Abstract
Purpose
Despite the wide spectrum of pediatric rhinitis, endotyping of rhinitis based on type 2 inflammation and bronchial hyper-responsiveness (BHR) is lacking. This study aimed to investigate endotypes of pediatric rhinitis using cluster analysis.
Methods
Cluster analysis was performed on data from 241 children with rhinitis by using 12 variables reflecting clinical characteristics of skin prick, laboratory, and pulmonary function tests. After extracting clusters, between-cluster differences in clinical features, such as nasal symptom scores and asthma comorbidity, were assessed to investigate the association between the endotypes and clinical features.
Results
Four clusters were extracted by hierarchical cluster analysis. Cluster 1 (n = 32 [13.3%]) was the non-allergic rhinitis dominant cluster with low type 2 inflammation and the lowest rate of BHR. Patients in cluster 1 had the mildest nasal symptoms and no asthma comorbidity. Cluster 2 (n = 114 [47.3%]) was the largest cluster and exhibited intermediate type 2 inflammation and low BHR. Cluster 3 (n = 65 [27.0%]) showed high type 2 inflammation and intermediate BHR. However, the severity of nasal symptoms and asthma comorbidity in this cluster were comparable with those in cluster 2. Cluster 4 (n = 30 [12.4%]) revealed high type 2 inflammation and BHR with potential functional airway impairment. Additionally, cluster 4 displayed the most severe nasal symptoms and frequent asthma comorbidity.
Conclusions
Four distinct endotypes of pediatric rhinitis based on allergen sensitization, type 2 inflammation, and BHR correlate to symptoms and asthma comorbidity. These endotypes may aid clinicians in understanding the wide spectrum of pediatric rhinitis.
Keywords: Allergic rhinitis, asthma, bronchial hyperreactivity, child, cluster analysis, rhinitis, bronchial hyper-responsiveness, pediatric rhinitis, type 2 inflammation
INTRODUCTION
Rhinitis is an inflammatory disease of the nasal mucosa characterized by nasal obstruction, rhinorrhea, nasal itching, and/or sneezing, and these symptoms can impair patients’ quality of life. Epidemiological correlations between rhinitis and asthma have been investigated and found that they are common comorbidities, which led to the acceptance of the “one airway disease” concept in clinical practice.1,2 Hence, rhinitis is considered a risk factor for the development of asthma,3,4,5 and the treatment of rhinitis may help reduce asthma morbidity.6 Allergic rhinitis (AR) is the most common type of chronic rhinitis and accounts for approximately 75% of all cases.7 Allergic Rhinitis and its Impact on Asthma guidelines indicate that persistence (intermittent/persistent) and severity (mild/moderate to severe) are 2 separate components of AR and that both components help define its phenotype.8 However, these components may not be sufficient to describe the heterogeneous features of AR. As asthma is an important comorbidity of rhinitis, associated factors, such as serum eosinophilia and bronchial hyper-responsiveness (BHR), should be considered when evaluating rhinitis.9 Moreover, sensitization to allergens may contribute to determining clinical features of rhinitis. Therefore, a comprehensive approach to investigating endotypes of rhinitis is required to distinguish groups of patients presenting homogeneous clinical characteristics and to establish management plans.
Unsupervised statistical techniques, such as cluster analysis, have recently emerged to provide data-driven disease stratification. Unsupervised statistical methods stratify a population with a wide distribution of related biomarkers and then identify possible underlying endotypes. Investigating endotypes of rhinitis using cluster analysis has been performed in previous studies10,11,12,13; however, cluster analysis for the pediatric population is limited. As a previous study demonstrated higher comorbidities of AR, including asthma, and different clinical features in children compared to adults,14 unsupervised statistical analysis may help elucidate diverse features of pediatric rhinitis. This study investigated endotypes of pediatric rhinitis using cluster analysis.
MATERIALS AND METHODS
Study participants
This study used the data from pediatric patients who were prospectively enrolled in the Allergic Rhinitis Cohort Study for Kids that had been conducted since 2009. Two hundred forty-one children with at least 2 rhinitis symptoms (nasal obstruction, rhinorrhea, sneezing, and itching sensation in the nose) who visited 2 tertiary hospitals between January 2009 and December 2011, underwent routine ear, nose, and throat endoscopic examinations, skin prick tests (SPTs), serum total immunoglobulin E (IgE) measurement, eosinophil counts, conventional spirometry, and methacholine bronchial provocation tests (MBPTs). Written informed consent was obtained from all patients and/or their parents. This study was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (IRB No. 0811-034-262) and Seoul National University Bundang Hospital (IRB No. B-0903-071-401).
