Abstract
Phycobilisomes (PBS) are massive structures that absorb and transfer light energy to photochemical reaction centres. Among the range of light harvesting systems, PBS are considered to be excellent solutions for absorption cross-sections but relatively inefficient energy transferring systems. This is due to the combination of a large number of chromophores with intermediate coupling distances. Nevertheless, PBS systems persisted from the origin of oxygenic photosynthesis to present-day cyanobacteria and red algae, organisms that account for approximately half of the primary productivity in the ocean. In this study, we modelled energy transfer through subsets of PBS structures, using a comprehensive dynamic Hamiltonian model. Our approach was applied, initially, to pairs of phycobilin hexamers and then extended to short rods. By manipulating the distances and angles between the structures, we could probe the dynamics of exciton transfer. These simulations suggest that the PBS chromophore network enhances energy distribution over the entire PBS structure—both horizontally and vertically to the rod axis. Furthermore, energy transfer was found to be relatively immune to the effects of distances or rotations, within the range of intermediate coupling distances. Therefore, we suggest that the PBS provides unique advantages and flexibility to aquatic photosynthesis.
Keywords: photosynthesis, phycobilisome, energy transfer, quantum effects
1. Introduction
In the photosynthetic process, light-harvesting pigment–protein complexes absorb light energy and transfer it to photosystems, where photochemical reactions occur [1]. While the structure of the photosystems is highly conserved in evolution, light-harvesting is done by a broad and diverse array of complexes [2]. One of the most prevalent light-harvesting systems is the phycobilisome (PBS), present in cyanobacteria and red algae. It is composed of soluble proteins anchored to the photosynthetic membrane surface and chromophores—tetrapyrrole molecules that covalently attach to conserved sites on the proteins [3]. The spectral properties of the chromophores are determined by their chemical nature and by their interaction with proteins [4]. The basic building blocks of PBS structures are phycobilin chromophore binding proteins which assemble into hexamers (see figure 1 for hexamer visualization). These hexamers either organize to form the core of the PBS antenna or stack into rod structures with the help of mostly unpigmented linker proteins [4]. Antenna rods channel excitonic energy to the PBS core for transfer to the photochemical reaction centres embedded in the thylakoid membranes [5].
Figure 1.
Creation of hexamer models. (a) Schematic showing the generation of models to simulate energy distribution in the PBS antenna system. We started out with the Synechococcus elongatus hexamer (PDB 4H0M), since the hexamer is the base unit of the antenna system. We then removed the scaffold proteins and left only the chromophores of the hexamer while maintaining their exact positions, as energy is transferred between the chromophores of the system. We aligned chromophore templates generated by Gaussian 09 to the hexamer chromophores according to type to determine the centres of mass and dipoles for the entire hexamer. Finally, we removed the chromophore structures and left only the centres of mass (COM) and dipole moment vectors (white arrows) for input into the simulation. (b) Models of the parallel hexamer pairs in both aligned (left) and rotated (right) orientations. Red: β-84 chromophores with a dipole vector magnitude of 9.836 Debye; blue: α-84 chromophores with a dipole vector magnitude of 12.816 Debye; green: β-155 chromophores with a dipole vector magnitude of 10.709 Debye. Hexamer 1 chromophores are labelled and coloured more brightly (on the left side of each model) and hexamer 2 chromophores are coloured in a faded shade (on the right side of each model). In the aligned model, β-155 chromophores from both hexamers are present in the interface. In the rotated model, hexamer 2 is rotated 65° so that its β-155 chromophores are staggered on either side of the interface (their positions are marked in dotted line boxes). (c) Hexamer pair model. Two copies of the S. elongatus hexamer, PDB 4H0M, were placed in parallel next to each other as close as possible without overlapping. The hexamers were then moved apart at increments of 10 Å until reaching a final distance of 220 Å. Simulations were run on each incremental shift. Purple: hexamer 1; rainbow spectrum: hexamer 2 shifted at 10 Å increments away from hexamer 1. This Figure shows only the original position of hexamer 2 (red) and the first five shifts that reached a distance of 50 Å.
The efficiency of excitation energy transfer (EET) through light-harvesting systems is a major determinant of the overall efficiency of the photosynthetic process. Ever since two-dimensional electronic spectroscopy revealed coherent beatings in the Fenna–Matthews–Olson complex [6], both the nature and the efficiency of EET have received much attention. Similar signatures have since been identified in antenna complexes of a purple bacterium and plant photosystems [7–9], triggering a continuing debate regarding the nature and role of the observed oscillations [9–11].
However, structures of PBS complexes do not feature prominently in this debate and also do not offer simple explanations to their observed efficiency [12]. The number of chromophores in a single antenna system can reach well above 1000 [13], creating one of the largest known absorption cross-sections for the photosynthetic unit [14]. According to FRET random walk principles, the diffusion length of an exciton increases with the number of pigments in a photosynthetic unit [15]. However, experimental results of energy transport in photosystems give a more nuanced picture wherein efficiency and yield are demonstrated to be constrained by the physical configuration of the system and its surroundings [16]. The chromophores of PBS are relatively far apart, generating a network of intermediately coupled chromophores. Furthermore, PBS rods can reach four hexamers in length, while FRET random walk calculations demonstrate that an antenna rod extending beyond three hexamers in length will exceed the limit for maintaining energy-efficient transfer [17]. However, it is important to note that high efficiency should not be considered a goal in and of itself for photosynthesis in natural environments [18]. Large dynamic range and robustness of energy transfer can be equally or even more important for the fitness of a photosynthetic organism.
