Summary
Chronic lymphocytic leukemia (CLL) that transforms into a more aggressive lymphoma has been termed Richter syndrome (RS). CLL with T-cell neoplasia is rarely reported; those with ALK+ anaplastic large cell lymphoma (ALCL) are also exceedingly rarely reported. A 63-year-old woman from the south of China presented with generalized lymphadenectasis and fever; she already had a prior diagnosis of CLL 9 years ago. As per her current diagnosis, it was CLL with ALK+ ALCL. The two-lymph node and bone marrow biopsies presented two types of cellular groups: i) left cervical lymph node biopsy suggested CLL (Ki67: 10%), along with bone marrow biopsy exhibited enhancement of the small lymphocytes (30%) with scant cytoplasm, round or irregular cell nuclei, and massive amounts of chromatin. Large cells (< 1%) that expressed CD30 and ALK were visible; The results of immunohistochemistry were as follows: CD20 (weak positive); PAX5 (positive); CD23 and CD5 (weak positive); and CD3, CD10, and CyclinD1 (negative); ii) left supraclavicular lymph node biopsy suggested ALK+ ALCL (Ki67: 70%). The final diagnosis was CLL with ALCL. The mechanisms of this condition are not fully understood, which might be associated with chronic stimulation of T cells by CLL cells along with immune dysfunction.
Keywords: chronic lymphocytic leukemia, Richter syndrome, ALK, anaplastic large cell lymphoma, fludarabine
1. Introduction
Chronic lymphocytic leukemia (CLL) that transforms into a more aggressive lymphoma has been termed Richter syndrome (RS) or Richter transformation (1). This occurs approximately in 5-10% of patients with CLL (2) and is commonly associated with a worse clinical outcome (3-5). Approximately 80-90% of RS cases are clonally related to CLL, whereas only 10-20% are clonally unrelated (6). Diffuse large B-cell lymphoma is the most common RS subtype, accounting for 95% of total RS cases, of which less than 5% may develop into classical Hodgkin's lymphoma (7). CLL with T-cell neoplasia is rarely reported; those with ALK+ anaplastic large cell lymphoma (ALCL) are also exceedingly rarely reported; however, whether CLL with ALK+ ALCL can be attributed to RS remains controversial (8).
2. Clinical manifestation of a rare case
A 63-year-old woman from the south of China was diagnosed with ALK+ ALCL 9 years after she was diagnosed as having CLL (Binet B) in 2011. She complained of lymphadenectasis throughout her body, including in the neck, axilla, and inguinal region. She was untreated (Binet B) as per the National Comprehensive Cancer Network (NCCN) guidelines (9) until 2018, when CLL progressed. She underwent six courses of fludarabine, cyclophosphamide, and rituximab chemotherapy and achieved alleviation of the symptoms. In 2020, she felt extreme fatigue with generalized lymphadenectasis. Pathological slices of the left cervical lymph node exhibited the morphological manifestation of CLL/SLL (Figure 1 A1). The results of immunohistochemistry were positive for CD20, CD5, CD23, Bcl-2, Bcl-6, CD21, and Ki67+ cells (10%) and negative for CD3, CD10, Mum1, CyclinD1, and TdT. The results of hybridization in situ were EBER negative. The immunophenotyping data of the bone marrow exhibited increased expression of CD5, CD22, CD19, CD79a, CD23, CD200, IgM, and Kappa and non-expression of CD10, FMC7, CD79b, and Lambda, which indicated that these cells were monoclonal mature B cells (CLL scoring 4-5). Fluorescence in situ hybridization of the marrow revealed the following: CEP12, 68%; D13S319/LAMP1, 85%; ATM/CEP11, P53/CEP17, and IGH/CCND1, negative; TP53 gene mutation, negative; IGH rearrangement, IGHV3-30; and IGH somatic mutation rate, 9.1%. The pathological slice of the bone marrow suggested recurrence of CLL (Figure 1 B1). The patient then underwent treatment with zanubrutinib. After 1 month of zanubrutinib treatment, the biopsy location of the left cervical lymph node began to swell accompanied by tenderness and fever of 39°C (Figure 1 C1).
Figure 1.
