Abstract
Ancient, species-poor lineages persistently occur across the Tree of life. These lineages are likely to contain unrecognized species diversity masked by the low rates of morphological evolution that characterize living fossils. Halecomorphi is a lineage of ray-finned fishes that diverged from its closest relatives before 200 Ma and is represented by only one living species in eastern North America, the bowfin, Amia calva Linnaeus. Here, we use double digest restriction-site-associated DNA sequencing and morphology to illuminate recent speciation in bowfins. Our results support the delimitation of a second living species of Amia, with the timing of diversification dating to the Plio-Pleistocene. This delimitation expands the species diversity of an ancient lineage that is integral to studies of vertebrate genomics and development, yet is facing growing conservation threats driven by the caviar fishery.
Keywords: bowfin, ddRAD, phylogenetics, biogeography, species delimitation
1. Introduction
The bowfin, Amia calva, is the sole living representative of Halecomorphi, an ancient lineage of ray-finned fishes with a cosmopolitan distribution in the fossil record that is classically labelled as a living fossil clade [1–5]. Bowfin and the seven species of gars (Lepisosteidae) compose the ancient and species-depauperate Holostei, which is the sister lineage of the hyper-diverse Teleostei [4,6,7]. Together with sturgeons, the paddlefish, and mooneyes, bowfin and gars contribute to a hotspot of ancient vertebrate biodiversity in the species-rich temperate freshwater fish fauna of eastern North America [1,2,6,8–11].
Because of its evolutionary history, the bowfin is important for understanding genomic, developmental, and immunological evolution in vertebrates [1,4,7]. The bowfin is also notable for its apparently low rates of molecular evolution and phenotypic similarity to species from the fossil record dated to more than 145 Ma [1,2,4]. In addition, the economic significance of bowfin is increasing with an intensifying demand for sources of caviar [12], putting pressure on extant populations already strained by the centuries-long reputation of Amia as a ‘rough fish’ [13].
The bowfin has a wide geographical distribution across eastern North America (figure 1a) [2,14–16]. Among the species-rich fauna of North American freshwater fishes [17–19], relatively few species have geographical distributions as large as the bowfin. This wide geographical range includes many areas characterized by both high species diversity and a sizeable number of endemic freshwater fish species [20–23], enhancing the possibility of additional species diversity masquerading as Amia calva [24].
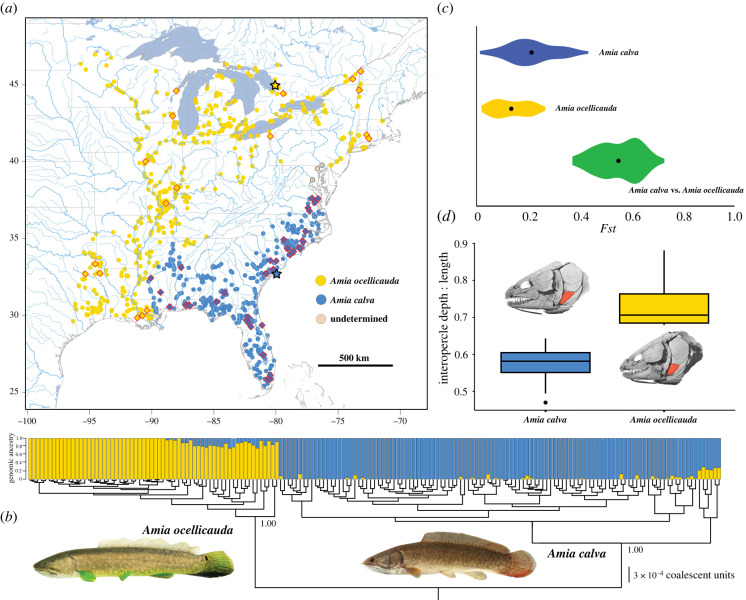
Figure 1.
