Summary
Spinal muscular atrophy (SMA) is a rare disease that has attracted considerable interest in China due to its severity and hefty treatment costs. Few studies have been conducted on Chinese patients. The objective of this study was to assess the quality of life of SMA patients in China and to investigate the real impact of new treatments. We used the Pediatric Quality of Life Inventory (PedsQL) to analyze the Health-related quality of life (HRQoL) of patients with SMA in China. Information on demographics, disease-specific characteristics, and treatment were collected using a child-reported or proxy-reported questionnaire. The mean scores of HRQoL for the Nusinersen treatment group and conventional treatment groups are 55.6 and 48.4, respectively. Patients with SMA type I have the lowest scores, while those with type III have the highest scores. A higher proportion of the medication group showed improvement in the condition in the past six months (56.9% vs. 17.1%). Our results show that the clinical type, motor function and treatment strategy have a significant influence on HRQoL. The findings imply that Nusinersen benefits patients by slowing the progression of the disease and increasing their quality of life in the real world.
Keywords: spina muscular atrophy, quality of life, patient-reported outcome, rare diseases, Nusinersen
1. Introduction
Spinal muscular atrophy (SMA) is a rare genetic disease that causes muscle weakness. For children with severe SMA, respiratory failure is a common cause of death. Patients with SMA type I are unable to sit by themselves, and significant muscle weakness impairs breathing and swallowing, they will not survive 2 years without respiratory support. Patients with SMA type II can achieve unassisted sitting, but they gradually lose mobility and live shorter than the general population. SMA III can gain independence in walking without assistance (1).
SMA causes a considerable deterioration in HRQoL, affecting both patients and their caregivers (2,3). According to López (2), the average EQ-5D social tariff score for SMA patients was 0.16, significantly lower than that of young Spanish people (0.987). Using the youth version of the EQ-5D and Australiana utility weights Chambers (1) measured the HRQoL for SMA patients and found that the average score was 0.115. A systematic review conducted by Erik (2019) (4) confirmed that poor physical health contributes to HRQoL impairment in SMA.
Before the approval of Nusinersen, the management of SMA was limited to symptomatic treatment, and the conventional treatment is supportive care. Nusinersen is the first disease-modifying medication for SMA, significantly improving motor function (5,6). A huge potential for improving HRQoL can be reached if the new therapeutic intervention results in a less severe course of the disease (7). The National Medical Products Administration approved Nusinersen in February 2019 to treat 5qSMA.
There is little knowledge of HRQoL in Chinese SMA patients. Only one study for Chinese patients was published: Yao (8) recruited 101 children aged 0-17 years with SMA in China and measured their HRQoL using the Pediatric Quality of Life Inventory 3.0 Neuromuscular Module. Only six patients received Nusinersen treatment in this study, and further comparison analysis was constrained by the limited sample size. We aimed to assess the HRQoL of patients with SMA in China, and explore the effectiveness of Nusinersen in the real world.
2. Methods
2.1. Study design and participants
Two groups of subjects were surveyed, namely medication and non-medication groups. The medication group included patients, who received Nusinersen during the study horizon, the non-medication group included patients, who received conventional treatment without Nusinersen. The allocation was non-randomized, and the patients' decision to receive Nusinersen was dependent on their own choices and willingness. The dosage and administration followed the instructions and medical prescription.
This study was approved by the Ethics Review Committee of The Chinese Academy of Medical Sciences and Peking Union Medical College Hospital (JS-1233), and informed consent was obtained prior to the investigation. Participants were enrolled from December 2019 to September 2020, and all participants were the members of Beijing Meier Advocacy & Support Center for SMA (Meier). We conducted three surveys in December 2019, April 2020, and September 2020 to evaluate the disease-specific characteristics and HRQoL. With the promotion of the new treatment, more patients were willing to choose the new drug. As an observational study, we did not set strict inclusion and exclusion criteria for participants. As a result, the total number of patients measured might change over time. The first measurement was considered as the baseline. We concentrated our further study on the third measurement in order to reflect the effectiveness of treatment more properly.
