This case series examines the outcomes experienced by pregnant patients prescribed nirmatrelvir and ritonavir to treat SARS-CoV-2 infection.
Key Points
Question
What outcomes are associated with nirmatrelvir and ritonavir for treatment of SARS-CoV-2 infection in pregnant patients?
Findings
In this case series of 47 pregnant patients who were treated with nirmatrelvir and ritonavir, the medication was well tolerated without evidence of an increase in complications affecting birthing parents or their offspring. Approximately half of deliveries after treatment with nirmatrelvir and ritonavir were via cesarean delivery.
Meaning
Results of this study suggest that pregnant patients with SARS-CoV-2 infection can be safely treated with nirmatrelvir and ritonavir.
Abstract
Importance
Pregnant people are at increased risk of poor outcomes due to infection with SARS-CoV-2, and there are limited therapeutic options available.
Objective
To evaluate the clinical outcomes associated with nirmatrelvir and ritonavir used to treat SARS-CoV-2 infection in pregnant patients.
Design, Setting, and Participants
This case series included pregnant patients who were diagnosed with SARS-CoV-2 infection, received nirmatrelvir and ritonavir, and delivered their offspring within the Johns Hopkins Health System between December 22, 2021, and August 20, 2022.
Exposures
Treatment with nirmatrelvir and ritonavir for SARS-CoV-2 infection during pregnancy.
Main Outcomes and Measures
Clinical characteristics and outcomes were ascertained through manual record review.
Results
Forty-seven pregnant patients (median [range] age, 34 [22-43] years) were included in the study, and the median (range) gestational age of their offspring was 28.4 (4.3-39.6) weeks. Medication was initiated at a median (range) of 1 (0-5) day after symptom onset, and only 2 patients [4.3%] did not complete the course of therapy because of adverse effects. Thirty patients (63.8%) treated with nirmatrelvir and ritonavir had a comorbidity in addition to pregnancy that could be a risk factor for developing severe COVID-19. Twenty-five patients [53.2%] delivered after treatment with nirmatrelvir and ritonavir. Twelve of these patients [48.0%] underwent cesarean delivery, 9 [75.0%] of which were scheduled. Two of 47 patients [4.3%] were hospitalized for conditions related to preexisting comorbidities.
Conclusions and Relevance
In this case series, pregnant patients who were treated with nirmatrelvir and ritonavir tolerated treatment well, although there was an unexpectedly high rate of cesarean deliveries. The lack of an increase in serious adverse effects affecting pregnant patients or offspring suggests that clinicians can use this drug combination to treat pregnant patients with SARS-CoV-2 infection.
Introduction
COVID-19 is associated with considerable morbidity and mortality, particularly among pregnant patients, which can affect the health of the developing fetus.1 Pregnant patients infected with SARS-CoV-2 have higher rates of severe COVID-19, enhanced immune response, higher preterm birth rates, and increased abnormal maternal vessels and thrombi in placental tissue compared with those without SARS-CoV-2 infection.2,3,4,5 Given the continued community transmission of SARS-CoV-2 despite vaccines being available, infection with SARS-CoV-2 will continue to affect pregnant patients, and treatment decisions pose challenges for maternal and fetal health clinicians.
The nirmatrelvir and ritonavir drug combination was granted an emergency use authorization by the US Food and Drug Administration (FDA) on December 22, 2021, for the treatment of mild to moderate COVID-19 in patients aged 12 years and older who are at high risk of progression to severe COVID-19.6 The drug is effective, acting as an inhibitor of the SARS-COV-2 main protease.7 The FDA states that there is inadequate knowledge of the effects of nirmatrelvir and ritonavir on the birthing parent and fetus to make a statement on its safety in pregnancy.6 Safety data for nirmatrelvir in rats and rabbits did not show either developmental toxic effects or detrimental effects on fertility.8 Data from the FDA show no increase in the risk of birth defects in pregnant individuals taking ritonavir.9
Nirmatrelvir and ritonavir showed no significant reduction in hospitalization and deaths in a subgroup analysis of patients with at least 1 risk factor for developing severe COVID-19.10 While there has been widespread adoption of nirmatrelvir and ritonavir as an oral option for outpatient treatment of SARS-CoV-2 infection, use of this drug combination in pregnancy is not well studied and may not be adequately used by patients and their clinicians due to lack of data in pregnant patients.
