Abstract
Behaviour and physiology are altered in reproducing animals, but neuronal circuits that regulate these changes remain largely unknown. Insights into mechanisms that regulate and possibly coordinate reproduction-related traits could be gleaned from the study of sex pheromones that can improve the reproductive success of potential mating partners. In Caenorhabditis elegans, the prominent male pheromone, ascr#10, modifies reproductive behaviour and several aspects of reproductive physiology in hermaphrodite recipients, including improving oocyte quality. Here we show that a circuit that contains serotonin-producing and serotonin-uptaking neurons plays a key role in mediating effects of ascr#10 on germline development and egg laying behaviour. We also demonstrate that increased serotonin signalling promotes proliferation of germline progenitors in adult hermaphrodites. Our results establish a role for serotonin in maintaining germline quality and highlight a simple neuronal circuit that acts as a linchpin that couples food intake, mating behaviour, reproductive output, and germline renewal and provisioning.
Keywords: serotonin, C. elegans, pheromone, germline, reproduction, coordination
1. Introduction
Males and females of the same species employ a rich repertoire of signals to improve reproductive success, including sex pheromones that can modulate behaviour and reproductive physiology of potential mates [1]. The case of the most abundant male-biased ascaroside pheromone in Caenorhabditis elegans, ascr#10 [2], is instructive. This small molecule alters several behaviours. Hermaphrodites exposed to physiological concentrations of ascr#10 reduce exploratory movement [3]. A shift from global to local exploration is generally associated with increased exploitation of local resources [4]. ascr#10 also increases mating receptivity in hermaphrodites and promotes egg laying in already reproducing animals [3].
In addition to these behavioural changes, ascr#10 alters hermaphrodite reproductive physiology. It improves sperm guidance [5] and affects several aspects of development of the oogenic germline. Exposure to ascr#10 increases mitotic proliferation of germline precursor cells (GPCs) [6]. In C. elegans hermaphrodites, GPCs proliferate and, following the irreversible switch from spermatogenesis to oogenesis, differentiate into oocytes; oocyte production can continue well into adulthood as long as sperm is available for fertilization [7]. The increased GPC proliferation in the presence of ascr#10 has no fewer than two consequences. First, it increases stores of GPCs as the worms age [8]. Second, it improves quality of the oogenic germline. The improvement is manifested in a more youthful oocyte morphology, decreased rates of chromosomal nondisjunction, and lower embryonic lethality both in the wild-type and mutant genetic backgrounds [6]. A likely mechanism responsible for the improved oocyte quality on ascr#10 is that the majority of the extra-generated GPCs undergo physiological cell death, and the salvaged components (nutrients, metabolites, organelles, etc.) are used to improve the quality of the surviving oocytes [6]. That an external signal can elicit these effects demonstrates that the nervous system can regulate germline quality, but the specific circuits are not known.
Some effects of ascr#10 are known to require the function of a specific serotonin circuit that contains NSM and HSN neurons that signal via the mod-1 receptor [3,9], the same circuit that regulates exploratory behaviour [10]. We therefore tested whether the same serotonin signalling regulates quality of the oogenic germline.
2. Results and discussion
(a) . The serotonin circuit that is required for ascr#10 improvement of oocyte quality
There are five classes of serotonergic neurons in C. elegans hermaphrodites; three of them (NSM, ADF and HSN) express a serotonin biosynthetic enzyme TPH-1 and can therefore produce serotonin, whereas two (AIM and RIH) can only uptake serotonin synthesized elsewhere [11]. Of the six annotated high-affinity and two low-affinity serotonin receptors, only one, MOD-1, is required for the known effects of ascr#10 [3,9,12]. Exposure to ascr#10 leads to increased serotonin signalling from two classes of producing neurons, NSM and HSN, that acts via the MOD-1 receptor [3,12] expressed in 40 classes of neurons [13]. We sought to test whether the NSM/HSN/MOD-1 neuronal circuit also mediates the beneficial effects of ascr#10 on the oogenic germline.
