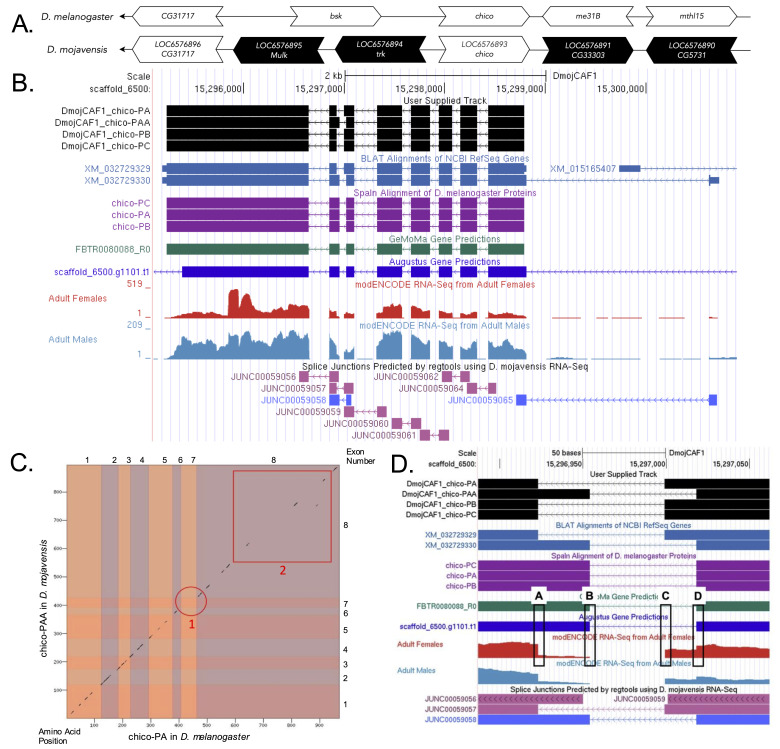
Figure 1.
(A) Synteny comparison of the genomic neighborhoods for chico in Drosophila melanogaster and D. mojavensis . Thin underlying arrows indicate the DNA strand within which the target gene– chico –is located on the negative strand in D. melanogaster (top) and D. mojavensis (bottom). The wide gene arrows pointing in the same direction as chico are on the same strand relative to the thin underlying arrows, while wide gene arrows pointing in the opposite direction of chico are on the opposite strand relative to the thin underlying arrows. White gene arrows in D. mojavensis indicate orthology to the corresponding gene in D. melanogaster , while black gene arrows indicate non-orthology. Gene symbols given in the D. mojavensis gene arrows indicate the orthologous gene in D. melanogaster , while the locus identifiers are specific to D. mojavensis . (B) Gene Model in GEP UCSC Track Data Hub (Raney et al ., 2014). The coding-regions of chico in D. mojavensis are displayed in the User Supplied Track (black); coding exons are depicted by thick rectangles and introns by thin lines with arrows indicating the direction of transcription. Subsequent evidence tracks include BLAT Alignments of NCBI RefSeq Genes (dark blue, alignment of Ref-Seq genes for D. mojavensis ), Spaln of D. melanogaster Proteins (purple, alignment of Ref-Seq proteins from D. melanogaster ), Transcripts and Coding Regions Predicted by TransDecoder (dark green), RNA-Seq from Adult Females and Adult Males (red and light blue, respectively; alignment of Illumina RNA-Seq reads from D. mojavensis ), and Splice Junctions Predicted by regtools using D. mojavensis RNA-Seq (Chen et al. , 2014; SRP006203). Splice junctions shown have a read-depth of 10-49 and 100-499 supporting reads in blue and pink. (C) Dot Plot of chico-PA in D. melanogaster ( x -axis) vs. the new PAA isoform in D. mojavensis ( y -axis). Amino acid number is indicated along the left and bottom; coding-exon number is indicated along the top and right, and exons are also highlighted with alternating colors. The red circle labeled 1, Regions which indicate a lack of similarity between the two sequences are highlighted in red (Region 1; Region 2). (D) Gene model in GEP UCSC displaying RNA-Seq and Splice Junctions data of exons six and seven of the ortholog in D. mojavensis . Boxes A and B indicate RNA-Seq drop-offs, boxes C and D indicate their corresponding splice acceptors and the region in between the boxes represent introns. The increase of RNA-Seq data in exons 6 and 7 and the existence of splice junction JUNC00059058 support that an additional isoform is present in D. mojavensis (chico-PAA).