More than 130 000 new SARS-CoV-2 infections were reported daily in the United States as of August 2022.11 The safety of vaccination against SARS-CoV-2 for pregnant individuals has been reported previously; rates of vaccine uptake among pregnant people, however, is lower than expected, and vaccinated individuals remain at risk of SARS-COV-2 infection, particularly with variants of concern, despite vaccination.12,13,14,15 There are multiple effective therapies for outpatient treatment of acute SARS-CoV-2, including remdesivir, convalescent plasma, molnupiravir, and monoclonal antibodies. Yet each of these options has limitations. Remdesivir, monoclonal antibodies, and convalescent plasma must be administered intravenously. Molnupiravir is an oral treatment for COVID-19 treatment; its mechanism of action, however, introduces errors into RNA replication, and it is contraindicated in pregnancy due to harm to the developing fetus.16 The aim of the study was to evaluate outcomes in pregnant patients who were treated with nirmatrelvir and ritonavir within a large hospital system to assess the outcomes associated with this drug combination to treat SARS-CoV-2 infection in this patient population.
Methods
This case series used the COVID-19 Precision Medicine Analytics Platform Registry (JH-CROWN), a database that includes outpatient and inpatient records for patients with SARS-CoV-2 within the Johns Hopkins Health System. The health system is located in Maryland and Washington, DC, and includes 6 hospitals and more than 40 outpatient facilities. The research was approved by the Core for Clinical Research Data Acquisition, which administers the JH-CROWN registry, and the Johns Hopkins Institutional Review Board, which waived the requirement for informed consent because only deidentified data were used. We followed the reporting guideline for case series in medicine.
Pregnant patients with a positive SARS-COV-2 test result within the Johns Hopkins Health System after FDA emergency use approval of nirmatrelvir and ritonavir from December 22, 2021, to August 20, 2022, were eligible for study inclusion. A query was written to identify patients with a diagnosis of pregnancy, a positive test result for SARS-CoV-2 between these dates, and a prescription for nirmatrelvir and ritonavir. Manual medical record review was performed on all medical records of patients who met these 3 criteria. Records were excluded if the patient was not pregnant, if there was no documentation of receipt of nirmatrelvir and ritonavir, or if records were duplicates. The medical records were reviewed at the time of the prescription, and subsequent notes were reviewed to assess patient outcomes and tolerance. Individual characteristics, including maternal age, gestational age, practitioner type, prepregnancy body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), gravidity and parity, vaccination status, prepregnancy comorbidities, adverse effects after administration of nirmatrelvir and ritonavir, delivery date, and delivery complications, were collected using REDCap, version 12.0.16 (Vanderbilt University).17,18 Race and ethnicity were classified by self-report in the electronic medical record and were collected to characterize the differences in socioeconomic factors associated with access to care. Age was defined as the age when the patient was treated with nirmatrelvir and ritonavir. Vaccination status was defined as having had no vaccination, having received the initial vaccination series, or having received the initial vaccination series with 1 or 2 additional boosters. Comorbidities were collected from the Epic Systems electronic health record problem list on initiation of antenatal care. We reviewed the medical record for specific comorbidities based on the Centers for Disease Control and Prevention (CDC) and Infectious Diseases Society of America (IDSA) indications for outpatient treatment with nirmatrelvir and ritonavir including patient age 65 years or older, chronic kidney disease, diabetes, BMI of 25 or greater, chronic lung disease, sickle cell disease, cardiovascular disease or hypertension, use of immunosuppressing medications, neurodevelopmental disorders, and medical technological dependence.19
Results
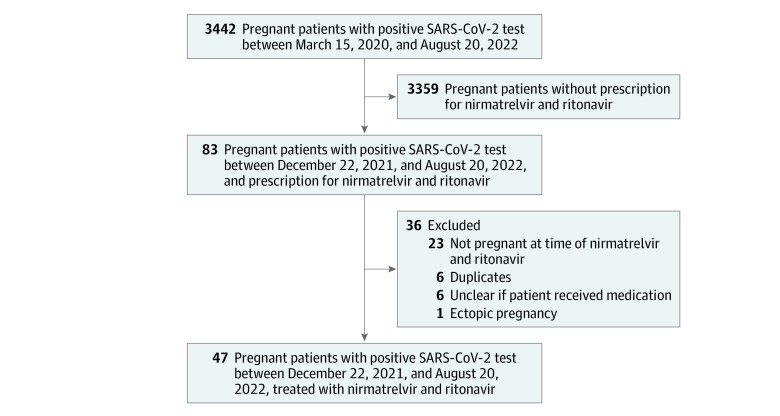
Forty-seven patients (median [range] age, 34 [22-43] years) were pregnant at the time they received nirmatrelvir and ritonavir from December 22, 2021, to August 20, 2022 (Figure), and the median (range) gestational age of their offspring was 28.4 (4.3-39.6) weeks. The median (range) time from symptom onset to receipt of medication was 1 (0-5) day. The baseline characteristics of pregnant patients receiving nirmatrelvir and ritonavir are presented in Table 1.
