Summary
As a consequence of breakthroughs in the area of guidelines research, the therapy for cholangiocarcinoma has significantly improved the efficacy rate of diagnosis and survival outcomes. We compared the most recently updated clinical practice guidelines and consensus to provide recommendations based on the diagnostic and therapeutic equipment available in various countries. Following a systematic review, we discovered that these guidelines and consensus had both similarities and differences in terms of what organizations or groups drafted the guidelines and the approach, applicability, content and recent updates of the guidelines as well as in terms of diagnostic and treatment algorithms. The disparities could be attributable to a variety of etiological factors, high risk patients, health resources, medical technology, treatment options, and income levels. Additionally, while complete adoption of guidelines may benefit physicians, patients, and authorities, there remains a disconnect between expected goals and implementation.
Keywords: cholangiocarcinoma, clinical practice guideline, diagnosis, treatment
1. Introduction
Cholangiocarcinoma (CCA) is a highly lethal, epithelial cell malignant tumor that can be derived from any point of the biliary tree. Due to the heterogeneity of malignancies, they are typically categorized according to primary anatomic subtype (intrahepatic, perihilar, and distal) (1,2). Intrahepatic cholangiocarcinoma (iCCA) is located proximally in the second-order bile ducts within the liver parenchyma. Perihilar cholangiocarcinoma (pCCA) arises between the second-order ducts and the insertion of the cystic duct. Distal cholangiocarcinoma (dCCA) is distal to the insertion of the cystic duct (3,4). Both pCCA and dCCA occur in the part of the bile duct outside the liver as an extrahepatic cholangiocarcinoma (ECC) (4). According to the 2019 WHO classification, mixed hepatocellular-cholangiocarcinoma (cHCC-CCA) was recently recognized as a distinct subtype of CCA (5). For each anatomic subtype, there is different epidemiology, biology, prognosis, and strategy for clinical management. Clinical practice recommendations for the management of CCA have been widely published globally. While the principle of the guidelines remains generally similar, the practice of different countries and the acceptance of recent research have presented a possible challenge to hepatobiliary surgeons. Several high-quality clinical practice guidelines have also been continually updated to reflect the most recent technological and drug advancement, as well as better understanding for the management of CCA. In addition to traditional treatments such as surgery and chemotherapy, targeted therapy and immunotherapy have made progress in the integrated management of CCA. The development of clinical trials and multicenter cross-regional collaboration provides high-level evidence-based medical evidence for new drug development and protocol optimization in CCA. Combined with the recent up-to-date guidelines, we have an updated review under the 2016 review version (6) to provide further treatment for the comprehensive management of CCA.
2. Literature search strategies
A literature search was conducted using PubMed, Embase, and Cochrane, as well as a bibliography search and manual search of association websites to identify guidelines and consensus for management of CCA. The search was limited to results with English or Chinese language since the year 2012 and is recent as of July 31, 2022. Key words included "cholangiocarcinoma", "biliary tract cancer", "hepatobiliary cancers", "guideline", and "consensus". Selected guidelines were extensively examined in order to extract topics, relevant recommendations, and conclusions to the questions. Between each guideline, these recommendations were compared and contrasted, and main differences and gaps were identified. The main characteristics of clinical practice guidelines and consensus are summarized in Table 1 (7-20). After screening, there are 14 current guidelines and consensus for CCA around the world, including 3 guidelines from the USA, 4 from Asia, and 7 from Europe. The National Comprehensive Cancer Network (NCCN) guideline and Chinese Society of Clinical Oncology (CSCO) guideline is distributed as a manual and it will be revised every year.
Table 1. Current guidelines and consesus on cholangiocarcinoma.
| Guidelines | Year | Country/Ge-ogrophical area | Language | Tumor | Ref. |
|---|---|---|---|---|---|
| NCCN Guideline | 2022 | USA | English | iCCA, pCCA, dCCA | (7) |
| CSCO Guideline | 2020 | China | Chinese | iCCA, pCCA, dCCA | (8) |
| ENS Guideline | 2020 | Europe | English | iCCA, pCCA, dCCA | (9) |
| SEOM Guideline | 2020 | Spain | English | PC, iCCA, pCCA, dCCA and GBC | (10) |
| Italian Guideline | 2020 | Italy | English | iCCA, pCCA, dCCA | (11,12) |
| JSHBPS Guideline | 2019 | Japan | English | iCCA, pCCA, dCCA | (13) |
| ESMO Guideline | 2016 | Europe | English | iCCA, pCCA, dCCA | (14) |
| CCHPBA Guideline | 2015 | China | Chinese | iCCA, pCCA, dCCA | (15) |
| AHPBA Guideline | 2015 | USA | English | iCCA, pCCA | (16,17) |
| ILCA Guideline | 2014 | Europe | English | iCCA | (18) |
| Asia-Pacific Guideline | 2013 | Asia-Pacific | English | pCCA | (20) |
| BSG Guideline | 2012 | UK | English | iCCA, pCCA, dCCA | (19) |
CCA, cholangiocarcinoma; pCCA, perihilar cholangiocarcinoma; iCCA, intrahepatic cholangiocarcinoma; dCCA, distal cholangiocarcinoma; GBC, gallbladder carcinoma; PC, Pancreatic cancer; HCC, hepatocellular carcinoma; NCCN, National Comprehensive Cancer Network; CSCO, Chinese Society of Clinical Oncology; SEOM, the European Network for the Study (ENS), the Spanish Society of Medical Oncology; JSHBPS, the Japanese Society of Hepato-Biliary-Pancreatic Surgery; ESMO, the European Society of Medical Oncology; CCHPBA, the Chinese Chapter of International Hepato-Pancreato-Biliary Association; AHPBA, the American International Hepato-Pancreato-Biliary Association; ILCA, the International Liver Cancer Association; BSG, the British Society of Gastroenterology
The initial CCA guideline was originally published in 2002 (21) and revised in 2012. Despite that the British Society of Gastroenterology (BSG) guideline had not renewed since 2012, we still incorporate the guideline into our review. Due to the lack of diagnosis and treatment for cHCC-CCA, current guidelines do not establish standard recommendations. Only the American International Hepato-Pancreato-Biliary Association (AHPBA) consensus published the management of cHCC-CCA separately. The following sections will discuss CCA management in the context of the new guidelines, and key differences between the guidelines will be highlighted.
3. Epidemiology and risk factors
It is well-known that there is significant heterogeneity among patients with CCA, and this heterogeneity is present even within variable clinicopathologic phenotypes and natural history. Thus, the incidence and mortality of CCA varies by subgroup and geographic region. According to epidemiologic studies (22-24), the age-standardized incidence rate for iCCA is growing, although the incidence rate for ECCs may be increasing or plateauing in the majority of countries. Internationally, recent studies have shown an annual incidence of CCA ranging from 0.3 cases per 100,000 in Costa Rica and Israel to 85 per 100,000 in northeast Thailand (25). However, the mortality rate from iCCA increased as a result of changes in risk variables and improved clinical classification. Following the rise of laparoscopic cholecystectomy, mortality from ECC has stabilized or declined (24).