Over the past few decades, different approaches have been applied to model and simulate EET in PBS systems [19–23]. In these studies, models of protein hexamers and short rods were used to simulate energy flow with calculations based on Förster coupling between the transition dipoles of the interacting chromophores as well as their spectral properties. While the authors of these studies were able to propose pathways for energy transfer in the system, the simulations were limited due to missing structural details of intact PBS systems, particularly the exact conformation of hexamer stacking along with the orientation of the rods relative to each other. In recent years, intact PBS structures have been resolved. The first intact structures were from red algae [13,24]. These structures are massive, containing 14 rods and 1598 light-absorbing chromophore molecules. More recently, the Kerfeld lab resolved the structure of a cyanobacterial PBS containing four orange carotenoid proteins (OCP) [25]. These structures provide a good deal of here-to-unknown structural information and a basis for understanding changes in PBS efficiency, through energy transfer simulations [25]. However, these simulations did not calculate dynamic quantum effects.
In this work, we present a framework for modelling energy transport with a microscopic Hamiltonian model. By combining structural PBS data with a comprehensive Hamiltonian model, we capture exciton dynamics relevant to fundamental biological processes. The model described here uses pairwise, distance-dependent dipole–dipole coupling (Förster) terms and includes the potential for coherence in the site basis as well as the energy basis, thereby describing a form of coherent energy migration. However, as we shall note, the timescales of interest in our dynamical model significantly exceed the lifetime of these dynamical coherences, which are therefore not of particular importance to our results. Using this approach, we were able to efficiently run large-scale calculations to simulate the distribution and efficiency of PBS energy transfer which provide insight into the design principles that govern this immensely complex photosynthetic antenna system.
2. Model
2.1. Physical model
The approach outlined in the following section is similar to previous approaches used to model light-harvesting antennae and iron-stress-induced-A (IsiA) proteins [5,26–30]. We reduce each chromophore in PBS down to an optical dipole located at the centre of mass from which we orientate the respective transition dipole vector. These values are influenced by their position in the photosystem and the corresponding protein environment. We therefore consider three different species of chromophore in this system: α-84, β-84 and β-155. The resulting description of the aforementioned hexamer structures in PBS is broken down into a collection of optical dipoles captured by the following Hamiltonian:
| 2.1 |
where the first term is the bare Hamiltonian of the chromophore sites, with species-dependent transition frequency at the jth site, and the second describes exciton hopping between the jth and the kth site, captured by the raising and lowering operators and . The strength of these hopping terms, the resonant Förster interactions, are given by Jj,k(rj,k),
| 2.2 |
for dipoles of oscillator strengths dj and dk and separated by the distance rj,k [28]. Being closely spaced relative to the wavelength of optical photons, the chromophores can be assumed to interact collectively with a shared optical bath [9]; this coupling is captured by the optical interaction Hamiltonian [31],
| 2.3 |
where is the annihilation (creation) operator for the pth optical mode and fp is the coupling strength between the pth mode and the chromophores. Furthermore, the dipoles are each assumed to be coupled to identical local phonon baths. Thus, we consider a vibrational interaction Hamiltonian of the form
| 2.4 |
where gq ≡ gk,q and are the coupling strength and annihilation (creation) operator for the qth phonon mode with the kth dipole, respectively. Lastly, the optical and phonon environmental modes are governed by the following Hamiltonian:
| 2.5 |
where ωp and are the frequencies of the pth photon modes and qth phonon modes at the kth dipole. The total Hamiltonian is then given by
| 2.6 |
describing the dynamics of chromophores as a collection of dipoles in interaction with each other, a shared optical bath and individual local phonon baths. We make the approximation that the interaction terms are weak and can therefore be used in a perturbative expansion wherein we truncate to the second-order terms [30,31]. The resulting Redfield quantum master equation describes the evolution of the density matrix ρ of only the chromophore system,
| 2.7 |
where and are the relaxation rates associated with the vibrational and optical baths, respectively.With equations (2.4) and (2.3) of the form the terms and are the non-secular Redfield dissipators for the photon and phonon fields associated with the jth dipole, taking the form
| 2.8 |
where h.c. denotes the Hermitian conjugate, An,j are the system operators associated with the dissipative process and the prefactors are environment correlation functions which depend on the relevant microscopic interactions with each environment [31]. As each vibrational environment is considered to be identical each correlation function is of the form
| 2.9 |
For simplicity, we choose a flat spectral density in order to construct the following rates for the vibrational dissipators:
| 2.10 |
whereas for the optical dissipators
| 2.11 |
where κvib is a phenomenologically motivated prefactor chosen to reflect the timescale of the phonon rates and is the spontaneous emission rate associated with jth dipole defined by the equation
| 2.12 |
where ωj and μj are the relevant dipole transition energy and moment. The functions nvib(ω) and nopt(ω) are the Bose–Einstein factors associated with the vibrational and optical baths, respectively, each at the appropriate temperature of the associated environment. In the final term of equation (2.7), , is a Lindblad dissipator term describing radiative recombination, where is the rate of this process, and takes the standard form
| 2.13 |
for the lowering (raising) operators () acting upon the kth site. We use equation (2.7) to resolve the system dynamics, in contrast to our previous work in [30] which analysed equilibrium properties for the same type of model applied to the IsiA complex. This allows us to here investigate dynamic energy transport processes emerging from a physically motivated model following the absorption of a photon.
It is important to highlight that the model presented in this work does not explore the limiting case, a strong interaction between chromophores and the surrounding vibrational environment. One could resolve this by moving to the polaron frame, which leads to an effective rescaling of the transition frequencies and hopping terms the bare Hamiltonian [29,32]. However, the complexity of phycobilisome complexes and the number of sites required for a meaningful associated transport model makes this computationally demanding. Furthermore, such a polaron approach would require more detailed knowledge of the particular spectral density present in PBS. For these reasons, we employ the presented weak coupling model, which is sufficient to explore the basic nature of exciton transport in certain physical regimes while still providing a framework upon which more complex approaches can be built.
