Abstract
Carbamoyl phosphate synthetase 1 (CPS1) deficiency is an autosomal recessive urea cycle disorder with varying presentations. Patients with a neonatal-onset phenotype are initially healthy but develop severe hyperammonemia days after birth and often have poor or lethal outcomes, while patients who present later in life may exhibit less severe clinical manifestations. CPS1 deficiency is rarely found on newborn screening because most states do not screen for this disease due to the technical difficulties. We report a case of an 11-year-old, previously healthy girl who presented with hyperammonemia and acute psychosis after eating large amounts of meat at summer camp. A diagnosis of carbamoyl phosphate synthetase type 1 deficiency was suspected by biochemical profiles and confirmed by molecular analysis. Subsequent follow up lab results revealed ammonia to be only 25–39 μmol/L shortly after glutamine reached levels as high as 770–1432 μmol/L with concurrent alanine elevations, highlighting the compensating mechanisms of the human body. Her initial hospital course also demonstrated the importance of continuous renal replacement therapy (CRRT) in avoiding rebound hyperammonemia and high glutamine and the benefits of intracranial pressure (ICP) monitoring, providing 3% hypertonic saline and temperature control to avoid fever in treating cerebral edema. Carglumic acid was not considered helpful in this case, with BUN levels ranging between 2 and 4 mg/dL after administration.
Keywords: Carbamoyl phosphate synthetase 1 deficiency, Urea cycle disorder, Nacetylglutamate synthetase deficiency, Hyperammonemia, Glutamine, Alphaketoglutarate
1. Introduction
Carbamoyl phosphate synthetase 1 (CPS1) deficiency is a rare, autosomal recessive urea cycle disorder with an incidence of approximately 1:1,300,000 in the United States [1]. CPS1 deficiency results from bi-allelic disease-causing variants in the CPS1 gene, which can cause combined effects of low enzyme level and decreased activity [2]. Biochemical findings in CPS1 deficiency include elevated ammonia, decreased plasma citrulline, and elevated plasma glutamine. These findings are often missed by newborn screen as CPS1, OTC, and NAGS are not included in the recommended uniform screening panel (RUSP) [3]. Most patients present as neonatal crisis with hyperammonemia but individuals with leaky variants can present at variable ages [4]. Previous research has suggested that responsiveness to carglumic acid in CPS1 deficiency is variable and may be variant-specific [5].
2. Case report
A previously healthy, 11-year-old girl presented to the emergency department due to the abrupt onset of altered mental status for one day. No fever or infectious symptoms, history of similar symptoms, recent tick bites, history of head trauma, or recent sick contacts were noted. The patient's parents reported that she was always thin and a picky eater, avoiding eating meat throughout her life. As a baby, she had poor weight gain and required hydroxylated formula. A review of her history revealed that the patient was often emotionally unstable growing up. Even though she had never been enrolled in an individualized educational program (IEP), she was held back in first grade and had difficulty learning.
Upon admission, she was 143 cm tall (33rd percentile) and weighed 26.6 kg (3rd percentile). During the previous week, she had been attending summer camp, where she ate more chicken than usual. She developed fatigue after returning from camp and became disoriented with bizarre behavior the following day; she began using her hands to eat rather than eating with utensils and urinated on the bathroom floor rather than in the toilet.
An elevated ammonia level was noted in the ER with a peak ammonia level of 494 μmol/L several hours after arrival. She was admitted to the PICU, protein intake was stopped and the patient's hyperammonemia quickly corrected within 12 h with the use of intravenous sodium benzoate (5.5 g/m2) and sodium phenylacetate (5.5 g/m2), intravenous L-arginine (600 mg/kg/day), high dose glucose infusion, insulin and hemodialysis. Initial labs showed glutamine 1289 μmol/L, citrulline 0 μmol/L, arginine 16 μmol/L and undetectable urine orotic acid. Total parenteral nutrition containing amino acids 0.5 g/kg/day and intralipid 1 g/kg/day was initiated on the second day of hospitalization to suppress catabolism.
