Abstract
Background:
Female athletes are at an increased risk of developing patellofemoral pain (PFP) relative to male athletes. The unique effects of maturation may compound that risk.
Hypothesis/Purpose:
The purpose was to evaluate the neuromuscular control mechanisms that are adaptive to pubertal maturation and determine their relative contribution to PFP development. It was hypothesized that aberrant landing mechanics (reduced sagittal-plane and increased frontal- and transverse-plane kinematics and kinetics) would be associated with an increased risk for PFP.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
There were 506 high school female athletes who completed a detailed medical history, the Anterior Knee Pain Scale, and a knee examination for the diagnosis of PFP and attended follow-up appointments. Athletes performed a drop vertical jump task instrumented with force plates, and biomechanical measures generated from standard 3-dimensional biomechanical analyses were used to classify participants into high- or low-risk knee and hip landing profiles for the development of PFP. The biomechanical measures used in the knee landing profile included sagittal-plane knee range of motion, peak knee abduction angle, peak knee abduction moment, and peak-to-peak transverse-plane knee moment. The biomechanical measures used in the hip landing profile included sagittal-plane hip range of motion, peak hip extensor moment, peak abductor moment, and peak hip rotator moment. Testing was conducted at sport-specific preseason appointments over the course of 2 years, and changes in pubertal status, landing profile, and PFP development were documented.
Results:
Female athletes with high-risk hip landing profiles experienced increased hip flexion and decreased abductor, rotator, and extensor moments. Participants with high-risk hip landing profiles who transitioned to postpubertal status at follow-up had higher odds (odds ratio, 2.1 [95% CI, 1.1–4.0]; P = .02) of moving to a low-risk hip landing profile compared with those who had not reached postpubertal status at follow-up. Participants with high-risk knee landing profiles experienced decreased knee flexion and increased knee abduction, external abductor, and external rotator moments. Pubertal maturation was not associated with a change in the high-risk knee landing profile at follow-up.
Conclusion:
The progression from prepubertal to postpubertal status may have a protective effect on high-risk hip mechanics but no similar adaptations in high-risk knee mechanics during maturation. These data indicate that before puberty, maladaptive hip mechanics may contribute to PFP, while aberrant knee mechanics associated with PFP are sustained throughout the maturational process in young female athletes.
Keywords: patellofemoral pain syndrome, anterior knee pain, biomechanics, neuromuscular control, knee valgus, puberty
Young female athletes are 2 to 10 times more likely to develop patellofemoral pain (PFP) than their male counterparts.17,18 Furthermore, athletes with PFP may reduce or completely cease participation in physical activity and have a greater likelihood of developing patellofemoral osteoarthritis later in life.34 PFP is commonly attributed to malalignment between the trochlea and the patella that results in increased contact pressures.36 Previous studies have proposed that shifts in the position of a relatively mobile patella in relation to a more fixed trochlea can lead to the development of PFP.19,20,40 However, Powers37 has proposed an alternative theory that suggests that the problems might arise from abnormal femoral movement in relation to a patella that is fixed by the patellar and quadriceps tendons. While some risk factors for PFP cannot be modified, such as patellar shape, soft tissue laxity, sex, or family history, other risk factors, including functional activity techniques and strength, can be adapted through training.1 The identification of altered muscle recruitment strategies for athletes with PFP could help clinicians identify high-risk biomechanics in other athletes before its onset and assist in the development of preventative training strategies.
Numerous retrospective biomechanical investigations have reported altered neuromuscular control strategies in male and female patients with PFP.4,5,11,12 For example, during a drop vertical jump (DVJ), female patients with PFP exhibit an increased external knee abduction moment (KAM) at initial contact on the most symptomatic limb and increased maximum and normalized knee abduction loading in the least symptomatic limb compared with matched controls.34 While these data indicate that neuromuscular control may lead to compromised loading mechanics (eg, hip vs knee loading)37 that contribute to PFP, it is unknown what effect pubertal maturation has on the incidence of PFP or on changes in lower extremity landing mechanics that influence its risk.
Female patients may be at an increased risk for PFP as they mature because of inadequate neuromuscular adaptation to the increased demands of the structural and inertial changes that develop after puberty.21,22,24,27,30,39 An increased body mass in conjunction with an increased limb length and consequently longer joint levers result in greater lower extremity joint forces that are difficult to balance during high-velocity maneuvers.21,22,24 Skeletal growth also changes the center of mass location, which makes muscular control of the trunk more challenging. Studies have shown that to compensate, female patients use frontal-plane force absorption strategies rather than sagittal-plane control strategies for lower extremity neuromuscular control.14,16 This frontal-plane compensation for altered patellofemoral joint loading might lead to an increased risk of developing PFP.21,34,37 Therefore, the purposes of this study were to identify the neuromuscular control mechanism profiles with regard to the PFP risk among young female athletes and to evaluate whether the risk profiles are adaptive to pubertal maturation. Prior literature drove our central hypothesis that reduced lower limb hip and knee neuromuscular control mechanisms (reduced sagittal-plane and increased frontal- and transverse-plane kinematics and kinetics) underlie the development of PFP in developing female athletes.
METHODS
Participants
The institutional review board approved the data collection procedures and consent forms. Parental consent and athlete assent were obtained before data collection. We defined the term “athlete” as a female member of a middle school or high school volleyball, soccer, or basketball team. All members of the team were included and scored based on answers to parental questions regarding the athlete’s pubertal status on the modified Pubertal Maturation Observational Scale (Figure 1).7,16 Positive answers to these questions were scored as a point, with ≤1 total points indicating prepubertal status, 2 to 4 total points indicating pubertal status, and ≥5 total points indicating postpubertal status. Of the 739 female athletes who completed baseline biomechanical measures at the start of the study, 506 completed ≥1 follow-up visits and were included in the final analyses. Table 1 presents the characteristics of the athletes at the baseline and follow-up visits. At baseline, the athletes had a mean (±SD) age of 13.6 ± 1.8 years, mean height of 159.4 ± 8.2 cm, and mean weight of 53.6 ± 12.1 kg. The mean body fat percentage was 22.9% ± 8.2%, and the mean body mass index was 20.9 ± 3.6 kg/m2. Of the 506 athletes who completed follow-up visits, 39% were postpubertal at baseline, and another 15% reached postpubertal by follow-up, which resulted in 54% postpubertal athletes at the follow-up visit.
Figure 1.

Pubertal Maturation Observational Scale. This checklist was used to determine the pubertal status of athletes included in the study. Positive answers were counted as 1 point: if an athlete had ≤1 point, she was considered as prepubertal; if an athlete had between 2 and 4 points, she was considered pubertal; and if an athlete had ≥5 total points, she was considered postpubertal.
TABLE 1.
Characteristics of Study Participantsa
| Baseline Visit for All Participants (N = 739) | Baseline Visitb (n = 506) | Follow-up Visitb (n = 506) | |
|---|---|---|---|
|
| |||
| Age, y | 13.6 ± 1.8 | 13.3 ± 1.6 | 14.5 ± 1.7 |
| Height, cm | 159.4 ± 8.2 | 158.8 ± 8.5 | 162.1 ± 7.5 |
| Weight, kg | 53.6 ± 12.1 | 52.3 ± 12.3 | 56.1 ± 11.4 |
| Fat percentage, % | 22.9 ± 8.2 | 22.2 ± 8.2 | 23.9 ± 7.5 |
| Body mass index, kg/m2 | 20.9 ± 3.6 | 20.5 ± 3.6 | 21.2 ± 3.4 |
| Pubertal status, n (%) | |||
| Prepubertal | 394 (53) | 310 (61) | 233 (46) |
| Postpubertal | 345 (47) | 196 (39) | 273 (54) |
Values are presented as mean ± SD unless otherwise specified.
Participants who completed follow-up visits.
Participants were tested before the start of their competitive season (baseline). Testing consisted of patient characteristics; questionnaires to determine medical history, familial anthropometric measurements, and indicators of pubertal development utilized for maturational estimates (as previously described); a clinical knee examination; and landing biomechanical analyses of DVJ performance.2,33 Teams of athletes were tested at preseason appointments over the course of 2 years.