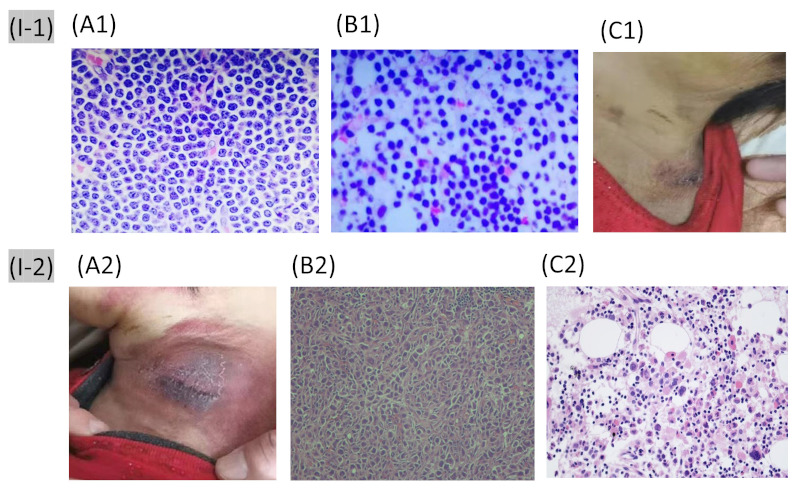
I-1, Images in the first hospitalization (2020); I-2, Images in the second hospitalization (2021). (A1) The pathological slices of the left cervical lymph node; (B1) The pathological slices of the bone marrow; (C1) The skin of the biopsy location of the left cervical lymph node during the first febrile episode; (A2) The skin of the biopsy location of the left cervical lymph node at the second febrile episode; (B2) The pathological slices of the left supraclavicular lymph node; (C2) The pathological slices of the bone marrow. (hematoxylin & eosin staining, 400×).
However, 1 week after antibiotics administration, fever recurred, which was now accompanied by cough, and the lymph node began to swell again. Antibiotics were again administered, but the symptoms did not improve. The skin in the left neck is shown in Figure 1 A2. Lymphadenectasis was noted at the bilateral neck, axilla, supraclavicular area, and inguinal area. She was then diagnosed with pulmonary infection and underwent several anti-infective treatments, including anti-virals and anti-fungals. The cough improved, but fever persisted. Hence, zanubrutinib treatment was maintained. A second biopsy of the left supraclavicular lymph node was conducted, which revealed heteromorphic cell nests. The cells were large with irregular nuclei and abundant karyokinesis (Figure 1 B2). The results of immunohistochemistry were positive for ALK, CD30, EMA, and Ki67+ cells (70%) and negative for AE1/ AE3, Bcl-2, Bcl-6, CD10, CD20, CD21, and CD3. The immunophenotyping data of the peripheral blood revealed that 88.41% of cells expressed CD19, CD200, and Kappa; weakly expressed CD5, CD20, CD23, and CD43; and did not express CD10, FMC7, CD79b, Lambda, CD22, CD103, CD38, and CD138. The results indicate that these cells were monoclonal mature B cells. The values of forward scatter and side scatter were small. The immunophenotyping assay of the bone marrow revealed that 86.15% of the cells expressed CD19 and Kappa; weakly expressed CD5, CD20, CD23, CD43, and CD200; and did not express CD10, FMC7, CD79b, Lambda, CD22, CD103, CD38, and CD138, thus also indicating that these cells were monoclonal mature B cells. The lymphoma gene rearrangement of the marrow fluids showed that TCRD, TCRβ, and TCRγ were negative. Human T-cell leukemia virus type I in the peripheral blood was negative. The pathological slices of the bone marrow revealed 60% bone morrow hyperplasia, enhancement of small lymphocytes (30%) with less cytoplasm, round or irregular nuclei, and massive amounts of chromatin. Large cells (< 1%) were visible and expressed CD30 and ALK (Figure 1 C2). The results of immunohistochemistry were as follows: CD20 (weak positive); PAX5+ (positive); and CD23 and CD5 (weak positive). Meanwhile, CD3, CD10, CyclinD1 were negative. Hence, the patient was finally diagnosed as CLL with ALK+ ALCL. She then underwent cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) plus zanubrutinib chemotherapy. After 4 days of treatment, fever abated. After two courses of treatment, generalized lymphadenectasis was resolved, and efficacy was evaluated as "partial remission". The patient is well and alive after the last follow-up (2022 August).