Identification of hidden bowfin species diversity. (a) Map of eastern North America showing museum specimen collection records of Amia calva (blue), Amia ocellicauda (yellow) and undetermined (tan), retrieved from fishnet2.net. Stars indicate type localities. Diamonds indicate specimens sampled in the ddRAD phylogenetic analysis. (b) Phylogeny and genomic structure analysis of 177 specimens of Amia based on 56 247 ddRAD loci. Photograph of A. ocellicauda from lower Tennessee River, Marshall County, Alabama, USA, YPM 035200, by J.M.M. and Amia calva from the Suwanee River, Gilchrist County, Florida, USA, UF 238466, by Z. Randall. (c) The comparison of pairwise Fst values for comparisons within A. calva (blue) and A. ocellicauda (yellow), and the comparisons between A. calva and A. ocellicauda (green). (d) Boxplot showing IO robusticity (ratio between maximum dorsoventral depth and maximum anteroposterior length) in A. calva and A. ocellicauda. CT-scanned skull of A. calva, TU 22613; CT-scanned skull of A. ocellicauda, TU 118772.
The taxonomic history of the bowfin provides another indication for the possibility of additional species in this clade. Amia calva was described by Linnaeus in 1766 [25] and in a period of 34 years between 1836 and 1870 [26–29], 12 species of Amia were described. At the close of the nineteenth century, all of these additional species were synonymized with A. calva without justification or reference to a study of variation among the named taxa [30, p. 113]. The only studies that explore genetic variation in A. calva sample a small part of the geographical distribution [24,31], and there is no study exploring variation in morphological traits among populations of A. calva across its entire geographical distribution [2,32] (figure 1a).
In this study, we analyse double-digest restriction-site-associated DNA (ddRAD) sequences from bowfin specimens sampled across their geographical distribution, using phylogenetic and population genetic techniques to look for the presence of distinct lineages (figure 1b). We also assess morphological variation in cranial features visualized with high-resolution computed tomography (CT) scans of Amia skulls and meristic trait data. The analysis of the genomic and phenotypic data is applied towards a delimitation of currently hidden species diversity in the sole living branch of the Halecomorphi.
2. Methods
(a) . Specimen sampling
Bowfin specimens were sampled over the course of several field seasons. Tissue samples were stored in 99% ethanol. Morphological voucher specimens were euthanized, fixed in an aqueous solution of formaldehyde for up to 21 days, soaked in tap water for up to 7 days, and transferred to 70% ethanol for long-term preservation in the ichthyology collection in the Yale Peabody Museum. Tissue samples were also obtained from museum collections. The sampling locations and museum collection records (if applicable) of all specimens used in the phylogenomic and morphological analyses are available on Dryad at http://doi.org/10.5061/dryad.8pk0p2nrf.
(b) . Generation of double-digest restriction-site-associated DNA loci
We extracted genomic DNA using a Qiagen DNeasy Tissue Extraction Kit (Qiagen, Valencia, CA, USA), following the manufacturer's protocols. The preparation of ddRAD libraries followed standard protocols [33], starting with approximately 400 ng of DNA from each specimen, and using PstI/MspI restriction enzymes. Size-selected libraries were sequenced using 100 bp single-end sequencing on an Illumina NovaSeq 6000 at the University of Oregon GC3F facility (http://gc3f.uoregon.edu). The demultiplexed reads were run through ipyrad v.0.9.68 [34], using default settings with the following exceptions: ‘reference’ for assembly method, ‘ddrad’ for datatype, ‘TGCAG, CCG’ for restriction overhang, ‘0.90’ for clustering threshold and ‘2’ for a stricter adapter filtration. The minimum number of specimens sharing a locus, hereafter referred to as ‘min’, was set to smallest numbers (184 and 75, see below) to reach a stationarity of loci dropout rate. ddRAD loci were aligned using the A. calva genome [4]. The raw sequence files for the ddRAD loci are available at Genbank (BioProject ID PRJNA868817).
(c) . Phylogenomic and population genomic analyses
The phylogenetic relationships among 177 sampled specimens of Amia were inferred from a concatenated DNA sequence dataset of the ddRAD loci. A posterior set of phylogenetic trees was generated using BEAST 2.6.4 [35] with a coalescent constant population size branching model, a GTR molecular evolutionary model with a gamma distribution of among-site rate variation, and a strict molecular clock model with a clock rate of 1.0. BEAST was run for 1.0 × 108 generations and log and tree files were updated every 1.0 × 104 generations. The convergence of parameter values was assessed by the effective sample sizes that were calculated using Tracer v.1.7 [36]. Generations sampled before convergence was attained were discarded as burn-in. The BEAST analyses were run three separate times and post-burn-in generations were pooled from all three runs using LogCombiner 2.8 [35]. A maximum clade credibility tree with median node heights was constructed for the post-burn-in species tree topologies using TreeAnnotator 2.6.4 [35].
