Highlights
-
•
A very high seroprevalence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies was found in rural north-eastern Tanzania.
-
•
Dynamic peaks of SARS-CoV-2 can be detected using seroprevalence measurement.
-
•
Age, adiposity, and socioeconomic status were found to be associated with anti-SARS-CoV-2.
-
•
Immune surveillance is vital to break down the burden of COVID-19 in rural populations.
Keywords: Anti-SARS-CoV-2, Seroprevalence, Low- and middle-income countries, Sub-Saharan Africa, Rural, Tanzania, Immune surveillance, Vaccination
Summary
Background
The reported infection rates and burden of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in low- and middle-income countries, including those in sub-Saharan Africa, are relatively low compared to the rates and burden in Europe and America, partly due to limited testing capability. Unlike many countries, Tanzania has implemented neither mass screening nor restrictive measures such as lockdowns to date. The prevalence of SARS-CoV-2 infection in rural mainland Tanzania is largely unknown.
Methods
A cross-sectional study was conducted between April and October 2021 to assess the anti-SARS-CoV-2 seroprevalence among mother–child pairs (n = 634 children, n = 518 mothers) in a rural setting in north-eastern Tanzania.
Results
A very high prevalence of anti-SARS-CoV-2 antibody titres was found, with seroprevalence rates ranging from 29% among mothers and 40% among children, with a dynamic peak in seropositivity incidence at the end of July/early August being revealed. Significant differences in age, socioeconomic status, and body composition were associated with seropositivity in mothers and children. No significant associations were observed between seropositivity and comorbidities, including anaemia, diabetes, malaria, and HIV.
Conclusions
The transmission of SARS-CoV-2 in a rural region of Tanzania during 2021 was high, indicating a much higher infection rate in rural Tanzania compared to that reported in the UK and USA during the same period. Ongoing immune surveillance may be vital to monitoring the burden of viral infection in rural settings without access to molecular genotyping, where the load of communicable diseases may mask COVID-19. Surveillance could be implemented in tandem with the intensification of vaccination strategies.
Introduction
Apart from South Africa [5], the epidemiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Africa is not well described, and infection rates and the disease burden of coronavirus disease 2019 (COVID-19) are poorly understood. In many low- and middle-income countries (LMICs), including Tanzania, diagnostic and epidemiological surveillance systems are suboptimal [23]. Thus, it is plausible that the transmission burden and dynamics have been underestimated, and the impact on vulnerable populations is largely unknown. Therefore, for effective control and management of COVID-19, there is an immediate need for reliable data on SARS-CoV-2 infection and transmission dynamics of the disease in LMICs.
While previous reports have indicated less clinical severity of COVID-19 in LMICs (Simeni [13,34]), a recent systematic review illustrated that the risk of infection and most significant impact of the pandemic occurred in poorer countries [16]. In sub-Saharan African (SSA) countries, COVID-19 presents a serious health threat in terms of morbidity and mortality, and has had a severe economic impact [39]. In Tanzania, more than 46% of the population live on less than $2 USD per day, and COVID-19 has adversely affected all economic sectors in the country [38,39]. The pandemic continues to inflict a heavy toll on the already fragmented healthcare system [40].
During the very early phases of the pandemic, the Tanzanian government initiated interventions to curtail SARS-CoV-2 transmission including suspending large gatherings and introducing facemasks. However, the majority of such efforts were short-lived due to logistical challenges. By May 2020, no SARS-CoV-2 data from Tanzania were being relayed to the Centers for Disease Control and Prevention (CDC) [20]. Furthermore, no mass screening, contact tracing, or restrictive measures such as lockdowns have been implemented in Tanzania to date. However, following the swearing-in of President Samia Suluhu Hassan in March 2021, COVID-19 preventive strategies were revitalized with the formation of a National COVID-19 Taskforce. As of July 27, 2022, a total of 36 886 confirmed cases of COVID-19, including 841 deaths, had officially been reported from Tanzania [42]. Nevertheless, there are no reliable estimates on the burden of SARS-CoV-2 infection in Tanzania, which has an estimated population size of over 60 million [42].
The serological detection of specific anti-SARS-CoV-2 antibodies is a valuable tool for understanding infection dynamics and the epidemiology of SARS-CoV-2 [10]. Few population-based anti-SARS-CoV-2 serological surveys have been conducted in the SSA countries [19,28,29,41]. The most extensive studies have been urban studies performed in South Africa (n = 1211)- and Zambia (n = 4258) [21]. In both studies, the estimated number of SARS-CoV-2 cases based on the presence of anti-SARS-CoV-2 antibodies was much higher than the number of reported COVID-19 cases using other surveillance platforms [5,32].
Currently, no estimate exists on the severity of the COVID-19 pandemic and the number of infections in rural Tanzania. This study was therefore performed to investigate the anti-SARS-CoV-2 seroprevalence, as well as antibody neutralizing capacity, in an unvaccinated population cohort of mothers and children in a rural Tanzanian setting, from April to October 2021. The potential socioeconomic and clinical factors influencing seropositivity were also investigated.
Methods
Study design
This population-based, cross-sectional study was conducted in the districts of Korogwe and Handeni, Tanga Region, north-eastern Tanzania, and covered 47 rural villages and seven peri-urban townships located in Korogwe village. Peri-urban was defined as a village/township with urban capacities including shops and schools, but retaining rural characteristics such as substantial reliance on agricultural production. The cohort included children aged 5–12 years and their mothers aged 21–59 years, who had previously participated in two longitudinal pregnancy studies: “Strategies TO Prevent Pregnancy Associated Malaria” (STOPPAM) [31] and “Foetal exposure and epidemiological transition: the role of anaemia in early life for non-communicable diseases in later life” (FOETALforNCD) [11].
From April 26 to October 27, 2021, when the children were between 5 and 6 years of age (FOETALforNCD) and between 11 and 12 years of age (STOPPAM), the child and mother pairs were invited to participate in a follow-up health examination study (the PONA2 study). Trained field workers made door-to-door visits to identify participants who had previously participated in the STOPPAM and/or FOETALforNCD study. The inclusion criteria were as follows: (1) mothers must have participated in the STOPPAM and/or FOETALforNCD study, (2) data on gestational age and/or birth weight should be available, (3) the child should be enrolled in the PONA2 study examination for the mother to be enrolled (mothers could not participate without a child). If eligible, detailed information of the study was provided, and those who provided informed consent to participate (parent(s) or legal guardian consented on behalf of the children) were enrolled in PONA2. To prevent viral transmission during the data collection, participants presenting with current common COVID-19 symptoms had their enrolment postponed until they were symptom-free.
Ethical considerations
The study received ethical approval from the Tanzania Medical Research Coordinating Committee (NIMR/HQ/R.8a/Vol. IX/3503, August 26 2020 and amendment number NIMR/HQ/R.8a/Vol. 1/970, November 12 2021). However, ethical approval for COVID-19 research was not approved before the data collection was finished. Hence, it was not possible to include COVID-19 testing and disease symptoms reporting as part of the clinical examination. All study procedures were performed according to good clinical and laboratory practices (GLC/GLP) and in accordance with the Declaration of Helsinki. All participants were treated according to the Tanzanian national guidelines, and the project assisted all participants in obtaining the best local medical care available if disease was diagnosed. The data sharing followed the Tanzanian Medical Research Coordinating Committee ethical guidelines.
Sociodemographic and clinical information
At enrolment, sociodemographic data, including profession, education, housing, main source of drinking water, and other lifestyle factors, were collected in structured interviews. The previous medical history of both mothers and children was documented. Body composition (body weight, body fat percentage, fat mass, and muscle mass) was estimated by bioimpedance analysis methodology using a Tanita DC-400M analyser (TANITA). Blood pressure was measured in a seated position on the right arm (Omron Health Care Europe). Height was measured with a stadiometer (precision 1 mm). Mid-upper arm circumference (MUAC) was measured on the upper right arm at the midpoint of the acromion process and the tip of the olecranon (precision 1 mm). Skinfold thickness was measured using a Harpenden skinfold calliper (BATY International). Waist circumference was measured just above the iliac crest in the horizontal plane and hip circumference was measured at the point yielding the maximum circumference over the buttocks, both using a standard measuring tape to the nearest 1 mm. All anthropometric measurements were collected using standard operating procedures. Finally, left and right hand grip strength was measured for all mothers and all children aged 11–12 years using a Saehan hand grip dynamometer (SAEHAN, Korea).
