]
- 17.Amorati R., Baschieri A., Valgimigli L. The role of sulfur and heavier chalcogens in the chemistry of antioxidants. Phosphorus Sulfur Silicon Relat. Elem. 2019;194:638–642. doi: 10.1080/10426507.2019.1602620. [DOI] [Google Scholar]
- 18.Yamago S. Photoactivation of organotellurium compounds in precision polymer synthesis: controlled radical polymerization and radical coupling reactions. Bull. Chem. Soc. Jpn. 2020;93:287–298. doi: 10.1246/bcsj.20190339. [DOI] [Google Scholar]
- 19.Chen Y., Deng X., Jing X., Zhou H. Tellurium-mediated organic reactions. Chin. J. Org. Chem. 2020;40:4147–4154. doi: 10.6023/cjoc202005024. [DOI] [Google Scholar]
- 20.Chen C., Liu G. In: Ma J.-A., Cahard D., editors. Vol. 1. Wiley-VCH Verlag GmbH & Co. KGaA; 2020. pp. 251–265. (Emerging Fluorinated Motifs). [Google Scholar]
- 21.Lee J.W., Lee K.N., Ngai M.-Y. In: Ma J.-A., Cahard D., editors. Vol. 1. Wiley-VCH Verlag GmbH & Co. KGaA; 2020. pp. 225–250. (Emerging Fluorinated Motifs). [Google Scholar]
- 22.Wang Q., Zhang X., Sorochinsky A.E., Butler G., Han J., Soloshonok V.A. Advances in the development of trifluoromethoxylation reagents. Symmetry. 2021;13:2380. doi: 10.3390/sym13122380. [DOI] [Google Scholar]
- 23.Hardy M.A., Chachignon H., Cahard D. Advances in asymmetric di-and trifluoromethylthiolation, and di- and trifluoromethoxylation reactions. Asian J. Org. Chem. 2019;8:591–609. doi: 10.1002/ajoc.201900004. [DOI] [Google Scholar]
- 24.Liu H., Ge H., Shen Q. In: Ma J.-A., Cahard D., editors. Vol. 2. Wiley-VCH Verlag GmbH & Co. KGaA; 2020. pp. 309–341. (Emerging Fluorinated Motifs). [Google Scholar]
- 25.Barthelemy A.-L., Magnier E., Dagousset G. Direct trifluoromethylthiolation reactions involving radical processes. Synthesis. 2018;50:4765–4776. doi: 10.1055/s-0037-1611278. [DOI] [Google Scholar]
- 26.Wang H.-N., Dong J.-Y., Shi J., Zhang C.-P. Trifluoromethylselenolation reactions using the versatile [Me4N] [SeCF3] reagent. Tetrahedron. 2021;99:132476. doi: 10.1016/j.tet.2021.132476. [DOI] [Google Scholar]
- 27.Wang Y., Ye Z., Zhang H., Yuan Z. Recent advances in the development of direct trifluoromethylselenolation reagents and methods. Adv. Synth. Catal. 2021;363:1835–1854. doi: 10.1002/adsc.202001508. [DOI] [Google Scholar]
- 28.Tlili A., Ismalaj E., Glenadel Q., Ghiazza C., Billard T. Synthetic approaches to trifluoromethylselenolated compounds. Chemistry. 2018;24:3659–3670. doi: 10.1002/chem.201704637. [DOI] [PubMed] [Google Scholar]
- 29.Umemoto T., Ishihara S. Power-variable electrophilic trifluoromethylating agents. S-Se-and Te-(trifluoromethyl)dibenzothio--seleno-and -tellurophenium salt system. J. Am. Chem. Soc. 1993;115:2156–2164. doi: 10.1021/ja00059a009. [DOI] [Google Scholar]
- 30.Pietrasiak E., Togni A. Synthesis and characterization of fluorinated hypervalent tellurium derivatives. Organometallics. 2017;36:3750–3757. doi: 10.1021/acs.organomet.7b00535. [DOI] [Google Scholar]
- 31.Haas A., Heuduk H., Monsé C., Yagupolskii L.M. Te-trifluoromethylarenetellurocarboxylates. J. Fluor. Chem. 1999;94:195–198. doi: 10.1016/S0022-1139(99)00014-7. [DOI] [Google Scholar]
- 32.Geri J.B., Wade Wolfe M.M., Szymczak N.K. Borazine-CF3− adducts for rapid, room temperature, and broad scope trifluoromethylation. Angew. Chem. Int. Ed. Engl. 2018;57:1381–1385. doi: 10.1002/anie.201711316. [DOI] [PubMed] [Google Scholar]
- 33.Tyrra W., Kirij N.V., Naumann D., Yagupolskii Y.L. Tetramethylammonium trifluoromethyltellurate(0), [NMe4]TeCF3, synthesis, characterisation and properties. J. Fluor. Chem. 2004;125:1437–1440. doi: 10.1016/j.jfluchem.2004.04.012. [DOI] [Google Scholar]
- 34.Tyrra W., Naumann D., Kremer S., Hermle J., Treutlein V., Pantenburg I., Fischer H.T.M., Scherer H. Synthesis and Characterization of methyl(perfluoroalkyl)tellurium(II) Compounds, MeTeRf (Rf = CF3, C2F5), the corresponding tellurium(IV) dihalides, MeTe(Rf)X2 (X = F, Cl, Br, I), and bis(trifluoroacetates), MeTe(Rf)(OCOCF3)2. Z. Anorg. Allg. Chem. 2012;638:580–588. doi: 10.1002/zaac.201100483. [DOI] [Google Scholar]
