Abstract
Background:
Chemotherapy-induced thrombocytopenia (CIT) is a known hematologic complication of oncology treatment. This single-institution study examines the degree with which CIT impacts specific pediatric solid tumor cohorts reflected by platelet transfusion burden and treatment modifications.
Procedure:
Data regarding clinically relevant CIT were obtained via a retrospective chart review of pediatric solid tumor patients treated at Memorial Sloan Kettering Cancer Center from 2013 to 2020. Patients were stratified based on histologic diagnoses as well as chemotherapy regimen. CIT impact was assessed through platelet transfusion means, chemotherapy dose reductions, and treatment delays.
Results:
A total of 150 patients were included with mean age 10.3 [0.2–21.0]. Patients receiving therapy for high-risk neuroblastoma and localized Ewing sarcoma, both of which included high-dose cyclophosphamide and doxorubicin, required the most platelet transfusions over the treatment course, with a mean of 13 and 9, respectively. Reduced relative dose intensity (RDI), due in part to CIT, was greatest for the patients receiving therapy for high-risk and intermediate-risk rhabdomyosarcoma. Fifty-six percent of high-risk patients experienced a reduced RDI during the final two cycles of treatment and 69% of intermediate-risk patients experienced one during the final four cycles of treatment.
Conclusions:
The impact of CIT varied by the administered chemotherapy regimens and dose intensity of chemotherapy agents. This study demonstrated that CIT causes both marked platelet transfusion burden as well as treatment reduction and delay within certain solid tumor cohorts. This can lend to future studies aimed at reducing the burden of CIT and targeting the most at-risk populations.
Keywords: chemotherapy-induced thrombocytopenia, pediatric oncology, platelet transfusions, relative reduced dose intensity
1 |. INTRODUCTION
Thrombocytopenia has long been recognized as a significant complication of pediatric cancer treatment. Standard induction regimens for many pediatric solid tumors are highly myelosuppressive and result in chemotherapy-induced thrombocytopenia (CIT).1 Although hematologic toxicity is well described in pediatric oncology literature, there are minimal data regarding its impact on treatment, specifically the platelet transfusion burden and chemotherapy delays and modifications. Profound CIT increases the risk of bleeding, need for platelet transfusions, and can cause interruptions in planned cancer therapy. The frequency and degree of CIT is dependent upon the chemotherapeutic regimen, including the drug timing and intensity, as well as the number of treatment cycles. The timing of the platelet nadir and kinetics of platelet recovery may differ significantly depending upon the specific chemotherapy regimen.2,3
Prior studies performed in adult oncology patients have estimated that approximately 10%–38% of patients with solid tumors and 40%–68% of patients with hematologic malignancies experience thrombocytopenia.4 Within these populations of patients, the incidence and prevalence of CIT varies greatly by specific chemotherapy regimens. Adult studies have shown that the majority of treatment-related thrombocytopenia occurred with regimens based upon taxanes, gemcitabine, and platinum agents.4 In a retrospective series of 609 adult solid tumor patients with CIT (as defined by platelets of <50,000/μl), a delay in subsequent chemotherapy occurred during 6% of cycles and a reduction in chemotherapy occurred in 15% of these patients when compared to those who did not experience CIT.5
Currently, there are no standardized guidelines in pediatrics for the prevention or treatment of CIT. To reduce the risk of bleeding in the setting of severe thrombocytopenia, platelet transfusions are the main supportive measure.6 Based on the updated 2018 American Society of Clinical Oncology platelet transfusion guidelines, prophylactic transfusions are recommended in the solid tumor, hematologic malignancy, and hematologic stem cell transplant populations once a platelet count reaches <10,000/μl.7 Depending on additional clinical risk factors such as age of patient, inpatient versus outpatient setting, and individual bleeding risk factors, a higher platelet level threshold may be utilized at the clinician’s discretion. However, the response to platelet transfusions is unreliable and the effect is short-lived. In addition, platelet transfusions are accompanied by their own risks. There is a reported incidence of 15%–25% platelet refractoriness in oncology patients utilizing leukocyte-reduced blood products. Platelet-reactive antibodies directed against the human leukocyte antigen (HLA) and human platelet antigen (HPA) present on the platelet surface are more likely to develop with repeated blood product exposure and are frequently associated with accelerated platelet destruction and transfusion failure.8 Further, up to 20%–30% of platelet transfusions result in febrile or allergic reactions, and more rare but serious complications may include septic reactions from contaminated blood and transfusion-related acute lung injury, estimated at one in 50,000 and one in 5000 transfusions, respectively.1
It is currently common practice to either reduce the dosage or delay the next cycle of chemotherapy in response to hematologic toxicity or inadequate count recovery from prior cycles, though this reduces the overall dose intensity of treatment. It has been shown that relative dose intensity (RDI) correlates with worse survival outcomes in both retrospective cohort studies and prospective clinical studies in numerous cancer types.9,10 In addition, data from the Ewing sarcoma randomized clinical trial run by the Children’s Oncology Group (COG) from 2003 to 2005, AEWS0031, showed that increased dose intensity of chemotherapy, either by increasing total dose given or decreasing interval between doses, led to improvements in both 5-year EFS (73% in intensified arm compared to 65% in standard arm) and 5year overall survival (OS) (83% in intensified arm compared to 77% in standard arm).11 Therefore, it is reasonable to hypothesize that decreasing dose intensity would lead to inferior outcomes in these patients.