Blood sample collection and processing
Venous blood was collected from both the children and mothers in EDTA-coated plain and heparinized vacutainer tubes, and transported at 2–8°C to the National Institute for Medical Research (NIMR) Korogwe Research Laboratory for further processing and laboratory analyses. Plasma was separated within 2 hours of collection in a refrigerated centrifuge. All samples were stored at −80°C and later shipped on dry ice to Rigshospitalet, Denmark for the detection of anti-SARS-CoV-2 antibodies by ELISA. High-sensitivity C-reactive protein (hs-CRP) was measured in the Department of Clinical Biochemistry, Copenhagen University Hospital, using the Cobas 8000 c702 system (Roche Diagnostics).
At the study site, point-of-care diagnostics were performed on the venous blood including the estimation of haemoglobin (Hb) (Sysmex KX-21N), fasting blood glucose (HemoCue 301), and malaria rapid diagnostic test (mRDT) (ParaHIT or CareStart Malaria Pf). For participants with a positive mRDT, the parasitaemia was confirmed by two independent expert microscopists. HIV infection was tested with the Determine HIV-1/2 test kit (Alere Ltd) and seropositive cases were confirmed using the Unigold test kit (Trinity Biotech Plc). All tests were done according to the manufacturer's instructions.
Detection of total anti-SARS-CoV-2 antibodies
The detection of total antibodies against SARS-CoV-2 spike (S) protein and receptor binding domain (RBD) as a proxy of previous infection in non-vaccinated individuals was performed using an in-house sandwich ELISA (S-ELISA) as described previously [10], with minor modifications. Briefly, Nunc MaxiSorp 96-well plates (Thermo Fisher Scientific) were coated with 0.5 µg/ml of RBD overnight at 4°C. The plates were blocked for 1 hour with phosphate-buffered saline (PBS) + 0.05% Tween (Merck) (PBS-T) followed by the addition of plasma samples at 1:2 dilution in PBS-T and incubated for 1 hour. A solution containing 0.5 µg/ml RBD biotinylated in PBS-T was applied and incubated for 1 hour. A solution of 1:16 000 horse radish peroxidase (HRP)-conjugated high-sensitivity streptavidin (Pierce) in PBS-T was applied and incubated for 1 hour. TMB-One (Kem-En-Tec) was used as a substrate and plates were revealed for 5 minutes. The reaction was stopped using 0.3 M H2SO4, and optical density (OD) was measured using a Synergy HT absorbance reader (Biotek Instruments) at 450–630 nm. A recombinant-human IgG monoclonal antibody against protein S/RBD (Genscript) diluted 1:2000 in PBS-T was used as a positive control. A pool of uninfected/unvaccinated normal human serum was used as a negative control. Plates were washed three times in PBS-T between steps, and incubations were performed while shaking at room temperature. The assay positivity threshold was set to OD >0.1585.
Quantitative determination of anti-SARS-CoV-2 antibody isotypes
Samples that were positive on the S-ELISA were further analysed for the quantitative determination of IgG, IgM, and IgA levels using an in-house ELISA-based assay, as described elsewhere [10,26].
ACE-2/RBD antibody inhibition measurement
A previously described in-house ELISA-based assay was used to study the ability of the measured antibodies to prevent the binding of the S protein and RBD to the host receptor angiotensin-converting enzyme 2 (ACE-2) [1]. A pool containing plasma from vaccinated individuals (previously quantified into international units per millilitre (IU/ml) using The Working Reagent for anti-SARS-CoV-2 immunoglobulin 21/234, NIBSC), diluted 1:50 in PBS-T, was used as a positive control. The assay positivity threshold was set to 420 IU/ml.
Statistical analysis
The assessment of antibody isotypes and neutralization levels was performed using GraphPad version 9.3.1 (GraphPad Software). IgG, IgM, IgA, and neutralization levels were interpolated using non-linear regression four-parameter curve fitting. IgG, IgM, and IgA results were given in arbitrary units per millilitre (AU/ml), where the highest concentration of the calibrator was given a value of 200 AU/ml. Neutralization results were given in IU/ml, where the highest concentration of the calibrator was given a value of 520 IU/ml.
In the univariate analyses, normally distributed data (mean ± standard deviation) were compared by Student t-test, while non-normally distributed data (median and interquartile range) were compared by Mann–Whitney U-test. Proportions of categorical data were compared using Fisher's exact test. Potential differences in socioeconomic and clinical characteristics between seronegative and seropositive cases were examined in separate analyses for the STOPPAM and FOETALforNCD groups, due to the apparent age differences between these participants.
Results
Anti-SARS-CoV-2 seroprevalence in 5–12-year-old children and mothers in rural north-eastern Tanzania
In total, 634 children and 513 mothers had a fasting venous blood sample collected and were included in the SARS-CoV-2 antibody study (n = 1147). Of note, 94 of the 634 children participated in the study without their mothers, since the mothers were not available due to either work, travelling, or being deceased. Among the 634 children, 45 were siblings, including 13 twin pairs. One mother participated with three children, of whom two were twins and one was a younger sibling. Hence, 21 sibling pairs and one sibling group of three children were included.
Among all participants (n = 1147), the overall anti-SARS-CoV-2 seroprevalence was 37.1%, with children having a seroprevalence of 39.0% (n = 634) and mothers having a seroprevalence of 29.0% (n = 513) (Table 1). Stratifying the children by age group and anti-SARS-CoV-2 seroprevalence, the seroprevalence was significantly higher among the 11–12-year-old children (43.3%) compared to the 5–6-year-old children (33.0%) (Fisher's exact test, p = 0.008). In the 11–12 years age group, female children were more likely to be seropositive than male children (50.5% vs 35.9%; Fisher's exact test, p = 0.006).
Table 1.
Anti-SARS-CoV-2 seroprevalence of total antibodies against SARS-CoV-2 protein receptor-binding domain in two mother–child cohorts from Korogwe District, Tanga Region, Tanzania—April 26 to October 27, 2021
| SARS-CoV-2 negative | SARS-CoV-2 positive | % positive | |
|---|---|---|---|
| Children | |||
| FOETALforNCD cohort, 5–6 years old (n = 267) | 179 | 88 | 33.0% |
| STOPPAM cohort, 11–12 years old (n = 367) | 208 | 159 | 43.3% |
| In total (n = 634) | 387 | 247 | 39.0% |
| Mothers | |||
| PONA1 [Au?13] cohort (mean age 34.5 years, n = 222) | 151 | 71 | 32.0% |
| STOPPAM cohort (mean age 39.9 years, n = 291) | 213 | 78 | 26.8% |
| In total (n = 513) | 364 | 149 | 29.0% |
Of the 513 mother–child pairs included, the majority had concordant seroprevalence (positive vs negative), with 253 (49.3%) pairs being negative and 89 (17.4%) pairs being positive. However, in 111 (21.6%) mother–child pairs, the mother was seronegative and the child seropositive; conversely, in 60 (11.7%) pairs, the mother was seropositive and the child was seronegative for anti-SARS-CoV-2 antibodies.
Of the sibling pairs/groups, 14 pairs had concordant seroprevalence and eight had divergent seroprevalence (negative vs positive). The majority (96%) of the mother–child pairs enrolled were living in the same households.
Date-specific and demographic effects on anti-SARS-CoV-2 seroprevalence
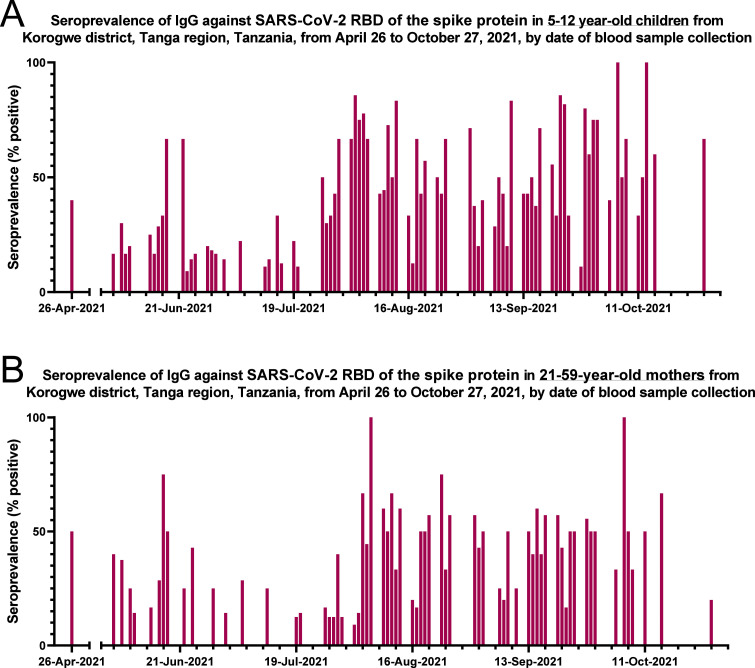
As shown in Figure 1 (further details in Supplementary Material Figure S1), a clear rise in anti-SARS-CoV-2 seropositivity had occurred among children (Figure 1A) and mothers (Figure 1B) by the end of July/early August 2021.