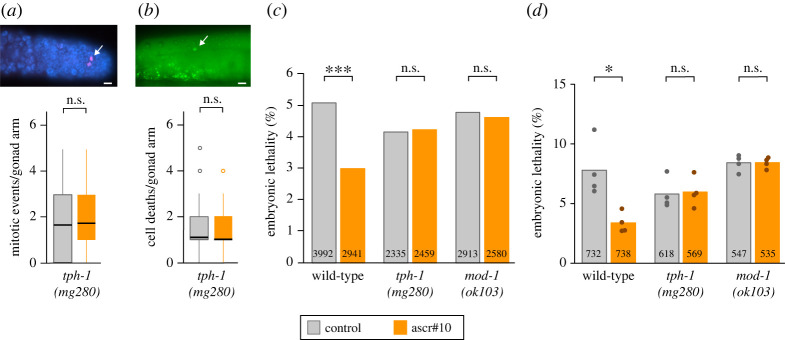
Exposure to ascr#10 (see Methods for details) increased GPC proliferation in wild-type N2 hermaphrodites [6], but no increase was seen in tph-1 (figure 1a) or mod-1 (electronic supplementary material, figure S1a) mutants. In the presence of ascr#10, incidence of physiological cell death in the germline increased only if GPC proliferation was increased [6]. Consistent with this results, tph-1 mutants showed no increase of germline cell death, likely because they were unable to increase germline proliferation in the presence of ascr#10 (figure 1b). The ultimate effect of ascr#10 on oocyte quality is increased probability of successful embryonic development [6]. Exposure to ascr#10 nearly halved embryonic lethality in broods of wild-type hermaphrodites that were mated just after exhausting their self-sperm supply (figure 1c). The same was the case in self-broods of older wild-type hermaphrodites (figure 1d). Because improvements occurred regardless of the source of sperm, these findings further support the idea that ascr#10 improves oocyte quality [6]. Loss-of-function alleles of tph-1 or mod-1 precluded this quality improvement by the pheromone (figure 1c,d). We concluded, that pheromone effects on the oogenic germline require serotonin signalling, specifically acting via the MOD-1 receptor.
Figure 1.
Serotonin signalling is required for the beneficial effects of ascr#10 on the oogenic germline. (a) Unlike wild-type N2 hermaphrodites [6], tph-1 hermaphrodites show no increase of germline precursor divisions in the presence of ascr#10. Germline proliferation was quantified as ‘mitotic events’ detected using phospho-Histone 3 (pH3) staining (magenta puncta indicated by an arrow in the inset) during day 2 of adulthood. Germline stained with DAPI, shown in blue. (b) Unlike wild-type N2 hermaphrodites [6], tph-1 hermaphrodites show no increase of germline cell death in the presence of ascr#10. Quantified using SYTO12 staining (indicated with an arrow in the inset) during day 3 of adulthood. In (a,b), black bars denote means. In (a,b), scale bars in the insets are 10 µm. (c) Percentage of unhatched embryos on versus off ascr#10 in the progeny of N2, tph-1 and mod-1 self-sperm depleted hermaphrodites mated on day 5 of adulthood to young males. (d) Percentage of unhatched embryos on versus off ascr#10 in the self-progeny of N2, tph-1 and mod-1 hermaphrodites during days 4 and 5 of adulthood. In (c,d), total numbers of tested embryos are indicated inside relevant bars. In (d), dots represent percentage of embryonic lethality in independent experiments. Asterisks indicate levels of statistical significance (* for p < 0.05, *** for p < 0.001). Kolmogorov–Smirnov test in (a,b), binomial test in (c,d). See electronic supplementary material, table S1 for primary data and details of statistical analyses. (Online version in colour.)
(b) . Increased serotonin signalling promotes germline proliferation
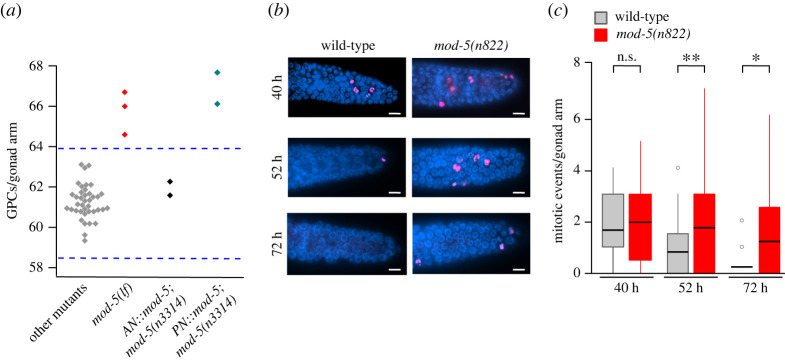
Serotonin signalling is increased following exposure to ascr#10 [3,12] and is required for ascr#10 effects on the oogenic germline (figure 1). Can increased serotonin signalling alone, without exogenous male pheromone, affect the hermaphrodite germline as does ascr#10? Loss of function mutations in the serotonin transporter gene mod-5 reduce serotonin uptake and therefore increase the amount of serotonin available at synapses effectively increasing serotonin signalling [14]. We first examined several strains for the number of GPCs, a measure that is a convenient proxy for elevated germline proliferation [6]. The number of GPCs in the otherwise untreated hermaphrodites carrying mod-5(lf) mutations was significantly higher than in the wild-type (average approx. equal to 61 [15]) or in any of the 31 other mutant strains we tested (figure 2a), suggesting that higher levels of serotonin signalling lead to the increase in the number of GPCs. The mod-5 gene is expressed in four classes of serotonin neurons including producing (NSM and ADF) and uptaking-only (AIM and RIH) cells [11,13]. Whereas expressing MOD-5 in the producing NSM and ADF neurons did not rescue the mod-5 defect, expression in a set of cells that included AIMs and RIH, but no serotonin-producing neurons, restored the wild-type GPC numbers (figure 2a).
