Abstract
In this study, the role of the hepatobiliary system in the early pathogenesis of Salmonella enteritidis infection was investigated in a rat model. Intravenous (i.v.) challenge with lipopolysaccharide (LPS) has previously been shown to enhance the translocation of normal gut flora. We first confirmed that LPS can similarly promote the invasion of S. enteritidis. Oral infection of outbred Australian Albino Wistar rats with 106 to 107 CFU of S. enteritidis led to widespread tissue invasion after days. If animals were similarly challenged after intravenous administration of S. enteritidis LPS (3 to 900 μg/kg of body weight), significant invasion of the livers and mesenteric lymph nodes (MLN) occurred within 24 h, with invasion of the liver increasing in a dose-dependent fashion (P < 0.01). If bile was prevented from reaching the intestine by bile duct ligation or cannulation, bacterial invasion of the liver and MLN was almost totally abrogated (P < 0.001). As i.v. challenge with LPS could induce the delivery of inflammatory mediators into the bile, biliary tumor necrosis factor alpha (TNF-α) concentrations were measured by bioassay. Biliary concentrations of TNF-α rose shortly after LPS challenge, peaked with a mean concentration of 27.0 ng/ml at around 1 h postchallenge, and returned to baseline levels (3.1 ng/ml) after 2.5 h. Although TNF-α cannot be directly implicated in the invasion process, we conclude that the invasiveness of the enteric pathogen S. enteritidis is enhanced by the presence of LPS in the blood and that this enhanced invasion is at least in part a consequence of the delivery of inflammatory mediators to the gastrointestinal tract by the hepatobiliary system.
Cell wall lipopolysaccharide (LPS) or endotoxin is frequently cited as a critical factor in the pathogenesis of infections with gram-negative bacteria. LPS is now accepted to be a potent inducer of a series of inflammatory mediators whose activities may explain much of the symptomatology of these infections (15, 48). Typhoid fever is a case in point where infection with Salmonella enteritidis serovar Typhi results in the symptoms of fever, cachexia and diarrhea. In the early phase of the infection, these symptoms can be ascribed in part to the inflammatory cytokines tumor necrosis factor alpha (TNF-α) (35, 39), interleukin-1 (IL-1) (1, 8), and IL-6 (14).
Less certainty surrounds the initiating phase of the infection. Infectious diseases are often characterized by incubation periods, which vary widely between individuals. It is recognized that the incubation period in typhoid fever, for example, may be as short as 3 days or extend to 56 days (17, 31). Several mechanisms which might influence the infectivity of the organism and thus the incubation period of the disease have been proposed. Most important of these are the dosage of the organism, their virulence, and the immune status of the host (18). Immunological factors include both innate mechanisms, such as colonization resistance offered by the microbial flora of the gut (42), and specific defenses of the adaptive immune system (27, 29).
Entry of the invasive salmonellae into tissues is considered to occur by organisms colonizing the intestinal epithelium and Peyer's patches (12), passing through into the submucosa and arriving at the mesenteric lymph nodes (MLN) via the draining lymphatic vessels (38, 47). From this point, the blood may be seeded and target organs such as the liver and spleen may be colonized (2, 19). Any disruption to the integrity of the epithelium may offer an opportunity for early entry by the organisms. Circulatory LPS is known to cause loss of intestinal epithelial integrity (24, 32) and has been shown to induce translocation of normal gut flora (4, 5). It is therefore likely that such LPS can similarly influence the invasiveness of gut pathogen such as invasive salmonellae.
The liver is the usual site of LPS clearance (10) and is an important site of production of cytokines (25, 33, 44) and other mediators of inflammation (46). Such mediators have been detected in bile (37, 40), and we have previously described a role for the hepatobiliary system in models of gastrointestinal inflammation (3). We therefore hypothesized that systemic LPS may lead to changes in biliary factors that could subsequently affect the integrity of the epithelial barrier, leading to enhanced bacterial invasion. A rat model of S. enteritidis infection was established to address these issues, and the results presented here provide strong evidence that the enhancement of invasion that follows systemic exposure to LPS is dependent on the integrity of the hepatobiliary system.
MATERIALS AND METHODS
Animals.
Outbred conventionally housed 9- to 11-week-old male Australian Albino Wistar rats, from the colony at the School of Microbiology and Immunology, University of New South Wales, were used in all experiments and were fed ad libitum with a commercial rodent diet (Allied Feeds Pty. Ltd., Sydney, Australia). All experiments were approved by the Animal Care and Ethics Committee of the University of New South Wales.
