Abstract
Background
Categorization of biopsy specimens into inflammatory reaction patterns is central to dermatopathologic assessment. Mixed inflammatory patterns are poorly characterized and may represent clinicopathologic challenges. The purpose of this study was to identify clinical and histopathologic findings associated with the mixed spongiotic‐interface dermatitis (SID) histopathologic pattern.
Methods
Fifty‐one institutional biopsy specimens of SID were identified over a 2‐year period by retrospective natural language search. Histopathologic and clinical features were identified.
Results
The most common histopathologic features associated with SID were mild spongiosis (51%), focal vacuolar interface change (72%), lymphocytic exocytosis (92%), and superficial–dermal lymphocytic infiltrate (94%) with variable eosinophils (61%). Clinically, 80% of subjects presented with a symmetric morbilliform eruption. Polypharmacy (94%), immunosuppression (47%), and history of malignancy (47%) were common. The most common diagnoses were drug reaction (37%), possible drug reaction (12%), and viral exanthem (12%). Drug reaction with eosinophilia and systemic symptoms represented 25% of all confirmed cutaneous adverse drug reactions (CADR). Average time from drug initiation to symptom initiation was 20 days (SD: 22.3, range: 0–90); median disease duration was 25.5 days. Spongiotic vesicles and Langerhans cells were less common in patients with a strong clinicopathologic diagnosis of drug reaction compared to non‐drug eruptions (p = 0.04).
Conclusions
The mixed SID pattern is commonly encountered in CADR but may represent a more subacute course, implying consideration for inciting medication(s) started before the typical 7‐ to 14‐day window.
Keywords: dermatopathology, drug eruptions, drug reaction with eosinophilia and systemic symptoms, interface, maculopapular eruption, spongiotic
1. INTRODUCTION
Histopathologic examination of biopsy specimens is essential to the practice of clinical dermatology. For inflammatory dermatoses, categorization of biopsy specimen findings into reaction patterns (e.g., interface dermatitis, psoriasiform dermatitis) is an important step in the algorithmic diagnostic approach of inflammatory skin disease. While a predominant histopathologic pattern is typically apparent, the presence of multiple patterns within the same specimen is sometimes encountered. These “mixed” histopathologic patterns (e.g., spongiotic‐interface, psoriasiform‐lichenoid) may pose an interpretative challenge requiring careful clinic‐pathologic correlation. Ackerman et al 1 delineated specific microscopic patterns in their algorithmic approach to inflammatory skin disease. These algorithms include mention and classification of several overlap phenomena, such as a “psoriasiform‐lichenoid” pattern. However, Ackerman et al agreed that the threshold for classifying a true overlap pattern is not explicitly delineated and requires some subjective judgment by the reading dermatopathologist.
Spongiotic dermatitis is characterized by intercellular edema and the resulting distension of desmosomal connections between adjacent keratinocytes. It has well‐established associations with eczematous conditions, including atopic, nummular and allergic contact dermatitis 2 and eczematous drug reactions, such as those triggered by calcium channel blockers or thiazides. 3 Interface dermatitis is characterized by a cytotoxic T‐cell attack on epidermal keratinocytes resulting in basal vacuolization and apoptosis. Interface dermatitis has a wide differential, including drug reactions, erythema multiforme, graft‐vs‐host disease (GvHD), and connective tissue disease. 4 The mixed pattern of spongiotic‐interface dermatitis (SID) is encountered in daily practice; yet, its clinical and histopathologic associations remain poorly defined. Histopathologic diagnosis thus falls outside of the typical algorithmic approaches for inflammatory reaction patterns. There are some reports of drug eruption displaying SID 4 , 5 , 6 but overall the literature appears limited.
In our dermatopathology practice at a tertiary‐care academic center, cutaneous adverse drug reactions (CADR) are a common clinical consideration for submitted biopsy specimens. CADRs are common, occurring in up to 8% of hospitalized patients and representing 10%–20% of dermatology hospital consultations. 7 , 8 Because CADR may occur with any drug class and mimic other pathologies, confirming the diagnosis is often difficult. 9 Several algorithms for diagnosing CADR by clinical parameters exist, including the Jones algorithm, 10 the Naranjo algorithm, 11 the WHO‐UMC, 12 and a new quantitative algorithm. 13 However, inter‐algorithm disagreement still occurs. 14 Histopathologic examination of biopsy specimens may be helpful but, currently, no established criteria to definitively diagnose CADR by histopathology exist. 5 Suggestive histopathologic findings include vacuolar interface dermatitis with superficial perivascular inflammation, the presence of eosinophils or neutrophils, papillary dermal edema, and endothelial swelling of superficial dermal vessels. 1 , 7 , 9 , 15 , 16 , 17 The purpose of this study was to retrospectively analyze the biopsy specimens and medical charts of patients who underwent biopsies initially categorized as SID, with the goal of establishing both histopathologic and clinical associations with this mixed inflammatory pattern.
2. METHODS
This institutional review board‐approved, monocenter, retrospective study was designed to capture skin biopsies with a mixed SID histopathologic pattern and correlate clinical and histopathologic features.
