Abstract
Aims
Using the principles of clinical governance, a patient-centred approach intended to promote holistic quality improvement, we designed a prospective, multicentre study in patients with acute coronary syndrome (ACS). We aimed to verify and quantify consecutive inclusion and describe relative and absolute effects of indicators of quality for diagnosis and therapy.
Methods and results
Administrative codes for invasive coronary angiography and acute myocardial infarction were used to estimate the ACS universe. The ratio between the number of patients included and the estimated ACS universe was the consecutive index. Co-primary quality indicators were timely reperfusion in patients admitted with ST-elevation ACS and optimal medical therapy at discharge. Cox-proportional hazard models for 1-year death with admission and discharge-specific covariates quantified relative risk reductions and adjusted number needed to treat (NNT) absolute risk reductions. Hospital codes tested had a 99.5% sensitivity to identify ACS universe. We estimated that 7344 (95% CI: 6852–7867) ACS patients were admitted and 5107 were enrolled—i.e. a consecutive index of 69.6% (95% CI 64.9–74.5%), which varied from 30.7 to 79.2% across sites. Timely reperfusion was achieved in 22.4% (95% CI: 20.7–24.1%) of patients, was associated with an adjusted hazard ratio (HR) for 1-year death of 0.60 (95% CI: 0.40–0.89) and an adjusted NNT of 65 (95% CI: 44–250). Corresponding values for optimal medical therapy were 70.1% (95% CI: 68.7–71.4%), HR of 0.50 (95% CI: 0.38–0.66), and NNT of 98 (95% CI: 79–145).
Conclusion
A comprehensive approach to quality for patients with ACS may promote equitable access of care and inform implementation of health care delivery.
Registration
ClinicalTrials.Gov ID NCT04255537
Keywords: acute coronary syndromes, clinical governance, quality improvement
Graphical Abstract
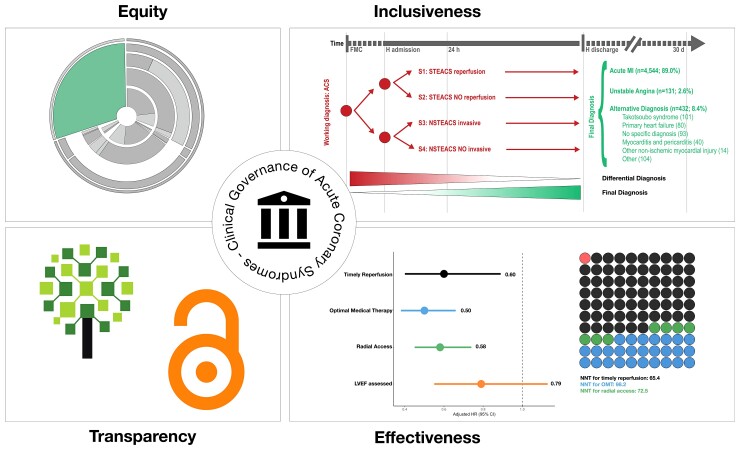
Graphical Abstract.
Introduction
To achieve the highest attainable standard of health,1 we must translate the best scientific evidence into clinical practice, hospital policies, and eventually patient care. An ideal model of care should provide equitable access to every eligible patient, govern the utilization of the limited existing resources, prioritize diagnostic and therapeutic interventions most likely to improve duration and quality of patients’ lives, and eventually share transparently not only the data that were collected but also every necessary information to optimally use them.
In this context, clinical governance has been proposed as a framework through which organizations are accountable for continuously improving the quality of care they provide by creating an environment in which excellence in clinical care will flourish.2 A comprehensive and transparent approach by which health systems are accountable may have enormous benefits to inform stakeholders, investments of resources, and eventually patients. This may be particularly relevant for patients admitted with acute coronary syndrome (ACS), a leading cause of premature death and impaired quality of life worldwide.
Herein, we present the primary results of the clinical governance programme in patients with ACS, a study designed to provide a unified and transparent approach to quality improvement intended to reach all patients admitted with ACS, quantify the degree of consecutive inclusion, measure multiple domains of care according to the evolving eligibility of patients (from admission to discharge), and identify priorities in their management toward the ideal model of care.
Methods
Study design, patients, and programme features
The study was designed to promote—and measure—the inclusion of the entire population of patients admitted with ACS in the participating hospitals.3 Eligibility was therefore set broad with minimal restrictions (protocol, see Supplementary material online). Briefly, all screened patients with evidence of suspected ACS in the clinical chart, as documented by symptoms of myocardial ischaemia, raised troponin values, or new electrocardiogram (ECG) changes (with or without ST elevation), were eligible. Eligibility for patients with negative troponins at baseline (i.e. unstable angina) required T-wave inversion ≥100 μV or ST segment depression ≥50 μV in leads with dominant R waves. Written informed consent to assess potential clinical outcome events was required only to patients discharged alive to allow the inclusion of patients who die in hospital.3
To quantify the completeness of the inclusion of patients admitted with ACS, we used a dedicated metric—the consecutive index. This is the ratio of the number of patients with ACS enrolled in each participating hospital during the study (numerator) to the estimated number of all ACS patients admitted during the same time (i.e. ACS Universe; denominator). The assumptions, derivation, and calculation performed for the estimation of the ACS Universe are detailed in the Supplementary material online. In brief, we tested two hospital codes based on the International Classification of Diseases Clinical Modification, 9th Revision (ICD-9 CM, which is the current administrative hospital coding system in Italy)—i.e. a procedure (invasive coronary angiography, ICA) and a diagnosis (acute myocardial infarction, AMI)—to identify patients admitted with ACS based on the assumption that ACS patients managed invasively will receive ICA while in patients managed medically without ICA a diagnosis of AMI will be assumed using a lower verification standard. The diagnostic performance of the two administrative codes was derived from the totality of unplanned admission to one institution over one calendar year (103 075 patients), and the diagnosis of ACS at admission was confirmed (adjudicated) by two study physicians.