Bacterial strain.
An invasive strain of S. enteritidis serovar Danyz was obtained from the culture collection of the School of Microbiology and Immunology, University of New South Wales, and was maintained on nutrient agar slopes. Suspensions for inoculation were prepared by growing organisms for 6 h in nutrient broth at 37°C. The concentration of organisms was then determined by spectrophotometer, and appropriate dilutions were prepared in Ringer's solution. The numbers of bacteria in the inocula were subsequently confirmed by plating onto nutrient agar.
Preparation of LPS.
S. enteritidis LPS was prepared by the phenol-water extraction procedure of Westphal and Jann (45) from organisms cultured for 48 h on nutrient agar in Roux bottles. The extracted LPS was lyophilized for storage. Phenol-water-extracted Escherichia coli O111:B4 LPS was purchased from Sigma Chemical Company (St. Louis, Mo.).
Inoculation of animals.
Animals were lightly anesthetized with diethyl ether (BDH, Kilsyth, Victoria, Australia) and then inoculated orogastrically with 1.0 ml of a 10% (wt/vol) solution of sodium bicarbonate, followed by 1.0 ml of the appropriate dose of bacteria. Doses of LPS in 0.5 ml of endotoxin-free saline were injected intravenously (i.v.) into a tail vein.
Surgical procedures.
Cannulation of the rat bile duct was carried out under ether anesthesia by the method of Lambert (22). Briefly, the abdomen was shaved, and the cavity was opened through a midline incision of 1.5 to 2 cm starting immediately below the sternum. The duodenum and the bile duct were located, and then approximately 1 to 2 cm of a 30-cm polyethylene cannulation tube (outer diameter, 0.61 mm; inner diameter, 0.28 mm; Dural Plastics, Sydney, New South Wales, Australia) was inserted into the bile duct and tied in place, allowing bile to flow freely. The other end of the tube was exteriorized through an opening in the right flank by means of an 18-gauge needle. The duodenal loop was then returned to the abdominal cavity, and the wound was closed. After recovery from anesthesia, the rats were placed in restraining cages to facilitate collection of bile. Bile samples were collected in chilled Eppendorf tubes and stored at −20°C for later analysis. Animals were provided with water ad libitum during the sample collection period.
For bile duct ligation, rats were prepared and the duodenum visualized. The bile duct was then carefully occluded by using 3/0 linen suture thread to prevent the flow of bile to the intestine. A sham operation involved the preparation of animals exactly as described above and then manipulation of the viscera around the bile duct for 5 min before the abdomen was closed.
Collection of tissues and bacterial enumeration.
The intra-abdominal structures of the rats were fully exposed under anesthesia, and the animals were killed by exsanguination, drawing blood either from the abdominal aorta or the inferior vena cava. Livers, spleens, and MLN were aseptically excised and placed in preweighed containers with 9 ml of sterile nutrient broth (Oxoid, Basingstoke, United Kingdom). Tissues were homogenized in an Ultra-Turrax machine (Janke and Kunkel, Staufen, Germany). Tenfold dilutions of homogenates and undiluted samples of blood were directly plated onto MacConkey agar (Oxoid) in duplicate and incubated aerobically for 24 to 48 h at 37°C. Isolated organisms were identified by Gram stain and slide agglutination with Salmonella agglutinating serum (Wellcome Diagnostics, Greenford, United Kingdom). Lactose-fermenting organisms were designated as such by the color of colonies on MacConkey agar. Numbers of bacteria were calculated and expressed as the mean number of CFU per gram of tissue.
Measurement of TNF-α.
Bile samples were collected from 15 rats challenged with LPS (1 mg/kg of body weight). Aliquots of bile were collected over a 3-h period, being saved from each 15-min period over the first hour and for 15 min every 30 min over the subsequent 2 h. TNF-α in bile samples was assayed by inhibition of the proliferation of WEHI 164 (clone 13) cells by the method of Espevik and Nissen-Meyer (9). Cell proliferation was measured by the metabolism of the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma). Recombinant murine TNF-α (Genzyme, Cambridge, Mass.) was used to generate a standard curve, and all samples were assayed in triplicate. Controls included samples incubated in the presence of neutralizing rabbit anti-mouse TNF-α serum (Genzyme), which has been shown to cross-react with rat TNF-α (28).
