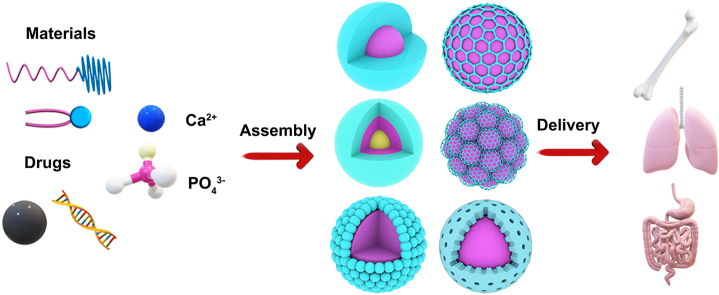
Abstract
Calcium phosphate nanoparticles represent promising materials for drug delivery because of its favorable properties, including biocompatibility, biodegradability and strong affinity for binding to nucleic acids (pDNA, siRNA, miRNA, etc.) and therapeutic drugs (cisplatin, carboplatin, paclitaxel, gefitinib, doxorubicin, etc.). Various strategies to prepare the size-controllable, stable, targeting and pH-responsive CaP nanocarriers have been extensively developed as the potential candidates in clinic. This review discusses the mostly recent developments in the design of calcium phosphate nanocarriers as drug delivery systems and therapeutic agents. Additionally, the advantage is unquestionably demonstrated and the obstacles are thoroughly examined in order to overcome future clinical issues.
Keywords: Calcium phosphate, Nanocarrier, Drug delivery, Preparation, Application
Graphical abstract
1. Introduction
Nanoparticles have been developed for drug delivery in vitro and in vivo rapidly due to its high surface-to-volume ratio which causes their specific physicochemical, biological, optical, electrical, and catalytic properties [1]. An ideal drug nanocarrier should be able to incorporate potential bioactive agents either physically or chemically and protect them in the bloodstream. Furthermore, the nanocarrier complex should de-assemble gradually and provide sustained and controllable drug release over prolonged period of time to increase therapeutic efficiency. In addition, it should provide a feasible mechanism to specifically bind to target cells or tissues, in order to reduce their off-target effects and enhance the on-site drug concentration [2]. Among different synthetic nanocarriers, calcium phosphate nanoparticles (CaP) have shown promising results toward the abovementioned criteria [[3], [4], [5]].
In contrast to many other kinds of inorganic nanoparticles (e.g., gold, magnetite, silica), calcium phosphate nanocarriers have gained increasing interest in nanomedicine because of their high biocompatibility and good biodegradability, which is due to the fact that calcium phosphate is the inorganic mineral of human bone and teeth [6,7]. Calcium phosphate nanoparticles are readily soluble at the low pH inside endo/lysosomes or phagosomes after cellular uptake, but stable at neutral pH, for example, the circulation in the body [8]. Furthermore, calcium phosphate nanoparticles meet many important requirements for an efficient delivery system, that is, the ability to incorporate drugs or biomolecules both inside and on the surface, either physically or covalently bound, the ability to retain such biomolecules until the particle has reached the target site and is dissolved, and its inherent biodegradation to harmless compounds (calcium and phosphate ions) [[9], [10], [11]].
In this review, we highlight a number of applications where calcium phosphate nanoparticles were successfully applied in biological systems for drug delivery. First, we shall introduce different preparations and characterization methods for calcium phosphate nanoparticles. Furthermore, we shall discuss those strategies of loading drugs and biomolecules functionalized in calcium phosphate nanoparticles. Compared with previous publications reviewing CaP design and applications in nanomedicine, our review provides a new materials science perspective, focusing more on the relationship of nanostructures and biological function of different CaP designed as therapeutics. This will be a major safety concern hindering clinical translation of different formulations of CaP nanocarriers developed for diseases therapy.
2. Preparation and characterization of calcium phosphate nanocarriers
Different methods for the preparations of calcium phosphate nanoparticles with different size, morphology, and composition were developed. These methods are focused on the preparation of calcium phosphate particles in the nanoscale dimension and several forms of calcium phosphate are prepared using a variety ratio of materials: hydroxyapatite (HAp) (Ca/P = 1.67); tricalcium phosphate (TCP) (Ca/P = 1.5); brushite (Ca/P = 1); and amorphous forms of CaP (ACP) (Ca/P = 1.5) and so on [12]. Current CaP coprecipitation synthesis protocols typically generate CaP particles using NaH2PO4, Na2HPO4, Na3PO4 or a combination of these three phosphate salts [[13], [14], [15]]. For example, CaP was synthesized as the following precipitation reaction [15]:
The supersaturation of different CaP phases in solution is dependent on many parameters, such as the calcium and phosphate ion concentrations in solution, pH and temperature. Table 1 lists the major members of the CaP family that are of interest to biomedical applications, according to Ca/P atomic ratio, pH stability range in aqueous solutions at 25 °C, and density. We want to stress that there is not only one calcium phosphate but a whole family of them, due to the different protonation states of the phosphate anion (PO43−, HPO42−, H2PO4−) and the ability of calcium phosphates to substitute anions and cations by other ions [12,16]. In various reports, the crystalline phase which is actually present is neither investigated nor known, and the material is often simply denoted as “calcium phosphate”. Thus, calcium phosphate nanomaterials often contain a mixture of different calcium phosphate phases. Given the fact that all of them are acid-soluble to calcium and phosphate ions, but this does affect the biomedical application mainly due to the degradability of different CaP phases in acidic buffers is different, which is sorted as follows (”≫” denotes much greater solubility): ACP ≫ α-TCP ≫ β-TCP > CDHAp ≫ HAp > fluorapatite [13]. Besides, among them, the most ubiquitous form of CaP is HAp. The process of HAp is formed in a neutral to basic solution by the interaction of calcium ions and phosphate ions to form a precursor amorphous phase (ACP) composed of Ca/P = 1.5 aqueous calcium phosphate (Ca3(PO4)2·xH2O), forming a spherical (Ca9(PO4)6) Posner's cluster (PCs) dense accumulation, which binds to water at the gap to form larger spherical particles [[17], [18], [19]]. However, HAp also is one of the most stable phases that can be generated under physiological conditions [20]. Hence it has been the model system that has been commonly developed for drug delivery.
Table 1.
| Ca/P Molar Ratio | CaP phase Name | Formula | pH Stability Range | Density (g/cm3) |
|---|---|---|---|---|
| 0.5 | MCPM (monobasic calcium phosphate monohydrate) | Ca(H2PO4)2·H2O | 0.0–2.0 | 2.22 |
| 1.0 | DCPA (dicalcium phosphate anhydrous) | CaHPO4 | 2.0–5.5 (>80 °C) | 2.929 |
| 1.0 | DCPD (dibasic calcium phosphate dehydrate) | CaHPO4·2H2O | 2.0–6.0 | 2.319 |
| 1.3 | OCP (octa calcium phosphate) | Ca8(HPO4)2(PO4)4·5H2O | 5.5–7.0 | 2.673 |
| 1.5 | TCP (tricalcium phosphate) | Ca3(PO4)2 | – | 2.814 |
| 1.2–2.2 | ACP (amorphous calcium phosphate) | CaxHy(PO4)z·nH2O n = 3–4.5 15%–20% H2O | 5–12 | – |
| 1.5–1.67 | CDHAp (calcium deficient hydroxyapatite) | Ca10-x (HPO4) x (PO4)6-x (OH)2-x (0 < x < 2) | 6.5–9.5 | – |
| 1.67 | HAp (Hydroxyapatite) | Ca10(PO4)6(OH)2 | 9.5–12.0 | 3.155 |
| 2.0 | TTCP (tetra calcium phosphate) | Ca4(PO4)2O | – | 3.056 |
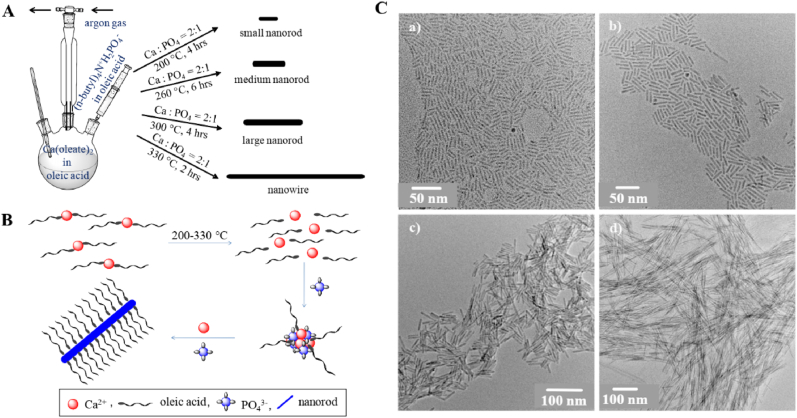
In general, calcium phosphate is synthesized by various methods like sol-gel chemistry [[27], [28], [29]], flame spray pyrolysis [20,30,31], solid-state reactions [32,33] and wet-chemical precipitation [[34], [35], [36]], as shown in Table 2. The sol–gel synthesis is based on the reaction of a calcium source and a phosphate source, usually in an organic solvent [37]. It offers different possibilities to fabricate a wide range of structured nanomaterials, including coatings on metallic implants [38]. As shown in Fig. 1, synthetic method involves injection of tetrabutylammonium phosphate into oleic acid solution of calcium oleate at 200–330 °C and controlling the nucleation-growth kinetics of calcium phosphate by temperature, molar ratio of calcium to phosphate, reaction time and solvent. Nanorods are formed at calcium to phosphate molar ratio of 2:1 and with increasing temperature more anisotropic nanowires are formed [39]. As the reaction progress at a certain growth condition, the length of nanorod/nanowire increases with time, attains a maximum and then length distribution becomes broad. These hydrophobic nanorods/nanowires are highly soluble, can be processed like conventional high quality hydrophobic nanoparticle and can be transformed into water soluble functional nanoparticle via ligand exchange or polymer coating approach. The sol-gel synthesis is advantageous due to its simplicity, high versatility, comparatively homogeneous product composition, and low synthesis temperature [27,40,41] (see Table 3).
Table 2.
Summary of main synthesis methods for CaP.
| Method | CaP phase | Size | Shape | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|
| sol-gel chemistry | ACP, β-TCP, HAp, DCPA | Nano/micron | Particle/sheet | narrow size distribution, comparatively low synthesis temperature | high cost, serious aggregation, usually needs special regents | [[27], [28], [29]], [37,38,40] |
| flame spray pyrolysis | β-TCP, HAp | submicron to hundreds of microns | particle | rapid synthesis, large-scale, usually resulted in spheic structure | difficult to obtain nano-size product, high energy consumption | [[42], [43], [44]] |
| solid-state reactions | β-TCP, α-TCP, HAp | micron | diverse | low cost, large-scale | serious aggregation, poor redispersability | [45,46] |
| wet-chemical precipitation in room temperature | Mainly HAp | nano | diverse | bulk synthesis, low cost, incorporation of compounds, often only water as solvent | upscaling can be difficult and requires a continuous process | [[47], [48], [49], [50], [51]] |
| wet-chemical precipitation under heating conditions | ACP, HAp | nano | Controllable shape | high reaction rate and efficiency | energy consumption; cannot load drugs (nucleic acids or proteins) | [[52], [53], [54], [55]] |
Fig. 1.
A) Synthesis approach for calcium phosphate nanorod and nanowire of controlled Length; B) Proposed mechanism of formation of calcium phosphate nanorod/nanowire; C) TEM images of CaP nanorod or nanowire with increasing length to width ratio [39].
Table 3.
Application of nanosized calcium phosphate in drug delivery.
| Structural form | Materials | Size/Shape | Drug | Features/Functions | Disease/Cells | Ref. |
|---|---|---|---|---|---|---|
| Mix-CaP | Sodium citrate | 129 nm Spherical | miR-133 | Synthesized via a straightforward one-pot protocol | Cardiovascular disease | [56] |
| Adenosine 5′-triphosphate | 150–240 nm Spherical | Doxorubicin | Sonochemical synthesis | human gastric carcinoma cancer | [57] | |
| Alendronate | ∼130 nm Spherical | pDNA ovalbumin | Mannose- and BP-dual modified | E.G7 tumor cells | [58] | |
| PDADMAC PAS | 72 nm Spherical | Docetaxel | Self-assembly by using two oppositely charged templates | Human gastric carcinoma cancer | [59] | |
| Sodium silicate | 50–70 nm Spherical and porous | ASODNs | Double reverse emulsion approach | Cervical carcinoma cancer | [60] | |
| PLGA | 207 nm Spherical | pDNA | Water-in–oil–in water (w/o/w) double emulsion solvent evaporation method | Human embryonic kidney 293 cancer | [61] | |
| Methylcellulose | 40–50 nm | – | One-pot synthesis to prepare thermo-sensitive hydrogel | Bone regeneration | [62] | |
| Polymer-Shell | LPEI | 304 nm Spherical | miR-34a | Coated by a long chain miRNA-34a conjugate and LPEI | Human prostate cancer | [51] |
| PEG-bisphosphonates | 160 nm Spherical | pDNA | Coated by PEG-bisphosphonates Stable over 72 h | Cervical carcinoma cancer | [63] | |
| PEG-alendronate | 260 nm Spherical | siBcl-2 | Coated by PEG- alendronate Stable over 1 month | Cervical carcinoma cancer | [35] | |
| PEG-PAsp(DET) | 40 nm Spherical |
siLuc | pH-responsive | Pancreatic cancer | [50,64] | |
| PEG-PAA | 140 nm Spherical |
siGL3 | pH-responsive | Human 293 cells | [65,66] | |
| PEG–SS–siRNA | 100 nm Spherical | siRNA | Redox-responsive | Huh-7 cells | [67] | |
| mPEG-b-PLG-g-GEM | 122 nm Spherical | Gemcitabine | cathepsin B- responsive | Pancreatic cancer | [68] | |
| Chitosan-glutamine | 119 nm Spherical | Noggin siRNA | Enhanced the cellular uptake | Stem cells | [69] | |
| Chitosan-dopamine | 131 nm Spherical | pDNA and siRNA | Enhanced the cellular uptake | COS-7 cells | [70] | |
| Carboxymethyl chitosan, KALA | 237 nm Spherical | Doxorubicin | Enhanced the cellular uptake by KALA | Hela cells | [71] | |
| Thiolated hyaluronic acid | 168 nm Vermicular/Spherical | siRNA | Disulfide cross-linked HA as an anionic shell | B16F10 cells | [36] | |
| Dopamine-hyaluronic acid | 63–278 nm Spherical | siRNA | Enhanced the cellular uptake by targeting CD44 | HT-29 cells | [72,73] | |
| Alendronate- hyaluronic acid | 170 nm Spherical | siEGFR | Enhanced the cellular uptake by targeting CD44 | A549 cells | [34] | |
| PLGA | 200 nm Spherical | pDNA | PLGA as the shell by W/O/W double emulsion method | HEK293 cells | [61] | |
| Heparin | <50 nm Spherical | Doxorubicin | Heparin/CaCO3/CaP mixed nanoparticles | Hela cells | [74] | |
| Citrus pectin, carboxymethyl-β-cyclodextrin | 108 nm Spherical | Paris saponin VII | Enhanced the cellular uptake, NPs stable within 18 days | Orthotopic drug-resistant colon cancer | [75] | |
| Arg-Gly-Asp peptide sequence RGD | 150/15 nm needle | siRNA | Enhanced the cellular uptake | A549 cancer cells | [76] | |
| Lipid-Shell | DOTAP, Cholesterol, DSPE-PEG-AA IGEPAL–CO–520 |
80 nm Spherical | siRNA | LCP-1: prepared by the reverse microemulsion method | H-460 cells | [77] |
| Cholesterol, DOTAP, DOPA, DSPE-PEG-AA |
20 nm Spherical | VEGF siRNA and gemcitabine | LCP-2: Codelivery of VEGF siRNA and gemcitabine in a single nanoparticle | Orthotopic xenograft NSCLC | [78] | |
| DOPA, DOPC, FA-DSPE-PEG |
40 nm Spherical | CD siRNA α-Tocopheryl succinate | Targeting combinate treatment of cancer | Melanoma | [79] | |
| CHOL-AA, DOTAP, DOPA, CHOL-AA-Cit |
80 nm Spherical | siBcl-2 | Charge reversible property to enhance the activity | A549 cancer cells | [80] | |
| PAMAM dendrimers DOPA, DOPC, DOTAP, SP94-DSPE-PEG |
110.5 nm Spherical | PD-L1 and pDNA | Dual-targeted immunogene therapy against cancer | Hepatocellular carcinoma | [81] | |
| DOTAP, DOPE, DOPE-CDM-PEG | 42 nm Spherical | miRNA-155 | Microemulsion to form miRNA loaded lipid coated CaP and then coating with PEG-lipid in organic phase | Murine sarcoma cancer | [82] | |
| PEI-Chol, DOPE, Pluronic F68 | 207 nm Spherical | siVEGF | Film dispersion hydrated method with CaP nanoparticle solution | MCF-7 cancer cells | [83] | |
| DOPA, PLGA-PEG | 30–80 nm Sphericity of cluster | miRi-221/222 and paclitaxel | Co-encapsulate miRi-221/222 and paclitaxel in different area for combination | MDA-MB-231 cancer cells | [84] | |
| Muti-Layer | Viral antigen hemagglutinin (HA) | 200–300 nm Spherical | CpG | Triple shell calcium phosphate-CpG-CaP-HA | Dendritic cells | [85,86] |
| PEI25k | 316 nm agglomeration | siTNF-a | Triple shell calcium phosphate-siRNA-CaP-PEI | MODE-K cells | [87] | |
| Octa-arginine (R8) | 220–580 nm agglomeration | pDNA | Triple shell calcium phosphate-DNA-CaP-R8 | hMSC and hOB cells | [88] | |
| poly-(l-lysine) (PLL) | 100–250 nm Spherical and polyelectrolyte films | shRNA | Triple shell calcium phosphate-DNA-CaP-PLL | human osteoblasts | [89] | |
| Chitosan, alginate | 35 nm Spherical | BSA or OVA | Triple shell CaP-Chi-Alg NPs as an oral vaccine delivery vehicle | Caco-2 and RAW264.7 cells | [90] | |
| CaP Shell | Gold nanoparticle Polydopamine (PDA) | ∼100 nm nanohybrids | glucose oxidase (GOx) | Gold nanoparticle core and calcium phosphate shell (Au@PDA@CaP) | – | [91] |
| mesoporous silica | ∼50 nm Spherical | doxorubicin | SiO2 core and calcium phosphate shell | Breast cancer MCF-7 cells | [92] | |
| mesoporous silica PNiPAM/AA |
∼100 nm Spherical | doxorubicin | SiO2 core coated with PNiPAM/AA and CaP, decorated with transferrin (Tf)/RGD ligand | HepG2 and HUVEC cells | [93] | |
| Fe3O4, PEI | 185–257 nm Spherical | DNA or doxorubicin | SPIONs@PEI-CPs/DNA or doxorubicin | A549/HepG2 cancer cells | [94] | |
| PEG-b-poly (benzoxaborole) (PEG-PBO) | 140 nm Spherical | siRNA | pH-responsive siRNA delivery | MDA-MB-231 cells | [95] | |
| PEG-PAA, poly (Pt (IV) prodrug | 85 nm Spherical | Pt (IV) | High drug loading efficiency and capacity | A549 cancer cells | [96] | |
| lipid-PEI, PAsp | ∼770 nm | pDNA | Mineralization responsibles for higher transfection efficiency | MC3T3-E1 cells | [97] | |
| PLLA, DOPA, DSPE-PEG | 170 nm Spherical | miR-21 and doxorubicin | The CaP-coated hybrid lipid-polymeric NPs | MDA-MB-231/A549 cancer cells | [98] | |
| NaCas | 150–200 nm Spherical | Curcumin | Robust stability, controlled release | A549 cancer cells | [99] | |
| Zn(II)-dipicolylamine, hyaluronic acid | 124 nm Spherical | siRNA and doxorubicin | Cationic derivatives to incorporate nucleic acid derivative. | OVCAR8/ADR cancer cells | [100] |
Flame-spray pyrolysis is a versatile method for the largescale synthesis of calcium phosphate nanoparticles [[42], [43], [44]]. A solution or a dispersion of the precursors is injected into a flame where the particle formation occurs at high temperature. The possibilities for precursor selection and reactor system engineering make this method suitable to produce particles with variable properties, also for biomedical applications. The applied solvents or precursors, the local temperature, and the residence time in the flame all influence the combustion reaction, giving some control over primary particle size and crystalline phase, but irreversible particle agglomeration is frequently observed. Although it requires specialized equipment, flame-spray pyrolysis is an efficient method to prepare larger quantities of nanoparticulate calcium phosphate with defined properties [101], but the high processing temperature prevents the incorporation of all organic or biological compounds, which limits the application in the medical drug delivery.