Literature studying the impact of clinically relevant CIT in pediatric oncology patients is limited. This observational study was performed to identify what specific pediatric cohorts, if any, are impacted by the effects of treatment-related thrombocytopenia in regards to both the degree of platelet transfusion requirements and treatment modifications.
2 |. METHODS
2.1 |. Study design and source population
This was a single-institution retrospective chart review of pediatric patients treated at Memorial Sloan Kettering Cancer Center (MSKCC) between January 1, 2013 and July 1, 2020. The studied population comprised patients ≤21 years of age who were treated with their initial chemotherapy at MSKCC during the designated time frame. Eligible patients for inclusion were those with one of the following solid tumor diagnoses: neuroblastoma, Ewing sarcoma, rhabdomyosarcoma, osteosarcoma, central nervous system (CNS)-germ cell tumor, medulloblastoma, and retinoblastoma. In addition to the specific diagnoses studied, particular chemotherapy regimens were chosen based on the variable expected degree of myelosuppression. Refer to Table 1 for the chosen regimens for study inclusion and Figure S1 for the comprehensive list of chemotherapy intensity within each regimen. Five patients received treatment dosing of enoxaparin for acute VTE during cancer therapy, which may have affected their platelet transfusion threshold. No one received prophylactic anticoagulation during their chemotherapy cycles. In order to eliminate confounding variables, excluded patients were those who received ≥40% of chemotherapy or supportive care at an outside institution, those who had been previously treated with a thrombopoietin (TPO) mimetic, those with a documented history of immune thrombocytopenia (ITP) or another previously diagnosed platelet disorder. Regimens with low-risk myelosuppressive agents for intermediate-risk rhabdomyosarcoma, standard-risk medulloblastoma, and intra-ocular retinoblastoma or regimens with less than five patients for high-risk rhabdomyosarcoma were also excluded. This study was approved by the Institutional Review Board at MSKCC. All data were collected from our institutional database and analyzed retrospectively.
TABLE 1.
Baseline patient demographics
| Characteristic | N = 150a |
|---|---|
| Histologic diagnosis and characteristics | |
| CNS-germ cell tumor | 11 (7%) |
| Ewing sarcoma (localized) | 28 (19%) |
| Medulloblastoma (high risk) | 9 (6%) |
| Neuroblastoma (high risk) | 28 (19%) |
| Bone marrow involvement | 20 (71%) |
| Osteosarcoma | 44 (29%) |
| Localized at diagnosis | 34 (77%) |
| Metastatic at diagnosis | 10 (23%) |
| Retinoblastoma (extraocular) | 14 (9%) |
| Bone marrow involvement | 4 (29%) |
| Rhabdomyosarcoma | 16 (11%) |
| Intermediate risk | 6 (37%) |
| High risk | 10 (6%) |
| Age at diagnosis (year) | 10.3 [0.2–21.0] |
| Chemotherapy regimens | |
| ACNS1123 stratum 1 (GCT) | 6 (4%) |
| ACNS1123 stratum 2 (GCT) | 5 (3%) |
| EFT (MSK-Ewing sarcoma) | 28 (19%) |
| ACNS0332 (medulloblastoma) | 9 (6%) |
| N8 (MSK-neuroblastoma) | 28 (19%) |
| MAP (osteosarcoma) | 44 (29%) |
| ARET0321 (retinoblastoma) | 14 (9%) |
| 03-099 (MSK-rhabdomyosarcoma) | 9 (6%) |
| D9803 (rhabdomyosarcoma) | 7 (5%) |
Statistics presented: n (%); mean [minimum–maximum].
2.2 |. Data collection and endpoints
Data collected included basic demographics as well as histologic diagnosis, chemotherapy regimen administered, and blood counts throughout treatment. The main analytic endpoints of the study included mean number of platelet transfusions (at a standard institutional dose of 7–10 ml/kg of apheresis, nonpathogen reduced platelets with maximum of one unit), mean number of delays, and mean number of chemotherapy dose reductions. Delays were defined as any time ≥4 days from expected completion of cycle (to account for nontreatment-related delays and reductions) and reductions defined as ≥20% decrease from expected dose as standardized in prior literature.3,5 An exploratory endpoint included relapse-free survival (RFS) on length of treatment.