Assessment of nasal symptoms
Parents of all children were asked to complete questionnaires regarding their children’s nasal symptoms. The questionnaire consisted of a visual analog scale (VAS) score of 0–10 (0: no complaints and 10: worst complaints) about nasal obstruction, rhinorrhea, sneezing, and itchy nose. The total nasal VAS score was calculated as the sum of all symptom scores.
SPTs
SPTs were performed using standardized allergen extracts (Allergo-Pharma, Reinbek, Germany). In this study, the allergens were categorized into 7 groups: house dust mites (HDMs; Dermatophagoides pteronyssinus and Dermatophagoides farinae), molds (Alternaria alternata and Aspergillus fumigatus), animal dander (cat and dog), cockroach (German cockroach), trees (mixture 1, mixture 2, and oak), grasses (mixture), and weeds (mugwort and ragweed). Histamine (1.7 mg/mL histamine dihydrochloride) and 0.9% saline were used as positive and negative controls, respectively. A wheal diameter of at least 3 mm was considered positive, and patients sensitized to one or more allergens were considered atopic. The number of sensitized allergens was calculated by summing positive results in each category rather than individual allergens. All participants were required to discontinue oral allergy medications at least 7 days before the test.
Pulmonary function tests (PFTs)
PFTs were performed according to the European Respiratory Society/American Thoracic Society (ATS) guidelines.15 Percent predicted values were used to adjust age, sex, and height on forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC ratio, and forced mid-expiratory flow (FEF25%–75%).16 MBPT was conducted based on the ATS guideline.17 PC20 means provocative concentration required to produce 20% fall in FEV1, and BHR was defined as a metacholine concentration < 16 mg/mL that caused a 20% decrease in FEV1.
Definition of the diseases
AR is diagnosed by a rhinologist and defined as the presence of at least 2 rhinitis symptoms (nasal obstruction, rhinorrhea, sneezing, and itching sensation in the nose) and positive SPT. Non-allergic rhinitis (NAR) is defined as the presence of at least 2 rhinitis symptoms and negative SPT. The diagnosis of asthma was made clinically by a pediatric allergist based on a history of episodic wheezing and/or dyspnea that was resolved after using bronchodilators.
Statistical analysis
Twelve cluster variables that reflect clinical characteristics of pediatric rhinitis were selected: age, sensitization for each allergen (HDM, animal, cockroach, fungus, tree, grass, and weeds), FEV1/FVC ratio, PC20 (log10), serum eosinophil percent, and total IgE. Continuous variables were standardized, and categorical variables were expressed as 0 or 1. For the patients whose BHR was not found with a provocative concentration < 25 mg/mL, MBPT is not performed for patient safety, and the PC20 value was imputed as 50. Due to the skewness of PC20 values in the original scale, data were log10 transformed to improve normality and homoscedasticity. Considering the wide range of total IgE levels, these data were also log10 transformed.
We performed cluster analysis on variables by using hierarchical cluster analysis based on the correlation ratio and exploratory factor analysis (EFA) of mixed data.18 Orthogonal rotation with the varimax method was performed, and only variables with factor loadings > 0.4 were retained. Ward’s minimum-variance was used for hierarchical clustering.19 For cluster analysis of individual cases, the Euclidean distance was calculated, which allows for both ordinal and binomial data. Next, the optimal number of clusters was determined by NbClust in R software package, which uses a variety of indices to find the optimal number of clusters ranging from 2 to 15 in a partitioning of a data set during the clustering process.20 To aid in interpreting cluster characteristics, a modified heat map was obtained to visualize the difference in parameters between the clusters using the ComplexHeatmap package.21
For continuous variables, results are expressed as median and interquartile range (IQR), or as box and whisker plots. In the multiple comparison analysis, between-cluster differences of continuous values were tested using the Kruskal-Wallis test, and categorical values were evaluated using the Fisher exact test or χ2 test. The Benjamini & Hochberg Procedure was performed for multiple tests.22 A Kaplan-Meier (KM) curve was generated to evaluate asthma comorbidity. Cox regression and Cox regression with Firth’s penalized likelihood23 were performed to elicit hazard ratio (HR) between the clusters. All statistical analyses were conducted using R for Windows version 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
A total of 241 pediatric patients with rhinitis aged 5.5–15.4 years were analyzed in the present study. The clinical characteristics of all participants are shown in Supplementary Table S1. The median value for the number of sensitized allergens was 2 (IQR: 1–2), and 84.6% of patients were sensitized to at least 1 allergen (atopic). The median value for serum eosinophil percent was 5.3 (IQR: 2.9–8.6), and BHR was found in 34.9% of all participants.