Pulsed laser ablation has also been applied to prepare calcium phosphate nanoparticles from synthetic and biological calcium phosphate substrates [45]. This method is based on the ablation of nanoparticles from a solid substrate and has turned out to be very versatile to prepare metallic and ceramic nanoparticles. However, its applicability to prepare calcium phosphate nanoparticles is probably limited because it is difficult to disperse the material in nanoparticulate form in the case of sinter [102].
According to the researches in recent decades, calcium phosphate designed to overcome the pharmacokinetic limitations of drugs delivery is mostly limited in the nano field due to the specific physicochemical, biological, optical, electrical, and catalytic properties of nanomaterials [103,104]. The CaP micromorphology of sol-gel chemistry, flame spray pyrolysis and solid-state reactions for drugs delivery often limited for external use or in vitro study. However, their preparation process involved high temperature or organic solvent often provides a controllable advantage over morphology and particle size, including zero-dimensional (0D) shape of particle and sphere, 1D shape of rod, fiber, wire and whisker, 2D shape of sheet, disk, plate, belt, ribbon and flake, 3D shape of porous, hollow, and biomimetic structures similar to biological bone and tooth [22,46], which resulted in the unique applications that some of these uniform nano-sized particles would be selected as pre-fabricated basic materials to constructure the CaP nanocarriers.
Among various methods for synthesis of calcium phosphate nanoparticles for biological application, wet-chemical precipitation from aqueous solutions has distinct advantages. Based on its sparingly soluble in neutral water, calcium phosphate tends to precipitate easily from supersaturated solutions, resulting in the easy and cost-efficient precipitation from water where no organic solvent is required in the process of wet-chemical precipitation [47]. Meanwhile, it has some advantages like the possibility to control particle crystallinity and size by varying pH, concentration, temperature, and precipitation time [48,49]. The methods of wet-chemical precipitation contained different refinement methods, such as the room-temperature coprecipitation method, the hydrothermal/solvothermal method, the microwave-assisted method, the sonochemical method and others [11].
In the room-temperature coprecipitation method, various organic/inorganic regulators and stabilizers are adopted to prevent the rapid nucleation and disordered growth of calcium phosphate, resulting in calcium phosphate-based nanocomposites or ion-doped nanocarriers. For example, Kazunori Kataoka et al. [50,64] presented a hybrid nanocarrier system composed of calcium phosphate comprising the block copolymer of poly (ethylene glycol) (PEG) and charge conversional polymer (CCP) as a siRNA vehicle. The “one-pot” method is described as following: a solution of CaCl2 was mixed with another solution containing PEG-PAsp(DET), siRNA and Na3PO4 in buffer (pH = 7.5) by pipetting for around 20 s. The TEM observations revealed hybrid nanoparticles with relatively homogenous spherical shape and average size of 40 nm. It allows to load biomolecules into the particles or/and to functionalize them on the surface, leading to reproducible and uniform nanoparticles in stable colloidal dispersions [50,51], and the lower-temperature processes avoids the denaturation of biomolecules like nucleic acids or proteins may occur in high temperature. However, the growth and crystallization of calcium phosphate are accelerated under heating conditions, so the hydrothermal, solvothermal and microwave heating methods were developed widely to prepare calcium phosphate nanoparticles [11]. Different from conventional hydrothermal/solvothermal methods that heat the reaction system from the outside to the inside by heat conduction, microwave heating could rapidly and uniformly heat the whole reaction system, and there was no obvious temperature gradient in the reaction system [[52], [53], [54], [55]]. Usually, the microwave-assisted method could complete the synthetic reactions in minutes rather than hours or days required by the traditional heating methods, so it had higher reaction rate and efficiency to save time and reduce energy consumption. Similarly, the sonochemical method could provide intense local heating, high pressure, and rapid cooling due to the continuous formation, growth and implosive collapse of bubbles in the reaction system, which also became a promising strategy for the preparation of nanostructured calcium phosphate [[105], [106], [107]].
Above all, regardless of the synthesis method, calcium phosphate nanoparticles need to be dispersed in water or biological media for most biological applications. And the colloidal stability, function and safety of nanoparticles directly depends on their surface characteristics [108]. Hence this is imperative if nanoparticles are acting as carriers where the particle composition must be exactly known, including those properties like size, charge, shape and the entrapment rate of drugs or biomolecules [109,110] which will conduct the comparative analysis in the following applications section. Presently, we will firstly discuss different methods which are generally applied for the characterization of calcium phosphate nanoparticles in colloidal dispersion.
Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) are universal choices for observing the morphology and particle size of calcium phosphate nanoparticles [111,112]. Electron microscopy is imaging in the dry state and often only a small number of particles can be analyzed. Attentionally, it is possible contaminated by salts or biomolecules from the dispersion medium and beam damage may cause artifacts, especially with hydrated or amorphous calcium phosphate phases. Besides electron microscopy, dynamic light scattering (DLS) is probably the most prominent method to analyze the size and the surface charge (zeta potential) of dispersed calcium phosphate nanoparticles if the particle size distribution is monomodal and narrow [113,114]. If the nanoparticles are not spherical or occur as a polydisperse mixture, DLS tends to produce artifacts due to the fact that large particles scatter the light much more intensely than smaller particles. Note that SEM or TEM probes the solid calcium phosphate core in the dry state whereas DLS probes the hydrodynamic diameter in the dispersed state, including possible aggregates of smaller primary particles [76,115].
For medical drugs delivery, the calcium phosphate nanoparticles must be thoroughly purified to remove excess reagents from the synthesis and unwanted synthesis by-products like the inorganic counter ions of calcium phosphate. Commonly applied purification techniques are centrifugation, nanofiltration, and dialysis. Centrifugation is the best option for calcium phosphate nanoparticles due to their density and comparatively large diameter (typically 50–200 nm) [113].
The amount of cargo also is an important character need to be quantitatively determined, which is more difficult than generally assumed. Auto fluorescent or fluorescently labelled drug molecules can be easily detected by UV-spectroscopy or (less accurately) by fluorescence spectroscopy [116,117]. This can be performed either with the cargo-loaded nanoparticles or with the supernatant that remains after nanoparticle purification. In the case of fluorescently labelled cargo molecules like proteins or antibodies, it is usually tacitly assumed that they have the same biological and physic-chemical properties as their non-labelled parent compounds. If cargo molecules are available only in small amounts, model cargo molecules can be used instead of the real cargo molecules. Additionally, HPLC is an option as the standard laboratory method after acidic dissolution of the nanoparticles. Moreover, the number or concentration of calcium phosphate nanoparticles in a dispersion can be determined by elemental analysis, such as atomic absorption spectroscopy (AAS) and inductively-coupled plasma-mass spectrometry (ICP-MS) [118].
3. Application of calcium phosphate nanocarriers
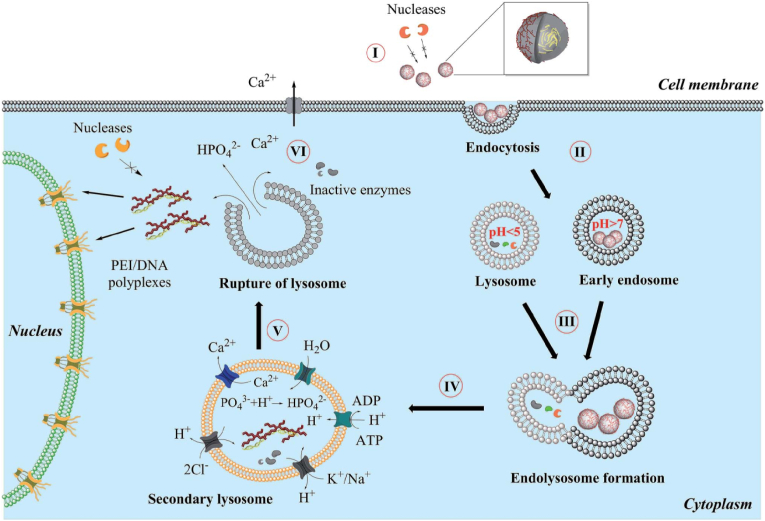
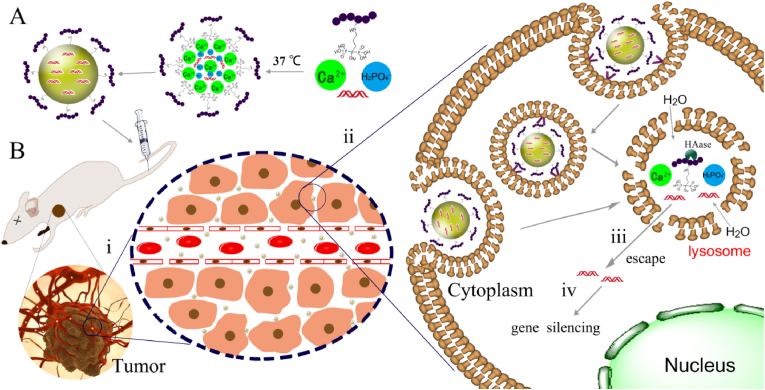
In recent decades, nanosized and nanostructured calcium phosphates have been used in several applications, including biomimetic remineralization, as fluorescent labels, bioactive coating for implants, especially non-viral vectors for gene and drug delivery. Fig. 2 shows the general mechanism which we propose for the uptake of calcium phosphate nanoparticles by eukaryotic cells [119]. The pDNA/CaP nanoparticle is regarded as the model and a five-step process needs to be implemented before giving effect. (I) Overcome biological barriers to obtain a good cellular uptake, (II) endosome loaded nanoparticles fuses with a lysosome (III), the acidification of lysosomes will degrade part of nanoparticles, and (IV) quickly endo/lysosomal escape is the key for subsequent effects, which is supported by an increased osmotic pressure inside the lysosome due to neutralization of acid by suitable basic compounds that the acid-soluble calcium phosphate will lead to a considerable number of calcium and hydrogen phosphate ions after dissolution. (V) The lysosome ruptures and released its cargo into the cytosol. Excess calcium is pumped out (VI) and pDNA enter the nucleus.
Fig. 2.
General mechanism proposed for the uptake and intracellular processing of calcium phosphate nanoparticles by cells [119].
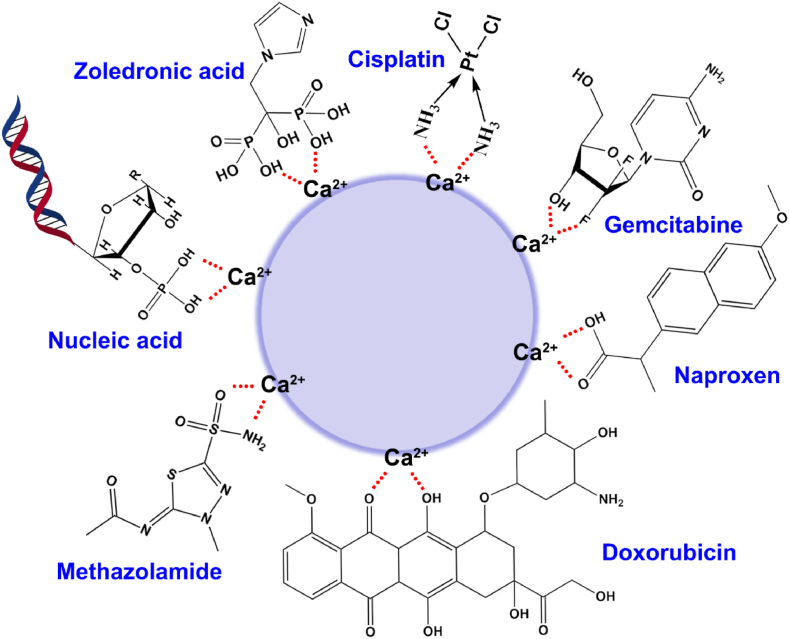
The mechanism of drug entrapment by calcium phosphate nanocarriers is the interaction between calcium ions and the functional groups of drug molecules. As shown in Fig. 3, the functional groups contain the phosphate group (-PO3H, nucleic acids, such as DNA, siRNA and microRNA or other small molecule compound, such as zoledronic acid [120]), the carboxyl group (-COOH, such as naproxen [121]), the sulfonic group (-SO3H, such as methazolamide [122]), the amino-group (-NHn, such as cisplatin [123]), the hydroxyl or fluorine group (-OH or –F, such as gemcitabine [68] and doxorubicin [59]), the sulfhydryl group (-SH) and so on. Theoretically, small molecule chemical drugs or macromolecular biological drugs containing the functional groups mentioned above may be delivered by the calcium phosphate nanocarriers for various medical applications.
Fig. 3.
Schematic illustration of the interaction between the group of representative molecules and the Ca2+ in CaP nanocarriers.
Note that the stability of calcium phosphate nanoparticles is closely related to the concentration of ions in the medium and the introduction of modified molecules will break this balance, which resulted in the unstable agglomeration or dissolution of calcium phosphate nanoparticles. Hence, the preparation of calcium phosphate nanoparticles should be controlled from various perspectives, such as the method (material dosage, pH, temperature, electrolyte and feeding order). Besides, surface functionalization of the therapeutic CaP's is necessary to prolong their circulation in blood or adding targeting molecules (e.g., peptides and antibodies) to their surface, which often enable CaP's enhanced permeation and retention (EPR) in nidus, leading to higher therapeutic efficacy with lower dosage of the administered nanoparticles [124]. Various methods have been used for surface functionalization of the CaP's, using covalent or non-covalent (electrostatic) conjugation of macromolecules (e.g., lipids, polymers, peptides and nucleic acids) to their surface. Briefly, a qualified calcium phosphate nanoparticle needs to maintain its nano-structural stability and have special functions at the same time.
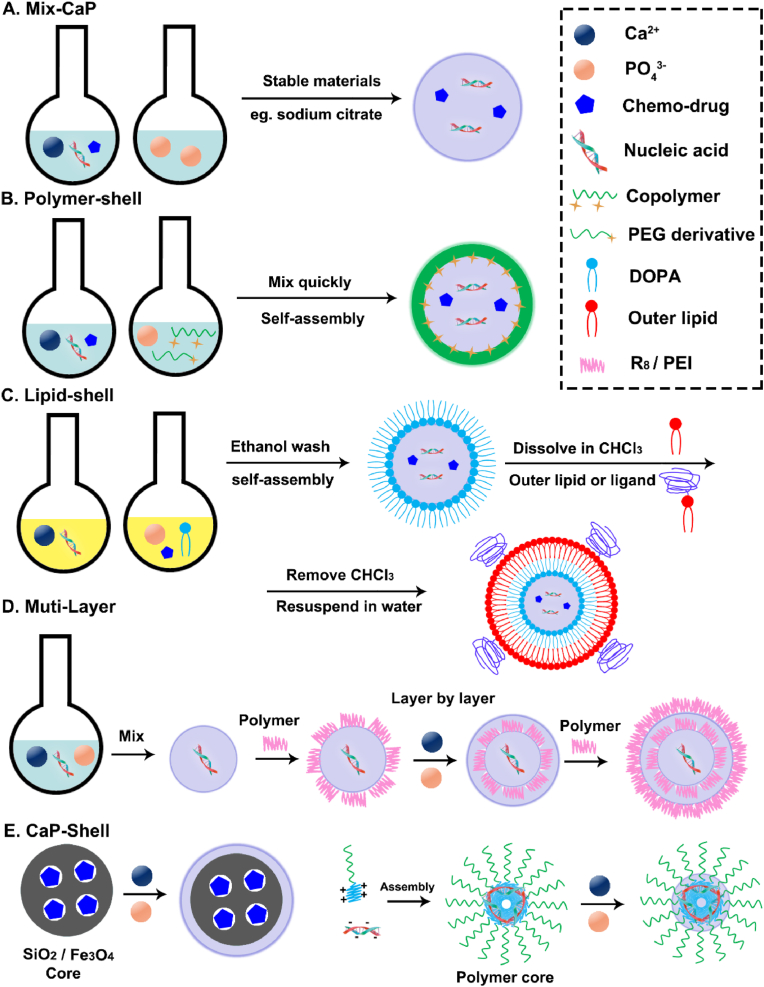
Based on this, scientists have devised a wide range of approaches to satisfy the two fundamental needs, mostly utilizing liposomes, polymers, and inorganic materials, which produce a variety of structural shapes using various techniques [9]. There were four main categories (Fig. 4): (1) To create a “Mix-CaP” as depicted in Fig. 4A, the Ca2+, phosphate water solutions, and chemotherapeutic or nucleic acid medicines were swiftly combined; (2) Calcium phosphate is first prepared as the core, then chemotherapy and nucleic acid drugs are adsorbed around it, or phosphate ions, calcium ions, and drugs precipitate to form a calcium phosphate core. Other materials are then modified or coated as the outer shell, such as “CaP Core” with a “Polymer Shell” (Fig. 4B) or “Lipid Shell” (Fig. 4C); (3) A “Muti-Layer” is created when calcium phosphate and pharmaceuticals or materials combine layer by layer (Fig. 4D); (4) A “CaP Shell” is created when medications molecules and materials first assemble into a nanocore and then phosphate ions and calcium ions precipitate to form the structure (Fig. 4E).
Fig. 4.
Schematic illustration of synthetic strategies to prepare different CaP nanocarriers. A) the “Mix-CaP” is prepared by mixing various materials directly; B) the hybrid CaP nanoparticles with a “Polymer-Shell” are prepared by “one-pot” method; C) the multifunctional CaP nanoparticles with a “Lipid-Shell” are prepared by micro-emulsion method; D) the “Muti-Layer” CaP nanoparticles are prepared by a layer-by-layer method and E) the “CaP-Shell” CaP nanoparticles are prepared by the external precipitation on the core of polymer or inorganic.