2.2. Structural model
From an extensive analysis of available structurally resolved red algae PBS, it is clear that the hexamer structures throughout the system are uniform and the stacking of hexamers into rods is repetitive. This means that within an individual hexamer or rod, there is little variation in the spacing and orientation between chromophores. However, the positioning of rods relative to each other compounds the variability of the structure, affecting the spacing and orientation of inter-rod chromophores. Therefore, in this research we focused on the transfer of energy between hexamers laterally, rather than within single rods or hexamers. For our models, we used the solved Synechococcus elongatus (S. elongatus) hexamer structure. We chose this hexamer, rather than one of the red algae rod hexamers, because of the simpler chemical properties of its chromophores and the smaller number of chromophores per hexamer. Owing to the computational power needed for our simulation, we needed to simplify the representation of these complex molecules in our models. The dipoles and centres of mass for each of the three chromophore types were calculated using Gaussian 09 [33]. These data were used with the model discussed in the preceding section of the simulation to construct a physically motivated description of system dynamics (figure 1).
To investigate the effect of distance and orientation on lateral energy transfer, we placed two parallel copies of the S. elongatus hexamer model as close together as possible without overlapping. For the aligned model, β-155 chromophores from both hexamers were positioned in the inter-hexamer interface. The rotated model was created by rotating the second hexamer of the aligned model 65° (figure 1). We ran the simulation for both models, shifting the parallel hexamers further apart at 10 Å increments until reaching a final distance of 220 Å between hexamers (figure 1).
The rod models were produced by adding a second hexamer below each of the original hexamers of the aligned and rotated hexamer models (figure 2). The purpose of this was to determine whether lateral energy transfer would be maintained in the presence of a vertical transfer option. The hexamers were stacked according to the stacking patterns found in the red algae Porphyridium purpureum PBS, one of the two publicly available sources for rod structures. A third rod model was created by aligning the S. elongatus hexamer model to two hexamers of two neighbouring rods of the P. purpureum PBS. This was done to represent a real-world spatial positioning of rod pairs relative to each other, in contrast to the very artificial, parallel set-ups for the other models used in this study. We used the artificial models to define control parameters for energy transfer in an intermediate coupled system, then evaluated the implementation of these parameters in the real-world structure.
Figure 2.
Rod pair models. Aligned (a), rotated (b), ‘real-world’ (c) and Synechocystis (d) rod pairs. Rod 1 for each pair is coloured in cyan, rod 2 is coloured in green. The β-155 chromophores in each model are coloured in purple and shown as spheres, and the distance between the closest inter-rod β-155 chromophore pair in the top and bottom hexamer pairs is marked. The rest of the system is shown in cartoon representation.
3. Results
3.1. Control parameters for energy transfer between hexamers
To evaluate the effect of distance and orientation on energy transfer, models were constructed to simulate the lateral transfer of energy between hexamer pairs. For each simulation, energy transfer was initiated by exciting one of the 18 chromophores in hexamer 1. Under physiological light conditions, the photon flux density is such that double excitation of a single PBS system are not expected and we therefore consider only the single excitation manifold [34,35]. The subsequent distribution of energy in both hexamer 1 and hexamer 2 is calculated across 100 ps following initial excitation. For these simulations we set the following phenomenologically motivated rates: indicative phonon relaxation rate κvib, 1.0 ps−1, and non-radiative recombination rate , 0.2 ns−1 [28–30]. The latter rate is chosen such that it competes with the optical rates, of the order of nanoseconds−1 and calculated using κopt, whereas the former is set such that the hierarchy of rates is appropriate, with vibrational rates being 103 faster than the optical rates.
Pymol [36] movies were used to visualize the energy distribution over time (see the Methods section for details). These movies are provided in the electronic supplementary material. Electronic supplementary material, Movie1 and Movie2 show the energy distribution upon initially exciting an α-84 chromophore in the aligned and rotated models, respectively. As can be seen in the movies, most of the energy of the system is distributed between all the α-84 chromophores in both aligned and rotated models, although slightly less is laterally transferred to the α-84 chromophores in hexamer 2, when compared with those in hexamer 1. There is even less but still significant energy transfer to the β-84 chromophores in hexamer 1, and a very small amount of transfer to the β-84 chromophores in hexamer 2. There is no significant distribution of energy to any of the β-155 chromophores.
Electronic supplementary material, Movie3 and Movie4 show examples of energy distribution upon initially exciting a β-84 chromophore in the aligned and rotated models, respectively. Here we see the opposite effect from what we saw after initially exciting the α-84 chromophores. Most of the energy in the system is distributed between the β-84 chromophores, and to a lesser extent to the α-84 chromophores. Here also, there is no significant distribution to the β-155 chromophores.
It is therefore not surprising that following initial excitation of each of the β-155 chromophores (see electronic supplementary material, Movie5 and Movie6 for the aligned and rotated models, respectively), energy distribution is isolated to the other β-155 chromophores of the system for both aligned and rotated models. Significantly, when an interface β-155 chromophore is initially excited in the aligned model, there is localization of energy. That is, despite the relatively weak coupling inherent to the system, almost all the energy remains trapped between four β-155 chromophores.