2.3 |. Definition of chemotherapy-induced thrombocytopenia
There is currently no universal definition of CIT in literature. We aimed to define CIT as more than just an absolute number as treatment-related thrombocytopenia is expected and not always considered relevant. Therefore, we defined “clinically relevant CIT” from a functional aspect to include thrombocytopenia severe enough to warrant a platelet transfusion or ultimately impact treatment delivery through dose reductions or delays.12
2.4 |. Statistical analyses
Data were described using mean and range for continuous variables and frequency and percentages for binary or categorical variables. RFS was defined as the time from the start of the last received cycle and the date of relapse or death from any cause. Patients alive without relapse were censored at their dates of last follow-up. The starting time was chosen to allow the analysis of the impact of length of treatment on the RFS, information that is not known until the last cycle. Survival rates were estimated using a Kaplan–Meier estimator, and survival curves were compared using log-rank tests.
3 |. RESULTS
3.1 |. Summary of baseline characteristics
Within the 150 patients, there were seven solid tumor diagnoses and nine chemotherapy regimens included. At the time of diagnosis, median age was 11 years (range: 0.2–21 years of age). Histologic diagnoses and chemotherapy regimens are depicted in Table 1.
3.2 |. Platelet transfusion burden per regimen
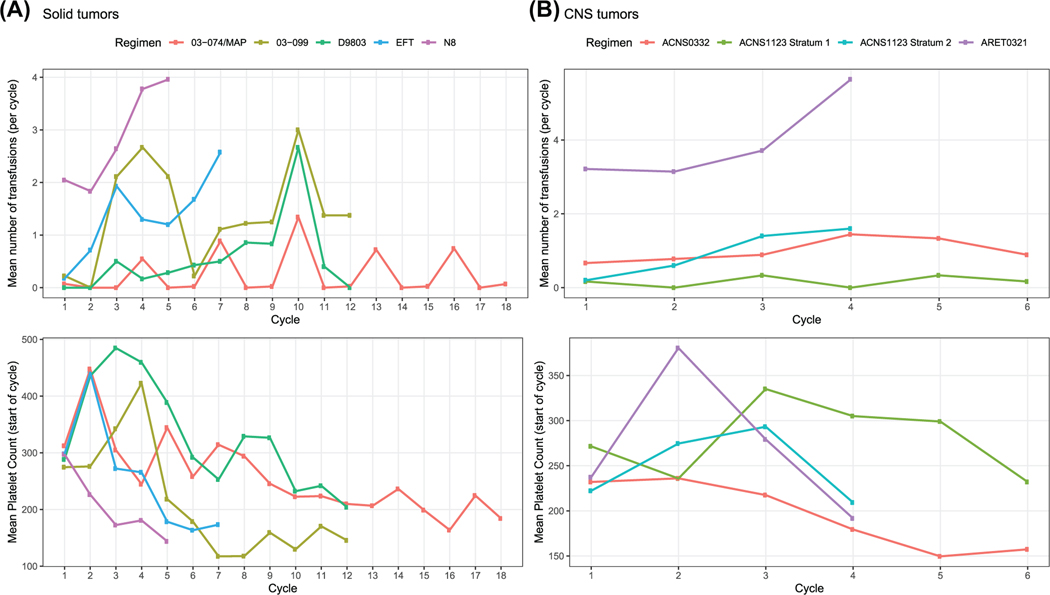
While all pediatric cohorts experienced some degree of anticipated treatment-related thrombocytopenia, certain regimens and chemotherapy agents were noted to cause more severe toxicity than others, which was illustrated through the variation in platelet transfusion requirements (Figure 1A,B).
FIGURE 1.
Aggregate data depicting mean platelet transfusions per chemotherapy regimen. (A) Non-central nervous system (CNS) solid tumor regimens. (B) CNS regimens
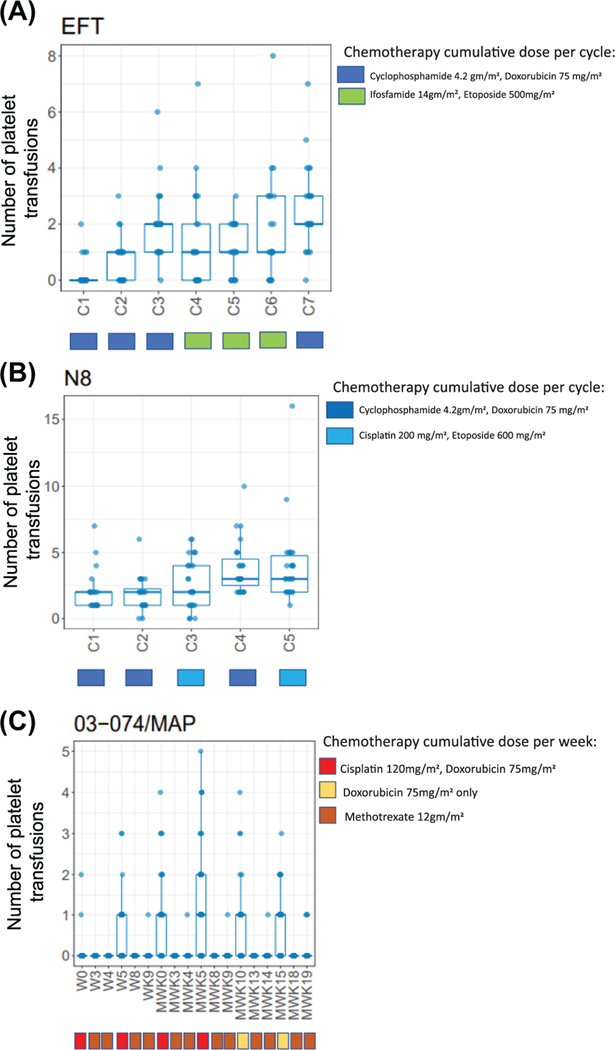
Several of the regimens led to a predictable increase in platelet transfusion requirements as cycles of chemotherapy progressed. Patients with Ewing sarcoma who were treated with the institution-specific EFT (Ewing’s family of tumors) regimen required a mean of 9.0 platelet transfusions throughout the regimen (range: 0–23), and this increased from a mean of 0.2 platelet transfusions during cycle 1 to a mean of 2.6 platelet transfusions during cycle 7 (Figure 2A). A similar increase in platelet transfusion requirement with progressive cycles of chemotherapy was observed in the cohort of neuroblastoma patients treated with the institution-specific N8 regimen. These patients required a mean of 13.1 (range: 5–35) platelet transfusions across all cycles of chemotherapy, and this varied from a mean of 2.0 platelet transfusions during cycle 1 to a mean of 4.0 platelet transfusions during cycle 5 (Figure 2B).
FIGURE 2.
Platelet transfusion means depicted over time for (A) Ewing sarcoma, (B) neuroblastoma, and (C) osteosarcoma cohorts. Chemotherapy cumulative dose shown for each cycle
