Abstract
After infection with a low-virulence strain of Mycobacterium avium, C57BL/6 and C57BL/10 mice had clear differences in the control of the infection in their livers and spleens. This difference in susceptibility was not associated with differences in the H-2 complex. It was dependent on the activity of CD4+ T cells but unrelated to the ability of these cells to secrete gamma interferon or to the development of delayed-type hypersensitivity responses at 3 weeks of infection. It was associated with lower total numbers of CD4+ cells present in infected spleens and was related to an earlier induction of protective T cells, as measured by adoptive-transfer assays. These data further strengthen the notion of gamma-interferon-independent mechanisms of protection against mycobacteria.
Mycobacterium avium is an opportunistic pathogen in humans affecting most frequently immunocompromised patients, namely, patients with AIDS (9). This intracellular mycobacterium proliferates inside macrophages and is controlled by as yet poorly understood effector mechanisms of these phagocytes, namely, after their activation by cytokines produced during innate and acquired immune responses (1). One of the major genetic determinants of resistance to infection by M. avium was identified in mice as the Nramp1 gene (formerly known as the Bcg/Ity/Lsh locus) (2). This gene is expressed mostly in macrophages and imparts an innate capacity to these cells to control M. avium infection, as well as other mycobacterial, Salmonella, and protozoal infections, even in the absence of external modulation by other immune cells and the cytokines produced by them (8). Although the mechanism of action of the encoded transmembrane polypeptide is still not clarified, it is possible that it is involved in the transport of iron out of the phagosome, thereby leading to the deprivation of such nutrients and consequent mycobacteriostasis (3, 6). Alternatively, it may work via the acidification of the mycobacterium-bearing phagosome (7).
It has been suggested by Orme and colleagues (13) that additional genetic determinants affecting the in vivo growth of M. avium are present in mice. Preliminary observations in our laboratory showed that strains of mice sharing the D169 mutant allele of Nramp1 (i.e., the susceptibility allele of the Bcg gene) differed in the control of the infection induced by an AIDS isolate of M. avium. We study here two such strains, C57BL/6 and C57BL/10, that differ in their susceptibilities to M. avium and show that the efficiency in mobilizing protective T cells against the infection by M. avium differs between these two strains.
MATERIALS AND METHODS
Animals.
Female C57BL/6J and C57BL/10ScSnOlaHsd mice were used when they were 6 to 8 weeks old. C57BL/6J mice were obtained from the Gulbenkian Institute (Oeiras, Portugal) and C57BL/10ScSnOlaHsd and B10.D2/nOlaHsd mice were purchased from Harlan (Oxon, United Kingdom). Mice were bred under similar conditions in our facilities and given sterile food and acidified drinking water. T-cell-depleted mice were obtained by thymectomizing 4- to 6-week-old mice and treating them with 0.2 mg of anti-CD4 monoclonal antibodies intraperitoneally (clone GK1.5; American Type Culture Collection [ATCC], Manassas, Va.) every 10 days starting on the day of the infection, initiated 2 weeks after surgery. Depletion of the targeted T cells was confirmed by flow cytometry and consistently led to a reduction in the percentage of CD4+ T cells below 1% of the total spleen population.
Bacterial preparations and infections.
M. avium 2447 SmT was isolated from an AIDS patient and given to us by F. Portaels, Institute of Tropical Medicine, Antwerp, Belgium. Mycobacterial inocula were obtained by growing the mycobacteria in Middlebrook 7H9 broth (Difco, Detroit, Mich.) containing 0.04% Tween 80 (Sigma, St. Louis, Mo.). Cultures were harvested during log phase, centrifuged, washed in saline containing 0.04% Tween 80, briefly sonicated, and stored in aliquots at −70°C until used. Mycobacterial antigen consisting of secreted M. avium proteins (CFP, for culture filtrate proteins) was prepared as described by Silva et al. (15). Mice were infected intravenously with 106 CFU of M. avium. At different time points, animals were sacrificed, and their livers, spleens, and lungs were collected. Serial dilutions of organ homogenates were plated on Middlebrook 7H10 agar medium, and the bacterial colonies were counted after culture for 10 days at 37°C.
Assessment of DTH.