Knee Examination
The initial injury screening process included the Anterior Knee Pain Scale (AKPS) questionnaire.6,28,46 The AKPS is composed of 13 items that evaluate subjective knee pain symptoms and functional limitations. Scores range from 0 to 100, with an athlete exhibiting no sign of anterior knee pain scoring 100. All participants with an AKPS score <100 underwent a further assessment, which included the International Knee Documentation Committee score26 to assess function in the right and left knees, a personal interview regarding current and prior knee symptoms and limitations, a medical history, and a knee physical examination by the same investigator. The standardized personal interview included questions regarding the participant’s severity of knee pain, sport participation time missed due to knee pain, timing of knee pain with activity, knee pain after play, duration of knee pain, symptoms of knee instability, and if the athlete had been evaluated by her primary care physician or a specialist for knee pain. The physical examination included palpation for tenderness along the region of the patellar attachment of the medial patellofemoral ligament, medial and lateral patellar facets, medial and lateral femoral-tibial joint lines, medial or lateral plica within the patellofemoral joint, Gerdy tubercle and iliotibial band, pes anserine bursa, distal pole of the patella, tibial tubercle, Hoffa fat pad, quadriceps tendon, and patellar tendon. In the absence of any additional knee injuries, participants were diagnosed as having active PFP if they had all of the following: AKPS score <100, knee pain with or shortly after activity, anterior knee tenderness at the patellar attachment of the medial patellofemoral ligament, tenderness at the medial patellar facet, and tenderness at the lateral patellar facet. All instances of PFP were treated as a positive PFP diagnosis, regardless of severity.
Landing Biomechanics
Three-dimensional hip, knee, and ankle kinematic and kinetic data were quantified for the initial ground contact phase of the DVJ. Each participant was instrumented with 37 retroreflective markers placed on the sacrum, left posterior superior iliac spine, and sternum and bilaterally on the shoulder, elbow, wrist, anterior superior iliac spine, greater trochanter, midthigh, medial and lateral knees, tibial tubercle, midshank, distal shank, medial and lateral ankles, heel, dorsal surface of the midfoot, lateral foot (fifth metatarsal), and central forefoot (between second and third metatarsals). First, a static trial was conducted in which the participant was instructed to stand still with foot placement standardized to the laboratory coordinate system. This static measurement was used as each participant’s neutral (zero) alignment; subsequent kinematic measures were referenced to this position. In addition, the static trial was used to calculate standing knee abduction angle measures. After the static trial, participants performed a minimum of 3 DVJ trials. The DVJ involved participants standing on top of a box (31 cm high) with their feet positioned 35 cm apart (Figure 2). Participants were instructed to drop directly down off the box and immediately perform a maximum vertical jump, raising both arms while jumping and reaching for a suspended basketball.15
Figure 2.
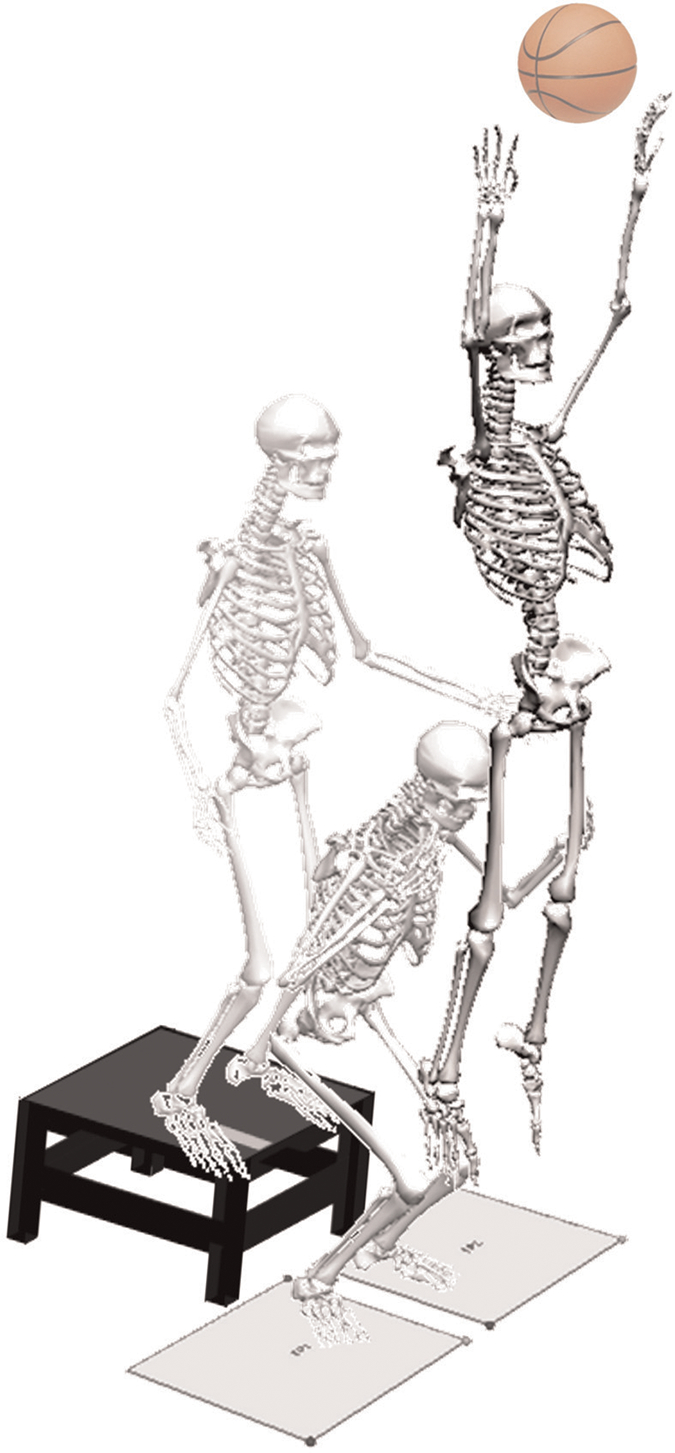
Drop vertical jump. Participants performed at least 3 trials of the drop vertical jump. This involved participants standing on top of a 31 cm–high box with their feet positioned 35 cm apart. Participants dropped straight down off the box. Upon making contact with the floor, participants were instructed to perform a vertical leap and reach for a basketball suspended from the ceiling.
Three trials were collected for each participant with EVaRT (Version 4; Motion Analysis) using a motion analysis system consisting of 10 digital cameras (Eagle cameras; Motion Analysis) positioned in the laboratory and sampled at 240 Hz. Two force platforms (AMTI) were sampled at 1200 Hz and time synchronized with the motion analysis system. The force platforms were embedded into the floor and positioned 8 cm apart so that each foot would contact a different platform during the stance phase of the DVJ.15
Biomechanical Data Analysis
The 3-dimensional Cartesian marker trajectories and ground-reaction forces (GRFs) from each DVJ trial were filtered through a low-pass filter at a matched cutoff frequency of 12 Hz.47 The raw, unfiltered vertical GRF data recorded for each limb were used to normalize the associated kinematic data from 0% to 100% of stance at 1% increments (n = 101), with initial contact defined as the instant when the vertical GRF first exceeded 10 N. From the 3-dimensional kinematic forces and GRFs, joint moments of force were computed using an inverse dynamics analysis.45
Athlete Surveillance and Follow-up
The athletes were monitored throughout their competitive season on a weekly basis for athletic onset of new PFP, or any other lower extremity injury with resultant time loss, by a certified athletic trainer. After the season, the AKPS was readministered to all participants. Athletes with a score indicating PFP on the AKPS (<100) and all athletes who had been examined at preseason underwent a further evaluation with a standardized personal interview and physical examination by the same physician. These data were compiled to calculate the preseason prevalence and in-season incidence of the development of PFP. At the end of surveillance, all study participants were classified into 1 of 4 groups: (1) PFP at preseason screening, (2) developed a new episode of PFP during the season, (3) developed PFP associated with another underlying knee condition, and (4) never developed PFP.
Statistical Analysis
A consultation between the biostatisticians and biomechanics experts resulted in a set of 70 biomechanical variables measured at the baseline visit (Appendix Tables A1a and A1b, available in the online version of this article) selected a priori that were considered clinically relevant to PFP. Each of these variables was examined as a potential predictor for PFP (either present or absent) using logistic regression, and those associated with PFP at a P value <.2 were identified. Correlation analysis among these potential risk factors further reduced the list of potential risk factors to ensure that they were not highly correlated with one another (Spearman r < 0.8 was used). If variables were highly correlated, the variable with the higher C-statistic, from the logistic regression using PFP as the outcome, was selected. From this further reduced list of potential PFP risk factors, we then selected biomechanical measures based on clinical relevance and research team opinion on relevance for modifiable risk factors to enter into a latent profile analysis (LPA) to define the PFP risk profile.