- 35.Huang C., Liang T., Harada S., Lee E., Ritter T. Silver-mediated trifluoromethoxylation of aryl stannanes and arylboronic acids. J. Am. Chem. Soc. 2011;133:13308–13310. doi: 10.1021/ja204861a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang C.-P., Vicic D.A. Oxidative trifluoromethylthiolations of aryl boronic acids using a copper/O2-based protocol. Chem. Asian J. 2012;7:1756–1758. doi: 10.1002/asia.201200347. [DOI] [PubMed] [Google Scholar]
- 37.Chen C., Xie Y., Chu L., Wang R.-W., Zhang X., Qing F.-L. Copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids with TMSCF3 and Elemental Sulfur. Angew. Chem. Int. Ed. Engl. 2012;51:2492–2495. doi: 10.1002/anie.201108663. [DOI] [PubMed] [Google Scholar]
- 38.Zhao M., Zhao X., Zheng P., Tian Y. Cu-mediated oxidative trifluoromethylthiolation of arylboronic acids with (bpy)CuSCF3. J. Fluorine Chem. 2017;194:73–79. doi: 10.1016/j.jfluchem.2017.01.007. [DOI] [Google Scholar]
- 39.Zhai L., Li Y., Yin J., Jin K., Zhang R., Fu X., Duan C. Copper-mediated oxidative trifluoromethylthiolation of aryl boronic acids with CF3CO2Na and elemental sulfur. Tetrahedron. 2013;69:10262–10266. doi: 10.1016/j.tet.2013.10.028. [DOI] [Google Scholar]
- 40.Wu W., Wang B., Ji X., Cao S. Direct copper-catalyzed oxidative trifluoromethylthiolation of aryl boronic acids with AgSCF3. Org. Chem. Front. 2017;4:1299–1303. doi: 10.1039/C7QO00198C. [DOI] [Google Scholar]
- 41.Kang K., Xu C., Shen Q. Copper-catalyzed trifluoromethylthiolation of aryl and vinyl boronic acids with a shelf-stable electrophilic trifluoromethylthiolating reagent. Org. Chem. Front. 2014;1:294–297. doi: 10.1039/C3QO00068K. [DOI] [Google Scholar]
- 42.Pluta R., Nikolaienko P., Rueping M. Direct catalytic trifluoromethylthiolation of boronic acids and alkynes employing electrophilic shelf-stable N-(trifluoromethylthio)phthalimide. Angew. Chem. Int. Ed. Engl. 2014;53:1650–1653. doi: 10.1002/anie.201307484. [DOI] [PubMed] [Google Scholar]
- 43.Glenadel Q., Alazet S., Tlili A., Billard T. Mild and soft catalyzed trifluoromethylthiolation of boronic acids: the crucial role of water. Chemistry. 2015;21:14694–14698. doi: 10.1002/chem.201502338. [DOI] [PubMed] [Google Scholar]
- 44.Lefebvre Q., Pluta R., Rueping M. Copper catalyzed oxidative coupling reactions for trifluoromethylselenolations–synthesis of R-SeCF3 compounds using air stable tetramethylammonium trifluoromethylselenate. Chem. Commun. 2015;51:4394–4397. doi: 10.1039/C4CC10212F. [DOI] [PubMed] [Google Scholar]
- 45.Glenadel Q., Ghiazza C., Tlili A., Billard T. Copper-catalyzed direct trifluoro- and perfluoroalkylselenolations of boronic acids with a shelf-stable family of reagents. Adv. Synth. Catal. 2017;359:3414–3420. doi: 10.1002/adsc.201700904. [DOI] [Google Scholar]
- 46.Gockel S., Haas A., Probst V., Boese R., Müller I. Contributions to bis(perfluoroalkyl) chalkogenide chemistry: preparation of (Rf)2SeO [Rf = C2F5, (CF3)2CF, n-C4F9], (Rf′)2TeX2 [X = F, Cl: Rf′ = n-C3F7, (CF3)2CF, n-C4F9; X = Br: Rf′ = n-C3F7, n-C4F9], (CF3)2Te(NSO)2 and (C2F5)2Te(OH)NO3. J. Fluorine Chem. 2000;102:301–311. doi: 10.1016/S0022-1139(99)00292-4. [DOI] [Google Scholar]
- 47.Kasemann R., Naumann D. Perfluororganotellur-verbindungen: die synthesen von C6F5TeTeC6F5, Hg(TeC6F5)2, CF3TeTeC6F5, CF3TeC6F5 und pentafluorphenylfuranen [1] J. Fluor. Chem. 1990;48:207–217. doi: 10.1016/S0022-1139(00)80433-9. [DOI] [Google Scholar]
- 48.Fischer H.T.M., Naumann D., Tyrra W. On the complexes of bis(trifluoromethyl)ditellurium, Te2(CF3)2, with halide Ions – a crystallographic study of the pseudo-trihalides [PNP] [(TeCF3)2X] (PNP = bis(triphenylphosphoranyliden)ammonium; X = Cl, Br, I) Z. Anorg. Allg. Chem. 2007;633:127–131. doi: 10.1002/zaac.200600234. [DOI] [Google Scholar]
- 49.Xu H.-J., Zhao Y.-Q., Feng T., Feng Y.-S. Chan–lam-type S-arylation of thiols with boronic acids at room temperature. J. Org. Chem. 2012;77:2878–2884. doi: 10.1021/jo300100x. [DOI] [PubMed] [Google Scholar]
- 50.Lam L.Y., Ma C. Chan–lam-type C–S coupling reaction by sodium aryl sulfinates and organoboron compounds. Org. Lett. 2021;23:6164–6168. doi: 10.1021/acs.orglett.1c02299. [DOI] [PubMed] [Google Scholar]
- 51.Armarego W.L.F., Chai C.L.L. Fivth edition. Butterworth Heinemann; 2003. Purification of Laboratory Chemicals. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Original data reported in this paper will be shared by the lead contact upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.