Genomic differences at a subsample of 26 305 single nucleotide polymorphisms (SNPs) among populations were visualized in a principal components (PCs) analysis implemented in iPyrad v.0.9.68 [34]. The missing portion of the dataset was filled by a sample imputation method using the function ‘impute_method=sample’. Relative genomic ancestry was assessed using the ‘snmf’ function implemented in the R package LEA v.3.0.0 [37]. With the R package hierfstat [38], we estimated the fixation index (Fst) for all pairs of specimens among the 177 sampled individuals.
(d) . Estimation of divergence times among living species of Amia
The divergence time of the two delimited species of Amia was estimated using a fossil tip dating strategy and the fossilized birth–death (FBD) branching model in BEAST 2.6.4 [35,39]. A total of 699 orthologous ddRAD loci were identified for the spotted gar (Lepisosteus oculatus) and the two delimited species of Amia. A single individual from each of the three sampled species was included in the FBD fossil tip dating analysis. The fossil lineages of Halecomorphi used in the tip dating analysis are listed in the electronic supplementary material, and their phylogenetic relationships were enforced with clade constraints that reflect relationships presented in phylogenetic analyses of living and extinct lineages in Holostei using morphological characters [2]. The prior settings for the FBD included an exponential distribution for the diversification rate and uniform distributions for the time of origin, sampling proportion and turnover parameters. The chain was run for 1.0 × 108 generations and log and tree files were updated every 1.0 × 104 generations. The convergence of parameter values was assessed by the effective sample sizes that were calculated using Tracer v.1.7. Generations sampled before convergence was attained were discarded as burn-in. The BEAST analyses were run three separate times and post-burn-in generations were pooled from all three runs using LogCombiner 2.8. A maximum clade credibility tree with median node heights was constructed for the post-burn-in species tree topologies using TreeAnnotator 2.6.4.
(e) . Assessment of disparity between delimited species of Amia in meristic traits
To investigate if disparity in meristic traits used to discover, delimit and describe species of fishes showed variation in Amia, we collected data from 225 specimens following standard protocols [2,40]. A PC analysis of the meristic traits was performed using the ‘prcomp’ function in R v.3.2.0 (http://www.R-project.org/). A cross-validation linear discriminant analysis (LDA) of the meristic data was conducted with the R package MASS (https://cran.r-project.org/web/packages/MASS/index.html).
(f) . Characterization of morphological differences in the skulls of delimited species of Amia
To further assess the presence of morphological differences between A. calva and delimited A. ocellicauda, we scanned eight specimens of A. calva and 12 specimens of A. ocellicauda using high-resolution CT with a Nikon XT H 225 ST system. All scan parameters are provided in the electronic supplementary material, table S1. Volume rendering was performed in VGStudio MAX 3.5.1 (volumegraphics.com). We used ImageJ to take digital measurements of CT scans digitally rendered in VGStudio MAX 3.5. Measurements were taken of the maximum depth and length of the subopercle and interopercle (IO), as well as of the number of alveoli in the dentary tooth row. All plots were made using ggplot2 in RStudio.
3. Results and discussion
The summarized posterior phylogeny resulting from the coalescent analysis of 56 247 ddRAD loci unambiguously resolves two major lineages in Amia (figure 1b): a clade that includes specimens from the type locality of A. calva Linnaeus [25, p. 500] in Charleston, South Carolina, USA and another that corresponds to a lineage for which the oldest available name is Amia ocellicauda Todd, in Richardson [29, p. 236]. The delimitation of the two species of Amia shows a break in the geographical distribution along the northern Gulf of Mexico (figure 1a,b). Amia calva is distributed from the Pearl River in Louisiana and Mississippi, USA to the Florida Peninsula, and the rivers draining to the Atlantic Ocean in Georgia, South Carolina, North Carolina and Virginia, USA (figure 1a). Amia ocellicauda was first described in 1836 from Lake Huron in Ontario, Canada [29] and is distributed from the Lake Pontchartrain system west in Gulf of Mexico draining rivers to the Colorado River system in Texas, USA, throughout the Mississippi River Basin, the Great Lakes Basin, the St Lawrence River system, including Lake Champlain, and the Atlantic draining Connecticut River system (figure 1a,b).