This study was approved by the ethics committee of the First Hospital of Putian City, and informed consent was signed to present her agreement reported in this study.
3. Experience and insights
CLL with ALCL is rarely reported. By far, only 16 cases have been reported in the literature (Table 1); of those, 10 are males and 6 are females. The median age of patients was 63.5 years (range, 47-86 years). The average time from CLL to diagnosis of ALCL was 4 years (range, 0-16 years). With respect to ALK expression, seven cases were ALK+, seven cases were ALK−, and two cases were unknown. In this case, the patient was 63 years old, and the time from CLL to diagnosis of ALK+ ALCL was 9 years.
Table 1. The included literature and patients.
| Literatures | Age,gender | Time from CLL to ALCL (years) | ALCL location | ALK (+ or −) | ALCL Immunophenotype | Chemotherapy before ALCL | Chemotherapy after ALCL | Outcome |
|---|---|---|---|---|---|---|---|---|
| Nai et al. 1998 (3) | 61, F | 3 | Spleen | UK | CD30 (+), CD3 (+), CD45(+), CD45RO (+), EMA (+); CD15 (+/−); CD20 (−), κ (−), λ (−) | Fludarabine | No | LTF |
| van den Berg et al. 2002 (4) | 76, M | 4 | Lymph node | − | CD30 (+), CD45 (+), TIA-1 (+), UCHL-1 (+); EMA (+/−); CD20 (−), CD3 (−), CD15 (−), ALK (−), TARC (−) | UK | UK | UK |
| Marschalkó et al. 2007 (5) | 75, M | 7 | Cutis | UK | CD30 (+), TIA-1(+); CD4 (+/−); CD3 (−), CD5 (), CD7 (-), CD8 (-), CD79a (-) | No | UK | MF appeared (1.5 years later) |
| Liu et al. 2008 (18) | 59, M | 8 | Lymph node | + | CD30 (+), CD45 (+), CD45RO (+), CD4(+), ALK (+); CD5 (-), CD8 (-), CD20 (-), CD23 (-), CD79a (-), EBER (-) | Chlorambucil/prednisone; fludarabine; fludarabine/rituximab; pentostatin/cyclophosphamide/rituximab; weekly rituximab | ICE | Dead (2 months) |
| Persad & Pang 2014 (19) | 47, M | 0 | Lymph node | − | CD30 (+), granzyme (+), CD43 (+), CD23 (+); CD2 (+/-), CD4 (+/-), CD45 (+/-), Bcl-2 (+/-); CD3 (-), CD5 (-), CD7 (-), CD8 (-), ALK (-), EBER (−), B-F1 (−), CD56 (−), CD57 (−), CD10 (−), CD15 (−), CD20 (−), PAX5 (−) | No | R-EPOCH; Autologous stem cell transplant | CR |
| Boyel et al. 2014 (8) | ||||||||
| Case 1 | 56, F | 0 | Lymph node | + | CD30 (+), Perforin (+), CD5 (+), EMA (+), ALK (+); granzyme (+/−), CD56 (+/−); CD3 (−), CD4 (−), CD8 (−), CD20 (−), EBER (−), Ki67: 80% | No | R-CHOP | ANED (15 months later) |
| Case 2 | 66, F | 0.7 | Lymph node/bone | + | CD30 (+), Perforin (+), CD5 (+), ALK (+); granzyme (+/−), CD4 (+/−), CD7 (+/−), CD45 (+/−); CD3 (−), CD8 (−), CD20 (−), EBER (−) | Cyclophosphamide, vincristine, prednisone, fludarabine, rituximab | Cisplatin, etoposide, cytarabine | Dead (7 months later) |
| Mant et al. 2015 (17) | ||||||||