Clustering outcomes
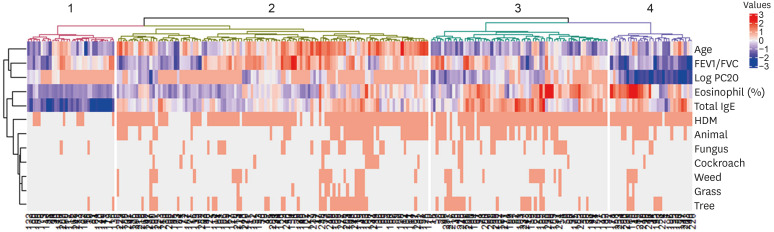
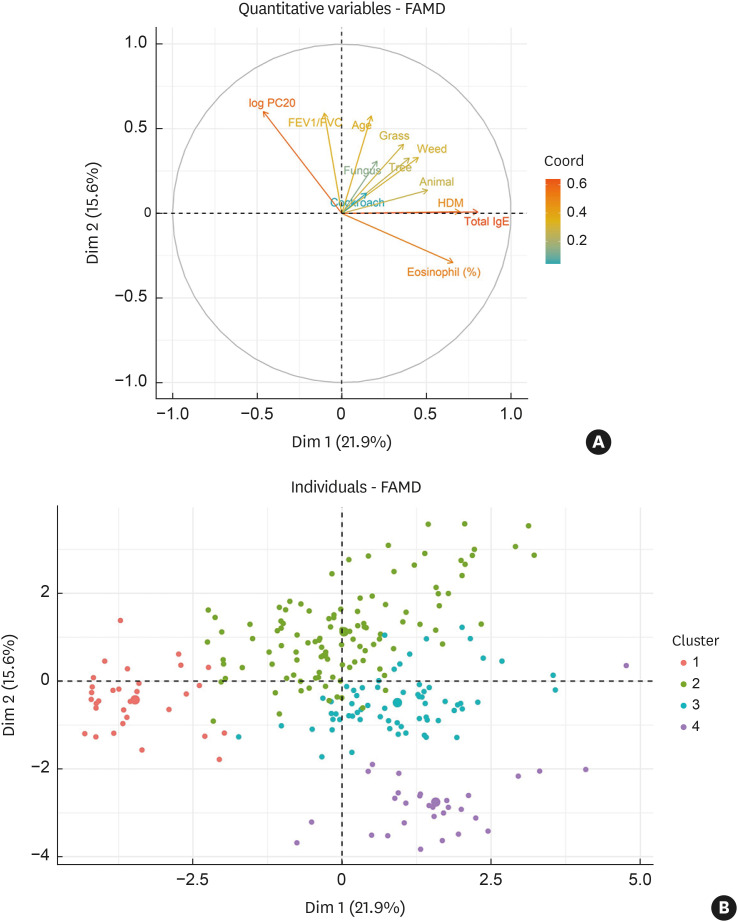
Four clusters were established as the optimal number through hierarchical clustering (Fig. 1). Relevant parameters in the generation of the diversity of pediatric rhinitis were driven by 4 variable groups based on the hierarchical clustering method (Fig. 2A): (1) age, and sensitization to HDM, animal, cockroach, fungus, tree, grass, and weed (first quadrant); (2) FEV1/FVC and PC20 (second quadrant); and (3) serum eosinophil percent, serum total IgE levels (fourth quadrant). Serum eosinophil percent and PC20 were negatively associated, indicating a positive relationship between type 2 inflammation and BHR. EFA retained 5 dimensions explaining 65.4% of all variances in the data (Supplementary Table S2) and the first and second dimensions accounted for 37.5% of the total variance. Individual patients were presented in the first and second dimensions categorized by cluster, and the clusters were separated from each other (Fig. 2B). A modified heatmap with clinical parameters was generated for cluster characterization (Fig. 3).
Fig. 1. Heatmap of the hierarchical clustering. The dendrogram on the left side shows cluster variables, and that on the bottom shows the 241 pediatric patients in this study. A total of 4 clusters of pediatric patients were obtained as illustrated on the top of dendrogram.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PC20, provocative concentration < 16 mg/mL that caused a 20% decrease in forced expiratory volume in one second; IgE, immunoglobulin E; HDM, house dust mite.
Fig. 2. Exploratory factor analysis based on principal component analysis and multiple correspondence analysis. The first and second principal components accounted for 21.9% and 15.6% of the total variance, respectively. (A) Cluster variables including age, type 2 inflammatory markers, parameters for bronchial hyper-responsiveness, and sensitization to allergens against the first 2 principal components. Arrows are displayed for cluster variables against the first 2 principal components. (B) Individual patients were categorized by the cluster against first 2 principal components. The dots are displayed for individual patients, and they are divided into 4 colors based on the cluster.
FAMD, factor analysis of mixed data; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; PC20, provocative concentration < 16 mg/mL that caused a 20% decrease in forced expiratory volume in one second; IgE, immunoglobulin E; HDM, house dust mite.