Figure. Cohort Flowchart.
Table 1. Baseline Characteristics of Pregnant Patients Receiving Nirmatrelvir and Ritonavir .
| Characteristic | Patients, No. (%) (n = 47) |
|---|---|
| Age, median (range), y | 34 (22-43) |
| Race | |
| Asian | 4 (8.5) |
| Black or African American | 8 (17.0) |
| White | 31 (66.0) |
| Othera | 2 (4.3) |
| Unknown | 2 (4.3) |
| Ethnicity | |
| Hispanic or Latino | 5 (10.6) |
| Not Hispanic or Latino | 41 (87.2) |
| Unknown | 1 (2.1) |
| Gestational age at treatment, median (range), wk | 28.4 (4.3-39.6) |
| Trimester of pregnancy at treatment | |
| First | 4 (8.5) |
| Second | 16 (34.0) |
| Third | 27 (57.4) |
| Prepregnancy BMI (n = 46)b | |
| <18.5 | 0 |
| 18.5-24.9 | 22 (47.8) |
| 25.0-29.9 | 14 (30.4) |
| ≥30.0 | 10 (21.7) |
| Gravidity | |
| 1 | 8 (17.0) |
| 2 | 14 (29.8) |
| 3 | 8 (17.0) |
| >3 | 17 (36.2) |
| Parity | |
| 0 | 16 (34.0) |
| 1 | 21 (44.7) |
| 2 | 5 (10.6) |
| >2 | 5 (10.6) |
| Prepregnancy medical comorbidities | |
| Mental health disorder | 21 (44.7) |
| Abnormal Papanicolaou test | 13 (27.7) |
| Obesity (BMI ≥30) | 12 (25.5) |
| Anemia | 12 (25.5) |
| Asthma | 11 (23.4) |
| Gastrointestinal disorderc | 8 (17.0) |
| Otherd | 6 (12.8) |
| Diabetes | 5 (10.6) |
| Substance use | 5 (10.6) |
| Thyroid disorder | 5 (10.6) |
| Venous thromboembolism | 3 (6.4) |
| Hypertension | 3 (6.4) |
| Hyperlipidemia | 2 (4.3) |
| Cardiac arrhythmia | 2 (4.3) |
| Inflammatory bowel disease | 2 (4.3) |
| Sickle cell disease | 1 (2.1) |
| Preeclampsia | 1 (2.1) |
| Antiphospholipid syndrome | 1 (2.1) |
| Indications for receipt of nirmatrelvir and ritonavire | |
| BMI ≥25 | 24 (51.1) |
| Pregnancy alone | 17 (36.2) |
| Chronic lung disease | 10 (21.3) |
| Diabetes | 5 (10.6) |
| Cardiovascular disease or hypertension | 4 (8.5) |
| Sickle cell disease | 1 (2.1) |
| COVID-19 vaccination status | |
| No vaccination | 7 (14.9) |
| Received initial vaccination series | 16 (34.0) |
| Received initial vaccination series and 1 booster | 21 (44.7) |
| Received initial vaccination series and 2 boosters | 3 (6.4) |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
No additional information was available in the data about the other races.
Data were missing for 1 patient.
Gastrointestinal disorders include gastroesophageal reflux disease, gastritis, nausea, and irritable bowel syndrome.
Other prepregnancy medical comorbidities include carotid artery dissection, endocarditis, vertebral dissection, thymoma, postural orthostatic tachycardia syndrome, and idiopathic thrombocytopenic purpura.
