Summary
Fragile X syndrome (FXS) is caused by the full mutation in the fragile x messenger ribonucleoprotein 1 (FMR1) gene leading to the absence of the fragile X protein (FXP). Previous studies show that individuals with FXS exhibit changing behavior over time; therefore, this study aimed to elucidate the aberrant behavior profile of FXS individuals. The Aberrant Behavior Checklist-Community (ABC-C) was used to measure the aberrant behavior profile of individuals with FXS, which was rated by the parent/caregiver combined with clinical impression. A total of 58 items were used to assess aberrant behaviors across five subscales. Forty-nine individuals with FXS were included (32 males, 17 females) with a mean age of 32.9 ± 14.62 years in males and 33.4 ± 13.98 years in females. The average score of irritability and hyperactivity was significantly higher in male FXS individuals (5.37 ± 6.231 and 10.28 ± 8.524) than in female individuals (3.24 ± 7.093 and 3.76 ± 3.327) with p = 0.046 and p = 0.001, respectively. Overall irritability in FXS individuals significantly decreased over time (ß = −0.141; p = 0.032). A modest worsening in lethargy/social withdrawal in males across age and a gentle improvement in hyperactivity/noncompliance in male of FXS individuals were observed. FXS males had higher hyperactivity problems than FXS female individuals across age.
Keywords: aberrant behaviors overtime, fragile X syndrome, hyperactivity, irritability
Fragile X syndrome (FXS) is the most frequent cause of inherited intellectual disability (ID), a well-known single-gene disorder associated with autism spectrum disorders (ASDs) (1). The frequency of FXS is approximately 1 in 4,000 males and 1 in 8,000 females worldwide (2). Males typically have a more severe phenotype in cognitive, physical, and behavioral features compared to females (3). Most males meet the criteria for severe ID and have more distinctive physical characteristics, macroorchidism, and more challenging behavior including ASD, ADHD, and self-injurious and aggressive behaviors (4). FXS is usually due to the expansion of CGG repeats of more than 200 units (full mutation) in the promotor region of the fragile x messenger ribonucleoprotein 1 (FMR1) gene that leads to transcriptional silencing and results in lack of fragile X protein (FXP) (5).
Developmental trajectories in speech, fine and gross motor, cognition, and behavior in FXS individuals have been studied to acknowledge the severity of the actual problem behavior and also to discover the need of services over time (6). Clinical manifestations change with age, some behaviors remain stable from childhood to adulthood, i.e., unusual eye contact, difficulty in showing emotion and unusual/inappropriate moods, inattentiveness, impulsivity, and social withdrawal. Other behaviors increase with age, i.e., overeating, social withdrawal, panic attack, and violent outburst/temper tantrums, and some appear to reduce post-puberty: sleep problems and hypersensitivity (7,8). Hustyi et al. carried out an interesting study in the United States, where clinical trials and standard interventions were available to address the longitudinal trajectories of aberrant behaviors (9).
In Indonesia, no specific therapy or standard intervention is available for children with FXS. Accordingly, documentation of persistent and nonpersistent behavioral problems in FXS individuals across age is needed to guide effective and strategic interventions. We conducted a study to obtain an aberrant behavior profile of individuals with FXS who did not undergo interventions to document the severity of behavioral problems that occur over time.
Study Design
Forty-nine individuals with FXS were included in this a cross-sectional study. The age of participants ranged from 6.56 to 60 years, and then were categorized and ordered from youngest to oldest by ten year intervals and analysis was done to obtain occurrence of behavioral problems in age categories. This study was approved by the Health Research Ethics Committee (Approval No.1.032/EC/ FK-RSDK/XII/2016). All parents/caregivers signed a consent form prior to the study.
Male and female individuals with FXS who had been confirmed genetically were recruited. The inclusion criteria for the respondents were as follows: having a child with FXS or caregivers who spent at least 6 h per day and were closely involved in daily living activities, health care, and social interactions for more than a year.
The Aberrant Behavior Checklist-Community (ABC-C) was used to assess the presence and severity of behavioral problems in children and adults with FXS. The ABC-C comprised five subscales that made up a 58-item questionnaire: irritability, lethargy/ social withdrawal, stereotypic behavior, hyperactivity/ noncompliance, and inappropriate speech (10). Each item was scored 0 (never a problem), 1 (slight problem), 2 (moderately serious problem), or 3 (severe problem). Each score was based on the parent or caregiver's perception of their child/client's behavior for the last 4 weeks.
The demographic characteristics of FXS individuals and respondents were obtained during the interview. The parent or caregiver completed the ABC-C through a semi-structured interview to rate their child/client's behavior conducted by experienced physicians (TIW and TAS). During and after the interview, TIW and TAS characterized the dominant aberrant behaviors to ensure that the information provided by the parent/caregiver was appropriate.