Description
Introduction The insulin signaling pathway is a highly conserved pathway in animals and is central to nutrient uptake (Hietakangas and Cohen 2009; Grewal 2009). A key component of the pathway, encoding a substrate of the Insulin Receptor (InR) protein, chico binds to the InR and thus is also referred to as the Insulin Receptor Substrate (IRS). The IRS chico is essential to the control of cell and organ size (Goderdhan et al. 1999; Bohni et al. 1999), overall growth (Bakopoulos et al. 2020), including the sex-biased control of cell size and growth (Millington et al. 2021; Kim and O’Connor 2021), and is involved in lifespan (Clancy et al. 2001). We propose a gene model for the D. mojavensis ortholog of the D. melanogaster chico (chico) gene. The genomic region of the ortholog corresponds to the uncharacterized protein LOC6576893 (RefSeq accession XP_032585220.1) in the dmoj_caf1 Genome Assembly of D. mojavensis (GenBank Accession: GCA_000005175.1 - Chen et al. , 2014; SRP006203). This model is based on RNA-Seq data from D. mojavensis (Chen et al., 2014; SRP006203) and chico in D. melanogaster using FlyBase release FB2022_04 (GCA_000001215.4; Larkin et al. , 2021). D. mojavensis is part of the mulleri complex in the repleta species group within the subgenus Drosophila of the genus Drosophila (Wasserman 1992, Durando et al, 2000 ). It was first described by Patterson (Patterson and Crow, 1940). D. mojavensis specializes on rotting cactus as its host and is found in the Mojave and Sonoran Deserts of the southwestern United States and northwestern Mexico including the Baja Peninsula, as well as on the channel-islands off the coast of California (https://www.taxodros.uzh.ch). The Genomics Education Partnership maintains a mirror of the UCSC Genome Browser (Kent WJ et al. , 2002; Gonzalez et al. , 2021), which is available at https://gander.wustl.edu .
Synteny
The target gene, chico, occurs on chromosome 2L in D. melanogaster and is flanked upstream by CG31717 and basket (bsk) and downstream by maternal expression at 31B (me31B) and methuselah-like 15 (mthl15) . The tblastn search of D. melanogaster chico-PA (query) against the D. mojavensis (GenBank Accession: GCA_000005175.1) Genome Assembly (database) placed the putative ortholog of chico within scaffold scaffold_6500 (CH933807.1) at locus LOC6576893 (XP_032585220.1)— with an E-value of 0.0 and a percent identity of 44.97%. Furthermore, the putative ortholog is flanked upstream by LOC6576896 (XP_015020894.1) LOC6576895 (XP_002002875.4) and LOC6576894 (XP_015020893.1), which correspond to CG31717, Multi-substrate lipid kinase (Mulk) and trunk (trk) in D. melanogaster (E-value: 2e-77, 3e-155 and 3e-100; identity: 65.64%, 54.44% and 65.58%, respectively, as determined by blastp ; Figure 1A, Altschul et al. , 1990). The putative ortholog of chico is flanked downstream by LOC6576891 (XP_002002871.1) and LOC6576890 (XP_002002870.1), which correspond to CG33303 and CG5731 in D. melanogaster (E-value: 0.0 and 0.0; identity: 65.78% and 84.56%, respectively, as determined by blastp ). The putative ortholog assignment for chico in D. mojavensis is supported by the following evidence: Although the genes surrounding the chico ortholog are not orthologous to the genes at the same locus in D. melanogaster , we conclude that LOC6576893 is the correct ortholog of chico in D. mojavensis (Figure 1A), supported by strong e-values and percent identities and the lack of other matches while performing the blastp search.