Based on guidelines from the US Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Twenty-seven patients (57.4%) received nirmatrelvir and ritonavir in the third trimester of pregnancy, 16 (34.0%) in the second trimester, and 4 (8.5%) in the first trimester. Forty patients (85.1%) had received some vaccination: 16 (34.0%) had received the initial vaccination series, 21 (44.7%) had received the initial vaccination series and 1 booster, and 3 (6.4%) had received the initial vaccination series and 2 boosters. Forty-three patients (91.5%) had any comorbidity, with mental health disorder (21 [44.7%]) being the most commonly reported. Thirty patients (63.8%) had a comorbidity other than pregnancy that qualified as a CDC and IDSA indication for outpatient treatment with nirmatrelvir and ritonavir, with the most common indication being a BMI of 25 or greater (24 [51.1%]).
All patients were either treated for COVID-19 as outpatients or were treated while hospitalized for reasons other than COVID-19. Thirty-seven prescriptions (78.7%) were written by obstetricians and gynecologists, and 10 (21.3%) were written by midlevel practitioners (Table 2). Twelve prescriptions (25.5%) were filled in May 2022, 17 (36.2%) in June 2022, 12 (25.5%) in July 2022, and 6 (12.8%) in August 2022. Viral sequencing data were available for 5 patients (10.6%) whose testing was performed within the Johns Hopkins Health System. Four of the samples (80.0%) were identified as Omicron (BA.4, BA.2, BA.5, unassigned) and 1 (20%) as Delta (21I).
Table 2. Patient Outcomes.
| Characteristic | Patients, No. (%) (n = 47) |
|---|---|
| Type of practitioner ordering nirmatrelvir and ritonavir | |
| Obstetrician and gynecologist | 37 (78.7) |
| Midlevel practitioner | 10 (21.3) |
| Time from symptom to receipt of medication, median (range), d | 1 (0-5) |
| Month of nirmatrelvir and ritonavir prescription | |
| December-April | 0 |
| May | 12 (25.5) |
| June | 17 (36.2) |
| July | 12 (25.5) |
| August | 6 (12.8) |
| Patient discontinued medication due to adverse effects | 2 (4.3) |
| Patient hospitalized after taking nirmatrelvir and ritonavir | 2 (4.3) |
| Patient delivered after taking nirmatrelvir and ritonavir | 25 (53.2) |
Two patients (4.3%) were hospitalized during pregnancy after treatment with nirmatrelvir and ritonavir: 1 for vomiting and dehydration in the setting of preexisting hyperemesis gravidarum and 1 for persistent cough in the context of sickle cell crisis; this patient was identified as having placenta accreta and experienced postpartum hemorrhage. Two patients (4.3%) discontinued the medication before completing treatment due to adverse effects. Three patients (6.4%) developed gestational hypertension. One patient (2.1%) developed excessive fetal growth and polyhydramnios. One patient (2.1%) developed oligohydramnios. One patient (2.1%) with a history of bicornuate uterus and multiple children with genetic disorders experienced the loss of a twin pregnancy at 12 weeks. A fetus that was the product of the pregnant patient’s in-vitro fertilization at age 40 years was noted to have absent ductus venosus as well as thickened nuchal fold. One fetus developed intrauterine growth restriction and syndactyly and was noted to have cystic hygroma before nirmatrelvir and ritonavir exposure. Twenty-five patients (53.2%) delivered after treatment with nirmatrelvir and ritonavir. Patient outcomes are summarized in Table 2.
Twelve of the 25 patients (48.0%) who delivered after treatment underwent cesarean delivery; 9 of these (75.0%) were scheduled. One unplanned cesarean delivery was performed during the birth of twins in which arrest of labor occurred after the first vaginal birth, 1 was in the context of fetal intolerance of labor, and 1 was in the context of oligohydramnios and breech presentation. Delivery outcomes are summarized in Table 3.
