Abstract
T helper-2 (Th2) cells and type 2 innate lymphoid cells (ILC2s) play crucial roles during type 2 immune responses; the transcription factor GATA3 is essential for the differentiation and functions of these cell types. It has been demonstrated that GATA3 is critical for maintaining Th2 and ILC2 phenotype in vitro; GATA3 not only positively regulates type 2 lymphocyte-associated genes, it also negatively regulates many genes associated with other lineages. However, such functions cannot be easily verified in vivo because the expression of the markers for identifying Th2 and ILC2s depends on GATA3. Thus, whether Th2 cells and ILC2s disappear after Gata3 deletion or these Gata3-deleted “Th2 cells” or “ILC2s” acquire an alternative lineage fate is unknown. In this study, we generated novel GATA3 reporter mouse strains carrying the Gata3 ZsG or Gata3 ZsG-fl allele. This was achieved by inserting a ZsGreen-T2A cassette at the translation initiation site of either the wild type Gata3 allele or the modified Gata3 allele which carries two loxP sites flanking the exon 4. ZsGreen faithfully reflected the endogenous GATA3 protein expression in Th2 cells and ILC2s both in vitro and in vivo. These reporter mice also allowed us to visualize Th2 cells and ILC2s in vivo. An inducible Gata3 deletion system was created by crossing Gata3 ZsG-fl/fl mice with a tamoxifen-inducible Cre. Continuous expression of ZsGreen even after the Gata3 exon 4 deletion was noted, which allows us to isolate and monitor GATA3-deficient “Th2” cells and “ILC2s” during in vivo immune responses. Our results not only indicated that functional GATA3 is dispensable for regulating its own expression in mature type 2 lymphocytes, but also revealed that GATA3-deficient “ILC2s” might be much more stable in vivo than in vitro. Overall, the generation of these novel GATA3 reporters will provide valuable research tools to the scientific community in investigating type 2 immune responses in vivo.
Keywords: GATA3, type 2 innate lymphoid cells, T helper cell 2, reporter mouse model, gene regulation, in vivo, allergic responses
Introduction
GATA3 is a critical transcription factor for the development of CD4 T cells and non-lymphoid tissue inducer (non-LTi) innate lymphoid cells (1, 2). Also, group 2 innate lymphoid cells (ILC2s) and type 2 T helper (Th2) cells rely on GATA3 expression for their fate determination and functions (3, 4). Both Th2 cells and ILC2s express high levels of GATA3 (5). Furthermore, GATA3 expression positively regulates the expression of several type 2 effector cytokines in both Th2 cells and ILC2s (2). Although ILC2s and Th2 cells secret similar effector cytokines and other regulatory molecules, signals that induce their development and activation are quite different (6). Nevertheless, ILC2s and Th2 crosstalk with each other during type 2 immune responses, including host defense against nematode infection and allergic inflammatory responses (7). A complete understanding of similar and differential functions of GATA3 in ILC2s and Th2 cells is important for gaining deep insights into type 2 immune responses.
The mouse Gata3 gene consists of six exons located on chromosome 2. We have previously generated a conditional Gata3 allele (Gata3 fl) by inserting loxP sequences on both ends of the exon 4 which encodes functional zinc finger domains (8). Using tamoxifen-inducible deletion of Gata3 gene under in vitro conditions, we have reported that many type 2 related genes (such as Il5, Il13, Areg, and Il1rl1) are positively regulated by GATA3 in both ILC2s and Th2 cells in an in vitro culture system (2). Since many known markers for identifying ILC2s or Th2 cells are regulated by GATA3, it is difficult to conclude whether “ILC2s” and/or “Th2” cells die after GATA3 deletion in vivo or they may adopt an alternative phenotype. For this reason, how GATA3 controls gene transcription in ILC2s and Th2 cells in vivo cannot be easily studied.
Mature Th2 cells express high levels of T1/ST2 (IL-33 receptor α chain) on their surface (9), which allows Th2 cells to respond to IL-33 to produce type 2 cytokines independent of T cell receptor-mediated signaling (10, 11). T1/ST2 has been widely used as a cell surface marker for isolating Th2 cells from non-Th2 cell populations that are generated in vivo in mice. ILC2s preferentially express several cell surface markers, including T1/ST2, CD25, ICOS, and KLRG1 (3, 12–14). While T1/ST2 is a reliable marker for identifying and isolating both Th2 cells and lung ILC2s, its expression is completely dependent on GATA3 expression (2). Because of this, whether GATA3-deficient “ILC2s” and “Th2” cells can persist in vivo is unknown.
It has been shown that GATA3 may self-regulate its own expression during Th2 cell differentiation in an overexpression study (15). However, it is also reported that Gata3 transcription does not depend on functional GATA3 under a Th2 environment (16). Thus, we speculate that a reporter under the control of the Gata3 gene could be a useful tool for identifying ILC2s and Th2 cells in vivo after Gata3 deletion. Although several GATA3 reporter systems are currently available (17–19), none of them offers the opportunity to study conditional Gata3 deletion in vivo.
In this study, we generated a novel GATA3 reporter system in combination with the conditional Gata3 allele (Gata3 ZsG-fl). The insertion of the ZsGreen reporter at the translation initiation site of GATA3 faithfully reflected the expression of endogenous GATA3. The two loxP sites flanking exon 4 encoding functional zinc finger domains allow us to delete the Gata3 gene by an inducible Cre. This novel mouse strain allowed us to identify, monitor and isolate Th2 cells and ILC2s after an acute GATA3 deletion in these cells in vivo. Through the experiments using these mice, we found that GATA3 is not required to regulate its own expression in mature type 2 lymphocytes and that GATA3-deficient “ILC2s”, defined by high levels of ZsGreen expression, might be relatively stable in vivo.
Another GATA3 reporter mouse strain carrying the Gata3 ZsG allele, in which ZsGreen insertion took place on the wild type Gata3 allele, can be used for general purposes in identifying, visualizing, and isolating Th2 cells and ILC2s. The ZsGeen insertion in both reporter systems does not affect the expression of endogenous GATA3 or impair the development of ILC2s and differentiation of Th2 cells. Therefore, our new GATA3 reporter mouse strains carrying the Gata3 ZsG or Gata3 ZsG-fl allele are valuable tools for studying Th2 cells and ILC2s during type 2 immune responses in vivo.
Material and methods
Mice
All mice were 8-12 weeks of age when they were used under a protocol (LISB-8E) approved by the National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee. Gata3 fl/fl mice (line 355) and Gata3 fl/fl-CreERT2 (line 8445) were obtained from the NIAID-Taconic repository. Gata3 ZsG/+ mice were generated on C57BL/6 background by the Mouse Genetics and Gene Modification section, NIAID. Gata3 ZsG-fl/fl mice were generated on the Gata3 fl/fl mice background by the Transgenic Core Facility, NHLBI. The details of the generation of these novel reporter strains are described below. Mice carrying the Gata3 ZsG-fl allele were also bred to the Gata3 fl/fl-CreERT2 mice to generate the Gata3 ZsG-fl/fl-CreERT2 strain. Gata3 ZsG/+ mice were bred to Cd4 Cre R26 tdTomato to generate Gata3 ZsG/+ Cd4 Cre R26 tdTomato mice. Mice were bred and/or maintained in the NIAID specific pathogen-free animal facilities.
BAC recombineering for Gata3-ZsGreen-T2A cassette insertion
The initial insertion of the ZsGreen-T2A gene cassette into the Gata3 gene on a BAC was accomplished via recombineering. galK positive and counterselection strategy was used to achieve ZsGreen-T2A insertion at the translation initiation site of the Gata3 gene. SW102 strain containing a BAC with the Gata3 gene was used for all BAC recombineering steps, and the detailed methodology for this process has been previously described (20). The gene galK encodes the enzyme galactokinase, which plays a key role in metabolizing galactose as a carbon source. The following galK primers (indicated in lowercase) with 50 bp Gata3 gene homology arms were used for amplifying galK gene.
Gata3-galK 5’F: CTCCCTACCCGCGAGGGTTCCGGGCCGGGCGAGAGGGCGCGAGCACAGGCGACGACcctgttgacaattaatcatcggca
Gata3-galK 3’R:
CGCGGGGTGATGGTGGCTCACCCAGCGCGGCTGGTCCGCAGTCACCTCCATtcagcactgtcctgctcctt
The resulting PCR product was introduced into SW102 competent cells by electroporation and the galK cassette was then inserted into the Gata3 gene-containing BAC by homologues recombination. The galK cassette insertion is critical for the positive selection of bacteria on minimal media containing galactose as the only carbon source. The galK insertion at the translation initiation site of Gata3 gene enables them to utilize galactose. Conversely, the non-transformed bacterial cells are eliminated due to the lack of ability to use galactose as a carbon source. This step is crucial for the positive selection of bacteria which are incorporated with galK insertion at the region of interest.
The following set of ZsGreen-T2A primers (indicated in lower case) with 50 bp length Gata3 gene homology arm was used for amplifying the ZsGreen-T2A gene.