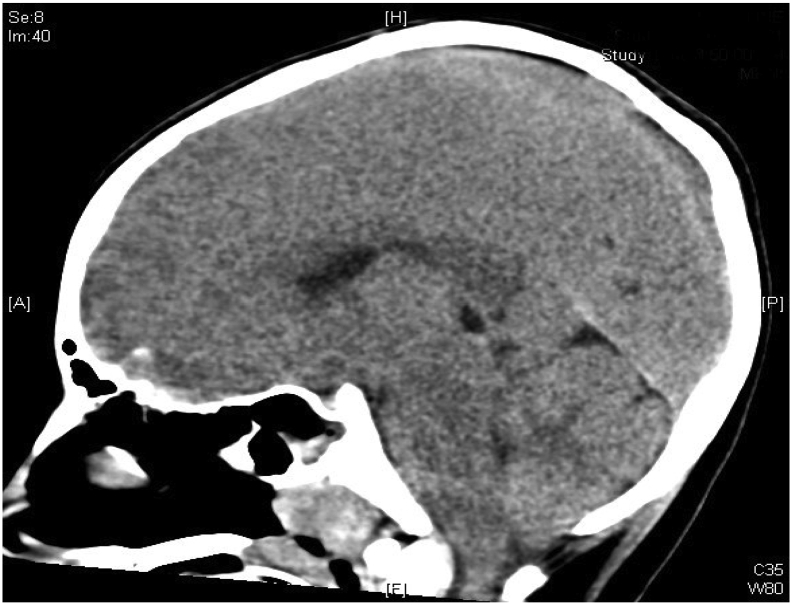
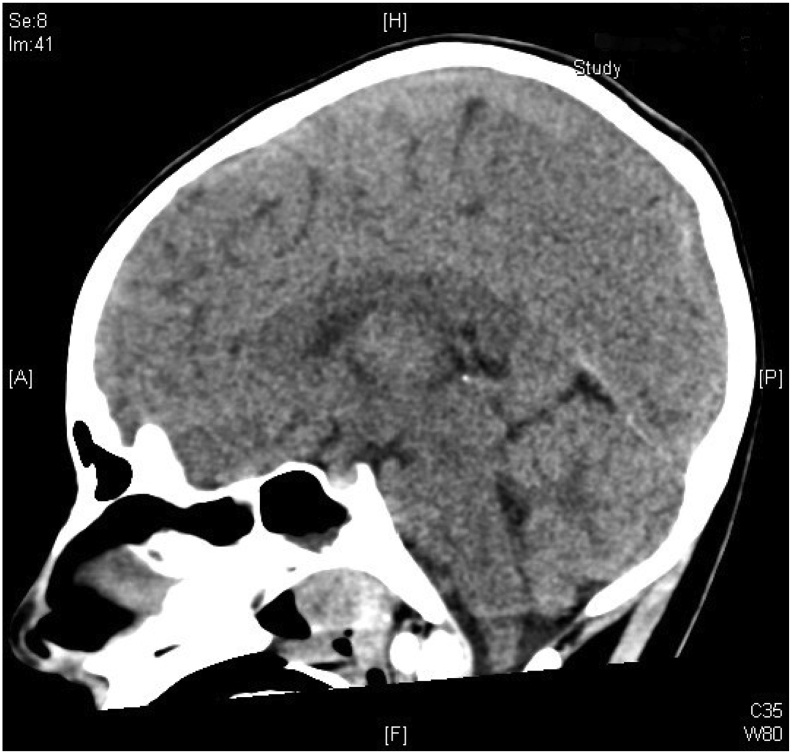
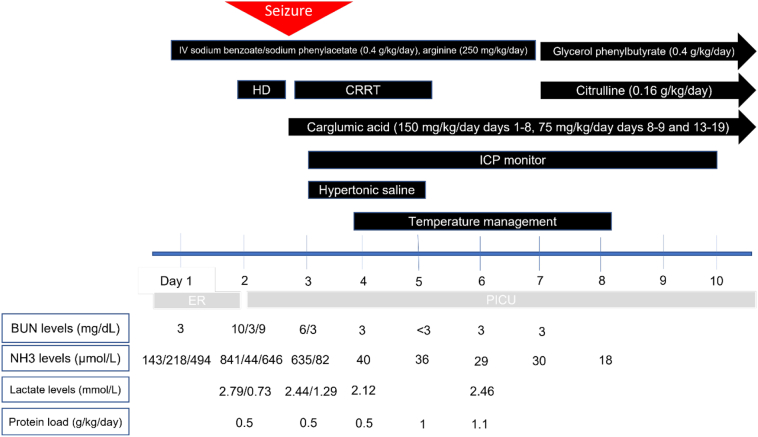
During her second day of hospitalization, she had a rebound hyperammonemia of 379 μmol/L; at that time, her glutamine measured 693 μmol/L. The patient was noted to have an irregular breathing pattern while on mechanical ventilation and desaturated to the 50's. She was seen actively seizing by the PICU night team. She received 1 mg of IV lorazepam followed by a second dose of 2 mg IV lorazepam. She also received two doses of IV levetiracetam 20 mg/kg (total 40 mg/kg). The patient was hypertensive, bradycardic, and had an abnormal breathing pattern with deep chest and abdominal inhalations followed by quick exhalations concerning for Cushing reflex due to increased intracranial pressure. She was given mannitol 1 g/kg, followed by propofol 50 mg and a second dose of propofol 25 mg for sedation and seizure control. STAT brain CT was done. Her CT was compatible with increasing cerebral and cerebellar edema with interval development of downward displacement of the cerebellar tonsils (Fig. 3) compared to prior CT performed 16 h earlier (Fig. 2). Electrolytes drawn during her seizure activity revealed a sodium of 134 mmol/L. She was given 5 mL/kg of hypertonic saline 3% twice and her sodium increased to 142 mmol/L. The patient's seizure stopped after hypertonic saline was administered. Continuous renal replacement therapy (CRRT) was initiated. Neurosurgery was consulted to place an ICP monitor and the patient was placed on continuous EEG monitoring and sedated with dexmedetomidine hydrochloride and fentanyl drips. She was treated with Artic Sun temperature management system to regulate her temperature for 5 days and IV sedation for a total of 10 days. Carglumic acid was initiated at a dose of 150 mg/kg/day and continued until hospital day 8, after which her carglumic acid dose was cut in half to 75 mg/kg/day. Her L-arginine infusion rate was decreased to 250 mg/kg/day on her second day of hospitalization. Parenteral nutrition amino acid content was adjusted to 1 g/kg/day on the 5th day of hospitalization and nasogastric tube feeding was started on her 6th day of hospitalization. She was started on a mixed formula that provided 47 kcal/kg and 1.1 g/kg/day of protein after she was weaned off total parenteral nutrition (TPN). On her 7th day of hospitalization, sodium benzoate, sodium phenylacetate and L-arginine infusions were discontinued and glycerol phenylbutyrate 9 mL/m2/day and citrulline at 270 mg/kg/day were initiated. (See Fig. 1.)