Table 3. Characteristics of Deliveries After Patient Receipt of Nirmatrelvir and Ritonavir.
| Maternal age, y | Gravidity | Parity | Trimester patient received nirmatrelvir and ritonavir | Vaccination status | Comorbiditiesa | Risk factors other than pregnancy for developing severe COVID-19b | BMI | Cesarean delivery | Indication for cesarean delivery | Newborn birth weight, g |
|---|---|---|---|---|---|---|---|---|---|---|
| 34 | 2 | 0 | 3 | Vaccinated | Preeclampsia, abnormal Papanicolaou test | BMI ≥25 | 25.7 | No | NA | 3620 |
| 30 | 4 | 2 | 3 | Not vaccinated | Asthma, mental health disorder, obesity, substance use | BMI ≥25, chronic lung disease | 31.7 | Yes | Scheduled repeat cesarean delivery | 3150 |
| 27 | >5 | 2 | 3 | Vaccinated | Mental health disorder, obesity | BMI ≥25 | 30.7 | No | NA | 3435 |
| 33 | 4 | 1 | 3 | Vaccinated with 1 booster | Diabetes, thyroid disorder | BMI ≥25, diabetes | 28.1 | Yes | History of breech presentation | 3270 |
| 43 | >5 | 2 | 3 | Vaccinated | Mental health disorder, obesity, history of venous thromboembolism, anemia | BMI ≥25 | 44.3 | Yes | History of breech presentation | 3657 |
| 22 | 2 | 1 | 3 | Vaccinated | Anemia | None | 24.8 | No | NA | 3910 |
| 39 | >5 | 0 | 3 | Vaccinated with 1 booster | Mental health disorder, sickle cell disease, anemia | Sickle cell disease | 21.6 | Yes | Planned due to placenta accreta | 2410 |
| 33 | 1 | 0 | 2 | Vaccinated with 1 booster | Obesity | BMI ≥25 | 40.4 | No | NA | 3080 |
| 39 | 2 | 1 | 2 | Vaccinated with 1 booster | History of venous thromboembolism, abnormal Papanicolaou test, antiphospholipid syndrome | None | 24.1 | No | NA | 3070 |
| 41 | 1 | 1 | 3 | Vaccinated with 1 booster | Diabetes, abnormal Papanicolaou test | BMI ≥25, diabetes | 27.9 | Yes | Elective primary cesarean delivery | 3033 |
| 38 | 5 | 4 | 3 | Vaccinated with 1 booster | Anemia | None | 23.7 | No | NA | 2940 |
| 36 | 5 | 3 | 3 | Vaccinated | Diabetes, obesity | BMI ≥25, diabetes | 37.5 | Yes | Scheduled cesarean delivery | 3770 |
| 30 | >5 | 1 | 3 | Not vaccinated | Mental health disorder, cardiac arrhythmia, abnormal Papanicolaou test, anemia | Cardiovascular disease | 19.6 | No | NA | 3670 |
| 30 | 1 | 0 | 3 | Vaccinated | Hyperlipidemia, obesity, anemia | BMI ≥25 | 30.4 | No | NA | 2540 |
| 34 | 4 | 1 | 3 | Vaccinated with 1 booster | Asthma, abnormal Papanicolaou test, anemia | BMI ≥25, chronic lung disease | 26.6 | Yes | Scheduled repeat cesarean delivery | 2840 |
| 35 | 3 | 0 | 3 | Vaccinated with 1 booster | Anemia | None | 22.9 | Yes | Fetal intolerance of labor | 3440 |
| 30 | 2 | 1 | 3 | Vaccinated | Abnormal Papanicolaou test, anemia, carotid artery dissection | None | 23.1 | No | NA | 3290 |
| 27 | 2 | 1 | 3 | Not vaccinated | Asthma | BMI ≥25, chronic lung disease | 25.4 | Yes | Cesarean delivery due to arrest of labor | 3000 |
| 33 | 2 | 1 | 3 | Vaccinated with 1 booster | Mental health disorder | None | 19.5 | No | NA | 3300 |
| 26 | 5 | 4 | 3 | Vaccinated | Asthma, mental health disorder, abnormal Papanicolaou test, anemia | BMI ≥25, chronic lung disease | 26.7 | Yes | Scheduled repeat cesarean delivery | 2190 |
| 38 | 2 | 1 | 3 | Vaccinated with 2 boosters | Diabetes, inflammatory bowel disease | Diabetes | 24.9 | Yes | Scheduled repeat cesarean delivery | 4650 |
| 34 | >5 | 1 | 3 | Not vaccinated | Mental health disorder, substance use disorder, endocarditis | Cardiovascular disease | 24.0 | Yes | Oligohydramnios, breech presentation | 3350 |
| 30 | 1 | 0 | 3 | Not vaccinated | Asthma, mental health disorder, inflammatory bowel disease | Chronic lung disease | 22.6 | No | NA | 3040 |
| 27 | >5 | 2 | 3 | Vaccinated | Mental health disorder, obesity | BMI ≥25 | 26.9 | No | NA | 3220 |
| 39 | 2 | 1 | 3 | Vaccinated | Mental health disorder, abnormal Papanicolaou test | None | 24.3 | No | NA | 3360 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Obesity is defined as having a BMI of 30 or greater.