Each individual score was assumed as a baseline score, considering that all individuals received no standard therapy. The Mann-Whitney U test was applied to calculate the differences in subscale I, II, III, IV, and V scores between males and females because the data were not normally distributed. The Mann Whitney U test was also applied to analyze the difference of mean ABC-C score according to sex, rater, seizure co-morbidity, and education. Regression analysis was used to analyze the rate of change of subscales across age. P-values < 0.05 were considered statistically significant.
Core research findings
Forty-nine FXS individuals were included consisting of 32 males (65.3%) and 17 females (34.7%), the majority of participants were adults (87%) and only 12.2% were children. The mean age was 32.9 ± 14.62 years in males and 33.4 ± 13.98 years in females; the difference of age between males and females was not significant. Approximately 10% of FXS individuals had a history of seizure, more than 20% had no education/training, and none of them were treated with medications.
According to sex, the average score of irritability was significantly higher in male FXS individuals (5.37 ± 6.231) than in female individuals (3.24 ± 7.093) (-2.000; p = 0.046), and hyperactivity was significantly higher in male FXS individuals (10.28 ± 8.524) than in female individuals (3.76 ± 3.327) (-3.382; p = 0.001). The difference of irritability and inappropriate speech according to education category were found significant, with z value = 2.136, p = 0.035 and z values -2.283, p = 0.030, respectively. The difference of age according to education category was found statistically significant (Kruskal-Wallis H = 15.697; df = 3; p = 0.001). The remaining subscales demonstrated no significant difference according to the role of rater and seizure comorbidity (Table 1).
Table 1. The mean and median ABC-C subscale scores according to sex, the role of rater, seizure comorbidity, and education/training.
| Characteristics | Irritability | Lethargy | Stereotypic Behavior | Hyperactivity | Inappropriate Speech | Total |
|---|---|---|---|---|---|---|
| Sex | 5.37 ± 6.231 | 14.81 ± 8.299 | 2.66 ± 2.659 | 10.28 ± 8.524 | 2.56 ± 2.873 | 59.63 ± 29.469 |
| Male | 4.50 (0-26) | 13.50 (1-36) | 2.00 (0-12) | 7.00 (1-34) | 2.00 (0-9) | 52.50 (14-132) |
| 3.24 ± 7.093 | 11.94 ± 10.183 | 2.00 ± 2.828 | 3.76 ± 3.327 | 1.24 ± 2.107 | 58.88 ± 41.041 | |
| Female | 1.00 (0-30) | 10.00 (1-38) | 1.00 (0-12) | 3.00 (0-14) | 0.00 (0-7) | 52.00 (3-134) |
| (-2.000; p = 0.046) | (-1.010; p = 0.312) | (-1.652; p = 0.098) | (-3.382; p = 0.001) | (-1.622; p = 0.105) | (-0.399; p = 0.690) | |
| (z value; p)* | ||||||
| Rater | ||||||
| Parents | 5.96 ± 7.815 | 13.25 ± 10.515 | 2.64 ± 3.082 | 8.39 ± 8.148 | 2.04 ± 2.755 | 59.54 ± 38.005 |
| 3.50 (0-30) | 12.00 (1-38) | 2.00 (0-12) | 5.50 (0-33) | 1.00 (0-9) | 51.50 (3-134) | |
| Caregiver | 2.86 ± 3.838 | 14.57 ± 6.630 | 2.14 ± 2.151 | 7.52 ± 7.414 | 2.19 ± 2.657 | 59.14 ± 27.273 |
| 1.00 (0-13) | 13.00 (4-29) | 2.00 (1-11) | 5.00 (1-34) | 1.00 (0-9) | 53.00 (19-115) | |
| (z value; p)* | (-1.893; p = 0.058) | (-0.891; p = 0.373) | (-0.405; p = 0.685) | (-0.122; p = 0.903) | (-0.203; p = 0.839) | (-0.344; p = 0.731) |
| Seizure Comorbidity | ||||||
| None | 4.71 ± 6.818 | 14.18 ± 9.161 | 2.53 ± 2.785 | 8.42 ± 7.976 | 2.27 ± 2.742 | 59.42 ± 32.442 |
| 2.00 (0-30) | 13.00 (1-38) | 2.00 (0-12) | 6.00 (0-34) | 1.00 (0-9) | 53.00 (3-134) | |
| Seizure | 3.75 ± 1.893 | 9.75 ± 6.344 | 1.25 ± 1.258 | 3.50 ± 2.082 | 0.25 ± 0.500 | 58.75 ± 50.222 |
| 4.50 (1-5) | 10.00 (2-17) | 1.00 (0-3) | 3.50 (1-6) | 0.00 (0-1) | 40.00 (22-133) | |
| (z value; p)* | (-0.573; p = 0.585) | (-0.823; p = 0.426) | (-1.145; p = 0.277) | (-1.337; p = 0.190) | (-1.487; p = 0.166) | (-0.511; p = 0.634) |
| Education/Training | ||||||