LPA is a statistical modeling technique that aims to identify distinct underlying (unobserved) groupings or profiles from a sample based on observed variables (the biomechanical measures in this particular analysis). In this study, LPA was used to create distinct profiles so that participants with similar patterns of biomechanical characteristics were grouped together, while those with different patterns of biomechanical characteristics were classified in different landing profiles. Initially, 2 profiles were specified for the LPA model. Then, in a stepwise fashion, the number of profiles specified was increased by one. At each step, the model fit was assessed by the change in the Bayesian information criterion, adjusted for sample size. A lower Bayesian information criterion indicates a better model fit. We also used the Lo-Mendell-Rubin adjusted likelihood ratio test,29 average posterior probability (entropy), and profile membership probabilities to guide the decision on the optimal number of profiles to be included. Separate LPAs were run for knee biomechanical measures and hip biomechanical measures. We then examined the knee and hip profiles as potential predictors for PFP in the logistic regression. The landing profiles were created using baseline preseason data.
At follow-up, participants were categorized into the landing profiles again using the biomechanical data from their follow-up visits. This was achieved by application of the landing profile parameters obtained from the baseline LPA. For athletes whose pubertal status had changed and advanced to postpubertal, we used data from the first visit at which they were defined as postpubertal. For athletes whose pubertal status had not changed during the entire follow-up period, we used data from the last follow-up visit. We evaluated the change in pubertal status in association with the change in profile membership. The LPAs were conducted in Mplus 7,31 and all other statistical analyses were conducted in SAS (Version 9.3; SAS Institute).
RESULTS
Knee and Hip Profiles
From the initial analyses, 4 knee biomechanical measures and 4 hip biomechanical measures were identified for inclusion in the LPA to define the landing profiles. The knee measures were sagittal-plane knee range of motion (knee flexion), peak knee abduction angle (knee abduction), peak external KAM, and peak-to-peak transverse-plane knee moment (eg, range between minimum and maximum values for knee rotator moment). The hip measures were sagittal-plane hip range of motion (hip flexion), peak hip extensor moment (hip extensor moment), peak hip abductor moment (hip abductor moment), and peak hip rotator moment (hip rotator moment). The LPA models yielded 2 distinct landing profiles for the knee biomechanical measures and 2 for the hip biomechanical measures. Table 2 shows the distribution of landing profile group membership and descriptive statistics of these biomechanical measures by profile. It should be noted that the PFP outcome was not included in the LPA models so that the knee and hip profiles were built independent of PFP. Each participant was classified either in “profile 1” (labeled as potentially high risk) or “profile 2” (labeled as potentially low risk) separately for knee analysis and for hip analysis.
TABLE 2.
Knee and Hip Landing Profiles for 506 Participants at Baseline and Follow-up Visitsa
| Risk Profile at Baseline | |||||
|
| |||||
| Hip | Athletes, n (%) | Sagittal-Plane Hip Range of Motion, deg | Peak Hip Extensor Moment, N·m | Peak Hip Abductor Moment, N·m | Peak Hip Rotator Moment, N·m |
|
| |||||
| 1 (high-risk hip) | 354 (70) | 33.42 ± 0.41 | 64.51 ± 0.85 | 11.99 ± 0.39 | 17.61 ± 0.27 |
| 2 (low-risk hip) | 152 (30) | 25.60 ± 0.60 | 89.28 ± 1.48 | 32.25 ± 1.04 | 31.95 ± 0.63 |
|
| |||||
| Knee | Athletes, n (%) | Sagittal-Plane Knee Range of Motion, deg | Peak Knee Abduction Angle, deg | Peak Knee Abduction Moment, N·m | Peak-to-Peak Transverse-Plane Knee Moment, N·m |
|
| |||||
| 1 (high-risk knee) | 210 (42) | −55.45 ± 0.47 | −12.01 ± 0.36 | −35.27 ± 0.92 | 0.95 ± 0.52 |
| 2 (low-risk knee) | 296 (58) | −65.45 ± 0.34 | −9.26 ± 0.26 | −19.35 ± 0.40 | 2.69 ± 0.25 |
|
| |||||
| Risk Profile at Follow-up | |||||
|
| |||||
| Hip | Athletes, n (%) | Sagittal-Plane Hip Range of Motion, deg | Peak Hip Extensor Moment, N·m | Peak Hip Abductor Moment, N·m | Peak Hip Rotator Moment, N·m |
|
| |||||
| 1 (high-risk hip) | 289 (57) | 35.20 ± 0.51 | 70.82 ± 1.07 | 14.10 ± 0.53 | 18.18 ± 0.37 |
| 2 (low-risk hip) | 217 (43) | 27.39 ± 0.67 | 94.60 ± 1.66 | 33.22 ± 1.10 | 33.23 ± 0.68 |
|
| |||||
| Knee | Athletes, n (%) | Sagittal-Plane Knee Range of Motion, deg | Peak Knee Abduction Angle, deg | Peak Knee Abduction Moment, N·m | Peak-to-Peak Transverse-Plane Knee Moment, N·m |
|
| |||||
| 1 (high-risk knee) | 241 (48) | −55.70 ± 0.53 | −10.95 ± 0.39 | −32.12 ± 0.98 | −0.07 ± 0.61 |
| 2 (low-risk knee) | 265 (52) | −65.60 ± 0.41 | −8.11 ± 0.33 | −18.66 ± 0.48 | 2.45 ± 0.34 |
Values are presented as mean ± standard error unless otherwise specified.
Hip Profile.
As illustrated in Figure 3, the profile group labeled as “profile 1” (henceforward referred to as high-risk hip profile) exhibited greater hip flexion angles combined with lower hip extensor, abductor, and rotator moments. Functionally, this was considered indicative of insufficient hip musculature recruitment during the DVJ. In contrast, the profile group labeled as “profile 2” (henceforward referred to as low-risk hip profile) was characterized by lower hip flexion in combination with higher hip extensor, abductor, and rotator moments. When we tested the hip risk profile as a predictor for PFP at baseline, athletes in the high-risk profile were more likely to have PFP compared with those in the low-risk profile (odds ratio [OR], 1.73 [95% CI, 1.05–2.85]; P = .03).
Figure 3.
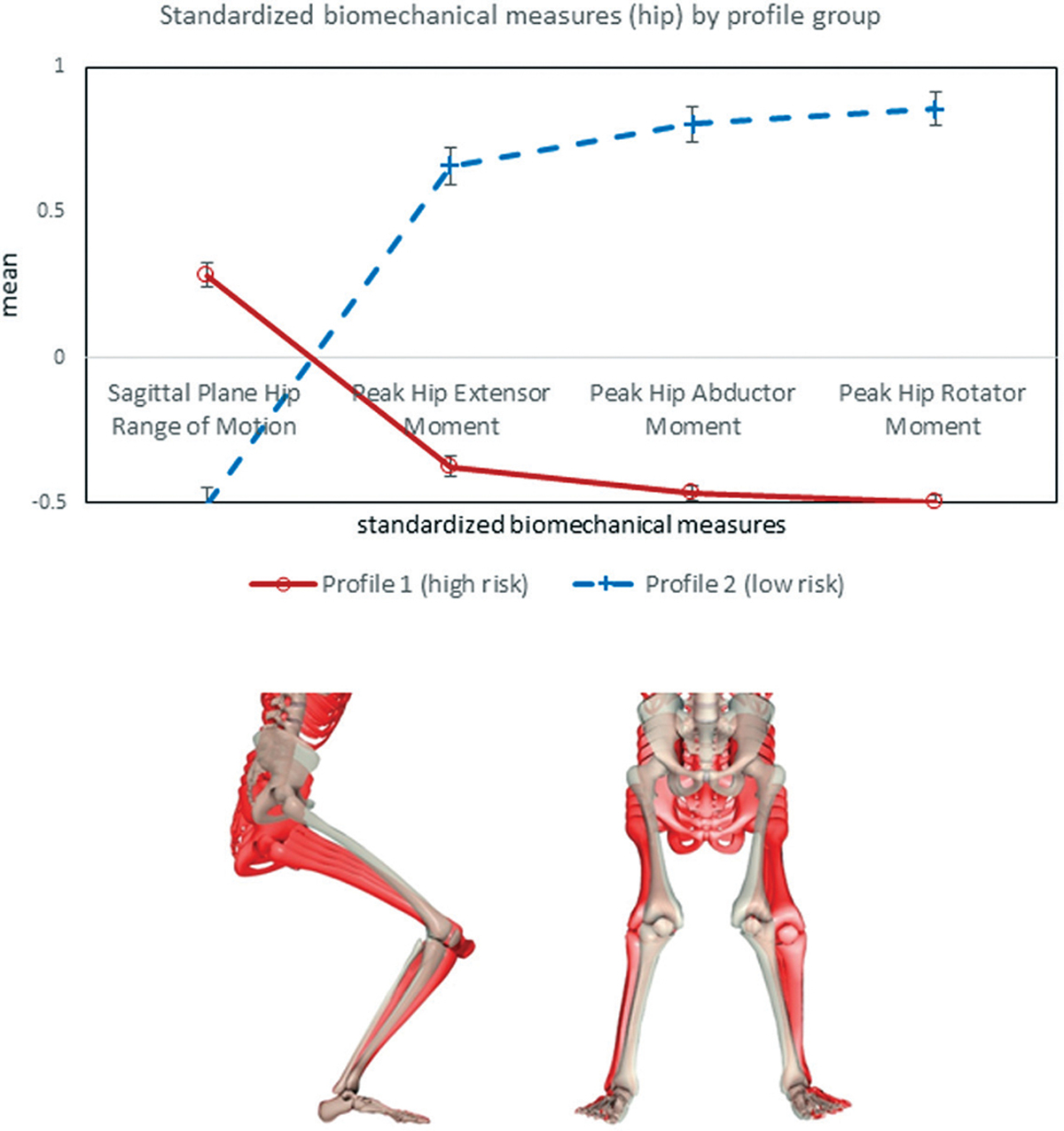
Hip risk profile at baseline. Red represents high-risk mechanics. White skeleton representing sagittal- and frontal-plane views of the low-risk landing mechanics, with red skeletons showing the difference in landing for the high-risk group mechanics. The directionality of the differences in low- and high-risk profile landing mechanics is emphasized by using 2, 3, 4, and 5 multipliers, respectively, in the hip high-risk postures.