Delayed-type hypersensitivity (DTH) was checked at 3 weeks of infection in groups of four animals after injection of 10 μg of M. avium CFP in phosphate-buffered saline (PBS) in one of the footpads and comparison of the swelling with that induced in the contralateral footpad by injection with PBS alone. Swelling was measured by using an appropriate caliper at 24, 48, and 72 h after injection.
In vitro stimulation of splenic cells.
Cells were obtained from the spleens of individual mice, washed once with PBS, and depleted of erythrocytes by using a hemolytic solution (155 mM NH4Cl, 10 mM KHCO3; pH 7.2). Cells were then plated in 96-well plates and incubated in triplicate in RPMI 1640 medium (Gibco, Paisley, United Kingdom) supplemented with 10% fetal calf serum (Gibco) either in medium alone or in the presence of mycobacterial antigen (4 μg/ml) or concanavalin A (4 μg/ml; Sigma). Supernatants from 3-day cultures were analyzed for the presence of gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay (ELISA).
Detection of IFN-γ in the serum and in culture supernatants.
IFN-γ was quantified through an ELISA method by using R4-6A2 and biotinylated-AN18 anti-IFN-γ monoclonal antibodies as the capture and detection antibodies, respectively. Recombinant IFN-γ from Genzyme (Cambridge, Mass.) was used as standard.
Flow cytometric analysis of spleen cell populations.
Spleen cells were obtained by gently teasing the organs. Staining was performed on ice-chilled suspensions of cells by using anti-CD4, anti-CD8, and anti-γδ monoclonal antibodies from Pharmingen (San Diego, Calif.). Cells were analyzed in a FACSort apparatus (Becton Dickinson, Mountain View, Calif.) after addition of propidium iodide to exclude dead cells.
Adoptive transfer of T-cell-enriched spleen cell suspensions.
Adoptive transfer was carried out as described previously (15). Spleen cells from uninfected C57BL/6 mice, as well as spleen cells from C57BL/6 and C57BL/10 mice infected for 2 or 4 weeks with M. avium 2447 were prepared by homogenizing the organs in metal sieves, depleting the suspension of erythrocytes after incubation in the hemolytic solution, and treating the cell suspension for 45 min with anti-CD24 monoclonal antibodies (clone J11d; ATCC) in the presence of rabbit complement (Serotec, Oxford, United Kingdom). The resulting cell suspension was depleted of adherent cells by incubation in nylon wool columns for 2 h. These T-cell-enriched suspensions were infused into previously irradiated C57BL/6 mice (5Gy, administered 24 h earlier) infected for 2 h with M. avium. Mycobacterial loads in the recipient mice were determined 32 days after infection.
Statistical analysis.
The Student's t test was used to compare pairs of data. The analysis of variance (ANOVA) test was used to compare the different groups or treatments.
RESULTS
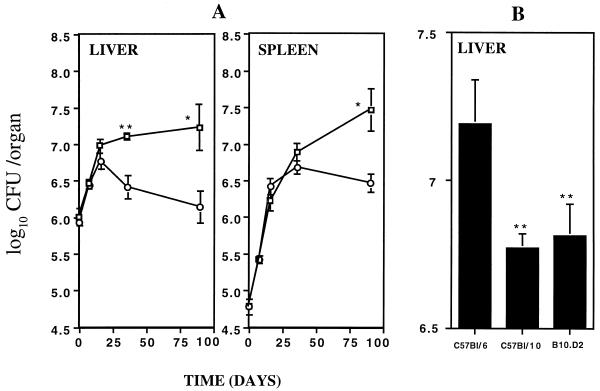
Preliminary experiments showed that the multiplication of M. avium 2447 SmT in the livers and spleens of four different strains of mice, sharing the same Nramp1D169 allele, varied (data not shown). We therefore studied the immunological basis of the different susceptibilities of two such strains, C57BL6 and C57BL/10. As shown in Fig. 1A, C57BL/10 mice controlled the growth of M. avium 2447 SmT better than the closely related strain C57BL/6. Such enhanced capacity to control the infection was observed after the second week of infection, and differences were retained for up to 3 months of infection. This was confirmed in seven independent experiments, where such differences between the two strains were consistently observed. We also compared the proliferation of M. avium in C57BL/6 and C57BL/10 strains with a C57BL/10 congenic strain with a distinct H-2 haplotype, the same found in BALB/c mice (d haplotype). Thus, although both C57Bl strains share the same H-2 haplotype, C57BL/10 mice were more resistant than the C57BL/6 mice (Fig. 1B). On the other hand, a C57BL/10 congenic strain which has the H-2 haplotype of the more susceptible BALB/c mice was not different in terms of control of M. avium proliferation from the C57BL/10 original strain (Fig. 1B).