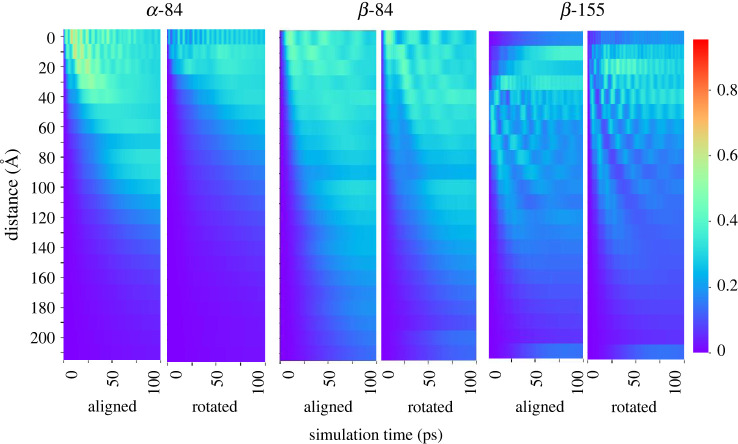
To break down what we are seeing in the simulation further, the heatmaps in figure 3 show the lateral energy transfer from hexamer 1 to hexamer 2 over time and distance following initial excitation of a sample α-84 chromophore, a sample β-84, and a sample β-155 on hexamer 1 of the aligned and rotated models. The results for all hexamer 1 α-84 chromophores can be viewed in electronic supplementary material, figure S1, panel A. At close proximity, we observe rapid oscillations of lateral transfer between the hexamers (chains A, C, G and I in the aligned and rotated models). Over larger distances, both oscillation frequency and overall lateral transfer declines. At close proximity in the aligned model, more energy is transferred to hexamer 2 in the first 50 ps of the simulation, and less is transferred to hexamer 2 as time continues, particularly in chains A, C, G and I. At larger distances, most energy is transferred to hexamer 2 in the second half of the simulation. Overall, the patterns of lateral energy transfer are similar in both the aligned and rotated models, although slightly more energy is transferred laterally in the aligned model.
Figure 3.
Lateral energy transfer over time and distance in hexamers. Heatmap showing the lateral energy transfer from hexamer 1 to hexamer 2 over time (x-axis) and distance (y-axis) following initial excitation of an α-84 chromophore (two left panels), a β-84 chromophore (two centre panels), and a β-155 chromophore (two right panels) in hexamer 1. The colour bar measures the amount of energy transferred out of the total energy of the system; here, 1 signifies all of the total energy, and 0 signifies none of the total energy. Thus, dark blue indicates low energy transfer, while red indicates high energy transfer. The two left panels correspond to Movie1 and Movie2, respectively, the two middle panels correspond to Movie3 and Movie4, and the two right panels correspond to Movie5 and Movie6. All movies can be found in electronic supplementary material.
Following initial excitation of the β-84 chromophores on hexamer 1 (figure 3 and electronic supplementary material, figure S2 panel B), lateral energy transfer is also characterized by rapid oscillation when hexamer 2 is in close proximity to hexamer 1, especially in chains D, F, J and L of the aligned model. However, this oscillation is not nearly as rapid as what was seen for the α-84 chromophores. The interior position of the β-84 chromophores in the core of the hexamer, in contrast to the more exterior position of the α-84 chromophores, could explain this reduced short-range oscillation 1. However, there is still very strong long-range lateral energy transfer to hexamer 2 at much greater distances following initial excitation of the β-84 chromophores, compared with what was simulated for the α-84 chromophores.
When either of the two hexamer 1 β-155 interface chromophores are initially excited (electronic supplementary material, figure S1 panel C), extremely rapid energy oscillation and energy transfer occurs. This can be seen in chains D and J of the aligned and rotated models. However, when any of the other four chromophores on hexamer 1 (chains B, F, H and L) are initially excited, there is very little energy transfer at distance 0, although energy transfer does increase between 10 Å and about 40 Å. This is the case in both aligned and rotated models.
After summing the total lateral energy transfer for each distance, we see that following initial excitation of many of the α-84 and β-155 chromophores, the maximum total energy transfer does not occur when the two hexamers are at distance 0 (see electronic supplementary material, figure S2). Rather, the optimal distance for lateral energy transfer can be as much as 30 Å from the original position. This means that while there is a general correlation between distance and lateral energy transfer (the closer the hexamers, the higher the lateral energy transfer), lateral energy transfer is somewhat diminished when the interface chromophores are too close. Further explanation can be found in the electronic supplementary material.
Overall, we can see from our results that for both aligned and rotated hexamer models energy transfer is not sensitive to moderate changes in orientation, provided the positioning of the chromophores in the interface does not result in strong energy localization. There is a small degree of difference in the optimal distances for lateral energy transfer between the aligned and rotated models. There is slightly more energy transfer in the aligned model following initial excitation of an α-84 chromophore, but in general energy transfer seems to be very robust and flexible, tolerating a fair amount of variation with relatively constant transfer patterns. Distance obviously controls the rate of transfer, but the correlation is not trivial. Very short hexamer–hexamer distances can be detrimental to transfer efficiency due to the trapping of the energy in local states between chromophore pairs in the interface.
3.2. Control parameters for energy transfer between rods
While simulating the energy transfer in hexamer pairs gave us a good start in understanding the control parameters of our system, most of the PBS is made up of rods along which energy is transferred from the periphery to the core of the structure. We therefore placed an additional hexamer below each of the original hexamers of our models to add the possibility of vertical transfer to our model (see §2). This procedure created simple rod models in both the aligned and rotated orientations.
We also created a ‘real-world’ model by locating two neighbouring rods in the publicly available electron microscopy (EM)-resolved P. purpureum structure (PDB 6KGX) and aligning our cyanobacteria hexamer to two red algae hexamers in each rod. We chose to create a homology-based model instead of using the P. purpureum hexamer itself, as the rods of the P. purpureum antenna system bind phycoerythrobilin and phycourobilin chromophores instead of phycocyanobilin chromophores. Moreover, the P. purpureum hexamers contain more chromophores than S. elongatus hexamers, which would increase the complexity of the simulation considerably. ‘Real-world’ rods are not parallel as in our aligned and rotated models, rather they have a staggered orientation (figure 2). As was done with the two hexamer model simulations, each of the 18 chromophores in hexamer 1 were initially excited, and energy distribution throughout the rod pairs was simulated. Here also, energy distribution movies were used to visualize the energy distribution in the rod systems (see the Methods section for details).