Figure 1.
Anti-SARS-CoV-2 seroprevalence of total immunoglobulin against SARS-CoV-2 RBD of the spike protein in (A) children aged 5–12 years, and (B) mothers aged 21–59 years. An average of seven children and six mothers were examined on each date. Exact numbers of individuals by date are shown in Supplementary Material Figure S1.
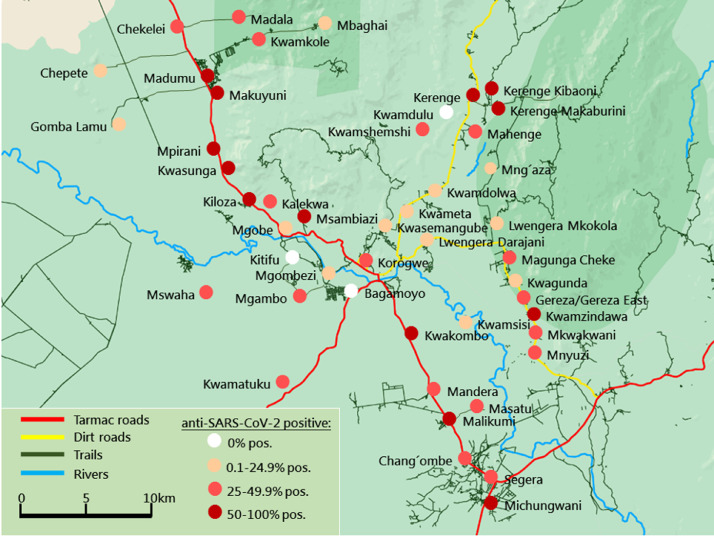
Since the participants lived in 54 smaller villages (47 rural and seven peri-urban, located in the periphery of Korogwe township), the numbers of positive versus negative cases across the different villages were examined. The anti-SARS-CoV-2 seroprevalence was heterogeneous across the villages, ranging from 0% in Kitifu and Kwamdulu, to 70% in Michungwani. As highlighted in Figure 2, the villages located along the main road between north and south through the study area, appeared to have higher seropositivity rates than the villages in the periphery of the main roads. The average anti-SARS-CoV-2 seroprevalence positivity in the 47 villages characterized as rural was 40% for children and 27.5% for mothers, and hence higher than in the seven villages characterized as peri-urban, with seropositivity of 25.5% and 21.5% for children and mothers, respectively.
Figure 2.
Overview of the villages included in the study. Korogwe village consists of seven smaller city areas that are not shown on the map: Kilole, Magundi, Majengo, Manundu, Masuguru, Mtonga, and Old Korogwe.
IgG, IgM, IgA antibody levels and neutralizing antibodies
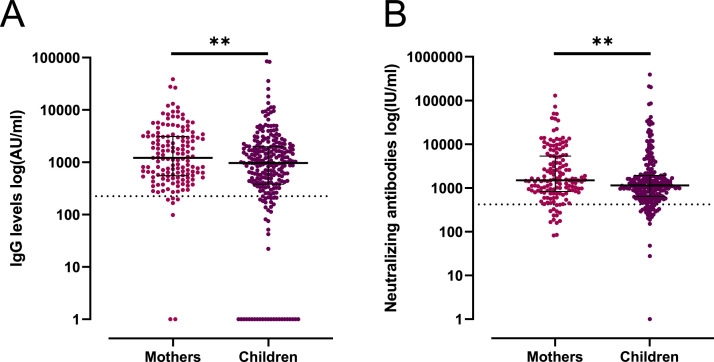
Levels of IgM, IgA, and IgG and neutralizing antibodies against SARS-CoV-2 were measured in plasma samples from the 396 individuals who were identified as positive for total SARS-CoV-2 Ig. IgG was the most abundant isotype when compared to IgM and IgA (Figure 3A, Supplementary Material Figure S2). The mothers had higher levels of antibodies and neutralizing antibody titres than the children (p ≤ 0.01) (Figure 3, Supplementary Material Figure S2).
Figure 3.
Quantitative determination of IgG antibody levels and neutralizing antibodies against RBD in anti-SARS-CoV-2 seropositive individuals. (A) IgG levels (log(AU/ml)) in mothers aged 21–59 years and children aged 5–12 years. (B) Levels of neutralizing antibodies (log(IU/ml)) measured in mothers aged 21–59 years and children aged 5–12 years. The horizontal dashed line represents the assay positivity threshold. A p-value <0.05 was considered statistically significant; **p < 0.01 by Mann–Whitney U-test.
Corresponding to the rise in seropositive cases experienced by the end of July/early August 2021 (Figure 1), the levels of IgG antibodies against SARS-CoV-2 were also higher in both children and mothers during this period of time (Supplementary Material Figure S3).
Associations between anti-SARS-CoV-2 seropositivity and socioeconomic and clinical characteristics, and comorbidities
Among the older mothers, with a mean age of approximately 40 years (STOPPAM), but not among the younger mothers, with a mean age of approximately 34.5 years (FOETALforNCD), several significant differences were observed between the seropositive versus seronegative women (Table 2). Specifically, it was observed that seropositive women were more likely to rely on a public domestic water source, such as a river, compared to the seronegative women, who were more likely to have tap/well water available (p = 0.04) (Table 2). There were no differences in ethnicity, rural vs peri-urban village, education level, profession, or monthly household income between the seropositive and seronegative mothers (Table 2). A higher weight and body fat percentage, more muscle mass, larger MUAC and skinfold thickness, and higher right hand grip strength were observed among the older seropositive mothers compared to the seronegative ones (p ≤ 0.04) (Table 2).
Table 2.