Additionally, depending on the intended use, the synthesis of calcium phosphate nanoparticles will include modifying agents to add specific functionalities, such as conjugated with target molecules (e.g., antibodies, peptides and polymer), labelled molecules (fluorescent dyes or photographic agents), and other materials, such as organosilane and methacrylate. Sometimes, the needle-shaped CaP may be a promising strategy for improving bioactivity of biomedical implants, promoting bone ingrowth and enhancing osseointegration, compared with spherical CaP [125]. And other CaP morphologies (nano-clusters [126] or porous structures [127]) have also been investigated as high-loading capacity candidates, but the relatively larger size and multiple synthesis steps are considered as the major drawback hindering their potential applications in clinical trials. However, most of the recent CaP-based in vivo therapy studies have used spherical calcium phosphate nanoparticles for delivery of the therapeutic agents to cancer tissues, which mainly because spherical nanoparticles provide highest possible specific surface area for loading drugs and round morphologies that are more stable thermodynamically [7].
3.1. Mix-CaP
The assembly of calcium phosphate nanoparticles mainly depends on the chemical bonding coprecipitation between Ca2+ and phosphate ion. Nucleic acid drugs have the backbone of phosphate group, such as DNA, siRNA, microRNA and antisense oligodeoxynucleotides. Hence the use of calcium phosphate for gene delivery was first demonstrated to delivery DNA in 1973 [128]. The nano-sized DNA loaded calcium phosphate particles were prepared by mixing Ca2+ with a DNA rich aqueous solution, which would lead to the spontaneous formation of the calcium phosphate structure due to the interfering of DNAs’ phosphate group with Ca2+. DNA, Ca2+ and phosphate group are distributed randomly in nanoparticles, like stars dotted in the night sky, which is the origin of the name “Mix-CaP” rather than the mix preparation process (Fig. 4A). However, the uncontrolled growth of calcium phosphate nanoparticles will lead to aggregation, which is the key to limit their application.
Control over the main reaction parameters (e.g., mixed manner, Ca/P ratio, temperature, pH, reaction time and precursor concentrations) is important to enable optimization of the particle properties to ensure stability and reproducibility [129]. Dana Olton et al. [130] reported a nanosized pDNA calcium phosphate nanoparticle by mixing the calcium and phosphate precursors in a controlled and regulated manner reproducibly. Meanwhile, the average particle size of the CaP/pDNA precipitates decreased from 2543 ± 667 nm with a Ca/P ratio of 30 to 19.30 ± 7.54 nm with a Ca/P ratio of 700. Moreover, CaP/microRNA nanoparticles were synthesized via a straightforward one-pot biomineralization-inspired protocol employing citrate as a stabilizing agent and regulator of crystal growth, which was efficiently delivered for cardiovascular disease therapy [56]. Similarly, citrate was also designed as a stabilizing agent and regulator to prepare CaP nanoparticles for delivering methazolamide to eye [122]. Ying-Jie Zhu et al. [59] prepared the CaP hybrid nanoparticles to deliver docetaxel for cancer therapy by using poly (diallyl-dimethyl ammonium chloride) (PDADMAC) and poly (acrylate sodium) (PAS) as the dual templates. First, the PAS/Ca2+ and PDADMAC/PO43− complexes form through electrostatic interactions and then two complexes self-assemble into CaP-HNPs after mixing them together, which can regulate the growth of CaP and reduce the size to the nanometer range. Additionally, arginine [76], sodium polymethacrylate [123] and trisodium citrate [120] were employed as stabilizing agent to constructure nano-CaP for the delivery of siRNA, cisplatin and zoledronic acid or other drugs, respectively.
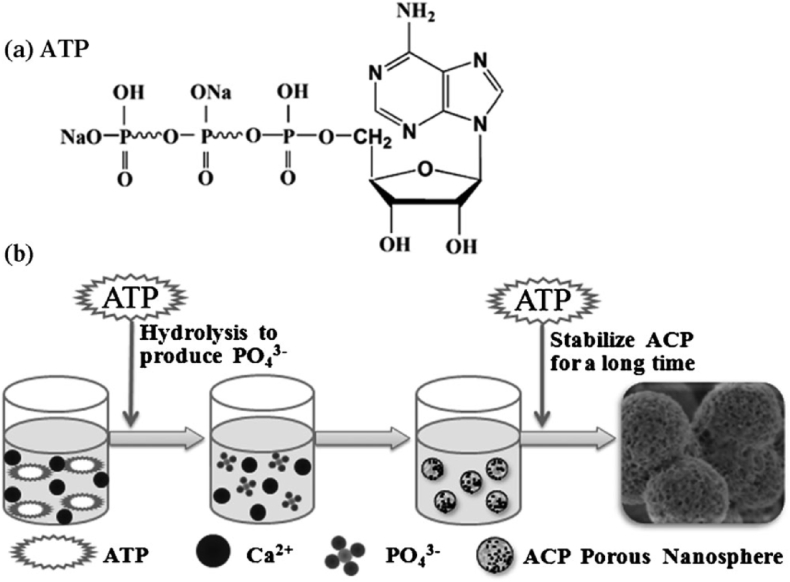
Notably, the phosphorus source is an important factor in the preparation of calcium phosphate nanoparticles. In the traditional synthetic methods, phosphate salts are usually used as the phosphorus source and the chemical reaction between free Ca2+ and PO43− are rapid, which often resulting in difficult control over the crystal growth of calcium phosphate [11]. Recently, the Zhu and Qi research groups developed a new method for the synthesis of calcium phosphate nanocarriers using biocompatible phosphorus-containing biomolecules (e.g., adenosine triphosphate [54,57], adenosine 5′-diphosphate [52], adenosine 5′-monophosphate [131], creatine phosphate [55,132], riboflavin-5′-phosphate [133], pyridoxal-5′-phosphate [77], fructose 1,6-bisphosphate [134]) as organic phosphorus sources. Different from the traditional methods, the phosphorus source was provided by the slow hydrolysis of phosphorus-containing biomolecules, so the hydrolysis rate of phosphorus-containing biomolecules could be used to regulate the morphology, size, and structure. For example, as shown in Fig. 5, Qi et al. [54] synthesized a highly stable amorphous calcium phosphate (ACP) porous nanospheres with a relatively uniform size and an average pore diameter of about 10 nm by a microwave-assisted hydrothermal method with adenosine 5′-triphosphate disodium salt (ATP) as the phosphorus source and stabilizer. The as-prepared ACP porous nanospheres have a high stability in the phosphate buffer saline (PBS) solution for more than 150 h without phase transformation to hydroxyapatite, and the morphology and size were essentially not changed.
Fig. 5.
Schematic diagrams: a) chemical structure of adenosine 5′-triphosphate disodium salt (ATP); b) formation of ACP porous nanospheres synthesized by a rapid microwave-assisted hydrothermal method using ATP as the phosphorus source and stabilizer [54].
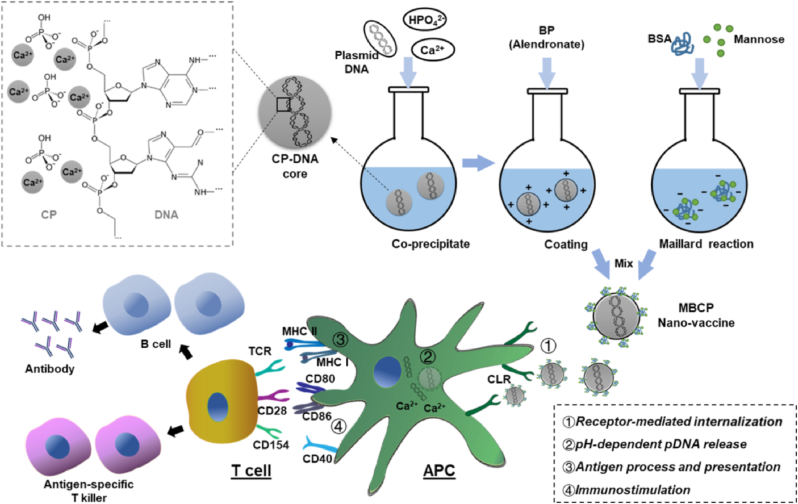
Besides, bisphosphonates (BPs) are a group of small-molecule drugs used in clinics for the first-line treatment of osteoporosis due to its phosphate group [135]. The Xu and Gu research groups developed several calcium phosphate nanocarriers using BPs as the stabilizer. For example, Gu et al. [136] prepared the bone-targeted drug delivery system (Ca-RISNPs) using a third-generation BP (risedronate) and drugs (plasmid and oligo double strand DNA). These Ca-RISNPs exhibited high specificity in killing tumor-associated macrophages (TAMs) and inhibit TAM-induced tumor cell migration. Similarly, they modified the calcium phosphate nanoparticles with a low amount of alendronate and bovine serum albumin (BSA) to overcome the poor chemical and colloidal stability for the effective delivery of plasmid DNA to macrophages [137]. Moreover, as shown in Fig. 6, they designed the mannose-modified and BP-stabilized calcium phosphate (MBCP) nanoparticles (∼130 nm) to deliver ovalbumin-encoded plasmid DNA vaccine for anti-tumor immunity [58].
Fig. 6.
Schematic diagram illustrates the design and function of mannose- and bisphosphonate-modified calcium phosphate (MBCP) nanoparticles as DNA nanovaccine.
Furthermore, changing the preparation method or introducing other materials is also an effective method to prepare uniform and stable CaP nanoparticles. Nengqin Jia et al. [60] used the double reverse emulsion approach to prepare a Mix-CaP nanoparticle (50–70 nm) as antisense oligodeoxynucleotides (ASODNs) delivery vehicle and achieved high cellular transfection efficiency. Similarly, Yu Zheng et al. [61] prepared the CaPi-pDNA-PLGA-NPs by a water-in–oil–in-water (w/o/w) double emulsion solvent evaporation method. Effect of various processing parameters and polymer characteristics on the mean diameter and entrapment efficiencies of the nanoparticles were discussed, including the MW of PVA, the Ca/P ratio, initial CaCl2 concentration, the composition of organic phase, sonication time and power and aqueous phase pH. CaPi-pDNA-PLGA-NPs produced by the optimal formulation exhibited spherical shape with a particle size of 207 ± 5 nm, zeta potential of −2.18 ± 0.17 mV, which had the remarkably increased transfection efficiency relative to pDNA-PLGA-NPs. Additionally, an injectable hydrogel contained CaP nanoparticles based on the thermo-reversible methylcellulose polymer was prepared by one pot reaction for bone regeneration [62]. The methylcellulose could inhibit the aggregation of CaP nanoparticles with stable size (40–50 nm) and it evenly distributed in the gel network. The resultant hydrogel with bioactive CaP nanoparticles exhibited the sol-gel transition in few seconds at below the body temperature, which showed a great potential as an injectable hydrogel for bone regeneration.
Although Mix-CaP nanoparticles frequently produce positive results when administered locally or in vitro, their use in vivo is severely constrained [138]. In the process of systemic circulation, numerous ions, proteins, and other substances intervene could cause the aggregation or dissolution of nanoparticles [139]. Larger nanoparticles usually have a short blood circulation time due to rapid accumulation in reticuloendothelial system (RES), while ultrasmall nanoparticles smaller than 8–10 nm have a fast renal clearance from the blood. Therefore, synthesis of CaP nanoparticles should be optimized to tune their sizes within the range of 10–80 nm in order to achieve longest blood circulation time and highest amount of uptake by target cells [[140], [141], [142]].
3.2. Polymer-Shell
In order to inhibit the uncontrolled aggregation and prevent the release of loaded drugs in the delivery process, the CaP nanoparticles are functionated by outer-coating a shell on the CaP core, in which polymers are mainly used (Fig. 4B), including polyethyleneimine (PEI), poly (lactic-co-glycolic acid) (PLGA), poly-ethylene-glycol (PEG) or their derivative.
Polyethyleneimine (PEI) is a hydrophilic positive polymer, widely used in conjunction with other materials as a nano-vector for cell transfection due to its favorable chemistry, such as enhancing the cellular uptake and endo/lysosomal escape of drugs [143]. PEI has been used as an outer shell for surface functionalization of calcium phosphate nanoparticles. Hyosook Jung et al. [51] prepared the linear polyethyleneimine (LPEI)-coated CaPs (LPEI-CaP) containing common miRNA-34a or lc-miRNA-34a, a long chain miRNA-34a conjugate prepared by crosslinking miRNA-34a with cleavable disulfide bonds. The particle size of LPEI-CaP/lc-miRNA (304 ± 10 nm) was smaller than that of LPEI-CaP/miRNA (433 ± 19 nm). LPEI-CaP/lc-miRNAs were observed to be successfully delivered into PC-3 cells to inhibit cancer cell proliferation. However, the PEI moderation is limited due to its high cytotoxic and PEI modification with PEG grafting is the most common approach to reduce the cytotoxicity consequently. Smith et al. [144] reported the degree of substitution for PEI-PEG polymers strongly decreased cytotoxicity of PEI. Meanwhile, the molecular weight of PEG affected the cellular uptake ability of PEG-PEI copolymers also became a new barrier.
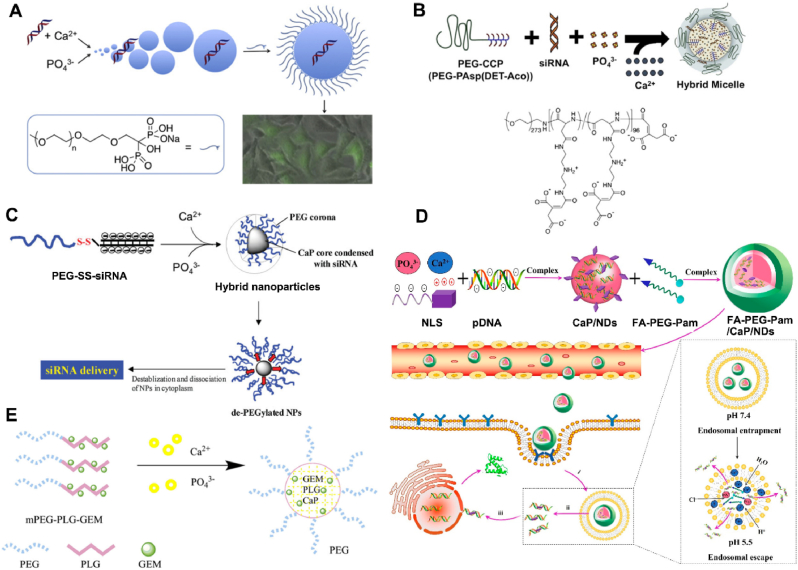
Poly-ethylene-glycol (PEG), a neutral hydrophilic polymer, not only prevents nanoparticle agglomeration by inhibiting particle growth, but also increases calcium phosphate biocompatibility by decreasing non-specific protein absorption in vivo [7]. Moreover, the use of a PEG shell also helps to minimize the calcium ion release from calcium phosphate following enzymatic degradation in the cytosol [145]. In order to obtain a PEG shell, various PEG derivatives containing the groups could interact with Ca2+ were synthesized. For this method, the bioactive compounds (e.g., siRNA or DNA) could be incorporated inside the core of CaP nanocarriers, while the surface shell of PEG could control the size and endow CaP nanocarriers with colloidal stability. Firstly, Giger et al. [63] synthesized the PEG-bisphosphonates to prepared DNA loaded calcium phosphate nanoparticles (Fig. 7A). The strength of the interaction between the bisphosphonate and the calcium phosphate enabled the formation of stable, but bioresorbable particles of around 200 nm, which exhibited physical stability over several days, proving the positive effect of surface functionalization of PEG in preventing particle agglomeration. Additionally, the nanoparticles revealed good and sustained ability to transfect cells while displaying low toxicity. Beside this, Giger et al. [35] also prepared the calcium phosphate-siRNA nanoparticles using the outer polymer of PEG-alendronate (ALE). The functionated CaP nanoparticles maintained the stable properties (∼260 nm, −17 mV) for over one month, which facilitated good transfection efficacy of siBcl-2 via clathrin-dependent endocytosis in PC-3 cells.
Fig. 7.
Schematic representation of the preparation and application of PEG-derivatives functionated calcium phosphate NPs: A) PEG-bisphosphonates [63], B) PEG-CCP [64], C) PEG–SS–siRNA [67], D) FA-PEG-Pam [146] and E) PEG-PLG-GEM [68].
PEG-carboxyl derivative was also synthesized to functionate the PEG shell on outer of CaP core. Kataoka et al. [50,64] designed a novel PEG-PAsp (DET) to present a hybrid “core-shell” calcium phosphate nanocarrier as a siRNA vehicle. Noted that PAsp (DET) is a good pH-responsive material because of the protonation behavior of its side chain, which leads to pH-selective membrane destabilization and its biodegradability in physiological conditions [64]. The hybrid nanoparticles (∼40 nm, −2.2 mV) indicated a higher in vivo gene silencing efficacy in the spontaneous pancreatic cancer cells (60% with ∼40 ng siRNA) (Fig. 7B). Similarly, they synthesized the poly (ethylene glycol)-block-poly (aspartic acid) (PEG-PAA) as the shell of calcium phosphate nanoparticles, which exhibited a mean particle size of 140 nm and obtained a significant improvement in transfection efficacy showing up to 60% silencing [65,66].
However, the PEG dilemma limited the application of PEG-ylation, such as cellular uptake and endosomal escape. Several approaches have been investigated to overcome this hurdle, including adding specific ligands to target the desired cells and introducing cleavable PEG systems. Based on this, an effective conjugate folate-polyethylene glycol-pamidronate (shortened as FA-PEG-Pam) was designed and coated on the surface of CaP/NLS/pDNA (CaP/NDs), forming a versatile gene carrier FA-PEG-Pam/CaP/NDs [146]. Inclusion of FA-PEG-Pam significantly reduced the size of CaP nanoparticles, thus inhibiting the aggregation of CaP nanoparticles (Fig. 7D). FA-PEG-Pam/CaP/NDs showed better cellular uptake than mPEG-Pam/CaP/NDs, which could be attributed to the high-affinity interactions between FA and highly expressed FR. Furthermore, in vivo studies revealed that the hybrid nanoparticles had supreme antitumor activity (IR% = 58.7%) among the whole preparations. Approximatively, to increase the speed of endo/lysosomal escape, Zhang et al. [67] designed a PEG-drug derivative (PEG–SS–siRNA) to constructure a redox-responsive CaP nanoparticle containing a disulphide bond between PEG and siRNA (Fig. 7C). The nanoparticle demonstrated instability when inserted into a reducing environment such as cytosol, allowing for effective siRNA release from lysosome. The resultant nanoparticles (∼100 nm) also confirmed the ability of PEG inhibiting calcium phosphate particle growth. Likewise, the polymeric material methoxy poly (-ethylene glycol)-block-poly (l-glutamic acid)-graft gemcitabine (mPEG-b-PLG-g-GEM) was synthesized by Xing Tang [68]. The amide bond linked gemcitabine polymer was able to protect GEM from cytidine deaminase degradation in vivo, and the skeleton formed by the calcium phosphate enhanced the stability and prolonged the half-life of GEM (Fig. 7E).