The time of enrolment was the first objective qualifying criterion, typically the time of diagnostic ECG for patients with ST-elevation ACS (STEACS) and the time of ECG or troponin, whatever was collected first, for patients without STEACS (NSTEACS). Therefore, unlike other initiatives,4 patients admitted for ACS but discharged with a diagnosis alternative to AMI or UA were included. Operationally, four prospective study groups (ACS strata) were distinguished at baseline on the basis of the presenting ECG (with or without ST elevation) and whether an urgent invasive strategy was intended or not, i.e. S1 or STEACS patients intended for urgent reperfusion; S2 or STEACS patients not intended for urgent reperfusion; S3 or NSTEACS patients intended for routine invasive management; and S4: NSTEACS not intended for routine invasive management.
Measures of quality process and follow up
The two co-primary quality indicators variables were (i) timely reperfusion in patients admitted with STEACS intended for urgent reperfusion (S1) and (ii) optimal medical therapy in patients discharged alive with a final diagnosis of AMI or UA, which were selected for their presumed relevance and complementarity.
Timely reperfusion was defined as the proportion of S1 patients with acceptable time delay, i.e. less than 60 min of door-to-arterial access for reperfusion for non-transfer patients and less than 30 min of door-in-door-out time for transfer patients.
Optimal medical therapy in patients discharged alive with a final diagnosis of AMI or UA was categorized as present if five individual indicators of therapy were concurrently prescribed (all-or-none) in patients with heart failure or left ventricular ejection fraction (LVEF) ≤0.40, i.e. (i) low-dose aspirin; (ii) P2Y12 inhibition considered adequate according to high bleeding risk status; (iii) high intensity statin regimen; (iv) ACE-inhibitor (or angiotensin receptor blocker if intolerant to ACE-I); and (v) beta-blockers, excluding for each indicators patients with documented drug-specific contraindications. For patients discharged without HF or LVEF≤0.40, optimal medical therapy was considered present if three individual indicators of therapy were concurrently prescribed: (i) low-dose aspirin, (ii) adequate P2Y12 inhibition, and (iii) high intensity statin regimen. The precise definition and derivation of each quality indicator assessed, including individual eligible population considered was specified a priori (see Supplementary material online).
Secondary quality processes included: initial use of radial access in ACS patients managed invasively (S1 and S3); assessment of left ventricular ejection fraction in patients discharged alive with a diagnosis of AMI or UA; and optimal medical therapy at discharge stratified by clinical evidence of heart failure or LVEF ≤0.40. Death and other non-fatal endpoints (myocardial infarction, bleeding, stroke) were adjudicated by a clinical events committee consisting of two physicians according to prespecified definitions. Follow-up visits (telephone or in person) were performed at 30-day and at 1 year (± 5 days).
Data collection, quality control, strategies for patients inclusion, and data sharing
Data collection was performed by dedicated staff at each site that was trained on the protocol, operations, and conventions for patients’ inclusion. To maximize consistency, we used guidelines for most common scenarios (see Supplementary material online). We used REDCap for data collection, a secure online web application with a central monitor providing guidance and technical advice for staff entering the data via a dedicated helpdesk. To minimize common errors, we designed consistency checks and error-checking routines, including queries for impossible or unlikely values. All entered data were verified independently by two observers (with one being a MD) to ensure consistency of conventions used, accuracy of data entry, and identify potential systematic errors. Completeness of the data set was routinely monitored to ensure that the fields required for the derivation of primary and secondary indicators and processes considered would have less than 5% of missing data. A data dictionary was embedded in the CRF, containing explanatory details and notes on all fields.
Each site was trained to screen all ACS patients managed invasively as well as ACS patients managed conservatively without ICA. Due to fragmentation of care of the latter group the inclusion of patients with ACS managed conservatively mostly focused on patients admitted to cardiac wards.
The complete set of de-identified participant data collected along with explicative documents, including annotated case report form and study protocol, are available upon request.