Most cholangiocarcinoma patients have no predisposing factors recognized, although there is evidence that some risk factors may be related to the disease in certain patients. The Italian guideline, the European Network for the Study of Cholangiocarcinoma (ENS-CCA) guideline and the BSG guideline summarized risk factors in table form, and the other guidelines did so in a description. All guidelines report that the development of CCA is associated with chronic inflammation. Leone et al. addressed how chronic inflammatory diseases favor hepatocyte malignant transformation, with a special focus on the immune cell compartment and oxidative stress, from the premalignant to full malignant stages (26). In western countries, primary sclerosing cholangitis (PSC) is the most well-known risk factor for CCA; nevertheless, some risk factors are recognized in all three subtypes. For instance, Caroli disease and choledochal cysts are strongly associated with all three CCA subtypes. In contrast, pancreaticobiliary maljunction (PBM), liver flukes, elderly people, non-alcoholic fatty liver disease (NAFLD), and hepatitis B and C are associated with iCCA, whereas choledocholithiasis is associated with ECCs. Globally increasing rates of obesity and NAFLD may be related to the rise in iCCA rates. A Japanese nationwide study of PBM revealed that the incidence of biliary tract cancer in adults was as high as 21.6% in patients with PBM with bile duct dilatation and 42.4% in patients with PBM without bile duct dilatation (27). Therefore, the Japanese Society of Hepato-Biliary- Pancreatic (JSHBP) guideline recommended that prophylactic surgical treatment should be performed at the earliest time after diagnosis to prevent cancer development. There is still controversy on the choice of treatment method for prophylactic surgery, which was not been mentioned in other guidelines.
Although there are multiple risk factors for CCA, the majority of CCAs lack an identifiable risk factor.
For patients with risk factors for CCA, both common and rare, targeted screening of high-risk individuals might be an alternative. Individuals with high-risk factors including PSC, liver cirrhosis, chronic inflammation of the biliary epithelium, cholestasis (25), and chronic hepatitis, by various tests should be considered for surveillance in the European Society for Medical Oncology (ESMO), the International Liver Cancer Association (ILCA) and BSG guidelines.
4. Screening and diagnosis
The lack of definitive diagnostic criteria and limited specificity of most diagnostic methods make cholangiocarcinoma challenging to diagnose. Early diagnosis of CCA is a critical point to improve the prognosis of patients. The guidelines and consensus contain distinctive diagnostic algorithms, which have been evaluated from a variety of viewpoints based on current guidelines. However, there is no accurate imaging examination that can be used for a comprehensive evaluation. All guidelines provide tests to diagnose CCA around the world include serological diagnosis, imaging diagnosis and histological diagnosis.
The clinical presentation of cholangiocarcinoma varies depending on the tumor stage, location, and growth pattern. ICCA patients usually have no specific clinical symptoms in the early stages, but as the disease progresses, abdominal discomfort, abdominal pain, fatigue, nausea, epigastric masses, malaise, night sweats, asthenia, weight loss and fever may occur (28-30). The most typical symptom of ECC is jaundice that is defined by the yellowing or greening of the skin and mucous membranes. From there, screening is theoretically the best way to detect asymptomatic CCA for early intervention.
The preliminary screening should include liver function tests, the carcinoembryonic antigen (CEA), the carbohydrate antigens 19-9 (CA19-9) (31-33), and abdominal ultrasound (US) in higher-risk groups. At present, CEA and CA19-9 are recommended as blood biomarkers for CCA, however their limited sensitivity and specificity make them ineffective for early identification. Serum CA 19-9 is neither highly sensitive nor specific for diagnosis, as CA 19-9 in patients with benign bile duct obstructions or acute cholangitis also could be slightly elevated (31). In the setting of bile duct obstruction, CA 19-9 levels should be reassessed after biliary intervention/drainage since the half-life of CA 19-9 is one to three days. Patients with iCCA who had either a high preoperative CA 19-9 or CEA had a very poor outcome with a 1-year survival of only 64.9% (34). Both pre- and postoperative serum CA 19-9 levels predict the survival of patients with resectable CCA, and may contribute to the establishment of a new therapeutic strategy (35). The descriptions of diagnostic tests with CA 19-9 reach a consensus in the current guidelines. The NCCN, CSCO, SEOM, CCHPBA and EASL also advise CEA for baseline blood tests. Only BSG guideline recommended CA125 to diagnose CCA. Other serum markers, such as cytokeratin-19 fragment (CYFRA 21-1), CA242, MK-1, Caudal homeobox 2(CDX2) and C-reactive protein, have been reported in a limited number of studies, but are not in routine clinical use (36-39). Thus, the first step to evaluate the usefulness of tumor biomarker (CA 19-9 and CEA) in the early diagnosis of CCA is to establish which high-risk population should be screened.
Several imaging examinations are also thought to be helpful in diagnosis, including Ultrasound (US), contrast-enhanced MRCP, contrast-enhanced CT, PET-CT scan, and endoscopic ultrasound (EUS). Their diagnostic accuracy is influenced by anatomic location and growth patterns of CCA.
US is the method of choice for the diagnosis of cholangiocarcinoma, which may appear as a limited intrahepatic mass, or as a portal tumor with dilated intrahepatic bile ducts and no dilated extrahepatic bile ducts. The advantage of US is that it can reliably differentiate between masses and stones and can initially identify the site of obstruction based on whether the bile ducts within or outside the liver are dilated. US can show lesions in and around the bile ducts and evaluate the degree of portal vein invasion. Testing of contrast enhanced ultrasound (CEUS) has been introduced into guidelines and recommendations for the diagnostic work-up of iCCA: the ENS guidelines, the Italy Society, and the EASL guidelines. CEUS increases the diagnostic performance in differentiation between iCCA and HCC significantly, in comparison with conventional ultrasound (40,41). US-screening is an effective technique for detecting CCA in its early stages, a comprehensive population-based program utilizing such screening in high incidence areas is recommended (42). A magnetic resonance cholangiopancreatography (MRCP) is considered the routine image study for staging CCA. Endoscopic ultrasound/fine needle aspiration EUS-FNA is effective to identify malignant regional lymph nodes (MRLNs) in patients with CCA, and should be routinely incorporated into staging of all CCA subtypes given the impact of MRLN on prognosis and management decisions (43). Due to difficulties in differential diagnosis between iCCA and liver metastases, fluorodeoxyglucose positron emission tomography (FDG-PET) is also commonly used to rule out a primary tumor.
The NCCN, CSCO, and ESMO guidelines emphasize that a multidisciplinary team (MDT) of experts including experienced radiologists and surgeons needs to review examination results in order to stage the disease and determine potential treatment options, and shared decision-making consultation (44,45). MDT should be involved in the whole management.
5. Staging and classification
Accurate staging is critical for establish the appropriate treatment strategy for all cancer types. The American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) staging system is the mostly commonly used staging system for CCA (46,47) (Table 2 and Table 3). Four of the eleven guidelines have been revised after publication. The JSHBPS guideline (3rd) did not revise the stage after it published the 3rd version of the Japanese classification of biliary tract cancers (48). The other 5 guidelines may not have been revised because the AJCC system was published in 2016 and made effective in 2018 (8th edition).
