Summary
Bleeding is a common complication after lower gastrointestinal surgery, and cases due to coagulation dysfunction are rare. The current authors encountered a 54-year-old Chinese man with refractory bleeding after endoscopic rectal polypectomy, and multiple endoscopic and surgical interventions failed to control that bleeding. An APTT mixing test could not be corrected and there was no evidence of autoimmune-related disease, so the presence of nonspecific antibodies was considered. After empiric therapy with a cyclophosphamide and glucocorticoid, APTT was corrected and gastrointestinal bleeding stopped. Based on laboratory results and therapeutic results, the patient was ultimately diagnosed with prolonged APTT induced by monoclonal gammopathy of undetermined significance (MGUS). MGUS and coagulopathy characterized by a prolonged APTT has rarely been reported. Here, studies noting elevated monoclonal immunoglobulins and coagulopathy have been reviewed. If a prolonged APTT of undetermined significance cannot be corrected with an APTT mixing test and if autoimmune-related factors are excluded, then plasma cell-related diseases such as MGUS need to be considered.
Keywords: lower gastrointestinal bleeding, postoperative bleeding, abnormal coagulation mechanism, MGUS
Lower gastrointestinal bleeding (LGIB) occurs in the small intestine, colon, or anorectum and accounts for 30 to 40% of gastrointestinal bleeding. Its incidence is approximately 33-87 per 100,000 (1,2). Endoscopic colorectal polypectomy is a routine clinical procedure, and bleeding is the most common postoperative complication. The incidence of bleeding after endoscopic polypectomy has been reported to be approximately 1-6% (3). Reported here is a case of post-polypectomy bleeding, and that bleeding recurred after multiple endoscopic and surgical interventions. Based on a multidisciplinary discussion, an APTT correction test could not be corrected, and there was no evidence of autoimmune-related disease. Relevant tests revealed the presence of nonspecific antibodies. After empiric therapy with a cyclophosphamide and glucocorticoid, APTT returned to normal and bleeding stopped. Relevant tests were conducted and the patient was ultimately diagnosed with monoclonal gammopathy of undetermined significance (MGUS).
MGUS refers to the clonal proliferation of plasma cells or B cells to secrete monoclonal immunoglobulins (4), and it is characterized by the presence of serum M-protein less than 3 g/dL, bone marrow (BM) clonal plasma cells less than 10%, an absence of myeloma-defining events or amyloidosis, and end-organ damage (5). A study has found that almost all patients with multiple myeloma (MM) suffered from MGUS before the onset of the disease (6,7). Other patients with MGUS may develop Waldenström macroglobulinemia, light chain amyloidosis, or related disorders (8). Although prolonged PT can occur in patients with MGUS, prolonged APTT occurs in only a few cases (9). Moreover, cases involving postoperative bleeding are rarer and harder to diagnose. The current case may offer clinicians insight into this condition.
Clinical manifestations: A 55-year-old male visited a local hospital for gastroscopy and rectal polypectomy. A routine examination before gastrointestinal endoscopy indicated that APTT was 42.4 seconds (24-36 seconds) and TT was 26.8 seconds (14-21 seconds). A slight abnormality of coagulation was considered as a possibility. The patient had a history of health and no history of abnormal bleeding, no family history of hemophilia, and no history of von Willebrand disease. The patient had not received antiplatelet or anticoagulant therapy. Before the examination was complete, gastrointestinal endoscopy was performed. Gastroscopy revealed atrophic gastritis and a gastric angle ulcer. Colonoscopy revealed a sessile polyp, located about 8 cm from the margin in the rectum, and 12 flat polyps in the rectum, with a size of about 0.2 cm × 0.3 cm. Endoscopic rectal polypectomy and argon ion coagulation were performed, and the procedures were successful.
On the fourth day after polypectomy, the patient had multiple dark red loose stools. Endoscopy revealed multiple blood clots in the rectal lumen and obvious bleeding. After treatment with endoscopic clips, bleeding persisted. The patient was treated with medications. Endoscopic clips were used and endoscopic electrocoagulation was performed three times for hemostasis, and the bleeding temporarily subsided. After 5 days, the patient suddenly had a large amount of bloody stools again. The patient was urgently referred to this hospital and treated with somatostatin and terlipressin to stop the bleeding. A hemostatic clip was applied with immediate obvious hemostatic effect. Bleeding recurred, and colonoscopy revealed bleeding at the polyp wounds and other margins of the polyp wounds. The hemostatic effect was still significant after re-hemostasis. When bleeding persisted after repeated hemostatic treatment (Figure 1A), the possibility of a vascular malformation was considered. Mesenteric arteriography and embolization were performed, and an arteriovenous malformation was considered.