2.2. Measures
The basic information was extracted from Meier's Chinese SMA patient registry database. The main fields included the patient's age, gender, SMA clinical subtypes, onset age, motor function, the use of nasal or gastric tube feeding, and whether a non-invasive or invasive ventilator is used regularly.
The PedsQL 4.0 Generic Core Scales (PedsQL GCS) and the PedsQL 3.0 Neuromuscular Module (PedsQL NMM) are primarily designed to measure the HRQoL for SMA patients, according to a systematic review conducted by Erik (2019) (4). The PedsQL (Pediatric Quality of Life Inventory) was designed to integrate the relative merits of generic and disease-specific approaches. It demonstrated feasibility, reliability, and validity in the SMA population. The PedsQL NMM was designed to measure HRQoL dimensions specific to children ages 2 to 25 years with neuromuscular disorders, including SMA. The feasibility, reliability, and validity of the PedsQL NMM in children with SMA were supported by Susan (9). Hu (10) evaluated the reliability and validity of the Chinese version of PedsQL NMM. The study came to the conclusion that the instrument would be useful for measuring the HRQoL of Chinese children with neuromuscular diseases. PedsQL GCS was designed for both healthy and patient populations, and utilized for children with numerous disorders (11). The PedsQL Infant Scales (PedsQL IS) were designed as a generic HRQoL instrument specifically for healthy and ill infants ages 1-24 months. It was the first and only instrument developed specifically for infants. James (12) demonstrated the initial measurement properties of the PedsQL IS in healthy and ill infants and concluded that these instruments might be utilized to evaluate HRQoL in infants ages 1-24 months.
In this study, PedsQL was used to evaluate the HRQoL. PedsQL NMM was applicable for ages 2-25 years and encompassed three dimensions: i) about my neuromuscular disease (17 items), ii) communication (3 items), and iii) about our family resources (5 items). The PedsQL IS was applicable for infants (ages 1-24 months), composed of 36 items for infants ages 1-12 months and 45 items for infants ages 13-24 months, comprising five dimensions: i) physical functioning, ii) physical symptoms, iii) emotional functioning, iv) social functioning, and v) cognitive functioning. For adults ages over 26, PedsQL GCS was used, which encompasses four sizes: i) physical functioning (8 items), ii) emotional functioning (5 items), iii) social functioning (5 items), and iv) school functioning (5 items). Each multiple-answer was scored using a 5-point Likert scale from 0 (never a problem) to 4 (almost always a problem). Item scores were reversed and transformed on a scale from 0 to 100 (0 = 100, 1 = 75, 2 = 50, 3 = 0). Therefore, higher scores indicate better HRQoL.
2.3. Statistical analysis
Descriptive statistics were used to describe the characteristics of patients at baseline and compare the difference across subgroups. Continuous variables were summarized as mean ± standard deviations (SDs), and categorical variables were summarized using frequencies with percentages. Differences between groups were compared using a two-sample t-test or one-way ANOVA for continuous variables. Normality test and homogeneity of variance test were performed before comparing. For categorical variables, differences between groups were compared using Pearson's chi-squared test. As for hierarchical variables, Cochran- Mantel-Haenszel chi-square (CMH χ2) test was used, and odds ratio (OR) was reported. Multiple linear regression was conducted to identify factors influencing HRQoL scores. The significance for all statistical tests was indicated by p <0.05 (two-tailed); all the analyses were performed using Stata 16 MP.
3. Results
3.1. General characteristics
Three hundred and twenty-two patients were enrolled in our study at the first measurement time, including 59 SMA type I, 197 SMA type II, and 76 SMA type III. There were 281 patients in the non-medication group and 51 patients in the medication group. The baseline number of patients who used each scale is shown in Figure 1. A total of 64 patients completed the PedsQL IS, 249 patients completed the PedsQL NMM, and 19 completed the PedsQL GCS.
Figure 1.