Statistical analysis.
Statistical analysis of data was by analysis of variance (ANOVA) using Statview 4.0 software (Abacus Concepts, Berkeley, Calif.). Significance was accepted at the 5% level.
RESULTS
Infection model.
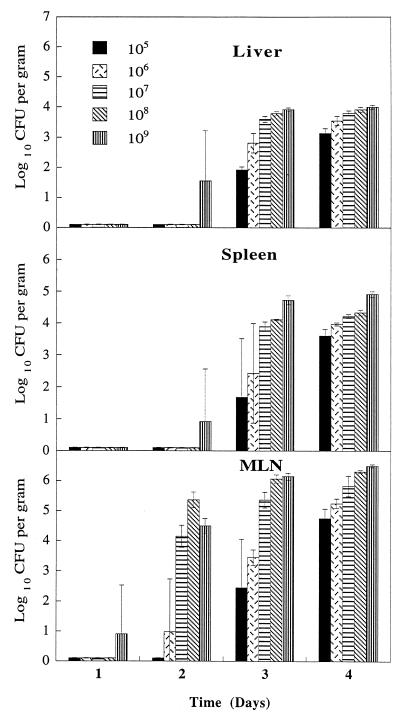
Groups of four rats were fed orogastrically with a range of doses (105 to 109 CFU) of S. enteritidis and examined over the period of a week for evidence of bacterial invasion of tissues. Bacterial cultures from liver, spleen, MLN, and blood samples were performed at days 1, 2, 3, 4, 5, and 7 postinfection. No organisms were isolated from blood samples collected at any stage of the experiment, but Fig. 1 shows that by day 4, all animals showed invasion of the liver, spleens, and MLN. Invasion of the MLN was seen from day 2 and of the spleen from day 3 at higher challenge doses, while invasion of the liver was seen over the whole dose range from day 3. From days 4 to 7, the number of organisms isolated from the various organs increased slowly but steadily (data not shown). By day 7, animals given the highest dose of S. enteritidis showed evidence of serious illness manifested by reduced intake of food, loss of weight, and diarrhea.
FIG. 1.
Isolation of S. enteritidis on different days from tissues of rats infected orogastrically with various doses of S. enteritidis CFU. Bars represent mean values (± standard deviations) from at least four animals.
Effect of LPS on invasion by S. enteritidis.
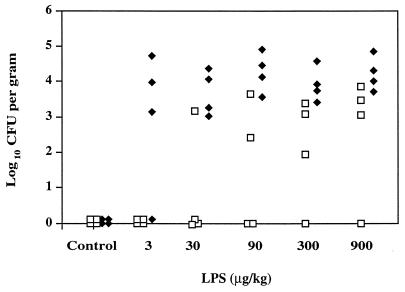
In a preliminary experiment, a sublethal i.v. dose of 900 μg of S. enteritidis LPS per kg was administered to a group of rats concomitantly fed with 1.5 × 107 CFU live organisms. Within 24 h, the animals became sick, and many died. Survivors were shown to have S. enteritidis present in all tissues tested. We then conducted experiments in which groups of rats were given a range of LPS doses up to 900 μg/kg and a lower dose of S. enteritidis (1.5 × 106 CFU organisms). Two further groups were given either bacteria or LPS alone. No animals given these regimens died during the course of the experiment. The numbers of organisms present in the liver and MLN after 24 h are presented in Fig. 2. A significant correlation was seen between LPS dose and invasion of the liver [r = 0.522, P < 0.01], and at LPS doses above 30 μg/kg, invasion of the MLN was seen in all animals. An E. coli O111:B4 LPS dose of 900 μg/kg given i.v. at the time of the oral challenge with 1.5 × 106 CFU S. enteritidis also caused invasion of the target tissues within 24 h (data not shown), suggesting that any potent endotoxin may be able to promote invasion. Preliminary results suggest that the LPS-induced enhancement of bacterial invasion is a transient phenomenon, for no bacterial invasion was seen in animals that were challenged with S. enteritidis organisms 16 h after LPS treatment (data not shown).
FIG. 2.
Isolation of S. enteritidis from liver (□) and MLN (⧫) of rats 24 h after i.v. injection with various doses of S. enteritidis LPS and orogastric challenge with 1.5 × 106 S. enteritidis CFU. The control group was given i.v. saline and orogastric bacteria.
