Abstract
Purpose
Respiratory viral infection increases the number of lung-resident T lymphocytes, which enhance cough sensitivity by producing interferon-γ (IFN-γ). It is poorly understood why IFN-γ-secreting T lymphocytes persist for a long time when the respiratory viruses have been removed.
Methods
Repeated pulmonary administration of IFN-γ and intraperitoneal injection with different inhibitors were used to study the effects of pulmonary IFN-γ in mice and guinea pigs.
Results
IFN-γ administration caused the increasing of IFN-γ-secreting T lymphocytes in both lung and blood, followed by the elevated physiological level of IFN-γ in the lung, the airway inflammation and the airway epithelial damage. IFN-γ administration also enhanced the cough sensitivity of guinea pigs. IFN-γ activated the STAT1 and extracellular signal-regulated kinase (ERK) pathways in lung tissues, released IFN-γ-inducible protein 10 (IP-10), and resulted in F-actin accumulation in lung-resident lymphocytes. The CXC chemokine receptor 3 (CXCR3) inhibitor potently suppressed all the IFN-γ-induced inflammatory changes. The STAT1 inhibitor mitigated IFN-γ-secreting T lymphocytes infiltration by inhibiting T lymphocytes proliferation. F-actin accumulation and the ERK1/2 pathway contributed to pulmonary IFN-γ-induced augmentation of the airway inflammation and increasing of IFN-γ-secreting T lymphocytes in blood.
Conclusions
High physiological levels of IFN-γ in the lung may cause pulmonary lymphocytic inflammation and cough hypersensitivity by increasing the number of IFN-γ-secreting T lymphocytes through the IP-10 and CXCR3 pathways.
Keywords: Interferon-gamma, T lymphocytes, cough, CXC chemokine receptor 3
INTRODUCTION
Post-infectious persistent cough and chronic refractory cough are common conditions associated with respiratory virus infection.1,2 The respiratory viruses generally become undetectable from the patient within one to two weeks after symptom onset.3,4,5 However, post-infectious persistent cough lasts for more than three weeks following a respiratory viral infection and even develops to chronic refractory cough. The respiratory viral infection can increase the number of lung-resident T lymphocytes, which mainly produce interferon-γ (IFN-γ).6 Both lymphocytes and IFN-γ are crucial factors for controlling acute viral infections during the acute stage of respiratory syncytial viral infection,7 but previous studies have discovered that IFN-γ can enhance the cough reflex sensitivity via calcium influx in vagal sensory neurons.8,9 Although the respiratory viruses have been removed, these patients with cough have a high level of IFN-γ and an increased number of T lymphocytes in the lung.10,11 An increased number of pulmonary lymphocytes are also found in multiple chronic airway diseases, including severe asthma, severe chronic obstructive pulmonary disease (COPD) and bronchiectasis.12 Pulmonary T lymphocyte infiltration, airway inflammation and chronic cough are common in patients with Sjögren’s syndrome, which are relevant to recurrent pulmonary infections.13 Why IFN-γ-secreting T lymphocytes persist for a long time is poorly understood.
IFN-γ-inducible protein 10 (IP-10) is a member of the chemokine ligands binding to CXC chemokine receptor 3 (CXCR3), which is expressed on numerous different cell types (including activated cluster of differentiation 4+ [CD4+] T and CD8+ T lymphocytes).14 IFN-γ stimulates the release of IP-10 by activating the Janus kinase/signal transducers and activators of transcription 1 (JAK/STAT1) signaling pathway in different cell types in mice liver in vivo and in airway epithelia in vitro. 15,16 IP-10 is involved in recruiting CXCR3+IFN-γ+ T and CXCR3+IFN-γ+CD8+ T lymphocytes to peripheral airways of smokers with COPD.17 The IP-10/CXCR3 pathway also contributes to IFN-γ-secreting T lymphocytes recruitment to the mouse lung after allogeneic stem cell transplantation.18 CXCR3-mediated extracellular signal-regulated kinase (ERK) activation in T lymphocytes is crucial for IP-10-induced T lymphocytes migration.19 ERK activation and filamentous actin (F-actin) polarization are involved in the migration of T lymphocytes into peripheral inflammatory sites.20 The intracellular F-actin levels are also associated with the migration of T lymphocytes.21
In view of the above information, the aim of this study was to investigate effects of pulmonary IFN-γ on IFN-γ-secreting T lymphocytes and cough sensitivity in vivo, and to identify the underlying mechanisms for the involvement of the JAK/STAT1 signaling pathway, the IP-10/CXCR3 axis, the ERK pathway and F-actin accumulation. Here we studied the effects of pulmonary IFN-γ on lymphocytic inflammation and the underlying mechanisms in the mice, and guinea pigs were used to study the effects of pulmonary IFN-γ on cough sensitivity.
MATERIALS AND METHODS
Animals
Eight-week-old male specific pathogen-free C57BL/6 mice weighing 20–25 g were purchased from Charles River Laboratories in ZheJiang province (certificate No. SCXK [Zhe] 2019-0001). Male closed colony Hartley guinea pigs weighing 350-400 g were purchased from Charles River Laboratories in Beijing province (certificate No. SCXK [Jing] 2016-0011). All animals were housed under controlled temperature (22 ± 2°C), humidity (50 ± 20%) and lighting (6:30 a.m.–6:30 p.m.) in solid bottom cages with food and water available ad libitum. We used the same batch of healthy animals in every experiment. Animals were excluded from our study if they became emaciated or died in the process of our study. We lost a few sample data due to the death of animals or the technical problems.
Ethics approval statement
Animal experiments received approval from the Animal Care and Use Committee of Guangzhou Medical University (animal ethical approval number: 2021109), and were performed in strict accordance with approved guidelines. All animal experiments were repeated for 3 times.
Compounds and materials
All compounds and materials are provided in Supplementary Table S1.
Study design
Mice were acclimatized for 2 weeks prior to experimentation, and then randomly divided into different groups in two separate experiments. All the sub-studies were of randomized and placebo-controlled design. The experimenter was blind to the treatment groups. In the first experiment, mice were divided into the control, low dose of IFN-γ, medium dose of IFN-γ, and high dose of IFN-γ groups. All the mice were anesthetized before the operation of intranasal instillation. Mice in the control or different doses of IFN-γ groups were intranasally instilled with 50 µL phosphate-buffered saline (PBS) or 3.125, 12.5 or 50 µg/kg recombinant murine IFN-γ dissolved in 50 µL PBS once a day for 5 consecutive days. In the second experiment, mice were divided into the control, IFN-γ model, JAK1 inhibitor (Filgotinib, 1.5 mg/kg), STAT1 inhibitor (Fludarabine, 15 mg/kg), CXCR3 inhibitor (AMG487, 5 mg/kg), Actin polymerization inhibitor (Cytochalasin D, 0.75 mg/kg), and ERK1/2 inhibitor groups (Ravoxertinib, 5 mg/kg). All mice except the control group were intranasally instilled with recombinant murine IFN-γ (50 µg/kg) dissolved in 50 µL PBS once a day for 5 consecutive days. Five minutes later after the instillation, mice in the control and IFN-γ model groups were intraperitoneally injected with the vehicle solution (5% DMSO + 40% PEG300 + 5% Tween-80 + 50% water, 0.2 mL), and mice in the inhibitor groups were intraperitoneally injected with the corresponding inhibitors dissolved in the vehicle solution. The experimental protocols were also supplied as Fig. 1A and Supplementary Tables S2 and S3.