Table 2. Definitions of American Joint Committee on Cancer (AJCC) staging system for iCCA, pCCA, and dCCA with 8th editions.
| iCCA | pCCA | dCCA | |
|---|---|---|---|
| T | Primary Tumor | Primary Tumor | Primary Tumor |
| TX | Primary tumor cannot be assessed | Primary tumor cannot be assessed | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor | No evidence of primary tumor | ― |
| Tis | Carcinoma in situ (intraductal tumor) | Carcinoma in situ/high-grade dysplasia | |
| T1 | Solitary tumor without vascular invasion, ≤ 5cm or >5 cm | Tumor confined to the bile duct, with extension up to the muscle layer or fibrous tissue | Tumor invades the bile duct wall with a depth less than 5 mm |
| T1a | Solitary tumor ≤ 5cm without vascular invasion | ||
| T1b | Solitary tumor >5 cm without vascular invasion | ||
| T2 | Solitary tumor with intrahepatic vascular invasion or multiple tumors, with or without vascular invasion | ||
| T2a | Tumor invades beyond the wall of the bile duct to surrounding adipose tissue | Tumor invades the bile duct wall with a depth of 5-12 mm | |
| T2b | Tumor invades beyond the wall of the bile duct to surrounding adipose tissue | ||
| T3 | Tumor perforation of the visceral peritoneum | Tumor invades unilateral branches of the portal vein or hepatic artery | Tumor invades the bile duct wall with a depth greater than 12 mm |
| T4 | Tumor involving local extrahepatic structures by direct invasion | Tumor invades the main portal vein or its branches bilaterally, or the common hepatic artery, or unilateral second order biliary radicals with contralateral portal vein or hepatic artery involvement | Tumor involves the celiac axis, the superior mesenteric artery, and/or common hepatic artery |
| N | Regional lymph nodes | Regional lymph nodes | Regional lymph nodes |
| NX | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
| N0 | No regional lymph node metastasis | No regional lymph node metastasis | No regional lymph node metastasis |
| N1 | Regional lymph node metastasis present | One to three positive lymph nodes typically involving the hilar, cystic duct, common bile duct, hepatic artery, posterior pancreatoduodenal, and portal vein lymph nodes | Metastasis to one to three regional lymph nodes |
| N2 | ― | Four or more positive lymph nodes from the sites described for N1 | Metastasis to four or more regional nodes |
| M | Distant Metastasis | Distant Metastasis | Distant Metastasis |
| M0 | No distant metastasis | No distant metastasis | No distant metastasis |
| M1 | Distant metastasis present | Distant metastasis present | Distant metastasis present |
Table 3. Prognositc Groups of American Joint Committee on Cancer (AJCC) staging system for iCCA, pCCA, and dCCA.
| iCCA | pCCA | dCCA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Stage 0 | Tis | N0 | M0 | Tis | N0 | M0 | Tis | N0 | M0 |
| Stage I | T1 | N0 | M0 | T1 | N0 | M0 | |||
| IA | T1a | N0 | M0 | ― | ― | ||||
| IB | T1b | N0 | M0 | ― | ― | ||||
| Stage II | T2 | N0 | M0 | T2a-b | N0 | M0 | |||
| IIA | ― | ― | T1N1M0 or T2N0M0 | ||||||
| IIB | ― | ― | T2-3N1M0 or T3N0M0 | ||||||
| Stage III | |||||||||
| IIIA | T3 | N0 | M0 | T3 | N0 | M0 | T1-3, N2M0 | ||
| IIIB | T4 or Any T | N0 or N1 | M0 | T4 | N0 | M0 | T4, any N, M0 | ||
| IIIC | ― | Any T | N1 | M0 | ― | ― | ― | ||
| Stage IV | Any T | Any N | M1 | Any T | Any N | M1 | |||
| IVA | ― | Any T | N2 | M0 | |||||
| IVB | Any T | Any N | M1 | ||||||
| Histologic Grade(G) | |||||||||
| GX | Grade cannot be assessed | ||||||||
| G1 | Well differentiated | ||||||||
| G2 | Moderately differentiated | ||||||||
| G3 | Poorly differentiated | ||||||||
In 2018, the AJCC/UICC published modifications of the staging system. The most significant alteration has been made in the N stage. Traditionally, the AJCC/ UICC classified regional lymph node stations depend on the anatomical site. In particular, in the 8th edition, the lymph node staging (N-category) of patients with CCA was altered, with the N1- and N2-stage categories based on the counts of positive lymph nodes (N1: one to three involved positive regional lymph nodes and N2: four or more involved positive regional lymph nodes). The 8th edition confirms that the anatomic extent of the tumor maintains to be the strongest predictor of outcome in CCA. The depth of tumor invasion is an independent predictor of prognosis in patients with dCCA and pCCA. Despite the fact that the present TNM classification provides a clinically useful categorization that is associated with prognosis, it has several drawbacks. A published study from two Western hepatobiliary centers evaluated the prognostic accuracy of the 8th TNM classification of the AJCC staging system in a cohort of 214 patients undergoing liver resection for CCA. In that study population, about 40% of patients changed their stages from the 7th to the 8th AJCC edition. The authors determined that the new 8th TNM edition was only slightly better than the previous 7th edition (49). The prognostic accuracy of the 8th edition of the AJCC staging system was similar to the 7th edition. Prognostic accuracy was particularly poor in unresectable patients (50). TNM classification has potential clinical implications during the preoperative stage, when it might still affect the choice to perform a resection or not. Thus, accuracy on imaging is therefore likely the most crucial variable. Future editions of the AJCC staging system should aim to improve the prognostic accuracy of the AJCC staging system on cross-sectional imaging.
The AHPBA guideline only suggests that staging laparoscopy should be routinely utilized in high-risk iCCA patients (i.e. patients with multicentric disease, high CA19-9, questionable vascular invasion or suspicion of peritoneal disease). The Italian guideline suggests against performing routine staging laparoscopy before surgery in CCA patients whose doctors will perform surgery (12). The others did not mention staging laparoscopy. Thus, staging laparoscopy is not recommended as a rule.
6. Treatment
Depending on CCA site of origin, each variety of CCA has different therapeutic strategies. A treatment algorithm is shown in Figure 1. This review is compared to recent research on guideline updated, treatment approaches, with an emphasis on treatment criteria and new therapy breakthroughs.
Figure 1.
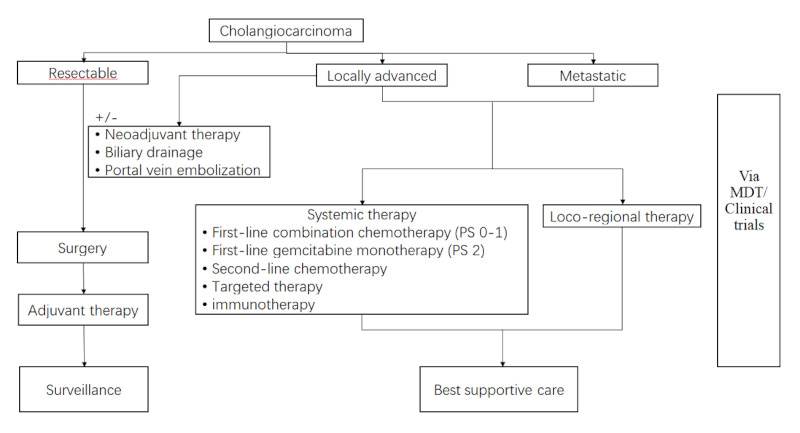
Algorithm for the management of patients with cholangiocarcinoma.
6.1. Biliary drainage and portal embolization
It is well-known that extended hepatectomy in patients with jaundice is related to a high risk of postoperative liver failure (PLF), morbidity and mortality (51-54). Therefore, preoperative biliary drainage and portal vein embolization (PVE) are frequently selected measures to prevent PLF.