2.1. Case selection and reporting
Candidate cases were selected from all biopsies performed at a large academic tertiary‐care center during the years 2018 and 2019 using a natural language search of our Laboratory Integration System (CoPath). Case inclusion required the words “spongiotic,” “spongiosis,” and “interface” together in either the diagnostic line, comments/notes, or microscopic description section of the final dermatopathology report. All qualifying cases were discussed at an internal institutional dermatopathology consensus conference, which included at least two dermatopathologists, and determined to have SID because they contained major histopathologic features of both interface and spongiotic reaction patterns. Cases with features of lichenoid dermatitis were excluded. Qualifying cases were also rereviewed by a board‐certified dermatopathologist (A.G. or D.M.), who confirmed 51 final cases. Cases were considered SID if most sections displayed both vacuolar interface change (with dyskeratotic cells if biopsy occurred later in the disease course) and spongiosis of at least the basal and lower spinous layers with a severity greater than the mild degree that typically accompanies interface dermatitis. Each case was assessed with consideration of biopsy site and timing within the disease course. We conducted a retrospective chart review of each case and recorded the histopathologic and clinical features listed below. The frequencies of these features were reported for the whole case population as well as by those receiving a final diagnosis of drug eruption versus non‐drug eruption so that a comparison could be established.
2.2. Histopathologic evaluation
Archival hematoxylin and eosin‐stained slides were used to evaluate 32 features from the stratum corneum (basket‐weave orthokeratosis; compact orthokeratosis; parakeratosis [at least focal retention of keratinocyte nuclei in the stratum corneum]; scale crust); epidermis (hyperplasia; region [basal and lower spinous layers only, basal and full spinous layers only, full epidermal thickness excluding stratum corneum], distribution [focal, continuous], and degree of spongiosis [mild = a degree of intercellular edema mildly greater than what would be typically expected for the interface change displayed within the specimen, moderate = moderate spongiotic change, severe = severe spongiotic change with vesicle or bullae formation]; spongiotic vesiculation; lymphocyte, eosinophil, and neutrophil exocytosis; Langerhans cells [>2 in focal aggregation]; mitotic figures [typically keratinocytes]; presence and distribution of dyskeratotic keratinocytes; distribution and degree of vacuolization of the dermoepidermal junction [DEJ]); dermis (papillary edema; fibrosis; papillary melanophages; erythrocyte extravasation; distribution and characterization of the dermal infiltrate, including presence of lymphocytes, histiocytes, neutrophils, and the number of eosinophils [counted linearly within the papillary dermis in four consecutive HPFs, which is equivalent to 1 mm2]; ectatic blood vessels with intravascular neutrophils or eosinophils). Features were counted as present regardless of distribution or severity, unless otherwise specified. Assessment of pathologic changes included consideration of biopsy anatomic site.
The histopathologic differential diagnoses were also extracted.
2.3. Clinical data
Medical record review was performed using an electronic medical record software. Longitudinal and updated clinical information for the cases of interest were collected through spring of 2020 to capture the full clinical course of each case. The following clinical data was documented: date and site of biopsy; timing of biopsy after symptom initiation; age, sex, and race; clinical description; total duration of rash; evidence of pruritus; dermatologic history; medication(s) initiated within 14 days of rash onset; total duration on new medication(s); duration between drug initiation and rash onset; concomitant systemic medications and illnesses; clinician's differential diagnosis; additional testing (e.g., blood test, patch test, allergy test, or immunofluorescence); treatment(s); clinician's final diagnosis; resolution occurrence; duration between discontinuation of medication(s) and rash resolution; and associated biopsies with their diagnoses.
2.4. Statistical analysis
Presence of clinical and histopathologic features were summarized using counts and rates. Numbers of drugs, time from drug initiation to symptoms, time from biopsy to final diagnosis, disease duration, and time from medication cessation to disease resolution were summarized using means and ranges. Rates of each feature were compared between drug and non‐drug eruptions using Fisher's exact test. Analyses were conducted using R version 4.1.1.
3. RESULTS
3.1. Histopathologic features
A total of 51 skin biopsy specimens from 51 unique patients were identified with the SID pattern. The histopathologic features are summarized in Table 1. The most common epidermal features were basket‐weave orthokeratosis (82% of cases), hyperplasia (65%), parakeratosis (37%), and compact orthokeratosis (20%). Spongiosis was typically mild (51%) or moderate (33%). Spongiotic vesicles were uncommon (14%) and found primarily in severe spongiosis cases, as expected. Epidermal lymphocyte exocytosis was nearly universal (92%) while epidermal neutrophils and eosinophils were rare. Individual necrotic keratinocytes were frequently present in the middle and lower layers but rare in the upper epidermis.
TABLE 1.