Clinical characterization of the mothers
| PONA1 [Au?13] mothers |
STOPPAM mothers |
|||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 Negative (n = 151) | SARS-CoV-2 Positive (n = 71) | p-Value | SARS-CoV-2 Negative (n = 213) | SARS-CoV-2 Positive (n = 78) | p-Value | |
| Age (years) | 34.3 ± 7.0 | 34.7 ± 6.4 | 0.69 | 39.8 ± 6.1 | 40.3 ± 7.0 | 0.58 |
| Ethnicity (n) | 0.59 | 0.99 | ||||
| Sambaa | 61 (40.4%) | 24 (33.8%) | 105 (49.3%) | 41 (52.6%) | ||
| Zigua | 50 (33.1%) | 30 (42.3%) | 47 (22.1%) | 15 (19.2%) | ||
| Pare | 7 (4.6%) | 5 (7.0%) | 0 (0.0%) | 0 (0.0%) | ||
| Bondei | 5 (3.3%) | 2 (2.8%) | 7 (3.3%) | 2 (2.6%) | ||
| Others | 28 (18.5%) | 10 (14.1%) | 53 (24.9%) | 20 (25.7%) | ||
| Main source of water (n) | 0.07 | 0.04* | ||||
| Tap | 100 (66.2%) | 57 (80.3%) | 147 (69.0%) | 46 (59.0%) | ||
| Well | 15 (9.9%) | 6 (8.5%) | 39 (18.3%) | 12 (15.4%) | ||
| River | 36 (23.8%) | 8 (11.3%) | 26 (12.2%) | 20 (25.6) | ||
| Rural vs peri-urban village (%) | Rural (95.4%) | Rural (97.2%) | 0.72 | Rural (82.2%) | Rural (82.1%) | 1.00 |
| Educational level | 0.35 | 0.14 | ||||
| None | 17 (11.3%) | 9 (12.7%) | 22 (10.3%) | 3 (3.9%) | ||
| Partial primary | 19 (12.6%) | 9 (12.7%) | 39 (18.3%) | 14 (18.0%) | ||
| Complete primary | 104 (69.9%) | 52 (73.2%) | 138 (64.8%) | 51 (65.4%) | ||
| Secondary or higher | 11 (7.3%) | 1 (1.4%) | 14 (6.6%) | 10 (12.8%) | ||
| Profession | 0.13 | 0.66 | ||||
| Housewife | 12 (8.0%) | 4 (5.6%) | 19 (8.9%) | 7 (9.0%) | ||
| Farmer/livestock keeper | 117 (77.5%) | 49 (69.0%) | 143 (67.1%) | 49 (62.8%) | ||
| Service | 6 (4.0%) | 2 (2.8%) | 10 (4.7%) | 5 (6.4%) | ||
| Business/professional | 14 (9.3%) | 16 (22.5%) | 38 (17.8%) | 16 (20.5%) | ||
| Other | 2 (1.3%) | 0 (0.0%) | 3 (1.4%) | 1 (1.3%) | ||
| Household monthly income (TZS)a | 150 000 (70 000, 200 000) | 120 000 (60 000, 200 000) | 0.78 | 100 000 (50 000, 200 000) | 150 000 (100 000, 300 000) | 0.18 |
| Height (cm) | 155.6 ± 5.8 | 154.9 ± 6.3 | 0.42 | 156.1 ± 5.9 | 157.0 ± 5.5 | 0.26 |
| Weight (kg) | 58.4 (50.3, 70.9) | 58.4 (50.7, 64.9) | 0.62 | 56.8 (49.8, 68.2) | 59.3 (54.1, 73.1) | 0.03* |
| BMI (kg/m2) | 24.0 (20.8, 29.3) | 24.4 (20.7, 27.7) | 0.88 | 23.2 (20.5, 28.2) | 25.2 (21.3, 29.2) | 0.051 |
| Skinfold (mm) | 20.0 (12.2, 30.0) | 21.8 (15.9, 28.3) | 0.40 | 19.8 (13.7, 28.1) | 21.2 (16.9, 31.4) | 0.04* |
| MUAC (cm) | 30.4 ± 5.1 | 30.2 ± 4.3 | 0.77 | 29.8 ± 4.4 | 31.1 ± 4.8 | 0.03* |
| Total body fat (%) | 31.9 ± 7.7 | 32.4 ± 6.8 | 0.67 | 32.0 ± 7.5 | 34.2 ± 6.8 | 0.02* |
| Muscle mass (kg) | 38.4 ± 5.4 | 37.7 ± 5.2 | 0.34 | 37.8 ± 4.9 | 39.5 ± 5.7 | 0.02* |
| Right hand grip strength (unit) | 61.9 ± 10.8 | 59.5 ± 9.5 | 0.11 | 59.0 ± 10.6 | 62.5 ± 10.7 | 0.01* |
| Left hand grip strength (unit) | 58.6 ± 11.3 | 57.5 ± 10.8 | 0.50 | 56.9 ± 9.8 | 58.1 ± 9.8 | 0.38 |
| Diastolic blood pressure (mmHg) | 78.6 ± 12.5 | 77.6 ± 12.3 | 0.60 | 81.8 ± 11.3 | 80.6 ± 11.8 | 0.42 |
| Systolic blood pressure (mmHg) | 121.0 (113.7, 130.5) | 118.0 (109.7, 128.5) | 0.13 | 123.5 (114.5, 134.0) | 123.0 (113.5, 137.3) | 0.99 |
| Fasting hs-CRP (pmol/l) | 1.55 (0.64, 3.70) | 1.48 (0.59, 2.87) | 0.46 | 1.29 (0.59, 3.14) | 1.36 (0.71, 4.26) | 0.22 |
| Haemoglobin (g/dl) | 12.5 ± 1.6 | 12.5 ± 1.4 | 0.94 | 12.7 ± 1.8 | 12.7 ± 1.3 | 0.93 |
| Anaemia (n) | 9 (6.0%) | 3 (4.2%) | 0.76 | 10 (4.7%) | 2 (2.6%) | 0.52 |
| Diabetes (n) | 15 (9.9%) | 6 (8.5%) | 0.81 | 17 (8.0%) | 3 (3.9%) | 0.30 |
| Malaria (n) | 3 (2.0%) | 0 (0.0%) | 0.55 | 4 (1.9%) | 1 (1.3%) | 1.00 |
| HIV (n) | 5 (3.3%) | 4 (5.6%) | 0.47 | 7 (3.3%) | 3 (3.9%) | 0.43 |
Data are presented as the mean ± standard deviation, median (interquartile range), or percentage. BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; MUAC, mid-upper arm circumference; TZS, Tanzanian Shilling. *Significant difference, p < 0.05.
Household monthly income was self-reported.
Among the older children, it was observed that seropositive children were slightly older, more likely to live in a rural compared to a peri-urban village, and to use a river as the main water source, when compared to the seronegative children (p ≤ 0.02). Furthermore, the seropositive children had a higher handgrip strength, similar to what was observed for the seropositive mothers (p = 0.03). Finally, children of the Sambaa ethnicity were more likely to be seropositive (p = 0.01).
Importantly, no associations were found between seropositivity and other comorbidities, including anaemia, diabetes, malaria, and HIV, for either the mothers or the children (Table 3).
Table 3.
Clinical characterization of the children
| PONA1 [Au?13] children (age 5–6 years) |
STOPPAM children (age 11–12 years) |
|||||
|---|---|---|---|---|---|---|
| SARS-CoV-2 Negative (n = 179) | SARS-CoV-2 Positive (n = 88) | p-Value | SARS-CoV-2 Negative (n = 208) | SARS-CoV-2 Positive (n = 159) | p-Value | |
| Age (years) | 5.4 ± 0.4 | 5.5 ± 0.4 | 0.16 | 11.6 ± 0.4 | 11.8 ± 0.4 | 0.001* |
| Sex, female | 85 (47.5%) | 46 (52.3%) | 0.52 | 92 (44.2%) | 94 (59.1%) | 0.006* |
| Ethnicity (n) | 0.52 | 0.01* | ||||
| Sambaa | 72 (40.2%) | 6 (40.9%) | 84 (44.4%) | 84 (52.8%) | ||
| Zigua | 3 (29.6%) | 20 (22.7%) | 40 (19.2%) | 37 (23.3%) | ||
| Pare | 10 (5.6%) | 7 (8.0%) | 12 (5.8%) | 9 (5.7%) | ||
| Bondei | 6 (3.4%) | 6 (6.8%) | 6 (2.9%) | 2 (1.3%) | ||
| Others | 38 (21.2%) | 19 (21.6%) | 66 (31.7%) | 27 (17.0%) | ||
| Main source of water (n) | 0.83 | 0.003* | ||||
| Tap | 126 (70.4%) | 64 (72.7%) | 147 (70.7%) | 100 (62.9%) | ||
| Well | 17 (9.5%) | 6 (6.8%) | 38 (18.3%) | 21 (13.2%) | ||
| River | 36 (20.1%) | 18 (20.5%) | 22 (10.6%) | 38 (23.9%) | ||
| Rural vs peri-urban village (%) | Rural (94.4%) | Rural (98.9%) | 0.11 | Rural (77.9%) | Rural (87.4%) | 0.02* |
| Household monthly income (TZS)a | 145 000 (70 000, 200 000) | 150 000 (70 000, 300 000) | 0.47 | 100 000 (50 000, 200 000) | 155 000 (100 000, 300 000) | 0.06 |
| Primary school attendance | NA | NA | - | 206 (99.0%) | 158 (99.4%) | 1.00 |
| Height (cm) | 106.9 ± 5.4 | 107.8 ± 5.0 | 0.20 | 138.2 ± 7.0 | 139.5 ± 7.3 | 0.10 |
| Weight (kg) | 16.4 ± 2.1 | 16.8 ± 2.4 | 0.20 | 29.9 (26.9, 32.9) | 30.2 (27.2, 33.9) | 0.32 |
| BMI (kg/m2) | 14.3 ± 1.2 | 14.4 ± 1.4 | 0.62 | 15.4 (14.7, 16.7) | 15.7 (14.6, 16.7) | 0.39 |
| Skinfold (mm) | 8.2 ± 1.9 | 8.9 ± 3.9 | 0.11 | 8.1 (6.5, 10.3) | 8.5 (7.0, 10.9) | 0.14 |
| MUAC (cm) | 16.0 ± 1.1 | 16.1 ± 1.3 | 0.23 | 19.1 (17.9, 20.3) | 19.4 (17.9, 20.6) | 0.50 |
| Total body fat (%) | 17.2 ± 3.4 | 17.9 ± 3.7 | 0.20 | 12.9 (10.7, 16.1) | 13.5 (11.0, 18.0) | 0.16 |
| Muscle mass (kg) | 12.7 ± 1.6 | 12.9 ± 1.6 | 0.35 | 24.5 ± 3.3 | 25.1 ± 3.9 | 0.12 |
| Right hand grip strength (unit) | N.A. | NA | - | 33.3 ± 7.3 | 35.0 ± 7.6 | 0.03* |
| Left hand grip strength (unit) | N.A. | NA | - | 31.4 ± 6.9 | 33.0 ± 7.4 | 0.03* |
| Diastolic blood pressure (mmHg) | 66.3 ± 9.2 | 66.2 ± 9.6 | 0.94 | 70.3 ± 8.2 | 70.4 ± 8.1 | 0.90 |
| Systolic blood pressure (mmHg) | 101.9 ± 10.2 | 100.5 ± 9.9 | 0.29 | 113.4 ± 9.5 | 114.0 ± 9.3 | 0.50 |
| Fasting hs-CRP (pmol/l) | 0.72 (0.30, 1.65) | 0.59 (0.30, 1.73) | 0.63 | 0.58 (0.30, 1.66) | 0.48 (0.30, 1.38) | 0.33 |
| Haemoglobin (g/dl) | 12.1 ± 1.3 | 11.9 ± 1.1 | 0.34 | 12.8 ± 1.3 | 12.8 ± 1.3 | 0.96 |
| Anaemia (n) | 5 (2.8%) | 7 (8.0%) | 0.07 | 2 (1.0%) | 5 (3.1%) | 0.25 |
| Malaria (n) | 15 (8.4%) | 6 (6.8%) | 0.81 | 13 (6.3%) | 16 (10.1%) | 0.24 |
| HIV (n) | 0 (0.0%) | 0 (0.0%) | - | 0 (0.0%) | 1 (0.6%) | 0.43 |
Data are presented as the mean ± standard deviation, median (interquartile range), or percentage. BMI, body mass index; hs-CRP, high sensitivity C-reactive protein; MUAC, mid-upper arm circumference; NA, not applicable; TZS, Tanzanian Shilling. *Significant difference, p < 0.05 [Au?14].