A different pattern of platelet transfusion requirements was seen among rhabdomyosarcoma patients, with transfusion burden differing based on specific chemotherapy protocol received. Patients treated on the past institution-specific high-risk protocol (03-099) required a higher number of platelet transfusions during cycles that contained high-dose alkylating agents. These patients required a mean of 15.6 platelet transfusions throughout their treatment course (range: 1–29), which varied from a mean of 0.0–2.0 platelet transfusions in cycles that did not contain high-dose alkylators to a mean of 2.0–3.0 platelet transfusions during cycles, which included high doses of cyclophosphamide and ifosfamide (Figure S1D). This pattern of platelet transfusion requirements differed from patients with rhabdomyosarcoma who received treatment with the COG D9803 regimen, who, more similarly to patients with Ewing sarcoma and neuroblastoma, required increasing numbers of platelet transfusions as chemotherapy progressed. During cycle 1, these patients required an average of 0.0 platelet transfusions, whereas during the final cycles (cycles 7–12), patients required an average of 5.3 platelet transfusions (range: 0–19). Overall, patients treated with D9803 required a mean of 5.3 platelet transfusions throughout their treatment course (range: 0–20) (Figure S1E).
Osteosarcoma patients receiving MAP chemotherapy exhibited yet another pattern of platelet transfusion requirements. Though these patients required relatively few platelet transfusions throughout their treatment course (mean 4.4, range: 0–11), during the cisplatin and doxorubicin-containing induction (preoperative) and maintenance (postoperative; consolidation) weeks, there were notably more transfusion requirements compared to the methotrexate-only weeks (Figure 2C). In addition, 50% (22/44) patients in the osteosarcoma cohort were noted to receive at least one transfusion during the first 9 weeks of induction therapy. These patients who required platelet transfusions during the induction stage continued to require notably more transfusions throughout their postoperative therapy than those who did not require transfusions during induction, suggesting a particular susceptibility to CIT in this cohort. For this impacted population, the average total transfusion burden during maintenance weeks had a mean of 4.9 (range: 1–9).
Of the CNS cohorts included, the retinoblastoma group receiving the COG ARET0321 exemplified a consistently high platelet transfusion requirement throughout this four-cycle regimen (Figure 1B). Patients experienced a mean of 15.7 platelet transfusions throughout their treatment course (range: 0–58). For cycle 1, there was a mean of 3.2 transfusions (range: 0–9); by cycle 4, the mean increased to 5.6 (range: 0–27). The remaining CNS cohorts were less significantly impacted by CIT as it pertains to transfusion burden (as depicted in Figure 1B).
For the medulloblastoma patients treated with COG ACNS0332, there were minimal platelet transfusions required (Figure S2G), with a mean of less than 1.0 transfusion per cycle throughout the course of treatment. And lastly, for the CNS germ cell tumor patients receiving COG ACNS1123, stratum 2 patients had more platelet transfusion requirements than stratum 1 patients (total platelet transfusion mean per stratum 2 was 3.8 [range: 0–5] compared to stratum 1 with total mean of 1.0 transfusions [range: 0–4]) (Figure S2H).