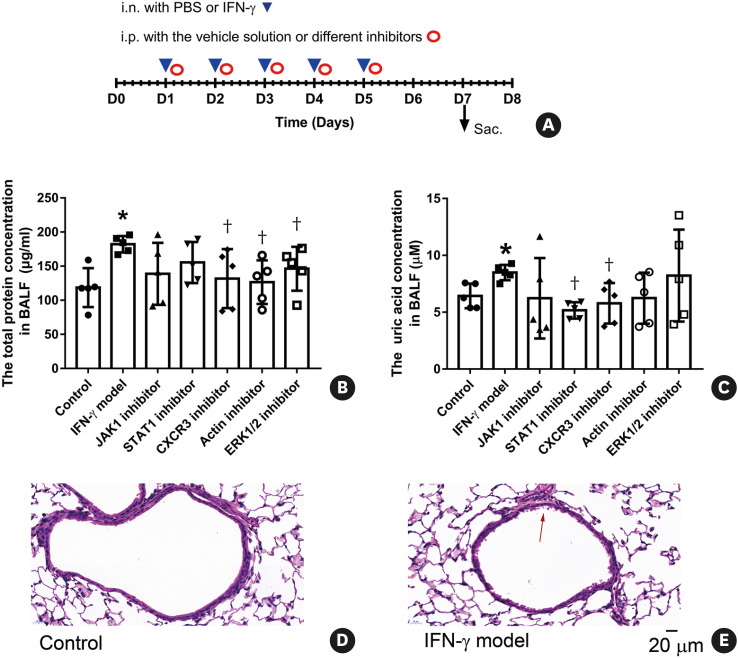
Fig. 1. (A) The experimental protocol for the mice that were treated with IFN-γ and different inhibitors. All the mice were sacrificed 2 days after the last treatment. (B) Effects of IFN-γ and different inhibitors on the total protein concentration in the supernatant of BALF (n = 5 per group). (C) Effects of IFN-γ and different inhibitors on the uric acid concentration in the supernatant of BALF (n = 5 per group). (D-E) Representative figures of pathological changes in hematoxylin and eosin-stained lung sections from the group of “Control” (D) and “IFN-γ model” (E). The symbol of “↑” marks the airway epithelial damage. Scale bars are 20 µm for the electron micrographs. Mice in different groups were treated as follows: the control group (i.n. with PBS + i.p. with the vehicle solution), IFN-γ model group (i.n. with 50 µg/kg IFN-γ + i.p. with the vehicle solution), JAK1 inhibitor group (i.n. with 50 µg/kg IFN-γ + i.p. with 1.5 mg/kg Filgotinib), STAT1 inhibitor group (i.n. with 50 µg/kg IFN-γ + i.p. with 15 mg/kg Fludarabine), CXCR3 inhibitor group (i.n. with 50 µg/kg IFN-γ + i.p. with 5 mg/kg AMG487), Actin polymerization inhibitor group (i.n. with 50 µg/kg IFN-γ + i.p. with 0.75 mg/kg Cytochalasin D), and ERK1/2 inhibitor group (i.n. with 50 µg/kg IFN-γ + i.p. with 5 mg/kg Ravoxertinib). Data are shown as mean ± SD.
i.n., intranasal; PBS, phosphate-buffered saline; IFN-γ, interferon-γ; Sac., sacrifice; BALF, bronchoalveolar lavage fluid; CXCR3, CXC chemokine receptor 3; ERK, extracellular signal-regulated kinase; i.p., intraperitoneal.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
On day 7, all the mice were anesthetized with pentobarbital sodium (150 mg/kg) by intraperitoneal injection. Deep anesthetic mice were bled from the orbits and kept with anticoagulant buffer. After the centrifugation of blood, the supernatant was extracted and stored at −80°C (used for cytokine measurement), and the pellet was resuspended with 1 mL D-Hank’s solution. Ten microliters of the blood cell suspension was spread on the glass slide for determining cell profiles; and other blood cell suspension was subjected to flow cytometry and lymphocytes chemotaxis tests. Bronchoalveolar lavage fluid (BALF) was collected from the left lung with PBS. After the centrifugation of BALF, the supernatant was extracted and used to measure the total protein concentration, the uric acid concentration and cytokine concentrations. The leukocytes in BALF were counted with the help of a counting slide. The hematoxylin-eosin stain was used to determine cell profiles by differential counts. At least 400 cells were classified into neutrophils, macrophages, lymphocytes or eosinophils per slide. After BALF collection, the left lung tissues were processed to single-cell suspensions and applied to flow cytometry. Half of the right lung tissues were fixed with 4% paraformoldehyde at 4°C for next immunofluorescence. The other half were stored at −80°C for western blotting and enzyme-linked immunosorbent assay (ELISA).
All mice were weighed on the first day before intranasal instillation and the seventh day. The body weight changes for 7 days were calculated for comparison and analysis.
Pre-processing of blood cells and lung tissues
Blood cell suspension was gently layered on the top of 1.5 mL of 100% Ficoll-Paque. After centrifugation (800 g, 25 minutes, no brake), peripheral blood mononuclear cells (PBMC) were collected.
The left lung tissues of mice were put into pre-warmed digest cocktail (1.5 mg/mL collagenase IV and 0.15 mg/mL DNase I in PBS), chopped into small pieces. Then the lung pieces were incubated at 37°C for 40 minutes, gently dispersed, filtered through 74-µm nylon meshes, and centrifuged. The resuspended lung single cell suspension was gently layered on the top of 1.5 mL of 50% Ficoll-Paque and 1.5 mL of 100% Ficoll-Paque. After centrifugation with no brake, lung-resident mononuclear cells (LRMCs) were collected.
After the centrifugation of the collected PBMC and LRMC, the residual erythrocytes in pellet were removed with erythrocyte lysis buffer. The extracted PBMC and LRMC were resuspended with PBS and subjected to flow cytometric analyses. Then, a part of the extracted PBMC was subjected to transwell migration assays.
Flow cytometry
For cell surface antigen staining, cells were first incubated with FVS780 for labeling dead cells. After washing, the cell pellets were resuspended with stain buffer, and then murine Fc receptor blocking was added and incubated for 15 minutes. After that surface antibodies, including CD3, CD4, CD8 and CXCR3, were added and incubated for 30 minutes. The flow cytometric gating strategy was described in Supplementary Fig. S1.
For intracellular cytokine staining, cells were first incubated for 2 hours at 37°C in the presence of Golgiplug. Following the incubation period, cells were washed and incubated with FVS780 and murine Fc receptor blocking consecutively. Then cells were incubated with a mixture of CD3, CD4 and CD8 antibodies. After washing, cells were fixed and permeabilized using Cytofix/Cytoperm. After washing, cells were incubated with murine Fc receptor blocking and intracellular antibodies (Ki-67 and IFN-γ). The flow cytometric gating strategy is described in Supplementary Fig. S2.
For staining of intracellular F-actin, the extracted LRMC was first incubated with FVS780. After washing, cells were fixed with 4% paraformoldehyde and permeabilized with 0.2% Triton X-100. Then cells were incubated with Fluorescein Phalloidin for 45 minutes. All flow cytometric analyses were performed using BD FACS VerseTM. All flow cytometric data were analyzed by using FlowJo software (TreeStar, Ashland, OR, USA).
Transwell migration assays
In vitro lymphocyte migration was measured with 200 ng/mL IP-10 in Transwell chambers (5 µm).
Western blotting
Homogenized lung tissues were subjected to Western blotting as described previously.22 The used primary antibodies were Phospho-ERK1/2 Antibody (1:2,000), Phospho-JAK1 Antibody (1:500), Phospho-STAT1 (1:200) and GAPDH Antibody (1:200). Horseradish peroxidase-conjugated immunoglobulin G (IgG) (1:2,500) was used as the secondary antibody. GAPDH was used as an internal standard. An enhanced chemiluminescence detection system was applied to detect the target proteins. The optical density of each band was quantified by using ImageJ software (NIH, Bethesda, MD, USA). Levels of targeted proteins were normalized against GAPDH.
Immunofluorescence staining
After fixation, one lobe of fixed lung of each mouse was frozen, sectioned at 4 µm, and adhered to the slide. The detailed procedures of immunofluorescence staining were described in our previous reports.23 Antibodies used were as follows: primary antibodies were Phospho-JAK1 Antibody (1:200) and Phospho-STAT1 Antibody (1:200); secondary antibodies were AF647-conjugated donkey anti-rabbit IgG (1:500) or AF488-conjugated goat anti-mouse IgG (1:500).
ELISA
The fully homogenized lung tissues were centrifuged at 10,000 g for 20 minutes at 4°C. The supernatant was extracted for ELISA assay. The levels of IP-10 and IFN-γ were measured using commercially available ELISA kits. Optical density was measured at 450 nm using an ELISA microplate reader (Multiskan Go; Thermo Fisher, Waltham, MA, USA). All ELISA results were normalized against the total protein concentration of the detected sample.
Cytokine measurement in the supernatant of BALF
Cytokine concentrations were measured in the supernatant of BALF according to the manufacturer’s instructions, using a Mouse Cytokine Magnetic Bead Panel (MCYTOMAG-70K, Merck, Darmstadt, Germany) on the Luminex-200 platform (Thermo Fisher, Waltham, MA, USA). Data were analyzed with the Milliplex Analyst Software (Millipore, Billerica, MA, USA). Eight detected cytokines were interleukin (IL)-2, IL-4, IL-5, IL-6, IL-10, IP-10, keratinocyte-derived chemokine and tumor necrosis factor-α.