Fig. 3.
Image of brain CT on the third day of hospitalization. The image shows increasing cerebral and cerebellar edema with interval development of downward displacement of the cerebellar tonsils.
Fig. 2.
Image of brain CT on the second day of hospitalization.
Fig. 1.
Timeline of medical interventions.
Next generation sequencing (NGS) was performed for a panel of urea cycle disorders. The test results revealed two heterozygous variants, c.4193_4206delinsG (p.Leu1398Argfs*25) and c.793C > A (p.Pro265Thr), in the CPS1 gene (NM_001875.4). These variants were classified as likely pathogenic and variant of uncertain significance respectively by the lab. Based on the molecular panel, carbamoyl phosphate synthetase 1 (CPS1) deficiency was diagnosed. The patient began oral feeds on day 10 of hospitalization with a goal of 12 g of natural protein and 18 g of essential amino acid mixture daily. Her neurocognitive function returned back to baseline and she was discharged after 19 days of hospitalization. She continued to be followed as an outpatient in the inborn errors of metabolism clinic.
At discharge, carglumic acid (75 mg/kg/day), L-citrulline (280 mg/kg/day) and glycerol phenylbutyrate (3 mL TID) were prescribed and her protein goal was reduced to 0.9 g/kg. Carglumic acid was discontinued immediately after discharge (day 19) due to severe vomiting that resolved promptly with medication discontinuation. The glycerol phenylbutyrate was discontinued for two weeks due to insurance issues, during which the patient was managed with protein restriction and L-citrulline supplementation alone. The patient's glutamine levels increased to 1432 μmol/L after glycerol phenylbutyrate and carglumic acid were stopped, with ammonia level being only 39 μmol/L (see Table 2). The glycerol phenylbutyrate was then restarted and the patient's glutamine levels improved to 686 μmol/L.
Table 2.
Biochemical testing after discharge.
| Days after hospitalization | 24 | 31 | 44 | 52 | 55 | 67 | 72 | Reference Range |
|---|---|---|---|---|---|---|---|---|
| Ammonia (μmol/L) |
N/A | N/A | N/A | N/A | 39 | 25 | 23 | 31–80 |
| BUN (mg/dL) | N/A | 3 | 2 | 3 | N/A | N/A | 2 | 7–18 |
| Glutamine (μmol/L) |
575 | 236 | 579 | 1432 | 770 | 870 | 686 | 254–823 |
| Glutamate (μmol/L) |
54 | 105 | 29 | 50 | 47 | 56 | 91 | 5–150 |
| Alanine (μmol/L) |
0 | 494 | 471 | 1250 | 916 | 1166 | 1081 | 152–547 |
| Arginine (μmol/L) |
89 | 93 | 103 | 158 | 30 | 94 | 62 | 10–140 |
| Citrulline (μmol/L) |
0 | 119 | 22 | 58 | 26 | 60 | 39 | 1–46 |
| Leucine (μmol/L) |
92 | 114 | 49 | 143 | 92 | 331 | 69 | 48–160 |
2.1. Molecular assay
The c.4193_4206delinsG variant is a 14-bp deletion and a 1 bp insertion located in exon 36 of the CPS1 gene. It creates a frameshift starting at residue 1398 and generates a stop codon at position 25 of the new reading frame (p.Leu1398Argfs*25). It is predicted to cause loss of normal protein function through protein truncation or nonsense-mediated mRNA decay. Loss-of-function variants in the CPS1 gene are known to be associated with CPS1 deficiency [6]. This variant has not been reported in the literature, nor has it been observed in the population database (gnomAD). Taken together, Invitae reported the c.4193_4206delinsG variant as pathogenic while University of Oklahoma's laboratory reported the variant as likely pathogenic. Previous studies have shown that C-terminal truncations more distal than the one caused by this frameshift variant result in a large decrease in activity of CPS1 at saturation of N-acetylglutamate (Vmax) and a significant increase in Km for N-acetylglutamate [7]. It can therefore be inferred that this variant likely results in near total lack of enzyme activity even without mRNA decay or protein degradation.
The c.793C > A variant in exon 8 of the CPS1 gene results in a missense substitution of proline with threonine at residue 265 (p.Pro265Thr), a highly conserved amino acid across species [8]. To our knowledge, this variant has not been reported in the literature; a different missense change affecting the same residue, p.Pro265Leu, has been identified in an infant with hyperammonemia [9]. The p.Pro265Thr variant in our patient with late-onset disease likely affects CPS1 less drastically than the p.Pro265Leu variant, which leads to neonatal-onset disease. This variant is absent from the population database gnomAD [10], indicating it is unlikely benign. Furthermore, the missense change has been observed in individual(s) with clinical features of carbamoyl phosphate synthetase I deficiency (Invitae unpublished data). Whereas the C-terminal moiety of CPS1 is involved in substrate binding and catalysis, the function of the N-terminal moiety of CPS1 remains undetermined. In silico analyses differ in their predictions of the variant's effect on protein function and structure. SIFT reports it as “deleterious”, PolyPhen-2 “Probably Damaging”, and Align-GVGD “Class C0”. Collectively, although both Invitae and the University of Oklahoma's laboratory reported the c.793C > A variant as a variant of uncertain significance, we consider this variant to be likely pathogenic.