Histopathologic features of the mixed spongiotic‐interface reaction pattern
| Characteristic | No. (%) of all patients | No. (%) of diagnosed non‐drug eruptions n = 31 | No. (%) of diagnosed drug eruptions n = 20 | p Value |
|---|---|---|---|---|
| Basket‐weave orthokeratosis | 42 (82) | 26 (81) | 16 (84) | 1.00 |
| Compact orthokeratosis | 10 (20) | 5 (16) | 5 (26) | 0.47 |
| Parakeratosis | 19 (37) | 13 (41) | 6 (32) | 0.56 |
| Scale crust/serum | 5 (10) | 3 (9) | 2 (11) | 1.00 |
| Acanthosis (epidermal hyperplasia) | 33 (65) | 22 (69) | 11 (58) | 0.55 |
| Region of spongiosis | ||||
| Basal and lower spinous layer only | 16 (31) | 11 (34) | 5 (26) | 0.76 |
| Basal and full spinous layer | 11 (22) | 7 (22) | 4 (21) | 1.00 |
| Full epidermal involvement | 24 (47) | 14 (44) | 10 (53) | 0.57 |
| Degree of spongiosis a | ||||
| Mild | 26 (51) | 16 (50) | 10 (53) | 1.00 |
| Moderate | 17 (33) | 9 (29) | 8 (42) | 0.54 |
| Severe | 8 (16) | 7 (22) | 1 (5) | 0.12 |
| Distribution of spongiosis b | ||||
| Focal | 26 (51) | 15 (47) | 11 (58) | 0.57 |
| Continuous | 25 (49) | 17 (53) | 8 (42) | 0.57 |
| Spongiotic vesicles | 7 (14) | 7 (22) c | 0 (0) c | 0.04 c |
| Lymphocyte exocytosis | 47 (92) | 29 (91) | 18 (95) | 1.00 |
| Eosinophilic exocytosis | 1 (2) | 1 (3) | 0 (0) | 1.00 |
| Neutrophilic exocytosis | 13 (25) | 9 (28) | 4 (21) | 0.74 |
| Langerhans's cells present d | 31 (61) | 23 (72) c | 8 (42) c | 0.04 c |
| Mitoses present e | 26 (51) | 18 (56) | 8 (42) | 0.39 |
| Dyskeratotic keratinocytes f | 42 (82) | 24 (75) | 18 (95) | 0.13 |
| Basal layer only | 13 (25) | 6 (19) | 7 (37) | 0.19 |
| Basal and spinous layers only | 21 (41) | 13 (41) | 8 (42) | 1.00 |
| All layers up to granular layer (no inclusion of corneum) | 8 (16) | 5 (16) | 3 (16) | 1.00 |
| DEJ/interface vacuolization b | ||||
| Focal | 37 (72) | 20 (62) c | 17 (89) c | 0.05 c |
| Continuous | 14 (28) | 12 (38) c | 2 (11) c | 0.05 c |
| Papillary edema | 18 (35) | 13 (41) | 5 (26) | 0.37 |
| Papillary fibrosis | 11 (22) | 8 (25) | 3 (16) | 0.50 |
| Melanophages in papillary dermis | 17 (33) | 9 (28) | 8 (42) | 0.37 |
| Dermal extravasation of erythrocytes | 19 (37) | 14 (44) | 5 (26) | 0.25 |
| Distribution of dermal infiltrate | 51 (100) | 32 (100) | 19 (100) | 1.00 |
| Superficial dermis | 48 (94) | 30 (94) | 18 (95) | 1.00 |
| Superficial and mid dermis | 3 (6) | 2 (6) | 1 (5) | 1.00 |
| Superficial—deep dermis | 1 (2) | 1 (3) | 0 | 1.00 |
| Composition of infiltrate | ||||
| Lymphocytes present | 51 (100) | 32 (100) | 19 (100) | 1.00 |
| Histiocytes present | 38 (75) | 25 (78) | 13 (68) | 0.51 |
| Eosinophils present | 31 (61) | 21 (66) | 10 (53) | 0.39 |
| No. of eosinophils per mm2, mean (SD) g | 4.75 (8.57) | 5.16 (9.29) | 3.79 (7.41) | |
| Neutrophils present | 29 (57) | 18 (56) | 10 (53) | 1.00 |
| Both eosinophils and neutrophils | 17 (33) | 13 (41) | 4 (21) | 0.22 |
| Perivascular infiltrate | 50 (98) | 32 (100) | 18 (95) | 0.37 |
| Periadnexal infiltrate | 21 (41) | 13 (41) | 8 (42) | 1.00 |
| Ectatic blood vessels | 32 (63) | 17 (53) | 15 (79) | 0.08 |
| Containing neutrophils | 28 (55) | 18 (56) | 10 (53) | 1.00 |
| Containing eosinophils | 3 (6) | 2 (6) | 1 (5) | 1.00 |
| Solar elastosis | 38 (75) | 24 (75) | 14 (74) | 1.00 |
| Mild | 24 (47) | 16 (50) | 8 (42) | 0.77 |
| Moderate to severe | 14 (28) | 8 (25) | 6 (32) | 0.75 |
Note: Features are reported here as their frequencies within all sampled patients as well as within those receiving a final diagnosis of drug eruption (n = 20) and within those receiving non‐drug‐related final diagnoses (n = 31). Uncertain final diagnoses were included in the non‐drug‐related group. A comparison of the frequency of each feature between the drug‐diagnosis and non‐drug‐diagnosis group was compared by Fisher's exact test and reported by p value. Unless otherwise specified, characteristics were counted as present regardless of severity or distribution; however, judgment was adjusted for biopsy site.
Mild = mild intercellular edema with minimal secondary change; moderate = moderate intercellular edema with notable secondary change; severe = severe edema with prominent secondary change including vesicle and/or bullae formation.
Focal = spotty, non‐continuous involvement; continuous = present along most or all of the specimen sections.
Significant difference in feature presence between groups.
>2 in focal arrangement, including when within a vesicle.
Typically keratinocytes, scattered, counted if more than expected were present after adjusting for biopsy location.
Counted if more than expected were present after adjusting for biopsy location, scattered.