Protein Model
Consistent with the blastp search result which shows 44.97% identity between D. melanogaster chico-PA and the D. mojavensis gene model as well as the low sensitivity parameters used to generate the dot plot (i.e., word size = 3; neighborhood threshold = 11), the dot plot of the two protein sequences contain multiple large gaps along the diagonal. chico in D. mojavensis has 3 identical protein-coding isoforms (chico-PA, chico-PB and chico-PC; Figure 1B). Isoform (chico-PA, chico-PB, chico-PC) contains 8 protein-coding exons. Relative to the ortholog in D. melanogaster , the coding-exon number is conserved. The sequence of chico-PA in D. mojavensis has 44.97% identity (E-value: 0.0) with the protein-coding isoform chico-PA in D. melanogaster , as determined by blastp (Figure 1C). While conducting our research, the presence of a new isoform of chico in D. mojavensis was discovered. The isoform identified as PA D. mojavensis is orthologous to the PA isoform in D. melanogaster and the PAA isoform is the novel isoform. Lower score Splice Junctions data due to lower levels of RNA-Seq data indicate the chico-PAA isoform is expressed at lower levels, or in fewer cells, than the chico-PA isoform (Figure 1D). Coordinates of this curated gene model are stored by NCBI at GenBank/BankIt (accession BK059533). These data are also archived in the CaltechDATA repository (see “Extended Data” section below).
Special characteristics of the protein model
Novel isoform: A new isoform of chico (chico-PAA) has been discovered based on the available RNA-Seq data and Splice Junctions data (Figure 1D). RNA-Seq data drops off in two regions, labeled A and B, and RNA-Seq data increase are displayed in two regions, labeled C and D. Blue and pink data tracks beneath the RNA-Seq data represent Splice Junctions, with pink splice junctions having a higher score than blue splice junctions (minimum read-depth scores found in above image caption of Figure 1A). The existence of splice junction JUNC00059058 as well as the decrease and sharp increase in RNA-Seq data in exons 6 and 7 which overlap the exons of isoform PA indicates an additional isoform (Figure 1D).
Extended Data
Description: FASTA. Resource Type: Model. DOI: 10.22002/n7ehe-xej65
Description: GFF. Resource Type: Model. DOI: 10.22002/26d2v-g8r63
Description: Peptide Sequence. Resource Type: Model. DOI: 10.22002/vbfq6-4z544
Acknowledgments
Acknowledgments
We would like to thank Wilson Leung for developing and maintaining the technological infrastructure that was used to create this gene model, and Madeline Gruys for retrofitting this model.
Funding
This material is based upon work supported by the National Science Foundation (1915544) and the National Institute of General Medical Sciences of the National Institutes of Health (R25GM130517) to the Genomics Education Partnership (GEP; https://thegep.org/; PI-LKR). Any opinions, findings, and conclusions or recommendations expressed in this material are solely those of the author(s) and do not necessarily reflect the official views of the National Science Foundation nor the National Institutes of Health.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bakopoulos D, Beadle LF, Esposito KM, Mirth CK, Warr CG, Johnson TK. Insulin-Like Signalling Influences the Coordination of Larval Hemocyte Number with Body Size in Drosophila melanogaster . . G3 (Bethesda) 2020 Jul 7;10(7):2213–2220. doi: 10.1534/g3.120.401313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böhni R, Riesgo-Escovar J, Oldham S, Brogiolo W, Stocker H, Andruss BF, Beckingham K, Hafen E. Autonomous control of cell and organ size by CHICO, a Drosophila homolog of vertebrate IRS1-4. Cell. 1999 Jun 25;97(7):865–875. doi: 10.1016/s0092-8674(00)80799-0. [DOI] [PubMed] [Google Scholar]