Patterns of genomic ancestry estimated in the snmf analysis demonstrate the genetic distinctiveness of the two species (figure 1b). Populations with signatures of admixture are those from the Gulf Coast that are reconstructed as early-branching in the coalescent model-inferred phylogenomic tree (figure 1a,b), which may result from incomplete lineage sorting or limited introgression following secondary contact. Most of the genetic variation in Amia was observed between the two species A. calva and A. ocellicauda (electronic supplementary material, figure S1). The mean pairwise Fst among all intraspecific comparisons of A. calva and A. ocellicauda were less than 0.25 and the average Fst value among all comparisons of A. calva and A. ocellicauda was greater than 0.55 (figure 1c), which is indicative of comparisons between species.
The geographical distribution of the two living species of Amia is suggestive of allopatric speciation associated with rivers draining into the Gulf of Mexico (figure 1a). The Bayesian FBD relaxed molecular clock analysis results in a mean posterior age estimate of 1.82 Ma, with a 95% credible interval ranging between 0.95 and 2.93 Ma, for the most recent common ancestor of the two species of Amia, suggesting the two species of Amia diverged during the Plio-Pleistocene. This timing of speciation is consistent with a pattern of glaciation-induced speciation along the Gulf Coast in other North American freshwater vertebrates [41–43].
The two delimited species are morphologically distinguished by a shape difference in the IO bone (figure 1d) and a diagnostic difference in the number of dentary teeth; A. ocellicauda has 15 teeth versus 16 or 17 teeth in A. calva (electronic supplementary material, table S2). Consistent with the observed lack of disparity in meristic traits (electronic supplementary material, tables S3 and S4), a PC analysis of the meristic traits shows substantial overlap of the two species when plotting PC2 versus PC1 (electronic supplementary material, figure S2). A cross-validation LDA of the meristic data shows that 88.9% of all A. calva specimens are correctly identified. By contrast, only 32.2% of the specimens of A. ocellicauda are correctly identified using the meristic trait data.
Our study reveals the presence of two recently diverged sibling species of bowfins. A recent study using ddRAD data, but with very limited sampling of A. calva and A. ocellicauda, concluded that there may be up to four living species of Amia [24]. With a more inclusive sampling of populations (figure 1a), our genomic analyses consistently delimit two species of Amia. The populations within either species that exhibit the greatest genetic divergence are those of A. calva from Florida and the Gulf Coast, which were not sampled in the other study [24]. In addition, the shape of the IO and the number of dentary teeth delimit two living species of Amia (figure 1d; electronic supplementary material, table S2).
Despite the extremely old age of their parent clade, A. calva and A. ocellicauda have diverged in the last two million years, contrasting with the view of the bowfin as an evolutionary ‘dead-end’ and recalling other ancient lineages that have more recently produced their standing species diversity [44,45]. A more accurate understanding of species diversity of bowfins will inform conservation decisions for this iconic living fossil lineage, which is the target of an emerging caviar fishery [12]. In turn, the illumination of hidden living diversity in bowfins demonstrates that North America has acted as both a cradle and refugium of ancient vertebrate diversity [2,4,6,9–11,46]. Along with evidence for deep splits in lineages of classic living fossils like coelacanths [47,48], the resurrection of A. ocellicauda reveals the potential for hidden species richness awaiting discovery in other deeply divergent and species-depauperate vertebrate lineages.
(a) . Taxonomy
Amia calva Linnaeus 1766 [25, p. 500]
Ruddy bowfin, proposed common name
Amia lintiginosa Valenciennes in Cuvier & Valenciennes 1847 [26, p. 426].
Amia cinerea Valenciennes in Cuvier & Valenciennes 1847 [26, p. 430].
Type material: Linnean Society of London LSL128, a dried skin taken from the left side of the specimen [2, fig. 6].
Diagnosis: A species of Amia as previously diagnosed [2, pp. 32–33]. Amia calva has 16 or 17 teeth on the left side of the primary dentary tooth row (electronic supplementary material, table S2). IO maximum anteroposterior width measures 60% or less of IO dorsoventral depth. Colour ranges from reddish brown, and nuptial males may exhibit slightly green fins. Caudal eyespot is weakly or moderately defined. Meristic traits are summarized and compared with A. ocellicauda in electronic supplementary material, tables S3 and S4.