| Not educated | 2.00 ± 3.682 | 14.00 ± 8.894 | 2.40 ± 3.169 | 7.30 ± 9.967 | 0.60 ± 1.578 | 60.90 ± 38.336 |
| 0.00 (0-11) | 14.00 (1-29) | 1.00 (0-11) | 3.50 (1-34) | 0.00 (0-5) | 49.50 (18-115) | |
| Educated | 5.31 ± 6.978 | 13.77 ± 9.138 | 2.44 ± 2.624 | 8.21 ± 7.255 | 2.49 ± 2.790 | 58.97 ± 32.695 |
| 3.00 (0-30) | 12.00 (1-38) | 2.00 (0-12) | 6.00 (0-33) | 2.00 (0-9) | 52.00 (3-134) | |
| (z value; p)* | (-2.136; p = 0.035) | -0.273; p = 0.798) | (-0.676; p = 0.517) | (-1.108; p = 0.274) | (-2.283; p = 0.030) | (-0.074; p = 0.951) |
Value in the table are mean ± SD, median (min-max). *Mann Whitney U test
The analysis revealed that the aberrant behavior in FXS individuals changed over time: the irritability, stereotypic behavior, and hyperactivity subscales were mildly improved in males and females, the lethargy somewhat increased in males, while the inappropriate speech subscale remained steady in males. However, only the trajectory of decreasing irritability in all FXS individuals across age groups was found significant (ß = −0.141; p = 0.032).
This study documented the behavioral changes based on ABC-C subscales according to sex and age. The five ABC-C subscales are presented in Figure 1. The graph shows the change (increase and decrease) in scores over time in male and female FXS individuals.
Figure 1.
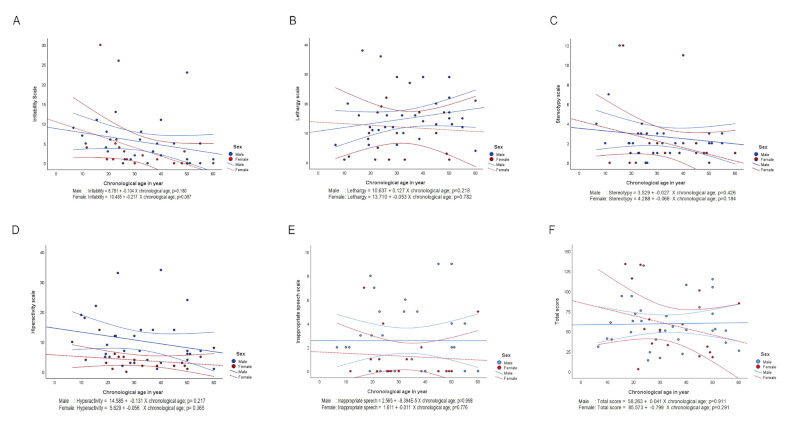
Trajectory score of five aberrant behavior subscales using the Aberrant Behavior Checklist-Community in FXS males and females across chronological age. (A) I, irritability; (B) II, lethargy/social withdrawal; (C) III, stereotypic behavior; (D) IV, hyperactivity/noncompliance; (E) V, inappropriate speech; and (F) Total score. Trajectory is presented in a blue dashed line for males with FXS and in a red dashed line for females with FXS, while a 95% confidence interval is presented in a blue solid line for males with FXS and in a red solid line for females with FXS.
Challenging behaviors are very common in individuals with FXS, and prior studies have indicated differences between sexes (9). In contrast with previous studies, the present study indicates that sex did not have a robust effect on the behavioral profile. Only the hyperactivity subscale was significantly more severe in males than in females. Males with full mutation allele will demonstrate complete penetrance of FXS phenotype, while females will demonstrate reduced penetrance of 50% with phenotype ranging from mild to moderate. The lack of FXP has been associated with FXS clinical characteristics, i.e., limited cognitive ability, behavioral problems, and social functioning (11).
This study revealed that age is an important factor in the behavioral phenotype. This study documented the variability in the aberrant behavior according to sex, especially with regards to irritability and hyperactivity subscales. Hyperactivity is a more significant problem in younger males than in females with FXS and it tended to decrease over time. The irritability in females and males (overall) across age groups significantly improved, while according to sex the change of irritability is modest. Previous studies reported improvement in hyperactivity and irritability subscales over time (9). From a parent and clinical perspective, irritability and hyperactivity behavior are their greatest concerns, including self-injurious behavior, aggression, disobedience, and noncompliance besides anxiety problems, because these behaviors negatively impact FXS individuals (12). There is an increased demand for medical care and support from very young ages, and financial problems arising from such behavioral challenges have been reported, however, Hatton et al., in 2002, found that no differences exist between FXS children on or off medication in externalizing behavior and attention problems. Moreover, those who were on medication had a higher score for problems (13).