Apart from PEG, natural polymers, including chitosan and hyaluronic acid, have also been used for the surface functionalization of calcium phosphate nanoparticles. Chitosan is a natural polysaccharide contains many amine groups and hydroxyl groups that can effectively control the synthesis of calcium phosphate nanoparticles by absorbing Ca2+ and forming chitosan/CaP nanoparticles [147]. Meanwhile, it has a high positive charge to enhance the electrostatic interactions with cell membrane, which contributed to the cellular uptake of nanoparticle. Choi et al. developed a stable CaP nanocarrier (CaP/Gln-Ochi, ∼119 nm) with enhanced intracellular uptake of siRNA by adding highly cationic chitosan-glutamine [69]. They subsequently loaded a Noggin siRNA into the calcium phosphate nanoparticles and evaluated transfection efficacy and ALP expression in adipose tissue derived stem cells, which obtained the transfection values comparable to Lipofectamine2000. Lee et al. [70] further functionalized these nanoparticles for DNA and siRNA delivery by adding dopamine, achieving enhanced nanoparticle stability. The catechol group of the dopamine molecule was crucial to particle stabilization, acting as a bridge between the Ca2+ and chitosan. The chitosan-dopamine-siRNA-calcium phosphate nanoparticles displayed enhanced target-gene silencing by siRNA when compared to the chitosan-siRNA-calcium phosphate nanoparticles. Likewise, the doxorubicin loaded calcium phosphate/carboxymethyl chitosan hybrid nanoparticles (CaP/CMC/KALA) were prepared by KALA, a polypeptide composed of 30 amino acids (Trp-Glu-Ala-Lys-Leu-Ala-Lys-Ala-Leu-Ala-Lys-Ala-Leu-Ala-Lys-His-Leu-Ala-Lys-Ala-Leu-Ala-Lys-Ala-Leu-Lys-Ala-Cys-Glu-Ala) [71]. The positively charged KALA was absorbed in the outer layer through the electrostatic interaction with the negatively charged CMC chains. The in vitro study showed that the cell inhibition effect could be significantly enhanced by the presence of KALA.
Compared with chitosan, hyaluronic acid performed a better potential for the functionalization of CaP nanoparticles because the negative material helps to maintain the stability of internal circulation and it could serve as a target agent due to its specific interactions with CD44 high-expressed on cell surface. Hence, various derivatives of hyaluronic acid were developed, such as the thiolate hyaluronic acid (HA-SH) [36], the alendronate-hyaluronic acid (AHA) [34], dopamine-hyaluronic acid and PLA-hyaluronic acid [148]. Lee et al. [72,73] demonstrated that increasing dopamine-hyaluronic acid functionalization resulted in increased particle stability and achieved significantly transfection in human bone marrow-derived MSCs (hMSCs) as a result of the specific interactions between hyaluronic acid and CD44 of hMSCs. Qiu et al. [34] developed a hyaluronan-functionalized calcium phosphate nanoparticle (CaP-AHA/siRNA NP) prepared by coating alendronate-hyaluronan graft polymer (AHA) around the surface of calcium phosphate-siRNA co-precipitates, as shown in Fig. 8. The prepared CaP-AHA/siRNA NPs had a uniform spherical core-shell morphology with approximate size of 170 nm and improved the physical stability of nanoparticles over one month based on the strong interaction between phosphonate and calcium. In vitro experiments demonstrated that the negatively charged CaP-AHA/siRNA NPs could effectively deliver EGFR-targeted siRNA into A549 cells through CD44-mediated endocytosis and significantly down-regulate the level of EGFR expression. Also, the internalized CaP-AHA/siRNA NPs exhibited a pH-responsive release of siRNA, indicating that the acidification of lysosomes probably facilitated the disassembling of nanoparticles and the resultant ions sharply increased the inner osmotic pressure and thus expedited the release of siRNA from late lysosomes to cytoplasm.
Fig. 8.
(A) Schematic illustration of the preparation of core-shell type CaP-AHA/siRNA nanoparticles; (B) After injection via intravenous route, i) the CaP-AHA/siRNA nanoparticles passively accumulated in tumor tissues by the enhanced permeation and retention effects (EPR effects), ii) Cell uptake through CD44-mediated endocytosis, iii) Lysosomal escape and release of siRNA into the cytoplasm with pH responsive disassembling of CaP core, iv) Gene silencing effects induced by the released siRNA in cytoplasm [34].
Similarly, Jie Tang et al. [61] prepared the CaP/pDNA PLGA nanoparticles coated with pDNA using PLGA as the shell by W/O/W double emulsion method. The experimental results showed that the particle size of the nanoparticles was controlled at about 200 nm, the pDNA loading efficiency was 95.7%. In addition, heparin, as an endogenous substance in the body, contains more carboxyl and sulfate groups, which can be tightly complexed with Ca2+. Ping Liang et al. [74] prepared the heparin/CaCO3/CaP mixed nanoparticles by mixing heparin sodium, CaCl2, Na2CO3 and Na2HPO4 in the aqueous phase and by dialysis purification. The scanning electron microscope results showed that the nanoparticles had small particle size (<50 nm), and the mixed nanoparticles of heparin/CaCO3/CaP loaded with doxorubicin could better solve the drug resistance of tumor cells. Similarly, PSVII carboxymethyl-β-cyclodextrin inclusion compound was successfully encapsulated in colon cancer targeting calcium phosphate nanoparticles (PSVII@MCP-CaP) by using modified citrus pectin as stabilizer agent and colon cancer cell targeting moiety [75].
Additionally, cationic and amphipathic cell-penetrating peptides (CPP) have been used to functionalize calcium phosphate nanoparticles for gene delivery applications [149]. Arginine has a guanidium head group that can bind to the negatively charged cell membrane through hydrogen bonding, which leads to cell penetration at physiological pH. Arginine-rich peptides, for example the Arg-Gly-Asp peptide sequence RGD, have been successfully used for calcium phosphate functionalization to improve siRNA delivery [76]. Sathy et al. [150] subsequently exploited the use of amphipathic RALA (a 30-amino acid cell penetrating amphipathic peptide made up of repeating arginine/alanine/leucine/alanine units) to enhance delivery of CaP nanoparticles, proving the positive effect of RALA-CaP in stimulating osteogenic markers and facilitating mineralization both in vitro and in vivo.
To sum up, the main method to solve the uncontrollable growth of calcium phosphate nanoparticles is to cover the outer layer with a protective shell, which is mainly divided into two categories [9,13]: 1) the shell is a self-assembling material (such as lipid) or the molecule contains groups (such as chitosan, hyaluronic acid, heparin, PLGA, etc.) that can closely complex with Ca2+. The size of these groups and their complexing force are as follows: the phosphate group > the carboxyl group > the hydroxyl group ≈the sulfhydryl group > the amino group. 2) Chemically modify macromolecules with those groups that can closely coordinate with Ca2+, such as phosphorylation or carboxylation of PEG. More importantly, when the above two methods are used together, the stability of calcium phosphate nanoparticles can be significantly improved.
3.3. Lipid-Shell
Liposomes are nano-sized to microsized vesicles comprising a phospholipid bilayer that surrounds an aqueous core, in which the core encapsulates the water-soluble drugs and the hydrophobic domain is responsible for entrapping insoluble agents [151]. And Liposomes can be modified to specifically interact with cellular membranes and fuse with the lipid bilayer to release their contents intracellularly. Progress in liposome technology, modulation of the lipid composition, size and charge of the vesicle and modification of their surface has enabled the shift from conventional vesicles to “smart-generation” liposomes and liposomes are widely used in the treatment of various diseases due to their multifunctional properties [152]. Certainly, liposomes have been used with greater success for the surface modification of calcium phosphate nanoparticles where they have effectively achieved nanoparticle stabilization.
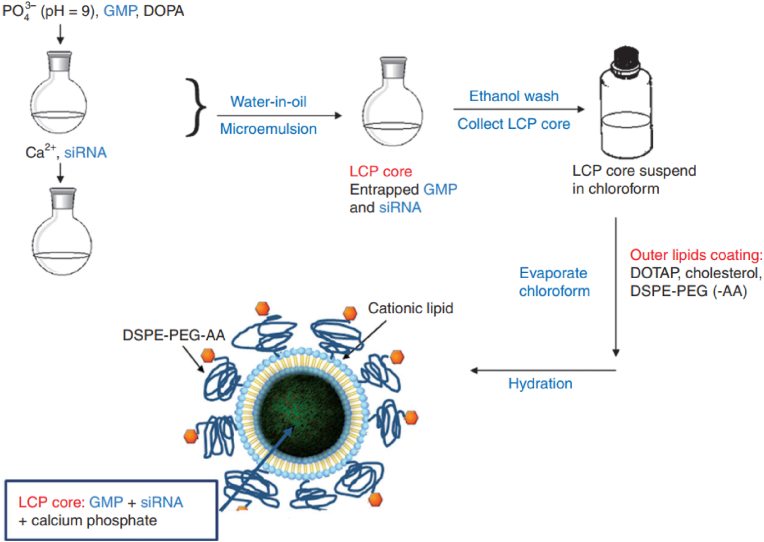
Leaf Huang's research group used the lipid layer to control the growth of calcium phosphate nanoparticles for the first time, and prepared (Lipid coated calcium phosphote-1, LCP-1) by the reverse microemulsion method (Fig. 4C) [153]. The reverse microemulsion is prepared by dispersing the aqueous solution into the cyclohexane oil phase solution containing nonylphenol polyoxymethylene ether (IGEPAL–CO–520). Disperse CaCl2 solution (pH = 9) and siRNA/Na2HPO4 (pH = 9) mixed solution into oil phase respectively, mix and stir the two phases, and microemulsion exchange reaction generates calcium phosphate precipitation containing siRNA; After that, sodium citrate was added until the solution was clarified and stabilized calcium phosphate nanoparticles (∼80 nm). At the same time, the surface of the nanoparticles was negatively charged, which was conducive to binding with cationic liposomes. Followed with ethanol water elution and silica gel column purification, it further interacts with cationic DOTAP/cholesterol to obtain LCP-1 nanoparticles with a particle size of about 150 nm. The mole ratio of DSPE-PEG modified nanoparticles encapsulated by liposomes with calcium phosphate nanoparticles as the core can be as high as 20%. The long cycle and target accumulation ability of LCP-1 can be improved by post modification of DSPE-PEG or DSPE-PEG-AA.
However, in the process of preparing LCP-1, citric acid stabilized calcium phosphate nanoparticles have strong polarity and could only be stored in water. Furthermore, 1,2-dioleoyl phosphatidic acid (DOPA) as an amphiphilic phospholipid with strong complexing ability with Ca2+ was used for modification [154]. Firstly, mixed CaCl2/siRNA solution (pH = 9) and Na2HPO4/DOPA (pH = 9), and DOPA would complex with calcium phosphate nanoparticles at the oil-water interface, forming a hydrophobic layer on the surface of the nanoparticles and stabilizing the growth of the nanoparticles. The ethanol was demulsified, centrifuged and redispersed in chloroform. DOPA modified calcium phosphate nanoparticles with uniform particle size (∼15 nm). DOTAP, cholesterol and DSPE-PEG self-assemble with DOPA modified calcium phosphate nanoparticles as the core through hydrophobic interaction to form core-shell calcium phosphate nanoparticles (LCP-2) (25–30 nm) with asymmetric lipid bilayer encapsulation, which are stable at −20 °C for upward of 1year. In H-460 cells and heterotopic tumor models, the gene silencing effect of LCP-2 is 40 times (in vitro) and 4 times (in vivo) that of LPD (cationic lipid direct delivery siRNA). Furthermore, they formulated vascular endothelial growth factor (VEGF) siRNA targeting VEGFs and gemcitabine monophosphate (GMP) into a single cell-specific, targeted lipid/calcium/phosphate (LCP) nanoparticle formulation (Fig. 9). Antitumor effect of the combination therapy using LCP loaded with both VEGF siRNA and GMP was evaluated in both subcutaneous and orthotopic xenograft models of NSCLC with systemic administration. The improved therapeutic response, as compared with either VEGF siRNA or GMP therapy alone, was supported by the observation of 30–40% induction of tumor cell apoptosis, eightfold reduction of tumor cell proliferation and significant decrease of tumor microvessel density (MVD) [78]. Similarly, LCP nanoparticles were developed for inhibiting lung metastasis by siRNAs or chemo-drugs [155,156], vaccination against advanced melanoma [157] and photodynamic therapy for head and neck cancer treatment [158].
Fig. 9.
(A) Schematic illustration of the preparation procedure of GMP- and/or VEGF siRNA-loaded LCP formulations [78].
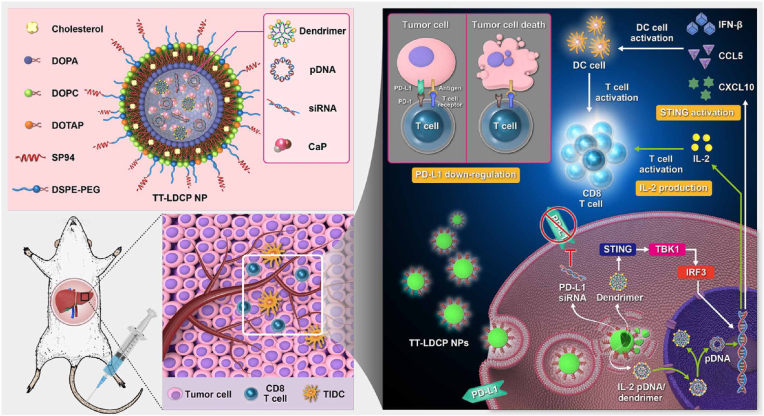
To improve the targeting of calcium phosphate nanoparticles and promote the cellular uptake, various modifications were designed, such as modified by targeted molecule (e.g., folic acid [79,159], antibody [160,161]), charge reversible property [80]. Huang et al. [81] engineered the nanoparticles (NPs) that contain an HCC-targeting peptide (sp94) and a unique dendrimer–calcium phosphate (CaP) core that harbors nucleic acids for gene delivery. Fig. 10 illustrates the design of a tumor targeted NP that carries siRNA against the immune checkpoint PD-L1 and pDNA encoding the immunostimulant cytokine IL-2 to promote antitumor immunity and increase the efficacy of whole-cell cancer vaccines. This nanoscale immunogenic therapy exhibits multifunctional characteristics, including (i) a tumor-targeting peptide (SP94) that enhances the tumor accumulation of NPs and increases the efficiency of intracellular delivery of the therapeutic pDNA/siRNA to HCC cells; (ii) a pH stimuli-responsive CaP core to achieve endosomal escape, along with enhanced release of the nucleic acid; (iii) thymine-capped polyamidoamine (PAMAM) dendrimers loaded in the CaP core to further enhance the endosomal escape and nuclear entry of pDNA, leading to promising gene transfection activity; and (iv) thymine-capped PAMAM dendrimers that can activate the stimulator of interferon genes (STING)–cyclic GMP-AMP synthase (cGAS) pathway and serve as immunotherapy adjuvants to promote cellular immunity. Xu et al. [79] synthesized a new folic acid (FA) receptor-targeted lipid-coated calcium carbonate/phosphate (LCCP) nanoparticle incorporating two often-used therapeutics, cell death siRNA and α-tocopherol succinate. The nanoparticles exhibited a high gene/drug loading efficiency (60%) with folic acid-enhanced cellular uptake and inhibited the growth of B16F0 melanoma cells. Chen et al. [82] developed a microRNA delivery system based on lipid-coated calcium phosphonate nanoparticles (CaP/miR@pMNPs) containing conjugated mannose and sterically shielded with a pH-responsive material. The nanocarrier could respond to the low pH in the tumor microenvironment and expose mannose to promote cellular internalization in TAMs, which is potential therapeutic strategy for tumor immunotherapy. Zhou et al. [80] reported a calcium phosphate lipid hybrid nanoparticle that possessed charge reversible property was prepared to enhance the activity of siBcl-2 in vivo. The average diameter and zeta potential of siBcl-2 loaded calcium phosphate lipid hybrid nanoparticles (LNPS@siBcl-2) were 80 nm and −13 mV at pH 7.4 whereas the diameter and zeta potential changed to 1506 nm and +9 mV at pH 5.0. LNPS@siBcl-2 could efficiently deliver siBcl-2 to the cytoplasm and decreased the expression of Bcl-2 in A549 cells. Apart from these, the CaP NPs of LCP form were also used for amyotrophic lateral sclerosis [162], kidney injury [163] and bone biomineralization [164].
Fig. 10.
Schematic representation of the mechanism of immunogenic therapy by TT-LDCP NPs containing siRNA against the immune checkpoint PD-L1 and pDNA encoding the immunostimulant cytokine IL-2 [81].
Further, LCP cores are readily amenable to the prototypical thin-film hydration process common to a variety of liposome formulations. A subset of cationic and helper lipids (DOTAP, dioleoyl phosphatidylcholine (DOPC)), stabilized by cholesterol (Chol) is dried under nitrogen gas and desiccated, and subsequently hydrated with the core solution to produce the LCP nanoparticles: a CaP core, encapsulated by a lipid bilayer, coated on its outer leaflet by both cationic and PEGylated lipids. Chen et al. [83] developed polycation liposome-encapsulated calcium phosphate nanoparticles (PLCP) for siVEGF delivery to MCF-7 cancer in vivo. PCLs were prepared by film dispersion method with a lipid mixture of PEI-Chol and DOPE (molar ratio 1:1), and then hydrated with CaP/siRNA nanoparticle solution followed by sonication and filtration through 0.22 μm filter. PLCP were constructed of CaP/siRNA nanoparticles as inner aqueous phase and PCL as outer lipid layer. Moreover, Lee et al. [84] designed the nanoparticle system could co-encapsulate and co-deliver a combination of therapeutic agents with different physicochemical properties (inhibitors for miRi-221/222 and paclitaxel). miRi-221/222 are hydrophilic and were encapsulated with calcium phosphate by co-precipitation in a water-in-oil emulsion. The precipitates were then coated with DOPA to co-encapsulate hydrophobic paclitaxel outside the hydrophilic precipitates and finally PLGA-PEG was coated as the outer layer. A single PLGA-b-PEG NP (30–80 nm) was found to encapsulate up to about 16 lipid/CaP/miRi complexes (10–25 nm).
3.4. Muti-Layer
In the process of constructing calcium phosphate nano delivery system, materials and drugs or calcium phosphate layer by layer self-assembly sandwich like structure can be obtained by controlling the ratio of added materials, adding order and reaction time, which is called “multi-layer” delivery system, as shown in Fig. 4D. Multilayer structure can significantly improve the drug loading efficiency, and achieve the purpose of controlled drug release by a layer-by-layer dissolution.
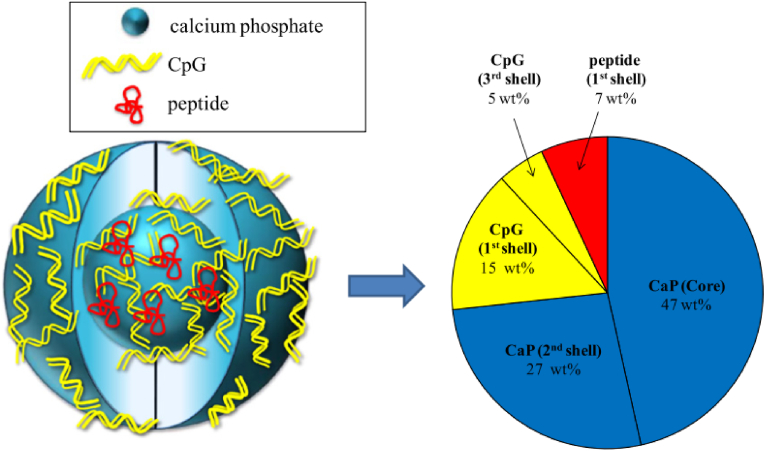
In 2006, Sokolova et al. [165] firstly prepared the stable colloids by coating the inorganic CaP nanoparticles with single- and double-stranded siRNA. The efficiency of such nanoparticles to specifically inhibit protein synthesis was tested on HeLa-EGFP cells whose green fluorescence was turned off by the coated nanoparticles (gene silencing with siRNA). Furthermore, they prepare the triple shell biodegradable calcium phosphate nanoparticles as carriers for the immunoactivity toll-like receptor ligands CpG and polyinosinic-polycytidylic acid for the activation of dendritic cells (DC) combined with the viral antigen hemagglutinin (HA) [85,86]. For the first time, an accurate quantification of the composition of such triple-shell nanoparticles was developed, as shown in Fig. 11, and the composition of triple-shell CaP nanoparticles is shown schematically. The core consists of CaP (47 wt%), the first shell consists of peptide (7 wt%) and CpG (15 wt%), both adsorbed on the CaP surface. The second shell consists of CaP (27 wt%), which forms the inorganic protective CaP layer. The third layer consists of CpG (5 wt%). Immunostimulatory effects of purified calcium phosphate nanoparticles on DC were demonstrated by increased expression of co-stimulatory molecules and MHC II and by cytokine secretion.