Fig. 3. Modified heat map of clinical and immunological parameters according to clusters. Rows consists of clinical and immunological parameters including variables used for cluster analysis, and columns define clusters of children with AR. Median values for each parameter are provided for each cluster. FEV1, FVC, FEV1/FVC, FEF25%–75% were adjusted for age, sex, height, and weight and presented as percent predicted values. For characterization of clusters, multiple group comparison for between-cluster differences is visualized with a color code, as shown in the legend. Because low values of FEV1, FVC, FEV1/FVC, FEF25%–75%, and PC20 indicate bronchial hyper-responsiveness and impaired lung function, the lower values of these parameters were colored for readability.
FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FEF25%–75%, forced mid-expiratory flow; HDM, house dust mite; PC20, provocative concentration < 16 mg/mL that caused a 20% decrease in forced expiratory volume in one second.
*The variables including in the clustering analysis.
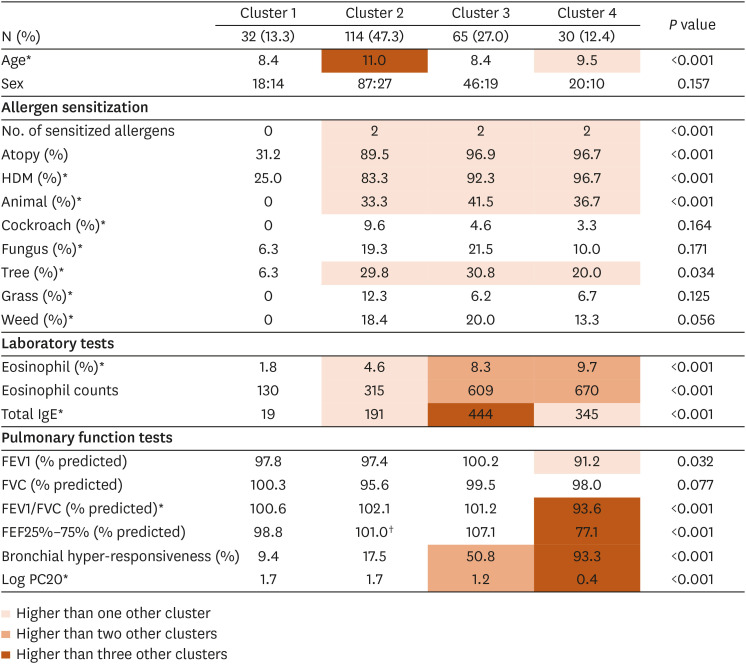
Cluster 1 (n = 32 [13.3%]): NAR dominant cluster with low type 2 inflammation
Cluster 1 was characterized by a large portion of NAR patients. As 68.8% were NAR patients, the number of sensitized allergens was lower in cluster 1 than in other clusters. In this cluster, the serum eosinophilia and total IgE levels were the lowest among all clusters. Furthermore, the rate of BHR was the lowest, and PC20 was the highest in this cluster, indicating that BHR was the lowest in cluster 1.
Cluster 2 (n = 114 [47.3%]): intermediate type 2 inflammation and low BHR
Cluster 2, the largest cluster, was characterized by intermediate type 2 inflammation (Fig. 3). Approximately 90% of the patients in cluster 1 were atopic and had higher serum eosinophilia and total IgE levels than those in cluster 1. The rate of BHR and PC20 were comparable to cluster 1, suggesting that BHR severity was low.
Cluster 3 (n = 65 [27.0%]): high type 2 inflammation and intermediate BHR
Nearly all participants in cluster 3 were atopic (96.9%). Cluster 3 exhibited high type 2 inflammation and intermediate BHR. Patients in this cluster had high serum eosinophilia and total IgE levels (Fig. 3). The rates of BHR and PC20 were intermediate, indicating intermediate BHR severity.
Cluster 4 (n = 30 [12.4%]): high type 2 inflammation and BHR
Cluster 4 mostly consisted of atopic patients (96.7%). Cluster 4 was characterized by high type 2 inflammation and BHR (Fig. 3). Patients in this cluster had the highest serum eosinophilia and total IgE levels. The BHR rate was the highest in this cluster (93.3%), and PC20 was the lowest, indicating high BHR. Furthermore, the low percent predicted FEV1/FVC and FEF25%–75% suggested functional airway impairment.