3.3 |. Treatment modifications per regimen
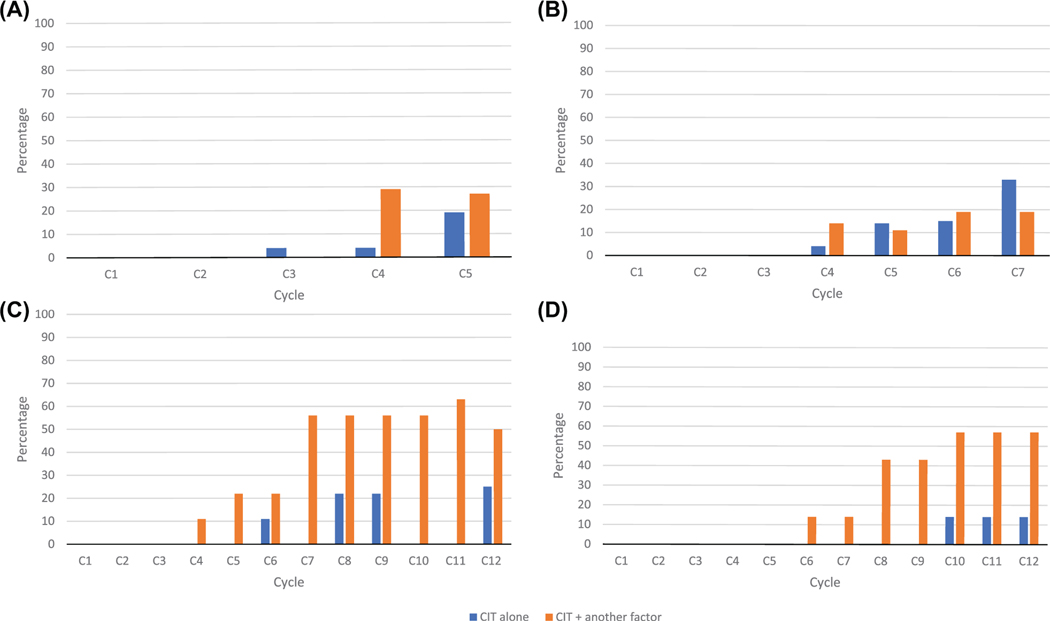
Of the regimens studied, all had some degree of treatment disruption in the form of either cycle delays or chemotherapy dose reductions (RDI). The etiology of such delays ranged from CIT alone, CIT + another factor (neutropenia, infection, surgery, mucositis, transaminitis, neuropathy, etc.) or lastly, for “other” reasons not including CIT.
As depicted in Table 2, the regimens noted to have highest number of treatment modifications due to CIT were within the neuroblastoma (N8), Ewing sarcoma (EFT), and rhabdomyosarcoma (03-099, D9803) regimen cohorts. Similarly, among these regimens, the dose reductions and treatment delays increased as treatment progressed. For the neuroblastoma N8 regimen, 4% of patients were noted to have a reduction or delay at cycle 3 due to isolated CIT and by cycle 5, this increased to 19% of patients (Figure 3A). In total, of the N8 chemotherapy cycles, 18% of total treatment disruptions were due to CIT alone and 38% due to CIT + another factor. For the EFT regimen, 4% of patients had a reduction or delay at cycle 4 due to isolated CIT and by cycle 7, this increased to 33% of patients (Figure 3B). In total, 34% of total treatment disruptions were due to CIT alone and 34% due to CIT + another factor. For the 03-099 rhabdomyosarcoma regimen, 11% of these patients had a reduction or delay at cycle 4 due to CIT + another factor and by cycles 10–12, this increased to up to 56% of patients (Figure 3C). In total, 12% of all treatment disruptions were due to CIT alone and 59% due to CIT + another factor. For the D9803 regimen, the first seven cycles of the regimen infrequently experienced treatment modifications, but by cycles 8–12, 69% of all patients had treatment disruptions, all of which were due, in some degree, to CIT (Figure 3D).
TABLE 2.
Reduced relative dose intensity (RDI) due to CIT per chemotherapy regimen
| Regimen | EFT | MAP | 03-099 | D9803 | N8 | ARET0321 | ACNS1123 stratum 1 | ACNS1123 stratum 2 | ACNS0332 |
|---|---|---|---|---|---|---|---|---|---|
| # Patients (n) | 28 | 44 | 9 | 7 | 28 | 14 | 6 | 5 | 9 |
| # Total cycles (# cycle in regimen × n) | 194 | 787b | 106 | 84 | 129 | 56 | 36 | 20 | 54 |
| Total reduced RDIa: N (%) | 53 (27%) | 175 (22%) | 58 (55%) | 29 (35%) | 40 (31%) | 34 (60%) | 8 (22%) | 6 (30%) | 36 (67%) |
| Of those with total RDI: | |||||||||
| Cycles of reduced RDI due, at least in part, to CIT | 36 (68%) | 29 (17%) | 41 (71%) | 23 (79%) | 22 (55%) | 10 (29%) | 1 (13%) | 3 (50%) | 5 (14%) |
| Cycles of reduced RDI due to CIT alone: N (%) | 18 (34%) | 15 (9%) | 7 (12%) | 3 (10%) | 7 (18%) | 2 (6%) | 1 (13%) | 3 (50%) | 1 (3%) |
| Cycles of reduced RDI due to CIT+factor: N (%) | 18 (34%) | 14 (8%) | 34 (59%) | 20 (69%) | 15 (38%) | 8 (24%) | 0 | 0 | 4 (11%) |
Reduced relative dose intensity: defined as a reduction in dose by 20% and/or delay ≥4 days from intended cycle length.