Electronic supplementary material, Movie7, Movie8 and Movie9 display the distribution in the system after initially exciting an α-84 chromophore in the aligned, rotated and real-world models, electronic supplementary material, Movie10, Movie11 and Movie12 show distribution after initially exciting a β-84 chromophore in the aligned, rotated and real-world models, and Movie13, Movie14 and Movie15 represent the distribution after initially exciting a β-155 chromophore in the aligned, rotated and real-world models, respectively. For the aligned and rotated models, these movies clearly show lateral energy transfer patterns that are very similar to those of their hexamer pair counterparts, despite the presence of a vertical transfer option.
To provide an overview of the energy distribution in all the rod systems after initial excitation of all 18 chromophores in hexamer 1, we created a static visualization similar to the movies described above. One difference in the static visualization, however, is that the size of the sphere indicates the average energy transferred to a given chromophore during the 100 ps simulation.
The most apparent differences in transfer patterns between models can be seen following initial excitation of all six of the β-155 chromophores (figures 4–6).
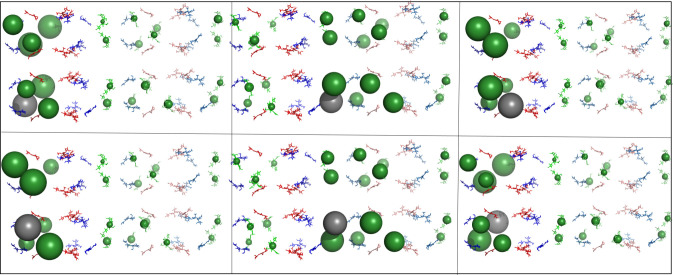
Figure 5.
Overall energy distribution after exciting β-155 chromophores in the rotated rods. Pymol visualization of energy distribution after initial excitation of all six β-155 chromophores in the rotated rod model. Each panel represents distribution after initialization of a different β-155 chromophore. Chromophores are shown as sticks and coloured according to type (α-84 chromophores in blue, β-84 chromophores in red and β-155 chromophores in green). The energy distributed to an individual chromophore is represented by a sphere, the size of which indicates the average amount of energy transferred to that specific chromophore during the 100 ps simulation. Sphere colours match chromophore type, except for the grey sphere, which marks the initially excited chromophore. See Methods section for more details.
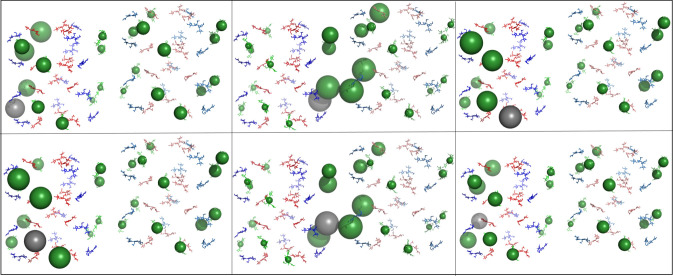
Figure 4.
Overall energy distribution after exciting β-155 chromophores in the aligned rods. Pymol visualization of energy distribution after initial excitation of all six β-155 chromophores in the aligned rod model. Each panel represents distribution after initialization of a different β-155 chromophore. Chromophores are shown as sticks and coloured according to type (α-84 chromophores in blue, β-84 chromophores in red and β-155 chromophores in green). The energy distributed to an individual chromophore is represented by a sphere, the size of which indicates the average amount of energy transferred to that specific chromophore during the 100 ps simulation. Sphere colours match chromophore type, except for the grey sphere, which marks the initially excited chromophore. See Methods section for more details.
Figure 6.
Overall energy distribution after exciting β-155 chromophores in the real-world rods. Pymol visualization of energy distribution after initial excitation of all six β-155 chromophores in the real-world rod model. Each panel represents distribution after initialization of a different β-155 chromophore. Chromophores are shown as sticks and coloured according to type (α-84 chromophores in blue, β-84 chromophores in red and β-155 chromophores in green). The energy distributed to an individual chromophore is represented by a sphere, the size of which indicates the average amount of energy transferred to that specific chromophore during the 100 ps simulation. Sphere colours match chromophore type, except for the grey sphere, which marks the initially excited chromophore. See Methods section for more details.
In the aligned models, the energy distribution remains in the area of the initially excited chromophore, and significant lateral transfer only occurs when the initially excited chromophore is located in the inter-rod interface. When this happens, energy is trapped between a few very local β-155 chromophores. These patterns play out in the rotated model as well, but the energy is trapped in the interface to a lesser degree and spreads out more into rod 2, most likely due to the larger distance between the β-155 chromophores in the interface. Significantly, in the real-world model, there is a much more even distribution between all the β-155 chromophores in both rods. This could be because the staggered orientation causes most of the β-155 chromophores in the interface to move slightly further apart, although there are still several interface β-155 chromophores that are as close to each other in the real-world model as they are in the rotated model. (For measurements of shortest inter-rod β-155 chromophore pair distances, see figure 2.) The effect of localization on energy transfer can be seen quantitatively in figure 7. More detailed results can be found in the electronic supplementary material.
Figure 7.
Lateral energy transfer over time and distance in rods. Heatmap showing the lateral energy transfer from rod 1 to rod 2 over time (x-axis) and distance (y-axis) following initial excitation of rod 1 β-155 chromophore in the aligned orientation (two left panels), the rotated orientation (two centre panels) and the real-world orientation (two right panels). The colour bar measures the proportion of energy transferred out of the total energy of the system. The panel for aligned chain B corresponds to electronic supplementary material, Movie9, the panel for rotated chain B corresponds to electronic supplementary material, Movie12, and the panel for real-world chain B corresponds most closely to electronic supplementary material, Movie15. All movies can be found in electronic supplementary material.