Gata3-Zsgreen-T2A 5’F:
CTCCCTACCCGCGAGGGTTCCGGGCCGGGCGAGAGGGCGCGAGCACAGGCGACGACatggcccagtccaagcac
Gata3-Zsgreen-T2A 3’R:
CGCGGGGTGATGGTGGCTCACCCAGCGCGGCTGGTCCGCAGTCACCTCCATtgggccaggattctcctc
The amplified cassette was purified and introduced into the bacterial cells by electroporation. During this step, the galK gene in the BAC was replaced by Zsgreen-T2A cassette by homologues recombination. The bacteria containing Gata3-ZsGreen-T2A BAC was then counter-selected on a media containing 2-deoxy-galactose. During this process, bacterial cells still expressing galK are eliminated due to the toxic substance generated by galactose kinase metabolizing 2-deoxy-galactose. This leads to the selection of bacteria with the Gata3-ZsGreen-T2A BAC.
Generation of the Gata3 ZsG/+ and Gata3 ZsG-fl/fl mouse strains
The ZsGreen-T2A sequence was introduced into the endogenous Gata3 locus in mice using CRISPR/Cas9 technology (21). Briefly, a sgRNA (GAGCACAGCCGAGGACATGG), which cuts near the Gata3 translation initiation codon (ATG), was made using ThermoFisher’s custom in vitro transcription service. The sgRNA (10 ng/ul) and donor Gata3-ZsGreen-T2A BAC DNA (5 ng/ul) were co-microinjected with Cas9 mRNA (20 ng/ul, purchased from Trilink Biotechnologies) into the pronuclei of zygotes collected from the Gata3 fl/fl mice (line 355) (8). Injected embryos were cultured in M16 medium (Millipore-Sigma) overnight in a 37°C incubator with 6% CO2. The following day, embryos that reached 2-cell stage of development were implanted into the oviducts of pseudo-pregnant surrogate mothers. Similarly, the same sgRNA (10 ng/μl), Cas9 mRNA (20 ng/μl) along with BAC clone (2 ng/μl) were injected into the pronuclei of normal C57BL/6 females from Taconic. The embryos were cultured overnight in KSOM medium to two cell stage and then transferred into the CD1 pseudo-pregnant females. Offspring born to the foster mothers were genotyped by PCR and Sanger Sequencing. The expression of ZsGreen in CD4 T cells was confirmed by FACS analysis. Following the establishment of new reporter lines, the Gata3 ZsG/+ mice (on the WT C57BL/6 background) or the Gata3 ZsG-fl/fl mice (on the C57BL/6 background but also carrying Gata3 floxed allele) were maintained as hemizygous through crossing with wild type C57BL/6 or Gata3 fl/fl mice.
Genotyping by PCR
The ZsGreen-T2A knock-in allele in the offspring mice was screened by standard PCR. Briefly, the pinna samples from weaned mice were collected and digested with 100 μl of proteinase K digestion buffer (100 mM Tris-HCl, pH 8.5, 5 mM EDTA, 0.2% SDS, 200 mM NaCl, and 1 mg/ml of proteinase K) at 55 °C overnight. The reaction was then diluted with distilled water in 1:10, and 1 μl was used as a template for PCR. The following set of primers was used to distinguish wild-type allele from knock-in allele. 5’-TAGTCAGTCCTGGGCTCCTG-3’ (WT F), 5’-TCCAGCACAAGCTGACCC-3’ (ZsGreenF), 5’-CAGCGGATACTGAGCTTCCATGT-3 (Common R). The annealing step in the PCR was conducted at 57 °C. After the completion of successful PCR, the amplified product for the knock-in gene allele appears at around 284 bp, and the wild-type allele appears at 700 bp.
Quantitative PCR for Gata3 and ZsGreen mRNA
Total RNAs were isolated using RNeasy kit (Cat# 74104, QIAGEN). cDNAs from isolated RNAs were prepared with the QuantiTect Reverse Transcription Kit (Cat# 205311, QIAGEN). Quantitative PCR was performed on QuantStudio™ 7, Applied Biosystems by using FastStart Universal SYBR Green Master mix (Rox) (Cat# 4913850001, Roche). The following set of primers are used for quantifying Gata3 and ZsGreen mRNA. 5’-CTCGGCCATTCGTACATGGAA-3’ (Gata3-exon 2 F), 5’-GGATACCTCTGCACCGTAGC-3’ (Gata3-exon 2 R), 5’-AAGGCAGGGAGTGTGTGAAC-3’ (Gata3-exon 4 F), 5’-TCGCTTGGGCTTGATAAGGG-3’ (Gata3-exon 4 R), 5’-CCCTTATCAAGCCCAAGCGA-3’ (Gata3-exon 4-5 F), 5’- CCCATTAGCGTTCCTCCTCC-3’ (Gata3-exon 4-5 R), 5’-AAGGCATCCAGACCCGAAAC-3’ (Gata3-exon 6 F), 5’-GGAGAGATGTGGCTCAGGGA-3’ (Gata3-exon 6 R), 5’-ATCTGCAACGCCGACATCAC-3’ (ZsGreen F), 5’-TGATCTTCTCGCAGGAGGGC-3’ (ZsGreen R).
In vitro polarization of T helper subsets
Naïve CD4 T cells from pooled lymph node cell suspension were purified using the naïve CD4 T cell isolation kit (Miltenyi Biotec, 130-104-453) according to the manufacturer’s instruction. All the T helper subset polarization experiments were performed on 24-well plates. Plate-bound anti-CD3 (1 μg/ml) and anti-CD28 (3 μg/ml) was used to polarize cells under Th1, Th17, and Treg conditions. For Th2 polarization conditions, the Dynabeads™ Mouse T-Activator anti-CD3/CD28 (Theromofisher, 11456D) microbeads were used due to an inefficient differentiation of Th2 cells using the plate-bound system. Additional cytokines and antibodies were supplied with the complete RPMI 1640 media under different polarization conditions: for Th1, 10 ng/ml IL-12, 10 μg/ml α-IL-4, and 100 U/ml IL-2; for Th2, 10 ng/ml IL-4, 10 μg/ml α-IFN-γ, and 100 U/ml IL-2; for Th17, 2 ng/ml TGF-β, 20 ng/ml IL-6, 10 ng/ml IL-1β, 10 μg/ml α-IFN-γ, and 10 μg/ml α-IL-4; for Treg, 5 ng/ml TGF-β, 10 μg/ml α-IFN-γ, 10 μg/ml α-IL-4, and 100 U/ml IL-2. All cultures were incubated at 37°C in 5% CO2. After 3-4 days of polarization, cells were washed and placed in a new culture plate with complete RPMI 1640 media with 100 U/ml IL-2.
Papain-induced airway inflammation
Protease papain is well known to induce airway inflammation through activating type 2 immune responses (22). Mice were briefly anesthetized with isoflurane followed by papain intranasal (i.n.) challenges (40 µg in 20 µl of PBS) on days 0, 1, and 2. Mice were then rested for 10 days and challenged again with papain on days 13, 14, and 15. Mice were analyzed with the BALF and lung tissues 24 hr after the final i.n. challenge.
Flow cytometry analysis
In vitro cultured cells or cells isolated from the BALF and lung tissues were stained with different antibody cocktails for identifying eosinophils, Th2 cells, and ILC2s, and analyzed by FACS LSR-II or Fortessa (BD Bioscience). Foxp3/transcription factor staining buffer set (eBioscience) was used for identifying intracellular markers. Eosinophils were identified as CD45+CD11c-Gr1-SiglecF+CD11b+; Th2 cells were identified as CD4+Foxp3-GATA3+T1/ST2+; ILC2s were identified as Lin-IL-7Rα+GATA3+T1/ST2+. All flow cytometric data analysis was performed with FlowJo software.
Live confocal microscopy of lung sections
Live confocal microscopy of lung sections was used for visualizing the distribution of ILC2s and Th2 cells in mouse lung tissue slices ex vivo. After euthanasia, mouse lungs were inflated with 1.5% of low-melt agarose in RPMI at 37°C. Inflated tissues were kept on ice, in 1% FBS in PBS, and sliced into 300-350 µm sections using Leica VT1000 S Vibrating Blade Microtome (Leica Microsystems) at speed 5, in ice-cold PBS. Tissue sections were stained with fluorescence labeled anti-EPCAM antibody (eBioscience) for 2 h on ice. After staining, sections were washed three times and cultured in complete lymphocyte medium (Phenol Red-free RPMI supplemented with 10% FBS, 25 mM HEPES, 50 μM β-ME, 1% Pen/Strep/L-Glu and 1% Sodium Pyruvate) in a humidified incubator at 37°C. Tissues were allowed to completely recover for 12 h prior to imaging. Sections were held down with tissue anchors (Warner Instruments) in 14 mm microwell dishes (MatTek) and imaged using Leica DMi8 inverted 5 channel confocal microscope equipped with an Environmental Chamber (NIH Division of Scientific Equipment and Instrumentation Services) to maintain 37°C and 5% CO2. Microscope configuration was set up for four-dimensional analysis (x,y,z,t) of cell segregation and migration within tissue sections. Diode laser for 405 nm excitation; Argon laser for 488 and 514 nm excitation, DPSS laser for 561; and HeNe lasers for 594 and 633 nm excitation wavelengths were tuned to minimal power (between 1 and 5%). Z stacks of images were collected (10 – 50 µm). Mosaic images of lung sections were generated by acquiring multiple Z stacks using a motorized stage to cover the whole section area and assembled into a tiled image using LAS X (Leica Microsystems) software. Post-acquisition mages were processed using Imaris (Bitplane) software.
Statistical analysis
Data analysis was performed with GraphPad Prism (GraphPad Software). The student’s t-test was used to determine the statistical significance between the two groups. Data were presented as mean ± SEM. A p-value <0.05 was considered statistically significant and indicated as *; p < 0.01 was indicated as **; p < 0.001 was indicated as ***; and p < 0.0001 was indicated as ****. Not statistically significant was indicated as ns.