Figure 2.
Increased serotonin signalling increases the number of germline precursors. (a) GPC counts from 39 experiments using 31 mutant strains tested in [3] and shown in the electronic supplementary material, table S2. Three experiments involving mod-5 hermaphrodites—two experiments using mod-5(n822), averages = 66.7 and 66, and one experiment using mod-5(n3314), average =64.6. Two experiments in which in mod-5(n3314) mutants MOD-5 function was restored in neurons including the absorbing AIM and RIH (AN::mod-5), and two experiments in which in mod-5(n3314) mutants MOD-5 function was restored in neurons including the producing NSM and ADF (PN::mod-5). Each diamond represents the mean value from one experiment. Dashed lines delimit three standard deviations above and below the mean of all strains except mod-5. See electronic supplementary material, figure S1b for more detail. (b) Representative images of pH3 staining (magenta) of gonads (DAPI stain in blue) in wild-type N2 and mod-5 hermaphrodites aged to mid-L4 (40 h), pre-reproductive adult (52 h) and day 2 of adulthood (72 h). Scale bars are 20 µm. (c) Quantification of cell divisions (pH3 staining) in the Progenitor Zone in the germlines of N2 and mod-5 hermaphrodites. In none of the experiments in this figure, were hermaphrodites treated with ascr#10. Asterisks indicate levels of statistical significance (* for p < 0.05, ** for p < 0.01; Kolmogorov–Smirnov test). See electronic supplementary material, table S1 for primary data and details of statistical analyses. (Online version in colour.)
Proliferation of the germline precursor cells is reduced in adult C. elegans hermaphrodites compared to late larvae [16]. The untreated mod-5(lf) adult hermaphrodites showed increased germline proliferation (figure 2b,c) that was comparable to that of wild-type worms exposed to ascr#10 [6].
(c) . The NSM/HSN/MOD-1 circuit regulates egg laying in response to ascr#10
Caenorhabditis elegans hermaphrodites continuously produce embryos as long as oocytes and sperm remain available. If the rate of egg laying is lower than the rate of fertilization, embryos accumulate in the uterus (figure 3a). Serotonin signal from HSN neurons stimulates egg laying [18], while the loss of tph-1 reduces egg laying and thus increases the number of embryos retained in the uterus [19]. Exposure to ascr#10 increases serotonin signalling from NSM and HSN neurons and stimulates egg laying [3]. We tested the role of the NSM/HSN/MOD-1 circuit in the pheromone effect on egg laying. The loss of the TPH-1 function in all neurons expressing this enzyme or specifically in NSM or HSN neurons completely negated the stimulating effects of ascr#10 on egg laying (figure 3b). Loss-of-function mod-1 mutants retained about the same number of embryos as the wild-type, as reported previously [20], but were unable to increase egg laying on ascr#10.
Figure 3.