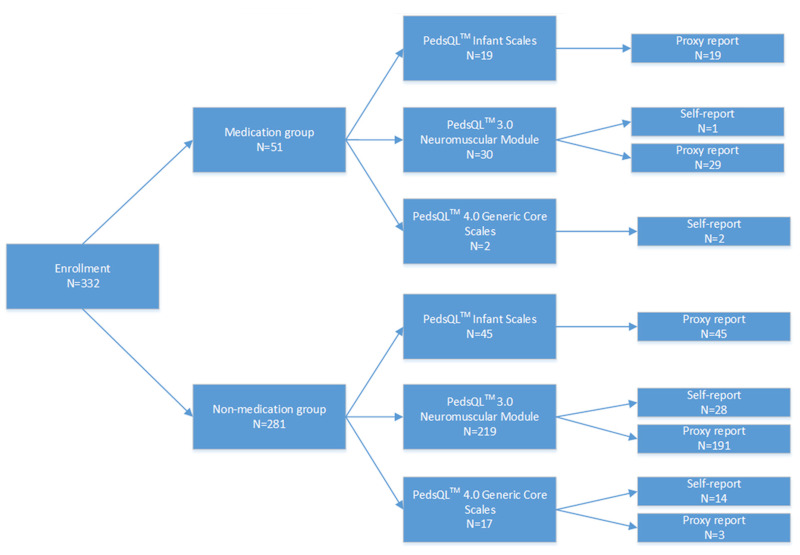
number of patients on each scale.332 patients were enrolled and divided into 2 groups: medication and non-medication groups. A total of 64 patients completed the PedsQL IS, 249 patients completed the PedsQL NMM, and 19 completed the PedsQL GC.
Two hundred seventy patients participated in the last survey, including 61 patients who received Nusinersen treatment. Compared to the baseline, the number of patients in the medication group increased, implying that acceptability of the new treatment increased.
Demographics and baseline results are provided in Table 1. More than 78.43% patients in the medication group received Nusinersen treatment at the first survey. It can be seen from the data that the medication group reported significantly higher HRQoL scores (55.6 vs. 48.4), and more patients achieved improvements in their condition (56.9% vs. 17.1%, p < 0.01). Also, the medication group shows that more patients used nasal or gastric tube feeding for nutrition support, and more patients used ventilators, implying that patients with more serious health conditions were more likely to use high-value treatments.
Table 1. Demographics and baseline results.
| Variable | Non-medication (n = 281) | Medication (n = 51) | p-value | test |
|---|---|---|---|---|
| Sex (male) | 121 (43.06%) | 19 (37.25%) | 0.44 | Pearson's χ2 |
| Clinical Subtypes | < 0.01 | CMH χ2 | ||
| SMA type I | 45 (16.01%) | 14 (27.45%) | - | |
| SMA type II | 167 (59.43%) | 30 (58.82%) | 0.58 (OR) | |
| SMA type III | 69 (24.56%) | 7 (13.73%) | 0.32 (OR) | |
| Onset age | < 0.01 | CMH χ2 | ||
| < 6 months old | 64 (22.78%) | 16 (31.37%) | - | |
| 7~18 months old | 170 (60.50%) | 33 (64.71%) | 0.78 (OR) | |
| 19 months~ 10 years old | 33 (11.74%) | 2 (3.92%) | 0.24 (OR) | |
| 10~30 years old | 13 (4.63%) | 0 (0.00%) | ||
| > 30 years old | 1 (0.36%) | 0 (0.00%) | ||
| Current age | < 0.01 | CMH χ2 | ||
| 1~12 months old | 11 (3.91%) | 4 (7.84%) | - | |
| 13~24 months old | 34 (12.10%) | 15 (29.14%) | 1.32 (OR) | |
| 2~4 years old | 77 (27.40%) | 16 (31.37%) | 0.62 (OR) | |
| 5~7 years old | 52 (18.51%) | 5 (9.80%) | 0.29 (OR) | |
| 8~12 years old | 38 (13.52%) | 5 (9.80%) | 0.39 (OR) | |
| 13~17 years od | 30 (10.68%) | 4 (7.84%) | 0.40 (OR) | |
| 18~25 years old | 22 (7.83%) | 0 (0.00%) | ||
| > 26 years old | 17 (6.05%) | 2 (3.92%) | 0.35 (OR) | |
| Motor function | < 0.05 | CMH χ2 | ||
| Non-sitter | 75 (26.69%) | 17 (33.33%) | ||
| Sitter | 148 (52.67%) | 28 (54.90%) | ||
| walker | 58 (20.64%) | 6 (11.78%) | ||
| Use of nasal tube or gastric tube feeding | 0 (0.00%) | 4 (7.84%) | < 0.01 | Pearson's χ2 |
| Use of non-invasive ventilator | < 0.01 | CMH χ2 | ||