Counted linearly along the papillary dermis in four representative HPFs (equivalent to 1 mm2).
In the dermis, a primarily lymphocytic and superficial perivascular inflammatory infiltrate was universal. Eosinophils were present in 61% of cases, neutrophils in 57%, and histiocytes in 75%. Infiltrate was both neutrophilic and eosinophilic in 59% of cases. Importantly, there was no significant difference in the average number of eosinophils per mm2 between cases diagnosed as drug eruption versus non‐drug eruption. Less than half had periadnexal infiltrate. Dilation of the superficial vascular plexus occurred in over half of cases, 55% of which contained neutrophils and 6% contained eosinophils. Edema and fibrosis of the papillary dermis were infrequent findings at 35% and 22%, respectively. Representative images of SID histopathologic patterns are presented in Figure 1.
FIGURE 1.
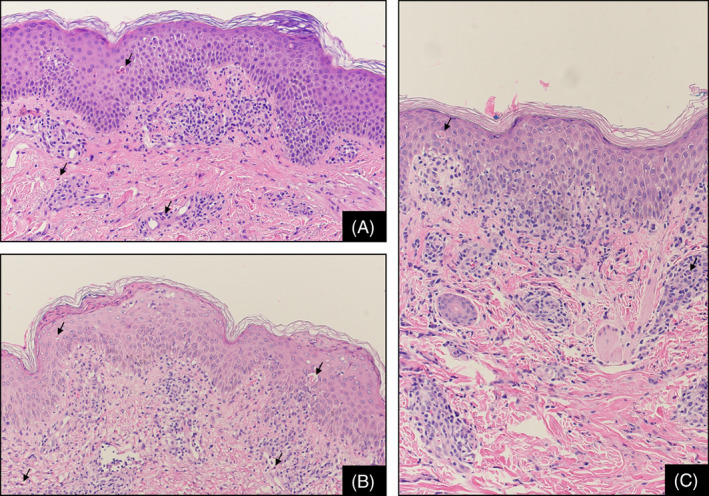
Representative examples of spongiotic‐interface dermatitis with characteristic histopathologic features, stained with H&E, at original magnification ×100. (A) This case presented with acanthosis, mild spongiosis and subtle interface changes; dyskeratotic keratinocytes (arrow in epidermis) are noted within the spinous layer; a moderate perivascular lymphocytic infiltrate with rare eosinophils (arrows in dermis) is noted. (B) Here, there is scattered parakeratosis above epidermal hyperplasia with mild spongiosis, interface changes and dyskeratotic keratinocytes (arrows in epidermis); the inflammatory infiltrate contains sparse eosinophils (arrows in dermis). (C) In this case acanthosis is mild with mild spongiosis, interface changes with a dyskeratotic keratinocyte (arrow in the spinous layer), lymphocytic exocytosis, and a mild perivascular lymphocytic inflammatory infiltrate with rare eosinophils and neutrophils (arrow in dermis)
The average number of diagnoses offered in the histopathologic differential was 2.5 (range: 1–4). Drug reaction (98%), viral exanthem (47%), eczematous process (35%), and GVHD (24%) were commonly considered (Table S1).
Histopathologic features of the 20 confirmed CADR cases are reported as a separate column in Table 1. Few significant histopathologic differences between CADR and non‐CADR samples were identified. Spongiotic vesicles and epidermal Langerhans cells were found more frequently in non‐drug eruption samples (p = 0.04 for both). Drug eruption was associated with focal DEJ vacuolization while continuous vacuolization was associated with non‐drug eruption.
3.2. Clinical features
Clinical features are shown in Table 2. Average patient age at biopsy was 53 years (range: 1–83). The female/male ratio was 1:1. Sixty‐five percent of the specimens were collected in the outpatient setting. Forty‐five percent reported a history of drug allergy. Polypharmacy was common, and 94% of patients were on chronic medications. Forty‐one of the 51 patients (80%) had started at least one “new” drug, the average being 4.5 (range: 1–28), defined as initiated within 2 weeks of cutaneous symptoms onset. Antibiotics were the most common (58%).
TABLE 2.