Results
Generation of novel Gata3-ZsGreen reporter mouse strains
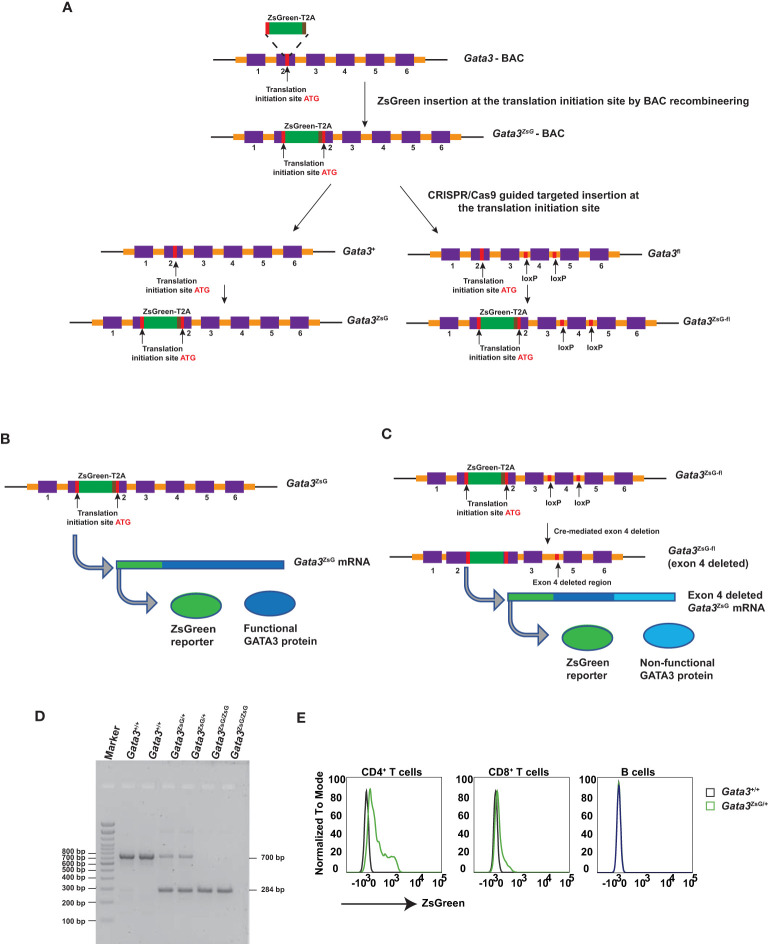
To generate a Gata3-ZsGreen reporter system in mice, we first inserted the ZsGreen-T2A cassette into a Gata3-containing bacterial artificial chromosome (BAC) at translation initiation site of the Gata3 gene ( Figure 1A ). T2A is a small self-cleavable peptide which allows co-expression of multiple proteins in a relatively equal ratio from the same messenger RNA (23, 24). Thus, the insertion of ZsGreen-T2A cassette at the ATG site of the endogenous GATA3 should result in the expression of separated ZsGreen and GATA3 protein and thus have a minimal effect on endogenous GATA3 functions. By using the CRISPR/Cas9 technology with a guided RNA targeting DNA fragment near the ATG site and the engineered BAC as the repairing template, the ZsGreen-T2A cassette was successfully inserted into the exon 2 region of either WT Gata3 allele or the Gata3 fl allele with conditional knockout potential ( Figure 1A ). The resulting Gata3-ZsGreen (Gata3 ZsG) reporter system should allow normal expression of the endogenous GATA3 protein ( Figure 1B ). On the other hand, the functional GATA3 protein on the Gata3 ZsG-fl reporter system could be converted into a non-functional form of GATA3 after Cre-mediated excision of the exon 4 since this exon encodes zinc fingers that are critical for GATA3 functions. Because the ZsGeen is at the 5’ of the Gata3 gene, its expression could be retained after Gata3 deletion if functional GATA3 has a minimal role on its own transcription ( Figure 1C ).
Figure 1.
Generation of Gata3-ZsGreen reporter system on WT (Gata3 ZsG) and on conditional Gata3 KO (Gata3 ZsG-fl) background. (A) Schematic representation of steps involved in the generation of mouse strains carrying the Gata3 ZsG or Gata3 ZsG-fl allele from the initial Gata3 ZsG-BAC construction to CRISPR/Cas9 targeted integration of ZsGreen-T2A reporter into the mouse genome. (B) Expected expression of ZsGreen reporter and functional GATA3 protein from the same mRNA. (C) Expected expression of ZsGreen reporter and non-functional GATA3 protein after exon 4 deletion. (D) Gel image of genotyping results distinguishing the Gata3 ZsG reporter allele from the WT Gata3 + allele. (E) Flow cytometry analysis of ZsGreen expression among CD4+ T cells, CD8+ T cells, and B lymphocytes in the blood. The results (D, E) are representative of more than two independent experiments.
Gata3 ZsG/+ hemizygous mice were bred with WT C57BL/6 to maintain a hemizygous state. Some hemizygous mice were also intercrossed to generate homozygous animals. The WT and ZsG alleles can be distinguished by PCR genotyping ( Figure 1D ). Since our initial testing indicated that the expression of ZsGreen from a single allele was bright enough, all the experiments in this study were carried out with the hemizygous Gata3 ZsG/+ or Gata3 ZsG-fl/fl mice. In naïve hemizygous Gata3 ZsG/+ mice, green fluorescence was readily detectable on blood CD4+ T cell population by flow cytometry analysis ( Figure 1E ). Low levels of ZsGreen were also observed in CD8 T cells. However, B lymphocytes did not show any green fluorescence. These results were expected based on our previous knowledge on the differential GATA3 expression in mature lymphocytes (25–27). Furthermore, ZsGreen expression was not detected in myeloid cell populations, including neutrophils, eosinophils, DCs, and alveolar macrophages ( Supplementary Figure 1 ). Therefore, these results confirm that the ZsGreen expression in our system is specific to T lymphocytes in which endogenous GATA3 is supposed to be expressed. Similar ZsGreen expression was also noted in the hemizygous Gata3 ZsG-fl/fl mice, which were mainly used in the follow-up in-depth analysis.
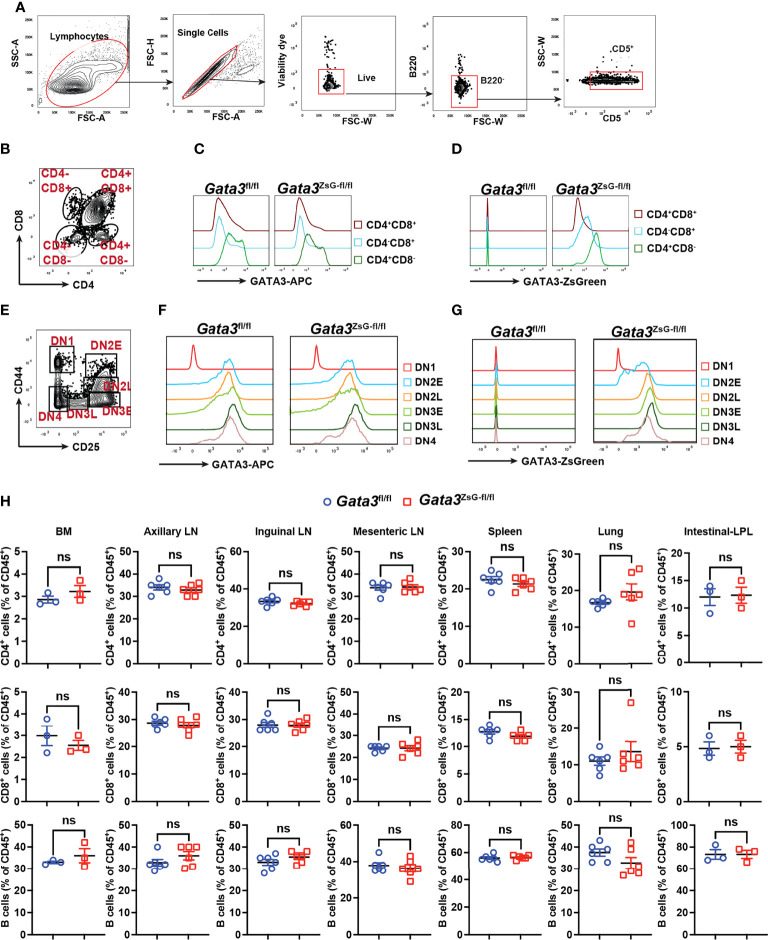
ZsGreen faithfully reflects GATA3 protein expression during thymic development
The expression of GATA3 protein is critical for the earliest T cell progenitor development, and its deficiency in hematopoietic stem cells leads to a complete absence of T cells (28, 29). The hemizygous Gata3 ZsG-fl/fl mice had a normal level of GATA3 protein expression across various stages of thymocyte development ( Figures 2A-G and Supplementary Figures 2A, B ). Importantly, the ZsGreen reporter expression correlated well with dynamic changes in GATA3 protein levels during CD4 and CD8 lineage commitment ( Figures 2C, D ). Furthermore, ZsGreen also correlated well with GATA3 protein levels at different stages of CD4/CD8 double negative (DN) cells during early thymic development ( Figures 2F, G ). These results indicate that the Gata3 ZsG-fl/fl mice have normal thymic development, and that the ZsGreen reporter faithfully reflects the expression of endogenous GATA3 throughout different stages of T cell development in the thymus. We next analyzed the distribution of T and B cells in secondary lymphoid organs and peripheral tissues. Consistent with normal T cell development in the thymus of the Gata3 ZsG-fl/fl mice, these mice had normal numbers of B cells, CD4 T cells, and CD8 T cells in axillary lymph nodes, inguinal lymph nodes, mesenteric lymph nodes, and spleen ( Figure 2H ). Normal distribution of these lymphocytes was also confirmed in the bone marrow, lung, and intestine lamina propria.