| Never | 270 (96.09%) | 46 (90.20%) | - | |
| Sometimes | 11 (3.91%) | 4 (7.84%) | 5.96 (OR) | |
| All-day | 0 (0.0%) | 1 (1.96%) | ||
| Use of invasive ventilator | < 0.01 | CMH χ2 | ||
| Never | 280 (99.64%) | 47 (92.16%) | - | |
| Sometimes | 1 (0.36%) | 1 (1.96%) | 2.13(OR) | |
| All-day | 0 (0.0%) | 3 (5.88%) | ||
| Change of disease condition in the past six months | < 0.01 | CMH χ2 | ||
| Significant regression | 51 (18.15%) | 7 (13.73%) | - | |
| Regression | 34 (12.10%) | 6 (11.76%) | 1.20 (OR) | |
| Slight regression | 89 (31.67%) | 4 (7.84%) | 0.32 (OR) | |
| No changes | 59 (21.00%) | 5 (9.80%) | 0.61 (OR) | |
| Slight improvements | 41 (14.59%) | 20 (39.22%) | 3.55 (OR) | |
| Improvements | 3 (1.07%) | 4 (7.84%) | 9.71 (OR) | |
| Significant improvements | 4 (1.42%) | 5 (9.80%) | 9.10 (OR) | |
| HRQoL score | 48.4 (16.1) | 55.6 (18.1) | < 0.01 | Two sample t-test |
3.2. HRQoL stratification by SMA clinical subtypes
Two hundred and seventy patients finished the third measurement. As shown in Table 2, the severity of the disease condition has a negative impact on the HRQoL. The type I patients have the lowest scores. For SMA type I, II, and III, the average scores are 46.17, 48.48, and 54.26, respectively.
Table 2. HRQoL stratified by (A) SMA clinical subtypes and (B) motor function.
| (A) | ||||
| Variable | SMA type I, Mean (SD) (n = 43) |
SMA type II, Mean (SD) (n = 173) |
SMA type III, Mean (SD) (n = 54) |
p-value (ANOVA) |
| HRQOL score | 46.17 (18.09) | 48.48 (15.78) | 54.26 (18.72) | 0.04 |
| (B) | ||||
| Variable | Non-sitter, Mean (SD) (n = 75) |
Sitter, Mean (SD) (n = 147) |
Walker, Mean (SD) (n = 48) |
p-value (ANOVA) |
| HRQoL score | 46.66 (18.08) | 48.60 (15.31) | 55.40 (18.57) | 0.02 |
3.3. HRQoL stratification by motor function
The average scores of HRQoL stratified by motor function are summarized in Table 2. There was a significant difference between the three conditions, patients who could walk scored the highest. The average scores are 46.66, 48.60, and 55.40 for non-sitter, sitter, and walker, respectively.
3.4. Impact of Nusinersen
Figure 2 shows an overview of changes in health conditions in the past six months for two treatment groups. The proportion of patients who achieved any condition improvement is higher in the medication group. Additionally, the disease condition deteriorates over time in the non-medication group, while the medication group has the opposite situation, more patients benefit from the treatment. In terms of HRQoL, Figure 3 shows that the medication group has higher HRQoL scores, and scores improve over time, and these results can be seen in all clinical subtypes.
Figure 2.