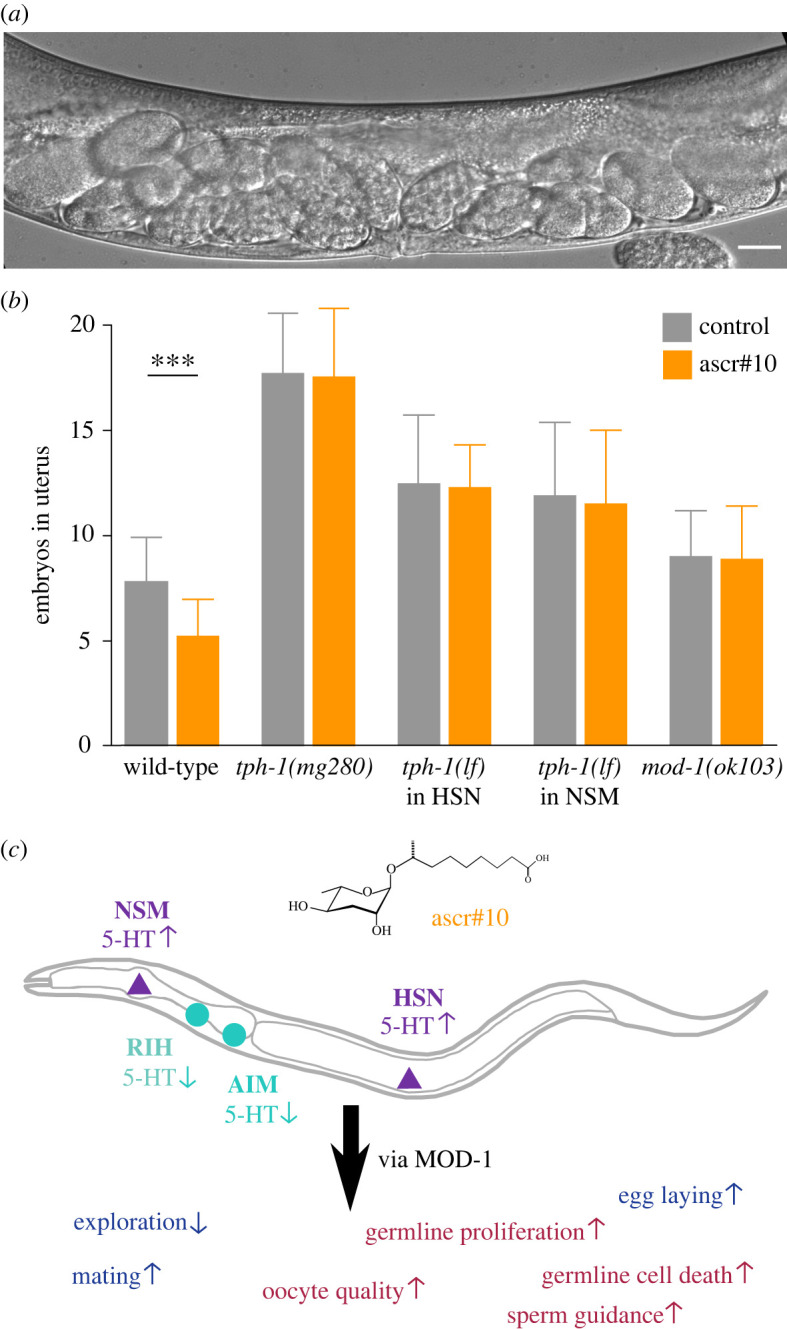
The serotonin circuit that coordinately regulates multiple reproductive functions in C. elegans. (a) An image of embryos accumulated in the uterus; tph-1(mg280) is shown. Scale bar is 20 µm. (b) The comparison of the number of embryos accumulated in the uterus on versus off ascr#10. Asterisks indicate statistical significance (*** for p < 0.001; Kolmogorov–Smirnov test). See electronic supplementary material, table S1 for primary data and details of statistical analyses. (c) A hypothesis regarding the role of serotonin in promoting reproductive behaviour and physiology. Increased serotonin (5-HT) signalling from tph-1-expressing neurons NSM and HSN (purple) affects a number of reproductive behaviours (blue) and germline physiology (red) via the MOD-1 receptor. Up and down arrows next to traits indicate whether they are up- or downregulated by increased serotonin signalling. RIH and AIM neurons reduce serotonin signalling. The male pheromone ascr#10 stimulates serotonin signalling from NSM and HSN. The way in which ascr#10 improves sperm guidance in the hermaphrodite reproductive tract [5] is not discussed in detail here, but sperm guidance is positively regulated by serotonin signalling [17]. (Online version in colour.)