Statistical analysis
We used Cox proportional-hazards regression to model time to death at 1 year for primary and key secondary quality indicators tested and Schoenfeld residuals to test the assumption of proportional hazards.5 Hazard ratios (and corresponding 95% confidence intervals) were estimated using multivariable models adjusted for known predictors of death, based on a set of covariates we specified a priori in the protocol. To examine different quality indicators in different eligible population we used two set of covariates for risk-adjustment: (i) admission covariates for patients with ACS and (ii) discharge covariates, for patients discharged alive with a diagnosis of AMI or UA (see Supplementary material online). Adjusted Kaplan–Meier estimates for 1-year death of primary and key secondary quality indicators according to time-specific (i.e. admission or discharge) covariates were also generated using a complete case analysis. To comprehensively quantify the absolute effect of primary and key secondary quality indicators on 1-year mortality, we calculated adjusted number needed to treat to prevent 1 death over time up to 1 year for quality indicators with significant association with mortality in multivariable analyses according to Altman.6 A P value of 0.05 or less was considered statistically significant. Due to the exploratory nature of the study, no adjustment for multiple comparison was performed.
Results
From September 2015 to January 2021, 5107 patients were enrolled from six Italian hospitals. Of these, 1524 were enrolled during the single-centre phase (September 2015 to December 2017) and 3583 during the multicentre phase (January 2018–2021).3 Patients’ characteristics by qualifying ACS stratum, and timing of principal diagnostic tests during hospitalization are presented in the Table 1; Supplementary material online, Tables S3–S6; Figure 1. An investigator-reported discharge diagnosis consistent with ACS, that is AMI or UA, was performed in 91.6% of enrolled patients while 8.4% had an alternative diagnosis, the most common being left ventricular apical ballooning syndrome (Graphical Abstract). At 1-year, 540 patients died (245 in-hospital) for a 1-year mortality rate of 10.6%. Of the 4567 who survived, 4473 (98%) had complete follow-up at 1-year and 94 (2%) incomplete follow-up. Of the latter group, 31 patients were lost-to follow-up at discharge (0.67%), while the remaining 63 were followed for a median of 113 days.
Table 1.
Characteristics of the patients at baseline by study group stratum
| STEACS reperfusion (N = 2402) |
STEACS NO reperfusion (N = 160) |
NSTEACS invasive (N = 2187) |
NSTEACS NO invasive (N = 358) |
Overall ACS population (N = 5107) |
||
|---|---|---|---|---|---|---|
| Age (years) |
N
Median (IQR) |
2402 65 (56–75) |
160 74 (63.8–83) |
2187 69 (59–78) |
358 77 (67–84) |
5107 68 (58–78) |
| Females | n (%) | 644 (26.8) | 67 (41.9) | 711 (32.5) | 163 (45.5) | 1585 (31) |
| Body mass index |
N
Median (IQR) |
2262 26 (24–29) |
141 25 (23–28) |
2141 27 (24–29) |
310 26 (23–29) |
4854 26 (24–29) |
| Body surface area |
N
Median (IQR) |
1646 1.9 (1.8–2.1) |
86 1.8 (1.7–2) |
1530 1.9 (1.8–2.1) |
234 1.9 (1.7–2) |
3496 1.9 (1.8–2.1) |
| Risk factors for coronary artery disease | ||||||
| Type 2 diabetes | n (%) | 404 (16.8) | 41 (25.6) | 556 (25.4) | 115 (32.1) | 1116 (21.9) |
| Hypertension | n (%) | 1438 (59.9) | 104 (65) | 1605 (73.4) | 277 (77.4) | 3424 (67.1) |
| Current smoker | n (%) | 998 (41.5) | 38 (23.8) | 567 (25.9) | 68 (19) | 1671 (32.7) |
| Family history of premature coronary artery disease | n (%) | 633 (26.4) | 35 (21.9) | 644 (29.5) | 77 (21.6) | 1389 (27.3) |
| Dyslipidaemia | n (%) | 1017 (42.3) | 62 (38.8) | 1160 (53.1) | 181 (50.7) | 2420 (47.4) |
| Cardiovascular history | ||||||
| Heart failure | n (%) | 35 (1.5) | 6 (3.8) | 127 (5.9) | 38 (10.8) | 206 (4.1) |