FIG. 1.
Proliferation of M. avium 2447 SmT in the organs of mice infected intravenously with 106 CFU. (A) M. avium growth was studied in the livers and spleens of C57BL/6 (□) and C57BL/10 (○) mice for up to 3 months. (B) The growth of M. avium was studied after 4 weeks of infection in C57BL/6, C57BL/10, and B10.D2 mice. Statistically significant differences are labeled (∗∗, P < 0.01) as determined by the Student's t test. Each group consisted of four or five mice.
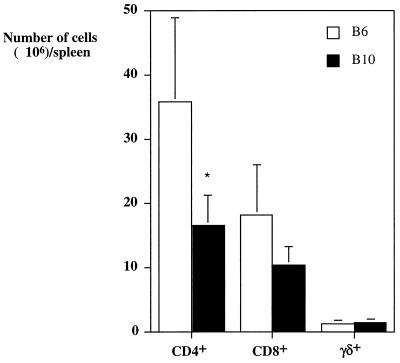
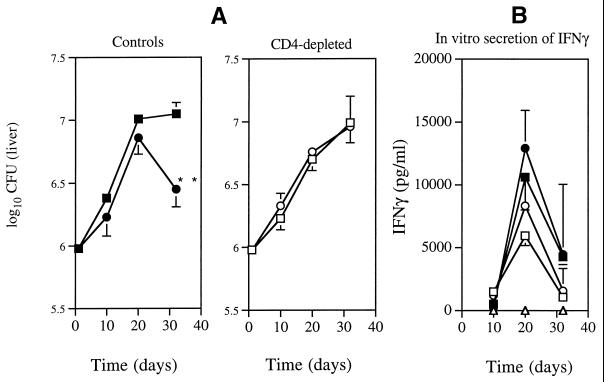
Preliminary experiments showed that macrophages isolated from either strain behaved similarly in vitro with regard to their ability to restrict M. avium growth (not shown). To assess whether T cells were involved in determining the increased resistance of the C57BL/10 strain, we first analyzed the numbers of the different T-cell populations in the spleens of mice infected with M. avium for 4 weeks (Fig. 2). No statistically significant differences between the CD8+ and γδ-TCR+ T cells were found between the two mouse strains, but higher numbers of CD4+ T cells appeared to be present in the spleens of the more-susceptible mouse strain, C57BL/6. Since the major T-cell subpopulation that leads to control of M. avium growth is the CD4+ subset (1), we depleted these cells by giving anti-CD4 monoclonal antibodies to previously thymectomized mice. As shown in Fig. 3A, CD4-depleted mice from either the C57BL/6 or the C57BL/10 strains allowed the mycobacteria to grow at the same rate, as opposed to the already-described differences that were evident in strains of mice that were left untreated before and during infection.
FIG. 2.
Numbers of CD4+, CD8+, and γδ-TCR+ T cells in the spleens of C57BL/6 (open columns) or C57BL/10 (solid columns) mice infected for 4 weeks with M. avium. Statistically significant differences between the two strains are labeled (∗, P < 0.05; as determined by the Student's t test). Cells from individual mice were analyzed, and four mice per group were used.
FIG. 3.
(A) Proliferation of M. avium 2447 SmT in the liver of C57BL/6 (squares) and C57BL/10 (circles) mice. Animals were either left untreated before or during infection (Controls) or thymectomized and given anti-CD4 monoclonal antibodies as described in Materials and Methods (CD4-depleted). Statistically significant differences are labeled “∗∗” for P < 0.01 as determined by the Student's t test. (B) In vitro secretion of IFN-γ by spleen cells from the same control animals after stimulation with M. avium CFP antigen (solid symbols) or concanavalin A (open symbols). Triangles represent data from nonstimulated cultures. There were no statistically significant differences in IFN-γ secretion between the two groups of mice as determined by the Student's t test. Four mice per group were examined.