Cycles are in weeks.
FIGURE 3.
Reduced relative dose intensity depicted over chemotherapy regimens for (A) neuroblastoma (N8), (B) Ewing sarcoma (EFT), (C) rhabdomyosarcoma (03-099), and (D) rhabdomyosarcoma (D9803)
Cohorts noted to have many treatment modifications due to etiologies “other than” CIT were mainly the osteosarcoma, medulloblastoma, and retinoblastoma groups. For the osteosarcoma cohort, a significant number of treatment disruptions were due to mucositis and renal impairment presumably due to high-dose methotrexate administration. But notably, during the maintenance weeks of therapy in which cisplatin and doxorubicin were administered, CIT appeared to contribute to delays and dose reductions, with up to 40% of weeks affected by thrombocytopenia. For the retinoblastoma cohort, while most treatment modifications were due to reasons other than CIT (i.e., infection), 25% of all treatment disruptions were due to CIT + another factor. For the medulloblastoma cohort, most treatment modifications were due to reasons other than CIT (i.e., ototoxicity, neuropathy) (Table 2).
3.4 |. Effect of treatment modifications on disease outcomes
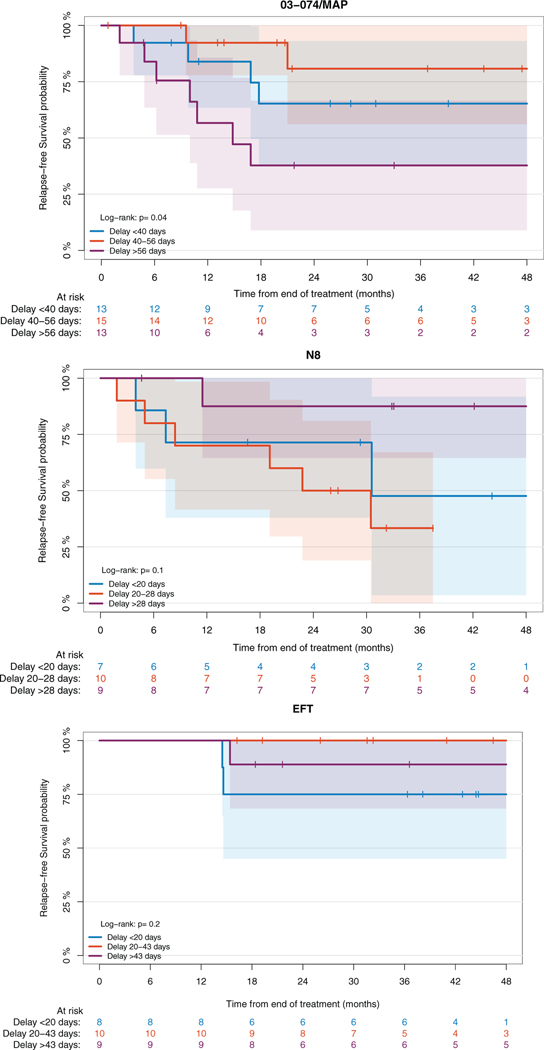
Survival was studied compared to length of treatment in the largest patient cohorts: neuroblastoma (N8), Ewing sarcoma (EFT), and osteosarcoma (MAP) patients. Length of treatment reflects the overall status of the patients regarding their ability to receive treatment and was impacted by various etiologies, including infection, lab abnormalities (including hematologic toxicities), mucositis, delays associated with surgery, and so forth. Within each cohort, patients were divided evenly into terciles. For the osteosarcoma cohort, expected length of treatment was approximately 217 days (28 weeks of therapy and 3 weeks built in for surgery as defined in institutional regimen). For the neuroblastoma cohort, expected length of treatment was approximately 105 days (five cycles of 21 days each). For the Ewing sarcoma cohort, the expected length of treatment was approximately 147 days (seven cycles of 21 days each). For the osteosarcoma cohort, length of treatment was significantly associated with RFS (p = .04), and patients with the greatest delay in treatment (>56 days from expected length) had the worst RFS (38% at 48 months) (Figure 4A). This suggests that taking measure to ensure the patients can go through their treatment as planned might impact their outcome. RFS did not appear to be associated to length of treatment in the neuroblastoma and Ewing sarcoma cohorts (Figure 4B,C).