| Prior myocardial infarction | n (%) | 323 (13.5) | 18 (11.2) | 584 (26.8) | 107 (30.1) | 1032 (20.2) |
| Prior PCI | n (%) | 318 (13.3) | 18 (11.2) | 619 (28.4) | 102 (28.6) | 1057 (20.7) |
| Prior CABG | n (%) | 54 (2.3) | 9 (5.6) | 176 (8.1) | 45 (12.7) | 284 (5.6) |
| Prior atrial fibrillation or flutter | n (%) | 110 (4.6) | 21 (13.1) | 236 (10.8) | 77 (21.8) | 444 (8.7) |
| Prior stroke | n (%) | 74 (3.1) | 9 (5.6) | 96 (4.4) | 29 (8.2) | 208 (4.1) |
| Prior TIA | n (%) | 48 (2) | 5 (3.1) | 59 (2.7) | 20 (5.6) | 132 (2.6) |
| Prior PAD | n (%) | 121 (5) | 10 (6.3) | 232 (10.6) | 80 (22.5) | 443 (8.7) |
| Prior ICD or PM implanted | n (%) | 24 (1) | 1 (0.6) | 70 (3.2) | 22 (6.2) | 117 (2.3) |
| Significant known comorbidity | ||||||
| Active cancer | n (%) | 61 (2.5) | 5 (3.1) | 80 (3.7) | 31 (8.8) | 177 (3.5) |
| Anemia | n (%) | 49 (2) | 9 (5.7) | 71 (3.3) | 39 (11.1) | 168 (3.3) |
| Chronic obstructive pulmonary disease | n (%) | 113 (4.7) | 4 (2.5) | 152 (7) | 37 (10.5) | 306 (6) |
| Chronic kidney disease | n (%) | 133 (5.6) | 19 (11.9) | 251 (11.5) | 85 (23.9) | 488 (9.6) |
| Pharmacological therapy prior to enrolment | ||||||
| Aspirin | n (%) | 521 (21.7) | 38 (23.8) | 829 (38) | 151 (42.4) | 1539 (30.2) |
| P2Y12 inhibitors | n (%) | 95 (4) | 8 (5) | 300 (13.8) | 49 (13.8) | 452 (8.9) |
| Oral anticoagulant | n (%) | 82 (3.4) | 13 (8.2) | 172 (7.9) | 58 (16.3) | 325 (6.4) |
| Beta blocker | n (%) | 499 (20.8) | 39 (24.4) | 811 (37.2) | 167 (47) | 1516 (29.8) |
| ACE-I/ARB/ARNI | n (%) | 793 (33.1) | 57 (35.6) | 1108 (50.7) | 170 (47.6) | 2128 (41.8) |
| Steroids | n (%) | 93 (3.9) | 8 (5.1) | 89 (4.1) | 30 (8.5) | 220 (4.3) |
| Loop diuretics | n (%) | 146 (6.1) | 14 (8.8) | 290 (13.3) | 96 (27.1) | 546 (10.7) |
| Statin | n (%) | 338 (20) | 22 (23.7) | 584 (37.8) | 99 (39.3) | 1043 (29.2) |
| PCSK9 inhibitors | n (%) | 1 (0.1) | 0 (0) | 6 (0.4) | 0 (0) | 7 (0.2) |
ACE-I: angiotensin-converting enzyme inhibitor; ARB: angiotensin II receptor blocker antagonist; ARNI: angiontensin receptor neprilysin inhibitor; ACS: acute coronary syndrome; CABG: coronary artery bypass graft; ICD: implantable cardioverter defibrillator; NSTEACS: without ST-elevation acute coronary syndrome; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; PM: pacemaker; STEACS: ST-elevation acute coronary syndrome; TIA: transient ischaemic attack.
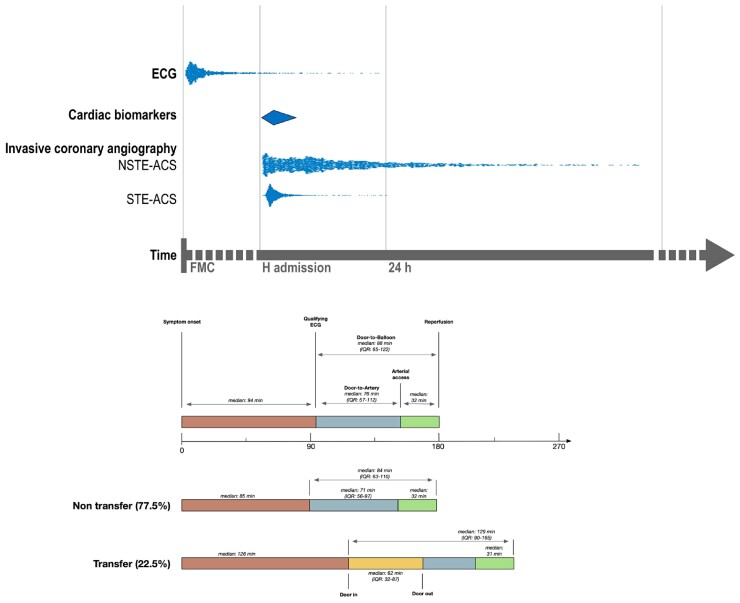
Figure 1.
Timing of principal diagnostic tests in acute coronary syndrome patients managed invasively (S1 and S3) and median delays in patients with ST-elevation acute coronary syndrome intended for urgent reperfusion (S1). Distribution of the timing of qualifying electrocardiogram and the timing of invasive coronary angiography in patients with and without ST elevation were measured and are represented as dots, while the timing of cardiac biomarkers (diamond) was not collected and is hypothetical. The lower part of the figure focuses on median time intervals in patients with ST elevation acute coronary syndrome intended for urgent reperfusion (S1). Door-to-balloon, door-to-artery, and door-in-door-out times are shown according to the type of patients: transfer (from spoke hospitals) or non-transfer. IQR, interquartile range.
Verification and quantification of consecutive inclusion
A strategy that combined administrative ICD-9 CM codes for ICA and for AMI had a 99.5% sensitivity to identify patients admitted with ACS (see Supplementary material online). We estimated that the totality of patients admitted for ACS during the study was 7344 (95% CI: 6852 to 7867) (see Supplementary material online). This means that 69.6% (95% CI 64.9–74.5%) of ACS patients admitted in the participating sites were included, i.e. global consecutive index, which varied from 30.7–79.2% across sites (see Supplementary material online, Table S2).