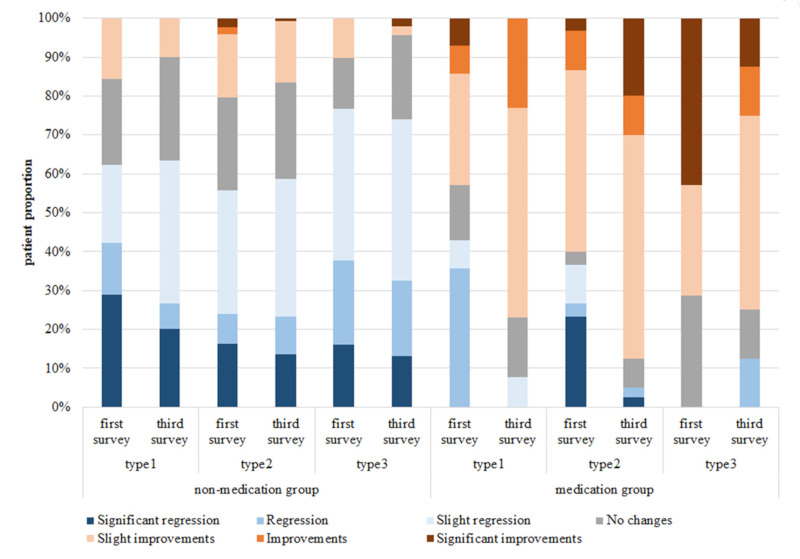
Change of disease condition in the past six months sorted by treatment group. The proportion of patients who achieved any condition improvement is higher in the medication group. In addition, the disease condition gets worse in the non-medication group over time.
Figure 3.
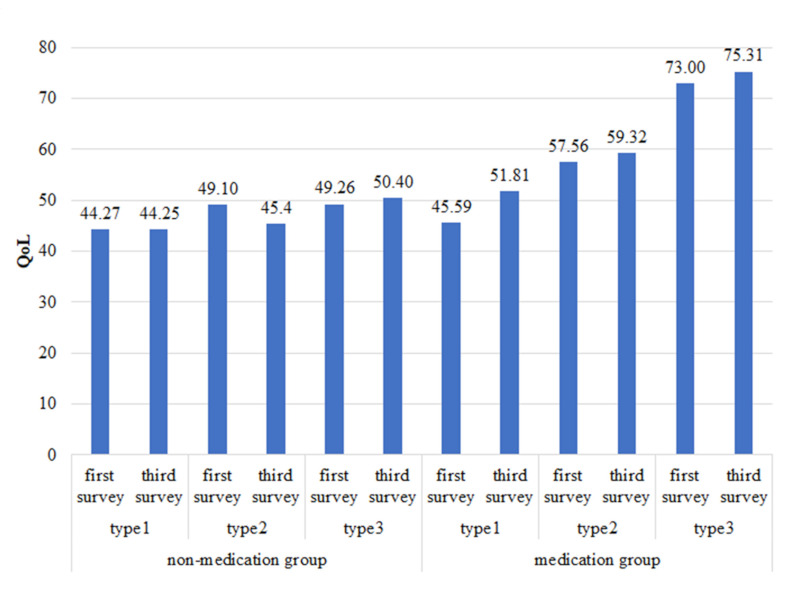
HRQoL score sorted by treatment group. Medication group has higher HRQoL scores, and the score increased with time. At the same time, the score decreased with time in the non-medication group.
3.5. Regression analysis
We performed a multiple linear regression analysis to identify factors influencing the HRQoL score. Factors for regression included demographics, disease-specific, and treatment variables. We also created dummy variables for multi categorical variables. The best model indicated the use of Nusinersen and SMA clinical subtype as significant variables, which explained 13.46% of the variance in HRQoL (Table 3). The use of Nusinersen increased HRQoL, and patients with SMA type 1 and type 2 reported lower HRQoL as compared to SMA type 3.
Table 3. Multivariate linear regression: predictors of HRQoL score.
| variables | Coef. | t | p > |t| | Beta |
|---|---|---|---|---|
| Use of Nusinersen | 12.53 | 5.36 | 0.000 | 0.31 |
| SMA type 1 | -9.59 | -2.90 | 0.004 | -0.21 |
| SMA type 2 | -6.82 | -2.74 | 0.007 | -0.19 |
| -cons | 52.41 | 23.86 | 0.000 | - |
4. Discussion
This study assessed HRQoL for patients with SMA in China. Consistent with the previous Chinese study conducted by Yao M, et al. (8), we confirmed that the HRQoL differed across SMA clinical subtype, motor function, and treatment regimen. Yao M, et al. (8) measured the HRQoL for children aged 0-17 years with SMA by PedsQL NMM. In our study, we chose a specific scale for each age group of patients according to the official description of PedsQL.