Clinical features of the mixed spongiotic‐interface reaction pattern
| Feature | No. (%) of all subjects n = 51 | No. (%) of diagnosed non‐drug eruptions n = 31 | No. (%) of diagnosed drug eruptions n = 20 | p value |
|---|---|---|---|---|
| Specimen collection setting | ||||
| Inpatient | 18 (35) | 10 (32%) | 8 (40%) | 0.76 |
| Outpatient | 33 (65) | 21 (68%) | 12 (60%) | 0.76 |
| Rash distribution | ||||
| Face/neck | 19 (37) | 11 (34) | 8 (42) | 0.77 |
| Trunk | 25 (49) | 10 (31) a | 15 (79) a | 0.0001 a |
| Upper extremities | 28 (55) | 12 (38) a | 16 (84) a | 0.0002 a |
| Lower extremities | 24 (47) | 11 (34) a | 13 (68) a | 0.002 a |
| Hand/feet | 7 (14) | 7 (22) a | 0 (0) a | 0.04 a |
| Symmetric | 45 (88) | 27 (84) | 18 (95) | 0.39 |
| Clinical rash pattern | ||||
| Maculopapular | 27 (53) | 10 (31) a | 17 (89) a | 0.0001 a |
| Eczematous/dry | 16 (31) | 14 (44) a | 2 (11) a | 0.02 a |
| Vesicular | 3 (6) | 2 (6) | 1 (5) | 1.00 |
| Targetoid | 1 (2) | 1 (3) | 0 (0) | 1.00 |
| Urticarial | 2 (4) | 2 (6) | 0 (0) | 0.52 |
| Multiple patterns | 5 (10) | 4 (12) | 1 (5) | 0.64 |
| Pruritis | 31 (61) | 17 (53) | 14 (74) | 0.24 |
| Medical history | ||||
| Pets or fleas | 15 (30) | 13 (41) a | 2 (11) a | 0.03 a |
| Eczema | 6 (12) | 6 (19) | 0 (0) | 0.07 |
| Urticaria | 3 (6) | 2 (6) | 1 (5) | 1.00 |
| Psoriasis | 2 (4) | 1 (3) | 1 (5) | 1.00 |
| Drug allergies | 23 (45) | 16 (50) | 7 (37) | 0.40 |
| Hematologic malignancy | 16 (31) | 10 (31) | 6 (32) | 1.00 |
| Solid organ malignancy | 8 (16) | 4 (12) | 4 (21) | 0.45 |
| Bone marrow transplant | 13 (25) | 7 (22) | 6 (32) | 0.51 |
| GvHD | 5 (10) | 3 (9) | 2 (11) | 1.00 |
| Organ transplant | 3 (6) | 2 (6) | 1 (5) | 1.00 |
| Immunocompromised | 22 (43) | 12 (38) | 10 (53) | 0.38 |
| Sepsis at time of rash | 5 (10) | 2 (6) | 3 (16) | 0.35 |
| Autoimmune disease | 4 (8) | 3 (9) | 1 (5) | 1.00 |
| On new medication | 41 (80) | 22 (69) a | 19 (100) a | 0.008 a |
| New antibiotic | 24 (47) | 14 (44) | 10 (53) | 0.57 |
| New chemotherapeutic | 6 (12) | 5 (16) | 1 (5) | 0.39 |
| Duration from drug initiation to symptom development | ||||
| Before 2 weeks | 19 (37) | 7 (22) a | 12 (63) a | 0.006 a |
| After 2 weeks | 19 (37) | 13 (41) | 6 (32) | 0.56 |
| Unclear or N/A | 13 (25) | 12 (38) a | 1 (5) a | 0.02 a |
Note: Features are reported here as their frequencies within all sampled patients as well as within those receiving a final diagnosis of drug eruption (n = 19) and within those receiving non‐drug‐related final diagnoses (n = 31). Uncertain final diagnoses were included in the non‐drug‐related group. A comparison of the frequency of each features between the drug‐diagnosis and non‐drug‐diagnosis group was compared by Fisher's exact test and reported by p value.
Difference in feature presence between groups.
Many patients had extensive medical histories. Nearly half (47%) were immunodeficient. Another 47% had a current or previous malignancy, including eight patients with lymphoma, seven with acute myeloblastic leukemia, one with acute lymphoblastic leukemia, and eight of various solid organs. Thirteen patients had a history of bone marrow transplant, ten of which presented for biopsy within 90 days of transplant. Three patients had previous solid‐organ transplants. Eight (16%) had autoimmune disease.
The most common clinical features were symmetric distribution (88%) and pruritus (61%). Involvement occurred similarly over the upper extremity (55%), trunk (49%), and lower extremity (47%). Face/neck (37%) and hands/feet (14%) involvement was less common. Drug reaction was the final diagnosis in 20 of 51 patients (37%), 5 of which had drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome (Table S1). Drug eruptions were significantly more likely to be distributed over the trunk and chest (p = 0.001), upper extremities (p = 0.002) and lower extremities (p = 0.02), and less likely to occur on the hands and feet (p = 0.04) compared to non‐drug eruptions. Clinical lesion descriptions were typically maculopapular/morbilliform (80%), eczematous (20%), vesicular (4%), urticarial (4%), and targetoid (1%). Six patients (12%) presented with two simultaneous rash patterns. Drug reactions were significantly more likely to present as morbilliform eruptions (p = 0.0001) and less likely than non‐drug eruptions to present as eczematous (p = 0.02).
The clinical differential diagnoses (up to three per case, n = 105) from the submitting dermatologists were wide (Table S2). Drug reaction (61%), eczema (23%), and GvHD (20%) were suspected most often. Among the 31 patients with clinically suspected drug reaction, the average number of suspected potential causative drugs, obtained directly from clinician notes, per patient was 1.9 (range: 1–5). The identities of suspected drugs were available for 18 of these patients. The most common was vancomycin (22%). Pantoprazole, lisinopril, allopurinol, cefepime, and penicillin were each suspected twice (11% each). Symptoms began an average of 20 days after suspected drug initiation (range: 0–90 days).
A definitive final diagnosis could not be made in 13 cases. Drug reaction was the favored or equally likely diagnosis in six of these cases and could not be ruled out in an additional three. The most common set of indistinguishable conditions was drug reaction versus viral exanthem, which occurred in six patients. After drug reaction, viral exanthem (12%) and eczema (10%) were the next most common final diagnoses. Of the 51 patients, two were given coincident diagnoses of viral exanthem and drug eruption. Final diagnoses were not available for four patients (8%).