Amia ocellicauda Todd in Richardson 1836 [29, p. 236]
Eyetail bowfin, proposed common name
Amia occidentalis De Kay 1842 [27, p. 269].
Amia marmorata Valenciennes in Cuvier & Valenciennes 1847 [26, p. 412].
Amia ornata Valenciennes in Cuvier & Valenciennes 1847 [26, p. 420].
Amia viridis Valenciennes in Cuvier & Valenciennes 1847 [26, p. 421].
Amia canina Valenciennes in Cuvier & Valenciennes 1847 [26, p. 424].
Amia subcoerulea Valenciennes in Cuvier & Valenciennes 1847 [26, p. 427].
Amia reticulata Valenciennes in Cuvier & Valenciennes 1847 [26, p. 431].
Amia thompsonii Duméril 1870 [28, p. 423].
Amia piquotii Duméril 1870 [28, p. 432].
Type material: There is no type specimen. In accordance with recommendations presented in Article 75 of the International Zoological Code of Nomenclature [49], we decline to designate a neotype specimen because there is no confusion regarding the taxonomic status of A. ocellicauda.
Diagnosis: A species of Amia as previously diagnosed [2, pp. 32–33]. Amia ocellicauda has 15 teeth on the left side of the dentary (electronic supplementary material, table S2). IO maximum anteroposterior width measures more than 60% of IO dorsoventral depth. Fin colour of nuptial males ranges from dull green to bright emerald green. Caudal eyespot is sharply defined in males. Meristic traits are summarized and compared with A. calva in electronic supplementary material, tables S3 and S4.
Acknowledgements
CT scanning support was provided by E. Paulson and B. Mercado at the Yale Chemical and Biophysical Instrumentation Center. This study used tissue holdings curated and maintained at the North Carolina Museum of Natural Sciences and the Yale Peabody Museum. Tissue specimens were provided by J. Stein and S. King (Illinois Natural History Survey, University of Illinois), B. Porter (Duquesne University), W. L. Smith and A. Bentley (University of Kansas), L. Page and R. Robins (University of Florida), M. Wagner (Mississippi Museum of Natural History), E. Hilton (Virginia Institute of Marine Science), P. Chrisman (South Carolina Department of Natural Resources) and the Royal Ontario Museum. Specimens and field support were provided by J. Ballenger and J. Archambault (South Carolina Department of Natural Resources), M. Beauchene, E. Machowski, C. McDowell and L. Glynos (Connecticut Department of Environmental Protection), A. E. Near, R. J. Near and Lake Champlain International.
Contributor Information
Chase D. Brownstein, Email: chase.brownstein@yale.edu.
Daemin Kim, Email: dae-min.kim@yale.edu.
Data accessibility
All ddRAD, meristic and measurement data are either in the electronic supplementary material or are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.8pk0p2nrf [50]. Comprehensive methods are in the manuscript and electronic supplementary material, text [51].
Authors' contributions
C.D.B.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization, writing—original draft, writing—review and editing; D.K.: conceptualization, data curation, formal analysis, investigation, methodology, software, validation, writing—review and editing; O.D.O.: data curation, formal analysis, investigation, project administration, supervision, writing—review and editing; G.M.H.: investigation, project administration, resources. writing—review and editing; B.H.T.: investigation, project administration, resources, writing—review and editing; M.W.P.: investigation, resources, validation, writing—review and editing; R.S.: investigation, resources, validation, writing—review and editing; C.M.-M.: investigation, resources, validation, writing—review and editing; J.M.M.: investigation, resources, writing—review and editing; J.W.S.: methodology, resources, validation, writing—review and editing; S.R.D.: investigation, resources, visualization, writing—review and editing; G.W.-C.: data curation, project administration, resources, supervision, writing—review and editing; E.A.H.: data curation, formal analysis, methodology, resources, software, visualization, writing—review and editing; T.J.N.: conceptualization, data curation, formal analysis, investigation, methodology, project administration, resources, supervision, validation, visualization, writing—original draft, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed herein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
The Bingham Oceanographic Fund maintained by the Peabody Museum, Yale University supported this work. C.D.B. was also supported by the Yale Peabody Museum summer 2021 internship, the Society of Systematic Biologists miniARTS award, and the Yale Pierson College Richter Fellowship for summer 2022.