Parental genetic test results show that the two variants are in different chromosomes (in trans). The c.4193_4206delinsG variant was inherited from the patient's mother and the c.793C > A variant was inherited from her father.
2.2. Biochemical assay
Initial plasma amino acid testing revealed a high glutamine and a citrulline level of zero (μmol/L). Orotic acid was not detected in urine. This biochemical profile was consistent with CPS1 deficiency or n-acetylglutamate synthetase (NAGS) deficiency. Serial amino acid and ammonia levels from admission to day 71 are summarized in the table below. Discharge occurred on day 19.
3. Discussion
CPS1 deficiency can be classified as neonatal- or late-onset, with neonatal-onset patients presenting with a potentially lethal hyperammonemia crisis days after birth, while late-onset CPS1 deficiency is usually associated with less severe manifestations [4]. This variation in disease may be due to the level of residual enzyme activity [11]. Patients diagnosed later in life often suffer cerebral insults and develop learning difficulties and mild intellectual impairment [[12], [13], [14]]. Furthermore, urea cycle disorders (UCDs) remain life-threatening conditions, with one study reporting a 10% mortality rate in a cohort of adult-onset UCDs and another case report of adult-onset CPS1 deficiency requiring liver transplantation shortly after diagnosis [15,16]. Therefore, proper recognition and management of UCDs is imperative to improve prognosis and quality of life. The low BUN levels with carglumic acid use in this patient suggests that carglumic acid may not be effective in CPS1 deficiency associated with this combination of genetic variants. Hypertonic saline was used to treat brain edema and had a successful outcome. Finally, ammonia remained low with glutamine elevation in this case, which we hypothesize was due to a large compensatory reservoir of alpha-ketoglutarate and alanine.
Carglumic acid, or N-carbamoyl-l-glutamate (NCG), is a synthetic analogue of N-acetyl-l-glutamate (NAG), an allosteric activator of CPS1 [17]. Previous reports have demonstrated variable clinical responsiveness to carglumic acid in patients with CPS1 deficiency, suggesting that the effect of carglumic acid may be variant-dependent [5,18]. CPS1 gene variants may decrease the stability of the CPS1 protein or lower the affinity of the enzyme for NAG [18]. Therefore, NCG supplementation may improve CPS1 function in some patients through saturation of NAG sites in partial CPS1 deficiency, maximizing CPS1 activation and protecting CPS1 from thermal and proteolytic inactivation [2,19]. However, in other CPS1 variants, NCG may compete with NAG binding instead and decrease residual ureagenesis [20]. Carglumic acid did not seem to be beneficial for our patient as her BUN levels remained at or below 3 mg/dL with carglumic acid administration. Since carglumic acid works by activating the urea cycle and increasing urea production, BUN levels can serve as a marker for the effectiveness of carglumic acid. This patient's low BUN levels indicate continued malfunction of the urea cycle and indicates that carglumic acid could not successfully restore her urea cycle [21]. The slightly elevated BUN before hemodialysis likely reflects dehydration (Table 1).
Table 1.
Biochemical testing during hospitalization.
| Days after hospitalization | 1 | 2 | 3 (AM) | 3 (PM) | 3 night | 4 | 5 | 6 | 7 | 8 | 18 | Reference Range |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ammonia (μmol/L) |
143 (outside hospital) 218 (after arrival) 494 (midnight) |
841 (before HD) 44 (after HD) 646 (rebound) |
635 (before CCRT) 82 (after starting CCRT) |
40 | 36 | 39 | 36 | 29 | 30 | 18 | N/A | 31–80 |
| BUN (mg/dL) | 3 | 10 (before HD) 3 (after HD) |
6 (before CCRT) 3 (after CCRT) |
3 | 3 | 2 | 3 | 3 | 3 | N/A | N/A | 7–18 |
| Glutamine (μmol/L) |
1289 | 1160 | 693 | 589 | 380 | 424 | 407 | 331 | 377 | 325 | 578 | 254–823 |
| Glutamate (μmol/L) |
50 | 435 | 143 | 76 | 34 | 70 | 95 | 130 | 142 | 156 | 77 | 5–150 |
| Alanine (μmol/L) |
216 | 371 | 593 | 506 | 190 | 313 | 252 | 202 | 263 | 235 | 616 | 152–547 |
| Arginine (μmol/L) |
16 | 2132 | 40 | 43 | 39 | 30 | 30 | 43 | 60 | 22 | 107 | 10–140 |
| Citrulline (μmol/L) |
0 | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 0 | 0 | 107 | 1–46 |
| Leucine (μmol/L) |
61 | 32 | 65 | 39 | 62 | 21 | 39 | 48 | 68 | 59 | 91 | 48–160 |
| Lactic acid (mmol/L) |
2.79 (before HD) 0.73 (after HD) |
2.44 (before CCRT) 1.29 (after CCRT) |
2.12 | 2.46 | 0.36–1.25 |
Ammonia levels can rise after hemodialysis and can be improved by early refeeding and protein reintroduction to prevent catabolism [22]. In our patient, rebound hyperammonemia occurred after hemodialysis, causing cerebral and cerebellar edema with interval development of downward displacement of the cerebellar tonsils. In addition to CRRT, nutrition administration, intracranial pressure monitor placement, hypertonic saline and temperature management were also used to decrease brain edema and prevent herniation.