4 |. DISCUSSION
CIT, a common and serious sequela of pediatric solid tumor chemotherapy regimens, may require platelet transfusion support or treatment modifications, potentially causing a decrease in treatment intensity. The prevalence of these complications appears to increase with time on treatment, which is likely due to the cumulative effect of chemotherapy on bone marrow recovery resulting in increased depth and duration of the platelet nadir. As these are potentially curative chemotherapy regimens and historically, dose intensity has been shown to be critical for cure, CIT may impact cure rates insofar as it heavily impacts RDI. While filgrastim has significantly reduced the burden of chemotherapy-induced neutropenia,13 at present, there is no standard approach to the management of CIT beyond what is currently practiced with supportive care measures.
The purpose of this chart review was to identify which subpopulations and chemotherapy regimens have the highest prevalence of CIT measured through the degree of platelet transfusions and dose reductions and delays. The modifications due to CIT were unique to specific treatment regimens. In our study, the localized Ewing sarcoma cohort and the high-risk neuroblastoma cohort were two of the most notable groups to experience prominent CIT from both a platelet transfusion burden and RDI perspective. This is most likely attributed to the high-dose cyclophosphamide (total 4.2 g/m2 per cycle) used in both regimens. The rhabdomyosarcoma cohorts experienced the effects of CIT from both a high platelet transfusion burden and significant dose reduction/cycle delay perspective. These regimens similarly contain high-dose cyclophosphamide as well as other myelosuppressive chemotherapeutic agents (such as carboplatin and ifosfamide) that can greatly impact regenerative bone marrow capabilities. In addition, the regimens used to treat rhabdomyosarcoma are substantial in length, with at least 12 cycles of therapy administered over course of treatment. To note, the institutional 03-099 regimen is no longer being used due to its notable treatment-related toxicities. Interestingly, analysis of the osteosarcoma cohort showed that treatment-related toxicities can affect some individuals more acutely than others. For example, for unknown reasons, only 50% (22/44) of the entire cohort was noted to experience significant CIT throughout their treatment course. While it may be uncommon for patients to require a high number of platelet transfusions during the induction period of treatment, of the 50% of individuals who did require such transfusion support, there appeared to be a correlation with significantly more CIT impact during the latter, maintenance weeks. For the CNS tumors studied, the retinoblastoma cohort regimen was noted to experience an extremely high platelet transfusion burden, which while likely is due to the high-dose alkylator administration, it may presumably be due to the fact that the patients being treated on this regimen have metastatic disease at treatment start and some have significant bone marrow involvement. Less impressive from a hematologic toxicity standpoint, both the medulloblastoma cohort and the CNS GCT cohort experienced minimal CIT impact, which is likely due to the less myelosuppressive chemotherapy regimens.
This study is to our knowledge, one of the first to analyze the hematologic effects associated with the treatment of such a heterogenous cohort of oncologic diagnoses and chemotherapeutic regimens within a single institution’s pediatric oncology population. While this study provides useful insight for chemotherapeutic intervention in pediatric oncology, there are notable limitations that are worth mentioning for potential future studies. The retrospective nature of this study is a disadvantage given the fact that accuracy and data collection were dependent on proper medical documentation and data extraction. To overcome this, multiple researchers collected data in a systematic manner to ensure that internal validity was upheld. In addition, given the overall rarity of pediatric oncologic diagnoses as a whole, while the overall study cohort number was substantial, when analyzing data within the specific diagnostic subdivisions, there are concerns that small sample size can affect the overall impact and significance of the data at hand. It is likely that patient factors such as age, comorbid infections (causing marrow suppression), and other factors (such as type of local control and/or location of primary tumor) play a part in influencing and modulating the incidence and severity of CIT, though full analysis of such factors is beyond the scope of this study. This study contains some institution-specific regimens and while comparable to COG protocols, there are notable differences that are clarified in Figure S1. In addition, while it is standard institutional practice to achieve a threshold of 75,000/μl prior to starting a cycle of chemotherapy, there is always chance in having provider variability in tolerating a lower threshold if felt to be clinically indicated. And lastly, deciphering between prophylactic versus symptomatic transfusions (due to bleeding) was difficult to assess given such variability in documentation.
While this study provides an initial description of CIT in pediatric patients treated for a variety of oncologic diagnoses at a single institution, additional data are required to determine potential interventions and expectations for impact on treatment success. While there are numerous studies currently being performed in adult oncology populations with thrombopoietin receptor agonists (TPO-R) in the setting of CIT, the potential beneficial use within the pediatric population is still unknown.14–16 To study this, we developed a clinical trial (currently open and enrolling patients) to study the prophylactic use of romiplostim in solid tumor patients (NCT04671901), which will hopefully provide insight into whether this approach reasonably mitigates CIT in high-risk patients. As always, the ultimate goal is to not only provide curative treatment options for children with cancer but to limit, as much as possible, treatment-related adverse effects in order to preserve quality of life. Given initial data provided in this study, there appears to be a potential opportunity for minimizing the effect of CIT in pediatric oncology patients leading to potentially greater treatment success.