Implementation of quality indicators
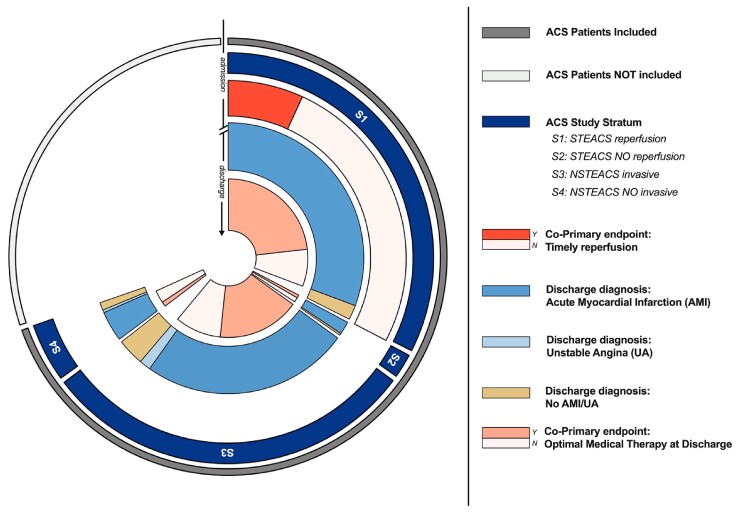
Primary and key secondary quality of care indicators delivered according to corresponding eligible population are illustrated in Figures 2 and 3, which illustrate the proportion of quality indicators per corresponding eligible population from hospital admission (outer circles, patients with ACS) to hospital discharge (inner circles, AMI, unstable angina, or an alternative diagnosis). In patients admitted with STEACS intended for reperfusion (S1, 47% of the enrolled population) the proportion of those that was reperfused timely was 22.4% (95% CI: 20.7–24.1%). In patients discharged alive with a diagnosis of AMI or UA (87% of the enrolled population), 70.1% (95% CI: 68.7–71.4%) received optimal medical therapy and in 87.6% (95%CI: 86.6–88.6%) left ventricular ejection fraction was measured.
Figure 2.
Implementation of primary quality indicators in eligible patients. The figure illustrates the proportion of co-primary quality indicators per corresponding eligible population from hospital admission (outer circles, patients with acute coronary syndrome) to hospital discharge (inner circles, acute myocardial infarction, unstable angina, or an alternative diagnosis) among the totality (universe) of patients with acute coronary syndrome, i.e. both those included and those estimated not to be included. Implementation to timely reperfusion is measured at admission in S1 (ST-elevation acute coronary syndrome patients managed invasively). Adherence to optimal medical therapy is measured in patients who survived hospital discharge with a diagnosis of acute myocardial infarction or unstable angina. Light coloured sectors for co-primary quality indicator (i.e. indicator not reached) quantify opportunities for quality improvements. Abbreviations are listed on the right side.
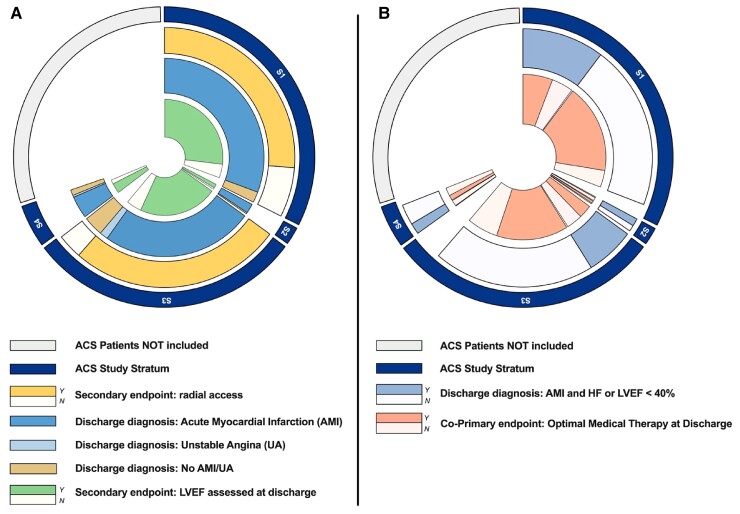
Figure 3.
Implementation of key secondary quality indicators in eligible patients. (A). Implementation of radial access and measurement of left ventricular ejection fraction. Adherence to radial access is measured at admission in acute coronary syndrome patients managed invasively (i.e. S1 and S3). Assessment of left ventricular ejection fraction is measured in patients who survived hospital discharge with a diagnosis of acute myocardial infarction or unstable angina. Light coloured sectors for quality indicator (i.e. indicator not reached) quantify opportunities for quality improvements. Abbreviations are listed on the right side. (B). Implementation of optimal medical therapy stratified by presence of heart failure or left ventricular systolic dysfunction. Optimal medical therapy considered three classes of drugs in patients without heart failure (HF) or left ventricular systolic dysfunction (LVSD), aspirin, adequate P2Y12 inhibition, and high-intensity statin and five classes of drugs (these 3, plus betablockers and angiotensin inhibitors) in patients with HF or LVSD. All therapies were assessed only in patients without reported contraindications. Light coloured sectors for quality indicator (i.e. indicator not reached) quantify opportunities for quality improvements. Abbreviations are listed on the right side.