Effect of bile duct cannulation or occlusion on LPS-induced invasion by S. enteritidis.
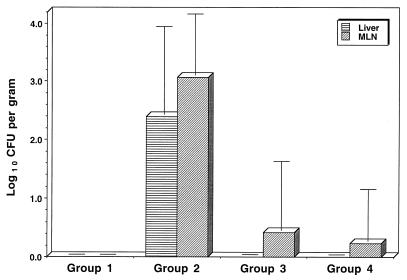
To investigate the role of biliary factors in the invasion process, groups of rats were either sham operated or bile duct cannulated or ligated. After the operation procedures were performed, the animals were injected with LPS (900 μg/kg) and then challenged orogastrically with 1.5 × 106 CFU of S. enteritidis. The organisms present in liver and MLN were assessed after 24 h. Logistical considerations necessitated the experiment being conducted in three stages, with typically four animals per group at each stage. Results were then combined and are shown as Fig. 3. To protect for the family-wise error rate, two separate planned contrast ANOVAs were used to analyze the results for the liver and MLN. For both analyses, three specific contrasts were undertaken, and all were found to be highly significant. LPS challenge led to significant invasion of both the MLN and liver: saline- and S. enteritidis-challenged controls versus LPS- and S. enteritidis-challenged animals [for MLN, F(1,39) = 129.7 and P < 0.001; for liver, F(1,39) = 47.7 and P < 0.001]. When normal bile flow to the intestine was interrupted, the reduction in invasion was highly significant: LPS- and S. enteritidis-challenged animals that had been sham operated versus animals that were bile duct ligated [for MLN, F(1,39) = 88.7 and P < 0.001; for liver, F(1,39) = 42.0 and P < 0.001] and LPS- and S. enteritidis-challenged animals that had been sham operated versus animals that were bile duct cannulated [for MLN, F(1,39) = 128.6 and P < 0.001; for liver, F(1,39) = 54.6 and P < 0.001]. No organisms were isolated from the livers of any of the bile duct ligated (n = 8) or cannulated animals (n = 13) and organisms were isolated from the MLN of only one animal each from the bile duct cannulated and ligated groups.
FIG. 3.
Isolation of S. enteritidis from liver and MLN of rats 24 h after orogastric challenge with 1.5 × 106 S. enteritidis CFU. Animals in group 1 (n = 10) were challenged i.v. with physiological saline, and those of groups 2, 3, and 4 were challenged with 900 μg of S. enteritidis LPS per kg at the time of bacterial challenge. In addition, animals in group 2 (n = 12) were sham operated prior to infection. Animals in group 3 (n = 8) had their bile ducts ligated; in group 4 (n = 13), the bile ducts were cannulated prior to challenge with bacteria and LPS.
In several of the earlier experiments where rats were cochallenged with LPS and S. enteritidis, the presence of lactose-fermenting gram-negative organisms (presumptive E. coli) were noted in cultures of tissue homogenates. Preliminary experiments suggest that while translocation to the MLN is seen in animals challenged concomitantly with LPS and bacteria, in animals that are bile duct ligated or cannulated prior to treatment, there is no evidence of bacterial translocation.
Measurement of TNF-α in bile.
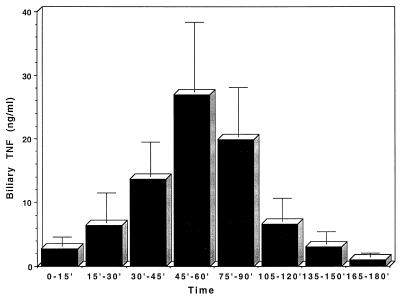
The presence of TNF-α was determined in bile samples collected from rats over a 3-h period following i.v. injection with 1 mg of LPS per kg of body weight. One set of data points, obtained from an animal that showed highly elevated levels of TNF throughout the measurement period, was excluded from analysis. This animal had a concentration of 145 ng/ml immediately after administration of LPS. The other animals (n = 14) had a mean concentration at this time point of 2.8 ng/ml, which was not significantly different from background levels (3.1 ng/ml; n = 11). The results are presented in Fig. 4. A repeated-measure ANOVA was used to analyze changing TNF-α concentrations over the 3-h period. A significant quadratic (curved) relationship was shown to occur [F(1,13) = 7.331, P = 0.02]. The highest levels of TNF-α were seen 45 to 60 min postchallenge, with a mean concentration of 27.0 ng/ml, and TNF-α was detectable in all samples at this time point. The values returned to base level around 2.5 h postchallenge.