Preoperative biliary drainage remains a matter for debate. Only 6 guidelines mention biliary drainage. In particular, the JSHPBS guideline emphasized that preoperative biliary drainage played an important role on the management of patients with CCA. And we summarized that the main selections of biliary drainage are i) cholangitis or sepsis originating from the biliary tract; ii) jaundice; iii) the need for preoperative anti-neoplastic therapy or PVE or ALPPS; iv) malnutrition, hepatic insufficiency; v) unresectable CCA. All 6 guidelines suggested biliary drainage, but only 3 mentioned total bilirubin concentration before drainage. The Italian guideline recommended patients biliary drainage with total bilirubin > 256.5μmol/L (mg/dL), and CCHPBA using a cut-off value of 200μmol/L. The CSCO guideline recommended that patients with hyperbilirubinemia more than 200μmol/L in pCCA and more than 380μmol/L in dCCA to perform biliary drainage. A randomized controlled trial was terminated because of higher all-cause mortality in the percutaneous transhepatic biliary drainage group in patients with pCCA (55). The results encourage further prospective trials and a reappraisal of the indications and approaches for biliary drainage. Despite the debate on whether to perform biliary drainage, PVE remains consensus according to all current guidelines for sufficient future liver remnant (FLR) with patients who will perform hepatectomy in pCCA. PVE could cause FLR hypertrophy, which could improve the safety of the extended hepatectomy.
6.2. Resection & transplantation
According to recent research, the only potentially curative treatment method that is recommended by all guidelines is surgical resection. Surgical management is based on the location and extent of the tumor. However, the surgical treatment for CCA recently have had little progress. The initial surgical examination should include evaluation for multifocal liver disease, lymph node metastases, distant metastases, and biopsy not required before surgery. In summary, the main selection of surgical procedures is: i) iCCA, segment or lobe resection. Extensive hepatic resections are usually needed to confirm R0 resection; ii) pCCA, extended right or left hepatectomy combined with caudate lobectomy, the extent of the involved biliary tract determines the range of hepatectomy; iii) pCCA, pancreatoduodenectomy is generally performed. Few patients with CCA in the middle part of the extrahepatic bile duct are cured with isolated resection of the bile duct. En-bloc resection of the caudate lobe is recommended because the tumor typically extends into the caudate lobe via small branches draining into the right or left hepatic ducts or the biliary confluence (56).
The AHPBA guideline recommended that regional lymphadenectomy be performed in patients undergoing resection. De Jong et al. demonstrated that among patients who underwent routine lymphadenectomy, patients with lymphadenectomy had a worse median survival (57). However, some studies reporting the number of lymph nodes (LNs) retrieved affects patient survival (58). However, owing to the lack of the randomized controlled trials, there is still no consensus about the prognostic significance in iCCA with or without lymphadenectomy. In addition, it is unclear what is a standard lymph node dissection (LND) given the multiple potential lymphatic pathways for intrahepatic malignancies. Nevertheless, the effects of lymphadenectomy remain controversial, and the majority of guidelines still recommend routine LND in CCA. Staging laparoscopy is recommended by ENS-CCA and Asia-Pacific guidelines, especially in patients with a high CA19-9 level or major vascular invasion (59). Wu et al. demonstrated laparoscopic liver resection (LLR) associated lymphadenectomy for iCCA is safe and feasible compared with open liver resection (OLR) (60). The study reported LLR was used to reduce intraoperative blood loss and postoperative hospital stay. In addition, laparoscopic surgery is useful to detect occult metastasis with the peritoneum. Their application in preoperative staging is controversial. Thus, laparoscopic surgery is not routinely recommend in most guidelines.
Liver transplantation (LT) for cholangiocarcinoma has been an absolute contraindication worldwide due to poor results. However, in recent years thanks to improvements of patient management and treatments of CCA patients, this indication has been revisited. The CSCO, ESMO and Italian guidelines (61) recommend LT for iCCA patients. LT may be considered in patients with unresectable pCCA who fulfill the Mayo Clinic protocol (tumor diameter ≤ 3 cm without lymph node or distant metastases in the staging laparotomy, after external beam radiation, chemotherapy based in 5-fluorouracil, intra biliary radiation, and oral capecitabine until LT). The diameter of the tumor is tightly associated with post-LT recurrence. Only single-nodule tumors ≤ 2 cm without vascular invasion would be acceptable (62). In this interim analysis of an initial case series, patients with stable intrahepatic cholangiocarcinoma before liver transplantation had an overall survival of 83% and a recurrence-free survival of 50% at 5 years. These findings suggest that tumor stability over time and response to therapy might serve as surrogate markers of favorable tumor biology for liver transplantation, and that the Methodist-MD Anderson selection criteria might identify subpopulations of patients with intrahepatic cholangiocarcinoma who would benefit most from liver transplantation (63).
6.3. Loco-regional therapies
Recent literature suggests an emerging role for loco-regional therapies in iCCA, including radiation therapy (RT), transcatheter arterial (TACE), radio embolization and radiofrequency ablation (RFA). While distant metastasis is a less frequent cause of mortality, many of these patients die of liver failure caused by tumor-related vascular involvement or biliary blockage. It is so necessary to try to obtain local control of the tumor to improve quality of life. Loco-regional treatment decisions must take into account both the conditions of patients (comorbidities, liver function, prior therapies) and the size of tumor, vascularity, and involvement of bile ducts, blood arteries, colon, and chest wall (64). All guidelines recommend loco-regional therapies for iCCA while guidelines encourage further research in these areas.
The palliative treatment of cholangiocarcinoma, with photodynamic therapy, is associated with an increased survival benefit, an improved biliary drainage, and a better quality of life (65). However, the quality of this evidence is low (66). Photodynamic therapy (PDT) is a new local-ablative, tumor-specific treatment that has shown promising results and is now the standard of care for unresectable cholangiocarcinoma. Moole et al. reported that PDT combined with biliary stenting improves the success of biliary drainage and improves the survival and quality of life in patients with nonresectable cholangiocarcinoma (67). Unfortunately, the combination of PDT with biliary stenting was reported to be associated with prolonged OS in patients with unresectable cholangiocarcinoma in relatively small sample studies. Photodynamic therapy (PDT) was recommended for routine use based on the most recent data by only BSG and NCCN guidelines.
6.4. Systemic therapies
CCA is a kind of digestive system tumor with high malignancy and poor prognosis. Despite significant advances in diagnostic modalities, the vast majority of patients present with metastases or with advanced locoregional disease that prevents surgical therapy. However, the recurrence rate is high, even for patients who have received treatment in the early stage, and the survival rate of patients with advanced cancer, including those who receive treatment, is poor. The main goals for the palliation of patients with advanced CCA are decompression of the biliary system and control of tumor growth. Currently, systemic therapies for advanced or metastatic CCA are ineffective due to molecular variants that define the biological characteristics of each CCA subtype.
At the time of assessment of patients with CCA for systemic therapies, the following three aspects need to be considered: patient fitness as assessed in terms of Eastern Cooperative Oncology Group Performance Status (ECOG PS), disease distribution and accessibility of tumor profiling. If patients with an ECOG PS ≥ 3 are unlikely to benefit from systemic treatment, guidelines recommend only supportive care.
6.4.1. Chemotherapy
Chemotherapy strategies for patients with CCA include: i) neoadjuvant chemotherapy; ii) post-operative adjuvant chemotherapy; iii) palliative chemotherapy for patients with unresectable or metastatic disease. A chemotherapy-based systemic treatment model has a proven clinical benefit in CCA, however, criteria of treatment remains controversial. Zhang et al. summarized that chemotherapy has a most significant effect on the systemic treatment of advanced or recurrent CCA (68). Appropriate patients are recommended to participate in clinical trials.
There is no evidence supporting the use of neoadjuvant systemic chemotherapy over upfront resection in patients with resectable iCCA (69). There is a lack of randomized controlled phase III clinical trials demonstrating the benefit of neoadjuvant therapy for CCA. This assessment of treatment response might be important in future trial designs (70). Interval from completion of neoadjuvant treatment to surgery varied from 3 days to 6 months. Resection was by hepatectomy with three studies reporting an R0 rate of 100%, 24% and 63%, respectively. Three studies reported histopathological evidence of prior treatment response. There were two treatment related deaths at 90 days. Median survival was 19 (95% CI: 9.9-28) months and 5-year survival 20% (70). In accordance with CSCO and ESMO guidelines, neoadjuvant treatment is also recommended for CCA. Nevertheless, neoadjuvant treatment for resectable CCA is not included in the guidelines: NCCN, SEOM, JSHBPS, ENS, Italian, AHPBA.