Interestingly, an increase of the lethargy/social withdrawal subscale in males over time was documented in this study. The score in the present study is higher (almost three to four times) compared to that in previous findings (9). Perhaps our findings are related to the lack of medical care in our country. The social withdrawal subscale consisted of 16 items representing lack of social interaction skills, which were found to be the strongest predictor of independence in adults with FXS and a marker of quality of life (QoL) (14). Our study shown some similar behavioral trajectories; however, sex differences have only been documented on the hyperactivity/noncompliance subscale, and problems on lethargy/social withdrawal behaviors are already prominent in younger males and females. In addition, the trend increased at older ages, especially in male FXS individuals.
Literature searches were conducted in the PubMed database and google search engine using two keywords i.e., fragile X behavioral profile and limited medical care, and we found this is the first study to investigate the aberrant behavioral profile in FXS individuals who had received inadequate care. Factors that may impact the developmental trajectory, that is, environmental factors such as parent/caregiver education level and responsivity, IQ, and FXP, were not measured.
In conclusion, this study shows the variability in aberrant behavior according to sex, specifically irritability and hyperactivity subscales. The hyperactivity subscale is a more significant problem in younger male FXS individuals and it tended to decrease over time, while, the irritability subscale in females and males (overall) improves with age.
Acknowledgements
We would like to thank all parents/caregivers who participated in this study.
Funding:
This study was supported by Universitas Diponegoro grant (118-02/UN7.6.6/PP/2021).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Reddy KS. Cytogenetic abnormalities and fragile-X syndrome in Autism Spectrum Disorder. BMC Med Genet. 2005; 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hagerman PJ. The fragile X prevalence paradox. Journal of Medical Genetics. 2008; 45:498-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Baker EK, Arpone M, Vera SA, et al. Intellectual functioning and behavioural features associated with mosaicism in fragile X syndrome. J J Neurodev Disord. 2019; 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Symons FJ, Byiers BJ, Raspa M, Bishop E, Bailey DB. Self-injurious behavior and fragile X syndrome: findings from the national fragile X survey. Am J Intellect Dev Disabil. 2010; 115:473-481. [DOI] [PubMed] [Google Scholar]
- 5. Coffee B, Zhang F, Warren ST, Reines D. Acetylated histones are associated with FMR1 in normal but not fragile X-syndrome cells. Nat Genet. 1999; 22:98-101. [DOI] [PubMed] [Google Scholar]
- 6. Will EA, Bishop SL, Roberts JE. Developmental divergence: motor trajectories in children with fragile X syndrome with and without co-occurring autism. J Neurodev Disord. 2019; 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cornish K. Behaviour and cognitive profiles in adults. In: A multiprofessional view in assiciation with fragile X society (Dew-Hughes D, ed. Taylor and Francis Group, New York, 2004. [Google Scholar]
- 8. Hagerman RJ. The Physical and Behavioral Phenotype. In Hagerman RJ and Cronister A (eds) Fragile X Syndrome: Diagnosis, Treatment and Research. Second edition. John Hopkins University Press, Baltimore, 1996; PP.3-87. [Google Scholar]
- 9. Hustyi KM, Hall SS, Jo B, Lightbody AA, Reiss AL. Longitudinal trajectories of aberrant behavior in fragile X syndrome. Res Dev Disabil. 2014; 35:2691-2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aman MG, Singh NN, Stewart AW, Field CJ. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985; 89:485-491. [PubMed] [Google Scholar]
- 11. Tassone F, Hagerman RJ, Ikle DN, Dyer PN, Lampe M, Willemsen R, Oostra BA, Taylor AK. FMRP expression as a potential prognostic indicator in fragile X syndrome. Am J Med Genet. 1999; 84:250-261. [PubMed] [Google Scholar]
- 12. Van Remmerden MC, Hoogland L, Mous SE, Dierckx B, Coesmans M, Moll HA, Lubbers K, Lincken CR, Van Eeghen AM. Growing up with fragile X syndrome: concerns and care needs of young adult patients and their parents. J Autism Dev Disord. 2020; 50:2174-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hatton DD, Hooper SR, Bailey DB, Skinner ML, Sullivan KM, Wheeler A. Problem behavior in boys with fragile X syndrome. Am J Med Genet. 2002; 108:105-116. [DOI] [PubMed] [Google Scholar]
- 14. Hartley SL, Seltzer MM, Raspa M, Olmstead M, Bishop E, Bailey DB. Exploring the adult life of men and women with fragile X syndrome: results from a national survey. Am J Intellect Dev Disabil. 2011; 116:16-35. [DOI] [PMC free article] [PubMed] [Google Scholar]