FIG. 4.
Measurement of TNF-α in rat bile after intravenous injection with 1 mg of LPS per kg. Results are presented as means and standard errors of the means from 14 animals at each time point.
DISCUSSION
Knowledge of the processes involved in the invasion of enteric pathogens from the gastrointestinal tract is central to an understanding of the pathogenesis of infections by these organisms. The key steps in the invasion of Salmonella species include attachment to the intestinal epithelium, proliferation, invasion, and movement to the major target tissues. These processes have been considered to be essentially dependent on bacterial virulence factors (18). Virulence factors, for example, promote entry into the tissues through Peyer's patches (11, 19) and between epithelial cells (23). However, little attention has been directed to a consideration of inducible host factors, which might contribute to these processes.
In this study we have shown that the hepatobiliary system can directly influence invasiveness of S. enteritidis in rats. Further, we have shown that TNF-α concentrations rise in bile after LPS challenge. Although it remains to be investigated whether TNF-α itself is directly involved in the enhancement of invasiveness, these results highlight the role of the hepatobiliary system in the delivery of inflammatory products of the liver to the gastrointestinal tract. Such factors could certainly be involved in the phenomenon observed.
The role of biliary factors in gastrointestinal pathology has received little attention to date. Bile continues to be viewed by most investigators as a secretion of the liver with a purely digestive function. In fact, a growing body of evidence points to the presence of a range of inflammatory mediators and other factors in bile. These factors include epidermal growth factor (21), cytokines TNF-α and IL-6 (37, 40), and IL-1α and IL-1β (M. Wiseman, W. Sewell, and G. D. F. Jackson, unpublished data), complement proteins, and acute-phase proteins (46). Although the harsh conditions in which they are found might argue against a functional role for these biliary mediators, recent evidence suggests they may remain active within the lumen of the gastrointestinal tract (13, 36; W. Sewell, Y. Dai, and G. D. F. Jackson, unpublished data).
Biliary factors could promote bacterial association with the epithelium, or might act upon lumenal microorganisms to enhance their invasive characteristics. There is evidence that TNF-α is able to directly affect the virulence properties of some organisms. For example, TNF-α enhances the invasion of cultured cells by Shigella species (26), and invasion can also be promoted by microbial proliferation, which may be influenced by cytokines. IL-1, for example, has been shown to act as a growth factor for uropathogenic E. coli (34). However, as it is reported here that S. enteritidis, as well as members of the regular gut microflora, showed enhanced invasiveness or translocation, it is more likely that this is the result of a direct and nonspecific action on the integrity of host defenses.
Fluid accumulation in the intestine of LPS-treated animals, which indicates an altered intestinal permeability, was a consistent observation in the course of these experiments. A number of other studies have also demonstrated that LPS can enhance intestinal permeability (7, 32). TNF-α has also been shown to directly affect the permeability of epithelial barriers (30), and such a cytokine-induced loosening of the tight junctions may facilitate the early entry of bacteria into the mesenteric lymph nodes.
In contrast to the results of this study, a previous report has shown evidence of bacterial translocation from the gut to the MLN after prolonged deprivation of the gut of bile in mice (6). Such prolonged occlusion of the normal bile flow to the gut following ligation of the bile duct for a week could lead to outcomes different from those seen in our studies of short-term bile deprivation. Long-term deprivation could compromise the mucosal integrity of the gut, due to the absence of factors such as biliary epidermal growth factor from the gastrointestinal tract (21). A prolonged absence of bile could also alter the invasiveness of the gut flora as a consequence of bacterial overgrowth, for it has been shown that 48 h following bile duct ligation, the number of coliform bacteria in the small bowel increases significantly (20).
The results reported here for a rat model lead us to propose that the invasion of viable organisms from the gut is strongly promoted by the action of LPS upon the hepatobiliary system and that this may be a significant factor in some human pathologies. Although the LPS concentrations used in this model may seem unnaturally high, it is well documented that rats are relatively refractory to LPS, while humans respond to nanogram-per-kilogram levels of exposure (41).
A variety of circumstances can lead to the uptake of low levels of gut-derived endotoxin that is then delivered to the liver (43). Although the concentrations of LPS must normally be too low to lead to deleterious events, if LPS concentrations rise as a consequence of some transient event, hepatobiliary factors might then drive subsequent events leading to invasion. Variability in the uptake and in the response to such LPS could help explain the variability in the incubation periods seen in some infections, such as in human Salmonella serovar Typhi infection. Investigations to elucidate the range of biliary factors induced by LPS and their roles in the process are now underway.