(d) . A model for coordinated effects of serotonin on Caenorhabditis elegans reproduction
Our results implicate NSM, HSN and AIM/RIH (i.e. the majority of serotonin neurons) in mediating reproductive functions in C. elegans hermaphrodites (figure 3c). Increased signalling from the serotonin-producing NSM and HSN promotes several reproductive traits, whereas AIM/RIH limit this signalling by removing serotonin. Downstream of the serotonin balance established by the NSM/HSN versus AIM/RIH antagonism is the MOD-1 receptor that is expressed in 40 classes of neurons. We infer that further downstream signalling from a currently unknown subset of these neurons modulates, via currently unknown but presumably neurotransmitter and/or neuropeptide signals, several reproductive traits described here. Determining which neurons and signals are involved in these processes promises to advance understanding of how the nervous system controls reproduction and reveal the extent to which reproductive processes are coordinately regulated.
The roles of NSM and HSN neurons in reproduction are particularly interesting. Serotonin release from HSN neurons stimulates egg laying [18]. Here we showed that increased serotonin signalling (due to the loss of serotonin uptake via MOD-5) increases germline proliferation. This suggests that the signal that promotes egg laying episodes also promotes production of germline precursors, presumably to replenish oocyte stores depleted by offspring production. The pharyngeal NSM are enteric sensory neurons that detect food ingestion and release serotonin as a signal [21]. Therefore, increased serotonin signalling from NSM likely promotes processes associated with greater food ingestion. Consistent with this idea, ascr#10 reduces exploration in a manner that depends on serotonin signalling from NSM [3,12].
These functions of NSM and HSN neurons suggest a model for the role of serotonin in orchestrating multiple reproduction-related events. On the one hand, increased serotonin signalling on ascr#10 reduces exploration leading to a focus on consumption of local resources and greater mating receptivity [3]. On the other hand, increased serotonin signalling increases egg laying and proliferation of germline precursors. The physiological cell death of the majority of extra germline precursors liberates nutrients, organelles, etc. that are used to improve the quality of surviving oocytes. In this way, the circuit that consists of the serotonin-producing NSM and HSN neurons, the MOD-5 transporter in AIM/RIH neurons, and the MOD-1 receptor coordinates resource consumption required for greater reproductive output, with reproduction-promoting behaviours, and matches quality and quantity of produced oocytes to demands imposed by germline expenditure and the presence of potential mates. As such, this circuit may serve as an important regulator of the trade-off between somatic maintenance and germline investment. As discussed elsewhere [6], ascr#10 promotes greater resource allocation to progeny production at the expense of the soma—although this pheromone improves the quality of the oogenic germline [6], it shortens the lifespan [8,22]. It is not currently clear whether male signalling to hermaphrodites via ascr#10 reflects sexual conflict or cooperation.
(e) . Is serotonin a conserved regulator of reproduction?
Evidence indicates that serotonin regulates reproduction-related traits in different species in ways that may be conserved. For example, serotonin signalling reduces exploratory movement in C. elegans [10], D. melanogaster [23] and mice [24]. In D. melanogaster, serotonin is involved in regulating a dietary switch that occurs in females following mating and manages the balance of nutrients, a role that may be conserved in other animals [25]. In Drosophila females, mating causes changes in the levels and distribution of serotonin in the termini of neurons that innervate reproductive organs [26]. In mosquitoes, serotonin promotes ovarian development [27]. In mammals, serotonin exerts complex effects on the germline [28] and on reproduction-related behaviours [29]. Some, of these effects may be shared with other vertebrates [30]. Future comparative work will benefit from standardizing experimental paradigms across species. We also suggest that precise definition of the circuits (sources of signal, receptors and sites of action) involved in regulating specific behaviours and physiological processes, as we reported here, will be particularly important for inferring conserved roles of serotonin in regulating reproductive functions in animals.