References
- 1.Braasch I, et al. 2016. The spotted gar genome illuminates vertebrate evolution and facilitates human-teleost comparisons. Nat. Genet. 48, 427-437. ( 10.1038/ng.3526) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grande L, Bemis WE. 1998. A comprehensive phylogenetic study of amiid fishes (Amiidae) based on comparative skeletal anatomy. An empirical search for interconnected patterns of natural history. J. Vertebr. Paleontol. 18(Suppl. 1), 4. ( 10.1080/02724634.1998.10011114) [DOI] [Google Scholar]
- 3.Lidgard S, Love AC. 2018. Rethinking living fossils. BioScience 68, 760-770. ( 10.1093/biosci/biy084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AW, et al. 2021. The bowfin genome illuminates the developmental evolution of ray-finned fishes. Nat. Genet. 53, 1373-1384. ( 10.1038/s41588-021-00914-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley SM. 1979. Macroevolution, pattern and process. San Francisco, CA: W. H. Freeman. [Google Scholar]
- 6.Grande L. 2010. An empirical and synthetic pattern study of gars (Lepisosteiformes) and closely related species, based mostly on skeletal anatomy. Lawrence, KS: American Society of Ichthyologists and Herpetologists. [Google Scholar]
- 7.Dornburg A, Near TJ. 2021. The emerging phylogenetic perspective on the evolution of actinopterygian fishes. Annu. Rev. Ecol. Evol. Syst. 52, 427-452. ( 10.1146/annurev-ecolsys-122120-122554) [DOI] [Google Scholar]
- 8.Abell R, et al. 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. Bioscience 58, 403-414. ( 10.1641/B580507) [DOI] [Google Scholar]
- 9.Grande L, Bemis WE. 1991. Osteology and phylogenetic relationships of fossil and recent paddlefishes (Polyodontidae) with comments on the interrelationships of Acipenseriformes. J. Vertebr. Paleontol. 11(Suppl. 001), 1. ( 10.1080/02724634.1991.10011424) [DOI] [Google Scholar]
- 10.Hilton EJ, Grande L. 2006. Review of the fossil record of sturgeons, family Acipenseridae (Actinopterygii: Acipenseriformes), from North America. J. Paleontol. 80, 672-683. ( 10.1666/0022-3360(2006)80[672:ROTFRO]2.0.CO;2) [DOI] [Google Scholar]
- 11.Hilton EJ, Grande L. 2008. Fossil mooneyes (Teleostei: Hiodontiformes, Hiodontidae) from the Eocene of western North America, with a reassessment of their taxonomy. Geol. Soc. Lond. Spec. Publ. 295, 221-251. ( 10.1144/SP295.13) [DOI] [Google Scholar]
- 12.Sinopoli DA, Stewart DJ. 2021. A synthesis of management regulations for bowfin, and conservation implications of a developing caviar fishery. Fisheries 46, 40-43. ( 10.1002/fsh.10526) [DOI] [Google Scholar]
- 13.Rypel AL, et al. 2021. Goodbye to ‘rough fish’: paradigm shift in the conservation of native fishes. Fisheries 46, 605-616. ( 10.1002/fsh.10660) [DOI] [Google Scholar]
- 14.Burgess GH, Gilbert CR. 1980. Amia calva Linnaeus. Bowfin. In Atlas of North American freshwater fishes (eds Lee DS, Gilbert CR, Hocutt CH, Jenkins RE, McAllister DE, Stauffer J Jr), pp. 53-54. Raleigh, NC: North Carolina State Museum. [Google Scholar]
- 15.Burr BM, Bennett MG. 2014. Amiidae: bowfins. In Freshwater fishes of North America, vol. 1 (eds Warren ML Jr, Burr BM), pp. 279-298. Baltimore, MD: Johns Hopkins Univeristy Press. [Google Scholar]
- 16.Boreske JR Jr. 1974. A review of the North American fossil amiid fishes. Bull. Mus. Comp. Zool. 146, 1-87. [Google Scholar]
- 17.Jenkins CN, Van Houtan KS, Pimm SL, Sexton JO. 2015. US protected lands mismatch biodiversity priorities. Proc. Natl Acad. Sci. USA 112, 5081-5086. ( 10.1073/pnas.1418034112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.April J, Mayden RL, Hanner RH, Bernatchez L. 2011. Genetic calibration of species diversity among North America's freshwater fishes. Proc. Natl Acad. Sci. USA 108, 10 602-10 607. ( 10.1073/pnas.1016437108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lundberg JG, Kottelat M, Smith GR, Stiassny MLJ, Gill AC. 2000. So many fishes, so little time: an overview of recent ichthyological discovery in continental waters. Ann. MO Bot. Gard. 87, 26-62. ( 10.2307/2666207) [DOI] [Google Scholar]