ACKNOWLEDGMENT
Abul F. M. Wali Ul Islam was supported by a WHO fellowship during this study.
REFERENCES
- 1.Cannon J G, Tompkins R G, Gelfand J A, Michie H R, Stanford G G, van der Meer J W M, Endres S, Lonnemann G, Corsetti J, Chernow B, Wilmore D W, Wolff S M, Burke J F, Dinarello C A. Circulating interleukin-1 and tumour necrosis factor in septic shock and experimental endotoxin fever. J Infect Dis. 1990;161:79–84. doi: 10.1093/infdis/161.1.79. [DOI] [PubMed] [Google Scholar]
- 2.Carter P B, Collins F M. The route of enteric infection in normal mice. J Exp Med. 1974;139:1189–1203. doi: 10.1084/jem.139.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collins A M, Leach S, Payne J, Mitchell A, Dai Y, Jackson G D F. A role for the hepatobiliary system in IgE-mediated intestinal inflammation in the rat. Clin Exp Allergy. 1999;29:262–270. doi: 10.1046/j.1365-2222.1999.00425.x. [DOI] [PubMed] [Google Scholar]
- 4.Deitch E A, Berg R, Specian R. Endotoxin promotes the translocation of bacteria from the gut. Arch Surg. 1987;122:185–190. doi: 10.1001/archsurg.1987.01400140067008. [DOI] [PubMed] [Google Scholar]
- 5.Deitch E A, Ma L, Ma W J, Grisham M B, Granger D N, Specian R D, Berg R D. Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Investig. 1989;84:36–42. doi: 10.1172/JCI114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deitch E A, Sittig K, Li M, Berg R D. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79–84. doi: 10.1016/s0002-9610(05)80610-5. [DOI] [PubMed] [Google Scholar]
- 7.Deitch E A, Specian R D, Berg R D. Endotoxin-induced bacterial translocation and mucosal permeability: role of xanthine oxidase, complement activation, and macrophage products. Crit Care Med. 1991;19:785–791. doi: 10.1097/00003246-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Dinarello C A, Cannon J G, Wolff S M, Bernheim H A, Beutler B, Cerami A, Figari I S, Palladino M A, O'Connor J V. Tumour necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986;163:1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumour necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 10.Fox E S, Thomas P, Broitman S A. Clearance of gut-derived endotoxins by the liver. Gastroenterology. 1989;96:456–461. doi: 10.1016/0016-5085(89)91571-0. [DOI] [PubMed] [Google Scholar]
- 11.Gaines S, Sprinz H, Tully J G, Tigertt W D. Studies on infection and immunity in experimental typhoid fever. VII. The distribution of Salmonella typhi in chimpanzee tissue following oral challenge, and the relationship between the numbers of bacilli and morphologic lesions. J Infect Dis. 1968;118:293–300. doi: 10.1093/infdis/118.3.293. [DOI] [PubMed] [Google Scholar]
- 12.Grutzkau A, Hanski C, Hahn H, Riecken E O. Involvement of M cells in the bacterial invasion of Peyer's patches: a common mechanism shared by Yersinia enterocolitica and other enteroinvasive bacteria. Gut. 1990;31:1011–1015. doi: 10.1136/gut.31.9.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton L J, Dai Y, Jackson G D F. Bile regulates the expression of major histocompatibility complex class II molecules on rat intestinal epithelium. Gastroenterology. 1997;113:1901–1905. doi: 10.1016/s0016-5085(97)70009-x. [DOI] [PubMed] [Google Scholar]
- 14.Helle M, Brakenhoff J P J, Groot E R D, Aarden L A. Interleukin-6 is involved in interleukin 1-induced activities. Eur J Immunol. 1988;18:957–959. doi: 10.1002/eji.1830180619. [DOI] [PubMed] [Google Scholar]
- 15.Hesse D G, Tracey K J, Fong Y, Manogue K R, Palladino M A, Cerami A, Shires T, Lowry S F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988;166:147–153. [PubMed] [Google Scholar]
- 16.Hormache C E. Dead salmonellae or their endotoxin accelerate the early course of a Salmonella infection in mice. Microb Pathog. 1990;19:213–218. doi: 10.1016/0882-4010(90)90023-j. [DOI] [PubMed] [Google Scholar]