Effects of repeated intratracheal administration of IFN-γ on cough sensitivity and airway inflammation in guinea pigs
Guinea pigs were acclimatized for 2 weeks prior to experimentation, and then randomly divided into the control group and IFN-γ group. All the guinea pigs were anesthetized before intratracheal instillation. Guinea pigs in the control group or the IFN-γ group were intratracheally instilled with 200 µL PBS or 12.5 µg/kg recombinant guinea pig IFN-γ dissolved in 200 µL PBS once a day for 7 consecutive days. Cough sensitivity was detected in all the guinea pigs before the first intratracheal instillation and two days later after the last intratracheal instillation. After completion of the cough responsiveness evaluation, BALF was collected to determine airway inflammation as described previously.8
For the detection of cough sensitivity, conscious unrestrained guinea pigs were placed in individual plastic transparent whole-body plethysmograph chambers (Buxco, St. Paul, MN, USA), and cough was evoked by exposing the animals to aerosolised citric acid (0.3 M) for 10 minutes. Cough events within 10 minutes and time from the citric acid spray to the first cough of animals (time to the first cough) were measured by both pressure changes and sound recorded by the analyzer, and confirmed by a trained observer. If a guinea pig did not cough within 10 minutes, the time to the first cough was recorded as 600 seconds.
Statistical analysis
Values are expressed as mean ± SD. Normality was assessed using Shapiro–Wilk and Kolmogorov–Smirnov tests. For normally distributed data, one-way ANOVA or unpaired student's t-tests (two-tailed) were applied to evaluate the difference between groups. Where data were not normally distributed, Kruskal-Wallis tests or Mann–Whitney tests were applied to evaluate the difference between groups. Values of P < 0.05 were considered statistically significant.
RESULTS
The total protein concentration and the uric acid concentration in BALF
Body weight did not significantly change between different groups (Supplementary Fig. S3), indicating that intranasal instillation of 50 µg/kg IFN-γ might not induce serious systemic inflammatory response. The increased concentration of total protein concentration and uric acid in the supernatant of BALF are associated with inflammation and cell injury in the airway.24 IFN-γ can also increase the permeability of endothelium and epithelium by modifying tight junctions in vitro. 25 Compared with the control group, intranasal instillation of 50 µg/kg IFN-γ significantly increased the total protein concentration and the uric acid concentration in the supernatant of BALF (Fig. 1B and C), implicating the augmentation of the airway inflammation and the airway epithelial damage. Consistently, more severe airway epithelial damage was observed in IFN-γ model mice than control mice (Fig. 1D and E). IFN-γ-induced increasing of the total protein concentration was significantly inhibited by treatment with CXCR3 inhibitor, Actin polymerization inhibitor and ERK1/2 inhibitor. The JAK1 inhibitor and STAT1 inhibitor showed a tendency to inhibit the total protein concentration. IFN-γ-induced increasing of the uric acid concentration was significantly inhibited by treatment with STAT1 inhibitor and CXCR3 inhibitor. The actin polymerization inhibitor, JAK1 inhibitor and ERK1/2 inhibitor showed a tendency to inhibit the uric acid concentration.
Lymphocytes in BALF and blood of mice
The proportion and number of lymphocytes in BALF were significantly increased by the administration of 12.5 or 50 µg/kg IFN-γ, but not by the administration of 3.125 µg/kg IFN-γ (Fig. 2A and Supplementary Fig. S4). These results demonstrate that high level of IFN-γ might induce the infiltration of lymphocytes in the lungs in vivo. CXCR3 inhibitor but no other inhibitors significantly reduced the proportion and number of lymphocytes in BALF (Fig. 2B and Supplementary Fig. S4).
Fig. 2. Effects of IFN-γ and different inhibitors on the proportion of lymphocytes in BALF and White Blood Cells, and the F-actin level in lung-resident lymphocytes. (A) Summarized data showing the proportion of lymphocytes in BALF are representative of three independent experiments. Mice in different groups were treated as follows: the control group (Intranasally instilled with PBS, n = 9), low IFN-γ group (intranasally instilled with 3.125 µg/kg IFN-γ, n = 9), medium IFN-γ group (intranasally instilled with 12.5 µg/kg IFN-γ, n = 10) and high IFN-γ group (intranasally instilled with 50 µg/kg IFN-γ, n = 10). (B) Group data showing the effects of IFN-γ instillation and different inhibitors on the proportion of lymphocytes in BALF (n = 5 per group). (C) Group data showing the effects of IFN-γ instillation and different inhibitors on the proportion of lymphocytes in White Blood Cells (n = 5 per group). (D) Summarized data representing the fluorescence value of F-actins in lung-resident lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
BALF, bronchoalveolar lavage fluid; IFN-γ, interferon-γ; CXCR3, CXC chemokine receptor 3; ERK, extracellular signal-regulated kinase.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
Surprisingly, IFN-γ instillation (50 µg/kg) significantly increased the proportion of lymphocytes in white blood cells, which was significantly reduced by all of the used inhibitors (Fig. 2C). IFN-γ instillation (50 µg/kg) significantly increased the F-actins level in lung-resident lymphocytes, which was significantly reduced by the actin polymerization inhibitor (Fig. 2D).
Flow cytometric analysis of lung-resident lymphocytes
Both CD8+ T lymphocytes and IFN-γ+ T lymphocytes can produce IFN-γ.26 Compared with the control group, IFN-γ instillation (50 µg/kg) significantly increased the proportion of CXCR3+CD8+ T lymphocytes (about 1.9 times the baseline), IFN-γ+ T lymphocytes (about 1.9 times the baseline), CD4+IFN-γ+ T lymphocytes (about 1.8 times the baseline), CD8+IFN-γ+ T lymphocytes (about 1.6 times the baseline) in lung-resident T lymphocytes (Fig. 3A and B, Supplementary Fig. S5). These results suggest that instilled IFN-γ might induce the infiltration of IFN-γ-secreting T lymphocytes in the lung in vivo. Treatment with STAT1 inhibitor and CXCR3 inhibitor significantly reduced the proportion of IFN-γ+ T lymphocytes, CD4+IFN-γ+ T lymphocytes and CD8+IFN-γ+ T lymphocytes in T lymphocytes (Fig. 3B, Supplementary Fig. S5).
Fig. 3. Effects of IFN-γ and different inhibitors on lung-resident T lymphocytes and blood T lymphocytes. (A) Summarized data showing the proportion of CXCR3+CD8+ T lymphocytes in lung-resident T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). (B) Summarized data showing the proportion of IFN-γ+ T lymphocytes in lung-resident T lymphocytes (n = 5 per group). (C) Summarized data showing the proportion of CD8+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). (D) Summarized data showing the proportion of CXCR3+CD8+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). (E) Summarized data showing the proportion of IFN-γ+ T lymphocytes in blood T lymphocytes (n = 5 per group). (F) Summarized data showing the proportion of CD4+IFN-γ+ T lymphocytes in blood T lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
CXCR3, CXC chemokine receptor 3; IFN-γ, interferon-γ; ERK, extracellular signal-regulated kinase.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
Flow cytometric analysis of lymphocytes in blood
Compared with the control group, IFN-γ instillation (50 µg/kg) significantly increased the proportion of CXCR3+CD8+ T lymphocytes (about 1.2 times of the baseline), CD8+ T lymphocytes (about 1.2 times the baseline), IFN-γ+ T lymphocytes (about 3.0 times the baseline), CD4+IFN-γ+ T lymphocytes (about 3.2 times of the baseline) in blood T lymphocytes (Fig. 3C-F, Supplementary Fig. S6). These results indicate that instilled IFN-γ might increase the proportion of IFN-γ-secreting T lymphocytes in blood in vivo. Except the JAK1 inhibitor, all other 4 inhibitors significantly reduced the proportion of IFN-γ+ T lymphocytes and CD4+IFN-γ+ T lymphocytes compared with the IFN-γ model group (Fig. 3E and F). Also, about 90% of T lymphocytes were positive to Ki-67 stain (Supplementary Fig. S2), indicating of cell proliferation.27 The ratio of CD8+IFN-γ+ T lymphocytes/CD4+IFN-γ+ T lymphocytes in lung-resident T lymphocytes was significantly higher than that in blood T lymphocytes (Supplementary Fig. S6D).
Chemotaxis analysis with IP-10
Compared with the negative medium, IP-10 attracted the migration of blood lymphocytes to the lower chambers. However, IFN-γ instillation (50 µg/kg) did not change the chemotaxis ability of blood lymphocytes to IP-10 (Supplementary Fig. S7).