Fig. 11.
Composition of triple-shell CaP nanoparticles functionalized with CpG and a peptide [85,86].
Based on this, Neuhaus et al. [87] developed the triple shell calcium phosphate-siRNA-CaP-PEI by three-steps: i) calcium-l-lactate and diammonium hydrogen phosphate firstly were pumped into a solution of siRNA to obtain the single-shell calcium phosphate nanoparticles with a shell of siRNA. ii) calcium-l-lactate and diammonium hydrogen phosphate solutions were subsequently added to the colloidal for the second shell of calcium phosphate. iii) The third shell of polyethyleneimine were added as follows. The triple shell could increase calcium phosphate stabilization and transfection efficacy, where the outer PEI shell provided a positive charge and calcium phosphate colloidal stability. Same method was applied in CaP/DNA/CaP/PEI nanoparticles for the apoptosis in corneal endothelial cells or others [[166], [167], [168]]. Similarly, Vanegas Sa'enz et al. [88] prepared a multi-shell octa-arginine functionalized DNA-loaded CaP nanoparticles for non-viral vector gene delivery in human mesenchymal stem cells (hMSC) and human osteoblasts (hOB). Compared with PEI, octa-arginine (R8) is a small cationic synthetic peptide with lower cellular toxicity [169,170]. Apart from these, poly-(l-lysine) (PLL) was also used to synthesize the triple shell calcium phosphate-shRNA nanoparticles [89].
The similar operation was also used for the oral administration of vaccines, Xu et al. [90] investigated calcium phosphate nanoparticles coated with polysaccharides as nanocarriers for oral protein antigen delivery. In this design, the CaP NP core had an optimized antigen encapsulation capacity of 90 mg (BSA-FITC)/g (CaP NPs). The polysaccharides chitosan and alginate were coated onto the CaP NPs layer-by-layer to protect the antigens against acidic degradation in the gastrointestinal environment and enhance the immune response in the small intestine. The antigen release profiles showed that alginate-chitosan-coated CaP NPs prevented antigen release in a simulated gastric fluid (pH 1.2), followed by sustained release in simulated intestinal (pH 6.8) and colonic (pH 7.4) fluids. Cellular uptake and macrophage stimulation data revealed that the chitosan coating enhanced antigen uptake by intestine epithelia cells (Caco-2) and macrophages and improved surface expression of costimulatory molecules on macrophages. In vivo test further demonstrated that oral administration of alginate-chitosan-coated CaP@OVA NPs significantly enhanced the mucosal IgA and serum IgG antibody responses as compared to naked OVA, indicating that the CaP-Chi-Alg nanoparticle can potentially be used as a promising oral vaccine delivery system.
3.5. CaP-shell
To prevent drug leakage during delivery, one important approach of modifying the surface is by coating with inert inorganic materials such as silica [171,172] and Au [173,174]. However, calcium phosphate mostly is selected as the coating material due to their excellent biocompatibility and non-inflammatory properties. More importantly, calcium phosphate is responsive to low pH in the endosomes or lysosomes, which contributes to the release of drugs. In this mode, drugs molecule and materials firstly assemble to nanocore, then phosphate ions and calcium ions precipitate to form a calcium phosphate shell, named as “CaP Shell” (Fig. 4E), which mainly divided into two common structural forms: inorganic nanoparticles/drugs or organic polymer/drug self-assembly as the core. However, drugs could be loaded inside the nanosized core and the CaP shell or hollow CaP nanospheres, respectively.
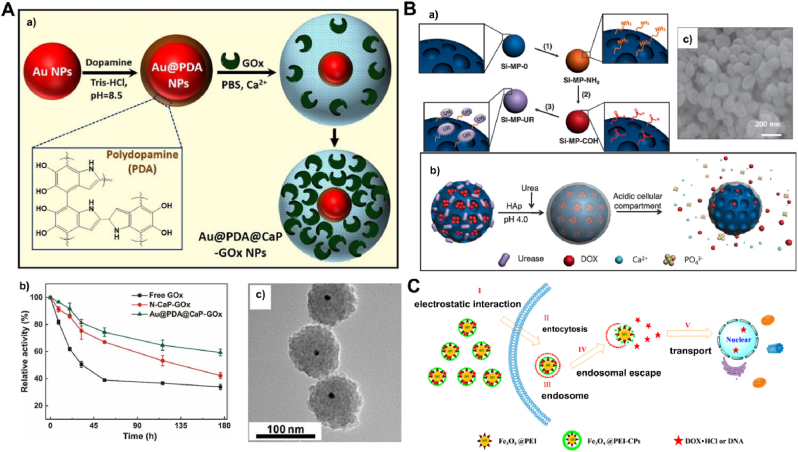
Calcium phosphates nanocarriers with large surface area and porous structure were considered to be ideal inorganic materials for enzyme immobilization. Liang et al. [91] reported a facile method of retaining full enzymatic activity by immobilizing glucose oxidase (GOx) into core-shell nanoparticles with polydopamine (PDA) sandwiched between gold nanoparticle (Au NP) core and calcium phosphate (CaP) shell (Au@PDA@CaP, Fig. 12A). The strong adhesion of PDA on Au NPs and its metal chelating properties directed the preferential growth of the CaP shell on the Au NPs, leading to well-dispersed and uniform nanohybrids (∼100 nm). As a result, Au@PDA@CaP-immobilized GOx had similar activity but better resistance against heating, long-term storage and repeated uses compared to free GOx. Moreover, mesoporous silica nanoparticles (MSNs) also attracted attention in drug loading and release with a CaP nanocoating shell. Lee et al. [92] reported a CaP covered mesoporous silica nanocontainers for controlled release of doxorubicin guest molecules. The doxorubicin was loaded into the pores of MSNs and then doxorubicin-loaded MSNs were capped by CaP coatings through the urease-mediated surface mineralization, and the final DOX-Si-MP-CaP was prepared (Fig. 12B). The doxorubicin-loading content inside MSNs was 4.2% by weight and facilitated DOX release was triggered under the low pH condition. DOX-Si-MP-CaP released a large amount of DOX (81.4%) even after 1 day. Meanwhile, Au and SiO2 were selected as the co-core with a CaP shell to prepared the Yolk–Shell nanoparticles for the dual-mode imaging and pH/NIR-responsive drug delivery [175]. Furthermore, Liang et al. [93] reported the design and fabrication of multifunctional mesoporous silica nanoparticles coated with poly (N-isopropylacrylamide)-co-acrylic acid and calcium phosphate (MSCNs) with pH-triggered doxorubicin release and dual-targeting transferrin (Tf)/RGD ligand functions. It was shown that Tf/RGD-MSCNs delivered the anti-tumor drug doxorubicin more efficiently into lysosomes and the resulting DOX-loaded nanoparticles (DOX-Tf/RGD-MSCNs) showed a stronger inhibitory effect towards tumor cell growth than free DOX and DOX delivered by unmodified MSNs. Besides, to explore the effect of pH on the synthesis and properties of SiO2/calcium phosphate core-shell nanoparticles, SiO2 cores with a particle diameter of 46 nm were successfully coated with an approximately 6-nm-thick Eu3+-doped calcium phosphate shell [176]. It has been established that the formation of a calcium phosphate shell is possible at pH below 4.5 and above 6.5. In the pH interval between 4.5 and 6.5, no shell growth but the formation of secondary NPs containing CaO and Ca(OH)2 was observed.
Fig. 12.
The CaP shell nanoparticles with different inorganic core: A) Au [91], B) SiO2 [92] and C) Fe3O4 [94], respectively.
Due to its natural magnetic properties, iron oxide is often used as the core of calcium phosphate drug delivery systems. Zhou et al. [94] fabricated a positively charged magnetic nano-formulation through the biomineralization of calcium phosphate on the surface of the superparamagnetic iron oxide nanoparticles with PEI, which was used to co-deliver DNA and doxorubicin for cancer therapy in A549 and HepG2 cells (Fig. 12C). Such a drug-loaded magnetic CaP nanoparticles showed a pH-dependent drug release behavior and inhibited tumor growth under an external magnetic field. Furthermore, Zhu et al. [177] prepared a magnetic, pH-responsive drug-delivery system based on magnetic iron oxide@amorphous calcium phosphate (MIO@ACP) core-shell hollow microspheres. The hollow structure was prepared by etching solid magnetic iron oxide microspheres (SMIOs) in hydrochloric acid/ethanol solution. After loading a drug into HMIOs, the drug-loaded HMIOs were coated by a protective layer of ACP by using Na2ATP as stabilizer, and an HMIOs/drug/ACP drug-delivery system based on MIO@ACP core-shell hollow microspheres was obtained, which showed a pH-responsive drug-release behavior. At pH 7.4, drug release was very slow, but was significantly enhanced at pH 4.5 due to dissolution of the ACP protective shell.
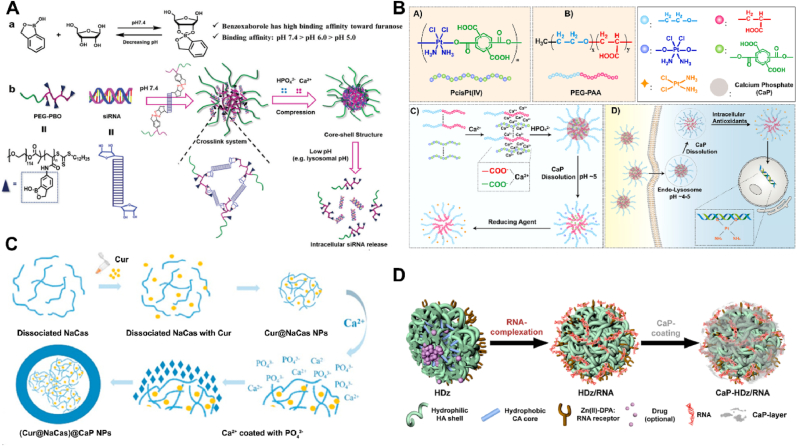
Another strategy is the CaP shell nanoparticles with different organic core, including PEG related polymer, lipid, hyaluronan and so on. The most common form is that calcium phosphate precipitates in the linking part of hydrophobic and hydrophilic regions of micelles, which will contribute to protect drugs from enzymatic degradation. For example, as shown in Fig. 13A, Shen et al. [95] demonstrated a non-anion stabilization strategy using a boroxole-containing block polymer, PEG-b-poly (benzoxaborole) (PEG-PBO), which forms pH-responsive boronic ester bonds with ribose rings of siRNA and also excellently adheres to the hydroxyapatite surface of CaP. The PEG-PBO/siRNA/CaP nanocomposites exhibited high siRNA loading efficiency, low cell cytotoxicity and excellent colloidal stability at neutral pH. The nanocomposites easily entered cancer cells mainly via clathrin-mediated endocytosis and transferred into acidic lysosomes, where the boronic esters broke, nanoparticles dissociated, and siRNA released and escaped from lysosomes. PEG-PBO/siRNA/CaP NPs showed significantly higher gene silencing efficacy than lipofectamine2000/siRNA lipoplex in multiple cancer cells. Similarly, Tao et al. [96] presented a unique strategy using a polymer (poly (Pt (IV) prodrug)) bearing numerous carboxyl side groups and incorporating redox-sensitive cisplatin Pt (IV) prodrugs in its backbone as the payload for encapsulation in CPNPs (Fig. 13B). The poly (Pt (IV) prodrug) was efficiently encapsulated in CPNPs (>90%), attributing to its improved solubility in alkaline water and strong binding affinity with CaP deriving from the plenty of carboxyl side groups. The CPNPs were stable and almost entirely inhibited the premature release of platinum drugs in the medium mimicking the pH condition of the bloodstream, whether there were reduction agents or not. While in an acidic condition with reduction agents, they released platinum drugs rapidly due to simultaneous reduction and hydrolysis of the poly (Pt (IV) prodrug). Further bioactivity experiments demonstrated the poly (Pt (IV) prodrug) encapsulated CPNPs were of higher efficacy against cancerous cells than free cisplatin and poly (Pt (IV) prodrug) owing to the enhanced platinum drugs uptake by cancerous cells via the CPNPs.
Fig. 13.
The CaP shell nanoparticles with different organic core: A) PEG-PBO [95], B) PEG-PAA [96], C) Curcumin/NaCas [99] and hyaluronic acid/Zn(II)-DPA [100], respectively.
Apart from PEG derivatives, Uludag et al. [97] used the lipid-modified PEI and poly (aspartic acid) (PAsp) to prepare a core loaded pDNA, followed to form the calcium phosphate shell by outer precipitating with phosphate and calcium ions. The modification enhanced the transfection efficiency and uptake of polyplexes in MC3T3-E1 cells. Similarly, Lee et al. [98] developed a calcium phosphate coated nanoparticle formulation by lipids to co-deliver miR-21 along with Dox. The NP formulation was confirmed to downregulate miR-21 levels and upregulate tumor suppressor gene levels.
Besides, Liang et al. [99] prepared a novel core-shell structure of NaCas@CaP as a nanodelivery system with NaCas (Sodium Caseinate) as the core for increasing solubility of curcumin and CaP as the shell for enhanced stability (Fig. 13C). After exposure to 80 °C for 2 h, the NaCas@CaP loaded curcumin still retained 80% stability while under the same conditions only 12% of free curcumin remained intact. UV-light stability was remarkably enhanced 8.56-fold by the protection of the core-shell structure. More importantly, pH responsive release was achieved owing to the CaP surface coating. The encapsulated curcumin by NaCas@CaP NPs exhibited an enhanced cellular and anti-cancer activity against A549 cancer cells than free curcumin. Moreover, Choi et al. [100] developed versatile RNAi nanoformulas (NFs) based on hyaluronan, conjugated with an artificial RNA receptor Zn (II)-dipicolylamine (DPA/Zn) for RNA loading, and stabilized with CaP layer (CaP-HDz/RNA-NF) (Fig. 13D). The doxorubicin could be encapsulated within the hydrophobic inner core of the nanocarriers, exhibiting pH-sensitivity and targeted drug delivery. By co-delivery doxorubicin and MDR1 gene target siRNA by this system could overcome the MDR of OVCAR8/ADR cancer cells to doxorubicin and improve the antitumor efficiency in vitro and in vivo.
No matter the core's shape, the coated or precipitated calcium phosphate structure's core-shell structure can efficiently encapsulate pharmaceuticals and subsequently release them in a way that is responsive to the environment, including pH sensitivity, redox sensitivity, temperature sensitivity, etc. It should be noted that calcium phosphate, when used as an outer layer, frequently exhibited instability when exposed to the medium. This instability could cause uncontrollable aggregation, which could increase particle size, even at the micron level. According to the findings of the literature review, modifying with PEG or other polymers in its outer layer to increase the stability of nanoparticles is a reasonably effective treatment approach.
4. Conclusion and future direction
Recent advances in the field of nanotechnology have opened up possibilities for the pleasant CaP-based therapy of nucleic acids and tiny molecules (Table 3). The CaP-based nanocarriers generally have a number of potential benefits for medication delivery: (1) readily available at low cost; (2) biocompatible and devoid of obvious toxicity or immune response; (3) biodegradable in biological environments; (4) responsive to low pH for a controlled release and endo/lysosomal escape of drugs; (5) stable biochemical properties that do not affect the bioactivity of payloads; and (6) flexible and modifiable features to create multifunctional nanoparticles.
This review gave an overview of the emerging trends of CaP nanoparticles and three key problems were discussed in detail: 1) preparation and characterization of CaP nanoparticles; 2) application of CaP nanoparticles based on multiple delivery systems, which have been relatively few reported on the relationship of nanostructures and biological function of different CaP designed as therapeutics. To achieve the satisfied therapeutic effects, four structural forms of CaP nanoparticles were concisely concluded following as: 1) the Ca2+, phosphate aqueous solutions and chemo-drugs or nucleic acids drugs mixed quickly to form a “Mix-CaP”; 2) calcium phosphate firstly prepared as the core, subsequently load drugs and finally other materials modified or coated as outer “Polymer Shell” or “Lipid Shell”; 3) calcium phosphate and drugs or materials integrate layer by layer to constructure a “Muti-CaP Layer”; 4) drugs molecule and materials firstly assemble to nanocore, then phosphate ions and calcium ions precipitate to form a “CaP Shell”, respectively. However, we consider that those CaP nanocarriers with an outer “Polymer Shell” or “Lipid Shell” will be the most promising delivery systems, which is supported by the realistic problems of nanoparticles. For in vivo applications, there is the general problem of the change of the dispersion medium from aqueous dispersion over inorganic physiological solutions (like PBS) to cell culture media (containing also proteins) and finally to blood or tissue [178,179]. Proteins are present in systemic circulation, and once they attach to the surface of nanoparticles and alter their charge, hydrophilicity and surface chemistry. And the ion strength of the solution increases, electrostatic repulsion between the nanoparticles decreases, resulting in agglomeration. Besides, the practical questions of sterilization and storage have to be solved for a final clinical application. Therefore, functional nanoparticles in blood or tissue will behave differently from them in vitro medium. The“Polymer Shell” or “Lipid Shell” could reduce proteins adsorption and avoid the CaP core dissolution, which is conducive to the stability of in vivo application. And their core-shell structure is more stable for storage, which can be confirmed by many other liposomal nanodrugs that have been used in clinical application, such as Doxil/Caelyx (PEGylated liposomal doxorubicin) [180], AmBisome (Liposomal amphotericin B) [181], Onpattro (Lipid-siRNA nanoparticles) [182] and so on. Notably, Calcium phosphate nanocarriers may make faster breakthroughs in local application or non-intravenous treatment, such as the calcium phosphate osteopontin particles used for controlling the development of dental caries [183]. Nevertheless, there are still many technical barriers which hindered the development in clinic, although with scientific advances over decades. Therefore, future efforts may be focusing on optimizing CaP nanocarriers for clinical applications, including large scale preparation, systemic toxicity evaluation of elevated intracellular calcium levels, pharmacokinetic and pharmacodynamic study of nanosized materials in vivo, as well as a deeper understanding of physiological characteristics.