Figure 1.
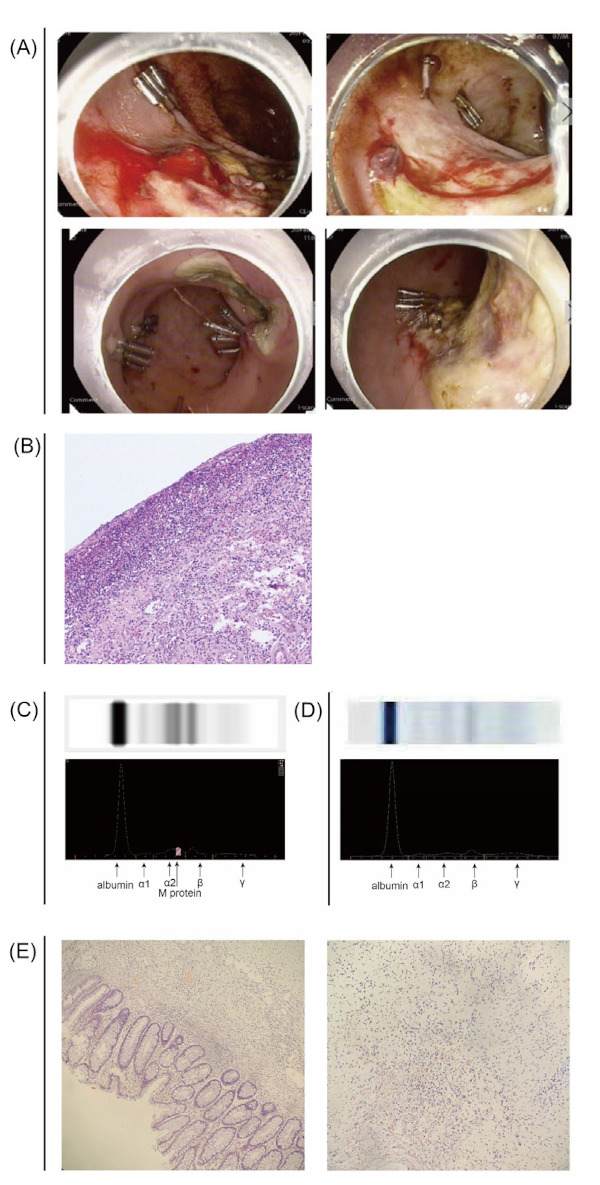
(A) Colonoscopy images. (B) Removed intestinal tissues (hematoxylin & eosin staining, medium magnification). (C) Serum M protein assay. Images of electrophoresis bands of serum protein and optical density scanning images of electrophoresis bands. (D) Normal protein electrophoresis. Images of electrophoresis bands of serum protein and optical density scanning images of electrophoresis bands. (E) Removed intestinal tissues (Congo red staining, medium magnification).
After embolization with gelatin sponge particles, blood in the stool was still present. Considering the possibility that a vascular malformation had caused the bleeding, after several multidisciplinary conferences the decision was made to perform surgery. The patient was subsequently transferred to Colorectal Surgery for laparoscopically assisted low anterior resection with prophylactic loop ileostomy. Pathology results suggested that there was an ulcer 3.5 cm × 2.2 cm in size on the surface of the intestinal mucosa. Microscopy revealed erosion necrosis of the surface mucosa, inflammatory exudation and hyperplasia of interstitial small blood vessels and fibrous tissue, and infiltration of a large number of acute and chronic inflammatory cells (Figure 1B). Pathology results revealed no vascular malformation. However, on the 7th and 10th days after surgery, the patient suffered from sudden gastrointestinal bleeding again. Additional colonoscopy revealed anastomotic oozing, and hemostasis was performed. Other treatments were provided, including repeated plasma transfusions, vitamin K supplementation, and aminocaproic acid hemostasis.