Previous reports have mostly focused on dialysis, ammonia scavengers, and glucose administration to control hyperammonemia-induced brain edema in urea cycle disorders [16,[22], [23], [24]]. Temperature management and fever prevention are neuroprotective by attenuating secondary injury after the primary neurological insult. The underlying mechanisms involve multiple cellular and molecular pathways. Brain injury activates the inflammatory and immune response causing additional neurotoxicity and apoptosis. There is strong evidence in experimental and clinical studies that lowering body temperatures suppresses multiple aspects of this inflammatory reaction [25,26]. Elevated body temperatures have also been found to independently increase mortality, ICU and hospital lengths of stay, and worsen discharge disposition in adult neurology intensive care unit patients [27]. Temperature management in encephalopathy due to UCDs has been explored and found to be feasible and safe [[28], [29], [30], [31]]. In our patient, temperature management was used to suppress fever and prevent an inflammatory response to fever. Our 11-year-old patient demonstrated a successful outcome using temperature control for cerebral edema secondary to hyperammonemia in CPS1 deficiency.
Hypertonic saline was used in this patient to control intracranial pressure. Both hypertonic saline and mannitol are hyper-osmolar agents commonly used to treat cerebral edema [32]. We hypothesize that hypertonic saline may be preferable to mannitol because 3% hypertonic saline has a more sustained effect on intracranial pressure and can effectively increase cerebral perfusion pressure [33].
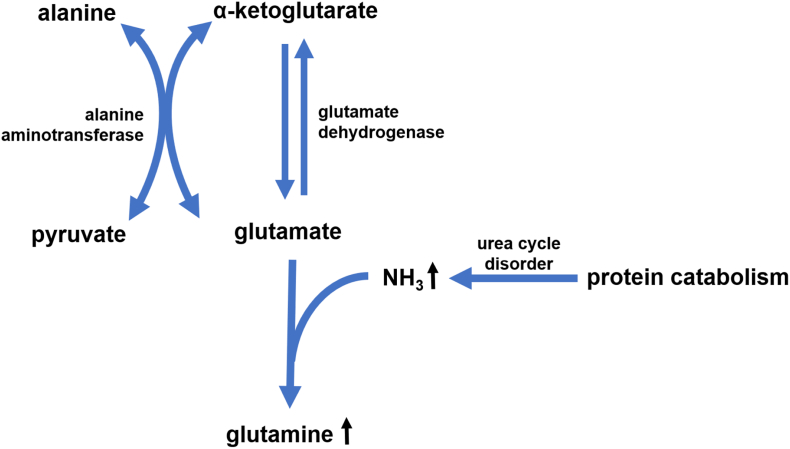
Despite the late identification of her diagnosis at 11 years of age, this patient self-selected low-protein foods and was spared clear of metabolic decompensation until her preteen years, suggesting that she possesses some measure of adequate protein tolerance. We hypothesize that the patient may have a large reservoir of alpha-ketoglutarate to compensate for chronically elevated ammonia levels. Alpha-ketoglutarate helps capture excess ammonia in the forms of glutamate and glutamine through glutamate dehydrogenase and glutamine synthetase, thereby keeping ammonia levels low [34]. Glutamine levels frequently rise in the setting of hyperammonemia in urea cycle disorders and can be used as a proxy for ammonia to monitor disease [34,35]. In our patient, glutamine levels were found to be elevated despite ammonia levels in the normal range. The patient felt well and had no obvious symptoms even at glutamine level of 1432 μmol/L. The concurrent elevation of alanine to 1250 μmol/L on Table 2 also demonstrated alanine being a reservoir to retain the NH3 to prevent hyperammonemia. These concepts can be visualized by Fig. 4 [[36], [37], [38]]. Even though glutamine formation from alpha-ketoglutarate helps detoxify ammonia, elevated glutamine has also been shown to have neurotoxic effects and should therefore be appropriately monitored and controlled [[36], [37], [38], [39]]. Also, once the patient has any illness, the balance could be immediately disrupted and result in hyperammonemia.
Fig. 4.
Compensating mechanism of hyperammonemia in urea cycle disorder.
Alanine also serves as a reservoir of ammonia as the amino group of glutamate is transferred to pyruvate by alanine aminotransferase which generates alanine and alpha-ketoglutarate [[36], [37], [38], [39]]. Therefore, alanine production allows the body to carry and store ammonia in a non-toxic form. The patient's recent onset of puberty and hunger resulted in too much protein intake and a glutamine level of 1164, concurrent alanine was 1646, and her ammonia was 13. It is not completely clear to us why alanine is elevated in CPS1 deficiency but not as much in other urea cycle disorders that cause hyperammonemia. Upon admission, her glutamine was 1289 and alanine was 216, her ammonia was 494. This again suggests alanine being the reservoir: when it is low, the ammonia goes up.
This patient's stable self-regulation for years also brings into question how stringent dietary restrictions need to be post-diagnosis. Although the patient was able to avoid hospitalizations in the past, she struggled with emotional regulation and academic performance was poor; it is not clear how much of this is related to her underlying metabolic diagnosis. Furthermore, failure to thrive was noted with weight at the 3rd percentile at admission to the hospital. Our aim during outpatient management of this patient was to maximize the amount of tolerated natural protein and add an appropriate nitrogen scavenger. She was prescribed glycerol phenylbutyrate, citrulline supplementation, and dietary modification of 0.9 g/kg/day total protein (with 7–12 g of essential amino acids and 19 g of natural protein). Dietary modification was then changed to natural protein at 0.9 g/kg/day to encourage adequate food intake. Under this regimen, the patient's weight and height improved gradually. One month after discharge, her weight increased to 30.2 kg (10th percentile) and her height was 142.6 cm (33th percentile). Thirteen months after discharge the patient reached 47 kg (67th percentile) and 152.8 cm (48th percentile). Ammonia levels remained well controlled, below 23 μmol/L. Prior to hospitalization, the patient's IQ was borderline normal and she never needed an individualized education program (IEP). Neuropsychological tests performed 6 months after discharge showed that the patient had returned to normal intellect. Her parents also reported that she had a more positive spirit and cried less with dietary and medical management.