Nasal symptoms score and asthma comorbidity
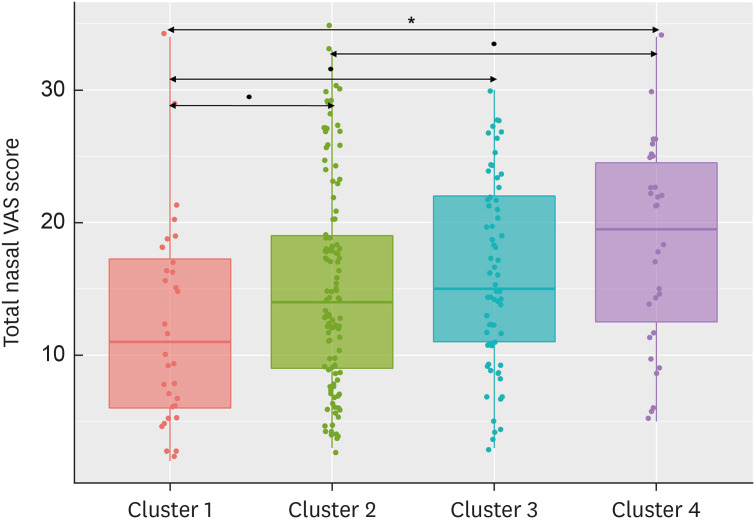
There was a significant difference in total nasal VAS scores across all clusters (P = 0.016, Fig. 4). The total nasal VAS score was the lowest in cluster 1 amongst all clusters, and was the highest in cluster 4. The VAS scores for 4 nasal symptoms are shown in Supplementary Fig. S1. Nasal obstruction and rhinorrhea were not significantly different across clusters (P = 0.441 and P = 0.111, respectively), while nasal itching and sneezing significantly differed across clusters (P = 0.050 and P = 0.002, respectively). Cluster characteristics are summarized in Table.
Fig. 4. Total nasal VAS score in each cluster. Total nasal VAS score was significantly different across all clusters (P = 0.016), and cluster 4 had the highest total nasal VAS score among all clusters.
VAS, visual analog scale.
*P < 0.05; †P < 0.10.
Table. Summary characterization of pediatric AR clusters.
| Cluster | Description | No. of sensitized allergens | Type 2 inflammation | BHR | Airway function impairment | Nasal symptoms | Asthma morbidity |
|---|---|---|---|---|---|---|---|
| 1 (13.3%) | NAR dominant cluster with low type 2 inflammation | +− | + | + | − | + | − |
| 2 (47.3%) | Intermediate type 2 inflammation and low BHR | ++ | ++ | + | − | ++ | + |
| 3 (27.0%) | High type 2 inflammation and intermediate BHR | ++ | +++ | ++ | − | ++ | + |
| 4 (12.4%) | High type 2 inflammation and BHR | ++ | +++ | +++ | + | ++ | ++ |
BHR, bronchial hyper-responsiveness; NAR, non-allergic rhinitis.
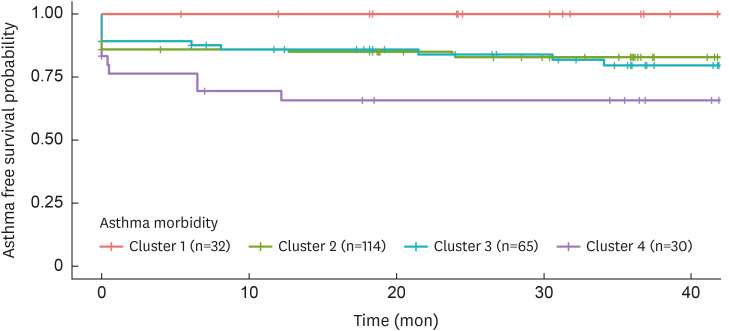
The median follow-up period to evaluate asthma morbidity was 37.5 months (IQR: 18.2–42.7). A KM curve showed a significant difference in asthma-free survival rates across clusters (P = 0.006; Fig. 5). At the time of enrollment, 0%, 14.0% (16/114), 10.8% (7/65), and 16.6% (5/30) of patients in each cluster had already been diagnosed with asthma, respectively. During the follow-up periods, 0%, 2.6% (3/114), 7.7% (5/65), and 16.6% (5/30) of patients in each cluster were additionally diagnosed with asthma, respectively. Therefore, clusters 2, 3, and 4 had higher asthma comorbidity compared to cluster 1 (cluster 2: HR of 11.71 with 95% confidence interval [CI] [1.61–inf], cluster 3: HR of 13.21 with 95% CI [1.74–inf], and cluster 4: HR of 25.21 with 95% CI [3.25–inf]). Additionally, cluster 4 tended to have higher asthma comorbidity compared to cluster 2 (HR of 2.13 with 95% CI [0.99–4.58].