Optimal medical therapy consisting of aspirin, a P2Y12 inhibitor, a high intensity statin, a beta-blocker, as well as an angiotensin inhibitor was prescribed to 59.2% (95% CI: 55.6–62.8%) of patients with left ventricular systolic dysfunction or heart failure; 75.8% (95% CI: 74.3–77.3%) of patients without left ventricular systolic dysfunction and heart failure (Figure 3B) received aspirin, a P2Y12 inhibitor, and a high intensity statin. In ACS patients managed invasively (with or without ST elevation, S1 and S3; 90% of the total), an initial radial access was attempted in 85.4% (95% CI: 84.4–86.5%) overall; in 81.6% (95% CI: 80–83.1%) of patients with ST elevation (S1) and 89.7% (95% CI: 88.3–90.9%) of patients without ST elevation (S3) (Figure 3A).
Relative and absolute effects of quality indicators
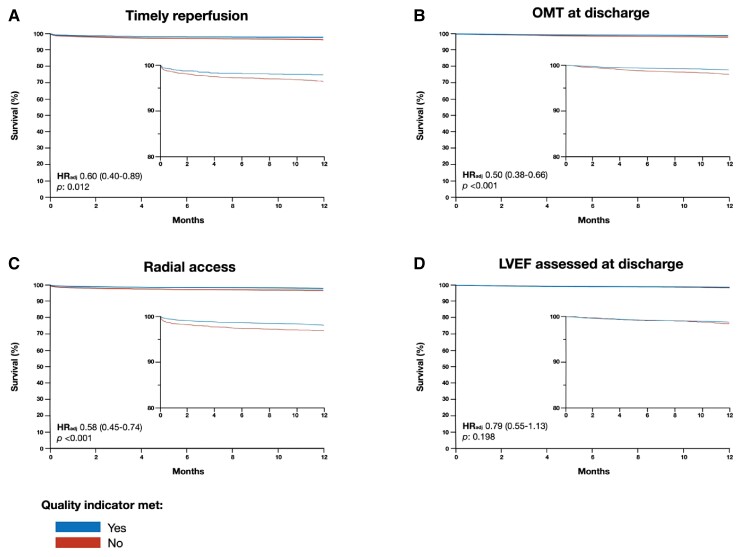
Univariate and multivariable association of primary and key secondary quality indicators with 1-year mortality are showed in Figure 4 and see Supplementary material online, Table S7. Both timely reperfusion and optimal medical therapy demonstrated significant association with 1-year mortality as well as radial access but not left ventricular ejection fraction assessment.
Figure 4.
Adjusted survival curves of primary and key secondary quality indicators at 1-year and corresponding hazard ratios. Adjusted Kaplan–Meier curves for timely reperfusion (A), optimal medical therapy (B), radial access (C), and assessment of left ventricular ejection fraction (D) with corresponding adjusted hazard ratio and 95% confidence intervals.
From the measured quality indicators that were significantly associated with 1-year mortality in multivariable analysis, we estimated number of patients that need to be treated to prevent 1 death at 1 year was 65 (95% CI: 44–250) for timely reperfusion, 98 (95% CI: 79–145) for optimal medical therapy, and 72 (95% CI: 55–119) for radial access (see Supplementary material online, Figure S3).
Discussion
The concept of clinical governance is a vision of the ideal system of care rather than a single testable hypothesis. In the present study we intended to structure this concept by proposing an integrated approach for health care delivery in patients with ACS that aim to universal patients’ inclusion and to identify priorities in the diagnosis and therapy in this population as so to reach the intended aim of clinical governance—promoting an equitable, impactful, and transparent system of care (Graphical Abstract).
The critical observations are the following: (i) a strategy that combine two administrative hospital codes, ICA and AMI, has an excellent sensitivity to identify patients admitted with ACS; (ii) despite standard training and screening procedures across participating hospitals, only 69.5% of patients admitted with ACS were included, and this proportion varied substantially among sites, from 30.7–79.2%; (iii) implementation of quality indicators also varied: it was only 22% for timely reperfusion while radial access exhibited the highest implementation; and (iv) despite this variability, timely reperfusion was associated with a high potential absolute benefit as indicated by the lowest measured number of patients need to be treated to prevent 1 death at 1 year.
Importance and implications of measuring consecutive inclusion
An ideal observational study should include all eligible patients, especially if the primary aim is quality improvement. Measuring and reporting the degree of consecutiveness is also necessary to interpret the generalizability of any observation. In turn, this is an essential requisite to verify the quality care that is achieved in patients with ACS, especially the most vulnerable, such as those managed medically without revascularization (and often admitted outside cardiology wards). Therefore, only if the highest proportion of eligible patients is included, equitable access to care is possible.
Consecutive inclusion is frequently reported but rarely verified. This introduces uncertainties on the external validity of any observation. Selective inclusion may also bias important characteristics of the population, such as the risk of mortality. By analyzing the totality of unplanned admission over one calendar year in the coordinating centre, we observed that a simple strategy combing two hospital codes, a procedure and a diagnosis, was able to identify >99% of patients admitted for ACS. Thus, complimenting local screening strategies with hospital-based administrative data may substantially increase the reach of patients with ACS.