- Chen ZX, Sturgill D, Qu J, Jiang H, Park S, Boley N, Suzuki AM, Fletcher AR, Plachetzki DC, FitzGerald PC, Artieri CG, Atallah J, Barmina O, Brown JB, Blankenburg KP, Clough E, Dasgupta A, Gubbala S, Han Y, Jayaseelan JC, Kalra D, Kim YA, Kovar CL, Lee SL, Li M, Malley JD, Malone JH, Mathew T, Mattiuzzo NR, Munidasa M, Muzny DM, Ongeri F, Perales L, Przytycka TM, Pu LL, Robinson G, Thornton RL, Saada N, Scherer SE, Smith HE, Vinson C, Warner CB, Worley KC, Wu YQ, Zou X, Cherbas P, Kellis M, Eisen MB, Piano F, Kionte K, Fitch DH, Sternberg PW, Cutter AD, Duff MO, Hoskins RA, Graveley BR, Gibbs RA, Bickel PJ, Kopp A, Carninci P, Celniker SE, Oliver B, Richards S. Comparative validation of the D. melanogaster modENCODE transcriptome annotation. Genome Res. 2014 Jul 1;24(7):1209–1223. doi: 10.1101/gr.159384.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy DJ, Gems D, Harshman LG, Oldham S, Stocker H, Hafen E, Leevers SJ, Partridge L. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001 Apr 6;292(5514):104–106. doi: 10.1126/science.1057991. [DOI] [PubMed] [Google Scholar]
- Drosophila 12 Genomes Consortium. Clark AG, Eisen MB, Smith DR, Bergman CM, Oliver B, Markow TA, Kaufman TC, Kellis M, Gelbart W, Iyer VN, Pollard DA, Sackton TB, Larracuente AM, Singh ND, Abad JP, Abt DN, Adryan B, Aguade M, Akashi H, Anderson WW, Aquadro CF, Ardell DH, Arguello R, Artieri CG, Barbash DA, Barker D, Barsanti P, Batterham P, Batzoglou S, Begun D, Bhutkar A, Blanco E, Bosak SA, Bradley RK, Brand AD, Brent MR, Brooks AN, Brown RH, Butlin RK, Caggese C, Calvi BR, Bernardo de Carvalho A, Caspi A, Castrezana S, Celniker SE, Chang JL, Chapple C, Chatterji S, Chinwalla A, Civetta A, Clifton SW, Comeron JM, Costello JC, Coyne JA, Daub J, David RG, Delcher AL, Delehaunty K, Do CB, Ebling H, Edwards K, Eickbush T, Evans JD, Filipski A, Findeiss S, Freyhult E, Fulton L, Fulton R, Garcia AC, Gardiner A, Garfield DA, Garvin BE, Gibson G, Gilbert D, Gnerre S, Godfrey J, Good R, Gotea V, Gravely B, Greenberg AJ, Griffiths-Jones S, Gross S, Guigo R, Gustafson EA, Haerty W, Hahn MW, Halligan DL, Halpern AL, Halter GM, Han MV, Heger A, Hillier L, Hinrichs AS, Holmes I, Hoskins RA, Hubisz MJ, Hultmark D, Huntley MA, Jaffe DB, Jagadeeshan S, Jeck WR, Johnson J, Jones CD, Jordan WC, Karpen GH, Kataoka E, Keightley PD, Kheradpour P, Kirkness EF, Koerich LB, Kristiansen K, Kudrna D, Kulathinal RJ, Kumar S, Kwok R, Lander E, Langley CH, Lapoint R, Lazzaro BP, Lee SJ, Levesque L, Li R, Lin CF, Lin MF, Lindblad-Toh K, Llopart A, Long M, Low L, Lozovsky E, Lu J, Luo M, Machado CA, Makalowski W, Marzo M, Matsuda M, Matzkin L, McAllister B, McBride CS, McKernan B, McKernan K, Mendez-Lago M, Minx P, Mollenhauer MU, Montooth K, Mount SM, Mu X, Myers E, Negre B, Newfeld S, Nielsen R, Noor MA, O'Grady P, Pachter L, Papaceit M, Parisi MJ, Parisi M, Parts L, Pedersen JS, Pesole G, Phillippy AM, Ponting CP, Pop M, Porcelli D, Powell JR, Prohaska S, Pruitt K, Puig M, Quesneville H, Ram KR, Rand D, Rasmussen MD, Reed LK, Reenan R, Reily A, Remington KA, Rieger TT, Ritchie MG, Robin C, Rogers YH, Rohde C, Rozas J, Rubenfield MJ, Ruiz A, Russo S, Salzberg SL, Sanchez-Gracia