3. Materials and methods
The following stains were used: wild-type N2 (CGC), MT15434 tph-1(mg280) (CGC), MT9668 mod-1(ok103) (CGC), AN::mod-5 mbr-1p::mod-5;mod-5(n3314) (Sze lab), PN::mod-5 tph-1p::mod-5;mod-5(n3314) (Sze lab), MT8944 mod-5(n822) (Koelle lab), MT9772 mod-5(n3314) (CGC), CX13576 tph-1(mg280) II; kySi56 IV; kyEx4107[egl-6::nCre] (aka tph-1(lf) in HSN) (Bargmann lab) and CX13572 tph-1(mg280) II; kySi56 IV; kyEx4057[ceh-2::nCre] (aka tph-1(lf) in NSM) (Bargmann lab). Standard, previously published methods were used [3,6,9,12,15]. All experimental treatments were processed in parallel with matched controls. Worms were synchronized by hypochlorite treatment and overnight incubation in M9, after which the synchronized L1 larvae were placed (in small populations, 10 or 30 worms, depending on the experiment) on agar plates seeded with E. coli OP50. Pre-reproductive adults (48 h post-release from L1 arrest) were transferred to OP50-seeded plates that were either control or conditioned with synthetic ascr#10 (gift of F. C. Schroeder). This protocol ensured that hermaphrodites were exposed to pheromone after the switch from spermatogenesis to oogenesis and during their pheromone-sensitive age [6]. For assessing embryonic lethality following mating, day 5 hermaphrodites (these have exhausted self-sperm) were singled and mated to one young male for 2 h. Numbers of live and dead progeny were counted for 3 days thereafter. For assessing embryonic lethality in self-broods, progeny produced during the last day of self-fertility (end of day 4 through day 5 of adulthood) was considered. More detailed protocol can be found in [6]. For quantifying germline proliferation, gonads were dissected and stained with Anti-Histone H3 (phospho S10) antibodies following a modified protocol of [31], as described in [6], and only prophase nuclei were counted. For counting cell death events, we used SYTO12 (Invitrogen) and the protocol by [32] as detailed in [6]. For GPC counts, on day 5 of adulthood, hermaphrodites were stained with DAPI (4′,6-diamidino-2-phenylindole) as described [8] and the germline precursor cells were counted. The boundary between the Progenitor Zone and the more proximal Transition Zone is defined by the appearance of crescent-shaped nuclei that have progressed to leptotene/zygotene stages of meiotic prophase [33,34]. The AN::mod-5 (absorbing neurons) and PN::mod-5 (producing neurons) strains (gift of J. Y. Sze) were reported in [11]. In these mod-5 mutant strains, the expression of the wild-type MOD-5 was restored under control of heterologous promoters. The expression of the AN::mod-5 was directed by a mbr-1 promoter. The expression of the mbr-1 gene can be detected in 28 classes of neurons [13], but the only overlap with the pattern of mod-5 expression is in AIM and RIH neurons. The expression of the PN::mod-5 was directed by the BCD region of the tph-1 promoter [35]. The overlap between the expression pattern of this promoter and the mod-5 gene is in two neurons: NSM and ADF. To quantify the number of embryos in the uterus, 72 h adult hermaphrodites were transferred to either control or ascr#10 plates for 1 h. After that time, the worms were placed individually in domed PCR caps containing 20 µl of hypochlorite solution and allowed to dissolve. After the hermaphrodite body disintegrated, the number of fertilized embryos was counted. Some strains were also tested at 96 h post-release from L1 arrest. The strains in which the tph-1 was deleted in either NSM or in HSN neurons were reported in [10].
Acknowledgements
We thank R. Morimoto for generous hospitality, C. Bargmann, M. Koelle and J. Y. Sze for strains, and F. Schroeder for ascr#10. We thank WormBase and the Caenorhabditis Genetics Center (CGC). WormBase is supported by grant U41 HG002223 from the National Human Genome Research Institute at the NIH, the UK Medical Research Council, and the UK Biotechnology and Biological Sciences Research Council.
Data accessibility
The data are provided in the electronic supplementary material [36].
Authors' contributions
E.Z.A.: conceptualization, data curation, formal analysis, investigation, methodology and writing—review and editing; S.D.: data curation and investigation; I.R.: conceptualization, data curation, formal analysis, funding acquisition, investigation, project administration, supervision, visualization and writing—original draft.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was funded in part by the NIH (grant no. R01GM126125) grant to I.R. The CGC is funded by the NIH Office of Research Infrastructure Programs (grant no. P40 OD010440).