Based on these data, we estimated the enrollment of an average of 69.5% of patients admitted with ACS. Despite standardized training and screening procedures across sites, this estimate varied widely: from 30.7 to 79.2%. This discloses the urgent necessity of measuring and monitoring consecutive inclusion in multicentre studies, especially in quality improvement initiatives and national registries. To this end, the requirement of a written informed consent for participation may be a potential barrier for consecutive inclusion. While restricted only to patients discharged alive, this requirement challenged the inclusion of ACS patients admitted outside cardiology wards, as we documented during a feasibility assessment. Waiving informed consent has been performed successfully in quality-improvement research4,7 and should be systematically considered by investigators and institutional review board to ensure an equitable and broad access of care while respecting the highest ethical standard.8
Relevance of prospective inclusion and impact of quality indicators: a call to define priorities
A further complexity in measuring the quality of ACS care is the dynamic nature of this diagnosis during hospitalization, with implications for study design as well as definition and eligibility of each indicator tested. We observed that one in twelve patients admitted with ACS was discharged with a diagnosis alternative to AMI or UA, the most common being left ventricular apical ballooning syndrome. This highlights the importance of the prospective and probabilistic nature of the diagnosis of ACS as well as the need to diagnose ACS before ICA and regardless of its findings, which may be also important to accurately measure ICA diagnostic yield.9
To optimally measure quality, we pre-defined each tested indicators, including the eligible population. The importance of defining the appropriate population is illustrated by the assessment of left ventricular ejection fraction, which was highly significant associated with mortality when considering all patients with AMI or UA, but not in patients discharged alive. We observed that implementation was extremely low for timely reperfusion in patients with STEACS, with only 22% of eligible patients receiving the intervention. While only applicable to a subgroup of ACS patients (i.e. S1, 47% of the total), this was the indicator with the highest absolute number of residual eligible patients. On the other hand, the implementation of radial access in ACS patients managed invasively (S1 and S3) was the highest implemented indicator (88% of all patients) but still associated with a substantial potential absolute benefit.
Therefore, considering both the absolute number of eligible patients and the absolute expected benefit10 may provide a broader and complimentary perspective of the eligibility, efficacy, and the effectiveness of the indicators tested. Such an approach could inform how to prioritize quality indicators among the several now proposed.11
Limitations
A study limitation may be related to the use of the ICD-9CM coding system for ACS screening and identification. This is the standard administrative coding system in Italy but it is obsolete in several other countries and is not currently recommended by the World Health Organization. While the assumptions for the codes we used are presumably valid with more updated ICD versions, the quantification of sensitivity for ACS diagnosis may need confirmation. Also, the high sensitivity for ACS diagnosis we observed may be reduced with the emerging use of coronary computed tomography angiography for the early diagnosis of patients with suspected ACS. Therefore, non-invasive tests of coronary anatomy may also need to be considered in future ACS screening programme.12
Conclusions
We describe an integrated approach to quality improvement in patients with ACS that verify and quantifies consecutive inclusion and could identify and mitigate across-hospitals variations in access to care, that measure and suggest an approach by priority of multiple indicators of quality for diagnosis and therapy, and that share transparently this information with the ultimate goal to translate evidence to clinical practice.
Author contributions
S.L. wrote the first draft of the manuscript, which was critically revised and checked for consistency by the steering committee members. All remaining authors critically revised the manuscript. S.L. submitted the manuscript for publication on behalf of the authors.
Supplementary material
Supplementary material is available at European Heart Journal: Acute Cardiovascular Care online.
Supplementary Material
Acknowledgements
Editorial support was provided by Ms. Daniela Civardi, Cardiovascular Clinical Research Center, Fondazione IRCCS Policlinico S.Matteo, Pavia.
Rasheed Gazmawi, Filippo Andrea Valenza, Francesco Alfio Russo, Sebastiano Carli, Francesco Matteo Dioniso, Alberto Barengo, Chiara Castelli, Federico Fortuni, Anna Peschiera, Pamela Candito, Marco Scorza, Mauro Acquaro, Rita Camporotondo, Ilaria Costantino, Massimiliano Gnecchi, Stefania Guida, Rossana Totaro, Alessandra Repetto, Marco Ferlini, Alessandro Mandurino Mirizzi, Barbara Marinoni, Maurizio Ferrario, Arianna Elia, Stefano Perlini, GianMarco Secco, Chiara Manzalini, Veronica Lodolini, Elisa Mosele, Filippo Flamigni, Giulia Sammarini, Emanuele Daniello, Roberto Carletti, Elisa Conficoni, Roberto Franco Enrico Pedretti, Tiziana Staine.
Contributor Information
Sergio Leonardi, Department of Molecular Medicine, University of Pavia, Pavia 27100, Italy; Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Claudio Montalto, Department of Molecular Medicine, University of Pavia, Pavia 27100, Italy.
Greta Carrara, Advice Pharma Group, Milan, Italy.
Gianni Casella, U.O.C. Cardiologia, Ospedale Maggiore, Bologna, Italy.
Daniele Grosseto, U.O.C. Cardiologia, Ospedale Infermi, Rimini, Italy.
Marco Galazzi, Department of Molecular Medicine, University of Pavia, Pavia 27100, Italy.