A, Saranga DJ, Sato H, Schaeffer SW, Schatz MC, Schlenke T, Schwartz R, Segarra C, Singh RS, Sirot L, Sirota M, Sisneros NB, Smith CD, Smith TF, Spieth J, Stage DE, Stark A, Stephan W, Strausberg RL, Strempel S, Sturgill D, Sutton G, Sutton GG, Tao W, Teichmann S, Tobari YN, Tomimura Y, Tsolas JM, Valente VL, Venter E, Venter JC, Vicario S, Vieira FG, Vilella AJ, Villasante A, Walenz B, Wang J, Wasserman M, Watts T, Wilson D, Wilson RK, Wing RA, Wolfner MF, Wong A, Wong GK, Wu CI, Wu G, Yamamoto D, Yang HP, Yang SP, Yorke JA, Yoshida K, Zdobnov E, Zhang P, Zhang Y, Zimin AV, Baldwin J, Abdouelleil A, Abdulkadir J, Abebe A, Abera B, Abreu J, Acer SC, Aftuck L, Alexander A, An P, Anderson E, Anderson S, Arachi H, Azer M, Bachantsang P, Barry A, Bayul T, Berlin A, Bessette D, Bloom T, Blye J, Boguslavskiy L, Bonnet C, Boukhgalter B, Bourzgui I, Brown A, Cahill P, Channer S, Cheshatsang Y, Chuda L, Citroen M, Collymore A, Cooke P, Costello M, D'Aco K, Daza R, De Haan G, DeGray S, DeMaso C, Dhargay N, Dooley K, Dooley E, Doricent M, Dorje P, Dorjee K, Dupes A, Elong R, Falk J, Farina A, Faro S, Ferguson D, Fisher S, Foley CD, Franke A, Friedrich D, Gadbois L, Gearin G, Gearin CR, Giannoukos G, Goode T, Graham J, Grandbois E, Grewal S, Gyaltsen K, Hafez N, Hagos B, Hall J, Henson C, Hollinger A, Honan T, Huard MD, Hughes L, Hurhula B, Husby ME, Kamat A, Kanga B, Kashin S, Khazanovich D, Kisner P, Lance K, Lara M, Lee W, Lennon N, Letendre F, LeVine R, Lipovsky A, Liu X, Liu J, Liu S, Lokyitsang T, Lokyitsang Y, Lubonja R, Lui A, MacDonald P, Magnisalis V, Maru K, Matthews C, McCusker W, McDonough S, Mehta T, Meldrim J, Meneus L, Mihai O, Mihalev A, Mihova T, Mittelman R, Mlenga V, Montmayeur A, Mulrain L, Navidi A, Naylor J, Negash T, Nguyen T, Nguyen N, Nicol R, Norbu C, Norbu N, Novod N, O'Neill B, Osman S, Markiewicz E, Oyono OL, Patti C, Phunkhang P, Pierre F, Priest M, Raghuraman S, Rege F, Reyes R, Rise C, Rogov P, Ross K, Ryan E, Settipalli S, Shea T, Sherpa N, Shi L, Shih D, Sparrow T, Spaulding J, Stalker J, Stange-Thomann N, Stavropoulos S, Stone C, Strader C, Tesfaye S, Thomson T, Thoulutsang Y, Thoulutsang D, Topham K, Topping I, Tsamla T, Vassiliev H, Vo A, Wangchuk T, Wangdi T, Weiand M, Wilkinson J, Wilson A, Yadav S, Young G, Yu Q, Zembek L, Zhong D, Zimmer A, Zwirko Z, Jaffe DB, Alvarez P, Brockman W, Butler J, Chin C, Gnerre S, Grabherr M, Kleber M, Mauceli E, MacCallum I. Evolution of genes and genomes on the Drosophila phylogeny. Nature. 2007 Nov 8;450(7167):203–218. doi: 10.1038/nature06341. [DOI] [PubMed] [Google Scholar]
- Durando CM, Baker RH, Etges WJ, Heed WB, Wasserman M, DeSalle R. Phylogenetic analysis of the repleta species group of the genus Drosophila using multiple sources of characters. Mol Phylogenet Evol. 2000 Aug 1;16(2):296–307. doi: 10.1006/mpev.2000.0824. [DOI] [PubMed] [Google Scholar]
- Goberdhan DC, Paricio N, Goodman EC, Mlodzik M, Wilson C. Drosophila tumor suppressor PTEN controls cell size and number by antagonizing the Chico/PI3-kinase signaling pathway. Genes Dev. 1999 Dec 15;13(24):3244–3258. doi: 10.1101/gad.13.24.3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Gonzalez J, Zweig AS, Speir ML, Schmelter D, Rosenbloom KR, Raney BJ, Powell CC, Nassar LR, Maulding ND, Lee CM, Lee BT, Hinrichs AS, Fyfe AC, Fernandes JD, Diekhans M, Clawson H, Casper J, Benet-Pagès A, Barber GP, Haussler D, Kuhn RM, Haeussler M, Kent WJ. The UCSC Genome Browser database: 2021 update. Nucleic Acids Res. 2021 Jan 8;49(D1):D1046–D1057. doi: 10.1093/nar/gkaa1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewal SS. Insulin/TOR signaling in growth and homeostasis: a view from the fly world. Int J Biochem Cell Biol. 2008 Oct 18;41(5):1006–1010. doi: 10.1016/j.biocel.