Figure 2.
Normal thymic development in the Gata3 ZsG-fl/fl mice. Thymocytes from Gata3 fl/fl and Gata3 ZsG-fl/fl mice were stained and analyzed by flow cytometry. (A) Gating strategy for distinguishing T cells from other cell population. (B) Gating strategy for analyzing different developmental stages of T lymphocytes. (C) Flow cytometric histograms showing GATA3 protein expression in CD4 and CD8 single or double positive lymphocytes. (D) ZsGreen expression in CD4 and CD8 single or double positive lymphocytes. (E) Gating strategy for analyzing different stages of CD4 and CD8 double negative (DN) cells. (F) Flow cytometric histograms showing GATA3 protein expression in DN cells at various developmental stages. (G) ZsGreen expression in DN cells at various developmental stages. (H) Distribution analysis of CD4+ T cells, CD8+ T cells, and B cells in various lymphoid organs and tissues based on flow cytometry data (Mean ± SEM; ns, not significant; Student t-test) . Results are representative of two independent experiments.
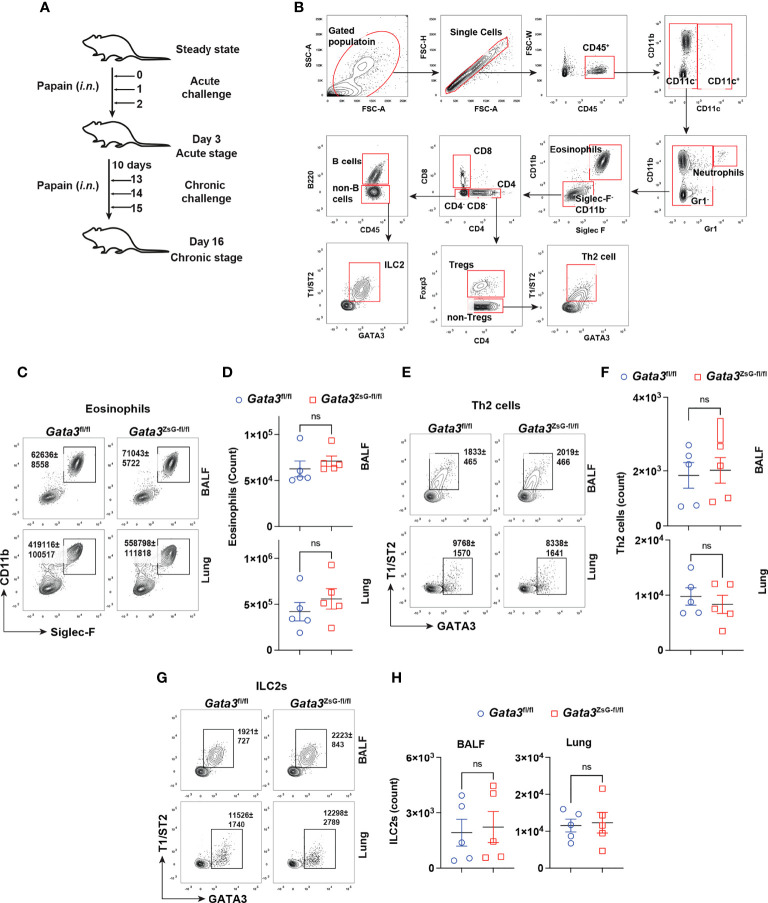
Gata3 ZsG-fl/fl mice have intact ILC2 and Th2 cell response
A coordinated activation of ILC2s and Th2 cells is important for generating robust type 2 immune responses (7). It has been observed that mice deficient in either ILC2s or Th2 cells show impaired immune responses (30, 31). We used a mouse model of airway inflammation by protease papain intranasal challenges, which activate both ILC2s and Th2 cells ( Figures 3A, B ). The disease severity was assessed by eosinophils enumeration ( Figures 3B-D ). Our results showed that Gata3 fl/fl and Gata3 ZsG-fl/fl mice had comparable numbers of eosinophils. In addition, both Gata3 fl/fl and Gata3 ZsG-fl/fl mice had identical numbers of ILC2s and Th2 cells ( Figures 3E-H ). Therefore, these results indicate that the insertion of ZsGreen reporter in the Gata3 ZsG-fl/fl mice does not affect the generation of ILC2s and Th2 cells that are capable of inducing eosinophil recruitment.
Figure 3.
Normal development of ILC2s and Th2 cells in the Gata3 ZsG-fl/fl mice. (A) Schematic representation of the mouse model of airway inflammation induced by chronic papain i.n. challenges. (B) Gating strategy for analyzing eosinophils, Th2 cells, and ILC2s from BALF and lung. (C) Gata3 fl/fl and Gata3 ZsG-fl/fl mice were challenged with papain followed by flow cytometric analysis of eosinophilic infiltration in BALF and lung. (D) The total numbers of eosinophils in lung in C were counted and plotted (Mean ± SEM; n = 5; ns = not significant, Student t-test). (E) Th2 cells in BALF and the lung were identified by flow cytometry. (F) The total numbers of Th2 cells in E were counted and plotted (Mean ± SEM; n = 5; ns, not significant, Student t-test). (G) ILC2s in BALF and the lung were identified by flow cytometry. (H) The total ILC2s in G were counted and plotted (Mean ± SEM; n = 5; ns, not significant, Student t-test). Results are representative of two independent experiments.
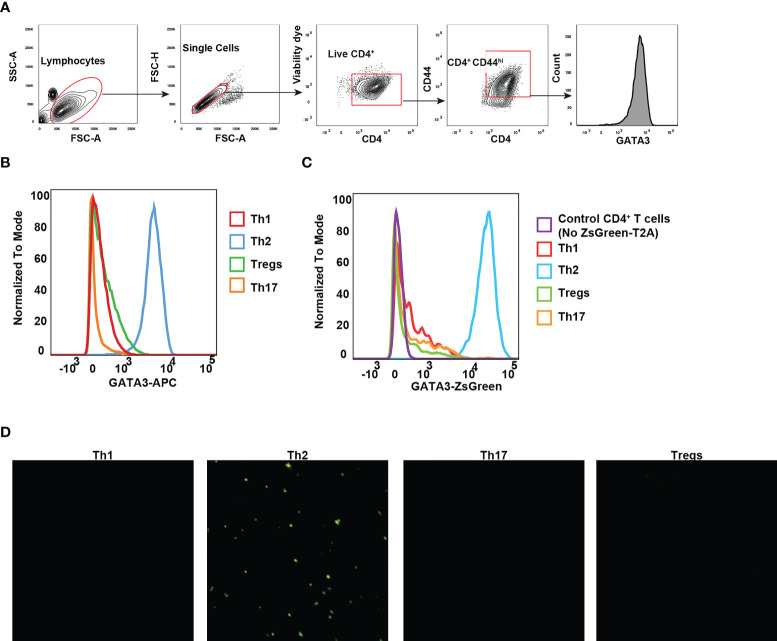
The ZsGreen reporter expression faithfully reflects the GATA3 protein levels in vitro
The differentiation of CD4 T helper cell subsets is tightly regulated by lineage specific expression of master transcription factors: T-bet for Th1, GATA3 for Th2, RORγt for Th17, and Foxp3 for Tregs (32). GATA3 expression in CD4 T cells is induced by TCR stimulation together with IL-4 signaling, and the induction of GATA3 is critical for effector Th2 differentiation (33). To evaluate whether ZsGreen expression can faithfully reflect the GATA3 protein levels, we chose to monitor ZsGreen expression across various effector T cell populations differentiated in vitro, particularly because in vitro generated Th2 cells express high levels of GATA3. Naïve CD4 T cells from Gata3 ZsG-fl/fl mice were isolated and cultured under different polarization conditions towards Th1, Th2, Th17, and Treg cells. As expected, Th2 cells expressed high levels of GATA3 protein ( Figures 4A, B ). Analysis of Th subsets for green fluorescence also confirmed the highest levels of ZsGreen expression in effector Th2 cells indicating that the ZsGreen reporter expression correlates with the GATA3 protein levels ( Figure 4C ). The green fluorescence expression was also preferentially detected in Th2 cells by fluorescence microscopic imaging ( Figure 4D ). These results demonstrate that the ZsGreen reporter expression in Th2 cells derived from the Gata3 ZsG-fl/fl mice can faithfully reflect the expression of GATA3 protein.
Figure 4.
GATA3 reporter expression reflects the GATA3 protein levels. Naïve CD4 T cells from Gata3 ZsG-fl/fl mice were polarized into different Th subsets in vitro. (A) Gating strategy for identifying live CD4+CD44hi effector population. (B) Flow cytometry histograms showing GATA3 protein expression by transcription factor staining. (C) Flow cytometry data showing the ZsGreen reporter expression on live CD4 T cell subsets. (D) Representative fluorescent microscopy photographs showing the presence of green fluorescence emitting cells with Th2 polarizing conditions. Results are representative of three (B, C) and two (D) independent experiments.