Knee Profile.
Of the 2 knee profile groups, the group labeled as “profile 1” (henceforward referred to as high-risk knee profile) exhibited lower knee flexion in conjunction with a higher knee abduction, external KAM, and rotator moment, which characterized a stiffer landing and a generally less absorptive biomechanical profile during the DVJ (Figure 4). The other “profile 2” (henceforward referred to as low-risk knee profile) exhibited higher knee flexion with a lower knee abduction, external KAM, and rotator moment, which likely indicated a more efficient landing pattern that allowed for the attenuation of GRFs via increased knee flexion. Athletes in the high-risk knee profile were more likely to have PFP at baseline compared with those in the low-risk knee profile (OR, 1.60 [95% CI, 1.02–2.50]; P = .04).
Figure 4.
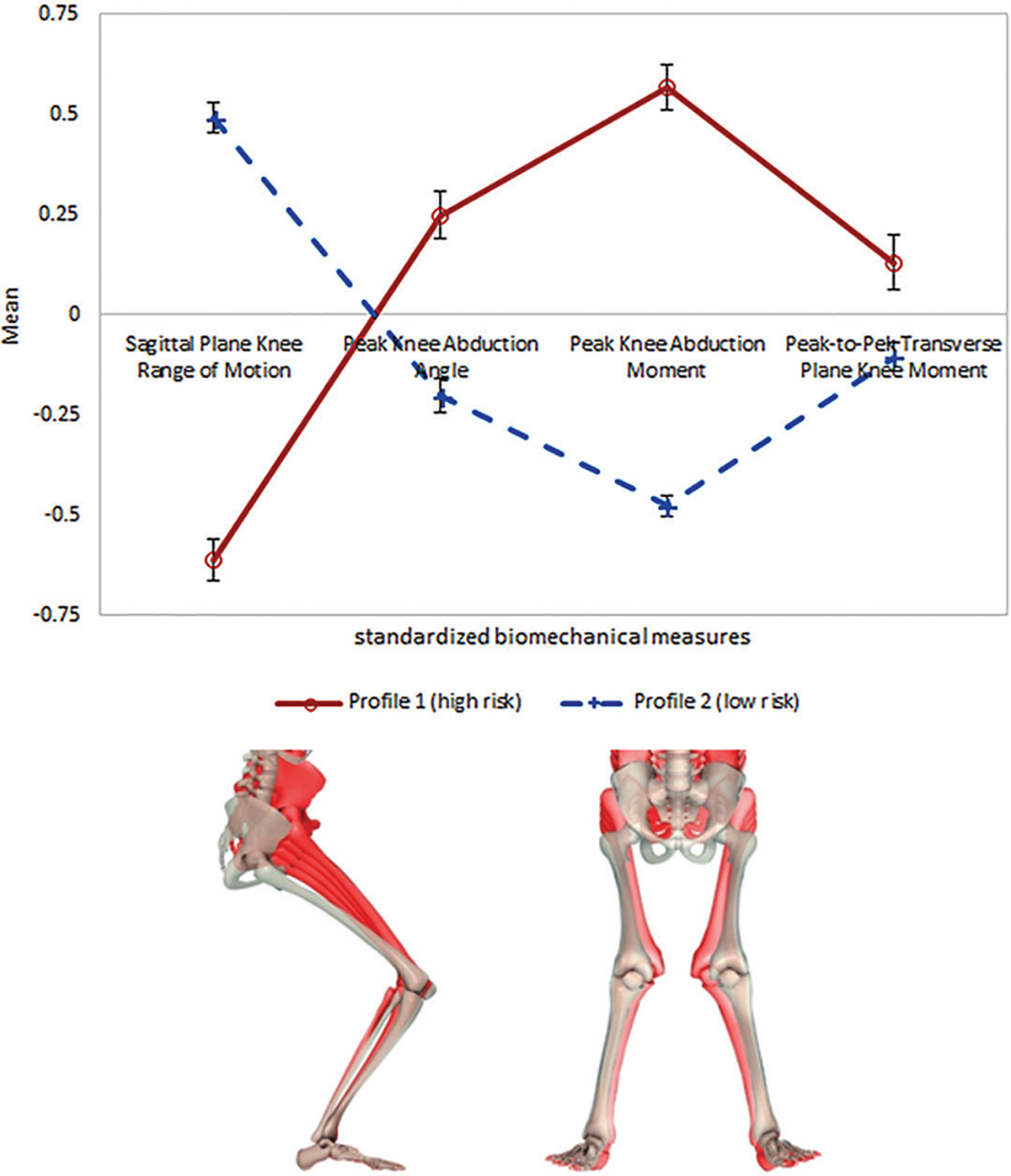
Knee risk profile at baseline. Red represents high-risk mechanics. White skeleton representing sagittal- and frontal-plane views of the low-risk landing mechanics, with red skeletons showing the difference in landing for the high-risk group mechanics. The directionality of the differences in low- and high-risk knee profile landing mechanics is emphasized by using 2, 3, 4, and 5 multipliers, respectively, in the hip high-risk postures.
Pubertal Status and Hip and Knee Profiles
For athletes who completed follow-up visits, their hip and knee risk profiles were identified at the follow-up visits and examined for a change in association with pubertal status. As presented in Figure 5, of the 506 athletes included in this follow-up analysis, 253 stayed in the high-risk hip profile group, 116 stayed in the low-risk hip profile group, while 101 moved from high-risk profile to low-risk profile, and 36 moved from low-risk profile to high-risk profile at the follow-up visit. With regard to the knee risk profile, 160 of the 506 stayed in the high-risk profile group, 215 stayed in the low-risk profile group, while 50 moved from high-risk profile to low-risk profile, and 81 moved from low-risk profile to high-risk profile at the follow-up visit. The change in the knee risk profile group is depicted in Figure 6.
Figure 5.