Alessandra Repetto, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Lorenzo Tua, Department of Molecular Medicine, University of Pavia, Pavia 27100, Italy.
Monica Portolan, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Filippo Ottani, U.O.C. Cardiologia, Ospedale G.B. Morgagni, Forlì—Fondazione Cardiologica ‘Myriam Zito Sacco’, Forlì, Italy.
Marcello Galvani, U.O.C. Cardiologia, Ospedale G.B. Morgagni, Forlì—Fondazione Cardiologica ‘Myriam Zito Sacco’, Forlì, Italy.
Leandro Gentile, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Laura Sofia Cardelli, Cardiology Unit, Azienda Ospedaliera Universitaria di Ferrara, Cona, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy.
Stefano De Servi, IRCCS MultiMedica, Milan, Italy; University of Pavia Medical School, Pavia, Italy.
Andrea Antonelli, IRCCS MultiMedica, Milan, Italy.
Gaetano Maria De Ferrari, Città della Salute e della Scienza, Torino.
Luigi Oltrona Visconti, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy.
Gianluca Campo, Cardiology Unit, Azienda Ospedaliera Universitaria di Ferrara, Cona, Italy; Maria Cecilia Hospital, GVM Care & Research, Cotignola, Italy.
ACS Clinical Governance Programme Investigators:
Rasheed Gazmawi, Filippo Andrea Valenza, Francesco Alfio Russo, Sebastiano Carli, Francesco Matteo Dioniso, Alberto Barengo, Chiara Castelli, Federico Fortuni, Anna Peschiera, Pamela Candito, Marco Scorza, Mauro Acquaro, Rita Camporotondo, Ilaria Costantino, Massimiliano Gnecchi, Stefania Guida, Rossana Totaro, Alessandra Repetto, Marco Ferlini, Alessandro Mandurino Mirizzi, Barbara Marinoni, Maurizio Ferrario, Arianna Elia, Stefano Perlini, GianMarco Secco, Chiara Manzalini, Veronica Lodolini, Elisa Mosele, Filippo Flamigni, Giulia Sammarini, Emanuele Daniello, Roberto Carletti, Elisa Conficoni, Roberto Franco Enrico Pedretti, and Tiziana Staine
Funding
The study was sponsored by Fondazione IRCCS Policlinico San Matteo in Pavia, was approved by the institutional review board of each participating hospital and was supported in part by a competitive grant: externally sponsored research of AstraZeneca (ESR-16–12480).
Data availability
The complete set of de-identified participant data collected along with explicative documents, including annotated case report form and study protocol will be shared. Data will be available upon request.
References
- 1. Hunter DJ. The complementarity of public health and medicine - achieving “the highest attainable standard of health”. N Engl J Med 2021;385:481–484. [DOI] [PubMed] [Google Scholar]
- 2. Scally G, Donaldson LJ. Looking forward: clinical governance and the drive for quality improvement in the new NHS in England. BMJ 1998;317:61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leonardi S, Montalto C, Casella G, Grosseto D, Repetto A, Portolan M, Fortuni F, Ottani F, Galvani M, Cardelli LS, De Servi S, Rubboli A, De Ferrari GM, Oltrona Visconti L, Campo G.. Clinical governance programme in patients with acute coronary syndrome: design and methodology of a quality improvement initiative. Open Heart 2020;7:e001415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Peterson ED, Roe MT, Chen AY, Fonarow GC, Lytle BL, Cannon CP, Rumsfeld JS.. The NCDR action registry-GWTG: transforming contemporary acute myocardial infarction clinical care. Heart 2010;96:1798–1802. [DOI] [PubMed] [Google Scholar]
- 5. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 6. Altman DG, Andersen PK. Calculating the number needed to treat for trials where the outcome is time to an event. BMJ 1999;319:1492–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Herrett E, Smeeth L, Walker L, Weston C, MINAP Academic Group. The myocardial ischaemia national audit project (MINAP). Heart 2010;96:1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller FG, Emanuel EJ. Quality-improvement research and informed consent. N Engl J Med 2008;358:765–767. [DOI] [PubMed] [Google Scholar]
- 9. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS.. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schiele F, Aktaa S, Rossello X, Ahrens I, Claeys MJ, Collet JP, Fox KAA, Gale CP, Huber K, Iakobishvili Z, Keys A, Lambrinou E, Leonardi S, Lettino M, Masoudi FA, Price S, Quinn T, Swahn E, Thiele H, Timmis A, Tubaro M, Vrints CJM, Walker D, Bueno H.. 2020. Update of the quality indicators for acute myocardial infarction: a position paper of the association for acute cardiovascular care: the study group for quality indicators from the ACVC and the NSTE-ACS guideline group. Eur Heart J Acute Cardiovasc Care 2021;10:224–233. [DOI] [PubMed] [Google Scholar]
- 12. Litt HI, Gatsonis C, Snyder B, Singh H, Miller CD, Entrikin DW, Leaming JM, Gavin LJ, Pacella CB, Hollander JE.. CT Angiography for safe discharge of patients with possible acute coronary syndromes. N Engl J Med 2012;366:1393–1403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The complete set of de-identified participant data collected along with explicative documents, including annotated case report form and study protocol will be shared. Data will be available upon request.