Author contributions
Writing-original draft preparation, Chong Qiu; writing-review & editing, Chong Qiu, Jigang Wang, Fei Xia, Yanyan Wu, Qiuyan Guo, Qiaoli Shi, Junzhe Zhang, and Yuqing Meng. All authors have read and agree to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the Fundamental Research Funds for the Beijing Natural Science Foundation (No.7214287), the Central Public Welfare Research Institutes (Grant Nos.: ZZ14-YQ-050, ZZ14-YQ-055, ZZ14-YQ-058, ZZ14-YQ-059, ZZ14-YQ-061, ZZ14-ND-010, ZZ15-ND-10, and ZZ14-FL-002) and the National Natural Science Foundation (No.82204322, 82141001, 82074098, 82274182); National Key Research and Development Program of China(2020YFA0908000); National Key R&D Program of China Key projects for international cooperation on science, technology and innovation (2020YFE0205100); the Innovation Team and Talents Cultivation Program of the National Administration of Traditional Chinese Medicine (No: ZYYCXTD-C-202002); the CACMS Innovation Fund (CI2021A05101 and CI2021A05104).
Contributor Information
Chong Qiu, Email: cqiu@icmm.ac.cn.
Fei Xia, Email: fxia@icmm.ac.cn.
Jigang Wang, Email: jgwang@icmm.ac.cn.
Data availability
No data was used for the research described in the article.
References
- 1.Wu J.L.Y., Stordy B.P., Nguyen L.N.M., Deutschman C.P., Chan W.C.W. A proposed mathematical description of in vivo nanoparticle delivery. Adv. Drug Deliv. Rev. 2022;189 doi: 10.1016/j.addr.2022.114520. [DOI] [PubMed] [Google Scholar]
- 2.Yang J., Wang X., Wang B., Park K., Wooley K., Zhang S. Challenging the fundamental conjectures in nanoparticle drug delivery for chemotherapy treatment of solid cancers. Adv. Drug Deliv. Rev. 2022;190 doi: 10.1016/j.addr.2022.114525. [DOI] [PubMed] [Google Scholar]
- 3.Ginebra M.P., Canal C., Espanol M., Pastorino D., Montufar E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012;64:1090–1110. doi: 10.1016/j.addr.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Sugawara A., Asaoka K., Ding S.J. Calcium phosphate-based cements: clinical needs and recent progress. J. Mater. Chem. B. 2013;1:1081–1089. doi: 10.1039/c2tb00061j. [DOI] [PubMed] [Google Scholar]
- 5.Qi C., Lin J., Fu L.H., Huang P. Calcium-based biomaterials for diagnosis, treatment, and theranostics. Chem. Soc. Rev. 2018;47:357–403. doi: 10.1039/c6cs00746e. [DOI] [PubMed] [Google Scholar]
- 6.Eliaz N., Metoki N. Calcium phosphate bioceramics: a review of their history, structure, properties, coating technologies and biomedical applications. Materials. 2017;10(4):334. doi: 10.3390/ma10040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levingstone T.J., Herbaj S., Redmond J., McCarthy H.O., Dunne N.J. Calcium phosphate nanoparticles-based systems for RNAi delivery: applications in bone tissue regeneration. Nanomaterials. 2020;10(1):146. doi: 10.3390/nano10010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yi L.J., Li J.F., Ma M.G., Zhu Y.J. Nanostructured calcium-based biomaterials and their application in drug delivery. Curr. Med. Chem. 2020;27:5189–5212. doi: 10.2174/0929867326666190222193357. [DOI] [PubMed] [Google Scholar]
- 9.Huang D., He B., Mi P. Calcium phosphate nanocarriers for drug delivery to tumors: imaging, therapy and theranostics. Biomater. Sci. 2019;7:3942–3960. doi: 10.1039/c9bm00831d. [DOI] [PubMed] [Google Scholar]
- 10.Bassett D.C., Robinson T.E., Hill R.J., Grover L.M., Barralet J.E. Self-assembled calcium pyrophosphate nanostructures for targeted molecular delivery. Biomater Adv. 2022;140 doi: 10.1016/j.bioadv.2022.213086. [DOI] [PubMed] [Google Scholar]
- 11.Cai A.-Y., Zhu Y.-J., Qi C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfac. 2020;7 [Google Scholar]
- 12.Sokolova V., Epple M. Biological and medical applications of calcium phosphate nanoparticles. Chemistry. 2021;27:7471–7488. doi: 10.1002/chem.202005257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorozhkin S.V. Functionalized calcium orthophosphates (CaPO4) and their biomedical applications. J. Mater. Chem. B. 2019;7:7471–7489. doi: 10.1039/c9tb01976f. [DOI] [PubMed] [Google Scholar]
- 14.Dorozhkin S.V. Synthetic amorphous calcium phosphates (ACPs): preparation, structure, properties, and biomedical applications. Biomater. Sci. 2021;9:7748–7798. doi: 10.1039/d1bm01239h. [DOI] [PubMed] [Google Scholar]
- 15.Kumta P.N., Sfeir C., Lee D.H., Olton D., Choi D. Nanostructured calcium phosphates for biomedical applications: novel synthesis and characterization. Acta Biomater. 2005;1:65–83. doi: 10.1016/j.actbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Lv C., Guo L., Ma M., Li X., Lan Z., Huo J., Dong H., Zhu X., Zhu Q., Gu Y., Liu Z., Liu J., Chen H., Guo X., Ma J. Surface stability and morphology of calcium phosphate tuned by pH values and lactic acid additives: theoretical and experimental study. ACS Appl. Mater. Interfaces. 2022;14:4836–4851. doi: 10.1021/acsami.1c18727. [DOI] [PubMed] [Google Scholar]
- 17.Wang L., Nancollas G.H. Calcium orthophosphates: crystallization and dissolution. Chem. Rev. 2008;108:4628–4669. doi: 10.1021/cr0782574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W., Wang G., Wu W., Shao C., Pan H., Chen Z., Tang R., Chen Z., Xie Z. The effect of calcium phosphate ion clusters in enhancing enamel conditions versus Duraphat and Icon. Aust. Endod. J. 2022 doi: 10.1111/aej.12689. Submitted for publication. [DOI] [PubMed] [Google Scholar]
- 19.Ma Y.X., Hoff S.E., Huang X.Q., Liu J., Wan Q.Q., Song Q., Gu J.T., Heinz H., Tay F.R., Niu L.N. Involvement of prenucleation clusters in calcium phosphate mineralization of collagen. Acta Biomater. 2021;120:213–223. doi: 10.1016/j.actbio.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 20.Uskokovic V., Uskokovic D.P. Nanosized hydroxyapatite and other calcium phosphates: chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. B Appl. Biomater. 2011;96:152–191. doi: 10.1002/jbm.b.31746. [DOI] [PubMed] [Google Scholar]
- 21.de Groot K., Wolke J.G., Jansen J.A. Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng. H. 1998;212:137–147. doi: 10.1243/0954411981533917. [DOI] [PubMed] [Google Scholar]
- 22.Lin K., Wu C., Chang J. Advances in synthesis of calcium phosphate crystals with controlled size and shape. Acta Biomater. 2014;10:4071–4102. doi: 10.1016/j.actbio.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Jun W., Lin L., Yurong C., Juming Y. Recent advances of calcium phosphate nanoparticles for controlled drug delivery. Mini Rev. Med. Chem. 2013;13:1501–1507. doi: 10.2174/13895575113139990059. [DOI] [PubMed] [Google Scholar]
- 24.Dorozhkin S.V., Epple M. Biological and medical significance of calcium phosphates. Angew Chem. Int. Ed. Engl. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 25.Palmer L.C., Newcomb C.J., Kaltz S.R., Spoerke E.D., Stupp S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008;108:4754–4783. doi: 10.1021/cr8004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dorozhkin S.V. Calcium orthophosphates in dentistry. J. Mater. Sci. Mater. Med. 2013;24:1335–1363. doi: 10.1007/s10856-013-4898-1. [DOI] [PubMed] [Google Scholar]
- 27.Beigoli S., Hekmat A., Farzanegan F., Darroudi M. Sol-gel synthesis of amorphous calcium phosphate nanoparticles in brown rice substrate and assessment of their cytotoxicity and antimicrobial activities. Avicenna J Phytomed. 2022;12:77–88. doi: 10.22038/AJP.2021.18930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joughehdoust S., Behnamghader A., Jahandideh R., Manafi S. Effect of aging temperature on formation of sol-gel derived fluor-hydroxyapatite nanoparticles. J. Nanosci. Nanotechnol. 2010;10:2892–2896. doi: 10.1166/jnn.2010.1397. [DOI] [PubMed] [Google Scholar]
- 29.Hanifi A., Fathi M.H., Sadeghi H.M., Varshosaz J. Mg2+ substituted calcium phosphate nano particles synthesis for non viral gene delivery application. J. Mater. Sci. Mater. Med. 2010;21:2393–2401. doi: 10.1007/s10856-010-4088-3. [DOI] [PubMed] [Google Scholar]
- 30.Sadat-Shojai M., Khorasani M.T., Dinpanah-Khoshdargi E., Jamshidi A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013;9:7591–7621. doi: 10.1016/j.actbio.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 31.Carmona F.J., Guagliardi A., Masciocchi N. Nanosized calcium phosphates as novel macronutrient. Nano-Fertilizers, Nanomaterials (Basel) 2022;12(15):2709. doi: 10.3390/nano12152709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimanovskaia E.V., Beznos O.V., Kliachko N.L., Kost O.A., Nikol'skaia, Pavlenko T.A., Chesnokova N.B., Kabanov A.V. [Production of timolol containing calcium-phosphate nanoparticles and evaluation of their effect on intraocular pressure in experiment] Vestn. Oftalmol. 2012;128:15–18. [PubMed] [Google Scholar]
- 33.Laonapakul T., Sutthi R., Chaikool P., Talangkun S., Boonma A., Chindaprasirt P. Calcium phosphate powders synthesized from CaCO3 and CaO of natural origin using mechanical activation in different media combined with solid-state interaction. Mater Sci Eng C Mater Biol Appl. 2021;118 doi: 10.1016/j.msec.2020.111333. [DOI] [PubMed] [Google Scholar]
- 34.Qiu C., Wei W., Sun J., Zhang H.T., Ding J.S., Wang J.C., Zhang Q. Systemic delivery of siRNA by hyaluronan-functionalized calcium phosphate nanoparticles for tumor-targeted therapy. Nanoscale. 2016;8:13033–13044. doi: 10.1039/c6nr04034a. [DOI] [PubMed] [Google Scholar]
- 35.Giger E.V., Castagner B., Raikkonen J., Monkkonen J., Leroux J.C. siRNA transfection with calcium phosphate nanoparticles stabilized with PEGylated chelators. Adv Healthc Mater. 2013;2:134–144. doi: 10.1002/adhm.201200088. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Z., Li H., Wang K., Guo Q., Li C., Jiang H., Hu Y., Oupicky D., Sun M. Bioreducible cross-linked hyaluronic acid/calcium phosphate hybrid nanoparticles for specific delivery of siRNA in melanoma tumor therapy. ACS Appl. Mater. Interfaces. 2017;9:14576–14589. doi: 10.1021/acsami.6b15347. [DOI] [PubMed] [Google Scholar]
- 37.Ishikawa K., Garskaite E., Kareiva A. Sol-gel synthesis of calcium phosphate-based biomaterials-A review of environmentally benign, simple, and effective synthesis routes. J. Sol. Gel Sci. Technol. 2020;94:551–572. [Google Scholar]
- 38.Bose S., Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: a review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Das P., Jana N.R. Length-controlled synthesis of calcium phosphate nanorod and nanowire and application in intracellular protein delivery. ACS Appl. Mater. Interfaces. 2016;8:8710–8720. doi: 10.1021/acsami.6b01667. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira L.K., Molina E.F., Moura A.L., de Faria E.H., Ciuffi K.J. Synthesis, characterization, and environmental applications of hybrid materials based on humic acid obtained by the sol-gel route. ACS Appl. Mater. Interfaces. 2016;8:1478–1485. doi: 10.1021/acsami.5b10810. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa K., Garskaite E., A.J.J.o.S.-G.S. Kareiva Technology, Sol-gel synthesis of calcium phosphate-based biomaterials-A review of environmentally benign, simple, and effective synthesis routes. J. Sol. Gel Sci. Technol. 2020;94:551–572. [Google Scholar]
- 42.Ramirez Caballero S.S., Ferri-Angulo D., Debret R., Granier F., Marie S., Lefevre F.X., Bouler J.M., Despas C., Sohier J., Bujoli B. Combination of biocompatible hydrogel precursors to apatitic calcium phosphate cements (CPCs): influence of the in situ hydrogel reticulation on the CPC properties. J. Biomed. Mater. Res. B Appl. Biomater. 2021;109:102–116. doi: 10.1002/jbm.b.34685. [DOI] [PubMed] [Google Scholar]
- 43.Goldberg M.A., Krohicheva P.A., Fomin A.S., Khairutdinova D.R., Antonova O.S., Baikin A.S., Smirnov V.V., Fomina A.A., Leonov A.V., Mikheev I.V., Sergeeva N.S., Akhmedova S.A., Barinov S.M., Komlev V.S. Insitu magnesium calcium phosphate cements formation: from one pot powders precursors synthesis to in vitro investigations. Bioact. Mater. 2020;5:644–658. doi: 10.1016/j.bioactmat.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho J.S., Ko Y.N., Koo H.Y., Kang Y.C. Synthesis of nano-sized biphasic calcium phosphate ceramics with spherical shape by flame spray pyrolysis. J. Mater. Sci. Mater. Med. 2010;21:1143–1149. doi: 10.1007/s10856-009-3980-1. [DOI] [PubMed] [Google Scholar]
- 45.Cleries L., Martinez E., Fernandez-Pradas J.M., Sardin G., Esteve J., Morenza J.L. Mechanical properties of calcium phosphate coatings deposited by laser ablation. Biomaterials. 2000;21:967–971. doi: 10.1016/s0142-9612(99)00240-9. [DOI] [PubMed] [Google Scholar]
- 46.Guo X., Yan H., Zhao S., Li Z., Li Y., Liang X. Effect of calcining temperature on particle size of hydroxyapatite synthesized by solid-state reaction at room temperature. Adv. Powder Technol. 2013;24:1034–1038. [Google Scholar]
- 47.Kusnieruk S., Wojnarowicz J., Chodara A., Chudoba T., Gierlotka S., Lojkowski W. Influence of hydrothermal synthesis parameters on the properties of hydroxyapatite nanoparticles. Beilstein J. Nanotechnol. 2016;7:1586–1601. doi: 10.3762/bjnano.7.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs E.E., Gronowicz G., Hurley M.M., Kuhn L.T. Biomimetic calcium phosphate/polyelectrolyte multilayer coatings for sequential delivery of multiple biological factors. J. Biomed. Mater. Res. 2017;105:1500–1509. doi: 10.1002/jbm.a.35985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Welzel T., Meyer-Zaika W., Epple M. Continuous preparation of functionalised calcium phosphate nanoparticles with adjustable crystallinity. Chem. Commun. 2004:1204–1205. doi: 10.1039/b402521k. [DOI] [PubMed] [Google Scholar]
- 50.Pittella F., Cabral H., Maeda Y., Mi P., Watanabe S., Takemoto H., Kim H.J., Nishiyama N., Miyata K., Kataoka K. Systemic siRNA delivery to a spontaneous pancreatic tumor model in transgenic mice by PEGylated calcium phosphate hybrid micelles. J. Contr. Release. 2014;178:18–24. doi: 10.1016/j.jconrel.2014.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Jung H., Kim S.A., Yang Y.G., Yoo H., Lim S.J., Mok H. Long chain microRNA conjugates in calcium phosphate nanoparticles for efficient formulation and delivery. Arch Pharm. Res. (Seoul) 2015;38:705–715. doi: 10.1007/s12272-014-0451-0. [DOI] [PubMed] [Google Scholar]
- 52.Qi C., Zhu Y.J., Sun T.W., Wu J., Chen F. Microwave-assisted hydrothermal rapid synthesis of amorphous calcium phosphate mesoporous microspheres using adenosine 5'-diphosphate and application in pH-responsive drug delivery. Chem. Asian J. 2015;10:2503–2511. doi: 10.1002/asia.201500667. [DOI] [PubMed] [Google Scholar]
- 53.Qi C., Zhu Y.J., Lu B.Q., Zhao X.Y., Zhao J., Chen F., Wu J. Hydroxyapatite hierarchically nanostructured porous hollow microspheres: rapid, sustainable microwave-hydrothermal synthesis by using creatine phosphate as an organic phosphorus source and application in drug delivery and protein adsorption. Chemistry. 2013;19:5332–5341. doi: 10.1002/chem.201203886. [DOI] [PubMed] [Google Scholar]
- 54.Qi C., Zhu Y.J., Zhao X.Y., Lu B.Q., Tang Q.L., Zhao J., Chen F. Highly stable amorphous calcium phosphate porous nanospheres: microwave-assisted rapid synthesis using ATP as phosphorus source and stabilizer, and their application in anticancer drug delivery. Chemistry. 2013;19:981–987. doi: 10.1002/chem.201202829. [DOI] [PubMed] [Google Scholar]
- 55.Yu W., Sun T.W., Ding Z., Qi C., Zhao H., Chen F., Shi Z., Zhu Y.J., Chen D., He Y. Copper-doped mesoporous hydroxyapatite microspheres synthesized by a microwave-hydrothermal method using creatine phosphate as an organic phosphorus source: application in drug delivery and enhanced bone regeneration. J. Mater. Chem. B. 2017;5:1039–1052. doi: 10.1039/c6tb02747d. [DOI] [PubMed] [Google Scholar]
- 56.Di Mauro V., Iafisco M., Salvarani N., Vacchiano M., Carullo P., Ramirez-Rodriguez G.B., Patricio T., Tampieri A., Miragoli M., Catalucci D. Bioinspired negatively charged calcium phosphate nanocarriers for cardiac delivery of MicroRNAs. Nanomedicine. 2016;11:891–906. doi: 10.2217/nnm.16.26. [DOI] [PubMed] [Google Scholar]
- 57.Qi C., Zhu Y.J., Zhang Y.G., Jiang Y.Y., Wu J., Chen F. Vesicle-like nanospheres of amorphous calcium phosphate: sonochemical synthesis using the adenosine 5'-triphosphate disodium salt and their application in pH-responsive drug delivery. J. Mater. Chem. B. 2015;3:7347–7354. doi: 10.1039/c5tb01340b. [DOI] [PubMed] [Google Scholar]
- 58.Sun B., Zhao X., Wu Y., Cao P., Movahedi F., Liu J., Wang J., Xu Z.P., Gu W. Mannose-functionalized biodegradable nanoparticles efficiently deliver DNA vaccine and promote anti-tumor immunity. ACS Appl. Mater. Interfaces. 2021;13:14015–14027. doi: 10.1021/acsami.1c01401. [DOI] [PubMed] [Google Scholar]
- 59.Zhao X.Y., Zhu Y.J., Chen F., Lu B.Q., Qi C., Zhao J., Wu J. Calcium phosphate hybrid nanoparticles: self-assembly formation, characterization, and application as an anticancer drug nanocarrier. Chem. Asian J. 2013;8:1306–1312. doi: 10.1002/asia.201300083. [DOI] [PubMed] [Google Scholar]