The simulations of short rod structures provide additional insight into energy transfer patterns. Importantly, both lateral and horizontal transfer are mostly unaffected by changes in the hexamer unit orientation, provided close localization of pigment pairs is avoided. In these cases, the distribution of energy is similar regardless of the initial hexamer location of the excited chromophore (see figure 6 and electronic supplementary material, figure S4). The staggered, angled positioning of the real-world rods seems specifically configured to avoid such localizations.
This effect is present in a simulation performed on the recently published Synechocystis sp. PCC6803 PBS structure [25]. Two neighbouring rods were selected, and the two hexamers proximal to the core from each rod were used to create a model for simulation by aligning template dipoles to the phycocyanin chromophores (figure 8). This structure also presents a staggered, angled positioning of the rod pair. The distribution of energy resembles that of the ‘real-world’ structure.
Figure 8.
Overall energy distribution after exciting β-155 chromophores in the Synechocystis rods. Pymol visualization of energy distribution after initial excitation of all six β-155 chromophores in the Synechocystis rod model. Each panel represents distribution after initialization of a different β-155 chromophore. Chromophores are shown as sticks and coloured according to type (α-84 chromophores in blue, β-84 chromophores in red and β-155 chromophores in green). The energy distributed to an individual chromophore is represented by a sphere, the size of which indicates the average amount of energy transferred to that specific chromophore during the 100 ps simulation. Sphere colours match chromophore type, except for the grey sphere, which marks the initially excited chromophore. See Methods section for more details.
4. Conclusion
At first glance, massively pigmented intermediately coupled PBS systems do not seem to fit as effective light harvesting systems. A β-155 ‘wire’ at an interface between rods, present in one of the cases studied here, can provide a path for high efficacy vertical energy transfer to the PBS core. However, these are absent from the ‘real-world’ models, studied here. Nevertheless, the simulation results demonstrate a different set of advantages for these intermediately coupled pigment systems. As long as closely localized hexamer pairs are avoided, as in the ‘real-world’ models, energy transfer is robust. Energy is distributed relatively evenly both horizontally and vertically to the rod axis, regardless of the type of chromophore excited and its location in the structure. A single PBS system is large enough to provide exciton energy to more than one photosystem [37]. Therefore, the ability to evenly distribute energy between rods and over the entire PBS can play an important biological role. Furthermore, energy transfer is relatively immune to the effects of distances or rotations, within the range of intermediate coupling distances. This robustness is critical in the context of large biological structures operating at room temperature in living cells. Considering the erratic nature of the light field in marine environments [38], it is easy to see how the robustness and the ability to distribute energy over the large volume of the PBS structure can convey advantages to marine photosynthesis.
5. Methods
5.1. Static Pymol visualization
The static Pymol visualization was used to create figures 4–8. Each chromophore was shown as sticks and coloured according to type (α-84 chromophores in blue, β-84 chromophores in red and β-155 chromophores in green), except for the grey sphere, which marks the chromophore that was initially excited during the simulation.
The amount of energy distributed to each individual chromophore was represented by a sphere located at its centre of mass coordinates. Ranges for sphere size were given according to the average proportion of energy that transferred to a given chromophore during the 100 ps simulation (table 1). If the average amount of energy (i.e. the exciton population) on a given chromophore was less than 0.0002, no sphere was generated to represent the chromophore.
Table 1.
Chart showing the Pymol sphere size for the proportion of energy transferred to a chromophore.
| proportion of energy | sphere size in Pymol |
|---|---|
| ≥0.9 | 12 |
| 0.8 → 0.9 | 11 |
| 0.7 → 0.8 | 10 |
| 0.6 → 0.7 | 9 |
| 0.5 → 0.6 | 8 |
| 0.4 → 0.5 | 7 |
| 0.3 → 0.4 | 6 |
| 0.2 → 0.3 | 5 |
| 0.1 → 0.2 | 4 |
| 0.05 → 0.1 | 3 |
| 0.025 → 0.05 | 2 |
| 0.002 → 0.025 | 1 |
| 0.0 → 0.002 | 0 |
5.2. Dynamic Pymol visualization
The dynamic Pymol visualization was used to create electronic supplementary material, movies 1–15. The methodology for generating the dynamic visualization using Pymol was similar to that of the static visualization, except that instead of using a sphere to represent the largest amount of energy distributed to each chromophore over the 100 ps simulation, the exact amount of energy transferred to each chromophore for every 0.1 ps timepoint was represented by a sphere in a separate video frame (table 1). The videos have a total of 1000 frames showing energy transfer over the course of the 100 ps simulation.
5.3. Transition dipole moment calculations
The magnitude and orientation of the transition dipole moment of each chromophore in the system are used in the equations (2.2) and (2.12) to calculate the interaction between dipoles. These calculations are necessary for our simulation of the overall energy flow of the PBS antenna system. The transition dipole moments for the three protonated phycocyanobilin conformers were obtained using quantum chemistry calculations. Heavy-atom coordinates were used from the Protein Data Bank [39] with explicit hydrogen atoms incorporated in a constrained geometry optimization. Density functional theory calculations were carried out at the B3LYP level using the 6-31G(d) basis, with the five lowest electronic excited states computed via time-dependent methods. All calculations used Gaussian 09 [33].
Acknowledgements
The authors acknowledge the University of Maryland supercomputing resources (http://hpcc.umd.edu) made available for conducting the research reported in this paper. Special thanks to Prof. Leah Dodson for her contribution and kind support and to Noa Hazony for her teamwork and technical support.
Contributor Information
Erik M. Gauger, Email: e.gauger@hw.ac.uk.
Nir Keren, Email: nir.ke@mail.huji.ac.il.
Data accessibility
All original data can be obtained from the corresponding author upon request. The data are provided in the electronic supplementary material [40].