In addition to in vitro differentiated T helper subsets, we also analyzed the expression of ZsGreen reporter by various ILCs in the gut lamina propria lymphocytes. The GATA3 protein judged by transcription factor staining was expressed by ILC2s at the highest levels ( Supplementary Figures 3A, B ). However, the remaining ILC subsets, including NK cells, ILC1s, ILC3s, and LTis, expressed moderate levels of GATA3 protein ( Supplementary Figures 3A, B ). Accordingly, the ZsGreen reporter expression in different ILC subsets correlated with the GATA3 protein levels with ILC2s expressing the highest level of ZsGreen among all ILC subsets ( Supplementary Figures 3C, D ). These results demonstrate that ZsGreen reporter expression from Gata3 ZsG-fl/fl mice faithfully reflects the GATA3 protein expression in both T cell and ILC subsets.
Functional GATA3 is dispensable for maintaining its own expression in mature ILC2s or Th2 cells
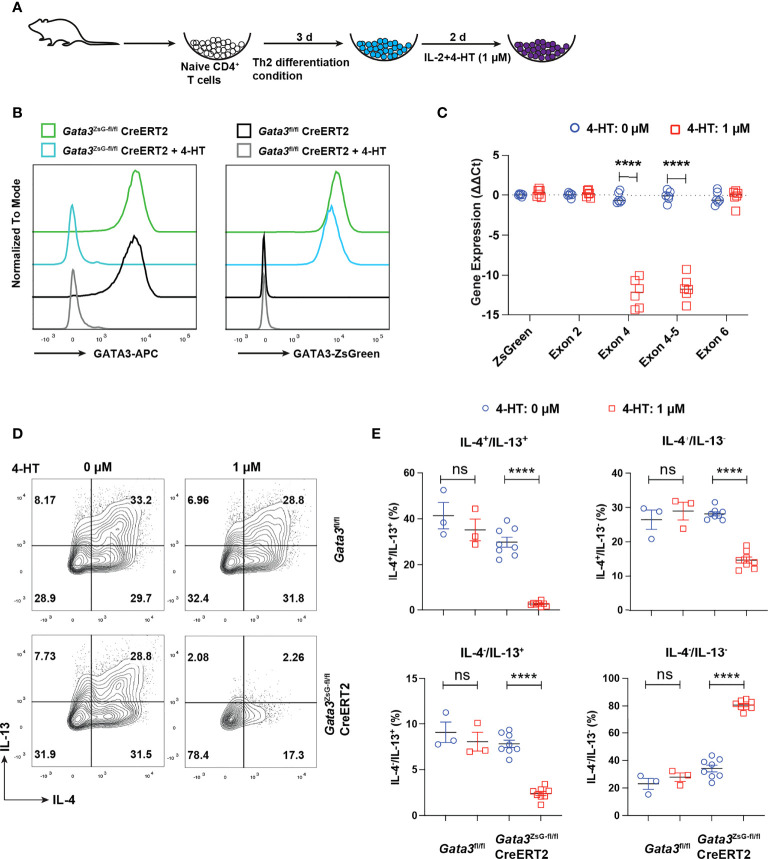
Our previous RNA-Seq analyses of Th2 cells from WT and GATA3-deficient (deletion of Gata3 exon 4 which encodes zinc finger domains required for GATA3 functions) mice suggest that functional GATA3 may not be required for maintaining its own transcription (2, 16, 34). To test whether ZsGreen inserted at the translation initiation site of Gata3 can still serve as a reporter after Gata3 gene exon 4 deletion, we isolated naïve CD4 T cells from the Gata3 fl/flCre-ERT2 and Gata3 ZsG-fl/flCre-ERT2 mice and cultured them under Th2 conditions in vitro for three days. The cultures were then treated with 4-hydroxy tamoxifen (4-HT) for two days ( Figure 5A ). The treatment with 4-HT, an active metabolite of tamoxifen that binds to CreERT2 to induce Cre activity, efficiently reduced the GATA3 protein in Th2 cells from both Gata3 fl/flCre-ERT2 and Gata3 ZsG-fl/flCre-ERT2 group. Nevertheless, 4-HT-treated Gata3 ZsG-fl/flCre-ERT2 “Th2” cells continuously expressed ZsGreen reporter after Gata3 gene deletion ( Figure 5B ). Moreover, the mRNA quantification by RT-qPCR confirmed that 4-HT treatment effectively removed the Gata3 exon 4. However, the expression of remaining Gata3 exons, including exon 2 and exon 6, as well as ZsGreen reporter mRNA remained intact ( Figure 5C ). The expression of both IL-4 and IL-13 by 4-HT-treated Gata3 ZsG-fl/flCre-ERT2 “Th2” cells was reduced confirmed functional Gata3 deletion ( Figures 5D, E ). We also differentiated naïve CD4 T cells from Gata3 ZsG-fl/flCre-ERT2 mice under Th2 conditions in the presence of 4-HT from the beginning of the culture and found that Gata3 ZsG-fl/flCre-ERT2 “Th2” cells had high levels of ZsGreen reporter expression even when the Gata3 deletion occurred before Th2 cell differentiation ( Supplementary Figure 4A ). These results further demonstrate that the induction of ZsGreen expression during Th2 differentiation does not require functional GATA3.
Figure 5.
ZsGreen continues to be expressed in the GATA3 KO “Th2” cultures. (A) Schematic diagram for Th2 cell differentiation using naïve CD4 T cells from Gata3 fl/flCreERT2, Gata3 fl/fl and Gata3 ZsG-fl/flCreERT2 mice followed by 4-HT treatment to delete Gata3 gene in CreERT2 positive cells. (B) Flow cytometry histograms showing GATA3 protein levels and ZsGreen reporter expression from Th2 cells with and without 4-HT treatment. (C) qPCR data for analyzing the expression of ZsGreen mRNA and mRNA of indicated Gata3 exons in the presence and absence of 4-HT (Mean ± SEM; n = 6; ****p < 0.0001, Student t-test). (D) Flow cytometry analysis of IL-4 and IL-13 expression by in vitro differentiated Th2 cells followed by 4-HT treatment. (E) The IL-4+ and IL-13+ Th2 cell population from (D) were counted and plotted (Mean ± SEM; n = 3-8; ns, not significant; ****p < 0.0001, Student t-test). Results are representative of two independent experiments.
We performed a similar experiment with ILC2s. The Gata3 fl/flCre-ERT2 and Gata3 ZsG-fl/flCre-ERT2 mice were chronically challenged with proteinase papain, and then ILC2s were purified and incubated with 4-HT for 2 days ( Supplementary Figure 5A ). 4-HT-treated ILC2s from either mouse strain no longer expressed GATA3 suggesting an efficient Gata3 deletion, however, these ILC2s from the Gata3 ZsG-fl/flCre-ERT2 mice continued to express ZsGreen reporter ( Supplementary Figure 5B ). These results indicate that GATA3 is dispensable for maintaining its own expression in mature ILC2s, and that ZsGreen inserted at the 5’ of Gata3 can be used as a valuable marker for identifying ILC2s and Th2 cells after Gata3 deletion.
GATA3-deficient “ILC2s” and “Th2” cells are identifiable by ZsGreen in vivo
To investigate the function of GATA3 in regulating its own expression in ILC2s and Th2 cells in vivo, we intranasally challenged mice with papain to induce airway inflammation as described in Figure 3A and treated mice with tamoxifen on day 14. Th2 cells induced in vivo in untreated mice expressed T1/ST2 as expected, and tamoxifen treatment resulted in the loss of T1/ST2-expressing CD4 T cell population ( Figure 6A ). A similar change was observed for the lung ILC2s after Gata3 deletion. Therefore, “Th2” cells and “ILC2s” after Gata3 deletion became unidentifiable without GATA3-ZsGreen reporter. However, in the Gata3 ZsG-fl/flCre-ERT2 mice while both Th2 cells and ILC2s co-expressed T1/ST2 and ZsGreen without tamoxifen treatment ( Figure 6B ), after Gata3 deletion induced by tamoxifen, these “type 2” cells still expressed high levels of ZsGreen (albeit with a slight reduction in MFI) despite of an expected loss of T1/ST2 expression. Thus, Gata3 ZsG-fl/flCre-ERT2 allows us to identify “Th2” cells and “ILC2s” after Gata3 deletion. These ZsGreen-expressing Gata3 KO type 2 lymphocytes also had a severe reduction in the expression of type 2 effector cytokines, including IL-5 and IL-13 ( Figure 6C ). These results not only confirmed the important role of GATA3 in maintaining type 2 phenotype that was observed in our previous in vitro studies (2, 16, 34), they also indicate that GATA3 plays a modest role in maintaining its own expression in mature type 2 lymphocytes in vivo. Therefore, our novel reporter system offers a valuable tool to identify and study Th2 cells and ILC2s generated in vivo with and without Gata3 deletion.
Figure 6.