| Case1 | 60, F | 5 | Lymph node/marrow/blood | − | CD30 (+), Perforin (+), CD2 (+), CD3 (+), CD43 (+); granzyme (+/−); ALK (−), CD4 (−), CD5 (−), CD8 (−), CD56 (−), EBER (−) | FCR | Palliative therapy | Dead (2 months later) |
| Case 2 | 44, M | 2 | Lymph node | − | CD30 (+), CD2 (+); granzyme (−), Perforin (−), ALK (−), CD4 (−), CD5 (−), CD8 (−), CD3 (−), EBER (−) | Fludarabine; CHOP | CHOP | Dead (1 months later) |
| Case 3 | 86, M | 1 | SNose | − | CD30 (+), CD43 (+); CD4 (+/−), granzyme (+/−); ALK (−), CD5 (−), CD8 (−), CD3 (−), CD45 (−), CD15 (−), CD56 (−), EBER (−) | UK | No | AD (3 months later) |
| Case 4 | 64, M | 8 | Lymph node/Ascitic fluid/CNS | + | CD30 (+), CD4 (+), granzyme (+), Perforin (+), ALK (+), EMA (+); CD43 (+/−), CD45 (+/−); CD2 (−), CD5 (−), CD8 (-), CD3 (-), EBER (-) | FCR | CHOP; High dose methotrexate; intrathecal chemotherapy | ANED (16 months later) |
| Case 5 | 63, F | 8 | Lymph node | + | CD30 (+), CD43 (+), Perforin (+), ALK (+); CD4 (+/-); CD45 (-), CD56 (-), CD5 (-), CD8 (-), CD3 (-), granzyme (-), EBER (−) | Chlorambucil and prednisone | CHOP | ANED (10 months later) |
| Thakra & Konoplev. 2017 (6) | 56, M | UK | Marrow | + | CD30 (+), ALK (+), CD4(+), CD5 (+), CD45 (+), CD43 (+); CD2 (+/−); CD3 (−), CD7 (−), CD8 (−), CD15 (−), Pax-5 (−) | UK | UK | UK |
| Van Der Nest et al. 2019 (11) | ||||||||
| Case 1 | 77, F | 4 | Lymph node | + | CD30 (+), MUM1 (+), granzyme (+), perforin (+), ALK1 (+); CD4 (+/−), CD45 (+/−); CD20 (−), PAX5 (−), CD79a (−), BOB1 (−), OCT2 (−), CD3(−), CD5 (−), CD138 (−), BCL2 (−), EBER (−), CD15 (−), C-MYC (−), CD34(−), CD117(−), TIA1(−) | UK | Mini-CHOP | A high-grade neuroendocrine tumor occurred |
| Case 2 | 74, M | 8 | Cutis | − | CD30 (+), CD3 (+), CD4 (+), granzyme (+); CD2 (+/−), CD5 (+/−), CD7 (+/−); CD20 (−), ALK (−), EBER (−), CD8 (−), BF1 (−) | No | CHOP, R-CHOP, dexamethasone, cytarabine, carboplatin, gemcitabine/vinorelbine, high dose methotrexate | Dead (3 years later) |
| Case 3 | 66, M | 16 | Cutis/Lymph node/Bone | − | CD30 (+), CD 4(+), CD43 (+), CD2 (+), CD7 (+), TIA (+), granzyme B (+), (focal) and EMA (+), (patchy); CD20 (−), CD45RO (−), CD3 (−), CD5 (−), CD8 (−), CD56 (−), ALK1 (−), CD163 (−), CD123 (−), BCL11A (−), CD2AP (−), CD303 (−), EBER (−), HHV8 (−) | No | Cyclophosphamide; vincristine/gemcitabine and brentuximab vedotin. | Dead (4 years) |
AD: Alive with disease; ANED: alive with no evidence of disease; CHOP: cyclophosphamide, doxorubicin, vincristine, prednisone; CR: complete remission; FCR: fludarabine, cyclophosphamide, rituximab; ICE: ifosfamide, carboplatin, and etoposide; LTF: loss to follow-up; MF: mycosis fungoides; R-EPOCH: rituximab, etoposide, prednisone, vincristine, doxorubicin, and cyclophosphamide; UK: unknown.