Patients with CCA are mostly unable to be cured due to recurrence after surgery, which is a significant basis for the options of adjuvant chemotherapy. NCCN guideline, CSCO guideline, ENS guideline, SEOM guideline, and Italian guideline recommend capecitabine as the first approach for patients with resectable CCA. Although the evidence of an optimal regimen has not yet been established in Japan, JSHBPS guideline noted adjuvant chemotherapy may be considered (13). It is with regret that most guidelines except for SEOM guideline and ENS guideline do not illustrate a duration of chemotherapy. Based on evidence from a phase III (BILCAP) randomized controlled trial, patients with resected BTC should be offered adjuvant capecitabine chemotherapy for a duration of 6 months (71,72). Besides, CSCO guideline, SEOM guideline, CCHPBA, and AHPBA guidelines recommend that radiotherapy for patients with lymph node-positive disease or with microscopically involved margins (R1 resection) could improve the poor prognosis. Findings from a nationwide retrospective study showed that adjuvant radiotherapy was associated with a survival benefit in patients with resected dCCA, regardless of pathological nodal involvement, resection margin status, and receipt of adjuvant chemotherapy (73).
Unresectable CCA is classified as locally advanced or metastatic disease. The combination of gemcitabine and cisplatin chemotherapy is still recommended as standard first-line treatment for advanced and metastatic CCA patients with an ECOG PS of 0-1. Durvalumab was approved as an orphan drug to treat BTC. Before that, a phase I study published results of combination therapy with durvalumab and tremelimumab to treat BTC (74). Based on the convincing data of the AC-02 trial which revealed a significantly increased median overall survival compared to gemcitabine monotherapy (11.7 vs. 8.1 months, respectively; hazard ratio 0.64; 95% CI: 0.52-0.8; p < 0.001). Additionally, the combination therapy had an 81.4% disease control rate compared to 71.8% for monotherapy (75). Some interesting trials such as a BTC trial, a phase II trial focused on triplet therapy cisplatin, gemcitabine and nab-paclitaxel (76), as well as the phase III trial of gemcitabine plus S1 (77). These provide a new option for patients with BTC as a convenient standard therapy. The most important independent prognostic factor for advanced BTC is ECOG PS, which can guide therapeutic choices. Indeed, patients with ECOG PS 2 should be preferred to gemcitabine monotherapy in CSCO, ENS, SEOM guidelines.
Patients with tumor progression under first-line chemotherapy might be suitable for a second-line treatment, especially young patients and those with a good performance status (78). However, there are no consensus guidelines that help in choosing an appropriate second-line therapy. In addition, a systematic review of 25 studies, which included 761 patients, evaluated the role of secondline therapy in advanced biliary tract cancer. The study showed an overall response of 8%, indicating that there could be a cohort of patients who might benefit from empirically selected secondline therapy (79). The ABC- 06 trial demonstrated the effectiveness of second-line chemotherapy (adjusted HR 0.69). Although variations in median OS between study arms were modest (5.3 vs. 6.2 months), differences in survival at 6 months (35.5% versus 50.6%) and 12 months (11.4% vs. 25.9%) were clinically significant. Based on these findings, FOLFOX can be considered a new standard of care in the second- line setting. FOLFOX was recommended for the treatment of advanced and metastatic patients by NCCN, CSCO, ENS, SEOM guidelines, but the JSHBPS guideline and Italian guideline respectively recommended fluoropyrimidine-based chemotherapy as second-line treatment.
6.4.2. Targeted therapy and immunotherapy for CCA
In terms of treatment algorithms, targeted therapy and immunotherapy have received considerable interest, and targeted therapy or immunotherapy as recommended by NCCN guideline, CSCO guideline, ENS guideline and SEOM guideline.
Because of the significant inter-tumoral and intra-tumoral heterogeneity of CCA, no effective targeted medicines are currently available for treating this disease. Alterations of isocitrate dehydrogenase (IDH)1, IDH2, fibroblast growth factor receptor FGFR1, FGFR2, FGFR3, epoxide hydrolase (EPH)A2, and biofilm-associated surface protein (BAP)1 genes have been reported in the intra-hepatic subtype, while in perihilar and dCCA genetic alterations of AT-rich interactive domain (ARID)1B, E74-like factor (ELF)3, protein polybromo-1 (PBRM1), protein kinase cAMP-activated catalytic subunit alpha (PRKACA), and PRKACB were described (80).
AG-120 (Ivosidenib) (81) was tested in 73 patients with IDH1-mutant advanced CCA in a phase I study. Four (5%) patients had a partial response, 56% experienced stable disease, and the median overall survival was 13.8 months. Results of the cross-over phase III study (ClarIDHy) of Ivosidenib compared to placebo were reported at ESMO 2019. Ivosidenib significantly improved PFS compared with placebo. The median OS was 10.8 months for Ivosidenib and 9.7 months for placebo, with 57% of placebo patients crossing over to Ivosidenib. In the intention to treat population, there was a trend in favor of Ivosidenib, but it was not yet significant. At present the drug has been recommended by CSCO and ENS guidelines. The distinguished genetic profile, histological characteristics, and clinical results observed in these different anatomical areas may lead one day to individualized treatment strategies.
Recently, immunotherapy has developed rapidly, especially the introduction of immune checkpoint inhibitors, which have achieved good efficacy in many solid tumors. Some phase I and II clinical studies on biliary system malignancies have demonstrated good safety and effectiveness of immunotherapy. There has been a surge of interest in targeted therapies and immune therapies for CCA. The anti-programmed cell death 1 (PD-1) antibody pembrolizumab has been approved by the United States Food and Drug Administration for previously treated patients with DNA mismatch repair (MMR) deficiency and/or microsatellite instability (MSI)-high advanced solid tumors, independent of histology, which would include those with CCA. Of note, MMR deficiency has been reported to occur in 5% to 10% of CCA (82,83). Pembrolizumab is a highly selective, humanized monoclonal antibody against PD-1 that is designed to block the interaction between PD-1 and its ligands, PD-L1 and PD-L2. On the basis of these results, this is similar to the recommendation in the updated NCCN, CSCO, JSHBPS, and ENS guidelines. The panelists of the consensus agree that all advanced, metastatic CCA in patients who are medically fit should be screened for MSI-H/dMMR, and those with MSI-H solid tumors should receive pembrolizumab monotherapy (84). The discovery of targeted therapies for this diverse and relatively uncommon cancer remains a challenging task. Precision medicine efforts have discovered the disease's underlying mutational landscape and prepared the way for targeted therapy and immunotherapy trials.
7. Future perspectives
According to our review, there are significant and notable variances. The goals of this review were to identify and emphasize the distinctions that lead to the development of these guidelines and to support the practicing surgeons in understanding these guidelines in order to provide superior treatment for patients with CCA. Unfortunately, comprehensive, randomized controlled trials comparing these guidelines in well-defined clinical studies do not exist. Therefore, it is challenging to recommend one guiding principle above others. The availability of various imaging modalities in addition to new biomarkers, enables the early detection of CCA, while developments in the treatment modalities have bolstered a multidisciplinary strategy for hepatobiliary surgeons. Identifying molecular biomarkers that indicate primary or secondary resistance to CCA remains an active research topic. Despite these developments, recurrence following curative treatment remains a significant drawback, and more effective adjuvant therapies are required. The potential efficacy of systematic therapy may revolutionize the systemic treatment protocol. As the number of effective systemic drugs continues to increase, the challenge is to determine which order of sequential systemic therapy can offer optimal efficacy with minimal toxicity. There are a few systemic chemotherapy studies dedicated to all anatomic subtypes of CCA, and the majority comprise GBC. These differences in guidelines also help to identify issues for future researches that will hopefully reconcile these controversial issues. Updated guidelines have had randomized controlled trials to improve prognosis of patients with iCCA, pCCA, dCCA separately.