- 20.Etnier DA, Starnes WC. 1993. The fishes of Tennessee. Knoxville, TN: University of Tennessee Press. [Google Scholar]
- 21.Boschung HT Jr, Mayden RL. 2004. Fishes of Alabama. Washington, DC: Smithsonian Books. [Google Scholar]
- 22.Robison HW, Buchanan TM. 2019. Fishes of Arkansas, 2nd edn. Fayetteville, NC: University of Arkansas Press. [Google Scholar]
- 23.Mettee MF Jr, O'Neil PE, Pierson JM. 1996. Fishes of Alabama and the Mobile basin. Birmingham, AL: Oxmoor House. [Google Scholar]
- 24.Wright JJ, Bruce SA, Sinopoli DA, Palumbo JR, Stewart DJ. 2022. Phylogenomic analysis of the bowfin (Amia calva) reveals unrecognized species diversity in a living fossil lineage. Scient. Rep. 12, 16514. ( 10.1038/s41598-022-20875-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linnaeus C. 1766. Systema naturae sive regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Holmiae (Stockholm), Sweden: Laurentii Salvii. [In Latin.] [Google Scholar]
- 26.Cuvier G, Valenciennes A. 1847. Histoire naturelle des poissons. Tome dix-neuvième. Suite du livre dix-neuvième. Brochets ou Lucioïdes. Livre vingtième. De quelques familles de Malacoptérygiens, intermédiaires entre les brochets et les clupes. [Natural history of fish. Volume 19. Continuation of the nineteenth book. Pikes or lucioids. Book 20. Of some families of Malacopterygians, intermediate between pike and herrings]. Paris, France: P. Bertrand. [In French.] [Google Scholar]
- 27.De Kay JE. 1842. Zoology of New-York, or the New-York fauna; comprising detailed descriptions of all the animals hitherto observed within the state of New-York, with brief notices of those occasionally found near its borders, and accompanied by appropriate illustrations. Part IV. Fishes. Albany, NY: W. & A. White and J. Visscher. [Google Scholar]
- 28.Duméril AHA. 1870. Histoire naturelle des poissons, ou, ichthyologie générale. Tome second. Ganoïdes, dipnés, lophobranches [Natural history of fishes, or, general ichthyology. Book II. Ganoids, lungfishes, lophobranchs]. Paris, France: Roret. [In French.] [Google Scholar]
- 29.Richardson J. 1836. Fauna Boreali-Americana; or the zoology of the northern parts of British America: containing descriptions of the objects of natural history collected on the late northern land expeditions, under the command of Sir John Franklin, R. N. Part 3. London, UK: J. Bentley. [Google Scholar]
- 30.Jordan DS, Evermann BW. 1896. The fishes of North and Middle America: a descriptive catalogue of the species of fish-like vertebrates found in the waters of North America, north of the Isthmus of Panama. Part I. Bull. US Nat. Mus. 47, 1-1240. [Google Scholar]
- 31.Bermingham E, Avise JC. 1986. Molecular zoogeography of freshwater fishes in the southeastern United States. Genetics 113, 939-965. ( 10.1093/genetics/113.4.939) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Funderburg JB, Gilbert ML. 1963. Observations of a probable new race of the bowfin, Amia calva, from central Florida. ASB Bull. 10, 28. [Google Scholar]
- 33.Peterson BK, Weber JN, Kay EH, Fisher HS, Hoekstra HE. 2012. Double digest RADseq: an inexpensive method for de novo SNP discovery and genotyping in model and non-model species. PLoS ONE 7, e37135. ( 10.1371/journal.pone.0037135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eaton DAR, Overcast I. 2020. ipyrad: Interactive assembly and analysis of RADseq datasets. Bioinformatics 36, 2592-2594. ( 10.1093/bioinformatics/btz966) [DOI] [PubMed] [Google Scholar]
- 35.Bouckaert R, et al. 2019. BEAST 2.5: an advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15, e1006650. ( 10.1371/journal.pcbi.1006650) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rambaut A, Drummond AJ, Xie D, Baele G, Suchard MA. 2018. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 67, 901-904. ( 10.1093/sysbio/syy032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frichot E, François O. 2015. LEA: an R package for landscape and ecological association studies. Methods Ecol. Evol. 6, 925-929. ( 10.1111/2041-210X.12382) [DOI] [Google Scholar]