IFN-γ activated the Jak1, STAT1 and ERK1/2 pathways in lung tissues
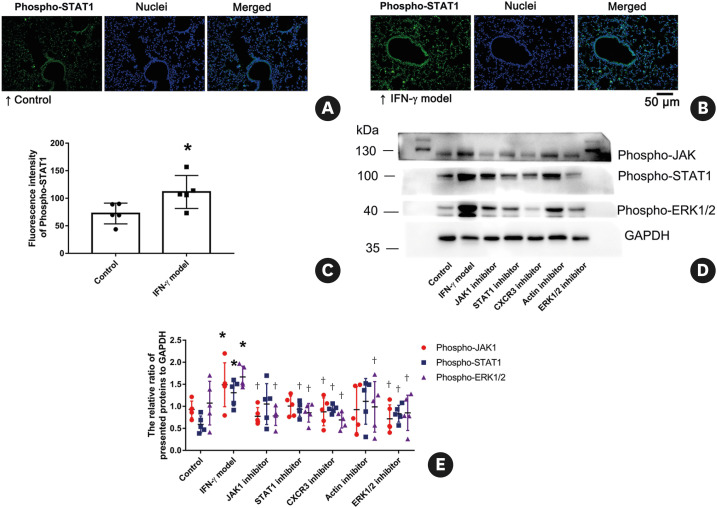
Compared with the control group, IFN-γ instillation (50 µg/kg) significantly increased the fluorescence intensity of Phospho-JAK1 (Fig. 4) and Phospho-STAT1 (Fig. 5A-C) in pulmonary cells. IFN-γ instillation (50 µg/kg) could also significantly increase the protein level of Phospho-JAK1, Phospho-STAT1 and Phospho-ERK1/2 (Fig. 5D and E). These findings suggest that instilled IFN-γ might primarily activate the JAK1 and STAT1 pathways in pulmonary cells, and activate the ERK1/2 pathway in lung tissues. IFN-γ-induced increasing of the fluorescence intensity and the protein level of Phospho-JAK1 were significantly inhibited by treatment with JAK1 inhibitor, CXCR3 inhibitor and ERK1/2 inhibitor, but not by treatment with STAT1 inhibitor and Actin polymerization inhibitor. IFN-γ-induced increasing of the Phospho-STAT1 protein level was significantly inhibited by STAT1 inhibitor, CXCR3 inhibitor and ERK1/2 inhibitor, but not by JAK1 inhibitor and Actin polymerization inhibitor. All of the used inhibitors significantly reduced the Phospho-ERK1/2 protein level.
Fig. 4. Effects of IFN-γ and different inhibitors on the fluorescence intensity of the Phospho-JAK1 level. (A-G) Representative lung tissues stained with anti-phospho-JAK1 (magenta) and Hoechst 33342 (blue) and imaged by the fluorescence microscope. Scale bar = 50 µm. (H) Summarized data showing the fluorescence intensity of the Phospho-JAK1 level in different groups. The images are representatives of 3 independent experiments. Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD. n = 5 per group.
IFN-γ, interferon-γ; CXC chemokine receptor 3; ERK, extracellular signal-regulated kinase.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
Fig. 5. (A-C) Effects of IFN-γ on the fluorescence intensity of the Phospho-Stat1 level. Lung tissues stained with anti-phospho-STAT1 (green) and Hoechst 33342 (blue) and imaged by a fluorescence microscope. Scale bar = 50 µm. n = 5 per group. (D-E) Effects of IFN-γ and different inhibitors on the expression of phospho-JAK1, phospho-STAT1 and phospho-ERK1/2 as indicated by the western blot. There are representative blots of phospho-JAK1, phospho-STAT1, phospho-ERK1/2 and GAPDH in lung tissues from seven different groups (D). Densitometric analyses are presented as the relative ratio of the targeted protein to GAPDH (E). The images are representatives of 3 independent experiments. n = 5 per group. Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
IFN-γ, interferon-γ; CXC chemokine receptor 3; ERK, extracellular signal-regulated kinase.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
Lung concentrations of IP-10 and IFN-γ
IFN-γ instillation dose-dependently increased the IP-10 concentration in homogenized lung tissues, which was only significantly reduced by the CXCR3 inhibitor (Fig. 6A and B). IFN-γ instillation (50 µg/kg) was also able to significantly increase the IFN-γ concentration in homogenized lung tissues, which was significantly reduced by the STAT1 inhibitor and CXCR3 inhibitor (Fig. 6C).
Fig. 6. (A) Summarized data showing the ratio of IP-10 concentrations to the total protein concentrations in homogenized lung tissues are representative of three independent experiments. Mice in different groups were treated as follows: the control group (Intranasally instilled with PBS, n = 10), low IFN-γ group (Intranasally instilled with 3.125 µg/kg IFN-γ, n = 9), medium IFN-γ group (Intranasally instilled with 12.5 µg/kg IFN-γ, n = 7) and high IFN-γ group (Intranasally instilled with 50 µg/kg IFN-γ, n = 10). (B-C) Group data showing the effects of IFN-γ instillation and different inhibitors on the ratio of IP-10 concentrations (B) and the IFN-γ concentrations (C) to the total protein concentrations in homogenized lung tissues (n = 5 per group). (D-E) Group data showing the effects of IFN-γ instillation on the IP-10 concentrations (D) and the IL-10 concentrations (E) in the supernatant of BALF (n = 10 per group). Results shown are representative of three independent experiments. Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
IFN-γ, interferon-γ; CXC chemokine receptor 3; ERK, extracellular signal-regulated kinase.
*P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.”
Cytokine concentration in the supernatant of BALF
In terms of the concentration of cytokines in the supernatant of BALF, IFN-γ instillation (50 µg/kg) significantly increased the IP-10 level, but reduced the IL-10 level (Fig. 6D and E). Other 6 cytokines were not significantly changed by IFN-γ instillation (Supplementary Fig. S8).
IFN-γ instillation enhanced the cough sensitivity and airway inflammation in guinea pigs in vivo
Compared with the control group, intratracheal instillation of 12.5 µg/kg recombinant guinea pig IFN-γ significantly increased the number of cough events within 10 minutes of exposing the animals to aerosolised citric acid (Fig. 7A and Supplementary Fig. S9). IFN-γ instillation could also significantly reduce the time to the first cough (Fig. 7B). IFN-γ instillation significantly increased the total number of leukocytes in BALF (Fig. 7C). These findings suggest that instilled IFN-γ might enhance the cough sensitivity and airway inflammation of guinea pigs in vivo.
Fig. 7. IFN-γ instillation enhanced the cough sensitivity and the total number of leukocytes in BALF of guinea pigs. It shows the effects of IFN-γ instillation on the number of cough events (A) and time to the first cough (B) within 10 minutes of exposing the animals to aerosolised citric acid (0.3 M). (C) Group data showing the effects of IFN-γ instillation on the total number of leukocytes in BALF. Guinea pigs in the control group (n = 8) or the IFN-γ group (n = 7) were intratracheally instilled with PBS or 12.5 µg/kg recombinant guinea pig IFN-γ once a day for 7 consecutive days. Results shown (mean ± SD) are representative of three independent experiments.
IFN-γ, interferon-γ; BALF, bronchoalveolar lavage fluid; PBS, phosphate-buffered saline.
*P < 0.05, compared with the “Control.”
DISCUSSION
After the intranasal IFN-γ administration (50 µg/kg) in our study, the mean IFN-γ concentration in homogenized lung tissues rose from 4.8 in the control group to 8.5 pg/mL in the IFN-γ model group. The average concentration of IFN-γ in sputum supernatant can rise from 32 pg/mL in healthy controls to 151 pg/mL in patients with chronic refractory cough.11 Furthermore, the IFN-γ levels in lung homogenates can rise to 5,000 pg/mL in mice intranasally infected with influenza A virus.28 Intranasal IFN-γ administration (about 600 µg/kg) in mice for 7 days was reported to suppress the virus replication.29 Thus, the dose of intranasal IFN-γ administration (50 µg/kg) in mice is not extremely high in our study. As far as IFN-γ levels are concerned, repeated pulmonary IFN-γ exposures in our study may have the physiological relevance.