Household monthly income was self-reported.
Discussion
This is the first epidemiological study on anti-SARS-CoV-2 seroprevalence performed in the Tanzanian mainland. Between April and October 2021, a very high anti-SARS-CoV-2 seroprevalence was found, with the seropositivity rate ranging from 29% to 40% in relatively young adult women and 5–12-year-old children. Important epidemiological differences in seropositivity rates were found, including increased seropositivity in female children and increased seropositivity with increasing age, adiposity, and handgrip strength, and with lower socioeconomic status. It was possible to document dynamic peaks in viral infections through anti-SARS-CoV-2 seroprevalence measurements, and the data support that this may be an important tool when assessing previous SARS-CoV-2 infections in rural and non-tested/unvaccinated settings, and is highly suitable for large-scale surveillance for SARS-CoV-2 antibodies [22].
High SARS-CoV-2 seroprevalence in rural north-eastern Tanzania
Tanzania reported its first laboratory-confirmed COVID-19 case on March 15, 2020 [37], and by July 27, 2022, there had been 36 886 confirmed COVID-19 cases in Tanzania according to the World Health Organization [42]. With a population of 59.7 million people [42], this corresponds to an overall percentage of 0.06%, when conservatively assuming that all confirmed SARS-CoV-2 cases were Tanzanian residents and none were diagnosed more than once. In an epidemiological setting, seroprevalence estimates are useful for understanding the burden of asymptomatic or subclinical infections that would not otherwise be detected. Based on the average anti-SARS-CoV-2 seroprevalence of 37.1% in the present study, the anti-SARS-CoV-2 seropositivity rate is hence more than 700 times higher than the reported cases when considering the results from the rural north-eastern region to be projectable to the entire country.
This seroprevalence of 37.1% is in line with the results of other studies performed in the same time period (January to August 2021) on unvaccinated individuals in other SSA countries, including Mali with a seropositivity of 58.5% (n = 2672) [28], Nigeria with a seropositivity of 42% (n = 802) [4], and Zambia where seropositivity increased from approximately 13.5% in December 2020 to 35% in March 2021 (n = 2977) [32]. A recent similar study that was conducted on the two main islands of Zanzibar showed a seroprevalence of almost 60% [30]. Finally, a very recently published article has reported the results of a malariometric and SARS-CoV-2- survey performed in July 2021, which was conducted in two villages in north-eastern Tanzania [18], one of which – Mkokola – was also included in the present study; this previously published study reported a seropositivity rate of 32.5% (76/234) in Mkokola, while in the present study the rate was 15.4% (16/104) for the participants from this village.
The seroprevalence rates observed in this study and in other studies from SSA countries are substantially higher than those reported in population-wide studies from the UK and the USA. One large US study (n >2 million), also conducted during the second wave of the COVID-19 pandemic, estimated approximately 18% of the entire US population to have infection-induced positive seroprevalence for SARS-CoV-2-19 [Au?8] [14]. However, it is worth noting that contrary to other SSA studies [4,28,41], the present study was conducted in a rural setting with little demographic movement. Therefore, in larger cities and urban regions with more opportunity for transmission, the anti-SARS-CoV-2 seroprevalence is likely to be much higher than in rural areas. In addition, our population was young compared to other large-scale SSA studies in which older age groups and men were also included [5,32].
Surprisingly, the anti-SARS-CoV-2 seroprevalence was higher among the children than among the mothers (39% vs 29%). This is in contrast to previous studies from the same time period in South Africa, Mali, and Zambia, which showed that seroprevalence increased with age and was generally higher in adults than children (Simeni [5,21,28,34]). In addition, the anti-SARS-CoV-2 seroprevalence in children in the present study was much higher than that reported in paediatric populations elsewhere. Specifically, a study conducted in South Africa after the second wave reported a seroprevalence of 18% in children less than 5 years of age [5]. Similarly, lower seroprevalence rates have been reported in children in Zambia (4.0%) [21], Denmark (3.2%) [7], Germany (2–10%) [35], and Canada (5.8%) [44]. However, recently a large study from South Africa published the results from the omicron wave, and showed that in both rural and urban areas, the largest increase in seroprevalence in this later time period was seen in children 13–18 years of age compared to both younger and older age groups [15]. Data on the SARS-CoV-2 seroprevalence among paediatric populations in rural African settings are still limited. It appears that the present study is the most extensive analysis showing individual-level paediatric SARS-CoV-2 infection in SSA, with detailed clinical and socioeconomic data available. However, it remains unknown why a higher seroprevalence among children was found in the rural setting of this study. It can be speculated to be related to socioeconomic status and access to clean water, perhaps in combination with different genetic backgrounds, which may increase vulnerability to infection.
The current approach to SARS-CoV-2 testing in Tanzania is missing most infections
The high anti-SARS-CoV-2 seropositivity in children and mothers reported here may have important implications for the silent burden of disease and transmission dynamics in the study area, where the majority of the population were, and may still be, ignorant about the disease. The uptake of COVID-19 vaccination in Tanzania is suspected to have remained low, but there are no good estimates. It is also important to note that the first SARS-CoV-2 vaccines reached Tanzania at the end of July 2021. At that time, only healthcare workers and elderly Tanzanian citizens were prioritized for vaccination. None of the participants in the present study were offered SARS-CoV-2 vaccination while the data collection was ongoing. - The study findings align with those of a recent meta-analysis on socioecological, biophysical, and public health interventions in 47 African countries, which estimated that the African region has had a similar number of SARS-CoV-2 infections when compared to the rest of the world [3].