- 17.Hornick R B, Greisman S E, Woodward T E, DuPont H L, Dawkins A T, Snyder M J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970;283:686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- 18.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 19.Jones B D, Nafisa G, Falkow S. Salmonella typhimurium initiates murine infection by penetrating and destroying the specialized epithelial M cells of the Peyer's patches. J Exp Med. 1994;180:15–24. doi: 10.1084/jem.180.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalambaheti T, Cooper G N, Jackson G D F. Role of bile in non-specific defence mechanisms of the gut. Gut. 1994;35:1047–1052. doi: 10.1136/gut.35.8.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kong W, Koldovsky O, Rao R K. Appearance of exogenous epidermal growth factor in liver, bile, and intestinal lumen of suckling rats. Gastroenterology. 1992;102:661–667. doi: 10.1016/0016-5085(92)90117-h. [DOI] [PubMed] [Google Scholar]
- 22.Lambert R. Surgery of the digestive system of the rat. Springfield, Ill: Charles C Thomas; 1965. pp. 113–139. [Google Scholar]
- 23.Lee C A, Falkow S. Entry of Salmonella into epithelial cells. In: Cabello F, Hormaeche C, Mastroeni P, Bonina L, editors. Biology of Salmonella. New York, N.Y: Plenum Press; 1993. pp. 169–180. [Google Scholar]
- 24.Li M, Specian R D, Berg R D, Deitch E A. Effects of protein malnutrition and endotoxin on the intestinal mucosal barrier to the translocation of indigenous flora in mice. J Parenter Enteral Nutr. 1989;13:572–578. doi: 10.1177/0148607189013006572. [DOI] [PubMed] [Google Scholar]
- 25.Lie J, Keiser J A, Scales W E, Kunkel S L, Kluger M J. Role of corticosterone in TNF and IL-6 production in isolated perfused rat liver. Am J Physiol. 1995;268:R699–R706. doi: 10.1152/ajpregu.1995.268.3.R699. [DOI] [PubMed] [Google Scholar]
- 26.Luo G, Niesel D W, Shaban R A, Grimm E A, Klimpel G R. Tumour necrosis factor alpha binding to bacteria: evidence for a high-affinity receptor and alteration of bacterial virulence properties. Infect Immun. 1993;61:830–835. doi: 10.1128/iai.61.3.830-835.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mastroeni P, Villarreal-Ramos B, Hormaeche C E. Adoptive transfer of immunity to oral challenge with virulent salmonellae in innately susceptible BALB/c mice requires both immune serum and T cells. Infect Immun. 1993;61:3981–3984. doi: 10.1128/iai.61.9.3981-3984.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merrick B A, He C Y, Craig W A, Corsini E, Rosenthal G J, Mansfield B K, Selkirket J K. Two dimensional gel electrophoresis of cellular and secreted proteins from rat alveolar macrophages after lipopolysaccharide treatment. Appl Theor Electrophor. 1992;2:177–187. [PubMed] [Google Scholar]
- 29.Michetti P, Porta N, Mahan M J, Slauch J M, Mekalanos J J, Blum A L, Kraehenbuhl J P, Neutra M R. Monoclonal immunoglobulin A prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology. 1994;107:915–923. doi: 10.1016/0016-5085(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 30.Mullin J M, Snock K V. Effect of tumour necrosis factor on epithelial tight junctions and transepithelial permeability. Cancer Res. 1990;50:2172–2176. [PubMed] [Google Scholar]
- 31.Naylor G R E. Incubation period and other features of food-borne and water-borne outbreaks of typhoid fever in relation to pathogenesis and genetics of resistance. Lancet. 1983;i:864–866. doi: 10.1016/s0140-6736(83)91395-8. [DOI] [PubMed] [Google Scholar]
- 32.O'Dwyer S T, Michie H R, Ziegler T R, Revhaug A, Smith R J, Wilmore D W. A single dose of endotoxin increases intestinal permeability in healthy humans. Arch Surg. 1988;123:1459–1464. doi: 10.1001/archsurg.1988.01400360029003. [DOI] [PubMed] [Google Scholar]