Supplementary Material
FIGURE 4.
Relapse-free survival based on days of delay from expected treatment length for (A) osteosarcoma, (B) neuroblastoma, and (C) Ewing sarcoma cohorts
ACKNOWLEDGMENTS
All authors at MSK are supported by the NCI Cancer Center Support Grant P30 CA008748. Editorial support was provided by Joseph Olechnowicz, MA, Senior Editor, Department of Pediatrics, MSKCC. Michael Ortiz acknowledges research and salary support from a Conquer Cancer Career Development Award and a Young Investigator Award from Cannonball Kids’ Cancer.
CONFLICT OF INTEREST
Mary Clouser is an employee and holds stock in Amgen Inc. Funding for this work was provided by Amgen Inc. Ira Dunkel currently serves on the advisory board and consultant for Astra Zeneca, Bristol Myers Squibb, Day One, and QED. Gerald Soff serves on advisory board and consultant for Amgen, Janssen Scientific Affairs, Novartis, Anthos Therapeutics, and Hengrui (USA) Ltd. Michael Ortiz has recently served as an expert advisor for Guidepoint Global.
Abbreviations:
- CIT
chemotherapy-induced thrombocytopenia
- CNS
central nervous system
- COG
Children’s Oncology Group
- RDI
relative dose intensity
- RFS
relapse-free survival
- TPO
thrombopoietin
Footnotes
SUPPORTING INFORMATION
Additional supporting information can be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Tamamyan G, Danielyan S, Lambert MP. Chemotherapy induced thrombocytopenia in pediatric oncology. Crit Rev Oncol Hematol. 2016;99:299–307. [DOI] [PubMed] [Google Scholar]
- 2.Liebman HA. Thrombocytopenia in cancer patients. Thromb Res. 2014;133:S63–S69. [DOI] [PubMed] [Google Scholar]
- 3.Mones JV, Soff G. Management of thrombocytopenia in cancer patients. Cancer Treat Res. 2019;179:139–150. [DOI] [PubMed] [Google Scholar]
- 4.Shaw JL, Nielson CM, Park JK, Marongiu A, Soff GA. The incidence of thrombocytopenia in adult patients receiving chemotherapy for solid tumors or hematologic malignancies. Eur J Haematol. 2021;106(5):662–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elting LS, Rubenstein EB, Martin CG, et al. Incidence, cost, and outcomes of bleeding and chemotherapy dose modification among solid tumor patients with chemotherapy-induced thrombocytopenia. J Clin Oncol. 2001;19(4):1137–1146. [DOI] [PubMed] [Google Scholar]
- 6.Bercovitz RS, Josephson CD. Thrombocytopenia and bleeding in pediatric oncology patients. Hematology Am Soc Hematol Educ Program. 2012;2012:499–505. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer CA, Bohlke K, Delaney M, et al. Platelet transfusion for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2018;36(3):283–299. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal N, Chatterjee K, Sen A, Kumar P. Prevalence of platelet reactive antibodies in patient’s refractory to platelet transfusions. Asian J Transfus Sci. 2014;8(2):126–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denduluri N, Patt DA, Wang Y, et al. Dose delays, dose reductions, and relative dose intensity in patients with cancer who received adjuvant or neoadjuvant chemotherapy in community oncology practices. J Natl Compr Canc Netw. 2015;13(11):1383–1393. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama G, Tanaka C, Uehara K, et al. The impact of dose/time modification in irinotecan- and oxaliplatin-based chemotherapies on outcomes in metastatic colorectal cancer. Cancer Chemother Pharmacol. 2014;73(4):847–855. [DOI] [PubMed] [Google Scholar]
- 11.Womer RB, West DC, Krailo MD, et al. Randomized controlled trial of interval-compressed chemotherapy for the treatment of localized Ewing sarcoma: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30(33):4148–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Samkari H, Soff GA. Clinical challenges and promising therapies for chemotherapy-induced thrombocytopenia. Expert Rev Hematol. 2021;14(5):437–448. [DOI] [PubMed] [Google Scholar]
- 13.Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–170. [DOI] [PubMed] [Google Scholar]
- 14.Al-Samkari H, Parnes AD, Goodarzi K, Weitzman JI, Connors JM, Kuter DJ. A multicenter study of romiplostim for chemotherapy-induced thrombocytopenia in solid tumors and hematologic malignancies. Haematologica. 2021;106(4):1148–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parameswaran R, Lunning M, Mantha S, et al. Romiplostim for management of chemotherapy-induced thrombocytopenia. Support Care Cancer. 2014;22(5):1217–1222. [DOI] [PubMed] [Google Scholar]
- 16.Soff GA, Miao Y, Bendheim G, et al. Romiplostim treatment of chemotherapy-induced thrombocytopenia. J Clin Oncol. 2019;37(31): 2892–2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.