Authors' contributions
E.J.D.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; N.W.: conceptualization, data curation, formal analysis, investigation, methodology, visualization, writing—original draft, writing—review and editing; Y.P.: conceptualization, supervision, writing—review and editing; E.M.G.: conceptualization, funding acquisition, methodology, project administration, supervision, writing—original draft, writing—review and editing; N.K.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, resources, software, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
N.K. and E.J.D. were supported by the Israel Science Foundation grant no. 1182/19 and the Zelman Cowen Academic Initiatives. N.W. and E.M.G. acknowledge funding from the EPSRC grant no. EP/T007214/1.
References
- 1.Glazer AN. 1985. Light harvesting by phycobilisomes. Annu. Rev. Biophys. Biophys. Chem. 14, 47-77. ( 10.1146/annurev.bb.14.060185.000403) [DOI] [PubMed] [Google Scholar]
- 2.Hohmann-Marriott MF, Blankenship RE. 2011. Evolution of photosynthesis. Annu. Rev. Plant Biol. 62, 515-548. ( 10.1146/annurev-arplant-042110-103811) [DOI] [PubMed] [Google Scholar]
- 3.MacColl R. 1998. Cyanobacterial phycobilisomes. J. Struct. Biol. 124, 311-334. ( 10.1006/jsbi.1998.4062) [DOI] [PubMed] [Google Scholar]
- 4.Adir N, Bar-Zvi S, Harris D. 2020. The amazing phycobilisome. Biochim. Biophys. Acta Bioenerg. 1861, 148047. ( 10.1016/j.bbabio.2019.07.002) [DOI] [PubMed] [Google Scholar]
- 5.van Grondelle R, Dekker JP, Gillbro T, Sundstrom V. 1994. Energy transfer and trapping in photosynthesis. BBA – Bioenergetics 1187, 1-65. ( 10.1016/0005-2728(94)90166-X) [DOI] [Google Scholar]
- 6.Engel GS, Calhoun TR, Read EL, Ahn T-K, Mančal T, Cheng Y-C, Blankenship RE, Fleming GR. 2007. Evidence for wavelike energy transfer through quantum coherence in photosynthetic systems. Nature 446, 782-786. ( 10.1038/nature05678) [DOI] [PubMed] [Google Scholar]
- 7.Hildner R, Brinks D, Nieder JB, Cogdell RJ, van Hulst NF. 2013. Quantum coherent energy transfer over varying pathways in single light-harvesting complexes. Science 340, 1448-1451. ( 10.1126/science.1235820) [DOI] [PubMed] [Google Scholar]
- 8.Romero E, Augulis R, Novoderezhkin VI, Ferretti M, Thieme J, Zigmantas D, van Grondelle R. 2014. Quantum coherence in photosynthesis for efficient solar-energy conversion. Nat. Phys. 10, 676-682. ( 10.1038/nphys3017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao J, et al. 2020. Quantum biology revisited. Sci. Adv. 6, eaaz4888. ( 10.1126/sciadv.aaz4888) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholes GD, et al. 2017. Using coherence to enhance function in chemical and biophysical systems. Nature 543, 647-656. ( 10.1038/nature21425) [DOI] [PubMed] [Google Scholar]
- 11.Duan H-G, Prokhorenko VI, Cogdell RJ, Ashraf K, Stevens AL, Thorwart M, Miller RJD. 2017. Nature does not rely on long-lived electronic quantum coherence for photosynthetic energy transfer. Proc. Natl Acad. Sci. USA 114, 8493-8498. ( 10.1073/pnas.1702261114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noji T, Watanabe M, Dewa T, Itoh S, Ikeuchi M. 2021. Direct energy transfer from allophycocyanin-free rod-type CpcL-phycobilisome to photosystem I. J. Phys. Chem. Lett. 12, 6692-6697. ( 10.1021/acs.jpclett.1c01763) [DOI] [PubMed] [Google Scholar]
- 13.Ma J, You X, Sun S, Wang X, Qin S, Sui S-F. 2020. Structural basis of energy transfer in Porphyridium purpureum phycobilisome. Nature 579, 146-151. ( 10.1038/s41586-020-2020-7) [DOI] [PubMed] [Google Scholar]
- 14.Mauzerall D, Greenbaum NL. 1989. The absolute size of a photosynthetic unit. Biochimica et Biophysica Acta (BBA) – Bioenergetics 974, 119-140. ( 10.1016/S0005-2728(89)80365-2) [DOI] [Google Scholar]
- 15.Feron K, Belcher WJ, Fell CJ, Dastoor PC. 2012. Organic solar cells: understanding the role of Förster resonance energy transfer. Int. J. Mol. Sci. 13, 17 019-17 047. ( 10.3390/ijms131217019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wientjes E, van Amerongen H, Croce R. 2013. Quantum yield of charge separation in photosystem II: functional effect of changes in the antenna size upon light acclimation. J. Phys. Chem. Part B: Condens. Matter Mater. Surfaces Interfaces Biophys. 117, 11 200-11 208. ( 10.1021/jp401663w) [DOI] [PubMed] [Google Scholar]
- 17.Chenu A, Cao J. 2017. Construction of multichromophoric spectra from monomer data: applications to resonant energy transfer. Phys. Rev. Lett. 118, 013001. ( 10.1103/PhysRevLett.118.013001) [DOI] [PubMed] [Google Scholar]
- 18.Keren N, Paltiel Y. 2018. Photosynthetic energy transfer at the quantum/classical border. Trends Plant Sci. 23, 497-506. ( 10.1016/j.tplants.2018.03.007) [DOI] [PubMed] [Google Scholar]