The higher than expected anti-SARS-CoV-2 seropositivity rate for mothers and children in the present study is likely to be attributable in part to continued viral transmission over the study period in this rural and peri-urban population, where no effective preventive strategies were in place [25]. Indeed, a progressive increase in seropositivity was found from April to August 2021 during the sample collection, indicating that active viral transmission from an unfolding COVID-19 wave was occurring in rural north-eastern Tanzania during this period. However, the majority of the anti-SARS-CoV-2 seropositive cases from this time period in our study may be speculated to have been asymptomatic, since participants presenting with any of the common COVID-19 symptoms had their enrolment and clinical examination postponed in order to prevent viral transmission during the data collection. Asymptomatic individuals have been shown to transmit SARS-CoV-2 at similar levels to symptomatic ones [5]. Therefore, there is a need for surveillance of hospitalizations, comorbidities, and scale-up of representative seroprevalence studies, as core response strategies. This continued viral transmission in rural areas in a context of low vaccine coverage creates room for the emergence of viral variants of concern, suggesting that interventions targeting asymptomatic individuals, including increased vaccine coverage to reach children, are needed [33].
Being female and of school age is associated with a higher risk of being SARS-CoV-2 seropositive
A higher prevalence of SARS-CoV-2 seropositivity was observed among older children (11–12 years) when compared to their younger counterparts (5–6 years). The older children were of school age, and at the time of data collection, the majority were attending school. Hence, the higher proportion of SARS-CoV-2 seropositivity among older children may reflect age-dependent susceptibility to infection and differences in contact patterns between younger children [6]. The virus might have predominantly spread from one child to another as they interacted with each other with less protection in place. Indeed, a trend towards an increased seropositivity rate was observed from April to June 2021 when schools were open, with the rate dipping again in July as schools were closed for the summer holidays. This is in line with a recent epidemiological study from Kenya, which found that reopening schools between the second and third waves led to only a minor increase in viral transmission and that socioeconomic and urban–rural population structures were critical determinants of the disease transmission [2]. Other factors such as socioeconomic status, occupation of the children and parents, and weather patterns might also have influenced transmission dynamics; but these were beyond the scope of the current analysis.
The impact of sex on SARS-CoV-2 infection is somewhat inconsistent and there is no general agreement about the impact of sex on antibody generation and the prognosis in SARS-CoV-2 infection [12,27]. While several studies involving adult populations have reported higher anti-SARS-CoV-2 antibody titres in women [9,27], other studies have reported equivalent levels in both sexes [17]. The present study found that SARS-CoV-2 seropositivity was higher in female children when compared to male children. Previous studies have suggested that the immune response to most pathogens is lower in men compared to women [36]. Therefore, an increased capacity to mount a greater magnitude of immune response against the infection in females as compared to males may partly explain the difference between the sexes observed in this study; however, this was not investigated. Other factors such as cultural practices, for example different gender-specific roles, may lead to more interaction among female children than among males, for instance, during indoor household work and sharing beds and bed nets.
Clinical and socioeconomic differences between the anti-SARS-CoV-2 seropositive and seronegative cases
Understanding of the pathophysiology of COVID-19 and how SARS-CoV-2 is transmitted and affects LMICs and vulnerable populations with low socioeconomic status levels is still poor, which is a cause for concern. This is not only due to the high and probably underestimated SARS-CoV-2 transmission rates in these regions [3,23], but also because such populations often live under a double burden of infectious diseases and increasing risks of non-communicable diseases, including hypertension, diabetes, and obesity [24]. There is a noticeable lack of association between seropositivity and comorbidities in this cohort, including HIV, malaria, anaemia, and diabetes, which is not immediately easy to explain. This may be due to the young age of the participants, or simply to sample size limitations, but could also reflect underlying important biological processes. Nevertheless, this should be examined in future investigations that include men and elderly individuals. However, it was found that participants who did not have a private water source but relied on drinking water from the river were more likely to have had a previous SARS-CoV-2 infection. Further, it was found that the older SARS-CoV-2 seropositive mothers had higher weight and body fat percentage, more muscle mass, larger MUAC and skinfold thickness, and higher right hand grip strength compared to the seronegative mothers. In addition, seropositive children also tended to be larger with more adiposity than the seronegative children. Available data show that obesity is a major risk factor for hospitalization and mortality related to COVID-19 [8,43], which is in line with the present study results showing increased adiposity among previously infected individuals. It could also be speculated that obesity may be a risk factor for disease and/or antibody acquisition too, which could explain why the results are not indicative of any association between seropositivity and other underlying conditions such as HIV and diabetes.
Limitations of the study
The data collection in this cross-sectional study took place from late April to late October 2021, and due to the limited knowledge of viral transmission in both Tanzania and neighbouring countries during this time period, it remains unclear when specific waves of pandemic transmissions were occurring in the country and region. This means that it is possible that mothers and children who participated in April may have seroconverted before October. Additionally, the present study involved only participants from a specific cohort and geographical area, hence their clinical and socioeconomic characteristics might differ from those of the general Tanzanian population. Furthermore, it is a limitation of this study that neither male adults nor elderly people were included. Therefore, the results should be interpreted with caution.
Conclusions
This study showed that SARS-CoV-2 transmission in Tanzania was higher than previously reported, even in rural areas. Furthermore, dynamic peaks in viral transmission can be documented through seroprevalence, which could be an important epidemiological tool when assessing previous SARS-CoV-2 infection in rural and non-tested/unvaccinated settings without access to rapid molecular diagnostics. Finally, and importantly, this study showed differences in age, socioeconomic status, and body composition between mothers and children who previously had SARS-CoV-2 infection.
In this setting, continued surveillance is vital to monitor new circulating viral variants, which pose a significant risk to vulnerable individuals, as well as to further investigate comorbidities and the pathophysiology of COVID-19 in populations such as the one studied here. This should be implemented in tandem with the intensification of vaccination strategies.
Acknowledgements
First and foremost, we greatly appreciate the participation of all of the women and the children in the study, and all committed healthcare workers assisting in the care of the women and their children. We are also thankful to the PONA2 study team members in Tanzania including Jaqueline Kichungo, Neema Malle, Regina Malugu, Celina Mzava, Eva Rimoy, Mohamed Mapondela, Rashid Mtumba, Walter Maranga, Emmanuel Kessy, Zeno Manjulungu, Gerson Maro, Francis Mkongo, Simba Athumani, Claud Tesha, and Humphrey Mathew, for their tremendous work efforts in recruitment, collecting data, and generating the biobank and database. We are thankful for the collaborations with the NIMR-Tanga Centre, Joint Malaria Programme, and the administration at Korogwe District Hospital, and all government staff working within KDH and satellite dispensaries. Finally, the authors would like to thank Mads Engelhardt Knudsen and Sif Kaas Nielsen from the Department of Clinical Immunology at Rigshospitalet for their excellent technical assistance.
Funding: The PONA2 study was funded by the “Læge Sofus Carl Emil Friis og Hustru Olga Doris Friis” Foundation and the Augustinus Foundation. L.H. is funded by the Danish Diabetes Academy supported by the Novo Nordisk Foundation, and the Danish Diabetes Association (Diabetesforeningen). P.G. is supported by funds from the Carlsberg Foundation (CF20-0045), the Novo Nordisk Foundation (NFF205A0063505 and NNF20SA0064201), and The Svend Andersen Research Foundation (SARF2021). C.S. is funded by the Danish Independent Research Fund: Clinician Scientist Positions, Medical Sciences. D.B. is supported by a National Health and Medical Research Council (Australia) Investigator Grant. D.L.C. is supported by grants from the Danish International Development Agency (DANIDA No. 17-03-KU and DANIDA No. 19-M06-KU). The Novo Nordisk Foundation Centre for Basic Metabolic Research is an independent research centre at the University of Copenhagen, partially funded by an unrestricted donation from the Novo Nordisk Foundation (NNF18CC0034900).
Conflict of interest: The authors declare no potential conflict of interest relevant to this study.
Footnotes
Abbreviations: AU, arbitrary units; BP, blood pressure; GA, gestational age; Hb, haemoglobin; hs-CRP, high sensitivity C-reactive protein; KDH, Korogwe District Hospital; LBW, low birthweight; LMIC, low- and middle-income countries; NCDs, non-communicable diseases.
Peter Garred and Line Hjort share last authorship.
Author contributions: O.A.M., D.T.R.M., S.G., G.M., L.G.G., D.L.C., I.C.B., D.B., C.S., and L.H. designed the PONA2 follow-up, and O.A.M., L.P-A., D.T.R.M., C.B.H., P.G., and L.H. designed the COVID-19 study. O.A.M., D.T.R.M., S.G., G.M., J.M., C.S., and L.H. performed the clinical study, and LP-A., V.M.L.L., and E.C.S.B. performed the COVID-19 antibody measurements. O.A.M., L.P-A., D.T.R.M., C.B.H., D.B., C.S., P.G., and L.H. analysed and interpreted the results. O.A.M. and L.H. wrote the manuscript, with contributions from all the co-authors who critically revised the manuscript and had access to the final version.