Most patients had clinical disease for less than a year with an average duration of 55 days (range: 6–210 days). Five patients (10%) had disease duration of over a year. For CADR cases, the average time from medication cessation to disease resolution was 45 days (range: 2–365 days).
4. DISCUSSION
We examined the clinical and histopathologic features of 51 cases displaying a combined spongiotic‐interface histopathologic pattern. Drug eruption was clinically suspected in 30 cases (49%) and later confirmed in 20. Five were DRESS syndrome. Interestingly, many specimens—regardless of final diagnosis—displayed typical features of CADR found in the literature. 16 The presence of vacuolar interface changes with superficial perivascular and interstitial infiltrate composed of neutrophils and/or eosinophils is strongly associated with drug eruption. 16 While less common than interface patterning, at least some degree of spongiosis is common among drug eruptions, even when it is not the predominant pattern. 16 Some have suggested CADR should be considered in cases containing multiple histopathologic patterns, such as SID, within a single tissue section. 15 , 16
Other studies have also identified SID on biopsies of morbilliform drug eruption. 5 , 17 Similar to our findings, spongiosis is typically mild to moderate. One suggested that severe spongiosis or spongiotic vesiculation could help differentiate spongiotic‐interface CADR from other spongiotic dermatoses, such as contact dermatitis and pityriasis rosea. 5 Our study also supports these findings.
GvHD and viral exanthem are challenging to distinguish from CADR because all three characteristically show superficial vacuolar interface changes with superficial infiltrate. 18 While eosinophilic infiltrate tends to favor a drug‐induced cause, it is not specific. 2 , 19 A quantitative study proposed that 16 eosinophils per 10 HPF can distinguish CADR from GvHD with 100% significance. Lower numbers may be sufficient (e.g., 3.6 eosinophils/10 HPF provides 92.9% specificity) 20 but there is no established consensus on this threshold number. 19 In our study, no differences were found in the presence or density of eosinophils between the CADR and non‐drug eruption diagnosis groups. Differentiating between GvHD and CADR was challenging because 10 patients (20%) had undergone BMT within 90 days of presentation. Because GvHD can present with a wide variety of histopathologic patterns, including psoriasiform, interface, and eczematous, clinical‐histopathologic correlation is paramount. 21 , 22
Few significant differences were found between the clinical and histopathologic features of CADR and non‐CADR cases. Spongiotic vesicles and intraepidermal Langerhans cells were both more common in non‐drug eruptions (p = 0.04). Focal interface change was more common in drug eruption, on the other hand, continuous interface change was more common in non‐drug eruption (Table 1). Clinical differences included rash distribution, clinical pattern, history of pet or flea exposure, and whether a new medication was started (Table 2). Antibiotics, specifically beta‐lactams (50%) and vancomycin (33%) were the most commonly implicated drugs and are a common CADR trigger. However, this may be a result of frequent prescription.
The spongiotic reaction pattern in some of our SID samples appeared subacute in nature, even among those with a final diagnosis of CADR, in which an acute reaction pattern would be expected. 16 Subacute histopathologic findings were present regardless of when in the disease course biopsy was collected. Subacute‐type changes included spongiosis confined to the lower epidermis with infrequent vesiculation, epidermal hyperplasia (65%), parakeratosis (37%), and presence of papillary dermal melanophages (33%). Clinical findings retrieved from longitudinal follow‐up were also subacute: drug initiation to rash onset was longer than typical for morbilliform CADRs, averaging 20 days (SD: 22.3, 0–90) or 18 days (SD: 22, 0–90) if DRESS patients, who characteristically have longer onset latency, were excluded. The median disease duration was also prolonged at 25.5 days (mean = 290 days, SD = 991) or 24 days if the five subjects with symptoms persisting over 1 year were excluded (SD = 51; median = 25 days). Excluding DRESS subjects increased the average duration to 38 days (SD = 45; median = 24 days). These findings are consistent with the Naim et al 5 study of morbilliform drug eruption, in which a combined spongiotic‐interface pattern with epidermal hyperplasia was present in all biopsies of patients with “long‐standing” eruptions, taken 2–8 weeks after symptom initiation.
Histopathologically similar cases were more likely to receive a diagnosis of CADR if a new drug had been initiated within 2 weeks of presentation (p = 0.006). Cases presenting over 2 weeks after initiation were equally likely to receive a CADR or non‐CADR diagnosis. This discrepancy, despite otherwise similar histopathologic and clinical features, may suggest an underdiagnosis of chronic spongiotic‐interface drug reaction and an overreliance on rash‐initiation timing during diagnosis.
Risk factors common to SID cases and typical CADR include high medication number, use of immunosuppressive agents, coincident infection, systemic autoimmune disease, and high number of secondary diagnoses. 23 , 24 , 25 , 26 , 27 Of note, the prevalence of immunosuppression, malignancy, and autoimmune disease in our patient population was quite high (47%, 47%, and 18%, respectively). While our case population was drawn exclusively from a single academic tertiary‐care facility, and thus likely overrepresents more complex and hospitalized patients, these numbers are still notably high, even compared to other studies of CADRs. 28 , 29 , 30 The immune dysregulation seen in these conditions is believed to be a major contributor to CADR development. 15 Determining specific diagnostic criteria for drug eruption is especially important for these patients, as CADR may present similarly to other serious conditions requiring specific management, including paraneoplastic syndromes, autoimmune diseases, GvHD, and infections. 24 , 26 , 31 , 32 Confirming our findings that these comorbidities are overrepresented in patients with SID may require a larger study.