Based on guidelines from the US Centers for Disease Control and Prevention and the Infectious Diseases Society of America.
Discussion
This study has several important findings. First, the nirmatrelvir and ritonavir combination was well tolerated and did not pose an immediate threat to the birthing parent or fetus in this study. In addition, 63.8% of pregnant patients who received nirmatrelvir and ritonavir were prescribed the drug combination for an indication other than pregnancy, and 78.7% of prescriptions were written by obstetricians and gynecologists. Most patients who received nirmatrelvir and ritonavir were vaccinated, and relatively few Black patients were included in the cohort, suggesting that disparities in vaccine uptake may also be reflected in the use of nirmatrelvir and ritonavir as a therapy in pregnancy.
Only 2 patients discontinued nirmatrelvir and ritonavir due to adverse effects, and no complications were associated with the drug. This finding was replicated in another descriptive study from the University of Connecticut of 7 pregnant patients treated with nirmatrelvir and ritonavir who all experienced symptomatic improvement and no adverse pregnancy outcomes.20 In accordance with our findings, 85.7% of patients in that study were vaccinated before nirmatrelvir and ritonavir use; racial demographic characteristics were not reported. In contrast to our findings, the 3 deliveries in that study were vaginal births. The present study had a relatively high rate of cesarean deliveries in patients who received nirmatrelvir and ritonavir and delivered.
The CDC and IDSA outpatient treatment guidelines include pregnant patients among populations at higher risk for developing severe COVID-19 and who are eligible to use nirmatrelvir and ritonavir.19,21 This recommendation is supported by the Society for Maternal-Fetal Medicine.22 In the present study, prescriptions for pregnant patients did not begin until May 2022 and increased in June 2022. Seventy-nine percent of prescriptions were from obstetricians, which likely reflects the diverse roles of obstetricians and gynecologists in treating their pregnant patients. In the present study, we found no serious adverse effects associated with nirmatrelvir and ritonavir in pregnant patients. Because COVID-19 will continue to present in a variety of settings, it is important for other health care practitioners to use this drug combination in this high-risk population.
Although Johns Hopkins Health System cares for a diverse patient population, the patients in this study were of advanced age, and few were of racial or ethnic minority groups. It was unclear if all pregnant patients with SARS-CoV-2 were offered treatment or if rates of refusal differed by age group. Future studies will need to be conducted to evaluate disparities in receipt of treatment.
Limitations
There are several limitations to this study, including the use of data from a single health system and a small population studied with mild illness. However, to our knowledge, this is the largest study to date in this population and found no serious adverse effects to the patients or their fetuses. The rise of at-home COVID-19 testing and use of registries designed for hospitalized patients means some patients were not included due to unrecorded testing and lack of standardized outpatient evaluation tools. Another limitation was the short follow-up period. Only 25 of 47 patients had undergone delivery at the time of this report. Treatment effects may not be apparent at delivery, and long-term effects of treatment will need to be followed up in both the birthing parents and children.
Conclusions
Findings of this case series support the safety and effectiveness of nirmatrelvir and ritonavir in pregnant patients with acute SARS-CoV-2. Although there are multiple effective therapies for outpatient treatment of acute SARS-CoV-2, all are either intravenous (remdesivir, convalescent plasma, and monoclonal antibodies) or contraindicated in pregnancy (molnupiravir). Nirmatrelvir and ritonavir can be easily used as a first-line outpatient treatment for pregnant patients. Future larger studies will be needed to evaluate for rare complications in neonates or birthing parents who are treated with this medication. The serious risk of morbidity from SARS-CoV-2 among pregnant patients is further justification for practitioners to encourage vaccination and use of nirmatrelvir and ritonavir to minimize the risk to pregnant patients and their fetuses.