8. Conclusion
Management of CCA remains a significant challenge, and as well as source of uncertainty and anxiety for both surgeons and patients. It's reassuring to notice such remarkable uniformity between various kinds of recommendations. However, no major advances in surgical treatment of CCA have occurred over the past 10 years. We analyzed the similarities and differences between the clinical practice guidelines for CCA from different countries to clarify the status of management. The systemic therapy of CCA remains a key clinical problem, and a promising breakthrough has not yet occurred. The management of locally advanced and metastatic CCA does need further research. Targeted therapy may become established in this field. Immunotherapy has obtained good results for treating CCA, but more clinical evidences are needed before these can be recommended for CCA. Distinctive treatment guidelines dependent on the regional scale, and clinical trials provide more evidence in the era of individualized treatment. The purpose of guidelines is not to replace doctor' expertise but to present physicians with the most up-to-date options for their patients. Therefore, they must address crucial issues and advise physicians on optimal treatment options for each specific individual. To advance standard management for CCA, governments should develop and implement domestic recommendations that are evidence-based, resource-constrained, appropriate to specific patients, and subject to systematic evaluation.
Acknowledgements
We are thankful for the support of the Department of Biliary Surgery, West China Hospital, Sichuan University.
Funding:
This work was supported by a grant from Sichuan Science and Technology Program (No. 2020YFS0098).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Razumilava N, Gores GJ. Cholangiocarcinoma. Lancet. 2014; 383:2168-2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kendall T, Verheij J, Gaudio E, Evert M, Guido M, Goeppert B, Carpino G. Anatomical, histomorphological and molecular classification of cholangiocarcinoma. Liver Int. 2019; 39 Suppl 1:7-18. [DOI] [PubMed] [Google Scholar]
- 3. Cardinale V. Classifications and misclassification in cholangiocarcinoma. Liver Int. 2019; 39:260-262. [DOI] [PubMed] [Google Scholar]
- 4. Valle JW, Kelley RK, Nervi B, Oh D-Y, Zhu AX. Biliary tract cancer. Lancet. 2021; 397:428-444. [DOI] [PubMed] [Google Scholar]
- 5. Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM, Carneiro F, Cree IA; WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology. 2019; 76:182-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cai Y, Cheng N, Ye H, Li F, Song P, Tang W. The current management of cholangiocarcinoma: A comparison of current guidelines. Biosci Trends. 2016; 10:92-102. [DOI] [PubMed] [Google Scholar]
- 7. Benson AB, D'Angelica MI, Abbott DE, et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021; 19:541-565. [DOI] [PubMed] [Google Scholar]
- 8. 2020 Guidelines of Chinese Society of Clinical Oncology (CSCO) for biliary tract cancer. (Liang HJ, Shen F, Qin SQ, eds). China. 2020.V1. 0, pp. 1-39. (in Chinese) [Google Scholar]
- 9. B a n a l e s J M, M a r i n J J G, Lamarca A, e t a l. Cholangiocarcinoma 2020: the next horizon in mechanisms and management. Nat Rev Gastroenterol Hepatol. 2020; 17:557-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gomez-Espana MA, Montes AF, Garcia-Carbonero R, Mercade TM, Maurel J, Martin AM, Pazo-Cid R, Vera R, Carrato A, Feliu J. SEOM clinical guidelines for pancreatic and biliary tract cancer (2020). Clin Transl Oncol. 2021; 23:988-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part I: Classification, diagnosis and staging. Dig Liver Dis. 2020; 52:1282-1293. [DOI] [PubMed] [Google Scholar]
- 12. Cholangiocarcinoma Working Group. Italian Clinical Practice Guidelines on Cholangiocarcinoma - Part II: Treatment. Dig Liver Dis. 2020; 52:1430-1442. [DOI] [PubMed] [Google Scholar]
- 13. Nagino M, Hirano S, Yoshitomi H, et al. Clinical practice guidelines for the management of biliary tract cancers 2019: The 3rd English edition. J Hepatobiliary Pancreat Sci. 2021; 28:26-54. [DOI] [PubMed] [Google Scholar]
- 14. Valle JW, Borbath I, Khan SA, Huguet F, Gruenberger T, Arnold D; ESMO Guidelines Committee. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016; 27:v28-v37. [DOI] [PubMed] [Google Scholar]
- 15. Chinese Chapter of International Hepato-Pancreato- Biliary Association, Hepatic Surgery Group, Chinese Society of Surgery, Chinese Medical Association. Diagnosis and treatment of cholangiocarcinoma:surgical expert consensus. Journal of Clinical Hepatology. 2015; 31:12-16. (in Chinese) [Google Scholar]
- 16. Mansour JC, Aloia TA, Crane CH, Heimbach JK, Nagino M, Vauthey JN. Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015; 17:691-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weber SM, Ribero D, O'Reilly EM, Kokudo N, Miyazaki M, Pawlik TM. Intrahepatic Cholangiocarcinoma: expert consensus statement. HPB (Oxford). 2015; 17:669-680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bridgewater J, Galle PR, Khan SA, Llovet JM, Park JW, Patel T, Pawlik TM, Gores GJ. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J Hepatol. 2014; 60:1268-1289. [DOI] [PubMed] [Google Scholar]
- 19. Khan SA, Davidson BR, Goldin RD, Heaton N, Karani J, Pereira SP, Rosenberg WMC, Tait P, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: an update. Gut. 2012; 61:1657-1669. [DOI] [PubMed] [Google Scholar]
- 20. Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013; 28:593-607. [DOI] [PubMed] [Google Scholar]
- 21. Khan SA, Davidson BR, Pereira SP, Rosenberg WMC, Taylor-Robinson SD, Thillainayagam AV, Thomas HC, Wasan H; British Society of Gastroenterology. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut. 2002; 51 Suppl 6:vi1-vi9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arnold M, Abnet CC, Neale RE, Vignat J, Giovannucci EL, McGlynn KA, Bray F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology. 2020; 159:335-349.e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Florio AA, Ferlay J, Znaor A, Ruggieri D, Alvarez CS, Laversanne M, Bray F, McGlynn KA, Petrick JL. Global trends in intrahepatic and extrahepatic cholangiocarcinoma incidence from 1993 to 2012. Cancer. 2020; 126:2666-2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019; 71:104-114. [DOI] [PubMed] [Google Scholar]
- 25. Khan SA, Tavolari S, Brandi G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019; 39 Suppl 1:19-31. [DOI] [PubMed] [Google Scholar]
- 26. Leone V, Ali A, Weber A, Tschaharganeh DF, Heikenwalder M. Liver Inflammation and Hepatobiliary Cancers. Trends Cancer. 2021; 7:606-623. [DOI] [PubMed] [Google Scholar]