5. CONCLUSION
The presence of multiple histopathologic patterns on a single tissue section—such as the spongiotic‐interface pattern discussed here—is a common yet often ambiguous finding in dermatopathology. In our retrospective analysis of 51 cases, we found that the SID pattern is characterized by mild to moderate spongiosis with typically focal vacuolar interface change. We also identified common ancillary histopathologic features, including lymphocytic intraepidermal infiltrate and superficial–dermal lymphocytic infiltrate with variable eosinophils. Clinically, SID occurred disproportionately in patients diagnosed with drug reaction, 25% of whom had DRESS syndrome. This leads us to suggest that SID should prompt consideration of CADR, especially in patients with immunosuppression. However, CADR and DRESS syndrome diagnoses rely on clinical parameters and cannot be made on histopathologic grounds alone. The histopathologic and clinical similarities between SID subjects receiving a CADR diagnosis compared to those who did not suggest that this pattern of CADR may be underrecognized. Furthermore, our data suggest that the SID pattern of CADR may be associated with a more acute to subacute disease course, implying that consideration for the inciting medication(s) should include agents started before the typical 7‐ to 14‐day window of CADR.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Appendix S1 Supporting information
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health's National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health's National Center for Advancing Translational Sciences.
Ernst M, Lundgren M, Evans MD, Miller D, Giubellino A. The mixed spongiotic and interface reaction pattern: A study of clinical and histopathologic findings. J Cutan Pathol. 2022;49(12):1051‐1059. doi: 10.1111/cup.14306
Daniel Miller and Alessio Giubellino are co‐senior authors.
Funding information National Institutes of Health's National Center for Advancing Translational Sciences, Grant/Award Number: UL1TR002494
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Ackerman A, Boer A, Bennin B. Histologic Diagnosis of Inflammatory Skin Diseases: An Algorithmic Method Based on Pattern Analysis. 3rd ed. Ardor Scribendi; 2005. [Google Scholar]
- 2. Billings S, Cotton J. Inflammatory Dermatopathology: A Pathologist's Survival Guide. Springer; 2011. [Google Scholar]
- 3. Summers EM, Bingham CS, Dahle KW, Sweeney C, Ying J, Sontheimer RD. Chronic eczematous eruptions in the aging: further support for an association with exposure to calcium channel blockers. JAMA Dermatol. 2013;149(7):814‐818. doi: 10.1001/jamadermatol.2013.511 [DOI] [PubMed] [Google Scholar]
- 4. LeBoit PE. Interface dermatitis. How specific are its histopathologic features? Arch Dermatol. 1993;129(10):1324‐1328. doi: 10.1001/archderm.129.10.1324 [DOI] [PubMed] [Google Scholar]
- 5. Naim M, Weyers W, Metze D. Histopathologic features of exanthematous drug eruptions of the macular and papular type. Am J Dermatopathol. 2011;33(7):695‐704. doi: 10.1097/DAD.0b013e31820a285d [DOI] [PubMed] [Google Scholar]
- 6. Ortonne N, Valeyrie‐Allanore L, Bastuji‐Garin S, et al. Histopathology of drug rash with eosinophilia and systemic symptoms syndrome: a morphological and phenotypical study. Br J Dermatol. 2015;173(1):50‐58. doi: 10.1111/bjd.13683 [DOI] [PubMed] [Google Scholar]
- 7. Valeyrie‐Allanore L, Obeid G, Revuz J. Drug reactions. In: Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:348‐375. [Google Scholar]
- 8. Kroshinsky D, Cotliar J, Hughey LC, Shinkai K, Fox LP. Association of dermatology consultation with accuracy of cutaneous disorder diagnoses in hospitalized patients: a multicenter analysis. JAMA Dermatol. 2016;152(4):477‐480. doi: 10.1001/jamadermatol.2015.5098 [DOI] [PubMed] [Google Scholar]
- 9. Patterson JW. Cutaneous drug reactions. In: Patterson JW, ed. Weedon's Skin Pathology. 5th ed. Elsevier; 2021:629‐645. [Google Scholar]
- 10. Jones JK. Adverse drug reactions in the community health setting: approaches to recognizing, counseling, and reporting. Fam Community Health. 1982;5(2):58‐67. [DOI] [PubMed] [Google Scholar]
- 11. Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239‐245. [DOI] [PubMed] [Google Scholar]
- 12. Koh Y, Li SC. A new algorithm to identify the causality of adverse drug reactions. Drug Saf. 2005;28(12):1159‐1161. doi: 10.2165/00002018-200528120-00010 [DOI] [PubMed] [Google Scholar]
- 13. Koh Y, Yap CW, Li SC. A quantitative approach of using genetic algorithm in designing a probability scoring system of an adverse drug reaction assessment system. Int J Med Inform. 2008;77(6):421‐430. [DOI] [PubMed] [Google Scholar]