Declaration of Competing Interest
We have no conflict of interests to declare.
Acknowledgements
We would like to show our gratitude to the patient depicted in this study and her family. We thank all the excellent IEM clinical team, ER, PICU, pediatric services of Oklahoma Children's Health and lab services of OU Health. This paper was supported by the Children Health Foundation's research fund.
Data availability
The authors are unable or have chosen not to specify which data has been used.
References
- 1.Summar M.L., Dasouki M.J., Schofield P.J., Krishnamani M.R., Vnencak-Jones C., Tuchman M., Mao J., Phillips J.A. 3rd, physical and linkage mapping of human carbamyl phosphate synthetase I (CPS1) and reassignment from 2p to 2q35. Cytogenet. Cell Genet. 1995;71:266–267. doi: 10.1159/000134124. [DOI] [PubMed] [Google Scholar]
- 2.Diez-Fernandez C., Häberle J. Targeting CPS1 in the treatment of carbamoyl phosphate synthetase 1 (CPS1) deficiency, a urea cycle disorder. Expert Opin. Ther. Targets. 2017;21:391–399. doi: 10.1080/14728222.2017.1294685. [DOI] [PubMed] [Google Scholar]
- 3.Recommended Uniform Screening Panel, Official Web Site of the U.S. Health Resources & Services Administration.
- 4.Funghini S., Thusberg J., Spada M., Gasperini S., Parini R., Ventura L., Meli C., De Cosmo L., Sibilio M., Mooney S.D., Guerrini R., Donati M.A., Morrone A. Carbamoyl phosphate synthetase 1 deficiency in Italy: clinical and genetic findings in a heterogeneous cohort. Gene. 2012;493:228–234. doi: 10.1016/j.gene.2011.11.052. [DOI] [PubMed] [Google Scholar]
- 5.Ah Mew N., McCarter R., Daikhin Y., Lichter-Konecki U., Nissim I., Yudkoff M., Tuchman M. Augmenting ureagenesis in patients with partial carbamyl phosphate synthetase 1 deficiency with N-carbamyl-L-glutamate. J. Pediatr. 2014;165:401–403.e403. doi: 10.1016/j.jpeds.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Häberle J., Shchelochkov O.A., Wang J., Katsonis P., Hall L., Reiss S., Eeds A., Willis A., Yadav M., Summar S., Urea Cycle Disorders Consortium, Lichtarge O., Rubio V., Wong L., Summar M. Molecular defects in human carbamoy phosphate synthetase I: mutational spectrum, diagnostic and protein structure considerations. Hum. Mutat. 2011 Jun;32(6):579–589. doi: 10.1002/humu.21406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cima S., Polo L.M., Díez-Fernández C., Martínez A.I., Cervera J., Fita I., Rubio V. Structure of human carbamoyl phosphate synthetase: deciphering the on/off switch of human ureagenesis. Sci. Rep. 2015;5 doi: 10.1038/srep16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent W.J., Sugnet C.W., Furey T.S., Roskin K.M., Pringle T.H., Zahler A.M., Haussler D. The human genome browser at UCSC. Genome Res. 2002 Jun;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kretz R., Hu L., Wettstein V., Leiteritz D., Häberle J. Phytohemagglutinin stimulation of lymphocytes improves mutation analysis of carbamoylphosphate synthetase 1. Mol. Genet. Metab. 2012;106:375–378. doi: 10.1016/j.ymgme.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Karczewski K.J., Francioli L.C., Tiao G., Cummings B.B., Alföldi J., Wang Q., Collins R.L., Laricchia K.M., Ganna A., Birnbaum D.P., Gauthier L.D., Brand H., Solomonson M., Watts N.A., Rhodes D., Singer-Berk M., England E.M., Seaby E.G., Kosmicki J.A., Walters R.K., Tashman K., Farjoun Y., Banks E., Poterba T., Wang A., Seed C., Whiffin N., Chong J.X., Samocha K.E., Pierce-Hoffman E., Zappala Z., O’Donnell-Luria A.H., Minikel E.V., Weisburd B., Lek M., Ware J.S., Vittal C., Armean I.M., Bergelson L., Cibulskis K., Connolly K.M., Covarrubias M., Donnelly S., Ferriera S., Gabriel S., Gentry J., Gupta N., Jeandet T., Kaplan D., Llanwarne C., Munshi R., Novod S., Petrillo N., Roazen D., Ruano-Rubio V., Saltzman A., Schleicher M., Soto J., Tibbetts K., Tolonen C., Wade G., Talkowski M.E., Salinas C.A. Aguilar, Ahmad T., Albert C.M., Ardissino D., Atzmon G., Barnard J., Beaugerie L., Benjamin E.J., Boehnke M., Bonnycastle L.L., Bottinger E.P., Bowden D.W., Bown M.J., Chambers J.C., Chan J.C., Chasman D., Cho J., Chung M.K., Cohen B., Correa A., Dabelea D., Daly M.J., Darbar D., Duggirala R., Dupuis J., Ellinor P.T., Elosua R., Erdmann J., Esko T., Färkkilä M., Florez J., Franke A., Getz G., Glaser B., Glatt S.J., Goldstein D., Gonzalez C., Groop L., Haiman C., Hanis C., Harms M., Hiltunen M., Holi M.M., Hultman C.M., Kallela M., Kaprio J., Kathiresan S., Kim B.