In our study, intranasal IFN-γ administration increased the airway inflammation and the airway epithelial damage. Consistently, intranasal IFN-γ administration increased the proportion and number of lymphocytes and the concentration of IFN-γ in the lungs of mice. In addition, intratracheal IFN-γ administration (12.5 µg/kg) enhanced the cough sensitivity and airway inflammation of guinea pigs in vivo, which was detected at 2 days later after the last time of IFN-γ administration. Lower doses of IFN-γ administration (0.2, 1, and 5 µg/kg) can enhance the cough sensitivity of guinea pigs without airway inflammation, which was detected at 5 hours later after the single intratracheal instillation.8 Flow cytometric results suggest that these infiltrated lymphocytes might be CXCR3+CD8+ T lymphocytes and IFN-γ+ T lymphocytes (including CD4+IFN-γ+ and CD8+IFN-γ+ T lymphocytes). In our study, IFN-γ (50 µg/kg) was intranasally instilled once a day for 5 consecutive days in IFN-γ model group, and the mice samples were also harvested at 2 days later. According to a previous biodistribution study of inhaled IFN-γ,30 less than 1.5% of the instilled or inhaled IFN-γ might maintain the biologic activity in the lung 2 days after the IFN-γ administration. We also discovered IFN-γ-induced pulmonary lymphocytic inflammation with the increase of IFN-γ levels, CXCR3+CD8+ T lymphocytes and IFN-γ+ T lymphocytes 7 days after the IFN-γ administration in mice (our unpublished data). Therefore, most of the increased IFN-γ concentration in lung might be secreted by the activated CXCR3+CD8+ T lymphocytes and IFN-γ+ T lymphocytes (including CD4+IFN-γ+ and CD8+IFN-γ+ T lymphocytes) in the lung of mice from IFN-γ model group in our study. The increased IFN-γ concentration in the lungs, which is produced by these IFN-γ-secreting T lymphocytes, might play an important role in cough hypersensitivity at 2 days later after the last time of IFN-γ administration.
Intranasal IFN-γ administration (50 µg/kg) caused the infiltration of IFN-γ-secreting T lymphocytes and inflammation in the mouse lungs. However, the lower dose of Intranasal IFN-γ administration (3.125 µg/kg) did not significantly raise the proportion or number of lymphocytes in mouse BALF. Similarly, intranasal administration of low dose IFN-γ can't cause obvious pulmonary inflammation or T-cell infiltration in mice.28,31 Transgenic mice with lung-targeted IFN-γ over-expression have lungs with the infiltration of lymphocytes and non-eosinophilic inflammation.32 Neutralization of IFN-γ protects against influenza-induced inflammatory lung damage.33 Clinically, the post-infectious chronic cough was reported to be 43% of participants after the influenza A virus infection.34 T lymphocytes infiltration into the airways after the influenza A virus infection can cause severe pulmonary immunopathology by producing proinflammatory cytokines (including IFN-γ).35 Moreover, the life span of T lymphocytes in adult humans is more than 10 times longer than the life span of T lymphocytes in adult mice.36 Thus, over-secreted IFN-γ might cause cough hypersensitivity, the infiltration of IFN-γ-secreting T lymphocytes and inflammation in the lung of some patients. These IFN-γ-secreting T lymphocytes could in turn produce IFN-γ in the lungs. The relationship between IFN-γ and IFN-γ-secreting T lymphocytes in the lung might partially explain why IFN-γ-secreting T lymphocytes persist for a long time, and might be relevant to chronic refractory cough and other chronic airway diseases. Further studies are needed to evaluate the role of IFN-γ in the mechanisms of post-infectious persistent cough in clinical practice.
In our study, intranasal IFN-γ administration increased the proportion of lymphocytes, CXCR3+CD8+ T lymphocytes, CD8+ T lymphocytes, IFN-γ+ T lymphocytes and CD4+IFN-γ+ T lymphocytes in blood of mice. Compared with lung-resident T lymphocytes, IFN-γ induced a higher increasing of IFN-γ+ T lymphocytes but a lower increasing of CXCR3+CD8+ T lymphocytes in blood T lymphocytes. IFN-γ also increased the IP-10 level in the lungs and BALF. Moreover, CXCR3 inhibitor significantly reduced the IFN-γ-induced pulmonary inflammation, IFN-γ-secreting T lymphocytes number in both lung and blood, and IP-10 level in the lungs. In line with the chemotaxis ability of blood lymphocytes to IP-10, the proportion of CXCR3+ T lymphocytes in blood was not changed by IFN-γ instillation in our study. These results imply that IFN-γ-secreting T lymphocytes in blood might be independent of that in lung, and CXCR3+CD8+ T lymphocytes might be recruited to the lung by IFN-γ-enhanced IP-10 in the mice lung. The source of IP-10 might be IFN-γ-activated pulmonary cells. Similarly, subcutaneous IFN-γ administration increases CD8+ T lymphocyte number in blood of patients with pulmonary tuberculosis.37 CD8+ T lymphocytes and the elevated serum IFN-γ are essential for developing lymphocytic choriomeningitic virus-induced hemophagocytic lymphohistiocytosis mouse model.38 Influenza A virus infection increases CD4+IFN-γ+ and CD8+IFN-γ+ T lymphocytes number in both blood and lung of pigs.6 The IFN-γ-producing CD8+ T lymphocytes in both blood and BALF are increased in smokers with COPD.39 How pulmonary IFN-γ affects T lymphocytes in blood is yet to be investigated in the future.
IFN-γ increases the expression of IP-10 via the JAK/STAT1 signaling pathway in airway epithelia.16 The elevated F-actin expression and ERK activation participate in IP-10-induced chemotaxis of T lymphocytes, especially CXCR3+ T lymphocytes.19,40 In our study, intranasal IFN-γ administration activated the JAK1, STAT1 and ERK1/2 pathways in lung tissues. IFN-γ instillation also increased the F-actins level in lung-resident lymphocytes in vivo.
In response to repeated house dust mite inhalation, ablation of IL-10 can increase IFN-γ and IP-10 levels in mouse lungs, followed by the airway epithelial disruption24. Exogenous IFN-γ reduced the IL-10 level in mouse BALF in our study. Consistently, IFN-γ can suppress lipopolysaccharide-induced IL-10 production in dendritic cells through the Stat1 pathway.41 Therefore, IFN-γ might act antagonistically with IL-10 in the process of lung inflammation.
In our study, results of treatment with the JAK1 inhibitor (Filgotinib) imply that the JAK1 pathway might participate in pulmonary IFN-γ-induced increasing of total lymphocytes in mice blood, but be irrelevant to IFN-γ-induced pulmonary inflammation, IFN-γ-secreting T lymphocytes infiltration in lung and blood. Similarly, the JAK inhibitor (SHR0302) can suppress the proportion of total B lymphocytes in the rat spleen.42 In contrast, systemic administration of ruxolitinib (an inhibitor of JAK1 and JAK2) can eliminate the infiltration of IFN-γ-producing CD8+ T lymphocytes and prevent the development of alopecia areata in vivo. 43 Therefore, JAK1 might not participate in pulmonary IFN-γ-induced pulmonary inflammation with IFN-γ-secreting T lymphocytes infiltration. Further studies are needed to demonstrate whether JAK2 participates in pulmonary IFN-γ-induced pulmonary inflammation.
Compared with the IFN-γ model group, treatment with the STAT1 inhibitor reduced the uric acid concentration in BALF, the proportion of IFN-γ+ T lymphocytes, the Phospho-STAT1 level and the IFN-γ concentration in the lungs, the proportion of total lymphocytes and IFN-γ-secreting T lymphocytes in blood. However, the STAT1 inhibitor did not significantly affect the total protein concentration or the proportion of total lymphocytes in BALF. These results suggest that blocking the STAT1 pathway might reduce IFN-γ-induced airway epithelial damage, and IFN-γ-secreting T lymphocytes number in both lung and blood. But it might not potently mitigate the airway inflammation and total lymphocytes infiltration in the lungs. Similarly, STAT1 is crucial to T lymphocytes expansion and T lymphocytes-mediated intestinal inflammation in mice.44 In our study, about 90% of IFN-γ+ T lymphocytes in the lungs and blood were positive to Ki-67 stain, indicating of cell proliferation.27 Therefore, pulmonary IFN-γ might induce the proliferation and expansion of IFN-γ-secreting T lymphocytes in blood and lung through the STAT1 pathway.
In the present study, intraperitoneal treatment with the CXCR3 inhibitor (AMG487) was potent in suppressing all the IFN-γ-induced lymphocytic inflammation in both lung and blood. These data implicate that CXCR3 might be involved in pulmonary IFN-γ-induced expansion of IFN-γ-secreting T lymphocytes in blood and lung, pulmonary IFN-γ-induced migration of CXCR3+CD8+ T lymphocytes to lung tissues, and lung inflammation. Consistently, AMG487 decreases the inflammatory damage with T lymphocyte infiltration in knee samples, and the proportion of CXCR3+ T lymphocytes in the spleen of collagen-induced mouse arthritis.45 IFN-γ can directly disrupt the mice airway epithelia and increase the uric acid concentration in BALF.24 Respiratory symptoms persist for many months in post-coronavirus patients, associated with ongoing airway epithelial injury, increased numbers of CD8+ T lymphocytes and increased concentrations of CXCR3 chemokines in the lungs.46 A chemokine antagonist (XC8) was reported to inhibit cough in guinea pigs induced by citric acid in combination with IFN-γ.47 The STAT1 inhibitor and CXCR3 inhibitor, which suppressed IFN-γ-induced increasing of the uric acid concentration in BALF, consistently decreased the IFN-γ level and the IFN-γ+ T lymphocytes infiltration in the lung in our study. We hope to determine whether the CXCR3 inhibitor could attenuate the cough hypersensitivity induced by IFN-γ or viral infection in guinea pigs in further studies.