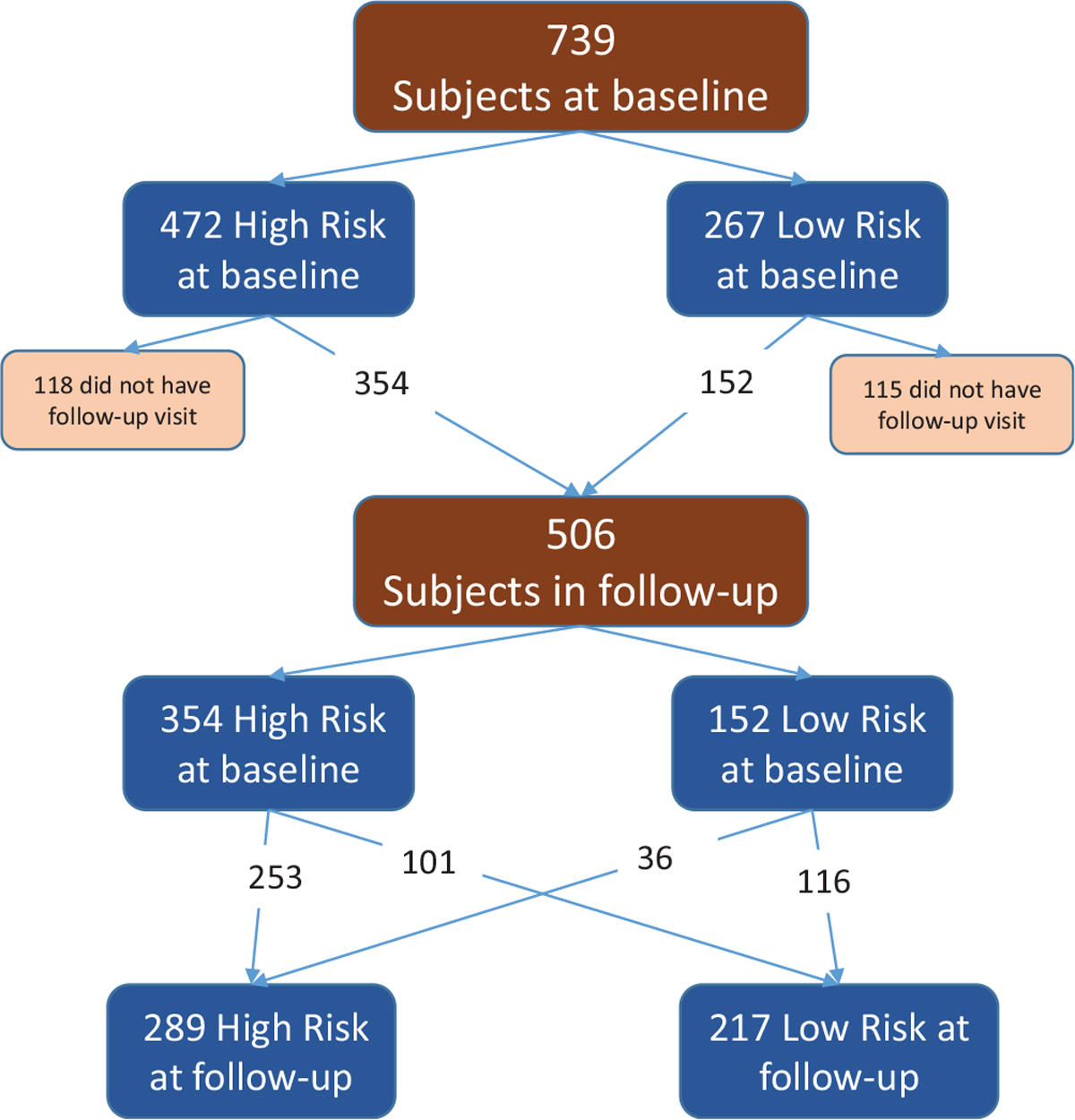
Hip risk profile. Of the original 739 study participants at baseline, 506 (68%) could attend follow-up appointments. Of those, 354 (70%) were high risk at baseline, and 152 (30%) were low risk at baseline. At the follow-up appointment, 101 (29%) of the original high-risk profile group progressed to low-risk profile, and 36 (24%) of the original low-risk profile group progressed to high-risk profile.
Figure 6.
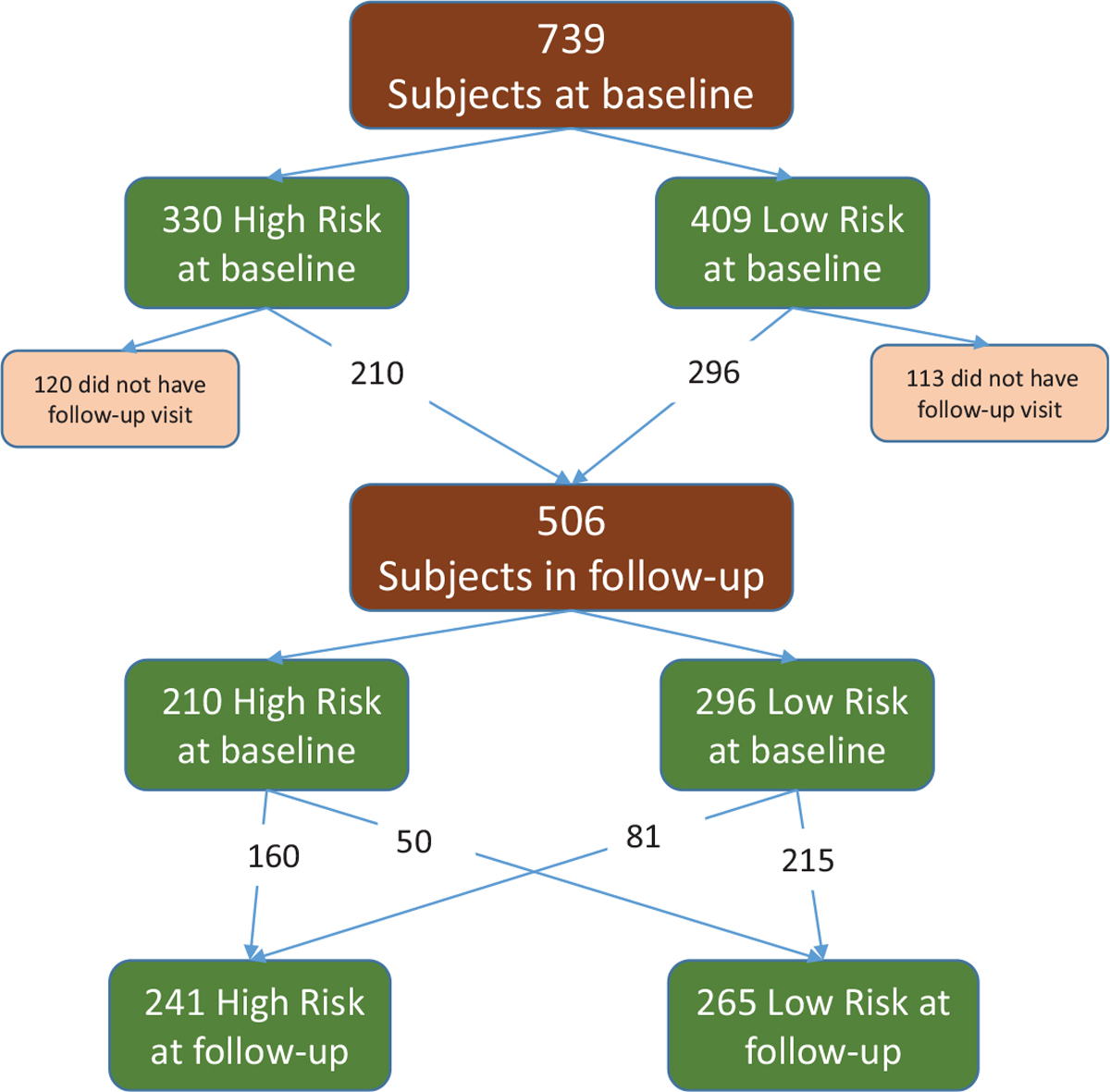
Knee risk profile. Of the original 739 study participants at baseline, 506 could attend follow-up appointments. Of those, 210 were high risk at baseline, and 296 were low risk at baseline. At the follow-up appointment, 50 of the original high-risk profile group progressed to low-risk profile, and 81 of the original low-risk profile group progressed to high-risk profile.
As shown in Figure 7, compared with athletes who matured over the course of the study (moved from prepubertal to postpubertal; change group), athletes whose maturational status remained unchanged from baseline to follow-up were at lower odds of moving to the low-risk hip profile (postpubertal at both baseline and follow-up group: OR, 0.56 [95% CI, 0.31–1.03], P = .06; prepubertal at both baseline and follow-up group compared with the change group: OR, 0.48 [95% CI, 0.26–0.89], P = .02). Further, a focus on athletes classified in the high-risk hip profile at baseline revealed that those who matured to postpubertal status had higher odds (OR, 2.1 [95% CI, 1.1–4.0]; P = .02) of moving to the low-risk hip profile compared with those who had not reached postpubertal status. Similar analyses that examined the change in the knee risk profile did not indicate that a change in pubertal status was associated with a change in the knee risk profile.