Data access: The datasets obtained and analysed in the current study are available from the last authors on reasonable request.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijregi.2022.11.011.
Appendix. Supplementary materials
References
- 1.Bayarri-Olmos Rafael, Idorn Manja, Rosbjerg Anne, Pérez-Alós Laura, Hansen Cecilie Bo, Johnsen Laust Bruun, Helgstrand Charlotte, et al. “SARS-CoV-2 Neutralizing Antibody Responses towards Full-Length Spike Protein and the Receptor-Binding Domain.”. Journal of Immunology (Baltimore, Md. : 1950) 2021;207(3):878–887. doi: 10.4049/jimmunol.2100272. [DOI] [PubMed] [Google Scholar]
- 2.Brand Samuel P C, Ojal John, Aziza Rabia, Were Vincent, Okiro Emelda A, Kombe Ivy K, Mburu Caroline, et al. “COVID-19 Transmission Dynamics Underlying Epidemic Waves in Kenya.”. Science (New York, N.Y.) 2021;374(6570):989–994. doi: 10.1126/science.abk0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabore Joseph Waogodo, Karamagi Humphrey Cyprian, Kipruto Hillary Kipchumba, Mungatu Joseph Kyalo, Asamani James Avoka, Droti Benson, Titi-Ofei Regina, et al. “COVID-19 in the 47 Countries of the WHO African Region: A Modelling Analysis of Past Trends and Future Patterns.”. The Lancet. Global Health. 2022;10(8):e1099–e1114. doi: 10.1016/S2214-109X(22)00233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chechet Gloria D, Kwaga Jacob K P, Yahaya Joseph, Noyes Harry, MacLeod Annette, Adamson Walt E. “SARS-CoV-2 Seroprevalence at Urban and Rural Sites in Kaduna State, Nigeria, during October/November 2021, Immediately Prior to Detection of the Omicron Variant.”. International Journal of Epidemiology. 2022 doi: 10.1093/ije/dyac141. June. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen Cheryl, Kleynhans Jackie, Gottberg Anne von, McMorrow Meredith L, Wolter Nicole, Bhiman Jinal N, Moyes Jocelyn, et al. “SARS-CoV-2 Incidence, Transmission, and Reinfection in a Rural and an Urban Setting: Results of the PHIRST-C Cohort Study, South Africa, 2020-21.”. The Lancet. Infectious Diseases. 2022;22(6):821–834. doi: 10.1016/S1473-3099(22)00069-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies Nicholas G, Klepac Petra, Liu Yang, Prem Kiesha, Jit Mark, CMMID COVID-19 working group, and Rosalind M Eggo “Age-Dependent Effects in the Transmission and Control of COVID-19 Epidemics.”. Nature Medicine. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 7.Espenhain Laura, Tribler Siri, Jørgensen Charlotte Sværke, Holm Hansen Christian, Wolff Sönksen Ute, Ethelberg Steen. “Prevalence of SARS-CoV-2 Antibodies in Denmark: Nationwide, Population-Based Seroepidemiological Study.”. European Journal of Epidemiology. 2021;36(7):715–725. doi: 10.1007/s10654-021-00796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frasca Daniela, Reidy Lisa, Cray Carolyn, Diaz Alain, Romero Maria, Kahl Kristin, Blomberg Bonnie B. “Influence of Obesity on Serum Levels of SARS-CoV-2-Specific Antibodies in COVID-19 Patients.”. PloS One. 2021;16(3) doi: 10.1371/journal.pone.0245424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grzelak Ludivine, Velay Aurélie, Madec Yoann, Gallais Floriane, Staropoli Isabelle, Schmidt-Mutter Catherine, Wendling Marie-Josée, et al. “Sex Differences in the Evolution of Neutralizing Antibodies to Severe Acute Respiratory Syndrome Coronavirus 2.”. The Journal of Infectious Diseases. 2021;224(6):983–988. doi: 10.1093/infdis/jiab127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen Cecilie Bo, Jarlhelt Ida, Pérez-Alós Laura, Landsy Lone Hummelshøj, Loftager Mette, Rosbjerg Anne, Helgstrand Charlotte, et al. “SARS-CoV-2 Antibody Responses Are Correlated to Disease Severity in COVID-19 Convalescent Individuals.”. Journal of Immunology (Baltimore, Md. : 1950) 2021;206(1):109–117. doi: 10.4049/jimmunol.2000898. [DOI] [PubMed] [Google Scholar]
- 11.Hjort Line, Møller Sofie Lykke, Minja Daniel, Msemo Omari, Nielsen Birgitte Bruun, Christensen Dirk Lund, Theander Thor, et al. “FOETAL for NCD-FOetal Exposure and Epidemiological Transitions: The Role of Anaemia in Early Life for Non-Communicable Diseases in Later Life: A Prospective Preconception Study in Rural Tanzania.”. BMJ Open. 2019;9(5) doi: 10.1136/bmjopen-2018-024861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho Jim Q, Sepand Mohammad Reza, Bigdelou Banafsheh, Shekarian Tala, Esfandyarpour Rahim, Chauhan Prashant, Serpooshan Vahid, Beura Lalit K, Hutter Gregor, Zanganeh Steven. “The Immune Response to COVID-19: Does Sex Matter?”. Immunology. 2022;166(4):429–443. doi: 10.1111/imm.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johns Hopkins University. 2020. “Understanding the COVID-19 Pandemic: In Insights from Johns Hopkins University Experts.” 2020. https://coronavirus.jhu.edu/.
- 14.Jones Jefferson M, Opsomer Jean D, Stone Mars, Benoit Tina, Ferg Robyn A, Stramer Susan L, Busch Michael P. “Updated US Infection- and Vaccine-Induced SARS-CoV-2 Seroprevalence Estimates Based on Blood Donations, July 2020-December 2021.”. JAMA. 2022;328(3):298–301. doi: 10.1001/jama.2022.9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleynhans Jackie, Tempia Stefano, Wolter Nicole, Gottberg Anne von, Bhiman Jinal N, Buys Amelia, Moyes Jocelyn, et al. “SARS-CoV-2 Seroprevalence after Third Wave of Infections, South Africa.”. Emerging Infectious Diseases. 2022;28(5):1055–1058. doi: 10.3201/eid2805.220278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levin Andrew T, Owusu-Boaitey Nana, Pugh Sierra, Fosdick Bailey K, Zwi Anthony B, Malani Anup, Soman Satej, et al. “Assessing the Burden of COVID-19 in Developing Countries: Systematic Review, Meta-Analysis and Public Policy Implications.”. BMJ Global Health. 2022;7(5) doi: 10.1136/bmjgh-2022-008477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo Chunhua, Liu Min, Li Qianyuan, Zheng Xiaoling, Ai Wen, Gong Feng, Fan Jinhong, Liu Shaowei, Wang Xi, Luo Jun. “Dynamic Changes and Prevalence of SARS-CoV-2 IgG/IgM Antibodies: Analysis of Multiple Factors.”. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2021;108(July):57–62. doi: 10.1016/j.ijid.2021.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyimo Eric, Fougeroux Cyrielle, Malabeja Anangisye, Mbwana Joyce, Hayuma Paul M, Liheluka Edwin, Turner Louise, et al. “Seroprevalence of SARS-CoV-2 Antibodies among Children and Adolescents Recruited in a Malariometric Survey in North-Eastern Tanzania July 2021.”. BMC Infectious Diseases. 2022;22(1):846. doi: 10.1186/s12879-022-07820-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandolo Jonathan, Msefula Jacquline, Henrion Marc Y R, Brown Comfort, Moyo Brewster, Samon Aubrey, Moyo-Gwete Thandeka, et al. “SARS-CoV-2 Exposure in Malawian Blood Donors: An Analysis of Seroprevalence and Variant Dynamics between January 2020 and July 2021.”. BMC Medicine. 2021;19(1):303. doi: 10.1186/s12916-021-02187-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mfinanga Sayoki G, Mnyambwa Nicholaus P, Minja Daniel T, Ntinginya Nyanda Elias, Ngadaya Esther, Makani Julie, Makubi Abel N. “Tanzania's Position on the COVID-19 Pandemic.”. Lancet (London, England) 2021;397(10284):1542–1543. doi: 10.1016/S0140-6736(21)00678-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mulenga Lloyd B, Hines Jonas Z, Fwoloshi Sombo, Chirwa Lameck, Siwingwa Mpanji, Yingst Samuel, Wolkon Adam, et al. “Prevalence of SARS-CoV-2 in Six Districts in Zambia in July, 2020: A Cross-Sectional Cluster Sample Survey.”. The Lancet. Global Health. 2021;9(6):e773–e781. doi: 10.1016/S2214-109X(21)00053-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuccetelli Marzia, Pieri Massimo, Gisone Francesca, Bernardini Sergio. “Combined Anti-SARS-CoV-2 IgA, IgG, and IgM Detection as a Better Strategy to Prevent Second Infection Spreading Waves.”. Immunological Investigations. 2022;51(2):233–245. doi: 10.1080/08820139.2020.1823407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Obande Godwin Attah, Bagudo Ahmad Ibrahim, Mohamad Suharni, Deris Zakuan Zainy, Harun Azian, Yean Chan Yean, Aziah Ismail, Singh Kirnpal Kaur Banga. “Current State of COVID-19 Pandemic in Africa: Lessons for Today and the Future.”. International Journal of Environmental Research and Public Health. 2021;18(19) doi: 10.3390/ijerph18199968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owino Victor O. “Challenges and Opportunities to Tackle the Rising Prevalence of Diet-Related Non-Communicable Diseases in Africa.”. The Proceedings of the Nutrition Society. 2019;78(4):506–512. doi: 10.1017/S0029665118002823. [DOI] [PubMed] [Google Scholar]
- 25.Patterson, Amy S. 2022. “The Tanzanian State Response to COVID-19 (2022).”