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Haas BJ, Papanicolaou A, Yassour M, Grabherr M, Blood PD, Bowden J, Couger MB, Eccles D, Li B, Lieber M, MacManes MD, Ott M, Orvis J, Pochet N, Strozzi F, Weeks N, Westerman R, William T, Dewey CN, Henschel R, LeDuc RD, Friedman N, Regev A. De novo transcript sequence reconstruction from RNA-seq using the Trinity platform for reference generation and analysis. Nat Protoc. 2013 Jul 11;8(8):1494–1512. doi: 10.1038/nprot.2013.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- Keilwagen J, Wenk M, Erickson JL, Schattat MH, Grau J, Hartung F. Using intron position conservation for homology-based gene prediction. Nucleic Acids Res. 2016 Feb 17;44(9):e89–e89. doi: 10.1093/nar/gkw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D. The human genome browser at UCSC. Genome Res. 2002 Jun 1;12(6):996–1006. doi: 10.1101/gr.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, O'Connor MB. Drosophila Activin signaling promotes muscle growth through InR/TORC1-dependent and -independent processes. . Development. 2021 Jan 10;148(1) doi: 10.1242/dev.190868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin A, Marygold SJ, Antonazzo G, Attrill H, Dos Santos G, Garapati PV, Goodman JL, Gramates LS, Millburn G, Strelets VB, Tabone CJ, Thurmond J, FlyBase Consortium. FlyBase: updates to the Drosophila melanogaster knowledge base. Nucleic Acids Res. 2021 Jan 8;49(D1):D899–D907. doi: 10.1093/nar/gkaa1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millington JW, Brownrigg GP, Basner-Collins PJ, Sun Z, Rideout EJ. Genetic manipulation of insulin/insulin-like growth factor signaling pathway activity has sex-biased effects on Drosophila body size. G3 (Bethesda) 2021 Mar 16;11(3) doi: 10.1093/g3journal/jkaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro Gonzalez J, Zweig AS, Speir ML, Schmelter D, Rosenbloom KR, Raney BJ, Powell CC, Nassar LR, Maulding ND, Lee CM, Lee BT, Hinrichs AS, Fyfe AC, Fernandes JD, Diekhans M, Clawson H, Casper J, Benet-Pagès A, Barber GP, Haussler D, Kuhn RM, Haeussler M, Kent WJ. The UCSC Genome Browser database: 2021 update. Nucleic Acids Res. 2021 Jan 8;49(D1):D1046–D1057. doi: 10.1093/nar/gkaa1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JT and JF Crow, 1940. Hybridization in the mulleri group of Drosophila. Univ. Texas Publs, 4032, 167-189
- Mahony JB, Brown IR. Characterization of poly(A) sequences in brain RNA. J Neurochem. 1975 Oct 1;25(4):503–507. doi: 10.1111/j.1471-4159.1975.tb04356.x. [DOI] [PubMed] [Google Scholar]
- Raney BJ, Dreszer TR, Barber GP, Clawson H, Fujita PA, Wang T, Nguyen N, Paten B, Zweig AS, Karolchik D, Kent WJ. Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC Genome Browser. Bioinformatics. 2013 Nov 13;30(7):1003–1005. doi: 10.1093/bioinformatics/btt637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rele CP, Sandlin KM, Leung W, Reed LK. (2020). Manual Annotation of Genes within Drosophila Species: the Genomics Education Partnership protocol. bioRxiv 2020.12.10.420521 doi: 10.1101/2020.12.12.420521
- Wasserman, M. (1992). Cytological evolution of the Drosophila repleta species group. Krimbas, Powell, 1992 : 455--552. FBrf0063954