GATA3 KO “Th2” cells and “ILC2s” are identifiable in the Gata3 ZsG-fl/flCreERT2 mice. Mice were challenged with papain as shown in Figure 3A and injected (i.p.) with tamoxifen on day 14. The mice were euthanized on day 16 to analyze ILC2 and Th2 cell responses. (A) Gata3 fl/fl and Gata3 fl/fl CreERT2 mice were challenged with papain and injected with tamoxifen followed by flow cytometric analysis of Th2 cells (left panel) and ILC2s (right panel) in lung. (B) Gata3 ZsG-fl/fl, and Gata3 ZsG-fl/fl CreERT2 mice were challenged with papain and injected with tamoxifen followed by flow cytometric analysis of Th2 cells (left panel) and ILC2s (right panel) in lung. (C) Flow cytometric analysis of IL-5 and IL-13-expressing Th2 cells (left panel) and ILC2s (right panel) among the ZsGreen+ cells from the Gata3 ZsG-fl/fl and Gata3 ZsG-fl/flCreERT2 mice challenged with papain and injected with tamoxifen. Results are representative of two independent experiments.
The role of GATA3 in maintaining “ILC2” population in vitro
We have previously reported that GATA3 is critical for maintaining the Th2 and ILC2 population in vitro, but its function in vivo is less clear. To confirm our previous results in this new reporter system, we set up in vitro culture experiments by mixing the WT ILC2s from the CD45.1 congenic C57BL/6 mice and CD45.2 ILC2s from the Gata3 ZsG-fl/flCre-ERT2 mice in the presence and absence of 4-HT ( Supplementary Figure 6A ). Consistent with our previous report, ILC2s originated from the Gata3 ZsG-fl/flCre-ERT2 mice reduced dramatically in number seven days after Gata3 deletion by 4-HT treatment as compared to the WT CD45.1 ILC2s in the same culture ( Supplementary Figures 6C, D ). Annexin V staining revealed that Gata3 exon 4 deletion resulted in a higher frequency of apoptotic cells even two days after 4-HT treatment compared to the untreated group ( Supplementary Figures 6F, G ). We also performed mixed culture experiments with CD4 T cells differentiated under Th2 conditions and found that the persistence of Th2 cells required functional GATA3, as previously reported ( Supplementary Figure 7 ). Therefore, GATA3 is indeed critical for maintaining ILC2s and Th2 cells in vitro.
GATA3-deficient ZsGreenhi ILCs can be maintained in vivo
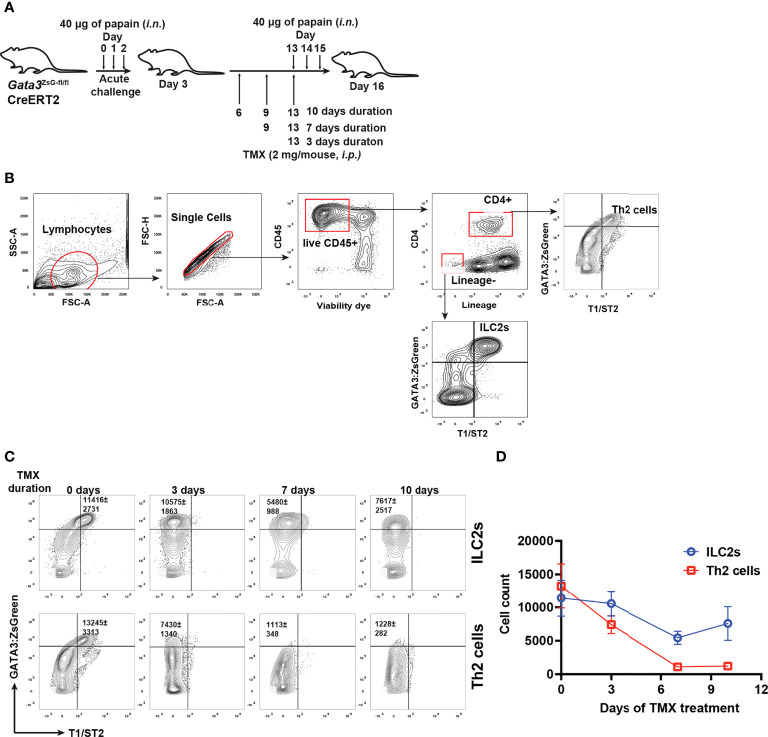
To assess whether GATA3 is also critical for maintaining ILC2s in vivo, we perform papain induced airway inflammation with Gata3 ZsG-fl/flCre-ERT2 mice that were treated with tamoxifen for various durations to assess the number of ILC2s and Th2 cells ( Figures 7A, B ). Unexpectedly, a substantial number of ZsGreenhi GATA3-deficient ILCs were still identified even 7-10 days after tamoxifen treatment, although Th2 cells were barely detectable under those conditions ( Figures 7C, D ). While Gata3 deletion in T cells early on may have blocked the generation of Th2 cells, these results also indicate that GATA3-deficient “ILC2s” might be more stable in vivo than in vitro.
Figure 7.
GATA3-deficient “ILC2s” are relatively stable in vivo. (A) Mice were challenged (i.n.) with papain and injected (i.p.) with tamoxifen as indicated in the figure for different durations. The mice were euthanized on day 16 to analyze the ILC2s and Th2 cells in the lung. (B) Gating strategy for identifying ILC2s and Th2 cells. (C) Flow cytometry data showing the ILC2s and Th2 cells at various time duration after TMX injection. (D) The total numbers of ILC2s and Th2 cells in (C) were counted, and the values were plotted. Results are representative of two independent experiments.
Gata3 ZsG/+ mice are also useful for visualizing ILC2s and Th2 cells by imaging
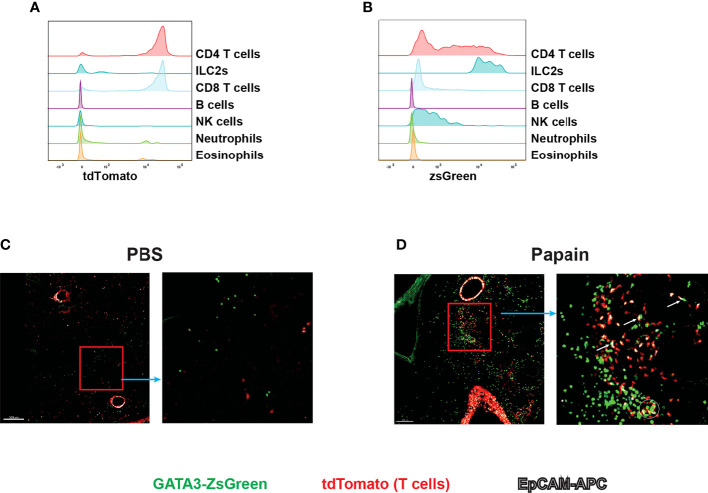
Live imaging is an important approach for understanding the behavior and interactions of immune cells during immune responses. The reporter system allows immune cells to be observed close to physiological conditions (35). Since GATA3 is highly expressed by ILC2s and Th2 cells, our Gata3 ZsG/+ mouse strain could be a valuable tool for live imaging of ILC2s and Th2 cells. To distinguish Th2 cells from ILC2s, we have further crossed our Gata3 ZsG/+ mice to a T cell fate mapping mouse strain CD4Cre R26 tdTomato. The resulting Gata3 ZsG/+ CD4 Cre R26 tdTomato mice allow us to distinguish GATA3hi ILC2s (ZsGreenhitdTomatoneg) from Th2 cells (ZsGreenhitdTomato+) during type 2 immune responses ( Figures 8A, B ). Indeed, papain induced dramatic expansion of ILC2s (marked by green color) in the lung of Gata3 ZsG/+ CD4 Cre R26 tdTomato mice, and the appearance of Th2 cells (marked by orange color, Figures 8C, D ). Some potential interactions between ILC2s and Th2 cells were also noted ( Figure 8D ). Therefore, our Gata3 ZsG reporter system is also suitable for live imaging of ILC2s and Th2 cells.
Figure 8.
Live imaging of Th2 cells and ILC2s with Gata3 ZsG/+ Cd4 Cre R26 tdTomato Gata3 ZsG/+ Cd4 Cre R26 tdTomato mice were chronically challenges with papain and lung tissues were sliced and visualized by confocal microscopy. The green fluorescence expressing cells represents the ZsGreen-expressing ILC2s. The red fluorescence expressing cells represents the total T cells population. The colocalization of green and red fluorescent cells represent Th2 cells indicated by orange color. The white fluorescence represents the EpCAM stained cells. (A) Flow cytometry histogram represents the fate mapping marker tdTomato expression among various lymphoid and myeloid population in the lung tissue. (B) ZsGreen expression analysis by flow cytometry across various lymphoid and myeloid population. (C) Confocal microscope image of lung tissue slice isolated from PBS challenged mice show the distribution of ILC2s and T cell population. The highlighted part of the image was enlarged and showed beside the main image. (D) Confocal microscope image of lung tissue slice isolated from papain challenged mice show the distribution of ILC2s and T cell population. The highlighted part of the image was enlarged and showed on the right. Several potential interactions between ZsGreenhitdTomatoneg cells (ILC2s) and ZsGreenhitdTomato+ (Th2) cells were indicated by a circle and arrows in the enlarged image. Results are representative of two independent experiments.
Discussion
ILC2s and Th2 cells are two critical lymphocyte subsets mediating type 2 immune responses (7, 36). Both populations share the master transcription factor GATA3 for their development and functions (2–4), while it is also important for T cell and ILC development (5, 37). In this report, we prepared novel GATA3 reporter strains (Gata3 ZsG/+ and Gata3 ZsG-fl/fl). By analyzing T cell development in these reporter mice, we have verified normal expression of endogenous GATA3 protein at various stages of T cell development, which also correlated with ZsGreen reporter. Among the Th subsets polarized in vitro, Th2 cells expressed the highest levels of ZsGreen as expected. High levels of ZsGreen reporter expression also correlated with GATA3 protein expression in vivo in Th2 cells and ILC2s in papain-induced lung inflammation. Thus, these novel GATA3 reporter strains allow us to study ILC2s and Th2 cells in vivo.