- 14. Doherty MJ. Algorithms for assessing the probability of an adverse drug reaction. Respir Med CME. 2009;2(2):63‐67. doi: 10.1016/j.rmedc.2009.01.004 [DOI] [Google Scholar]
- 15. Justiniano H, Berlingeri‐Ramos AC, Sánchez JL. Pattern analysis of drug‐induced skin diseases. Am J Dermatopathol. 2008;30(4):352‐369. doi: 10.1097/DAD.0b013e3181722ef4 [DOI] [PubMed] [Google Scholar]
- 16. Weyers W, Metze D. Histopathology of drug eruptions—general criteria, common patterns, and differential diagnosis. Dermatol Pract Concept. 2011;1(1):33‐47. doi: 10.5826/dpc.0101a09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gerson D, Sriganeshan V, Alexis JB. Cutaneous drug eruptions: a 5‐year experience. J Am Acad Dermatol. 2008;59(6):995‐999. doi: 10.1016/j.jaad.2008.09.015 [DOI] [PubMed] [Google Scholar]
- 18. Marra DE, McKee PH, Nghiem P. Tissue eosinophils and the perils of using skin biopsy specimens to distinguish between drug hypersensitivity and cutaneous graft‐versus‐host disease. J Am Acad Dermatol. 2004;51(4):543‐546. doi: 10.1016/j.jaad.2004.02.019 [DOI] [PubMed] [Google Scholar]
- 19. Nghiem P. The “drug vs graft‐vs‐host disease” conundrum gets tougher, but there is an answer: the challenge to dermatologists. Arch Dermatol. 2001;137(1):75‐76. doi: 10.1001/archderm.137.1.75 [DOI] [PubMed] [Google Scholar]
- 20. Weaver J, Bergfeld WF. Quantitative analysis of eosinophils in acute graft‐versus‐host disease compared with drug hypersensitivity reactions. Am J Dermatopathol. 2010;32(1):31‐34. doi: 10.1097/DAD.0b013e3181a85293 [DOI] [PubMed] [Google Scholar]
- 21. Vernali S, Vertes George E, Gonzalez Santiago T, Motaparthi K. Psoriasiform graft‐versus‐host disease: report and brief review of the literature. Am J Dermatopathol. 2018;40(7):511‐514. doi: 10.1097/dad.0000000000001078 [DOI] [PubMed] [Google Scholar]
- 22. Creamer D, Martyn‐Simmons CL, Osborne G, et al. Eczematoid graft‐vs‐host disease: a novel form of chronic cutaneous graft‐vs‐host disease and its response to psoralen–UV‐A therapy. Arch Dermatol. 2007;143(9):1157‐1162. doi: 10.1001/archderm.143.9.1157 [DOI] [PubMed] [Google Scholar]
- 23. Renn CN, Straff W, Dorfmüller A, Al‐Masaoudi T, Merk HF, Sachs B. Amoxicillin‐induced exanthema in young adults with infectious mononucleosis: demonstration of drug‐specific lymphocyte reactivity. Br J Dermatol. 2002;147(6):1166‐1170. doi: 10.1046/j.1365-2133.2002.05021.x [DOI] [PubMed] [Google Scholar]
- 24. Roudier C, Caumes E, Rogeaux O, Bricaire F, Gentilini M. Adverse cutaneous reactions to trimethoprim‐sulfamethoxazole in patients with the acquired immunodeficiency syndrome and pneumocystis carinii pneumonia. Arch Dermatol. 1994;130(11):1383‐1386. [PubMed] [Google Scholar]
- 25. Dunagin WG, Milikan LE. Drug eruptions. Med Clin North Am. 1980;64(5):983‐1003. doi: 10.1016/s0025-7125(16)31578-4 [DOI] [PubMed] [Google Scholar]
- 26. Mitsuyasu R, Groopman J, Volberding P. Cutaneous reaction to trimethoprim‐sulfamethoxazole in patients with AIDS and Kaposi's sarcoma. N Engl J Med. 1983;308(25):1535‐1536. doi: 10.1056/NEJM198306233082512 [DOI] [PubMed] [Google Scholar]
- 27. Liao P, Shih C, Mao C, Deng S, Hsieh M, Hsu K. The cutaneous adverse drug reactions: risk factors, prognosis and economic impacts. Int J Clin Pract. 2013;67(6):576‐584. doi: 10.1111/ijcp.12097 [DOI] [PubMed] [Google Scholar]
- 28. Machoń N, Lewandowska J, Zdanowska N, Placek W, Owczarczyk‐Saczonek A. Cutaneous adverse drug reactions (CADRs)—statistical analysis of the causal relationship between the drug, comorbidities, cofactors, and the cutaneous reaction—a single‐centered study. Int J Environ Res Public Health. 2022;19(13):7982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liao P‐J, Mao C‐T, Chen T‐L, Deng S‐T, Hsu K‐H. Factors associated with adverse drug reaction occurrence and prognosis, and their economic impacts in older inpatients in Taiwan: a nested case–control study. BMJ Open. 2019;9(5):e026771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Woo S‐D, Yoon J, Doo G‐E, et al. Common causes and characteristics of adverse drug reactions in older adults: a retrospective study. BMC Pharmacol Toxicol. 2020;21:87. doi: 10.1186/s40360-020-00464-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. da Silva JA, de Carvalho Mesquita K, de Souza Machado Igreja AC, et al. Paraneoplastic cutaneous manifestations: concepts and updates. An Bras Dermatol. 2013;88(1):9‐22. doi: 10.1590/s0365-05962013000100001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ely JW, Stone MS. The generalized rash: part I. differential diagnosis. Am Fam Physician. 2010;81(6):726‐734. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supporting information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