- 27. Morine Y, Shimada M, Takamatsu H, Araida T, Endo I, Kubota M, Toki A, Noda T, Matsumura T, Miyakawa S, Ishibashi H, Kamisawa T, Shimada H. Clinical features of pancreaticobiliary maljunction: update analysis of 2nd Japan-nationwide survey. J Hepatobiliary Pancreat Sci. 2013; 20:472-480. [DOI] [PubMed] [Google Scholar]
- 28. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvise M, Lamarca A. Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 2019; 39 Suppl 1:98-107. [DOI] [PubMed] [Google Scholar]
- 29. Plentz RR, Malek NP. Clinical presentation, risk factors and staging systems of cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 2015; 29:245-252. [DOI] [PubMed] [Google Scholar]
- 30. Blechacz B, Komuta M, Roskams T, Gores GJ. Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011; 8:512-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macias RIR, Banales JM, Sangro B, Muntane J, Avila MA, Lozano E, Perugorria MJ, Padillo FJ, Bujanda L, Marin JJG. The search for novel diagnostic and prognostic biomarkers in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:1468-1477. [DOI] [PubMed] [Google Scholar]
- 32. Rahnemai-Azar AA, Weisbrod A, Dillhoff M, Schmidt C, Pawlik TM. Intrahepatic cholangiocarcinoma: Molecular markers for diagnosis and prognosis. Surg Oncol. 2017; 26:125-137. [DOI] [PubMed] [Google Scholar]
- 33. Macias RIR, Kornek M, Rodrigues PM, Paiva NA, Castro RE, Urban S, Pereira SP, Cadamuro M, Rupp C, Loosen SH, Luedde T, Banales JM. Diagnostic and prognostic biomarkers in cholangiocarcinoma. Liver Int. 2019; 39 Suppl 1:108-122. [DOI] [PubMed] [Google Scholar]
- 34. Moro A, Mehta R, Sahara K, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2020; 27:2888-2901. [DOI] [PubMed] [Google Scholar]
- 35. Kondo N, Murakami Y, Uemura K, Sudo T, Hashimoto Y, Sasaki H, Sueda T. Elevated perioperative serum CA 19-9 levels are independent predictors of poor survival in patients with resectable cholangiocarcinoma. J Surg Oncol. 2014; 110:422-429. [DOI] [PubMed] [Google Scholar]
- 36. Guowei H, Yuan L, Ma L, Zhongyang L, Zhixing S, Lin L, Minqi L. The diagnostic efficacy of CYFRA21-1 on intrahepatic cholangiocarcinoma: A meta-analysis. Clin Res Hepatol Gastroenterol. 2019; 43:266-272. [DOI] [PubMed] [Google Scholar]
- 37. Huang L, Chen W, Liang P, Hu W, Zhang K, Shen S, Chen J, Zhang Z, Chen B, Han Y, Meng F, DeMorrow S, Yin X, Lai J, Liang L. Serum CYFRA 21-1 in Biliary Tract Cancers: A reliable biomarker for gallbladder carcinoma and intrahepatic cholangiocarcinoma. Dig Dis Sci. 2015; 60:1273-1283. [DOI] [PubMed] [Google Scholar]
- 38. Wang B CL, Chang HT. Potential diagnostic and prognostic biomarkers for cholangiocarcinoma in serum and bile. Biomark Med. 2016; 10:613-619. [DOI] [PubMed] [Google Scholar]
- 39. Tang H, Wang Z, Lv W, Meng X. The expression and clinicopathological role of CDX2 in intrahepatic cholangiocarcinoma. Intractable Rare Dis Res. 2018; 7:106-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guo LH, Xu HX. Contrast-enhanced ultrasound in the diagnosis of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: Controversy over the ASSLD Guideline. Biomed Res Int. 2015; 2015:349172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen LD, Xu HX, Xie XY, Xie XH, Xu ZF, Liu GJ, Wang Z, Lin MX, Lu MD. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: differential diagnosis with contrast-enhanced ultrasound. Eur Radiol. 2010; 20:743-753. [DOI] [PubMed] [Google Scholar]
- 42. Khuntikeo N, Koonmee S, Sa-Ngiamwibool P, et al. A comparison of the proportion of early stage cholangiocarcinoma found in an ultrasound-screening program compared to walk-in patients. HPB (Oxford). 2020; 22:874-883. [DOI] [PubMed] [Google Scholar]
- 43. Malikowski T, Levy MJ, Gleeson FC, Storm AC, Vargas EJ, Topazian MD, Abu Dayyeh BK, Iyer PG, Rajan E, Gores GJ, Roberts LR, Chandrasekhara V. Endoscopic ultrasound/fine needle aspiration is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology. 2020; 72:940-948. [DOI] [PubMed] [Google Scholar]
- 44. Morement H, Harrison R, Taylor-Robinson S. The multidisciplinary team meeting in the UK from the patients perspective: comments and observations from cholangiocarcinoma patients and their families. Int J Gen Med. 2017; 10:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sapisochin G, Ivanics T, Subramanian V, Doyle M, Heimbach JK, Hong JC. Multidisciplinary treatment for hilar and intrahepatic cholangiocarcinoma: A review of the general principles. Int J Surg. 2020; 82S:77-81. [DOI] [PubMed] [Google Scholar]
- 46. Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018; 25:845-847. [DOI] [PubMed] [Google Scholar]
- 47. Amin MB, Greene FL, Byrd DR, Brookland RK, Washington MK. AJCC Cancer Staging Manual (8th edn). Springer: New York. 2017. [Google Scholar]
- 48. Miyazaki M, Ohtsuka M, Miyakawa S, e t a l. Classification of biliary tract cancers established by the Japanese Society of Hepato-Biliary-Pancreatic Surgery: 3(rd) English edition. J Hepatobiliary Pancreat Sci. 2015; 22:181-196. [DOI] [PubMed] [Google Scholar]
- 49. Ruzzenente A, Bagante F, Ardito F, Campagnaro T, Scoleri I, Conci S, Iacono C, Giuliante F, Guglielmi A. Comparison of the 7th and 8th editions of the American Joint Committee on Cancer Staging Systems for perihilar cholangiocarcinoma. Surgery. 2018; 164:244-250. [DOI] [PubMed] [Google Scholar]
- 50. Gaspersz MP, Buettner S, van Vugt JLA, de Jonge J, Polak WG, Doukas M, Ijzermans JNM, Koerkamp BG, Willemssen F. Evaluation of the New American Joint Committee on Cancer Staging Manual 8th Edition for Perihilar Cholangiocarcinoma. J Gastrointest Surg. 2020; 24:1612-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Iacono C, Ruzzenente A, Campagnaro T, Bortolasi L, Valdegamberi A, Guglielmi A. Role of preoperative biliary drainage in jaundiced patients who are candidates for pancreatoduodenectomy or hepatic resection: highlights and drawbacks. Ann Surg. 2013; 257:191-204. [DOI] [PubMed] [Google Scholar]
- 52. Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro). HPB (Oxford). 2008; 10:130-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Higuchi R, Yamamoto M. Indications for portal vein embolization in perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014; 21:542-549. [DOI] [PubMed] [Google Scholar]
- 54. Farges O, Regimbeau JM, Fuks D, Le Treut YP, Cherqui D, Bachellier P, Mabrut JY, Adham M, Pruvot FR, Gigot JF. Multicentre European study of preoperative biliary drainage for hilar cholangiocarcinoma. Br J Surg. 2013; 100:274-283. [DOI] [PubMed] [Google Scholar]