Figure 7.
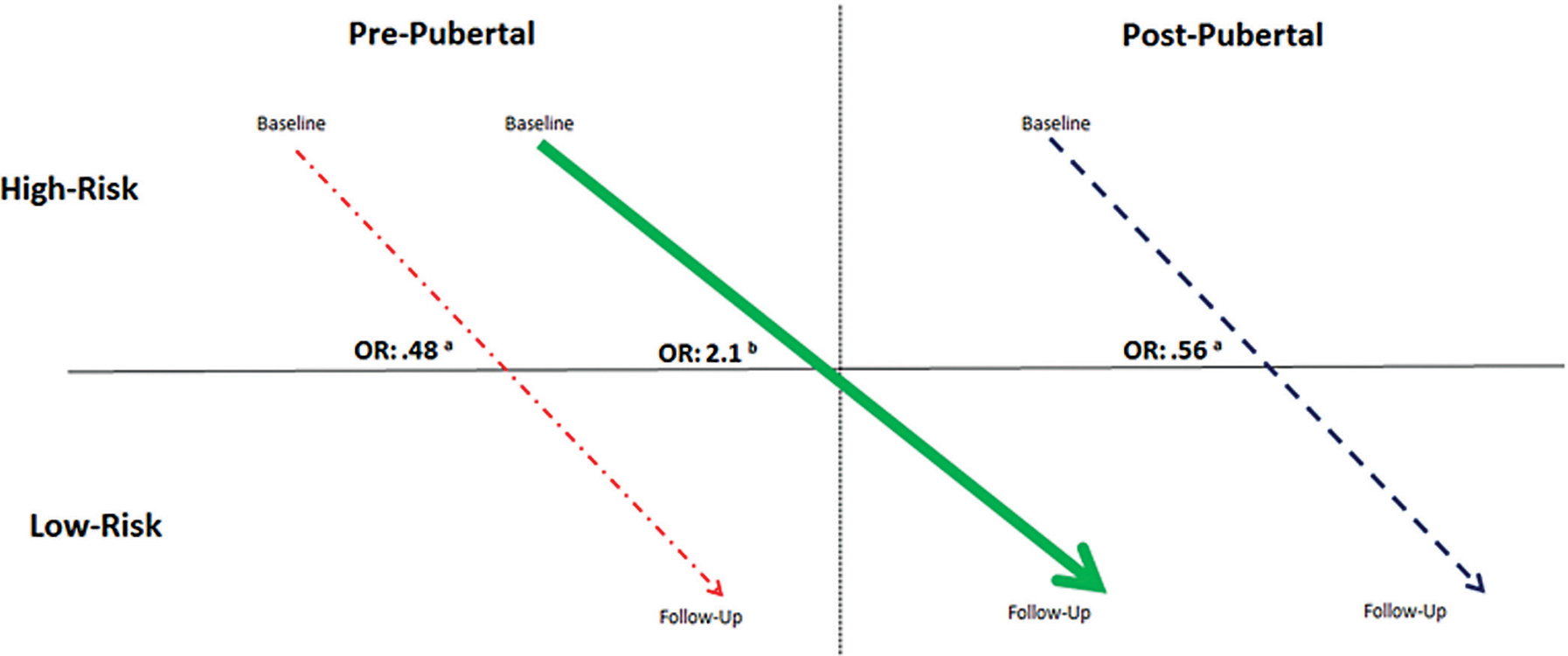
Pubertal maturation in hip profile. When compared with athletes in the change group (thick line), athletes whose maturational status remained unchanged over the course of the study (dotted and dashed line = prepubertal from baseline to follow-up; dashed line = postpubertal from baseline to follow-up) had lower odds of moving to the low-risk profile. aOdds ratio comparing athletes who matured over the course of the study to those who did not mature over the course of the study. bOdds ratio comparing athletes with a high-risk profile who matured over the course of the study to athletes with a high-risk profile who did not reach postpubertal status.
DISCUSSION
The goals of the current study were to identify the neuromuscular control mechanism profiles with regard to the PFP risk among young female athletes and to evaluate whether the risk profiles are adaptive to pubertal maturation. The investigation revealed that high-risk hip mechanics were likely to normalize toward a low risk with maturation. Among the athletes with a high-risk hip landing profile, those who matured to postpubertal status by the end of the study were more likely to progress to the low-risk hip landing profile than athletes who either did not mature or were postpubertal for the study’s duration (baseline and follow-up). However, no such protective influence was observed in the change in the knee landing profile, suggesting that pubertal advancement did not help resolve high-risk knee mechanics.
Hip Risk Profile
As shown in Figure 3, female athletes in the high-risk hip profile exhibited increased hip flexion and decreased rotator, abductor, and extensor moments. One possible reason for the observed increase in hip flexion was insufficient sagittal-plane hip eccentric extensor recruitment. The observed lower abductor, rotator, and extensor moments might indicate that a greater portion of the absorption load is transferred to passive lower limb structures. These mechanics could be indicative of an “out-of-plane” loading strategy that puts greater emphasis on control in the frontal and transverse planes. Uneven distribution of this nature would cause malalignment between the patella and femur.
While current theories posit that patellar maltracking secondary to abnormal patellar motion contributes to the development of PFP, research by Powers et al38 and Souza et al42 suggests that femoral translation and rotation beneath a patella that is contained within the extensor mechanisms are an important factor. During weightbearing activities, the patella is tethered to the tibia by the patellar tendon and is suspended by quadriceps contraction. Medial-lateral stability is provided by the trochlear shape and soft tissue restraints. With the patella fixed in this position, the decreased external rotator moment of the high-risk group suggests a decreased ability to resist inward rotation of the hip in response to GRFs. This in turn might indicate inefficient femoral transverse-plane control mechanisms that could alter the contact surface area between the femur and patella, thereby resulting in the localization of stress to a smaller area.
Further, greater uncontrolled hip flexion and lower hip extensor moments may transfer greater compressive force to the patella, thereby necessitating the dissipation of higher order reaction forces. The increased force on the patella, and greater use of “out-of-plane” control mechanisms in the high-risk profile, would require the patella to dissipate higher levels of force through inefficient control mechanisms. Uneven distribution of stress of this nature has the potential to cause peripatellar and retropatellar pain indicative of PFP.
Knee Risk Profile
As shown in Figure 4, female athletes in the high-risk knee profile exhibited decreased knee flexion and an increased abductor angle, KAM, and rotator moment. This group was generally characterized by stiffer landings and poorer knee biomechanics that mirror high-risk knee injury behavior as well as a previously investigated PFP risk (such as greater knee medial rotation).23,34 The smaller degree of knee flexion during the DVJ emphasizes the reduction of sagittal-plane control mechanics in athletes with a high-risk knee profile.24 Similar results were documented by Boling et al,4,5 who found that a decreased knee flexion angle and knee flexor strength were risk factors for PFP. Because the large lower limb muscles dampen force most efficiently during sagittal-plane movement, this decreased reliance might demand a greater proportion of the GRFs to be attenuated by smaller sagittal-plane motions or in alternative planes of motion. The findings of KAM and rotator moment would indicate the latter of those 2 options as increases in these variables suggest greater reliance on frontal- and transverse-plane control mechanics to diminish additional landing forces.21,34 In addition, decreased knee flexion signals an increased activation of knee extensors, which in turn can lead to a fixed patella. This finding, coupled with those of the high-risk hip profile, ties into the model of PFP development caused by deleterious hip mechanics in concert with a fixed patella during weightbearing activities.
All the factors that contribute to the high-risk knee profile result in femoral adduction and tibial abduction similar to the mechanics found in dynamic knee valgus. Alternating valgus translations and rotations decrease frontal- and transverse-plane control and result in a greater quadriceps angle (Q-angle) during dynamic movements.37 Q-angle alterations during dynamic movements are theorized to affect the magnitude of the lateral force vector acting on the patella.37 Larger Q-angles can lead to increased lateral force on the patella, which can cause greater lateral patellar tracking.37 This generates wear on the articular cartilage by means of increased patellofemoral joint stress due to inefficient force attenuation mechanics.37 Poor landing mechanics, in conjunction with internal joint pressure, may lead to uneven force distribution during high maneuver tasks that have the potential to disrupt patellar tracking and cause more localized patellofemoral stress.34,37
Maturation
Athletes with a high-risk hip landing profile at baseline who transitioned to postpubertal status at follow-up were more likely to also move to a low-risk hip landing profile compared with those who had not reached postpubertal status at follow-up. While the change in the hip profile classification was affected by maturation, the change in the knee profile classification was not. This finding indicates that puberty may have a protective effect on hip stabilization mechanics but no such effect on knee stabilization mechanics.