- 60.Hou S., Ma H., Ji Y., Hou W., Jia N. A calcium phosphate nanoparticle-based biocarrier for efficient cellular delivery of antisense oligodeoxynucleotides. ACS Appl. Mater. Interfaces. 2013;5:1131–1136. doi: 10.1021/am3028926. [DOI] [PubMed] [Google Scholar]
- 61.Tang J., Chen J.Y., Liu J., Luo M., Wang Y.J., Wei X.W., Gao X., Wang B.L., Liu Y.B., Yi T., Tong A.P., Song X.R., Xie Y.M., Zhao Y., Xiang M., Huang Y., Zheng Y. Calcium phosphate embedded PLGA nanoparticles: a promising gene delivery vector with high gene loading and transfection efficiency. Int. J. Pharm. 2012;431:210–221. doi: 10.1016/j.ijpharm.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 62.Park H., Kim M.H., Yoon Y.I., Park W.H. One-pot synthesis of injectable methylcellulose hydrogel containing calcium phosphate nanoparticles. Carbohydr. Polym. 2017;157:775–783. doi: 10.1016/j.carbpol.2016.10.055. [DOI] [PubMed] [Google Scholar]
- 63.Giger E.V., Puigmartí-Luis J., Schlatter R., Castagner B., Dittrich P.S., Leroux J.-C. Gene delivery with bisphosphonate-stabilized calcium phosphate nanoparticles. J. Contr. Release. 2011;150:87–93. doi: 10.1016/j.jconrel.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 64.Pittella F., Zhang M., Lee Y., Kim H.J., Tockary T., Osada K., Ishii T., Miyata K., Nishiyama N., Kataoka K. Enhanced endosomal escape of siRNA-incorporating hybrid nanoparticles from calcium phosphate and PEG-block charge-conversional polymer for efficient gene knockdown with negligible cytotoxicity. Biomaterials. 2011;32:3106–3114. doi: 10.1016/j.biomaterials.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 65.Kakizawa Y., Furukawa S., Kataoka K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. J. Contr. Release. 2004;97:345–356. doi: 10.1016/j.jconrel.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 66.Kakizawa Y., Furukawa S., Ishii A., Kataoka K. Organic-inorganic hybrid-nanocarrier of siRNA constructing through the self-assembly of calcium phosphate and PEG-based block aniomer. J. Contr. Release. 2006;111:368–370. doi: 10.1016/j.jconrel.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 67.Zhang M., Ishii A., Nishiyama N., Matsumoto S., Ishii T., Yamasaki Y., Kataoka K. PEGylated calcium phosphate nanocomposites as smart environment-sensitive carriers for siRNA delivery. Adv. Mater. 2009;21:3520–3525. [Google Scholar]
- 68.Chu W., Tian P., Ding N., Cai Q., Li J., Zhuo X., Tang Z., Gou J., Yin T., Zhang Y., He H., Tang X. Improving plasma stability and bioavailability in vivo of gemcitabine via nanoparticles of mPEG-PLG-GEM complexed with calcium phosphate. Pharm. Res. (N. Y.) 2018;35:230. doi: 10.1007/s11095-018-2506-2. [DOI] [PubMed] [Google Scholar]
- 69.Choi B., Cui Z.K., Kim S., Fan J., Wu B.M., Lee M. Glutamine-chitosan modified calcium phosphate nanoparticles for efficient siRNA delivery and osteogenic differentiation. J. Mater. Chem. B. 2015;3:6448–6455. doi: 10.1039/C5TB00843C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee K., Oh M.H., Lee M.S., Nam Y.S., Park T.G., Jeong J.H. Stabilized calcium phosphate nano-aggregates using a dopa-chitosan conjugate for gene delivery. Int. J. Pharm. 2013;445:196–202. doi: 10.1016/j.ijpharm.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Wang J., Chen B., Zhao D., Peng Y., Zhuo R.X., Cheng S.X. Peptide decorated calcium phosphate/carboxymethyl chitosan hybrid nanoparticles with improved drug delivery efficiency. Int. J. Pharm. 2013;446:205–210. doi: 10.1016/j.ijpharm.2013.02.028. [DOI] [PubMed] [Google Scholar]
- 72.Lee M.S., Lee J.E., Byun E., Kim N.W., Lee K., Lee H., Sim S.J., Lee D.S., Jeong J.H. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Contr. Release. 2014;192:122–130. doi: 10.1016/j.jconrel.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 73.Lee J.E., Yin Y., Lim S.Y., Kim E.S., Jung J., Kim D., Park J.W., Lee M.S., Jeong J.H. Enhanced transfection of human mesenchymal stem cells using a hyaluronic acid/calcium phosphate hybrid gene delivery system. Polymers. 2019;11(5):798. doi: 10.3390/polym11050798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liang P., Zhao D., Wang C.Q., Zong J.Y., Zhuo R.X., Cheng S.X. Facile preparation of heparin/CaCO3/CaP hybrid nano-carriers with controllable size for anticancer drug delivery. Colloids Surf. B Biointerfaces. 2013;102:783–788. doi: 10.1016/j.colsurfb.2012.08.056. [DOI] [PubMed] [Google Scholar]
- 75.Bai S., Sun Y., Cheng Y., Ye W., Jiang C., Liu M., Ji Q., Zhang B., Mei Q., Liu D., Zhou S. MCP mediated active targeting calcium phosphate hybrid nanoparticles for the treatment of orthotopic drug-resistant colon cancer. J. Nanobiotechnol. 2021;19:367. doi: 10.1186/s12951-021-01115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bakan F., Kara G., Cokol Cakmak M., Cokol M., Denkbas E.B. Synthesis and characterization of amino acid-functionalized calcium phosphate nanoparticles for siRNA delivery. Colloids Surf. B Biointerfaces. 2017;158:175–181. doi: 10.1016/j.colsurfb.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 77.Zhao X.-Y., Zhu Y.-J., Lu B., Chen F., Qi C., Zhao J., Wu J.J.M.R.B. Hydrothermal synthesis of hydroxyapatite nanorods using pyridoxal-5′-phosphate as a phosphorus source. Mater. Res. Bull. 2014;55:67–70. [Google Scholar]
- 78.Zhang Y., Schwerbrock N.M., Rogers A.B., Kim W.Y., Huang L. Codelivery of VEGF siRNA and gemcitabine monophosphate in a single nanoparticle formulation for effective treatment of NSCLC. Mol. Ther. 2013;21:1559–1569. doi: 10.1038/mt.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yilun Wu W.G., Xu Zhi Ping. Enhanced combination cancer therapy using lipid-calcium carbonate/phosphate nanoparticles as a targeted delivery platform. Nanomedicine. 2019;14:77–92. doi: 10.2217/nnm-2018-0252. [DOI] [PubMed] [Google Scholar]
- 80.Cai R.Q., Liu D.Z., Cui H., Cheng Y., Liu M., Zhang B.L., Mei Q.B., Zhou S.Y. Charge reversible calcium phosphate lipid hybrid nanoparticle for siRNA delivery. Oncotarget. 2017;8:42772–42788. doi: 10.18632/oncotarget.17484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang K.W., Hsu F.F., Qiu J.T., Chern G.J., Lee Y.A., Chang C.C., Huang Y.T., Sung Y.C., Chiang C.C., Huang R.L., Lin C.C., Dinh T.K., Huang H.C., Shih Y.C., Alson D., Lin C.Y., Lin Y.C., Chang P.C., Lin S.Y., Chen Y. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aax5032. eaax5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zang X., Zhang X., Zhao X., Hu H., Qiao M., Deng Y., Chen D. Targeted delivery of miRNA 155 to tumor associated macrophages for tumor immunotherapy. Mol. Pharm. 2019;16:1714–1722. doi: 10.1021/acs.molpharmaceut.9b00065. [DOI] [PubMed] [Google Scholar]
- 83.Chen J., Sun X., Shao R., Xu Y., Gao J., Liang W. VEGF siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017;12:6075–6088. doi: 10.2147/IJN.S142739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou Z., Kennell C., Lee J.Y., Leung Y.K., Tarapore P. Calcium phosphate-polymer hybrid nanoparticles for enhanced triple negative breast cancer treatment via co-delivery of paclitaxel and miR-221/222 inhibitors. Nanomedicine. 2017;13:403–410. doi: 10.1016/j.nano.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 85.Sokolova V., Knuschke T., Buer J., Westendorf A.M., Epple M. Quantitative determination of the composition of multi-shell calcium phosphate-oligonucleotide nanoparticles and their application for the activation of dendritic cells. Acta Biomater. 2011;7:4029–4036. doi: 10.1016/j.actbio.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 86.Sokolova V., Knuschke T., Kovtun A., Buer J., Epple M., Westendorf A.M. The use of calcium phosphate nanoparticles encapsulating Toll-like receptor ligands and the antigen hemagglutinin to induce dendritic cell maturation and T cell activation. Biomaterials. 2010;31:5627–5633. doi: 10.1016/j.biomaterials.2010.03.067. [DOI] [PubMed] [Google Scholar]
- 87.Neuhaus B., Frede A., Westendorf A.M., Epple M. Gene silencing of the pro-inflammatory cytokine TNF-alpha with siRNA delivered by calcium phosphate nanoparticles, quantified by different methods. J. Mater. Chem. B. 2015;3:7186–7193. doi: 10.1039/c5tb01377a. [DOI] [PubMed] [Google Scholar]
- 88.Vanegas Saenz J.R., Tenkumo T., Kamano Y., Egusa H., Sasaki K. Amiloride-enhanced gene transfection of octa-arginine functionalized calcium phosphate nanoparticles. PLoS One. 2017;12 doi: 10.1371/journal.pone.0188347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang X., Kovtun A., Mendoza-Palomares C., Oulad-Abdelghani M., Fioretti F., Rinckenbach S., Mainard D., Epple M., Benkirane-Jessel N. SiRNA-loaded multi-shell nanoparticles incorporated into a multilayered film as a reservoir for gene silencing. Biomaterials. 2010;31:6013–6018. doi: 10.1016/j.biomaterials.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 90.Cao P., Han F.Y., Grondahl L., Xu Z.P., Li L. Enhanced oral vaccine efficacy of polysaccharide-coated calcium phosphate nanoparticles. ACS Omega. 2020;5:18185–18197. doi: 10.1021/acsomega.0c01792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li D., Fang Z., Duan H., Liang L. Polydopamine-mediated synthesis of core-shell gold@calcium phosphate nanoparticles for enzyme immobilization. Biomater. Sci. 2019;7:2841–2849. doi: 10.1039/c9bm00283a. [DOI] [PubMed] [Google Scholar]
- 92.Rim H.P., Min K.H., Lee H.J., Jeong S.Y., Lee S.C. pH-Tunable calcium phosphate covered mesoporous silica nanocontainers for intracellular controlled release of guest drugs. Angew Chem. Int. Ed. Engl. 2011;50:8853–8857. doi: 10.1002/anie.201101536. [DOI] [PubMed] [Google Scholar]
- 93.Liu J., Hu X., Jin S., Liang X.J., Ma X. Enhanced anti-tumor activity of a drug through pH-triggered release and dual targeting by calcium phosphate-covered mesoporous silica vehicles. J. Mater. Chem. B. 2022;10:384–395. doi: 10.1039/d1tb02540f. [DOI] [PubMed] [Google Scholar]
- 94.Tang Z., Zhou Y., Sun H., Li D., Zhou S. Biodegradable magnetic calcium phosphate nanoformulation for cancer therapy. Eur. J. Pharm. Biopharm. 2014;87:90–100. doi: 10.1016/j.ejpb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Zhou Q., Wang Y., Xiang J., Piao Y., Zhou Z., Tang J., Liu X., Shen Y. Stabilized calcium phosphate hybrid nanocomposite using a benzoxaborole-containing polymer for pH-responsive siRNA delivery. Biomater. Sci. 2018;6:3178–3188. doi: 10.1039/c8bm00575c. [DOI] [PubMed] [Google Scholar]
- 96.Yu Y., Sun L., Tang Y., Zhu H., Wang H., Xiao H., Wang F., Tao W. Preparation of cisplatin delivery calcium phosphate nanoparticles using poly(Pt(IV) prodrug) as the payload. Mater. Today Commun. 2022;33 [Google Scholar]
- 97.T A.D., Uludag H. Mineralized polyplexes for gene delivery: improvement of transfection efficiency as a consequence of calcium incubation and not mineralization. Mater Sci Eng C Mater Biol Appl. 2021;129 doi: 10.1016/j.msec.2021.112419. [DOI] [PubMed] [Google Scholar]
- 98.Sriram V., Lee J.Y. Calcium phosphate-polymeric nanoparticle system for co-delivery of microRNA-21 inhibitor and doxorubicin. Colloids Surf. B Biointerfaces. 2021;208 doi: 10.1016/j.colsurfb.2021.112061. [DOI] [PubMed] [Google Scholar]
- 99.Wu Q., Gao H., Vriesekoop F., Liu Z., He J., Liang H. Calcium phosphate coated core-shell protein nanocarriers: robust stability, controlled release and enhanced anticancer activity for curcumin delivery. Mater Sci Eng C Mater Biol Appl. 2020;115 doi: 10.1016/j.msec.2020.111094. [DOI] [PubMed] [Google Scholar]
- 100.Choi K.Y., Silvestre O.F., Huang X., Min K.H., Howard G.P., Hida N., Jin A.J., Carvajal N., Lee S.W., Hong J.I., Chen X. Versatile RNA interference nanoplatform for systemic delivery of RNAs. ACS Nano. 2014;8:4559–4570. doi: 10.1021/nn500085k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hild N., Schneider O.D., Mohn D., Luechinger N.A., Koehler F.M., Hofmann S., Vetsch J.R., Thimm B.W., Muller R., Stark W.J. Two-layer membranes of calcium phosphate/collagen/PLGA nanofibres: in vitro biomineralisation and osteogenic differentiation of human mesenchymal stem cells. Nanoscale. 2011;3:401–409. doi: 10.1039/c0nr00615g. [DOI] [PubMed] [Google Scholar]
- 102.Surmenev R.A., Surmeneva M.A., Ivanova A.A. Significance of calcium phosphate coatings for the enhancement of new bone osteogenesis--a review. Acta Biomater. 2014;10:557–579. doi: 10.1016/j.actbio.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 103.Sonksen M., Kerl K., Bunzen H. Current status and future prospects of nanomedicine for arsenic trioxide delivery to solid tumors. Med. Res. Rev. 2022;42:374–398. doi: 10.1002/med.21844. [DOI] [PubMed] [Google Scholar]
- 104.Tawfik M., Chen F., Goldberg J.L., Sabel B.A. Vol. 395. Naunyn Schmiedebergs Arch Pharmacol; 2022. Nanomedicine and Drug Delivery to the Retina: Current Status and Implications for Gene Therapy; pp. 1477–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Poinern G.E., Brundavanam R.K., Mondinos N., Jiang Z.T. Synthesis and characterisation of nanohydroxyapatite using an ultrasound assisted method. Ultrason. Sonochem. 2009;16:469–474. doi: 10.1016/j.ultsonch.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 106.Xu H., Zeiger B.W., Suslick K.S. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013;42:2555–2567. doi: 10.1039/c2cs35282f. [DOI] [PubMed] [Google Scholar]
- 107.Fu L.H., Qi C., Liu Y.J., Cao W.T., Ma M.G. Sonochemical synthesis of cellulose/hydroxyapatite nanocomposites and their application in protein adsorption. Sci. Rep. 2018;8:8292. doi: 10.1038/s41598-018-25566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Christian P., Von der Kammer F., Baalousha M., Hofmann T. Nanoparticles: structure, properties, preparation and behaviour in environmental media. Ecotoxicology. 2008;17:326–343. doi: 10.1007/s10646-008-0213-1. [DOI] [PubMed] [Google Scholar]
- 109.Goesmann H., Feldmann C. Nanoparticulate functional materials. Angew Chem. Int. Ed. Engl. 2010;49:1362–1395. doi: 10.1002/anie.200903053. [DOI] [PubMed] [Google Scholar]
- 110.Vergallo C., Torrieri G., Provenzani R., Miettinen S., Moslova K., Varjosalo M., Cristiano M.C., Fresta M., Celia C., Santos H.A., Cilurzo F., Di Marzio L. Design, synthesis and characterization of a PEGylated stanozolol for potential therapeutic applications. Int. J. Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118826. [DOI] [PubMed] [Google Scholar]
- 111.Kozlova D., Sokolova V., Zhong M., Zhang E., Yang J., Li W., Yang Y., Buer J., Westendorf A.M., Epple M., Yan H. Calcium phosphate nanoparticles show an effective activation of the innate immune response in vitro and in vivo after functionalization with flagellin. Virol. Sin. 2014;29:33–39. doi: 10.1007/s12250-014-3379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldschmidt G.M., Krok-Borkowicz M., Zybala R., Pamula E., Telle R., Conrads G., Schickle K. Biomimetic in situ precipitation of calcium phosphate containing silver nanoparticles on zirconia ceramic materials for surface functionalization in terms of antimicrobial and osteoconductive properties. Dent. Mater. 2021;37:10–18. doi: 10.1016/j.dental.2020.09.018. [DOI] [PubMed] [Google Scholar]
- 113.Li S., Wang B., Jiang S., Pan Y., Shi Y., Kong W., Shan Y. Surface-functionalized silica-coated calcium phosphate nanoparticles efficiently deliver DNA-based HIV-1 trimeric envelope vaccines against HIV-1. ACS Appl. Mater. Interfaces. 2021;13:53630–53645. doi: 10.1021/acsami.1c16989. [DOI] [PubMed] [Google Scholar]
- 114.Chu W., Huang Y., Yang C., Liao Y., Zhang X., Yan M., Cui S., Zhao C. Calcium phosphate nanoparticles functionalized with alendronate-conjugated polyethylene glycol (PEG) for the treatment of bone metastasis. Int. J. Pharm. 2017;516:352–363. doi: 10.1016/j.ijpharm.2016.11.051. [DOI] [PubMed] [Google Scholar]
- 115.Haedicke K., Kozlova D., Grafe S., Teichgraber U., Epple M., Hilger I. Multifunctional calcium phosphate nanoparticles for combining near-infrared fluorescence imaging and photodynamic therapy. Acta Biomater. 2015;14:197–207. doi: 10.1016/j.actbio.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 116.Sokolova V., Epple M. Synthetic pathways to make nanoparticles fluorescent. Nanoscale. 2011;3:1957–1962. doi: 10.1039/c1nr00002k. [DOI] [PubMed] [Google Scholar]
- 117.Ruedas-Rama M.J., Walters J.D., Orte A., Hall E.A. Fluorescent nanoparticles for intracellular sensing: a review. Anal. Chim. Acta. 2012;751:1–23. doi: 10.1016/j.aca.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 118.Yuan W., Chen J.B., Birck J.L., Yin Z.Y., Yuan S.L., Cai H.M., Wang Z.W., Huang Q., Wang Z.H. Precise analysis of gallium isotopic composition by MC-ICP-MS. Anal. Chem. 2016;88:9606–9613. doi: 10.1021/acs.analchem.6b02317. [DOI] [PubMed] [Google Scholar]
- 119.Neuhaus B., Tosun B., Rotan O., Frede A., Westendorf A.M., Epple M. Nanoparticles as transfection agents: a comprehensive study with ten different cell lines. RSC Adv. 2016;6:18102–18112. [Google Scholar]
- 120.Anoop M., Nambiar A.R., Nair S.V., Koyakutty M., Ashokan A. Zoledronic acid conjugated calcium phosphate nanoparticles for applications in cancer immunotherapy. Mater. Today Commun. 2022;30 [Google Scholar]