- 55. Coelen RJS, Roos E, Wiggers JK, et al. Endoscopic versus percutaneous biliary drainage in patients with resectable perihilar cholangiocarcinoma: a multicentre, randomised controlled trial. Lancet Gastroenterol Hepatol. 2018; 3:681-690. [DOI] [PubMed] [Google Scholar]
- 56. Neuhaus P, Thelen A, Jonas S, Puhl G, Denecke T, Veltzke-Schlieker W, Seehofer D. Oncological superiority of hilar en bloc resection for the treatment of hilar cholangiocarcinoma. Ann Surg Oncol. 2012; 19:1602-1608. [DOI] [PubMed] [Google Scholar]
- 57. de Jong MC, Nathan H, Sotiropoulos GC, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011; 29:3140-3145. [DOI] [PubMed] [Google Scholar]
- 58. Kim DH, Choi DW, Choi SH, Heo JS, Kow AW. Is there a role for systematic hepatic pedicle lymphadenectomy in intrahepatic cholangiocarcinoma? A review of 17 years of experience in a tertiary institution. Surgery. 2015; 157:666-675. [DOI] [PubMed] [Google Scholar]
- 59. Doussot A, Groot-Koerkamp B, Wiggers JK, Chou J, Gonen M, DeMatteo RP, Allen PJ, Kingham TP, D'Angelica MI, Jarnagin WR. Outcomes after resection of intrahepatic cholangiocarcinoma: External validation and comparison of prognostic models. J Am Coll Surg. 2015; 221:452-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu J, Han J, Zhang Y, Liang L, Zhao J, Han F, Dou C, Zhang Y, Liu J, Wu W, Hu Z, Zhang C. Safety and feasibility of laparoscopic versus open liver resection with associated lymphadenectomy for intrahepatic cholangiocarcinoma. Biosci Trends. 2020; 14:376-383. [DOI] [PubMed] [Google Scholar]
- 61. Sapisochin G, Javle M, Lerut J, Ohtsuka M, Ghobrial M, Hibi T, Kwan NM, Heimbach J. Liver transplantation for cholangiocarcinoma and mixed hepatocellular cholangiocarcinoma: working group report from the ILTS Transplant Oncology Consensus Conference. Transplantation. 2020; 104:1125-1130. [DOI] [PubMed] [Google Scholar]
- 62. Rodriguez-Peralvarez M, Gomez-Bravo MA, Sanchez- Antolin G, De la Rosa G, Bilbao I, Colmenero J; Spanish Society of Liver Transplantation (SETH) Consensus Panel. Expanding indications of liver transplantation in Spain: Consensus statement and recommendations by the Spanish Society of Liver Transplantation. Transplantation. 2021; 105:602-607. [DOI] [PubMed] [Google Scholar]
- 63. Lunsford KE, Javle M, Heyne K, et al. Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018; 3:337-348. [DOI] [PubMed] [Google Scholar]
- 64. Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, Vibert E, Castaing D, Adam R, Cherqui D, Sa Cunha A. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018; 105:839-847. [DOI] [PubMed] [Google Scholar]
- 65. Ortner MA. Photo dynamic therapy for cholangiocarcinoma. Lasers Surg Med. 2011; 43:776-780. [DOI] [PubMed] [Google Scholar]
- 66. Lu Y, Liu L, Wu JC, Bie LK, Gong B. Efficacy and safety of photodynamic therapy for unresectable cholangiocarcinoma: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015; 39:718-724. [DOI] [PubMed] [Google Scholar]
- 67. Moole H, Tathireddy H, Dharmapuri S, Moole V, Boddireddy R, Yedama P, Dharmapuri S, Uppu A, Bondalapati N, Duvvuri A. Success of photodynamic therapy in palliating patients with nonresectable cholangiocarcinoma: A systematic review and meta-analysis. World J Gastroenterol. 2017; 23:1278-1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhang W, Zhou H, Wang Y, Zhang Z, Cao G, Song T, Zhang T, Li Q. Systemic treatment of advanced or recurrent biliary tract cancer. Biosci Trends. 2020; 14:328-341. [DOI] [PubMed] [Google Scholar]
- 69. Mazzaferro V, Gorgen A, Roayaie S, Droz Dit Busset M, Sapisochin G. Liver resection and transplantation for intrahepatic cholangiocarcinoma. J Hepatol. 2020; 72:364-377. [DOI] [PubMed] [Google Scholar]
- 70. Baltatzis M, Jegatheeswaran S, Siriwardena AK. Neoadjuvant chemoradiotherapy before resection of perihilar cholangiocarcinoma: A systematic review. Hepatobiliary Pancreat Dis Int. 2020; 19:103-108. [DOI] [PubMed] [Google Scholar]
- 71. Shroff RT, Kennedy EB, Bachini M, Bekaii-Saab T, Crane C, Edeline J, El-Khoueiry A, Feng M, Katz MHG, Primrose J, Soares HP, Valle J, Maithel SK. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019; 37:1015-1027. [DOI] [PubMed] [Google Scholar]
- 72. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019; 20:663‐673. [DOI] [PubMed] [Google Scholar]
- 73. Kamarajah SK, Bednar F, Cho CS, Nathan H. Survival benefit with adjuvant radiotherapy after resection of distal cholangiocarcinoma: A propensity-matched National Cancer Database analysis. Cancer. 2021; 127:1266-1274. [DOI] [PubMed] [Google Scholar]
- 74. Cheng H, Zhou D, Wang S, Ding J, Ma F. Orphan drugs in different countries and development of new drugs to treat biliary tract cancer. Intractable Rare Dis Res. 2021; 10:146-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010; 362:1273-1281. [DOI] [PubMed] [Google Scholar]
- 76. Shroff RT, Javle MM, Xiao L, Kaseb AO, Varadhachary GR, Wolff RA, Raghav KPS, Iwasaki M, Masci P, Ramanathan RK, Ahn DH, Bekaii-Saab TS, Borad MJ. Gemcitabine, cisplatin, and nab-paclitaxel for the treatment of advanced biliary tract cancers: A phase 2 clinical trial. JAMA Oncol. 2019; 5:824-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Morizane C, Okusaka T, Mizusawa J, et al. Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: The FUGA-BT (JCOG1113) Randomized Phase III Clinical Trial. Ann Oncol. 2019; 12:1950-1958. [DOI] [PubMed] [Google Scholar]
- 78. Schweitzer N, Kirstein MM, Kratzel AM, Mederacke YS, Fischer M, Manns MP, Vogel A. Second-line chemotherapy in biliary tract cancer: Outcome and prognostic factors. Liver Int. 2019; 39:914-923. [DOI] [PubMed] [Google Scholar]
- 79. Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014; 25:2328-2338. [DOI] [PubMed] [Google Scholar]
- 80. Nakamura H, Arai Y, Totoki Y, et al. Genomic spectra of biliary tract cancer. Nat Genet. 2015; 47:1003-1010. [DOI] [PubMed] [Google Scholar]
- 81. Abou-Alfa GK, Macarulla T, Javle MM, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020; 21:796-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Fontugne J, Augustin J, Pujals A, Compagnon P, Rousseau B, Luciani A, Tournigand C, Cherqui D, Azoulay D, Pawlotsky JM, Calderaro J. PD-L1 expression in perihilar and intrahepatic cholangiocarcinoma. Oncotarget. 2017; 8:24644-24651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Silva VW, Askan G, Daniel TD, Lowery M, Klimstra DS, Abou-Alfa GK, Shia J. Biliary carcinomas: pathology and the role of DNA mismatch repair deficiency. Chin Clin Oncol. 2016; 5:62. [DOI] [PubMed] [Google Scholar]
- 84. Lee SH, Lee HS, Lee SH, Woo SM, Kim DU, Bang S. Efficacy and safety of pembrolizumab for gemcitabine/ cisplatin-refractory biliary tract cancer: A multicenter retrospective study. J Clin Med. 2020; 9:1769. [DOI] [PMC free article] [PubMed] [Google Scholar]