Pubertal maturation is associated with rapid growth and increases in center of mass as well as bone length. While male patients see an increase in their vertical height and a decrease in their landing forces during maturation, female patients experience relatively little to no change in either measure.22,24 In female patients, the lateral component of these landing forces (the vector that contributes to shear force on the patella) is correlated with larger knee torques.41 Although both male and female patients demonstrate similar valgus motion before puberty, male patients display less frequent valgus relative to female patients after pubertal maturation.22,25 This might indicate that male patients go through a neuromuscular spurt during puberty that helps to correct for valgus and decreases dynamic knee valgus, but female patients undergo no such development, which helps explain why their landing forces remain unchanged after pubertal maturation.24 Through puberty, male patients maintain a sagittal-plane landing strategy through increases in quadriceps strength that matches their growth in both stature and mass.8,22
Clinical Implications
The PFP risk increases during adolescence and experiences a sharp decline once athletes enter high school.34 The current study identified high-risk hip and knee mechanics that put female athletes at a higher risk of developing PFP. While the hip and knee profiles are represented as their own separate entities, they are not mutually exclusive. When mechanics from the knee and hip risk profiles are coupled together, they present an out-of-plane landing strategy that shifts joint stabilization from a safe, purely sagittal-plane alignment to one controlled through transverse- and frontal-plane mechanics. In doing so, it is possible for the patella, under weightbearing conditions, to be fixed in place and the femur to rotate in directions that decrease the contact surface area, thereby increasing the point stress within the femoral trochlear groove. Over time, this increased point stress causes wear on the articular cartilage and results in the development of patellofemoral arthritis. The short- and long-term pain that can be caused by increased point stress makes it vital to identify methods to correct for high-risk behavior.
Previous biomechanical analyses have identified altered or decreased neuromuscular control in athletes with symptomatic PFP.3,43 In addition, athletes with PFP have demonstrated primary motor cortex (M1) reorganization that may result in a loss of intermuscle coordination and the implementation of oversimplified movement mechanics during dynamic tasks.44 Such neuroplastic reorganization could further inhibit those with the high-risk knee profile as a loss of M1 organization could lead to further decreases in knee flexion. These possible changes make it vital to determine the optimal time to introduce female athletes to protective neuromuscular interventions to properly modify muscle recruitment strategies during puberty and prevent alterations in neural configuration.
Our findings indicate that the optimal time to begin training female athletes is when adolescence begins and athletes experience peak growth rates for height. Such strategies should emphasize protective knee mechanics as the current study found that while maturation may contribute a protective effect on hip mechanics, it does not seem to have such an effect on knee mechanics. Previous studies have revealed that real-time biofeedback integrated with plyometric and neuromuscular training is effective for resolving risky joint stabilization mechanics associated with anterior cruciate ligament injuries.9,10,13 While these strategies can prove useful if implemented within the high-risk knee profile, the hip profile would benefit from the infusion of more targeted hip and trunk exercises to promote safer mechanics.32 The utilization of a comprehensive neuromuscular training regimen allows athletes to implement controlled sagittal-plane mechanics during high-velocity maneuvers to evenly distribute joint and reaction forces across the medial and lateral compartments of the knee and decrease lateral patellar stresses.35
A limitation of the current study is that static alignment and muscle strength were not assessed as part of the data set, and as such, it could not be determined for certain how muscle power was altered during maturation. Future research could prospectively evaluate how these variables changed during maturation as these factors were not included in the current study but might still relate to the development of PFP. The current study indicates that decreased hip extensor and knee flexor power are risk factors for PFP, but without a specific examination of these factors, this cannot be determined with certainty.
CONCLUSION
In this analysis of longitudinally collected data, we found that the progression from prepubertal to postpubertal status may have a protective effect on high-risk hip mechanics but no such effect on high-risk knee mechanics. Female athletes entering puberty who demonstrate high-risk landing mechanics may benefit from neuromuscular training targeted to their deficits.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Oliver Faul and Tron Krosshaug for their support in the development of the images for the current article.
One or more of the authors has declared the following potential conflict of interest or source of funding: Funding support was received from the National Institute of Arthritis and Musculoskeletal and Skin Diseases/National Institutes of Health (grants R21AR065068-01A1, U01AR067997, R01-AR049735, R01-AR055563, and R01-AR056259).
Contributor Information
Ryan T. Galloway, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Duke University School of Medicine, Durham, North Carolina, USA.
Yingying Xu, Division of General and Community Pediatrics, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Timothy E. Hewett, Orthopedic Biomechanics Laboratory, Mayo Clinic, Rochester, Minnesota, USA; Departments of Orthopedic Surgery, Physical Medicine & Rehabilitation, and Physiology & Biomedical Engineering, Mayo Clinic, Rochester, Minnesota, USA.
Kim Barber Foss, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Rocky Mountain University of Health Professions, Provo, Utah, USA; Department of Allied Health, Northern Kentucky University, Highland Heights, Kentucky, USA.
Adam W. Kiefer, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; Center for Cognition, Action & Perception, University of Cincinnati, Cincinnati, Ohio, USA.
Christopher A. DiCesare, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Robert A. Magnussen, Department of Orthopaedics, The Ohio State University College of Medicine, Columbus, Ohio, USA; Sports Health and Performance Institute, The Ohio State University Wexner Medical Center, Columbus, Ohio, USA.
Jane Khoury, Division of Biostatistics and Epidemiology, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Kevin R. Ford, Department of Physical Therapy, High Point University, High Point, North Carolina, USA.
Jed A. Diekfuss, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA.
Dustin Grooms, Ohio Musculoskeletal and Neurological Institute, Ohio University, Athens, Ohio, USA; Division of Athletic Training, School of Applied Health Sciences and Wellness, College of Health Sciences and Professions, Ohio University, Athens, Ohio, USA.
Gregory D. Myer, Division of Sports Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; Department of Orthopaedic Surgery, University of Cincinnati College of Medicine, Cincinnati, Ohio, USA; The Micheli Center for Sports Injury Prevention, Waltham, Massachusetts, USA.
Alicia M. Montalvo, Department of Athletic Training, Nicole Wertheim College of Nursing and Health Sciences, Florida International University, Miami, Florida, USA..