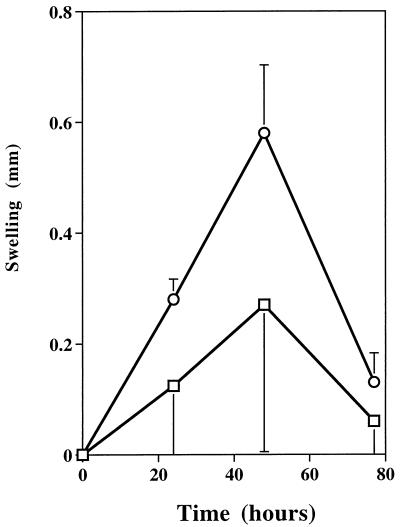
Mice infected for 3 weeks with M. avium were injected in their footpads with M. avium CFP as antigen, and the swellings of the injected footpad and the contralateral footpad injected with the vehicle alone were evaluated for 3 days. As shown in Fig. 4, C57BL/10 mice mounted DTH responses to M. avium antigens similar to those of the C57BL/6 mice. Histological analysis of the development of granulomas in the infected livers also failed to reveal any major difference between the two mouse strains (data not shown).
FIG. 4.
DTH responses at 3 weeks of infection in C57BL/6 (□) and C57BL/10 (○) mice. Footpad swelling was measured at different time points after injection of either 10 μg of M. avium CFP antigen or PBS. Data are shown as the means of the difference between the swellings in the footpad injected with antigen and in the contralateral one, injected with PBS alone, ± one standard deviation. Differences between the two strains were not statistically significant as determined by the Student's t test.
Given the major role played by IFN-γ in mediating immunity to M. avium, we analyzed whether differences between the two C57BL strains were associated with different amounts of secretion of this cytokine. Sera from C57BL/6 and C57BL/10 mice showed similar amounts of IFN-γ during infection by M. avium (Table 1). Also, spleen cells from infected animals of either strain produced similar amounts of IFN-γ upon in vitro stimulation with M. avium-specific CFP antigen or concanavalin A, and such responses had similar kinetics during in vivo infection (Fig. 3B).
TABLE 1.
Presence of immunoreactive IFN-γ in sera from mice infected with M. aviuma
| Expt | Time postinfection (days) | Mean concn (pg/ml) in sera from:
|
|
|---|---|---|---|
| C57BL/6 mice | C57BL/10 mice | ||
| 1 | 15 | 153.6 ± 63.7 | 134.3 ± 52.1 |
| 30 | 689.7 ± 284.7 | 433.3 ± 95.0 | |
| 2 | 30 | 280.6 ± 58.6 | 219.3 ± 76.2 |
| 90 | 614.5 ± 360.8 | 265.9 ± 70.9 | |
Data represent the mean concentrations of IFN-γ in the sera from groups of four mice ± one standard deviation. No statistically significant differences were found between the two strains.
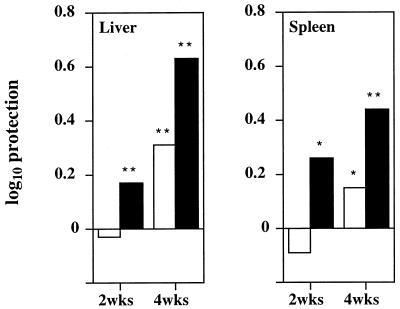
To further characterize the T-cell function in the two mouse strains, we evaluated the ability of T cells to adoptively transfer protection. Spleen cells from C57BL/6 or C57BL/10 mice infected for 2 or 4 weeks with M. avium were isolated and enriched for T cells. The resulting cell preparations were infused into sublethally irradiated C57BL/6 mice that were infected with the same strain of M. avium, and the mycobacterial proliferation was assessed 32 days later. As shown in Fig. 5, protection afforded by T-cell-enriched spleen cells from C57BL/10 mice was bigger than that afforded by cells from C57BL/6 mice at both time points of infection of the donors. Thus, CFU values were significantly lower in the livers of mice receiving spleen cells from C57BL/10 mice than those receiving spleen cells from C57BL/6 mice after both 2 and 4 weeks of infection of the donors (P < 0.01 for recipients of either 2- or 4-weeks immune spleen cells according to the ANOVA test). CFU values were also significantly lower in the spleens of mice receiving spleen cells from C57BL/10 mice than those receiving spleen cells from C57BL/6 mice at 4 weeks of infection of the donors (P < 0.01 according to the ANOVA test). These data were confirmed in a second independent experiment (not shown).
FIG. 5.