This study shows a meaningful difference in HRQoL across SMA clinical subtypes. Patients with SMA type I have the lowest scores, while those with type III have the highest scores. HRQoL decreases with disease severity, consistent with earlier publications (8,13,14). The HRQoL stratified by motor function lends support to the hypothesis that motor function affects the HRQoL. Patients with less motor disability showed better scores, and the walkers had the highest HRQoL scores.
Another important finding was that the patients could benefit from Nusinersen treatment. This result supports evidence from previous observations (5,8,15). Compared to the conventional treatment, patients receiving Nusinersen showed higher HRQoL scores, more patients achieved improvement in their health condition in the past six months, and the benefits grew over time.
Our regression analysis confirmed the association between SMA clinical subtype, Nusinersen treatment, and HRQoL. SMA type1 or type 2 would lower the HRQoL scores. While the Nusinersen treatment would increase the scores.
The main strength of our study is that we enrolled 332 SMA patients, a relatively large sample size, especially for rare diseases. Our sample covered the main age groups of SMA, and we adapted the survey instruments to the patients' ages, which can reflect their survival condition more accurately. This was the first study to explore the real effectiveness of Nusinersen for SMA patients in China, especially the patient-reported outcomes like improvement of motor functions and HRQoL.
The study is limited by the lack of HRQoL information on family or caregivers. SMA is a severe disease with high caregiver demands, with implications across all aspects of the family (2). Farrar (16) gained insights into the effects of caring for a child with SMA from the carer's perspective. The impact of taking care of an SMA patient includes changes in career choices and ongoing physical, social, and psychological consequences (17). These costs are high, and the impact on families can be devastating. Yao (8) measured the caregiver's HRQoL by Pediatric Quality of Life Inventory Family Impact Module (PedsQL FIM), they concluded that the more severe the SMA disorder, the lower the average scores on PedsQL FIM, which was consistent with the HRQoL of patients with SMA. The second limitation of this study is the time horizon. We conducted 3 surveys in ten months, which means the longest observation time is less than 1 year. SMA is so far a non-curable disease in China needing continuous treatment. We were unable to trend the quality longitudinally due to the short time horizon. Thus, the long-term effects of Nusinersen are still unknown. Lastly, the three Scales we used to measure HRQoL have not been validated in children with SMA in China.
In conclusion, the HRQoL differed across SMA clinical subtype, motor function, and treatment regimen. The HRQoL scores decreased with increasing disease severity. SMA patients can benefit from Nusinersen treatment, by slowing disease progression and improving HRQoL.
Acknowledgements
We thank all the patients and their parents for their contribution to this work. We also thank Huanping Xing, Bin Ma and China Alliance for Rare Diseases for support of this study.
Funding:
This work was supported by China Alliance for Rare Diseases and sponsored by Biogen. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing, and the submission of this paper.