REFERENCES
- 1.Alentorn-Geli E, Myer GD, Silvers HJ, et al. Prevention of non-contact anterior cruciate ligament injuries in soccer players, part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee Surg Sports Traumatol Arthrosc. 2009;17(8):859–879. [DOI] [PubMed] [Google Scholar]
- 2.Barber Foss KD, Myer GD, Chen SS, Hewett TE. Expected prevalence from the differential diagnosis of anterior knee pain in adolescent female athletes during preparticipation screening. J Athl Train. 2012;47(5):519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolgla LA, Malone TR, Umberger BR, Uhl TL. Comparison of hip and knee strength and neuromuscular activity in subjects with and without patellofemoral pain syndrome. Int J Sports Phys Ther. 2011;6(4):285. [PMC free article] [PubMed] [Google Scholar]
- 4.Boling M, Padua D, Marshall S, Guskiewicz K, Pyne S, Beutler A. Gender differences in the incidence and prevalence of patellofemoral pain syndrome. Scand J Med Sci Sports. 2010;20(5):725–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boling MC, Padua DA, Alexander Creighton R. Concentric and eccentric torque of the hip musculature in individuals with and without patellofemoral pain. J Athl Train. 2009;44(1):7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crossley KM, Bennell KL, Cowan SM, Green S. Analysis of outcome measures for persons with patellofemoral pain: which are reliable and valid? Arch Phys Med Rehabil. 2004;85(5):815–822. [DOI] [PubMed] [Google Scholar]
- 7.Davies PL, Rose JD. Motor skills of typically developing adolescents: awkwardness or improvement? Phys Occupational Ther Pediatr. 2000;20(1):19–42. [PubMed] [Google Scholar]
- 8.DiStefano LJ, Martinez JC, Crowley E, et al. Maturation and sex differences in neuromuscular characteristics of youth athletes. J Strength Cond Res. 2015;29(9):2465–2473. [DOI] [PubMed] [Google Scholar]
- 9.Ericksen HM, Thomas AC, Gribble PA, Armstrong C, Rice M, Pietrosimone B. Jump-landing biomechanics following a 4-week real-time feedback intervention and retention. Clin Biomech. 2016;32:85–91. [DOI] [PubMed] [Google Scholar]
- 10.Ericksen HM, Thomas AC, Gribble PA, Doebel SC, Pietrosimone BG. Immediate effects of real-time feedback on jump-landing kinematics. J Orthop Sports Phys Ther. 2015;45(2):112–118. [DOI] [PubMed] [Google Scholar]
- 11.Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46(2):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finnoff JT, Hall MM, Kyle K, Krause DA, Lai J, Smith J. Hip strength and knee pain in high school runners: a prospective study. PM R. 2011;3(9):792–801. [DOI] [PubMed] [Google Scholar]
- 13.Ford KR, DiCesare CA, Myer GD, Hewett TE. Real-time biofeedback to target risk of anterior cruciate ligament injury: a technical report for injury prevention and rehabilitation. J Sport Rehabil. 2015;Technical Notes 13:2013–0138. [DOI] [PubMed] [Google Scholar]
- 14.Ford KR, Myer GD, Hewett TE. Longitudinal effects of maturation on lower extremity joint stiffness in adolescent athletes. Am J Sports Med. 2010;38(9):1829–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford KR, Myer GD, Hewett TE. Valgus knee motion during landing in high school female and male basketball players. Med Sci Sports Exerc. 2003;35(10):1745–1750. [DOI] [PubMed] [Google Scholar]
- 16.Ford KR, Shapiro R, Myer GD, Van Den Bogert AJ, Hewett TE. Longitudinal sex differences during landing in knee abduction in young athletes. Med Sci Sports Exerc. 2010;42(10):1923–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fulkerson JP. Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med. 2002;30(3):447–456. [DOI] [PubMed] [Google Scholar]
- 18.Fulkerson JP, Arendt EA. Anterior knee pain in females. Clin Orthop Relat Res. 2000;372:69–73. [DOI] [PubMed] [Google Scholar]
- 19.Goodfellow J, Hungerford D, Woods C. Patello-femoral joint mechanics and pathology, 2: chondromalacia patellae. J Bone Joint Surg Br. 1976;58(3):291–299. [DOI] [PubMed] [Google Scholar]
- 20.Grana W, Kriegshauser L. Scientific basis of extensor mechanism disorders. Clin Sports Med. 1985;4(2):247–257. [PubMed] [Google Scholar]
- 21.Hewett TE, Myer GD. The mechanistic connection between the trunk, hip, knee, and anterior cruciate ligament injury. Exerc Sport Sci Rev. 2011;39(4):161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hewett TE, Myer GD, Ford KR. Decrease in neuromuscular control about the knee with maturation in female athletes. J Bone Joint Surg Am. 2004;86(8):1601–1608. [DOI] [PubMed] [Google Scholar]
- 23.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. [DOI] [PubMed] [Google Scholar]
- 24.Hewett TE, Myer GD, Ford KR, Slauterbeck JR. Preparticipation physical exam using a box drop vertical jump test in young athletes: the effects of puberty and sex. Clin J Sport Med. 2006;16(4):298–304. [DOI] [PubMed] [Google Scholar]
- 25.Hewett TE, Stroupe AL, Nance TA, Noyes FR. Plyometric training in female athletes: decreased impact forces and increased hamstring torques. Am J Sports Med. 1996;24(6):765–773. [DOI] [PubMed] [Google Scholar]
- 26.Higgins LD, Taylor MK, Park D, et al. Reliability and validity of the International Knee Documentation Committee (IKDC) subjective knee form. Joint Bone Spine. 2007;74(6):594–599. [DOI] [PubMed] [Google Scholar]
- 27.Kellis E, Tsitskaris GK, Nikopoulou MD, Moiusikou KC. The evaluation of jumping ability of male and female basketball players according to their chronological age and major leagues. J Strength Cond Res. 1999;13(1):40–46. [Google Scholar]
- 28.Kujala UM, Jaakkola LH, Koskinen SK, Taimela S, Hurme M, Nelimarkka O. Scoring of patellofemoral disorders. Arthroscopy. 1993;9(2):159–163. [DOI] [PubMed] [Google Scholar]
- 29.Lo YT, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika. 2001;88(3):767–778. [Google Scholar]
- 30.Malina RM, Bouchard C. Growth, Maturation, and Physical Activity. Champaign, Illinois: Human Kinetics; 1991. [Google Scholar]
- 31.Muthen LK, Muthen BO. Mplus User’s Guide, Version 7. Los Angeles, CA: Mathén & Mathén; 2007. [Google Scholar]
- 32.Myer GD, Chu DA, Brent JL, Hewett TE. Trunk and hip control neuromuscular training for the prevention of knee joint injury. Clin Sports Med. 2008;27(3):425–448, ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myer GD, Faigenbaum AD, Foss KB, et al. Injury initiates unfavourable weight gain and obesity markers in youth. Br J Sports Med. 2014;48(20):1477–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Myer GD, Ford KR, Barber Foss KD, et al. The incidence and potential pathomechanics of patellofemoral pain in female athletes. Clin Biomech (Bristol, Avon). 2010;25(7):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myer GD, Ford KR, McLean SG, Hewett TE. The effects of plyometric versus dynamic stabilization and balance training on lower extremity biomechanics. Am J Sports Med. 2006;34(3):445–455. [DOI] [PubMed] [Google Scholar]
- 36.Powers CM. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40(2): 42–51. [DOI] [PubMed] [Google Scholar]
- 37.Powers CM. The influence of altered lower-extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33(11):639–646. [DOI] [PubMed] [Google Scholar]
- 38.Powers CM, Ho KY, Chen YJ, Souza RB, Farrokhi S. Patellofemoral joint stress during weight-bearing and non-weight-bearing quadriceps exercises. J Orthop Sports Phys Ther. 2014;44(5):320–327. [DOI] [PubMed] [Google Scholar]
- 39.Quatman CE, Ford KR, Myer GD, Hewett TE. Maturation leads to gender differences in landing force and vertical jump performance: a longitudinal study. Am J Sports Med. 2006;34(5):806–813. [DOI] [PubMed] [Google Scholar]
- 40.Sanchis-Alfonso V, Rosello-Sastre E, Martinez-Sanjuan V. Pathogenesis of anterior knee pain syndrome and functional patellofemoral instability in the active young. Am J Knee Surg. 1999;12(1): 29–40. [PubMed] [Google Scholar]
- 41.Sigward SM, Cesar GM, Havens KL. Predictors of frontal plane knee moments during side-step cutting to 45° and 110° men and women: implications for ACL injury. Clin J Sport Med. 2015;25(6):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Souza RB, Draper CE, Fredericson M, Powers CM. Femur rotation and patellofemoral joint kinematics: a weight-bearing magnetic resonance imaging analysis. J Orthop Sports Phys Ther. 2010;40(5):277–285. [DOI] [PubMed] [Google Scholar]
- 43.Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sports Phys Ther. 2009;39(1):12–19. [DOI] [PubMed] [Google Scholar]
- 44.Te M, Baptista AF, Chipchase LS, Schabrun SM. Primary motor cortex organization is altered in persistent patellofemoral pain. Pain Med. 2017;18(11):2224–2234. [DOI] [PubMed] [Google Scholar]
- 45.van den Bogert AJ. Analysis and simulation of mechanical loads on the human musculoskeletal system: a methodological overview. Exerc Sport Sci Rev. 1994;22:23–51. [PubMed] [Google Scholar]
- 46.Watson CJ, Propps M, Ratner J, Zeigler DL, Horton P, Smith SS. Reliability and responsiveness of the lower extremity functional scale and the anterior knee pain scale in patients with anterior knee pain. J Orthop Sports Phys Ther. 2005;35(3):136–146. [DOI] [PubMed] [Google Scholar]
- 47.Woltring HJ, Huiskes R, de Lange A, Veldpaus FE. Finite centroid and helical axis estimation from noisy landmark measurements in the study of human joint kinematics. J Biomech. 1985;18(5):379–389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