-J., Kim Y.J., Kirov G., Kooner J., Koskinen S., Krumholz H.M., Kugathasan S., Kwak S.H., Laakso M., Lehtimäki T., Loos R.J.F., Lubitz S.A., Ma R.C.W., MacArthur D.G., Marrugat J., Mattila K.M., McCarroll S., McCarthy M.I., McGovern D., McPherson R., Meigs J.B., Melander O., Metspalu A., Neale B.M., Nilsson P.M., O’Donovan M.C., Ongur D., Orozco L., Owen M.J., Palmer C.N.A., Palotie A., Park K.S., Pato C., Pulver A.E., Rahman N., Remes A.M., Rioux J.D., Ripatti S., Roden D.M., Saleheen D., Salomaa V., Samani N.J., Scharf J., Schunkert H., Shoemaker M.B., Sklar P., Soininen H., Sokol H., Spector T., Sullivan P.F., Suvisaari J., Tai E.S., Teo Y.Y., Tiinamaija T., Tsuang M., Turner D., Tusie-Luna T., Vartiainen E., Vawter M.P., Ware J.S., Watkins H., Weersma R.K., Wessman M., Wilson J.G., Xavier R.J., Neale B.M., Daly M.J., MacArthur D.G., C. Genome Aggregation Database The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu L., Diez-Fernandez C., Rüfenacht V., Hismi B., Ünal Ö., Soyucen E., Çoker M., Bayraktar B.T., Gunduz M., Kiykim E., Olgac A., Pérez-Tur J., Rubio V., Häberle J. Recurrence of carbamoyl phosphate synthetase 1 (CPS1) deficiency in Turkish patients: characterization of a founder mutation by use of recombinant CPS1 from insect cells expression. Mol. Genet. Metab. 2014;113:267–273. doi: 10.1016/j.ymgme.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Brusilow S.W., Horwich A.L. McGraw-Hill; New York: 2001. The Metabolic and Molecular Bases of Inherited Disease. [Google Scholar]
- 13.Braissant O. Current concepts in the pathogenesis of urea cycle disorders. Mol. Genet. Metab. 2010;100(Suppl. 1):S3–s12. doi: 10.1016/j.ymgme.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Walker V. Ammonia toxicity and its prevention in inherited defects of the urea cycle. Diabetes Obes. Metab. 2009;11:823–835. doi: 10.1111/j.1463-1326.2009.01054.x. [DOI] [PubMed] [Google Scholar]
- 15.Toquet S., Spodenkiewicz M., Douillard C., Maillot F., Arnoux J.B., Damaj L., Odent S., Moreau C., Redonnet-Vernhet I., Mesli S., Servais A., Noel E., Charriere S., Rigalleau V., Lavigne C., Kaphan E., Roubertie A., Besson G., Bigot A., Servettaz A., Mochel F., Garnotel R. Adult-onset diagnosis of urea cycle disorders: results of a French cohort of 71 patients. J. Inherit. Metab. Dis. 2021;44:1199–1214. doi: 10.1002/jimd.12403. [DOI] [PubMed] [Google Scholar]
- 16.Bates T.R., Lewis B.D., Burnett J.R., So K., Mitchell A., Delriviere L., Jeffrey G.P. Late-onset carbamoyl phosphate synthetase 1 deficiency in an adult cured by liver transplantation. Liver Transpl. 2011;17:1481–1484. doi: 10.1002/lt.22407. [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration Drug Approval Package: Carbaglu (Carglumic Acid) Tablets. 2010. [Google Scholar]
- 18.Yap S., Gougeard N., Hart A.R., Barcelona B., Rubio V. N-carbamoylglutamate-responsive carbamoyl phosphate synthetase 1 (CPS1) deficiency: a patient with a novel CPS1 mutation and an experimental study on the mutation's effects. JIMD Rep. 2019;48:36–44. doi: 10.1002/jmd2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sugiyama Y., Shimura M., Ogawa-Tominaga M., Ebihara T., Kinouchi Y., Isozaki K., Matsuhashi T., Tajika M., Fushimi T., Ichimoto K., Matsunaga A., Ishida T., Mizutani K., Tsuruoka T., Murayama K. Therapeutic effect of N-carbamylglutamate in CPS1 deficiency. Mol. Genet. Metab. Rep. 2020;24 doi: 10.1016/j.ymgmr.2020.100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi D., Zhao G., Ah Mew N., Tuchman M. Precision medicine in rare disease: mechanisms of disparate effects of N-carbamyl-l-glutamate on mutant CPS1 enzymes. Mol. Genet. Metab. 2017;120:198–206. doi: 10.1016/j.ymgme.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barmore W., Azad F., Stone W.L. StatPearls Publishing; Treasure Island (FL): 2022 Jan. Physiology, Urea Cycle StatPearls [Internet] [PubMed] [Google Scholar]
- 22.Summar M.L., Mew N.A. Inborn errors of metabolism with Hyperammonemia: urea cycle defects and related disorders. Pediatr. Clin. N. Am. 2018;65:231–246. doi: 10.1016/j.pcl.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Redant S., Empain A., Mugisha A., Kamgang P., Attou R., Honoré P.M., De Bels D. Management of late onset urea cycle disorders-a remaining challenge for the intensivist? Ann. Intensive Care. 2021;11:2. doi: 10.1186/s13613-020-00797-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Summar M. Current strategies for the management of neonatal urea cycle disorders. J. Pediatr. 2001;138 doi: 10.1067/mpd.2001.111834. S30–39. [DOI] [PubMed] [Google Scholar]