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Chambers GM, Settumba SN, Carey KA, Cairns A, Menezes MP, Ryan M, Farrar MA. Prenusinersen economic and health-related quality of life burden of spinal muscular atrophy. Neurology. 2020; 95:e1-e10. [DOI] [PubMed] [Google Scholar]
- 2. López-Bastida J, Peña-Longobardo LM, Aranda-Reneo I, Tizzano E, Sefton M, Oliva-Moreno J. Social/economic costs and health-related quality of life in patients with spinal muscular atrophy (SMA) in Spain. Orphanet J Rare Dis. 2017; 12:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peña-Longobardo LM, Aranda-Reneo I, Oliva-Moreno J, Litzkendorf S, Durand-Zaleski I, Tizzano E, López- Bastida J. The economic impact and health-related quality of life of spinal muscular atrophy. An analysis across Europe. Int J Environ Res Public Health. 2020; 17:5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Landfeldt E, Edström J, Sejersen T, Tulinius M, Lochmüller H, Kirschner J. Quality of life of patients with spinal muscular atrophy: A systematic review. Eur J Paediatr Neurol. 2019; 23:347-356. [DOI] [PubMed] [Google Scholar]
- 5. Thimm A, Brakemeier S, Kizina K, Munoz Rosales J, Stolte B, Totzeck A, Deuschl C, Kleinschnitz C, Hagenacker T. Assessment of health-related quality of life in adult spinal muscular atrophy under nusinersen treatment-a pilot study. Front Neurol. 2022; 12:812063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Albrechtsen SS, Born AP, Boesen MS. Nusinersen treatment of spinal muscular atrophy - a systematic review. Dan Med J. 2020; 67:A02200100. [PubMed] [Google Scholar]
- 7. Klug C, Schreiber-Katz O, Thiele S, Schorling E, Zowe J, Reilich P, Walter MC, Nagels KH. Disease burden of spinal muscular atrophy in Germany. Orphanet J Rare Dis. 2016; 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yao M, Ma Y, Qian R, Xia Y, Yuan C, Bai G, Mao S. Quality of life of children with spinal muscular atrophy and their caregivers from the perspective of caregivers: a Chinese cross-sectional study. Orphanet J Rare Dis. 2021; 16:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Iannaccone ST, Hynan LS, Morton A, Buchanan R, Limbers CA, Varni JW; AmSMART Group. The PedsQL in pediatric patients with Spinal Muscular Atrophy: feasibility, reliability, and validity of the Pediatric Quality of Life Inventory Generic Core Scales and Neuromuscular Module. Neuromuscul Disord. 2009; 19:805-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu J, Jiang L, Hong S, Cheng L, Kong M, Ye Y. Reliability and validity of the Chinese version of the Pediatric Quality Of Life InventoryTM (PedsQLTM) 3.0 neuromuscular module in children with Duchenne muscular dystrophy. Health Qual Life Outcomes. 2013; 11:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mah JK, Thannhauser JE, Kolski H, Dewey D. Parental stress and quality of life in children with neuromuscular disease. Pediatr Neurol. 2008; 39:102-107. [DOI] [PubMed] [Google Scholar]
- 12. Varni JW, Limbers CA, Neighbors K, Schulz K, Lieu JE, Heffer RW, Tuzinkiewicz K, Mangione-Smith R, Zimmerman JJ, Alonso EM. The PedsQL™ Infant Scales: feasibility, internal consistency reliability, and validity in healthy and ill infants. Qual Life Res. 2011; 20:45-55. [DOI] [PubMed] [Google Scholar]
- 13. Weaver MS, Hanna R, Hetzel S, Patterson K, Yuroff A, Sund S, Schultz M, Schroth M, Halanski MA. A prospective, crossover survey study of child- and proxy-reported quality of life according to apinal muscular atrophy type and medical interventions. J Child Neurol. 2020; 35:322-330. [DOI] [PubMed] [Google Scholar]
- 14. Vega P, Glisser C, Castiglioni C, Amézquita MV, Quirola M, Barja S. Quality of life in children and adolescents with Spinal Muscular Atrophy. Rev Chil Pediatr. 2020; 91:512-520. [DOI] [PubMed] [Google Scholar]
- 15. Mix L, Winter B, Wurster CD, Platen S, Witzel S, Uzelac Z, Graf H, Ludolph AC, Lulé D. Quality of life in SMA patients under treatment with nusinersen. Front Neurol. 2021; 12:626787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Farrar MA, Carey KA, Paguinto SG, Chambers G, Kasparian NA. Financial, opportunity and psychosocial costs of spinal muscular atrophy: an exploratory qualitative analysis of Australian carer perspectives. BMJ Open. 2018; 8:e020907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Callan A, Nallagangul TK, Jawla S, de Pommerol HJ, Risson V. Neurology. 2019; 92 (15 Supplement):P4.4-016. [Google Scholar]