Protective efficacy of T-cell-enriched spleen cell suspensions from C57BL/6 (open columns) or C57BL/10 (solid columns) mice infected for 2 or 4 weeks with M. avium compared to cells isolated from untreated animals. Recipient mice were sublethally irradiated C57BL/6 mice. Mycobacterial loads in the recipient animals were evaluated 32 days after infection and spleen cell transfusion. Data are shown as log10 decrease in CFU (protection), calculated by subtracting the geometric mean in the immune groups from that of the nonimmune controls. Statistically significant protection (recipients of immune cells were compared to those receiving nonimmune cells) is labeled “∗” for P < 0.05 or “∗∗” for P < 0.01 (according to the ANOVA test).
DISCUSSION
We used here an infection model with a strain of M. avium which induces a demonstrable T-cell-dependent protective immune response to characterize the immunological basis of the difference in control of M. avium infection by the C57BL/6 and C57BL/10 mouse strains. The studies with these two strains and one congenic strain did not support a major role of the H-2 locus in the different controls of the infection. The emergence of acquired immunity in C57BL/10 mice occurred at earlier time points than in the more susceptible C57BL/6 mice when the course of mycobacterial infections was studied in those animals. This pointed to a major role of T cells, which was also suggested by the ablation of the phenotype after thymectomy and depletion of CD4+ T cells. The difference in phenotype could not be explained by an increased production of the major protective cytokine, IFN-γ. Neither serum levels of this cytokine nor the ability of isolated immune spleen cells to secrete IFN-γ in vitro in response to specific antigen were different between the two strains. Also, a difference in the T-cell populations in terms of total numbers was not found to explain the results obtained, since the total number of CD4+ T cells was even higher in the more susceptible strain. This may be related to higher mycobacterial loads at week 4 of infection in the C57BL/6 strain than in the C57BL/10 strain. In any case, a higher expansion of CD4+ T cells in the latter strain was probably not the cause of the increased resistance. The emergence of DTH, measured at 3 weeks of infection, or the formation of granulomas were not significantly different between the two strains. However, the ability to transfer protective immunity, measured at 2 and 4 weeks of infection, seemed to develop faster in the more resistant C57BL/10 mice than in the C57BL/6 animals. It is not known what mechanisms underlie these differences in T-cell activity, but it is curious that they are not related to the capacity of T cells to secrete IFN-γ. In this context, we should point out that we have recently postulated that IFN-γ-independent mechanisms of protection exist during M. avium infections (5), and therefore we propose that the present model may allow us to address the nature of those mechanisms.
Genetic differences have already been identified between the C57BL/6 and C57BL/10 strains. Restriction fragment length polymorphisms were detected at multiple loci on chromosome 4 (12). Additionally, genetic differences on chromosomes 2, 11, 13, and 16 were detected by studying microsatellite markers (16). One gene that was found to have different allelic forms in the two mouse strains is the one coding for δ-aminolevulinate dehydratase, an enzyme involved in the synthesis of porphobilinogen. The form found in C57BL/6 has lower activity than that found in C57BL/10 and other mouse strains (4, 14). Whether this gene has any effect on T-cell function is not known, but a stimulatory effect of heme on the immune system has been demonstrated (17). Finally, it was shown that DTH responses differ between the two mouse strains during infection with mouse hepatitis virus. However, the relationship between DTH and protective immunity is controversial, and some researchers have claimed that they are not causally related (10).
The advantage in the control of proliferation of M. avium exhibited by C57BL/10 mice compared to C57BL/6 mice is rather small and does not confer much survival benefit as far as this infection is concerned. However, it would be interesting to study these two strains with regard to other infectious agents, namely, those that both depend on T cells for their control and have a faster course, thereby requiring a faster emergence of the T-cell-dependent mechanisms of immunity for survival.
In conclusion, we described here differences in protective antimycobacterial immunity that are likely unrelated to the H-2 locus or the Nramp1 gene and are independent of the secretion of IFN-γ. We postulate that such a mechanism affects the rate of emergence of protective T cells, namely, of IFN-γ-independent mechanisms of induction of protective immunity.
ACKNOWLEDGMENTS
This work was supported by contract P/SAU58/96 from the PRAXIS XXI programme (Lisbon, Portugal). Irene S. Leal, Manuela Flórido, Jorge Pedrosa, and Teresa F. Pais received fellowships from the PRAXIS XXI programme.