Actin polymerization and ERK activation can regulate the migration of lymphocytes.19,48 In our study, both the actin polymerization inhibitor (Cytochalasin D) and ERK1/2 inhibitor (Ravoxertinib) significantly reduced the total protein concentration in BALF, the proportion of total lymphocytes and IFN-γ-secreting T lymphocytes in blood. These results suggest that F-actin accumulation and the ERK1/2 pathway-involved migration of T lymphocytes might play an important role in pulmonary IFN-γ-induced augmentation of the airway inflammation and increasing of IFN-γ-secreting T lymphocytes in blood.
Collectively, high physiological levels of pulmonary IFN-γ might cause airway epithelial disruption, airway inflammation and cough hypersensitivity in vivo. IFN-γ might activate the STAT1 and ERK pathways in lung tissues, release IP-10, and result in F-actin accumulation in lung-resident lymphocytes. The CXCR3 pathway might participate in pulmonary IFN-γ-induced increasing of IFN-γ-secreting T lymphocytes in both blood and lung. Further studies are needed to demonstrate this cascade.
There are limitations in this study that should be mentioned. A major limitation is that the involvement of IFN-γ in the mechanisms of post-infectious persistent cough is lack of clinical validation. Because influenza A virus infection can produce IFN-γ in the lung of mice,28 there is the need for complementary measures involving with influenza A virus infection and intrapulmonary administration of IFN-γ to investigate the immunopathological effects of IFN-γ in animal models. We wish to investigate the immunopathological effects of IFN-γ in animal models with influenza A virus infection in further studies. Another limitation of this study is that we focus on the IP-10/CXCR3 pathway that participates in pulmonary IFN-γ-induced increasing of IFN-γ-secreting T lymphocytes in the lungs. Actually, many cell types produce a wide range of chemokines (including IP-10, monokine induced by IFN-γ, Interferon-inducible T-cell alpha chemoattractant and so on) upon IFN-γ stimulus. And CXCR3 is not only expressed on lymphocytes, but also on natural killer cells and other cells.14 Type I IFNs, the other predominant cytokines after influenza virus infection, may also play a role in viral infection-induced coughing and IP-10 production.28,49
In conclusion, high physiological levels of IFN-γ in the lung may cause pulmonary lymphocytic inflammation and cough hypersensitivity by increasing the number of IFN-γ-secreting T lymphocytes, and be relevant to chronic refractory cough and other chronic airway diseases. IFN-γ and CXCR3 may be good targets for treating these diseases.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC 81970013, 82100034), Guangzhou Science and Technology Planning Project (202002030151, 202102010168), and Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020003).
Footnotes
Disclosure: Zheng Deng, Kefang Lai and Wenbin Ding are the named inventors on the pending patent for “CXCR3 may be used as the drug target of cough” (China Patent Application 202110907136.0). Fengying Li, Shuirong Shen and Chuqin Huang have no conflict of interests to declare.
Data Availability Statement
Data available on request from the authors.
SUPPLEMENTARY MATERIALS
Compounds and materials used in this study
Methods of treatment with mice in the first experiment and the second experiment
Methods of treatment with guinea pigs
Multigating strategy for the analysis of surface antigens of mononuclear cells. Lymphocytes (R1) were identified based on gating of forward scatter area (FSC-A) and side scatter area (SSC-A). And then forward scatter height (FSC-H) and FSC-A gating was used to obtain single lymphocytes (R2). Total live lymphocytes (R3) were then identified based on the exclusion of a Live/Dead dye (FVS780). Positive staining for CD3 was then used to distinguish live T lymphocytes (R4). Subsequently, representative plots showed percentage of CD4+ T lymphocytes (R5), CD8+ T lymphocytes (R6), CXCR3+ T lymphocytes (R7), CXCR3+CD4+ T lymphocytes (R8) and CXCR3+CD8+ T lymphocytes (R9) within all the live T lymphocytes.
Multigating strategy for the analysis of intracellular cytokines of mononuclear cells. Lymphocytes (R1) were identified based on gating of FSC-A and SSC-A. And then FSC-H and FSC-A gating was used to obtain single lymphocytes (R2). Total live lymphocytes (R3) were then identified based on the exclusion of a live/dead dye (FVS780). Positive staining for CD3 was then used to distinguish live T lymphocytes (R4). Subsequently, representative plots showed percentage of CD4+ T lymphocytes (R5), CD8+ T lymphocytes (R6), Ki-67+ T lymphocytes (R7), IFN-γ+ T lymphocytes (R8) and CD4+IFN-γ+ T lymphocytes (R9) within all the live T lymphocytes.
Body weight changes of mice. Values greater than zero represent weight gain, and values less than zero represent weight loss. Group data showing that there is no significant difference between different groups (n = 5 per group).
Effects of IFN-γ and different inhibitors on the number of lymphocytes in BALF. (A) Summarized data showing the number of lymphocytes in BALF are representative of three independent experiments. Mice in different groups were treated as follows: the control group (Intranasally instilled with PBS, n = 9), low IFN-γ group (Intranasally instilled with 3.125 µg/kg IFN-γ, n = 9), medium IFN-γ group (Intranasally instilled with 12.5 µg/kg IFN-γ, n = 10) and high IFN-γ group (Intranasally instilled with 50 µg/kg IFN-γ, n = 10). (B) Group data showing the effects of IFN-γ instillation and different inhibitors on the number of lymphocytes in BALF (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
Effects of IFN-γ and different inhibitors on the proportion of CD8+ T lymphocytes, CXCR3+CD4+ T lymphocytes, CD4+IFN-γ+ T lymphocytes, CD8+IFN-γ+ T lymphocytes in lung-resident T lymphocytes. (A) Summarized data showing the proportion of CD8+ T lymphocytes in lung-resident T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (B) Summarized data showing the proportion of CXCR3+CD4+ T lymphocytes in lung-resident T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN- γ model” group. (C) Summarized data showing the proportion of CD4+IFN-γ+ T lymphocytes in lung-resident T lymphocytes (n = 5 per group). (D) Summarized data showing the proportion of CD8+IFN-γ+ T lymphocytes in lung-resident T lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
Effects of IFN-γ and different inhibitors on the proportion of CD4+ T lymphocytes, CXCR3+CD4+ T lymphocytes, CD8+IFN-γ+ T lymphocytes in blood T lymphocytes. (A) Summarized data showing the proportion of CD4+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (B) Summarized data showing the proportion of CXCR3+CD4+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (C) Summarized data showing the proportion of CD8+IFN-γ+ T lymphocytes in blood T lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD. *P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.” (D) Summarized data showing the ratio of CD8+IFN-γ+ T lymphocytes/CD4+IFN-γ+ T lymphocytes in lung-resident T lymphocytes and blood T lymphocytes (n = 35 per group). *P < 0.05, compared with the “Lung-resident lymphocytes.”
Effects of IFN-γ instillation on IP-10-induced migration of blood lymphocytes in vitro. Summarized data showing the relative ratio of IP-10- induced migration of blood lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10).
Group data showing the effects of IFN-γ instillation on the IL-2 concentrations (A), IL-4 concentrations (B), IL-5 concentrations (C), IL-6 concentrations (D), keratinocyte-derived chemokine concentrations (E) and tumor necrosis factor-α concentrations (B2) in the supernatant of BALF. There is no significant difference between the “Control” group and the “IFN-γ model” group (n = 10 per group).
Summarized data showing the number of cough events in the “Control” group (n = 8) and the “IFN-γ group” (n = 7) at the time of “Baseline” and “After treatment”. The number of cough events were recorded within 10 minutes of exposing guinea pigs to aerosolised citric acid (0.3 M). “Baseline” means the day before the first intratracheal instillation; “After treatment” means two days later after the last intratracheal instillation.