Several cytokine reporters have been made to study Th cell differentiation, ILC development, and activation in vivo. However, not all the ILC or Th subsets express their signature cytokines at any given time point and such process usually requires cell activation. Therefore, transcription factor reporters are more reliable than cytokine reporters in identifying ILC and Th subsets. For lymphocytes involved in type 2 immune responses, while reporters for Th2 cytokines, including IL-4, IL-5, and IL-13, are quite valuable (38–40), GATA3 reporter should be more definitive in identifying Th2 cells and ILC2s in vivo.
We have previously generated T-bet and RORγt reporters by preparing BAC transgenic mice (41–43). Since these transgenic BACs contain sufficient genomic elements to mimic the endogenous T-bet or RORγt expression but are independent of natural Tbx21 and Rorc gene loci, the reporters worked quite well. There is also an opportunity to breed these reporters onto the gene knockout background to study Th1 or Th17 wannabe cells. However, by using the same BAC strategy, we failed to generate a faithful GATA3 reporter strain which is consistent with the notion that the regulatory elements at the Gata3 locus span more than 1Mb, a size that is impossible to be included in a BAC clone. Therefore, a knock-in strategy is more appropriate for preparing the GATA3 reporter. Even though we have used an engineered BAC as the repairing template in CRISPR/Cas9-mediated knock-in hoping that the very long homologous arms on the BAC can increase the efficiency of site-specific insertion, recent advances in the CRISPR/Cas9 technology allow much shorter synthetic DNAs to be used as the template.
Several GATA3 reporter strains, including lacZ expression system with GATA3 protein (28), GFP cassette within the Gata3 gene locus (18), EGFP-GATA3 fusion protein expression system (17), and most recently the IRES-YFP at the 3’-UTR of Gata3 gene locus (19), have been made. It is important to note that there is a dose effect of GATA3 in various cells. GATA3 haploinsufficiency has been reported both in mice and in humans (44, 45). Therefore, the knock-in/knock-out strategy in making a GATA3 reporter is not an ideal situation. In addition, any increased or reduced expression of endogenous GATA3 by reporter insertion could possibly have a functional impact. Both IRES and T2A have been used to introduce a reporter gene without affecting the endogenous gene expression. However, it has also been reported that the insertion of IRES sometimes may result in overexpression of the linked gene, whereas T2A has a very minimal if any effect. Therefore, we chose to use T2A over IRES in preparing our reporter mice. Nevertheless, the insertion of IRES-YFP at the 3’ of Gata3 gene doesn’t seem to alter GATA3 expression in another report (19). Therefore, some of the previous GATA3 reporter strains have their limitations, and none of them has the potential to report GATA3 expression after conditionally deleting functional GATA3 (i.e. conditional GATA3 KO).
GATA3 regulates the expression of type 2 associated genes, including Il5, Il13, and Il1rl1 in both Th2 cells and ILC2s (2). GATA3 is also critical for the expansion of Th2 cells and ILC2s in vitro. Such a conclusion was made based on the experiments of deleting GATA3 in vitro. In vivo, while GATA3 expression is critical for the development of ILC2s and Th2 cell differentiation, how GATA3 regulates its target genes in mature ILC2s and Th2 cells in vivo is difficult to address. This is partly because lack of a suitable reporter system to identify “ILC2s” and “Th2” cells after inducible Gata3 gene deletion. It remains uncertain whether ILC2s and Th2 cells will die after GATA3 removal in vivo. By using the mouse strain Gata3 ZsG-fl/flCreERT2 generated in this study, we have now shown clearly that GATA3-deficient “ILC2s” and “Th2” cells expressing ZsGreen reporter can be identified in vivo, and these cells indeed fail to express type 2 cytokines and T1/ST2. The continuous expression of ZsGreen after GATA3 deletion also indicates that GATA3 is not essential for regulating its own expression in mature Th2 cells and ILC2s. While ILC2s die after GATA3 removal in vitro consistent with previous reports, by monitoring ZsGreenhi ILCs in vivo after GATA3 removal by tamoxifen, unexpectedly, we were still able to detect ZsGreenhi ILCs ten days after tamoxifen treatment. Further investigations are needed to explain the difference between in vitro and in vivo. Can other ILCs upregulate ZsGreen expression to fill the niche if ILC2s are indeed depleted? Is the type 2 environment responsible for maintaining the survival of ZsGreenhi ILCs after GATA3 removal? Are these remaining cells pre-existing ILC2s or newly generated ILC2-like cells from the ILC2 progenitors? If these remaining ZsGreenhi ILCs are indeed pre-existing ILC2s before tamoxifen treatment, what are the in vivo signals that support their survival? It will be also interesting to test whether these “ILC2s” will gain a phenotype of other ILCs in vivo in the absence of GATA3.
Our new reporter strain with the conditional GATA3 knockout potential will allow the research to study GATA3-deficient ILC2s and Th2 cells in vivo. A similar GATA3-GFP reporter mouse strain has been previously made with the IRES-GFP cassette inserted into the 3’ of the Gata3 exon 4, which was also flanked by two loxp sites (46). Indeed, GFP-expressing GATA3-deficient nephric duct cells can be detected in the embryos of these mice. However, it is difficult to know whether our new strain is better than this previous strain in studying lymphocytes without directly comparing them. Since the authors only reported GFP expression after GATA3 deletion, the GFP reporter is likely non-functional in the presence of floxed exon 4 given that the IRES-GFP cassette is located within the intron of endogenous Gata3. Therefore, when this GFP reporter is used in the GATA3-sufficient settings with a presumable Gata3 gfp/+ strain, the GFP-expressing cells from these mice are basically GATA3 heterozygous and thus express only half amounts of GATA3.
In addition, we used the ZsGreen, which is an enhanced version of GFP. Because of its brightness, ZsGreen offers an advantage in sensitivity in reporting gene expression, which also makes it suitable for live cell imaging. Indeed, after crossing the Gata3 ZsG/+ mice with the Cd4 Cre R26 tdTomato mice, in which all T cells are marked red, the resulting Gata3 ZsG/+ Cd4 Cre R26 tdTomato mice could be used to visualize ILC2s (ZsGreen+) and Th2 cells (ZsGreen+tdTomato+) during type 2 immune responses. Therefore, our reporter system will also facilitate future research on investigating the crosstalk between ILC2s and Th2 cells, as well as their appearance, migration, and tissue distributions under physiological conditions in vivo. Furthermore, because GATA3 plays an important role in early T cell and ILC development, our reporter system should also be suitable for lymphocyte progenitor studies.
Data availability statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was reviewed and approved by National Institute of Allergy and Infectious Diseases (NIAID) Animal Care and Use Committee.
Author contributions
JZ conceived the project. RG performed most of the experiments. DW and MB performed some experiments related to in vitro differentiation of Th subsets; QY performed some experiments related to papain-induced airway inflammation. OK performed lung tissue slicing and confocal imaging of live lung tissue. HC and MZ performed experiments analyzing ZsGreen expression by various ILCs in the gut. JKa analyzed the confocal microscopy data and produced the final images. CL and JKh supervised the generation of mouse strains carrying the Gata3 ZsG-fl and Gata3 ZsG allele using CRISPR/Cas9. RG and JZ wrote the manuscript. JZ supervised the project. All authors contributed to the article and approved the submitted version.