Fig. 5. Kaplan-Meier plot for asthma morbidity according to clusters. Twenty-four patients (11.6%) had been diagnosed with asthma prior to time of enrollment (cluster 1: 0%, cluster 2: 14%, cluster 3: 10.8%, and cluster 4: 16.6%). During the follow-up periods, 13 (5.4%) patients were additionally diagnosed with asthma (cluster 1: 0%, cluster 2: 2.6%, cluster 3: 7.7%, and cluster 4: 16.6%). Clusters 2, 3, and 4 had higher asthma comorbidity compared with cluster 1 (cluster 2: HR of 11.71 with 95% CI [1.61–inf], cluster 3: HR of 13.21 with 95% CI [1.74–inf], and cluster 4: HR of 25.21 with 95% CI [3.25–inf]). In addition, cluster 4 tended to have higher asthma comorbidity compared with cluster 2 (HR of 2.13 with 95% CI [0.99–4.58]).
HR, hazard ratio; CI, confidence interval.
In summary, cluster 1 had mild rhinitis symptoms and no asthma comorbidity; however, the VAS score for nasal obstruction in cluster 1 was comparable to other clusters. Clusters 2 and 3 had moderate rhinitis symptoms and asthma comorbidity. Cluster 4 had severe rhinitis symptoms and asthma comorbidity.
DISCUSSION
In this study, 4 endotypes of pediatric rhinitis were obtained using cluster analysis based on allergen sensitization, type 2 inflammation, and BHR, unbiased by symptoms and comorbidity data. Interestingly, these endotypes also correlated with rhinitis symptoms and asthma comorbidity, supporting their clinical relevance. Cluster analysis, an unsupervised statistical method, provided the characterization of pediatric rhinitis without preconceived assumption bias, and these endotypes may be helpful in understanding baseline pathogenesis and the mechanisms of pediatric rhinitis.
A NAR-dominant cluster and 3 AR dominant clusters were obtained from this analysis. Cluster 1 was the NAR-dominant cluster with the lowest type 2 inflammation and BHR, and accounted for 13.3% of all patients. Although NAR is more frequently found in adults, it can also be present in children.24 However, due to a lack of pediatric studies regarding the classification of NAR, most concepts of NAR have been derived from the pathology of adult NAR. Cluster analysis revealed distinct characteristics of rhinitis symptoms in cluster 1, including low nasal itching and sneezing score, but showed a comparable nasal obstruction score to other clusters. Although childhood asthma is commonly associated with allergies,25 asthma can also be observed in NAR patients. However, no asthmatic patients in cluster 1 were observed during the follow-up period in this study. This may have been attributed to the fact that non-atopic asthma is usually late-onset asthma, although further studies with a large population of NAR are needed to confirm our results
Cluster 2, the largest cluster, accounted for approximately half of the patients (47.3%) and was characterized as an AR dominant cluster with intermediate type 2 inflammation and low BHR. This cluster exhibited the lowest type 2 inflammation among the AR dominant clusters. Although the level of type 2 inflammatory markers (serum eosinophilia and total IgE) in cluster 2 was higher than cluster 1 (NAR dominant cluster with low type 2 inflammation and BHR), it was lower than in clusters 3 and 4. This low type 2 inflammation might be associated with mild rhinitis symptoms.
Clusters 3 and 4 consisted of almost all AR patients and exhibited high type 2 inflammation. However, cluster 4 was distinguished from cluster 3 with high BHR and impaired airway function, indicating possible lower airway inflammation. Despite the high type 2 inflammation in cluster 3, interestingly, the severity of rhinitis symptoms and asthma comorbidity were not as high as in cluster 4. It is speculated that cluster 3 might progress to cluster 4. In contrast to the significant difference in type 2 inflammatory markers and BHR between clusters 2 and cluster 3, the severity of rhinitis symptoms and asthma comorbidity were comparable. However, continuation or progress of type 2 inflammation and BHR may cause impaired airway function that presents as severe rhinitis symptoms and airway function impairment, which was observed in cluster 4. The development of asthma in pediatric rhinitis patients with high serum eosinophilia levels should be examined with further studies.
Rhinitis is known as a risk factor for asthma development; however, the distinct characteristics of rhinitis that can develop into asthma have not been well-established.5,26 This cluster analysis provides insight into which types of pediatric patients with rhinitis are likely to have asthma comorbidity: patients with high type 2 inflammation and BHR. This suggests that physicians should carefully monitor the development of asthma in children with rhinitis presenting with high eosinophilia levels and BHR. Importantly, the correlation between the endotypes and rhinitis symptoms or asthma comorbidity was analyzed after extracting clusters, and symptom and comorbidity data were not used as cluster variables.