To date, mechanisms of the CLL complicating aggressive T lymphoma remain unclear. Some authors believe that it is associated with the chronic stimulation of T cells by CLL cells along with immune dysfunction (10-12). In addition, abnormal proliferation of T cells might cause new mutations that may develop into aggressive T-cell lymphoma (12). This theory is also supported by a previous report that the cytotoxic T-cell expansions in the peripheral blood are closely associated with the occurrence of T-cell lymphoma (13). In total, 12 of 16 CLL patients that developed ALCL expressed cytotoxic T lymphocyte-related genes such as TIA-1, granzyme, and perforin (Table 1). Unfortunately, the expression of these genes was not investigated in the patient in this study. The immune dysregulation inherent to CLL also contributes to T-cell oncogenesis due to its capacity to induce mixed neoplastic clones (10). A high occurrence of lymphoma is also observed in other diseases with immune dysregulation, such as sicca syndrome, rheumatoid arthritis, and chronic lymphocytic thyroiditis. All of this evidence indicates that immune dysregulation plays a role in the occurrence of lymphoma. Moreover, T cells from patients with CLL (vs. healthy subjects) seem to be more resistant against cellular apoptosis (14). All these factors might have contributed to the development of aggressive T lymphomas. Another factor that must be seriously considered is that whether the treatment for CLL has secondarily induced the occurrence of ALCL. Indeed, this is a controversial issue. The present case underwent six courses of fludarabine treatment before the occurrence of ALK+ ALCL. Of all the 16 patients included in the literature review, 5 underwent treatment with fludarabine. Gassner et al. reported that the administration of fludarabine continuously reduced the number of CD4+ and CD8+ T cells, which then correlates with the cytotoxic effects of fludarabine on T cells in vitro. Moreover, fludarabine plays a role in immune modulation, which might be a double-edged sword to T-cell oncogenesis (15). The role of CLL treatment in T-cell oncogenesis requires further investigation.
Before this patient could be diagnosed with ALCL, fever was a predominant symptom, and lymph node swelling recurred after zanubrutinib treatment, which we considered as both being due to the skin and soft tissue infections. Unfortunately, the subsequent anti-infective therapy was ineffective. We had to determine the final diagnosis by performing lymph node and bone marrow biopsies. When the patient had fever and recurrence was considered, the lymph node biopsy exhibited ALK+ cells. Moreover, the bone marrow biopsy found that there were < 1% large cells (Figure 1 C2). These results suggested that the lymph nodes and bone marrow were involved.
Generalized ALCL has a poor clinical outcome. Patients with ALK+ may have better outcomes than those of ALK− with the development of treatments such as brentuximab vedotin (BV) and crizotinib. The 5-year survival rate of ALK+ ALCL is 70-80%; in contrast, those with ALK− ALCL is only 40-60% (16). Our case is well and alive. However, due to the small sample size and the heterogeneity of the included studies, the effects of ALK expression on the prognosis of CLL complicating ALCL as well as the efficacy of the related treatments require further investigation.
Taken together, CLL with ALCL is rare, and the mechanisms involved are not fully understood. Chronic stimulation of T cells by CLL cells, along with immune dysfunction in CLL, might play a role. Several clinical problems, such as whether fludarabine treatment will increase the risk of CLL developing ALCL and the difference in the prognosis between idiopathic ALCL and ALCL complicated from CLL, have not been elucidated. Further investigation is required in the future.