Funding
This work is supported by the Division of Intramural Research of the NIAID (grant 1ZIA-AI-001169).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2022.975958/full#supplementary-material
References
- 1. Pai SY, Truitt ML, Ting CN, Leiden JM, Glimcher LH, Ho IC. Critical roles for transcription factor GATA-3 in thymocyte development. Immunity (2003) 19:863–75. doi: 10.1016/s1074-7613(03)00328-5 [DOI] [PubMed] [Google Scholar]
- 2. Yagi R, Zhong C, Northrup DL, Yu F, Bouladoux N, Spencer S, et al. The transcription factor GATA3 is critical for the development of all IL-7Ralpha-expressing innate lymphoid cells. Immunity (2014) 40:378–88. doi: 10.1016/j.immuni.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoyler T, Klose CS, Souabni A, Turqueti-Neves A, Pfeifer D, Rawlins EL, et al. The transcription factor GATA-3 controls cell fate and maintenance of type 2 innate lymphoid cells. Immunity (2012) 37:634–48. doi: 10.1016/j.immuni.2012.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zheng W, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4 T cells. Cell (1997) 89:587–96. doi: 10.1016/S0092-8674(00)80240-8 [DOI] [PubMed] [Google Scholar]
- 5. Zhu J. GATA3 regulates the development and functions of innate lymphoid cell subsets at multiple stages. Front Immunol (2017) 8:1571. doi: 10.3389/fimmu.2017.01571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinette ML, Colonna M. Immune modules shared by innate lymphoid cells and T cells. J Allergy Clin Immunol (2016) 138:1243–51. doi: 10.1016/j.jaci.2016.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gurram RK, Zhu J. Orchestration between ILC2s and Th2 cells in shaping type 2 immune responses. Cell Mol Immunol (2019) 16:225–35. doi: 10.1038/s41423-019-0210-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhu J, Min B, Hu-Li J, Watson CJ, Grinberg A, Wang Q, et al. Conditional deletion of Gata3 shows its essential function in T(H)1-T(H)2 responses. Nat Immunol (2004) 5:1157–65. doi: 10.1038/ni1128 [DOI] [PubMed] [Google Scholar]
- 9. Lohning M, Stroehmann A, Coyle AJ, Grogan JL, Lin S, Gutierrez-Ramos JC, et al. T1/ST2 is preferentially expressed on murine Th2 cells, independent of interleukin 4, interleukin 5, and interleukin 10, and important for Th2 effector function. Proc Natl Acad Sci United States America (1998) 95:6930–5. doi: 10.1073/pnas.95.12.6930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guo L, Huang Y, Chen X, Hu-Li J, Urban JF, Jr., Paul WE. Innate immunological function of TH2 cells in vivo . Nat Immunol (2015) 16:1051–9. doi: 10.1038/ni.3244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guo L, Wei G, Zhu J, Liao W, Leonard WJ, Zhao K, et al. IL-1 family members and STAT activators induce cytokine production by Th2, Th17, and Th1 cells. Proc Natl Acad Sci United States America (2009)106:13463–8. doi: 10.1073/pnas.0906988106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halim TY, MacLaren A, Romanish MT, Gold MJ, McNagny KM, Takei F. Retinoic-acid-receptor-related orphan nuclear receptor alpha is required for natural helper cell development and allergic inflammation. Immunity (2012) 37:463–74. doi: 10.1016/j.immuni.2012.06.012 [DOI] [PubMed] [Google Scholar]
- 13. Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature (2010) 464:1367–70. doi: 10.1038/nature08900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wong SH, Walker JA, Jolin HE, Drynan LF, Hams E, Camelo A, et al. Transcription factor RORalpha is critical for nuocyte development. Nat Immunol (2012) 13:229–36. doi: 10.1038/ni.2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ouyang W, Lohning M, Gao Z, Assenmacher M, Ranganath S, Radbruch A, et al. Stat6-independent GATA-3 autoactivation directs IL-4-independent Th2 development and commitment. Immunity (2000) 12:27–37. doi: 10.1016/S1074-7613(00)80156-9 [DOI] [PubMed] [Google Scholar]
- 16. Wei G, Abraham BJ, Yagi R, Jothi R, Cui K, Sharma S, et al. Genome-wide analyses of transcription factor GATA3-mediated gene regulation in distinct T cell types. Immunity (2011) 35:299–311. doi: 10.1016/j.immuni.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hosoya T, Kuroha T, Moriguchi T, Cummings D, Maillard I, Lim KC, et al. GATA-3 is required for early T lineage progenitor development. J Exp Med (2009) 206:2987–3000. doi: 10.1084/jem.20090934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Panayi H, Panayiotou E, Orford M, Genethliou N, Mean R, Lapathitis G, et al. Sox1 is required for the specification of a novel p2-derived interneuron subtype in the mouse ventral spinal cord. J Neurosci (2010) 30:12274–80. doi: 10.1523/JNEUROSCI.2402-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rao TN, Kumar S, Pulikkottil AJ, Oliveri F, Hendriks RW, Beckel F, et al. Novel, non-Gene-Destructive knock-in reporter mice refute the concept of monoallelic Gata3 expression. J Immunol (2020) 204:2600–11. doi: 10.4049/jimmunol.2000025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Warming S, Costantino N, Court DL, Jenkins NA, Copeland NG. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res (2005) 33:e36. doi: 10.1093/nar/gni035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell (2013) 153 910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kamijo S, Takeda H, Tokura T, Suzuki M, Inui K, Hara M, et al. IL-33-mediated innate response and adaptive immune cells contribute to maximum responses of protease allergen-induced allergic airway inflammation. J Immunol (2013) 190:4489–99. doi: 10.4049/jimmunol.1201212 [DOI] [PubMed] [Google Scholar]
- 23. Fang D, Cui K, Mao K, Hu G, Li R, Zheng M, et al. Transient T-bet expression functionally specifies a distinct T follicular helper subset. J Exp Med (2018) 215:2705–14. doi: 10.1084/jem.20180927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu Z, Chen O, Wall JBJ, Zheng M, Zhou Y, Wang L, et al. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci Rep (2017) 7:2193. doi: 10.1038/s41598-017-02460-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banerjee A, Northrup D, Boukarabila H, Jacobsen SE, Allman D. Transcriptional repression of Gata3 is essential for early b cell commitment. Immunity (2013) 38:930–42. doi: 10.1016/j.immuni.2013.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Garcia-Ojeda ME, Klein Wolterink RG, Lemaitre F, Richard-Le Goff O, Hasan M, Hendriks RW, et al. GATA-3 promotes T-cell specification by repressing b-cell potential in pro-T cells in mice. Blood (2013) 121:1749–59. doi: 10.1182/blood-2012-06-440065 [DOI] [PubMed] [Google Scholar]
- 27. Wang L, Wildt KF, Zhu J, Zhang X, Feigenbaum L, Tessarollo L, et al. Distinct functions for the transcription factors GATA-3 and ThPOK during intrathymic differentiation of CD4(+) T cells. Nat Immunol (2008) 9:1122–30. doi: 10.1038/ni.1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hendriks RW, Nawijn MC, Engel JD, van Doorninck H, Grosveld F, Karis A. Expression of the transcription factor GATA-3 is required for the development of the earliest T cell progenitors and correlates with stages of cellular proliferation in the thymus. Eur J Immunol (1999) 29:1912–8. doi: [DOI] [PubMed] [Google Scholar]
- 29. Ting CN, Olson MC, Barton KP, Leiden JM. Transcription factor GATA-3 is required for development of the T-cell lineage. Nature (1996) 384:474–8. doi: 10.1038/384474a0 [DOI] [PubMed] [Google Scholar]
- 30. Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity (2014) 40:425–35. doi: 10.1016/j.immuni.2014.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Harris NL, Loke P. Recent advances in type-2-Cell-Mediated immunity: Insights from helminth infection. Immunity (2017) 47:1024–36. doi: 10.1016/j.immuni.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 32. Fang D, Zhu J. Dynamic balance between master transcription factors determines the fates and functions of CD4 T cell and innate lymphoid cell subsets. J Exp Med (2017) 214:1861–76. doi: 10.1084/jem.20170494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol (2010) 10:225–35. doi: 10.1038/nri2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fang D, Cui K, Hu G, Gurram RK, Zhong C, Oler AJ, et al. Bcl11b, a novel GATA3-interacting protein, suppresses Th1 while limiting Th2 cell differentiation. J Exp Med (2018) 215:1449–62. doi: 10.1084/jem.20171127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Jong OG, Murphy DE, Mager I, Willms E, Garcia-Guerra A, Gitz-Francois JJ, et al. A CRISPR-Cas9-based reporter system for single-cell detection of extracellular vesicle-mediated functional transfer of RNA. Nat Commun (2020) 11:1113. doi: 10.1038/s41467-020-14977-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhu J. T Helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine (2015) 75:14–24. doi: 10.1016/j.cyto.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ho IC, Tai TS, Pai SY. GATA3 and the T-cell lineage: essential functions before and after T-helper-2-cell differentiation. Nat Rev (2009) 9:125–35. doi: 10.1038/nri2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, et al. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity (2001) 14:1–11. doi: 10.1016/S1074-7613(01)00084-X [DOI] [PubMed] [Google Scholar]
- 39. Mohrs M, Shinkai K, Mohrs K, Locksley RM. Analysis of type 2 immunity in vivo with a bicistronic IL-4 reporter. Immunity (2001) 15:303–11. doi: 10.1016/S1074-7613(01)00186-8 [DOI] [PubMed] [Google Scholar]
- 40. Ricardo-Gonzalez RR, Van Dyken SJ, Schneider C, Lee J, Nussbaum JC, Liang HE, et al. Tissue signals imprint ILC2 identity with anticipatory function. Nat Immunol (2018) 19:1093–9. doi: 10.1038/s41590-018-0201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yu F, Sharma S, Edwards J, Feigenbaum L, Zhu J. Dynamic expression of transcription factors T-bet and GATA-3 by regulatory T cells maintains immunotolerance. Nat Immunol (2015) 16:197–206. doi: 10.1038/ni.3053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhong C, Cui K, Wilhelm C, Hu G, Mao K, Belkaid Y, et al. Group 3 innate lymphoid cells continuously require the transcription factor GATA-3 after commitment. Nat Immunol (2016) 17:169–78. doi: 10.1038/ni.3318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity (2012) 37:660–73. doi: 10.1016/j.immuni.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Klein Wolterink RG, Serafini N, van Nimwegen M, Vosshenrich CA, de Bruijn MJ, Fonseca Pereira D, et al. Essential, dose-dependent role for the transcription factor Gata3 in the development of IL-5+ and IL-13+ type 2 innate lymphoid cells. Proc Natl Acad Sci United States America (2013) 110:10240–5. doi: 10.1073/pnas.1217158110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, et al. GATA3 haplo-insufficiency causes human HDR syndrome. Nature (2000) 406:419–22. doi: 10.1038/35019088 [DOI] [PubMed] [Google Scholar]
- 46. Grote D, Souabni A, Busslinger M, Bouchard M. Pax 2/8-regulated gata 3 expression is necessary for morphogenesis and guidance of the nephric duct in the developing kidney. Development (2006) 133:53–61. doi: 10.1242/dev.02184 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/ Supplementary Material . Further inquiries can be directed to the corresponding author.