Footnotes
Corresponding author. Mailing address: Laboratory of Microbiology and Immunology of Infection, Institute for Molecular and Cell Biology, Rua do Campo Alegre 823, 4150 Porto, Portugal. Phone: 351.2.6074952. Fax: 351.2.6099157. E-mail: rappelb@ibmc.up.pt.
REFERENCES
- 1.Appelberg R. Immune response to atypical mycobacteria. Res Immunol. 1996;147:560–564. doi: 10.1016/s0923-2494(97)85222-4. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R, Sarmento A M. The role of macrophage activation and of Bcg-encoded macrophage function(s) in the control of Mycobacterium avium infection in mice. Clin Exp Immunol. 1990;80:324–331. doi: 10.1111/j.1365-2249.1990.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atkinson P G P, Barton C H. High level expression of Nramp1G169 in RAW264.7 cell transfectants: analysis of intracellular iron transport. Immunology. 1999;96:656–662. doi: 10.1046/j.1365-2567.1999.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doyle D, Schimke R T. The genetic and developmental regulation of hepatic δ-aminolevulinate dehydratase in mice. J Biol Chem. 1969;244:5449–5459. [PubMed] [Google Scholar]
- 5.Flórido M, Gonçalves A S, Silva R A, Ehlers S, Cooper A M, Appelberg R. Resistance of virulent Mycobacterium avium to gamma interferon-mediated antimicrobial activity suggests additional signals for induction of mycobacteriostasis. Infect Immun. 1999;67:3610–3618. doi: 10.1128/iai.67.7.3610-3618.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes M S, Appelberg R. Evidence for a link between iron metabolism and Nramp1 gene function in innate resistance to Mycobacterium avium. Immunology. 1998;95:165–168. doi: 10.1046/j.1365-2567.1998.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Govoni, G., F. Canonne-Hergaux, C. G. Pfeifer, S. L. Marcus, S. D. Mills, D. J. Hackam, S. Grinstein, D. Malo, B. B. Finlay, and P. Gros. Functional expression of Nramp1 in vitro in the murine macrophage line RAW264.Infect. Immun. 67:2225–2232. [DOI] [PMC free article] [PubMed]
- 8.Govoni G, Gros P. Macrophage NRAMP1 and its role in resistance to microbial infections. Inflamm Res. 1998;47:277–284. doi: 10.1007/s000110050330. [DOI] [PubMed] [Google Scholar]
- 9.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 10.Johnson C M, Cooper A M, Frank A A, Orme I M. Adequate expression of protective immunity in the absence of granuloma formation in Mycobacterium tuberculosis-infected mice with a disruption in the intracellular adhesion molecule 1 gene. Infect Immun. 1998;66:1666–1670. doi: 10.1128/iai.66.4.1666-1670.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyuwa S, Yamaguchi K, Fujiwara K, Yamanouchi K. Genetic control of delayed-type hypersensitivity in mice infected with mouse hepatitis virus. Prog Leukocyte Biol. 1985;3:159–167. [Google Scholar]
- 12.McClive P J, Huang D, Morahan G. C57BL/6 and C57BL/10 inbred mouse strains differ at multiple loci on chromosome 4. Immunogenetics. 1994;39:286–288. doi: 10.1007/BF00188793. [DOI] [PubMed] [Google Scholar]
- 13.Orme I M, Stokes R W, Collins F M. Genetic control of natural resistance to nontuberculous mycobacterial infections in mice. Infect Immun. 1986;54:56–62. doi: 10.1128/iai.54.1.56-62.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell R L, Coleman D L. Genetic control of hepatic δ-aminolevulinate dehydratase in mice. Genetics. 1963;48:1033–1039. doi: 10.1093/genetics/48.8.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva R A, Pais T F, Appelberg R. Evaluation of interleukin 12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 16.Slingsby J H, Hogarth M B, Simpson E, Walport M J, Morley B J. New microsatellite polymorphisms identified between C57BL/6, C57BL/10, and C57BL/KsJ inbred mouse strains. Immunogenetics. 1995;43:72–75. doi: 10.1007/BF00186607. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji A, Wang J, Stenzel K H, Novogrodsky A. Immune stimulatory and anti-tumour properties of haemin. Clin Exp Immunol. 1993;93:308–312. doi: 10.1111/j.1365-2249.1993.tb08177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]