Funding: None.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. De Mello RB, Do Vale ECS. Ulcerated cutaneous Richter syndrome. Dermatol Online J. 2020; 26:13030/qt1nt555qc. [PubMed] [Google Scholar]
- 2. Reda G, Cassin R, Fabris S, Ciceri G, Fattizzo B, Sciume M, Orofino N, Gianelli U, Neri A, Cortelezzi A. Biological and molecular characterization of a rare case of cutaneous Richter syndrome. Hematol Oncol. 2017; 35:869-874. [DOI] [PubMed] [Google Scholar]
- 3. Nai GA, Cabello-Inchausti B, Suster S. Anaplastic large cell lymphoma of the spleen. Pathol Res Pract. 1998; 194:517-522. [DOI] [PubMed] [Google Scholar]
- 4. van den Berg A, Maggio E, Rust R, Kooistra K, Diepstra A, Poppema S. Clonal relation in a case of CLL, ALCL, and Hodgkin composite lymphoma. Blood. 2002; 100:1425-1429. [PubMed] [Google Scholar]
- 5. Marschalko M, Csomor J, Eros N, Szigeti A, Harsing J, Szakonyi J, Desaknai M, Matolcsy A, Demeter J, Karpati S. Coexistence of primary cutaneous anaplastic large cell lymphoma and mycosis fungoides in a patient with B-cell chronic lymphocytic leukaemia. Br J Dermatol. 2007; 157:1291-1293. [DOI] [PubMed] [Google Scholar]
- 6. Thakral B, Konoplev S. "Soccer ball" cells to "donut" cells: an unusual case of Richter syndrome. Blood. 2017; 130:2358. [DOI] [PubMed] [Google Scholar]
- 7. Rossi D. Richter syndrome and fludarabine: a controversial relationship. Leuk Lymphoma. 2013; 54:213-214. [DOI] [PubMed] [Google Scholar]
- 8. Boyer DF, Lindeman NI, Harris NL, Ferry JA. Peripheral T-cell lymphomas with cytotoxic phenotype in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Am J Surg Pathol. 2014; 38:279-288. [DOI] [PubMed] [Google Scholar]
- 9. Wierda WG, Byrd JC, Abramson JS, et al. Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma, Version 4.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2020; 18:185-217. [DOI] [PubMed] [Google Scholar]
- 10. Campidelli C, Sabattini E, Piccioli M, Rossi M, De Blasi D, Miraglia E, Rodriguez-Abreu D, Franscini LL, Bertoni F, Mazzucchelli L, Cavalli F, Zucca E, Pileri SA. Simultaneous occurrence of peripheral T-cell lymphoma unspecified and B-cell small lymphocytic lymphoma. Report of 2 cases. Hum Pathol. 2007; 38:787-792. [DOI] [PubMed] [Google Scholar]
- 11. Van Der Nest BM, Leslie C, Joske D, Radeski D, White R, Cheah CY. Peripheral T-cell lymphoma arising in patients with chronic lymphocytic leukemia. Am J Clin Pathol. 2019; 152:818-827. [DOI] [PubMed] [Google Scholar]
- 12. Roncati L. Richter's syndrome of T-cell lineage (T rex lymphoma). Curr Med Res Opin. 2020; 36:473-476. [DOI] [PubMed] [Google Scholar]
- 13. Martinez A, Pittaluga S, Villamor N, Colomer D, Rozman M, Raffeld M, Montserrat E, Campo E, Jaffe ES. Clonal T-cell populations and increased risk for cytotoxic T-cell lymphomas in B-CLL patients: clinicopathologic observations and molecular analysis. Am J Surg Pathol. 2004; 28:849-858. [DOI] [PubMed] [Google Scholar]
- 14. Campas C, Lopez JM, Santidrian AF, Barragan M, Bellosillo B, Colomer D, Gil J. Acadesine activates AMPK and induces apoptosis in B-cell chronic lymphocytic leukemia cells but not in T lymphocytes. Blood. 2003; 101:3674-3680. [DOI] [PubMed] [Google Scholar]
- 15. Gassner FJ, Weiss L, Geisberger R, Hofbauer JP, Egle A, Hartmann TN, Greil R, Tinhofer I. Fludarabine modulates composition and function of the T cell pool in patients with chronic lymphocytic leukaemia. Cancer Immunol Immunother. 2011; 60:75-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015; 126:17-25. [DOI] [PubMed] [Google Scholar]
- 17. Mant S, Taylor G, Dutton D, Butler A, Browett P, Ganly P. Development of T-cell lymphomas with an activated cytotoxic immunophenotype, including anaplastic large cell lymphomas, in patients with chronic lymphocytic leukemia: a series of six cases. Leuk Lymphoma. 2015; 56:774-778. [DOI] [PubMed] [Google Scholar]
- 18. Liu T, He M, Carlson DL, Hedvat C, Teruya-Feldstein J. ALK-positive anaplastic large cell lymphoma in a patient with chronic lymphocytic leukemia. Int J Surg Pathol. 2010; 18:424-428. [DOI] [PubMed] [Google Scholar]
- 19. Persad P, Pang CS. Composite ALK-negative anaplastic large cell lymphoma and small lymphocytic lymphoma involving the right inguinal lymph node. Pathol Res Pract. 2014; 210:127-129. [DOI] [PubMed] [Google Scholar]