- 19.Figueroa M, Martínez-Oyanedel J, Matamala AR, Dagnino-Leone J, Mella C, Fritz R, Sepúlveda-Ugarte J, Bunster M. 2012. In silico model of an antenna of a phycobilisome and energy transfer rates determination by theoretical Förster approach. Protein Sci. 21, 1921-1928. ( 10.1002/pro.2176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matamala AR, Almonacid DE, Figueroa MF, Martínez-Oyanedel J, Bunster MC. 2007. A semiempirical approach to the intra-phycocyanin and inter-phycocyanin fluorescence resonance energy-transfer pathways in phycobilisomes. J. Comput. Chem. 28, 1200-1207. ( 10.1002/jcc.20628) [DOI] [PubMed] [Google Scholar]
- 21.Padyana AK, Ramakumar S. 2006. Lateral energy transfer model for adjacent light-harvesting antennae rods of c-phycocyanins. Biochim. Biophys. Acta 1757, 161-165. ( 10.1016/j.bbabio.2006.02.012) [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, et al. 2013. Understanding the electronic energy transfer pathways in the trimeric and hexameric aggregation state of cyanobacteria phycocyanin within the framework of Förster theory. J. Comput. Chem. 34, 1005-1012. ( 10.1002/jcc.23221) [DOI] [PubMed] [Google Scholar]
- 23.Debreczeny MP, Sauer K, Zhou J, Bryant DA. 1995. Comparison of calculated and experimentally resolved rate constants for excitation energy transfer in c-phycocyanin. 1. Monomers. J. Phys. Chem. 99, 8412-8419. ( 10.1021/j100020a080) [DOI] [Google Scholar]
- 24.Zhang J, Ma J, Liu D, Qin S, Sun S, Zhao J, Sui S-F. 2017. Structure of phycobilisome from the red alga Griffithsia pacifica. Nature 551, 57-63. ( 10.1038/nature24278) [DOI] [PubMed] [Google Scholar]
- 25.Domínguez-Martín MA, Sauer PV, Kirst H, Sutter M, Bína D, Greber BJ, Nogales E, Polívka T, Kerfeld CA. 2022. Structures of a phycobilisome in light-harvesting and photoprotected states. Nature 609, 835-845. ( 10.1038/s41586-022-05156-4) [DOI] [PubMed] [Google Scholar]
- 26.Dexter DL. 1953. A theory of sensitized luminescence in solids. J. Chem. Phys. 21, 836-850. ( 10.1063/1.1699044) [DOI] [Google Scholar]
- 27.Ishizaki A, Fleming GR. 2009. Theoretical examination of quantum coherence in a photosynthetic system at physiological temperature. Proc. Natl Acad. Sci. USA 106, 17 255-17 260. ( 10.1073/pnas.0908989106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Curutchet C, Mennucci B. 2017. Quantum chemical studies of light harvesting. Chem. Rev. 117, 294-343. ( 10.1021/acs.chemrev.5b00700) [DOI] [PubMed] [Google Scholar]
- 29.Brown WM, Gauger EM. 2019. Light harvesting with guide-slide superabsorbing condensed-matter nanostructures. J. Phys. Chem. Lett. 10, 4323-4329. ( 10.1021/acs.jpclett.9b01349) [DOI] [PubMed] [Google Scholar]
- 30.Schoffman H, Brown WM, Paltiel Y, Keren N, Gauger EM. 2020. Structure-based Hamiltonian model for IsiA uncovers a highly robust pigment-protein complex. J. R. Soc. Interface 17, 20200399. ( 10.1098/rsif.2020.0399) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Breuer HP, Petruccione F. 2002. The theory of open quantum systems. Oxford, UK: Oxford University Press. [Google Scholar]
- 32.Nazir A, McCutcheon DPS. 2016. Modelling exciton–phonon interactions in optically driven quantum dots. J. Condens. Matter Phys. 28, 103002. ( 10.1088/0953-8984/28/10/103002) [DOI] [PubMed] [Google Scholar]
- 33.Frisch MJ, et al. 2016. Gaussian 09 Revision E.01. Wallingford, CT: Gaussian Inc.
- 34.Pollock FA, McCutcheon DPS, Lovett BW, Gauger EM, Nazir A. 2013. A multi-site variational master equation approach to dissipative energy transfer. New J. Phys. 15, 075018. ( 10.1088/1367-2630/15/7/075018) [DOI] [Google Scholar]
- 35.Nazir A. 2009. Correlation-dependent coherent to incoherent transitions in resonant energy transfer dynamics. Phys. Rev. Lett. 103, 146404. ( 10.1103/PhysRevLett.103.146404) [DOI] [PubMed] [Google Scholar]
- 36. Schrödinger, Inc. 2015 Pymol: the Pymol molecular graphics system, version 1.8. See https://www.schrodinger.com/products/pymol .
- 37.Ueno Y, Aikawa S, Kondo A, Akimoto S. 2016. Energy transfer in cyanobacteria and red algae: confirmation of spillover in intact megacomplexes of phycobilisome and both photosystems. J. Phys. Chem. Lett. 7, 3567-3571. ( 10.1021/acs.jpclett.6b01609) [DOI] [PubMed] [Google Scholar]
- 38.Kolodny Y, Avrahami Y, Zer H, Frada MJ, Paltiel Y, Keren N. 2022. Phycobilisome light-harvesting efficiency in natural populations of the marine cyanobacteria Synechococcus increases with depth. Commun. Biol. 5, 727. ( 10.1038/s42003-022-03677-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berman H, Henrick K, Nakamura H. 2003. Announcing the worldwide protein data bank. Nat. Struct. Biol. 10, 980. ( 10.1038/nsb1203-980) [DOI] [PubMed] [Google Scholar]
- 40.Dodson EJ, Werren N, Paltiel Y, Gauger EM, Keren N. 2022. Large scale FRET simulations reveal the control parameters of phycobilisome light harvesting complexes. Figshare. ( 10.6084/m9.figshare.c.6296401) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All original data can be obtained from the corresponding author upon request. The data are provided in the electronic supplementary material [40].