During those treatments, laboratory results indicated that APTT was prolonged and that von Willebrand factor was normal. An APTT mixing test revealed that APTT could not be corrected to the normal range, and coagulation factor XI and XII activity decreased (Table 1). After a multidisciplinary discussion, postoperative bleeding was deemed to have been caused by abnormal coagulation. After consultation with an experienced hematologist, the presence of immediately acting clotting inhibitors was considered (including heparin, lupus anticoagulants, or several clotting factor antibodies). Combined with negative autoimmune results, the presence of non-specific antibodies resulting in abnormal coagulation was considered. The patient's TT was prolonged, and the clotting time was normal after treatment with protamine. Thus, the presence of heparin antibodies was excluded. Due to repeated bleeding, the patient's condition was critical, and hemoglobin decreased from normal to 61g/L within 1 week. After empiric therapy with a cyclophosphamide and glucocorticoid, APTT and TT returned to their normal range, and gastrointestinal bleeding did not recur.
Table 1. Basic coagulation profile and coagulation factor activity assay.
| Items | Before disease | After endoscopic hemostasis | After surgery | After CTX | Normal range |
|---|---|---|---|---|---|
| Basic coagulation profile | |||||
| PT | 13 | 14.1 | 14.5 | 12.2 | 11-15s |
| APTT | 42.4 (24-36) | 57.1 | 52.3 | 40.1 | 28-42s |
| Normal reference APTT | NA | 35.2 | 31.2 | 31.5 | |
| APTT 50/50 mixed immediately | NA | 46.8 | 44.9 | 36.1 | |
| APTT 50/50 mixed within 2 h | NA | 50.8 | 45.6 | 38.4 | |
| Fibrinogen | 3.02 | 3.42 | 4.82 | 2.63 | 2-4g/L |
| Coagulation factor activity assay | |||||
| Plasma coagulation factor VIII activity assay | - | 201 | 113 | NA | 60-150% |
| Plasma coagulation factor IX activity assay | - | 165 | 173 | NA | 60-150% |
| Plasma coagulation factor X activity assay | - | 92 | 91 | NA | 70-120% |
| Plasma coagulation factor XI activity assay | - | 43 | 45 | 114 | 60-150% |
| Plasma coagulation factor XII activity assay | - | 51 | 43 | 110 | 60-150% |
| Von Willebrand factor | - | 231 | NA | NA | 50-160% |
PT, prothrombin time. APTT, activated partial thromboplastin time. CTX, cyclophosphamide.
Since the cause of bleeding due to a prolonged APTT was not clear, the relevant tests were completed. Immunofixation electrophoresis revealed monoclonal IgG and light chain λ in blood and urine. M protein in blood was 2.8 g/L. Serum free light chain λ was 165.27 mg/L. Serum free light chain κ was 10.06 mg/L. The free light chain ratio λ/κ was 16.4:1 (Figure 1C and D). The proportion of plasma cells in bone marrow was 0.5%, and there were no abnormalities in bone marrow pathology, MM FISH, or flow cytometry. Plain body bone radiographs revealed no significant abnormalities. Bone marrow pathology, an abdominal wall skin biopsy, and the pathology of the removed intestine were negative for Congo red staining (Figure 1E). Based on comprehensive clinical data, the patient was diagnosed with MGUS and a prolonged APTT. At present, the patient has a normal APTT without bleeding, and factor XI and XII activity has returned to its normal range. Elective enterostomy was subsequently performed, and the patient was followed up. This study was approved by the ethics committee of the Fujian Medical University Union Hospital, and informed consent was obtained prior to this study.
Experience and insights: The patient reported in this case suffered from refractory bleeding at the site of rectal polyp resection after colonoscopy due to slight prolongation of APTT and an absence of medical attention. After surgery, bleeding continued at the surgical site. Repeated endoscopic hemostasis was ineffective, and a vascular malformation was considered, but postoperative bleeding persisted after surgery. Pathology revealed no vascular malformation. One week after surgery, bleeding recurred. A multidisciplinary conference deemed that postoperative bleeding was not related to the procedure. Thus, repeated lower gastrointestinal bleeding was considered to have been mainly related to coagulopathy. The APTT mixing test suggested the presence of non-specific antibodies, causing an acquired coagulation dysfunction. Although the antibodies were unclear, the patient's condition was critical, and the only option was to eliminate autoantibodies to anticoagulation factors through empirical use of immunosuppressive therapy. Fortunately, the patient's APTT has returned to the normal range and postoperative bleeding stopped.