References
- 1.Capristo C, Rossi GA. Post-infectious persistent cough: pathogenesis and therapeutic options. Minerva Pediatr. 2017;69:444–452. doi: 10.23736/S0026-4946.17.04958-1. [DOI] [PubMed] [Google Scholar]
- 2.Gibson PG, Vertigan AE. Management of chronic refractory cough. BMJ. 2015;351:h5590. doi: 10.1136/bmj.h5590. [DOI] [PubMed] [Google Scholar]
- 3.To KK, Chan KH, Li IW, Tsang TY, Tse H, Chan JF, et al. Viral load in patients infected with pandemic H1N1 2009 influenza A virus. J Med Virol. 2010;82:1–7. doi: 10.1002/jmv.21664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, et al. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Talker SC, Stadler M, Koinig HC, Mair KH, Rodríguez-Gómez IM, Graage R, et al. Influenza A virus infection in pigs attracts multifunctional and cross-reactive T cells to the lung. J Virol. 2016;90:9364–9382. doi: 10.1128/JVI.01211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YM, Miyahara N, Takeda K, Prpich J, Oh A, Balhorn A, et al. IFN-gamma production during initial infection determines the outcome of reinfection with respiratory syncytial virus. Am J Respir Crit Care Med. 2008;177:208–218. doi: 10.1164/rccm.200612-1890OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng Z, Zhou W, Sun J, Li C, Zhong B, Lai K. IFN-γ enhances the cough reflex sensitivity via calcium influx in vagal sensory neurons. Am J Respir Crit Care Med. 2018;198:868–879. doi: 10.1164/rccm.201709-1813OC. [DOI] [PubMed] [Google Scholar]
- 9.Undem BJ, Sun H. Molecular/ionic basis of vagal bronchopulmonary C-fiber activation by inflammatory mediators. Physiology (Bethesda) 2020;35:57–68. doi: 10.1152/physiol.00014.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mund E, Christensson B, Grönneberg R, Larsson K. Noneosinophilic CD4 lymphocytic airway inflammation in menopausal women with chronic dry cough. Chest. 2005;127:1714–1721. doi: 10.1378/chest.127.5.1714. [DOI] [PubMed] [Google Scholar]
- 11.Sun J, Zhan C, Deng Z, Luo W, Chen Q, Jiang M, et al. Expression of interferon-γ and its effect on cough hypersensitivity in chronic refractory cough patients. Thorax. 2022;77:621–624. doi: 10.1136/thoraxjnl-2021-218403. [DOI] [PubMed] [Google Scholar]
- 12.Regard L, Martin C, Teillaud JL, Lafoeste H, Vicaire H, Ladjemi MZ, et al. Effective control of Staphylococcus aureus lung infection despite tertiary lymphoid structure disorganisation. Eur Respir J. 2021;57:2000768. doi: 10.1183/13993003.00768-2020. [DOI] [PubMed] [Google Scholar]
- 13.Flament T, Bigot A, Chaigne B, Henique H, Diot E, Marchand-Adam S. Pulmonary manifestations of Sjögren’s syndrome. Eur Respir Rev. 2016;25:110–123. doi: 10.1183/16000617.0011-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skinner D, Marro BS, Lane TE. Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunol. 2019;32:25–37. doi: 10.1089/vim.2018.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaruga B, Hong F, Kim WH, Gao B. IFN-gamma/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 16.O’Connell D, Bouazza B, Kokalari B, Amrani Y, Khatib A, Ganther JD, et al. IFN-γ-induced JAK/STAT, but not NF-κB, signaling pathway is insensitive to glucocorticoid in airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2015;309:L348–L359. doi: 10.1152/ajplung.00099.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, et al. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 18.Hildebrandt GC, Corrion LA, Olkiewicz KM, Lu B, Lowler K, Duffner UA, et al. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J Immunol. 2004;173:2050–2059. doi: 10.4049/jimmunol.173.3.2050. [DOI] [PubMed] [Google Scholar]
- 19.Lee JH, Kim B, Jin WJ, Kim HH, Ha H, Lee ZH. Pathogenic roles of CXCL10 signaling through CXCR3 and TLR4 in macrophages and T cells: relevance for arthritis. Arthritis Res Ther. 2017;19:163. doi: 10.1186/s13075-017-1353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas MS, Mitchell JS, DeNucci CC, Martin AL, Shimizu Y. The p110gamma isoform of phosphatidylinositol 3-kinase regulates migration of effector CD4 T lymphocytes into peripheral inflammatory sites. J Leukoc Biol. 2008;84:814–823. doi: 10.1189/jlb.0807561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cecchinato V, Bernasconi E, Speck RF, Proietti M, Sauermann U, D’Agostino G, et al. Impairment of CCR6+ and CXCR3+ Th cell migration in HIV-1 infection is rescued by modulating actin polymerization. J Immunol. 2017;198:184–195. doi: 10.4049/jimmunol.1600568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deng Z, Gao ZC, Ge HQ, Zhang LR, Zhou JJ, Zhu ZP, et al. Treatment responses of procaterol and CD38 inhibitors in an ozone-induced airway hyperresponsiveness mice model. Biol Pharm Bull. 2013;36:1348–1355. doi: 10.1248/bpb.b13-00290. [DOI] [PubMed] [Google Scholar]
- 23.Deng Z, Zhou JJ, Sun SY, Zhao X, Sun Y, Pu XP. Procaterol but not dexamethasone protects 16HBE cells from H2O2-induced oxidative stress. J Pharmacol Sci. 2014;125:39–50. doi: 10.1254/jphs.13206fp. [DOI] [PubMed] [Google Scholar]
- 24.Branchett WJ, Stölting H, Oliver RA, Walker SA, Puttur F, Gregory LG, et al. A T cell-myeloid IL-10 axis regulates pathogenic IFN-γ-dependent immunity in a mouse model of type 2-low asthma. J Allergy Clin Immunol. 2020;145:666–678.e9. doi: 10.1016/j.jaci.2019.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rahman MT, Ghosh C, Hossain M, Linfield D, Rezaee F, Janigro D, et al. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem Biophys Res Commun. 2018;507:274–279. doi: 10.1016/j.bbrc.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 26.Nicolet BP, Guislain A, van Alphen FP, Gomez-Eerland R, Schumacher TN, van den Biggelaar M, et al. CD29 identifies IFN-γ-producing human CD8+ T cells with an increased cytotoxic potential. Proc Natl Acad Sci U S A. 2020;117:6686–6696. doi: 10.1073/pnas.1913940117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice. Gastroenterology. 2017;153:1621–1633.e6. doi: 10.1053/j.gastro.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Verzele NA, Chua BY, Law CW, Zhang A, Ritchie ME, Wightman O, et al. The impact of influenza pulmonary infection and inflammation on vagal bronchopulmonary sensory neurons. FASEB J. 2021;35:e21320. doi: 10.1096/fj.202001509R. [DOI] [PubMed] [Google Scholar]
- 29.Yamamoto N, Shibamori M, Ogura M, Seko Y, Kikuchi M. Effects of intranasal administration of recombinant murine interferon-gamma on murine acute myocarditis caused by encephalomyocarditis virus. Circulation. 1998;97:1017–1023. doi: 10.1161/01.cir.97.10.1017. [DOI] [PubMed] [Google Scholar]
- 30.Virgolini I, Kurtaran A, Leimer M, Smith-Jones P, Agis H, Angelberger P, et al. Inhalation scintigraphy with iodine-123-labeled interferon gamma-1b: pulmonary deposition and dose escalation study in healthy volunteers. J Nucl Med. 1997;38:1475–1481. [PubMed] [Google Scholar]
- 31.Empey KM, Orend JG, Peebles RS, Jr, Egaña L, Norris KA, Oury TD, et al. Stimulation of immature lung macrophages with intranasal interferon gamma in a novel neonatal mouse model of respiratory syncytial virus infection. PLoS One. 2012;7:e40499. doi: 10.1371/journal.pone.0040499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim YS, Choi SJ, Choi JP, Jeon SG, Oh S, Lee BJ, et al. IL-12-STAT4-IFN-gamma axis is a key downstream pathway in the development of IL-13-mediated asthma phenotypes in a Th2 type asthma model. Exp Mol Med. 2010;42:533–546. doi: 10.3858/emm.2010.42.8.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verma AK, Bauer C, Palani S, Metzger DW, Sun K. IFN-γ drives TNF-α hyperproduction and lethal lung inflammation during antibiotic treatment of postinfluenza Staphylococcus aureus pneumonia. J Immunol. 2021;207:1371–1376. doi: 10.4049/jimmunol.2100328. [DOI] [PubMed] [Google Scholar]