- 121.Rodriguez L., Cini M., Fini A., Orienti I. Modulation of the release of naproxen from calcium phosphate monoliths. J. Pharm. Belg. 1987;42:364–370. [PubMed] [Google Scholar]
- 122.Chen R., Qian Y., Li R., Zhang Q., Liu D., Wang M., Xu Q. Methazolamide calcium phosphate nanoparticles in an ocular delivery system. Yakugaku Zasshi. 2010;130:419–424. doi: 10.1248/yakushi.130.419. [DOI] [PubMed] [Google Scholar]
- 123.Cheng X., Kuhn L. Chemotherapy drug delivery from calcium phosphate nanoparticles. Int. J. Nanomed. 2007;2:667–674. [PMC free article] [PubMed] [Google Scholar]
- 124.Khalifehzadeh R., Arami H. Biodegradable calcium phosphate nanoparticles for cancer therapy. Adv. Colloid Interface Sci. 2020;279 doi: 10.1016/j.cis.2020.102157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sutthavas P., Habibovic P., van Rijt S.H. The shape-effect of calcium phosphate nanoparticle based films on their osteogenic properties. Biomater. Sci. 2021;9:1754–1766. doi: 10.1039/d0bm01494j. [DOI] [PubMed] [Google Scholar]
- 126.Xiaoyu M., Xiuling D., Chunyu Z., Yi S., Jiangchao Q., Yuan Y., Changsheng L. Polyglutamic acid-coordinated assembly of hydroxyapatite nanoparticles for synergistic tumor-specific therapy. Nanoscale. 2019;11:15312–15325. doi: 10.1039/c9nr03176f. [DOI] [PubMed] [Google Scholar]
- 127.Ansari L., Derakhshi M., Bagheri E., Shahtahmassebi N., Malaekeh-Nikouei B. Folate conjugation improved uptake and targeting of porous hydroxyapatite nanoparticles containing epirubicin to cancer cells. Pharmaceut. Dev. Technol. 2020;25:601–609. doi: 10.1080/10837450.2020.1725045. [DOI] [PubMed] [Google Scholar]
- 128.Graham F.L., van der Eb A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973;52:456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- 129.Jin L., Zeng X., Liu M., Deng Y., He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4:240–255. doi: 10.7150/thno.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Olton D., Li J., Wilson M.E., Rogers T., Close J., Huang L., Kumta P.N., Sfeir C. Nanostructured calcium phosphates (NanoCaPs) for non-viral gene delivery: influence of the synthesis parameters on transfection efficiency. Biomaterials. 2007;28:1267–1279. doi: 10.1016/j.biomaterials.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 131.Qi C., Zhu Y.-J., Ding G.-J., Wu J., Chen F.J.R.A. Solvothermal synthesis of hydroxyapatite nanostructures with various morphologies using adenosine 5′-monophosphate sodium salt as an organic phosphorus source. RSC Adv. 2015;5:3792–3798. [Google Scholar]
- 132.Qi C., Zhu Y.J., Wu C.T., Sun T.W., Chen F., Wu J. Magnesium phosphate pentahydrate nanosheets: microwave-hydrothermal rapid synthesis using creatine phosphate as an organic phosphorus source and application in protein adsorption. J. Colloid Interface Sci. 2016;462:297–306. doi: 10.1016/j.jcis.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 133.Zhao X., Zhu Y.-J., Chen F., Lu B.-Q., Qi C., Zhao J., Wu J. Hydrothermal synthesis of hydroxyapatite nanorods and nanowires using riboflavin-5′-phosphate monosodium salt as a new phosphorus source and their application in protein adsorption. CrystEngComm. 2013;15:7926. [Google Scholar]
- 134.Qi C., Zhu Y.J., Chen F. Fructose 1,6-bisphosphate trisodium salt as a new phosphorus source for the rapid microwave synthesis of porous calcium-phosphate microspheres and their application in drug delivery. Chem. Asian J. 2013;8:88–94. doi: 10.1002/asia.201200901. [DOI] [PubMed] [Google Scholar]
- 135.Kubicek V., Rudovsky J., Kotek J., Hermann P., Vander Elst L., Muller R.N., Kolar Z.I., Wolterbeek H.T., Peters J.A., Lukes I. A bisphosphonate monoamide analogue of DOTA: a potential agent for bone targeting. J. Am. Chem. Soc. 2005;127:16477–16485. doi: 10.1021/ja054905u. [DOI] [PubMed] [Google Scholar]
- 136.Lin Y., Villacanas M.G., Zou H., Liu H., Carcedo I.G., Wu Y., Sun B., Wu X., Prasadam I., Monteiro M.J., Li L., Xu Z.P., Gu W. Calcium-bisphosphonate nanoparticle platform as a prolonged nanodrug and bone-targeted delivery system for bone diseases and cancers. ACS Appl. Bio Mater. 2021;4:2490–2501. doi: 10.1021/acsabm.0c01455. [DOI] [PubMed] [Google Scholar]
- 137.Sun B., Gillard M., Wu Y., Wu P., Xu Z.P., Gu W. Bisphosphonate stabilized calcium phosphate nanoparticles for effective delivery of plasmid DNA to macrophages. ACS Appl. Bio Mater. 2020;3:986–996. doi: 10.1021/acsabm.9b00994. [DOI] [PubMed] [Google Scholar]
- 138.Kollenda S.A., Klose J., Knuschke T., Sokolova V., Schmitz J., Staniszewska M., Costa P.F., Herrmann K., Westendorf A.M., Fendler W.P., Epple M. In vivo biodistribution of calcium phosphate nanoparticles after intravascular, intramuscular, intratumoral, and soft tissue administration in mice investigated by small animal PET/CT. Acta Biomater. 2020;109:244–253. doi: 10.1016/j.actbio.2020.03.031. [DOI] [PubMed] [Google Scholar]
- 139.K. Raza, P. Kumar, N. Kumar, R. Malik, 9 - pharmacokinetics and biodistribution of the nanoparticles, in: S. Nimesh, R. Chandra, N. Gupta (Eds.) Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids, Woodhead Publishing2017, pp. 165-186.
- 140.Li S.D., Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol. Pharm. 2008;4:496–504. doi: 10.1021/mp800049w. [DOI] [PubMed] [Google Scholar]
- 141.Lewinski N., Colvin V Fau - Drezek R., Drezek R. Cytotoxicity of nanoparticles. Small. 2008;4:14. doi: 10.1002/smll.200700595. [DOI] [PubMed] [Google Scholar]
- 142.Arami H., Khandhar A., Liggitt D., Krishnan K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015;44:8576–8607. doi: 10.1039/c5cs00541h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Varkouhi A.K., Scholte M., Storm G., Haisma H.J. Endosomal escape pathways for delivery of biologicals. J. Contr. Release. 2011;151:220–228. doi: 10.1016/j.jconrel.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 144.Smith R.J., Beck R.W., Prevette L.E. Impact of molecular weight and degree of conjugation on the thermodynamics of DNA complexation and stability of polyethylenimine-graft-poly(ethylene glycol) copolymers. Biophys. Chem. 2015;203–204:12–21. doi: 10.1016/j.bpc.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 145.Mok Z.H., Mylonas P., Austin R., Proctor G., Pitts N., Thanou M. Calcium phosphate nanoparticles for potential application as enamel remineralising agent tested on hydroxyapatite discs. Nanoscale. 2021;13:20002–20012. doi: 10.1039/d1nr05378g. [DOI] [PubMed] [Google Scholar]
- 146.Zhao M., Li J., Chen D., Hu H. A valid bisphosphonate modified calcium phosphate-based gene delivery system: increased stability and enhanced transfection efficiency in vitro and in vivo. Pharmaceutics. 2019:11. doi: 10.3390/pharmaceutics11090468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qi C., Musetti S., Fu L.H., Zhu Y.J., Huang L. Biomolecule-assisted green synthesis of nanostructured calcium phosphates and their biomedical applications. Chem. Soc. Rev. 2019;48:2698–2737. doi: 10.1039/c8cs00489g. [DOI] [PubMed] [Google Scholar]
- 148.Yang Q., Liu D.Z., Liu M., Ji Q.F., Mei Q.B., Cheng Y., Zhou S.Y. Bone-targeted calcium phosphate-polymer hybrid nanoparticle Co-deliver zoledronate and docetaxel to treat bone metastasis of prostate cancer. J. Pharmacol. Sci. 2021;110:876–887. doi: 10.1016/j.xphs.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 149.Wang F., Wang Y., Zhang X., Zhang W., Guo S., Jin F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Contr. Release. 2014;174:126–136. doi: 10.1016/j.jconrel.2013.11.020. [DOI] [PubMed] [Google Scholar]
- 150.Sathy B.N., Olvera D., Gonzalez-Fernandez T., Cunniffe G.M., Pentlavalli S., Chambers P., Jeon O., Alsberg E., McCarthy H.O., Dunne N., Haut Donahue T.L., Kelly D.J. RALA complexed alpha-TCP nanoparticle delivery to mesenchymal stem cells induces bone formation in tissue engineered constructs in vitro and in vivo. J. Mater. Chem. B. 2017;5:1753–1764. doi: 10.1039/c6tb02881k. [DOI] [PubMed] [Google Scholar]
- 151.De Leo V., Maurelli A.M., Giotta L., Catucci L. Liposomes containing nanoparticles: preparation and applications. Colloids Surf. B Biointerfaces. 2022;218 doi: 10.1016/j.colsurfb.2022.112737. [DOI] [PubMed] [Google Scholar]
- 152.Sikora M.D.E. Liposomes as biocompatible and smart delivery systems - the current state. Adv. Colloid Interface Sci. 2022;309 doi: 10.1016/j.cis.2022.102757. [DOI] [PubMed] [Google Scholar]
- 153.Li J., Chen Y.C., Tseng Y.C., Mozumdar S., Huang L. Biodegradable calcium phosphate nanoparticle with lipid coating for systemic siRNA delivery. J. Contr. Release. 2010;142:416–421. doi: 10.1016/j.jconrel.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li J., Yang Y., Huang L. Calcium phosphate nanoparticles with an asymmetric lipid bilayer coating for siRNA delivery to the tumor. J. Contr. Release. 2012;158:108–114. doi: 10.1016/j.jconrel.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang Y., Li J., Liu F., Huang L. Systemic delivery of siRNA via LCP nanoparticle efficiently inhibits lung metastasis. Mol. Ther. 2012;20:609–615. doi: 10.1038/mt.2011.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang Y., Peng L., Mumper R.J., Huang L. Combinational delivery of c-myc siRNA and nucleoside analogs in a single, synthetic nanocarrier for targeted cancer therapy. Biomaterials. 2013;34:8459–8468. doi: 10.1016/j.biomaterials.2013.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu Z., Wang Y., Zhang L., Huang L. Nanoparticle-delivered transforming growth factor-beta siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano. 2014;8:3636–3645. doi: 10.1021/nn500216y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lecaros R.L., Huang L., Lee T.C., Hsu Y.C. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol. Ther. 2016;24:106–116. doi: 10.1038/mt.2015.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tang J., Howard C.B., Mahler S.M., Thurecht K.J., Huang L., Xu Z.P. Enhanced delivery of siRNA to triple negative breast cancer cells in vitro and in vivo through functionalizing lipid-coated calcium phosphate nanoparticles with dual target ligands. Nanoscale. 2018;10:4258–4266. doi: 10.1039/c7nr08644j. [DOI] [PubMed] [Google Scholar]
- 160.Tang J., Li B., Howard C.B., Mahler S.M., Thurecht K.J., Wu Y., Huang L., Xu Z.P. Multifunctional lipid-coated calcium phosphate nanoplatforms for complete inhibition of large triple negative breast cancer via targeted combined therapy. Biomaterials. 2019;216 doi: 10.1016/j.biomaterials.2019.119232. [DOI] [PubMed] [Google Scholar]
- 161.Jebali A., Kalantar S.M., Hekmatimoghaddam S., Saffarzadeh N., Sheikha M.H., Ghasemi N. Surface modification of tri-calcium phosphate nanoparticles by DOPE and/or anti-E6 antibody to enhance uptake of antisense of E6 mRNA. Colloids Surf. B Biointerfaces. 2015;126:297–302. doi: 10.1016/j.colsurfb.2014.12.040. [DOI] [PubMed] [Google Scholar]
- 162.Chen L., Watson C., Morsch M., Cole N.J., Chung R.S., Saunders D.N., Yerbury J.J., Vine K.L. Improving the delivery of SOD1 antisense oligonucleotides to motor neurons using calcium phosphate-lipid nanoparticles. Front. Neurosci. 2017;11:476. doi: 10.3389/fnins.2017.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu H., Zhang H., Yin N., Zhang Y., Gou J., Yin T., He H., Ding H., Zhang Y., Tang X. Sialic acid-modified dexamethasone lipid calcium phosphate gel core nanoparticles for target treatment of kidney injury. Biomater. Sci. 2020;8:3871–3884. doi: 10.1039/d0bm00581a. [DOI] [PubMed] [Google Scholar]
- 164.Favarin B.Z., Bolean M., Ramos A.P., Magrini A., Rosato N., Millan J.L., Bottini M., Costa-Filho A.J., Ciancaglini P. Lipid composition modulates ATP hydrolysis and calcium phosphate mineral propagation by TNAP-harboring proteoliposomes. Arch. Biochem. Biophys. 2020;691 doi: 10.1016/j.abb.2020.108482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Sokolova V., Kovtun A., Prymak O., Meyer-Zaika W., Kubareva E.A., Romanova E.A., Oretskaya T.S., Heumann R., Epple M. Functionalisation of calcium phosphate nanoparticles by oligonucleotides and their application for gene silencing. J. Mater. Chem. 2007;17:721–727. [Google Scholar]
- 166.Hu J., Kovtun A., Tomaszewski A., Singer B.B., Seitz B., Epple M., Steuhl K.P., Ergun S., Fuchsluger T.A. A new tool for the transfection of corneal endothelial cells: calcium phosphate nanoparticles. Acta Biomater. 2012;8:1156–1163. doi: 10.1016/j.actbio.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 167.Sokolova V.V., Radtke I., Heumann R., Epple M. Effective transfection of cells with multi-shell calcium phosphate-DNA nanoparticles. Biomaterials. 2006;27:3147–3153. doi: 10.1016/j.biomaterials.2005.12.030. [DOI] [PubMed] [Google Scholar]
- 168.Xu Z.X., Zhang R., Wang Y.X., Hu Q.L. A facile approach to construct hybrid multi-shell calcium phosphate gene particles. J. Zhejiang Univ. - Sci. B. 2010;11:292–297. doi: 10.1631/jzus.B0900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tenkumo T., Vanegas Saenz J.R., Takada Y., Takahashi M., Rotan O., Sokolova V., Epple M., Sasaki K. Gene transfection of human mesenchymal stem cells with a nano-hydroxyapatite-collagen scaffold containing DNA-functionalized calcium phosphate nanoparticles. Gene Cell. 2016;21:682–695. doi: 10.1111/gtc.12374. [DOI] [PubMed] [Google Scholar]
- 170.Kong X., Xu J., Yang X., Zhai Y., Ji J., Zhai G. Progress in tumour-targeted drug delivery based on cell-penetrating peptides. J. Drug Target. 2022;30:46–60. doi: 10.1080/1061186X.2021.1920026. [DOI] [PubMed] [Google Scholar]
- 171.Li C., Ma C., Wang F., Xil Z., Wang Z., Deng Y., Hel N. Preparation and biomedical applications of core-shell silica/magnetic nanoparticle composites. J. Nanosci. Nanotechnol. 2012;12:2964–2972. doi: 10.1166/jnn.2012.6428. [DOI] [PubMed] [Google Scholar]
- 172.Xiao D., Hu J.J., Zhu J.Y., Wang S.B., Zhuo R.X., Zhang X.Z. A redox-responsive mesoporous silica nanoparticle with a therapeutic peptide shell for tumor targeting synergistic therapy. Nanoscale. 2016;8:16702–16709. doi: 10.1039/c6nr04784j. [DOI] [PubMed] [Google Scholar]
- 173.Wang R., Ponsard B., Wolterbeek H., Denkova A. Core-shell structured gold nanoparticles as carrier for (166)Dy/(166)Ho in vivo generator. EJNMMI Radiopharm Chem. 2022;7:16. doi: 10.1186/s41181-022-00170-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mills A.M., Strzalka J., Bernat A., Rao Q., Hallinan D.T., Jr. Magnetic-core/gold-shell nanoparticles for the detection of hydrophobic chemical contaminants. Nanomaterials. 2022;12 doi: 10.3390/nano12081253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Li G., Chen Y., Zhang L., Zhang M., Li S., Li L., Wang T., Wang C. Facile approach to synthesize gold Nanorod@Polyacrylic acid/calcium phosphate yolk-shell nanoparticles for dual-mode imaging and pH/NIR-responsive drug delivery. Nano-Micro Lett. 2018;10:7. doi: 10.1007/s40820-017-0155-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dembski S., Milde M., Dyrba M., Schweizer S., Gellermann C., Klockenbring T. Effect of pH on the synthesis and properties of luminescent SiO2/calcium phosphate:Eu3+ core-shell nanoparticles. Langmuir. 2011;27:14025–14032. doi: 10.1021/la2021116. [DOI] [PubMed] [Google Scholar]
- 177.Lu B.Q., Zhu Y.J., Chen F., Qi C., Zhao X.Y., Zhao J. Core-shell hollow microspheres of magnetic iron oxide@amorphous calcium phosphate: synthesis using adenosine 5'-triphosphate and application in pH-responsive drug delivery. Chem. Asian J. 2014;9:2908–2914. doi: 10.1002/asia.201402319. [DOI] [PubMed] [Google Scholar]
- 178.Grainger D.W., Castner D.G. Nanobiomaterials and nanoanalysis: opportunities for improving the science to benefit biomedical technologies. Adv. Mater. 2008;20:867–877. [Google Scholar]
- 179.Epple M., Ganesan K., Heumann R., Klesing J., Kovtun A., Neumann S., Sokolova V. Application of calcium phosphatenanoparticles in biomedicine. J. Mater. Chem. 2010;20:18–23. [Google Scholar]
- 180.Gabizon A.A., Lyass O., Berry G.J., Wildgust M. Cardiac safety of pegylated liposomal doxorubicin (Doxil/Caelyx) demonstrated by endomyocardial biopsy in patients with advanced malignancies. Cancer Invest. 2004;22:663–669. doi: 10.1081/cnv-200032899. [DOI] [PubMed] [Google Scholar]
- 181.Heinemann V., Bosse D., Jehn U., Kahny B., Wachholz K., Debus A., Scholz P., Kolb H.J., Wilmanns W. Pharmacokinetics of liposomal amphotericin B (Ambisome) in critically ill patients. Antimicrob. Agents Chemother. 1997;41:1275–1280. doi: 10.1128/aac.41.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X., Hope M.J., Madden T.D., Mui B.L., Semple S.C., Tam Y.K., Ciufolini M., Witzigmann D., Kulkarni J.A., van der Meel R., Cullis P.R. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 183.Schlafer S., Ibsen C.J., Birkedal H., Nyvad B. Calcium-phosphate-osteopontin particles reduce biofilm formation and pH drops in in situ grown dental biofilms. Caries Res. 2017;51:26–33. doi: 10.1159/000451064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.