- 25.Muengtaweepongsa S., Srivilaithon W. Targeted temperature management in neurological intensive care unit World. J. Methodol. 2017 Jun 26;7(2):55–67. doi: 10.5662/wjm.v7.i2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.González-Ibarra F.P., Varon J., López-Meza E.G. Therapeutic hypothermia: critical review of the molecular mechanisms of action. Front. Neurol. 2011;2:4. doi: 10.3389/fneur.2011.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diringer M.N., Reaven N.L., Funk S.E., Uman G.C. Elevated body temperature independently contributes to increased length of stay in neurologic intensive care unit patients. Crit. Care Med. 2004;32(7):1489–1495. doi: 10.1097/01.ccm.0000129484.61912.84. [DOI] [PubMed] [Google Scholar]
- 28.Ninković D., Mustapić Ž., Bartoniček D., Benjak V., Ćuk M., Buljević A.D., Grčić B.F., Fumić K., Grizelj R., Lehman I., Ramadža D.P., Sarnavka V., Slaviček J., Kastelić J.S., Barišić N., Barić I. The therapeutic hypothermia in treatment of Hyperammonemic encephalopathy due to urea cycle disorders and organic Acidemias. Klin. Padiatr. 2019;231:74–79. doi: 10.1055/a-0855-4001. [DOI] [PubMed] [Google Scholar]
- 29.Whitelaw A., Bridges S., Leaf A., Evans D. Emergency treatment of neonatal hyperammonaemic coma with mild systemic hypothermia. Lancet. 2001;358:36–38. doi: 10.1016/S0140-6736(00)05269-7. [DOI] [PubMed] [Google Scholar]
- 30.Lichter-Konecki U., Nadkarni V., Moudgil A., Cook N., Poeschl J., Meyer M.T., Dimmock D., Baumgart S. Feasibility of adjunct therapeutic hypothermia treatment for hyperammonemia and encephalopathy due to urea cycle disorders and organic acidemias. Mol. Genet. Metab. 2013;109:354–359. doi: 10.1016/j.ymgme.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 31.Vargha R., Möslinger D., Wagner O., Golej J. Venovenous hemodiafiltration and hypothermia for treatment of cerebral edema associated with hyperammonemia. Indian Pediatr. 2012;49:60–62. [PubMed] [Google Scholar]
- 32.Cook A.M., Morgan Jones G., Hawryluk G.W.J., Mailloux P., McLaughlin D., Papangelou A., Samuel S., Tokumaru S., Venkatasubramanian C., Zacko C., Zimmermann L.L., Hirsch K., Shutter L. Guidelines for the acute treatment of cerebral edema in neurocritical care patients. Neurocrit. Care. 2020;32:647–666. doi: 10.1007/s12028-020-00959-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rockswold G.L., Solid C.A., Paredes-Andrade E., Rockswold S.B., Jancik J.T., Quickel R.R. Hypertonic saline and its effect on intracranial pressure, cerebral perfusion pressure, and brain tissue oxygen. Neurosurgery. 2009 Dec;65(6) doi: 10.1227/01.NEU.0000359533.16214.04. (1035–41; discussion 1041–2) [DOI] [PubMed] [Google Scholar]
- 34.Batshaw M.L., Walser M., Brusilow S.W. Plasma alpha-ketoglutarate in urea cycle enzymopathies and its role as a harbinger of hyperammonemic coma. Pediatr. Res. 1980;14:1316–1319. doi: 10.1203/00006450-198012000-00008. [DOI] [PubMed] [Google Scholar]
- 35.Häussinger D., Schliess F. Glutamine metabolism and signaling in the liver. Front. Biosci. 2007;12:371–391. doi: 10.2741/2070. [DOI] [PubMed] [Google Scholar]
- 36.Gariani K., Klauser A., Vargas M.I., Lazeyras F., Tran C. New insight in hyperinsulinism/hyperammonemia syndrome by magnetic resonance imaging and spectroscopy. Brain Sci. 2022;12:389. doi: 10.3390/brainsci12030389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y.H., Hou X.L., Qi Q., Wang L., Luo L., Yang S.Q., Zhang Y.M., Miao Z.H., Zhang Y.L., Wang F., Wang H.Y., Huang W.D., Wang Z.H., Shen Y., Wang Y. Scavenging of blood glutamate for enhancing brain-to-blood glutamate efflux. Mol. Med. Rep. 2014;9:305–310. doi: 10.3892/mmr.2013.1793. [DOI] [PubMed] [Google Scholar]
- 38.Limón I.D., Angulo-Cruz I., Sánchez-Abdon L., Patricio-Martínez A. Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage. Comprom. Memory Funct. Front. Neurosci. 2021;15 doi: 10.3389/fnins.2021.578922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albrecht J., Norenberg M.D. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44:788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are unable or have chosen not to specify which data has been used.