References
- 1.Wyatt TD. 2014. Pheromones and animal behavior: chemical signals and signatures, 2nd edn. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 2.Izrayelit Y, Srinivasan J, Campbell SL, Jo Y, Von Reuss SH, Genoff MC, Sternberg PW, Schroeder FC. 2012. Targeted metabolomics reveals a male pheromone and sex-specific ascaroside biosynthesis in Caenorhabditis elegans. ACS Chem. Biol. 7, 1321-1325. ( 10.1021/cb300169c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aprison EZ, Ruvinsky I. 2019. Coordinated behavioral and physiological responses to a social signal are regulated by a shared neuronal circuit. Curr. Biol. 29, 4108-15 e4. ( 10.1016/j.cub.2019.10.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charnov EL. 1976. Optimal foraging, the marginal value theorem. Theor. Popul. Biol. 9, 129-136. ( 10.1016/0040-5809(76)90040-x) [DOI] [PubMed] [Google Scholar]
- 5.Aprison EZ, Ruvinsky I. 2015. Sex Pheromones of C. elegans males prime the female reproductive system and ameliorate the effects of heat stress. PLoS Genet. 11, e1005729. ( 10.1371/journal.pgen.1005729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aprison EZ, Dzitoyeva S, Angeles-Albores D, Ruvinsky I. 2022. A male pheromone that improves quality of the oogenic germline. Proc. Natl. Acad. Sci. USA 119, e2015576119. ( 10.1073/pnas.2015576119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubbard EJA, Schedl T. 2019. Biology of the Caenorhabditis elegans germline stem cell system. Genetics 213, 1145-1188. ( 10.1534/genetics.119.300238) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aprison EZ, Ruvinsky I. 2016. Sexually antagonistic male signals manipulate germline and soma of C. elegans hermaphrodites. Curr. Biol. 26, 2827-2833. ( 10.1016/j.cub.2016.08.024) [DOI] [PubMed] [Google Scholar]
- 9.Aprison EZ, Ruvinsky I. 2022. The roles of several sensory neurons and the feedback from egg laying in regulating the germline response to a sex pheromone in C. elegans hermaphrodites. MicroPubl. Biol. ( 10.17912/micropub.biology.000523) [DOI]
- 10.Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. 2013. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell 154, 1023-1035. ( 10.1016/j.cell.2013.08.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jafari G, Xie Y, Kullyev A, Liang B, Sze JY. 2011. Regulation of extrasynaptic 5-HT by serotonin reuptake transporter function in 5-HT-absorbing neurons underscores adaptation behavior in Caenorhabditis elegans. J. Neurosci. 31, 8948-8957. ( 10.1523/JNEUROSCI.1692-11.2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aprison EZ, Ruvinsky I. 2019. Dynamic regulation of adult-specific functions of the nervous system by signaling from the reproductive system. Curr. Biol. 29, 4116-23 e3. ( 10.1016/j.cub.2019.10.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taylor SR, et al. 2021. Molecular topography of an entire nervous system. Cell 184, 4329-47 e23. ( 10.1016/j.cell.2021.06.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ranganathan R, Sawin ER, Trent C, Horvitz HR. 2001. Mutations in the Caenorhabditis elegans serotonin reuptake transporter MOD-5 reveal serotonin-dependent and -independent activities of fluoxetine. J. Neurosci. 21, 5871-5884. ( 10.1523/JNEUROSCI.21-16-05871.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aprison EZ, Ruvinsky I. 2017. Counteracting ascarosides act through distinct neurons to determine the sexual identity of C. elegans Pheromones. Curr. Biol. 27, 2589-99 e3. ( 10.1016/j.cub.2017.07.034) [DOI] [PubMed] [Google Scholar]
- 16.Roy D, Michaelson D, Hochman T, Santella A, Bao Z, Goldberg JD, Hubbard EJA. 2016. Cell cycle features of C. elegans germline stem/progenitor cells vary temporally and spatially. Dev. Biol. 409, 261-271. ( 10.1016/j.ydbio.2015.10.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edmonds JW, Prasain JK, Dorand D, Yang Y, Hoang HD, Vibbert J, Kubagawa HM, Miller MA. 2010. Insulin/FOXO signaling regulates ovarian prostaglandins critical for reproduction. Dev. Cell 19, 858-871. ( 10.1016/j.devcel.2010.11.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schafer WF. 2006. Genetics of egg-laying in worms. Annu. Rev. Genet. 40, 487-509. ( 10.1146/annurev.genet.40.110405.090527) [DOI] [PubMed] [Google Scholar]