Moreover, an examination revealed elevated monoclonal IgG and lambda light chains, an abnormal serum free light chain ratio (κ/λ), M protein less than 30g/L, bone marrow (BM) clonal plasma cells less than 10%, and an absence of end-organ damage. Thus, the patient was diagnosed with low-risk MGUS. Based on Mayo risk stratification, the International Myeloma Working Group (IMWG) developed clinical follow-up guidelines for MGUS patients in 2010, recommending re-examination of M protein 6 months after initial diagnosis. According to those guidelines (10), the current patient is in the low-risk group and should only be followed up. Due to repeated bleeding after lower gastrointestinal surgery and failure to correct the prolonged APTT using normal plasma, the presence of non-specific antibodies and reduced factor XI activity was considered. Thus, empirical therapy with a cyclophosphamide and glucocorticoid was provided. The APTT returned to normal, bleeding stopped, and treatment was effective. Factor XI and XII activity decreased slightly, and those factors are only slightly correlated with bleeding (9). Whether M protein was involved in the prolonged APTT and whether M protein was the direct cause of bleeding is still uncertain. A handful of studies have suggested that MGUS is associated with prolonged APTT. McCaughan et al. reported that a patient with MGUS and detectable IgG and kappa also had uncorrectable APTT and reduced factor XI activity (9). However, that patient did not suffer from obvious bleeding.
Studies have found that patients with MGUS may develop an acquired bleeding disorder similar to congenital von Willebrand disease. These patients have low plasma levels of factor VIII or von Willebrand factor, and measures to improve hemostasis should be taken to prevent or treat bleeding. Two patients with MGUS and severe recurrence of chronic gastrointestinal bleeding were treated with long-term IVIg, and their laboratory results improved and chronic gastrointestinal bleeding stopped (11). Monoclonal binding proteins may bind to von Willebrand factor in the body.
MGUS is a precancerous lesion that may become a plasma cell disease. For patients with MM, prolonged PT or prolonged APTT is an independent factor for a poor prognosis in patients with newly diagnosed MM (12,13). Five to 86% of patients with MM have abnormal PT, and 8.9-69% of patients have abnormal APTT (14-16). A study on abnormal coagulation in patients with plasma cell tumors such as MM and MGUS found that prolonged PT was more common and that prolonged APTT alone accounted for less than 1% (17). Laboratory results can detect coagulation abnormalities in more than half of patients with AL amyloidosis, with prolonged APTT and bleeding. Abnormal deletion of factor X is the most common cause, which is due to the selective adsorption of factor X by amyloid fibrils. However, a deficiency in factor X is not the only cause of bleeding in patients with AL amyloidosis because vascular wall amyloidosis, fibrinogen abnormalities, abnormal platelet aggregation, and deficiencies in other factors including factors II, VII, IX, and V can lead to bleeding. An acquired coagulation factor deficiency can be explained by coagulation factor adsorption by amyloid fibrils, but the specific pathophysiological mechanism remains unclear (18). The current patient was negative for Congo red staining, and AL amyloidosis was ruled out by pathology.
The pathogenic mechanisms involved in MGUS are diverse, manifesting as autoantibody activity against tissue antigens, immune complex formation, and complement activation (11). The current patient required long-term follow-up, and more studies need to be conducted to examine the correlation between APTT prolongation and reduced factor XI activity and MGUS in order to determine the underlying cause of bleeding.
Based on the principles of perioperative management of hemophilia and the current authors' experience, alternative therapy is required to treat and prevent perioperative bleeding in a patient with a coagulation factor deficiency alone and the absence of specific or non-specific antibodies. Depending on the type of surgery, the plasma level of factors needs to be increased to at least 50-100% of the normal value, thereby reducing the risk of bleeding. For patients with specific and non-specific antibodies and an uncorrectable APTT undergoing elective surgery, the cause needs to be ascertained, the condition needs to be controlled, coagulation abnormalities need to be corrected, and surgery needs to be performed. If emergency surgery is performed, immunosuppressive drugs can be added as appropriate via fresh frozen plasma transfusions to reduce the risk of postoperative bleeding. If routine examinations fail to identify the cause, further testing for disorders associated with the clonal proliferation of plasma cells or B cells should be performed.