- 38.Goudet J. 2005. hierfstat, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 5, 184-186. ( 10.1111/j.1471-8286.2004.00828.x) [DOI] [Google Scholar]
- 39.Gavryushkina A, Heath TA, Ksepka DT, Stadler T, Welch D, Drummond AJ. 2017. Bayesian total-evidence dating reveals the recent crown radiation of penguins. Syst. Biol. 66, 57-73. ( 10.1093/sysbio/syw060) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hubbs CL, Lagler KF, Smith GR. 2004. Fishes of the Great Lakes region. Ann Arbor, MI: University of Michigan. [Google Scholar]
- 41.Soltis DE, Morris AB, McLachlan JS, Manos PS, Soltis PS. 2006. Comparative phylogeography of unglaciated eastern North America. Mol. Ecol. 15, 4261-4293. ( 10.1111/j.1365-294X.2006.03061.x) [DOI] [PubMed] [Google Scholar]
- 42.Near TJ, Kassler TW, Koppelman JB, Dillman CB, Philipp DP. 2003. Speciation in North American black basses, Micropterus (Actinopterygii: Centrarchidae). Evolution 57, 1610-1621. ( 10.1111/j.0014-3820.2003.tb00368.x) [DOI] [PubMed] [Google Scholar]
- 43.Kim D, Taylor AT, Near TJ. 2022. Phylogenomics and species delimitation of the economically important black basses (Micropterus). Scient. Rep. 12, 9113. ( 10.1038/s41598-022-11743-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagalingum NS, Marshall CR, Quental TB, Rai HS, Little DP, Mathews S. 2011. Recent synchronous radiation of a living fossil. Science 334, 796-799. ( 10.1126/science.1209926) [DOI] [PubMed] [Google Scholar]
- 45.Near TJ, Dornburg A, Tokita M, Suzuki D, Brandley MC, Friedman M. 2014. Boom and bust: ancient and recent diversification in bichirs (Polypteridae: Actinopterygii), a relictual lineage of ray-finned fishes. Evolution 68, 1014-1026. ( 10.1111/evo.12323) [DOI] [PubMed] [Google Scholar]
- 46.Patterson C. 1981. The development of the North American fish fauna—a problem of historical biogeography. In The evolving biosphere (ed. Forey PL), pp. 265-281. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 47.Holder MT, Erdmann MV, Wilcox TP, Caldwell RL, Hillis DM. 1999. Two living species of coelacanths? Proc. Natl Acad. Sci. USA 96, 12 616-12 620. ( 10.1073/pnas.96.22.12616) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sugeha HY, et al. 2020. A thirteen-million-year divergence between two lineages of Indonesian coelacanths. Scient. Rep. 10, 192. ( 10.1038/s41598-019-57042-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.International Commission on Zoological Nomenclature. 1999. International code of zoological nomenclature, 4th edn. London, UK: International Trust for Zoological Nomenclature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brownstein CD, et al. 2022. Data from: Hidden species diversity in an iconic living fossil vertebrate. Dryad Digital Repository. ( 10.5061/dryad.8pk0p2nrf) [DOI] [PMC free article] [PubMed]
- 51.Brownstein CD, et al. 2022. Hidden species diversity in an iconic living fossil vertebrate. Figshare. ( 10.6084/m9.figshare.c.6307499) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Brownstein CD, et al. 2022. Data from: Hidden species diversity in an iconic living fossil vertebrate. Dryad Digital Repository. ( 10.5061/dryad.8pk0p2nrf) [DOI] [PMC free article] [PubMed]
- Brownstein CD, et al. 2022. Hidden species diversity in an iconic living fossil vertebrate. Figshare. ( 10.6084/m9.figshare.c.6307499) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All ddRAD, meristic and measurement data are either in the electronic supplementary material or are available from the Dryad Digital Repository: http://doi.org/10.5061/dryad.8pk0p2nrf [50]. Comprehensive methods are in the manuscript and electronic supplementary material, text [51].