Concerning allergen sensitization, some points were noted in this study. The number of sensitized allergens did not reflect the nasal symptoms and proportion of asthma in patients with AR. Although AR-dominant clusters had more severe symptoms and frequent comorbid asthma than NAR-dominant cluster, there was no significant difference in the number of sensitized allergens among the AR-dominant clusters, which was consistent with our previous study findings.27 HDM might play a major role as a dominant allergen in the Korean population, as described in our previous study.27 In addition, while sensitization to HDM seemed to be strongly associated with type 2 inflammation, sensitization to other allergens did not seem to be associated with type 2 inflammation and did not contribute to classifying AR-dominant clusters. This result suggests that other factors such as type 2 inflammation and BHR are more important to determine AR endotypes than sensitization to other allergens.
The present study has several limitations. First, pharmacotherapy of rhinitis at the time of enrollment may influence the clinical parameters. Second, although cluster analysis revealed the characteristics of the endotypes of pediatric rhinitis, data on the natural course of the endotypes is limited. Furthermore, patients visited clinics during a median of 37.5 months (IQR: 18.2–42.7); however, a longer follow-up period is required to evaluate asthma comorbidity more thoroughly. Lastly, although this study provides a concept for endotyping pediatric rhinitis, closely related to type 2 inflammation and BHR, specific criteria for endotyping should be developed for classifying actual pediatric patients with rhinitis in future studies. Despite these limitations, the present study investigated 4 endotypes of pediatric rhinitis and suggests that pediatric rhinitis with high type 2 inflammation and BHR may have severe rhinitis symptoms and asthma comorbidities, which indicates the “one airway, one disease” concept. Rhinitis can be classified according to the atopic status: AR and NAR. However, this cluster analysis revealed further stratification of AR based on type 2 inflammation and BHR beyond the simple rhinitis classification, which correlated with symptoms severity and asthma comorbidity. In this study, some cluster variables were related to each other (e.g., serum eosinophilia and PC20), and some had a well-known association with allergic symptoms. However, we integrated these clinical parameters using an unbiased method beyond a simple association and showed rhinitis endotypes that correlated with nasal symptoms and comorbid asthma. In the future, the clinical course with controlled treatment tools for patients in these clusters should be assessed, and treatment responses based on the clusters should also be evaluated to determine the optimal treatment regimens for each endotype.
In conclusion, this cluster analysis derived 4 distinct endotypes of pediatric rhinitis based on sensitization to allergens, type 2 inflammation, and BHR, unbiased by symptoms and comorbidity. These endotypes also correlated to the severity of rhinitis symptoms and asthma comorbidity. Although the natural course of these clusters should be assessed in the future, these endotypes reflecting the type 2 inflammation and BHR may help clinicians understand the wide spectrum of pediatric rhinitis.
ACKNOWLEDGMENTS
This study was supported by the Centers for Disease Control and Prevention, Ministry of Health and Welfare, Republic of Korea (grant number: 2012-E33003-00).
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Characteristics of the study participants
Coordinates of given variables in the first 5 dimensions from the results of factor analysis for mixed data
Nasal visual analog scale score in each cluster. (A) Nasal obstruction. (B) Rhinorrhea. (C) Nasal itching. (D) Sneezing. Nasal obstruction and rhinorrhea were not significantly different across all clusters (P = 0.441 and P = 0.111, respectively), while nasal itching and sneezing significantly differed between clusters (P = 0.050 and P = 0.002, respectively).
References
- 1.de Benedictis FM, del Giudice MM, Jr, Severini S, Bonifazi F. Rhinitis, sinusitis and asthma: one linked airway disease. Paediatr Respir Rev. 2001;2:358–364. doi: 10.1053/prrv.2001.0172. [DOI] [PubMed] [Google Scholar]
- 2.Grossman J. One airway, one disease. Chest. 1997;111:11S–6S. doi: 10.1378/chest.111.2_supplement.11s. [DOI] [PubMed] [Google Scholar]
- 3.Rochat MK, Illi S, Ege MJ, Lau S, Keil T, Wahn U, et al. Allergic rhinitis as a predictor for wheezing onset in school-aged children. J Allergy Clin Immunol. 2010;126:1170–1175.e2. doi: 10.1016/j.jaci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 4.van den Nieuwenhof L, Schermer T, Bosch Y, Bousquet J, Heijdra Y, Bor H, et al. Is physician-diagnosed allergic rhinitis a risk factor for the development of asthma? Allergy. 2010;65:1049–1055. doi: 10.1111/j.1398-9995.2009.02316.x. [DOI] [PubMed] [Google Scholar]