Funding: This work was supported by the Startup Fund for Scientific Research, Fujian Medical University (Grant number 2018QH1022), National Key Clinical Specialty Discipline Construction Program (2021-76), Fujian Provincial Clinical Research Center for Hematological Malignancies (2020Y2006) and the National Clinical Key Specialty Construction Project (General Surgery) of China (No. 2012-649).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Kim HS. Delayed postpolypectomy bleeding. J Korean Soc Coloproctol. 2011; 27:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hreinsson JP, Gumundsson S, Kalaitzakis E, Bjornsson ES. Lower gastrointestinal bleeding: Incidence, etiology, and outcomes in a population-based setting. Eur J Gastroenterol Hepatol. 2013; 25:37-43. [DOI] [PubMed] [Google Scholar]
- 3. Gibbs DH, Opelka FG, Beck DE, Hicks TC, Timmcke AE, Gathright JB, Jr. Postpolypectomy colonic hemorrhage. Dis Colon Rectum. 1996; 39:806-810. [DOI] [PubMed] [Google Scholar]
- 4. Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Offord JR, Dispenzieri A, Katzmann JA, Melton LJ, 3rd. Prevalence of monoclonal gammopathy of undetermined significance. N Engl J Med. 2006; 354:1362-1369. [DOI] [PubMed] [Google Scholar]
- 5. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014; 15:e538-548. [DOI] [PubMed] [Google Scholar]
- 6. Sigurdardottir EE, Turesson I, Lund SH, Lindqvist EK, Mailankody S, Korde N, Bjorkholm M, Landgren O, Kristinsson SY. The role of diagnosis and clinical follow-up of monoclonal gammopathy of undetermined significance on survival in multiple myeloma. JAMA Oncol. 2015; 1:168-174. [DOI] [PubMed] [Google Scholar]
- 7. Landgren O, Kyle RA, Pfeiffer RM, Katzmann JA, Caporaso NE, Hayes RB, Dispenzieri A, Kumar S, Clark RJ, Baris D, Hoover R, Rajkumar SV. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: A prospective study. Blood. 2009; 113:5412-5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hallek M, Bergsagel PL, Anderson KC. Multiple myeloma: Increasing evidence for a multistep transformation process. Blood. 1998; 91:3-21. [PMC free article] [PubMed] [Google Scholar]
- 9. McCaughan G, Jarvis S, Joseph J. Abnormal coagulation profiles in monoclonal gammopathy of undetermined significance: A case series. Pathology. 2021; 53:798-800. [DOI] [PubMed] [Google Scholar]
- 10. Kyle RA, Durie BG, Rajkumar SV, et al. International Myeloma Working G. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010; 24:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Federici AB, Stabile F, Castaman G, Canciani MT, Mannucci PM. Treatment of acquired von Willebrand syndrome in patients with monoclonal gammopathy of uncertain significance: Comparison of three different therapeutic approaches. Blood. 1998; 92:2707-2711. [PubMed] [Google Scholar]
- 12. Geng C, Yang G, Wang H, Zhang Z, Zhou H, Chen W. The prognostic role of prothrombin time and activated partial thromboplastin time in patients with newly diagnosed multiple myeloma. Biomed Res Int. 2021; 2021:6689457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fermand JP, Bridoux F, Dispenzieri A, Jaccard A, Kyle RA, Leung N, Merlini G. Monoclonal gammopathy of clinical significance: A novel concept with therapeutic implications. Blood. 2018; 132:1478-1485. [DOI] [PubMed] [Google Scholar]
- 14. Coppola A, Tufano A, Di Capua M, Franchini M. Bleeding and thrombosis in multiple myeloma and related plasma cell disorders. Semin Thromb Hemost. 2011; 37:929-945. [DOI] [PubMed] [Google Scholar]
- 15. Kyle RA, Gertz MA, Witzig TE, Lust JA, Lacy MQ, Dispenzieri A, Fonseca R, Rajkumar SV, Offord JR, Larson DR, Plevak ME, Therneau TM, Greipp PR. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin Proc. 2003; 78:21-33. [DOI] [PubMed] [Google Scholar]
- 16. Gogia A, Sikka M, Sharma S, Rusia U. Hemostatic abnormalities in multiple myeloma patients. Asian Pac J Cancer Prev. 2018; 19:127-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pandey S, Post SR, Alapat DV, Smock KJ, Post GR. Prolonged prothrombin time correlates with serum monoclonal protein concentration in patients with plasma cell dyscrasia. Int J Lab Hematol. 2013; 35:421-427. [DOI] [PubMed] [Google Scholar]
- 18. Choufani EB, Sanchorawala V, Ernst T, Quillen K, Skinner M, Wright DG, Seldin DC. Acquired factor X deficiency in patients with amyloid light-chain amyloidosis: incidence, bleeding manifestations, and response to high-dose chemotherapy. Blood. 2001; 97:1885-1887. [DOI] [PubMed] [Google Scholar]