- 26.Pérez-Alós Laura, Armenteros Jose Juan Almagro, Madsen Johannes Roth, Hansen Cecilie Bo, Jarlhelt Ida, Hamm Sebastian Rask, Heftdal Line Dam, et al. “Modeling of Waning Immunity after SARS-CoV-2 Vaccination and Influencing Factors.”. Nature Communications. 2022;13(1):1614. doi: 10.1038/s41467-022-29225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poustchi Hossein, Darvishian Maryam, Mohammadi Zahra, Shayanrad Amaneh, Delavari Alireza, Bahadorimonfared Ayad, Eslami Saeid, et al. “SARS-CoV-2 Antibody Seroprevalence in the General Population and High-Risk Occupational Groups across 18 Cities in Iran: A Population-Based Cross-Sectional Study.”. The Lancet. Infectious Diseases. 2021;21(4):473–481. doi: 10.1016/S1473-3099(20)30858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sagara Issaka, Woodford John, Kone Mamady, Hamady Assadou Mahamadoun, Katile Abdoulaye, Attaher Oumar, Zeguime Amatigue, et al. “Rapidly Increasing SARS-CoV-2 Seroprevalence and Limited Clinical Disease in Three Malian Communities: A Prospective Cohort Study.”. MedRxiv : The Preprint Server for Health Sciences. 2021 doi: 10.1101/2021.04.26.21256016. April. [DOI] [Google Scholar]
- 29.Salako A O, Amoo O S, Odubela O O, Osuolale K A, James A B, Oladele D A, Musa A Z, et al. “Prevalence and Clinical Characteristics of Coronavirus Disease 2019 Seen at a Testing Centre in Lagos Nigeria.”. West African Journal of Medicine. 2021;38(1):54–58. [PubMed] [Google Scholar]
- 30.Salum Salum Seif, Sheikh Mohammed Ali, Hebestreit Antje, Kelm Sørge. “Anti SARS-CoV-2 Seroprevalence in Zanzibar in 2021 before the Omicron Wave.”. IJID Regions (Online) 2022;4(September):120–122. doi: 10.1016/j.ijregi.2022.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmiegelow Christentze, Scheike Thomas, Oesterholt Mayke, Minja Daniel, Pehrson Caroline, Magistrado Pamela, Lemnge Martha, et al. “Development of a Fetal Weight Chart Using Serial Trans-Abdominal Ultrasound in an East African Population: A Longitudinal Observational Study.”. PloS One. 2012;7(9):e44773. doi: 10.1371/journal.pone.0044773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanaube K, Schaap A, Klinkenberg E, Floyd S, Bwalya J, Cheeba M, de Haas P, et al. “SARS-CoV-2 Seroprevalence and Associated Risk Factors in Periurban Zambia: A Population-Based Study.”. International Journal of Infectious Diseases : IJID : Official Publication of the International Society for Infectious Diseases. 2022;118(May):256–263. doi: 10.1016/j.ijid.2022.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shiri Tinevimbo, Evans Marc, Talarico Carla A, Morgan Angharad R, Mussad Maaz, Buck Philip O, McEwan Phil, Strain William David. “Vaccinating Adolescents and Children Significantly Reduces COVID-19 Morbidity and Mortality across All Ages: A Population-Based Modeling Study Using the UK as an Example.”. Vaccines. 2021;(10):9. doi: 10.3390/vaccines9101180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Njonnou Simeni, Raoul Sylvain, Anangmo Nadia Christelle Noumedem, Lekpa Fernando Kemta, Noukeu Njinkui Diomede, Enyama Dominique, Ouankou Christian Ngongang, Balti Eric Vounsia, Eloumba Esther Astrid Mbono Samba, Tapouh Jean Roger Moulion, Choukem Simeon Pierre. “The COVID-19 Prevalence among Children: Hypotheses for Low Infection Rate and Few Severe Forms among This Age Group in Sub-Saharan Africa.”. Interdisciplinary Perspectives on Infectious Diseases. 2021;2021 doi: 10.1155/2021/4258414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorg Anna-Lisa, Bergfeld Leon, Jank Marietta, Corman Victor, Semmler Ilia, Goertz Anna, Beyerlein Andreas, et al. “Cross-Sectional Seroprevalence Surveys of SARS-CoV-2 Antibodies in Children in Germany, June 2020 to May 2021.”. Nature Communications. 2022;13(1):3128. doi: 10.1038/s41467-022-30482-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi Takehiro, Ellingson Mallory K, Wong Patrick, Israelow Benjamin, Lucas Carolina, Klein Jon, Silva Julio, et al. “Sex Differences in Immune Responses That Underlie COVID-19 Disease Outcomes.”. Nature. 2020;588(7837):315–320. doi: 10.1038/s41586-020-2700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tarimo Clifford Silver, Wu Jian. “The First Confirmed Case of COVID-19 in Tanzania: Recommendations Based on Lesson Learned from China.”. Tropical Medicine and Health. 2020;48:25. doi: 10.1186/s41182-020-00214-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The World Bank. 2020. “Tanzania Economic Update : Addressing the Impact of COVID-19.”
- 39.Tripathi Hemant G., Smith Harriet E., Sait Steven M., Sallu Susannah M., Whitfield Stephen, Jankielsohn Astrid, Kunin William E., Mazibuko Ndumiso, Nyhodo Bonani. “Impacts of COVID-19 on Diverse Farm Systems in Tanzania and South Africa.”. Sustainability. 2021;13(17):9863. doi: 10.3390/su13179863. [DOI] [Google Scholar]
- 40.USAID. 2021. “Tanzania Overview 2021.” 2021. https://www.usaid.gov/tanzania/newsroom/fact-sheets.
- 41.Uyoga Sophie, Adetifa Ifedayo M O, Karanja Henry K, Nyagwange James, Tuju James, Wanjiku Perpetual, Aman Rashid, et al. “Seroprevalence of Anti-SARS-CoV-2 IgG Antibodies in Kenyan Blood Donors.”. Science (New York, N.Y.) 2021;371(6524):79–82. doi: 10.1126/science.abe1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.WHO, The World health Organization. 2022. “WHO Coronavirus (COVID-19) Dashboard.” 2022. https://covid19.who.int/.
- 43.Wu Zunyou, McGoogan Jennifer M. “Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention.”. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 44.Zinszer Kate, McKinnon Britt, Bourque Noémie, Pierce Laura, Saucier Adrien, Otis Alexandra, Cheriet Islem, et al. “Seroprevalence of SARS-CoV-2 Antibodies Among Children in School and Day Care in Montreal, Canada.”. JAMA Network Open. 2021;4(11) doi: 10.1001/jamanetworkopen.2021.35975. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.