- 5.Bousquet J, Vignola AM, Demoly P. Links between rhinitis and asthma. Allergy. 2003;58:691–706. doi: 10.1034/j.1398-9995.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 6.de Groot EP, Nijkamp A, Duiverman EJ, Brand PL. Allergic rhinitis is associated with poor asthma control in children with asthma. Thorax. 2012;67:582–587. doi: 10.1136/thoraxjnl-2011-201168. [DOI] [PubMed] [Google Scholar]
- 7.Settipane RA, Charnock DR. Epidemiology of rhinitis: allergic and nonallergic. Clin Allergy Immunol. 2007;19:23–34. [PubMed] [Google Scholar]
- 8.Bousquet J, Schünemann HJ, Samolinski B, Demoly P, Baena-Cagnani CE, Bachert C, et al. Allergic Rhinitis and its Impact on Asthma (ARIA): achievements in 10 years and future needs. J Allergy Clin Immunol. 2012;130:1049–1062. doi: 10.1016/j.jaci.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 9.Magnan A, Fourre-Jullian C, Jullian H, Badier M, Lanteaume A, Vervloet D, et al. Rhinitis alone or rhinitis plus asthma: what makes the difference? Eur Respir J. 1998;12:1073–1078. doi: 10.1183/09031936.98.12051073. [DOI] [PubMed] [Google Scholar]
- 10.Kurukulaaratchy RJ, Zhang H, Patil V, Raza A, Karmaus W, Ewart S, et al. Identifying the heterogeneity of young adult rhinitis through cluster analysis in the Isle of Wight birth cohort. J Allergy Clin Immunol. 2015;135:143–150. doi: 10.1016/j.jaci.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meng Y, Lou H, Wang Y, Wang X, Cao F, Wang K, et al. Endotypes of chronic rhinitis: a cluster analysis study. Allergy. 2019;74:720–730. doi: 10.1111/all.13640. [DOI] [PubMed] [Google Scholar]
- 12.Burte E, Bousquet J, Varraso R, Gormand F, Just J, Matran R, et al. Characterization of rhinitis according to the asthma status in adults using an unsupervised approach in the EGEA study. PLoS One. 2015;10:e0136191. doi: 10.1371/journal.pone.0136191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bousquet PJ, Devillier P, Tadmouri A, Mesbah K, Demoly P, Bousquet J. Clinical relevance of cluster analysis in phenotyping allergic rhinitis in a real-life study. Int Arch Allergy Immunol. 2015;166:231–240. doi: 10.1159/000381339. [DOI] [PubMed] [Google Scholar]
- 14.Izquierdo-Dominguez A, Jauregui I, Del Cuvillo A, Montoro J, Davila I, Sastre J, et al. Allergy rhinitis: similarities and differences between children and adults. Rhinology. 2017;55:326–331. doi: 10.4193/Rhino17.074. [DOI] [PubMed] [Google Scholar]
- 15.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 16.Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: the global lung function 2012 equations. Eur Respir J. 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am J Respir Crit Care Med. 2000;161:309–329. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 18.Husson F, Josse J, Le S, Mazet J. Package ‘FactoMineR’. An R package. 2016;96:698. [Google Scholar]
- 19.Murtagh F, Contreras P. Algorithms for hierarchical clustering: an overview. Wiley Interdiscip Rev Data Min Knowl Discov. 2012;2:86–97. [Google Scholar]
- 20.Charrad M, Ghazzali N, Boiteau V, Niknafs A. NbClust: an R package for determining the relevant number of clusters in a data set. J Stat Softw. 2014;61:1–36. [Google Scholar]
- 21.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics. 2016;32:2847–2849. doi: 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 22.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 23.Nagashima K, Sato Y. Information criteria for Firth’s penalized partial likelihood approach in Cox regression models. Stat Med. 2017;36:3422–3436. doi: 10.1002/sim.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poddighe D, Gelardi M, Licari A, Del Giudice MM, Marseglia GL. Non-allergic rhinitis in children: epidemiological aspects, pathological features, diagnostic methodology and clinical management. World J Methodol. 2016;6:200–213. doi: 10.5662/wjm.v6.i4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pakkasela J, Ilmarinen P, Honkamäki J, Tuomisto LE, Andersén H, Piirilä P, et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm Med. 2020;20:9. doi: 10.1186/s12890-019-1040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan DA. Allergic rhinitis and asthma: epidemiology and common pathophysiology. Allergy Asthma Proc. 2014;35:357–361. doi: 10.2500/aap.2014.35.3794. [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Han DH, Kim DY, Park SK, Rhee CS. Does new sensitization correlate with nasal symptoms in children with allergic rhinitis? Laryngoscope. 2020;130:1864–1871. doi: 10.1002/lary.28267. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics of the study participants
Coordinates of given variables in the first 5 dimensions from the results of factor analysis for mixed data
Nasal visual analog scale score in each cluster. (A) Nasal obstruction. (B) Rhinorrhea. (C) Nasal itching. (D) Sneezing. Nasal obstruction and rhinorrhea were not significantly different across all clusters (P = 0.441 and P = 0.111, respectively), while nasal itching and sneezing significantly differed between clusters (P = 0.050 and P = 0.002, respectively).