- 33.O'Neil S, Hunt J, Filkins J, Gamelli R. Obstructive jaundice in rats results in exaggerated hepatic production of tumour necrosis factor-alpha and systemic and tissue tumour necrosis factor-alpha levels after endotoxin. Surgery. 1997;122:281–286. doi: 10.1016/s0039-6060(97)90019-2. [DOI] [PubMed] [Google Scholar]
- 34.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of growth of virulent strains of Escherichia coli by interleukin-1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 35.Remick D G, Kunkel R G, Larrick J W, Kunkel S L. Acute in vivo effects of human recombinant tumour necrosis factor. Lab Investig. 1987;56:583–590. [PubMed] [Google Scholar]
- 36.Rollwagen F M, Baqar S. Oral cytokine administration. Immunol Today. 1996;17:548–555. doi: 10.1016/s0167-5699(96)30065-0. [DOI] [PubMed] [Google Scholar]
- 37.Rosen H R, Winkle P J, Kendall B J, Diehl D L. Biliary interleukin-6 and tumour necrosis factor-alpha in patients undergoing endoscopic retrograde cholangiopancreatography. Digest Dis Sci. 1997;42:1290–1294. doi: 10.1023/a:1018822628096. [DOI] [PubMed] [Google Scholar]
- 38.Takeuchi A. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am J Pathol. 1967;50:109–136. [PMC free article] [PubMed] [Google Scholar]
- 39.Tracey K J, Wei H, Manogue K R, Fong Y, Hesse D G, Nguyen H T, Kuo G C, Beutler B, Cotran R S, Cerami A, Lowry S F. Cachectin/tumour necrosis factor induces cachexia, anaemia, and inflammation. J Exp Med. 1988;167:1211–1227. doi: 10.1084/jem.167.3.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Umeshita K, Monden M, Tono T, Hasuike Y, Kanai T, Gotoh M, Mori T, Shaked A, Busuttil R W. Determination of the presence of IL-6 in bile after orthotropic liver transplantation. Its role in the diagnosis of acute rejection. Ann Surg. 1996;223:204–211. doi: 10.1097/00000658-199602000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van der Poll T, Calvano S E, Kumar A, Coyle S M, Lowry S F. Epinephrine attenuates down-regulation of monocyte tumor necrosis factor receptors during human endotoxemia. J Leukoc Biol. 1997;61:156–160. doi: 10.1002/jlb.61.2.156. [DOI] [PubMed] [Google Scholar]
- 42.van der Waaij D, Berghuis-De Vries J M, Lekkerkerk-van der Wees J E C. Colonization resistance of the digestive tract in conventional and antibiotic-treated mice. J Hyg Camb. 1971;69:405–411. doi: 10.1017/s0022172400021653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Deventer S J H, ten Cate J W, Tytgat G N J. Intestinal endotoxemia. Clinical significance. Gastroenterology. 1988;94:825–831. doi: 10.1016/0016-5085(88)90261-2. [DOI] [PubMed] [Google Scholar]
- 44.Wang P, Ba Z F, Chaudry I H. Mechanism of hepatocellular dysfunction during early sepsis. Key role of increased gene expression and release of proinflammatory cytokines tumour necrosis factor and interleukin-6. Arch Surg. 1997;132:364–369. doi: 10.1001/archsurg.1997.01430280038005. [DOI] [PubMed] [Google Scholar]
- 45.Westphal O, Jann K. Bacterial lipopolysaccharides extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]
- 46.Wilton P B, Dalmasso A P, Allen M O. Complement in local biliary tract defences: dissociation between bile complement and acute phase reactants in cholecystitis. J Surg Res. 1987;42:434–439. doi: 10.1016/0022-4804(87)90179-x. [DOI] [PubMed] [Google Scholar]
- 47.Worton K J, Candy D C A, Wallis T S, Clarke G J, Osborne M P, Haddon S J, Stephen J. Studies on early association of Salmonella typhimurium with intestinal mucosa in vivo and in vitro: relationship to virulence. J Med Microbiol. 1989;29:283–294. doi: 10.1099/00222615-29-4-283. [DOI] [PubMed] [Google Scholar]
- 48.Zanetti G, Heumann D, Gerain J, Kohler J, Abbet P, Barras C, Lucas R, Glauser M, Baumgartner J. Cytokine production after intravenous or peritoneal gram-negative bacterial challenge in mice. Comparative protective efficacy of antibodies to tumour necrosis factor-α and to lipopolysaccharide. J Immunol. 1992;148:1890–1897. [PubMed] [Google Scholar]