- 19.Tanis JE, Moresco JJ, Lindquist RA, Koelle MR. 2008. Regulation of serotonin biosynthesis by the G proteins Galphao and Galphaq controls serotonin signaling in Caenorhabditis elegans. Genetics 178, 157-169. ( 10.1534/genetics.107.079780) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hapiak VM, Hobson RJ, Hughes L, Smith K, Harris G, Condon C, Komuniecki P, Komuniecki RW. 2009. Dual excitatory and inhibitory serotonergic inputs modulate egg laying in Caenorhabditis elegans. Genetics 181, 153-163. ( 10.1534/genetics.108.096891) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rhoades JL, et al. 2019. ASICs mediate food responses in an enteric serotonergic neuron that controls foraging behaviors. Cell 176, 85-97 e14. ( 10.1016/j.cell.2018.11.023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ludewig AH, et al. 2019. An excreted small molecule promotes C. elegans reproductive development and aging. Nat. Chem. Biol. 15, 838-845. ( 10.1038/s41589-019-0321-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pooryasin A, Fiala A. 2015. Identified serotonin-releasing neurons induce behavioral quiescence and suppress mating in Drosophila. J. Neurosci. 35, 12 792-12 812. ( 10.1523/JNEUROSCI.1638-15.2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lottem E, Banerjee D, Vertechi P, Sarra D, Lohuis MO, Mainen ZF. 2018. Activation of serotonin neurons promotes active persistence in a probabilistic foraging task. Nat. Commun. 9, 1000. ( 10.1038/s41467-018-03438-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vargas MA, Luo N, Yamaguchi A, Kapahi P. 2010. A role for S6 kinase and serotonin in postmating dietary switch and balance of nutrients in D. melanogaster. Curr. Biol. 20, 1006-1011. ( 10.1016/j.cub.2010.04.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heifetz Y, Lindner M, Garini Y, Wolfner MF. 2014. Mating regulates neuromodulator ensembles at nerve termini innervating the Drosophila reproductive tract. Curr. Biol. 24, 731-737. ( 10.1016/j.cub.2014.02.042) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling L, Raikhel AS. 2018. Serotonin signaling regulates insulin-like peptides for growth, reproduction, and metabolism in the disease vector Aedes aegypti. Proc. Natl Acad. Sci. USA 115, E9822-E9E31. ( 10.1073/pnas.1808243115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dube F, Amireault P. 2007. Local serotonergic signaling in mammalian follicles, oocytes and early embryos. Life Sci. 81, 1627-1637. ( 10.1016/j.lfs.2007.09.034) [DOI] [PubMed] [Google Scholar]
- 29.Angoa-Perez M, Kuhn DM. 2015. Neuroanatomical dichotomy of sexual behaviors in rodents: a special emphasis on brain serotonin. Behav. Pharmacol. 26, 595-606. ( 10.1097/FBP.0000000000000157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prasad P, Ogawa S, Parhar IS. 2015. Role of serotonin in fish reproduction. Front. Neurosci. 9, 195. ( 10.3389/fnins.2015.00195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crittenden SL, Seidel HS, Kimble J. 2017. Analysis of the C. elegans germline stem cell pool. Methods Mol. Biol. 1463, 1-33. ( 10.1007/978-1-4939-4017-2_1) [DOI] [PubMed] [Google Scholar]
- 32.Gumienny TL, Lambie E, Hartwieg E, Horvitz HR, Hengartner MO. 1999. Genetic control of programmed cell death in the Caenorhabditis elegans hermaphrodite germline. Development 126, 1011-1022. ( 10.1242/dev.126.5.1011) [DOI] [PubMed] [Google Scholar]
- 33.Hansen D, Hubbard EJ, Schedl T. 2004. Multi-pathway control of the proliferation versus meiotic development decision in the Caenorhabditis elegans germline. Dev. Biol. 268, 342-357. ( 10.1016/j.ydbio.2003.12.023) [DOI] [PubMed] [Google Scholar]
- 34.Crittenden SL, Leonhard KA, Byrd DT, Kimble J. 2006. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol. Biol. Cell 17, 3051-3061. ( 10.1091/mbc.e06-03-0170) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sze JY, Zhang S, Li J, Ruvkun G. 2002. The C. elegans POU-domain transcription factor UNC-86 regulates the tph-1 tryptophan hydroxylase gene and neurite outgrowth in specific serotonergic neurons. Development 129, 3901-3911. ( 10.1242/dev.129.16.3901) [DOI] [PubMed] [Google Scholar]
- 36.Aprison EZ, Dzitoyeva S, Ruvinsky I. 2022. The serotonin circuit that coordinates germline proliferation and egg laying with other reproductive functions in C. elegans. Figshare. ( 10.6084/m9.figshare.c.6307528) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Aprison EZ, Dzitoyeva S, Ruvinsky I. 2022. The serotonin circuit that coordinates germline proliferation and egg laying with other reproductive functions in C. elegans. Figshare. ( 10.6084/m9.figshare.c.6307528) [DOI] [PMC free article] [PubMed]
Data Availability Statement
The data are provided in the electronic supplementary material [36].