References
- 1.Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 2021;175(8):817-826. doi: 10.1001/jamapediatrics.2021.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Narasimhan H, Chudnovets A, Burd I, Pekosz A, Klein SL. Animal models of congenital zika syndrome provide mechanistic insight into viral pathogenesis during pregnancy. PLoS Negl Trop Dis. 2020;14(10):e0008707. doi: 10.1371/journal.pntd.0008707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherer ML, Lei J, Creisher PS, et al. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol. 2021;225(3):301.e1-301.e14. doi: 10.1016/j.ajog.2021.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanes ED, Mithal LB, Otero S, Azad HA, Miller ES, Goldstein JA. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154(1):23-32. doi: 10.1093/ajcp/aqaa089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McClymont E, Albert AY, Alton GD, et al. ; CANCOVID-Preg Team . Association of SARS-CoV-2 infection during pregnancy with maternal and perinatal outcomes. JAMA. 2022;327(20):1983-1991. doi: 10.1001/jama.2022.5906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.US Food and Drug Administration . Fact sheet for healthcare providers: emergency use authorization for Paxlovid. Revised September 26, 2022. Accessed July 13, 2022. https://www.fda.gov/media/155050/download
- 7.Owen DR, Allerton CMN, Anderson AS, et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science. 2021;374(6575):1586-1593. doi: 10.1126/science.abl4784 [DOI] [PubMed] [Google Scholar]
- 8.Catlin NR, Bowman CJ, Campion SN, et al. Reproductive and developmental safety of nirmatrelvir (PF-07321332), an oral SARS-CoV-2 Mpro inhibitor in animal models. Reprod Toxicol. 2022;108:56-61. doi: 10.1016/j.reprotox.2022.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.US Food and Drug Administration . Norvir: full prescribing information. Revised October 2020. Accessed July 6, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/020659s072,022417s024,209512s007lbl.pdf
- 10.Pfizer. Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study. November 5, 2021. Accessed July 6, 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate
- 11.Johns Hopkins University & Medicine . Coronavirus resource center: United States. 2022. Accessed August 21, 2022. https://coronavirus.jhu.edu/region/united-states
- 12.Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327(15):1469-1477. doi: 10.1001/jama.2022.3271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327(15):1478-1487. doi: 10.1001/jama.2022.4255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun H. On preliminary findings of mRNA COVID-19 vaccine safety in pregnant persons. N Engl J Med. 2021;385(16):1535-1536. doi: 10.1056/NEJMc2113516 [DOI] [PubMed] [Google Scholar]
- 15.Galanis P, Vraka I, Siskou O, Konstantakopoulou O, Katsiroumpa A, Kaitelidou D. Uptake of COVID-19 vaccines among pregnant women: a systematic review and meta-analysis. Vaccines (Basel). 2022;10(5):766. doi: 10.3390/vaccines10050766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabinger F, Stiller C, Schmitzová J, et al. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat Struct Mol Biol. 2021;28(9):740-746. doi: 10.1038/s41594-021-00651-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium . The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Infectious Diseases Society of America . COVID-19 outpatient treatment guidelines roadmap. Updated June 21, 2022. Accessed July 11, 2022. https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/covid-19-outpatient-treatment--guidelines-roadmap/
- 20.Loza A, Farias R, Gavin N, Wagner R, Hammer E, Shields A. Short-term pregnancy outcomes after nirmatrelvir–ritonavir treatment for mild-to-moderate coronavirus disease 2019 (COVID-19). Obstet Gynecol. 2022;140(3):447-449. doi: 10.1097/AOG.0000000000004900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.US Centers for Disease Control and Prevention . Underlying medical conditions associated with higher risk for severe COVID-19: information for healthcare professionals. Updated June 15, 2022. Accessed July 11, 2022. https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html
- 22.Society for Maternal-Fetal Medicine . FDA issues EUA for the treatment of mild-to-moderate COVID-19: maternal-fetal medicine subspecialists support use in pregnant patients. News release published December 22, 2021. Accessed July 11, 2022. https://s3.amazonaws.com/cdn.smfm.org/media/3287/Treatment_1.10.pdf