- 34.Ryan NM, Vertigan AE, Ferguson J, Wark P, Gibson PG. Clinical and physiological features of postinfectious chronic cough associated with H1N1 infection. Respir Med. 2012;106:138–144. doi: 10.1016/j.rmed.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 35.Duan S, Thomas PG. Balancing immune protection and immune pathology by CD8(+) T-cell responses to influenza infection. Front Immunol. 2016;7:25. doi: 10.3389/fimmu.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Boer RJ, Perelson AS. Quantifying T lymphocyte turnover. J Theor Biol. 2013;327:45–87. doi: 10.1016/j.jtbi.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SK, Cho S, Lee IH, Jeon DS, Hong SH, Smego RA, Jr, et al. Subcutaneously administered interferon-gamma for the treatment of multidrug-resistant pulmonary tuberculosis. Int J Infect Dis. 2007;11:434–440. doi: 10.1016/j.ijid.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Jordan MB, Hildeman D, Kappler J, Marrack P. An animal model of hemophagocytic lymphohistiocytosis (HLH): CD8+ T cells and interferon gamma are essential for the disorder. Blood. 2004;104:735–743. doi: 10.1182/blood-2003-10-3413. [DOI] [PubMed] [Google Scholar]
- 39.Hodge G, Nairn J, Holmes M, Reynolds PN, Hodge S. Increased intracellular T helper 1 proinflammatory cytokine production in peripheral blood, bronchoalveolar lavage and intraepithelial T cells of COPD subjects. Clin Exp Immunol. 2007;150:22–29. doi: 10.1111/j.1365-2249.2007.03451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito T, Hashizume H, Shimauchi T, Funakoshi A, Ito N, Fukamizu H, et al. CXCL10 produced from hair follicles induces Th1 and Tc1 cell infiltration in the acute phase of alopecia areata followed by sustained Tc1 accumulation in the chronic phase. J Dermatol Sci. 2013;69:140–147. doi: 10.1016/j.jdermsci.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 41.Flores RR, Diggs KA, Tait LM, Morel PA. IFN-gamma negatively regulates CpG-induced IL-10 in bone marrow-derived dendritic cells. J Immunol. 2007;178:211–218. doi: 10.4049/jimmunol.178.1.211. [DOI] [PubMed] [Google Scholar]
- 42.Wu H, Yan S, Chen J, Luo X, Li P, Jia X, et al. JAK1-STAT3 blockade by JAK inhibitor SHR0302 attenuates inflammatory responses of adjuvant-induced arthritis rats and decreases Th17 and total B cells. Joint Bone Spine. 2016;83:525–532. doi: 10.1016/j.jbspin.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 43.Xing L, Dai Z, Jabbari A, Cerise JE, Higgins CA, Gong W, et al. Alopecia areata is driven by cytotoxic T lymphocytes and is reversed by JAK inhibition. Nat Med. 2014;20:1043–1049. doi: 10.1038/nm.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kang YH, Biswas A, Field M, Snapper SB. STAT1 signaling shields T cells from NK cell-mediated cytotoxicity. Nat Commun. 2019;10:912. doi: 10.1038/s41467-019-08743-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bakheet SA, Ansari MA, Nadeem A, Attia SM, Alhoshani AR, Gul G, et al. CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell Signal. 2019;64:109395. doi: 10.1016/j.cellsig.2019.109395. [DOI] [PubMed] [Google Scholar]
- 46.Vijayakumar B, Boustani K, Ogger PP, Papadaki A, Tonkin J, Orton CM, et al. Immuno-proteomic profiling reveals aberrant immune cell regulation in the airways of individuals with ongoing post-COVID-19 respiratory disease. Immunity. 2022;55:542–556.e5. doi: 10.1016/j.immuni.2022.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romanova J, Rydlovskaya A, Mochalov S, Proskurina O, Gorokh Y, Nebolsin V. The effect of anti-chemokine oral drug XC8 on cough triggered by the agonists of TRPA1 but not TRPV1 channels in guinea pigs. Pulm Ther. 2022;8:105–122. doi: 10.1007/s41030-022-00183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson SB, Waldman MM, Jacobelli J. Polymerization power: effectors of actin polymerization as regulators of T lymphocyte migration through complex environments. FEBS J. 2021 doi: 10.1111/febs.16130. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Patil MJ, Ru F, Sun H, Wang J, Kolbeck RR, Dong X, et al. Acute activation of bronchopulmonary vagal nociceptors by type I interferons. J Physiol. 2020;598:5541–5554. doi: 10.1113/JP280276. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Compounds and materials used in this study
Methods of treatment with mice in the first experiment and the second experiment
Methods of treatment with guinea pigs
Multigating strategy for the analysis of surface antigens of mononuclear cells. Lymphocytes (R1) were identified based on gating of forward scatter area (FSC-A) and side scatter area (SSC-A). And then forward scatter height (FSC-H) and FSC-A gating was used to obtain single lymphocytes (R2). Total live lymphocytes (R3) were then identified based on the exclusion of a Live/Dead dye (FVS780). Positive staining for CD3 was then used to distinguish live T lymphocytes (R4). Subsequently, representative plots showed percentage of CD4+ T lymphocytes (R5), CD8+ T lymphocytes (R6), CXCR3+ T lymphocytes (R7), CXCR3+CD4+ T lymphocytes (R8) and CXCR3+CD8+ T lymphocytes (R9) within all the live T lymphocytes.
Multigating strategy for the analysis of intracellular cytokines of mononuclear cells. Lymphocytes (R1) were identified based on gating of FSC-A and SSC-A. And then FSC-H and FSC-A gating was used to obtain single lymphocytes (R2). Total live lymphocytes (R3) were then identified based on the exclusion of a live/dead dye (FVS780). Positive staining for CD3 was then used to distinguish live T lymphocytes (R4). Subsequently, representative plots showed percentage of CD4+ T lymphocytes (R5), CD8+ T lymphocytes (R6), Ki-67+ T lymphocytes (R7), IFN-γ+ T lymphocytes (R8) and CD4+IFN-γ+ T lymphocytes (R9) within all the live T lymphocytes.
Body weight changes of mice. Values greater than zero represent weight gain, and values less than zero represent weight loss. Group data showing that there is no significant difference between different groups (n = 5 per group).
Effects of IFN-γ and different inhibitors on the number of lymphocytes in BALF. (A) Summarized data showing the number of lymphocytes in BALF are representative of three independent experiments. Mice in different groups were treated as follows: the control group (Intranasally instilled with PBS, n = 9), low IFN-γ group (Intranasally instilled with 3.125 µg/kg IFN-γ, n = 9), medium IFN-γ group (Intranasally instilled with 12.5 µg/kg IFN-γ, n = 10) and high IFN-γ group (Intranasally instilled with 50 µg/kg IFN-γ, n = 10). (B) Group data showing the effects of IFN-γ instillation and different inhibitors on the number of lymphocytes in BALF (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
Effects of IFN-γ and different inhibitors on the proportion of CD8+ T lymphocytes, CXCR3+CD4+ T lymphocytes, CD4+IFN-γ+ T lymphocytes, CD8+IFN-γ+ T lymphocytes in lung-resident T lymphocytes. (A) Summarized data showing the proportion of CD8+ T lymphocytes in lung-resident T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (B) Summarized data showing the proportion of CXCR3+CD4+ T lymphocytes in lung-resident T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN- γ model” group. (C) Summarized data showing the proportion of CD4+IFN-γ+ T lymphocytes in lung-resident T lymphocytes (n = 5 per group). (D) Summarized data showing the proportion of CD8+IFN-γ+ T lymphocytes in lung-resident T lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD.
Effects of IFN-γ and different inhibitors on the proportion of CD4+ T lymphocytes, CXCR3+CD4+ T lymphocytes, CD8+IFN-γ+ T lymphocytes in blood T lymphocytes. (A) Summarized data showing the proportion of CD4+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (B) Summarized data showing the proportion of CXCR3+CD4+ T lymphocytes in blood T lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10). There is no significant difference between the “Control” group and the “IFN-γ model” group. (C) Summarized data showing the proportion of CD8+IFN-γ+ T lymphocytes in blood T lymphocytes (n = 5 per group). Mice in different groups were treated as the same as that in Fig. 1. Data are shown as mean ± SD. *P < 0.05, compared with the “Control”; †P < 0.05, compared with the “IFN-γ model.” (D) Summarized data showing the ratio of CD8+IFN-γ+ T lymphocytes/CD4+IFN-γ+ T lymphocytes in lung-resident T lymphocytes and blood T lymphocytes (n = 35 per group). *P < 0.05, compared with the “Lung-resident lymphocytes.”
Effects of IFN-γ instillation on IP-10-induced migration of blood lymphocytes in vitro. Summarized data showing the relative ratio of IP-10- induced migration of blood lymphocytes from the “Control” group (n = 9) and the “IFN-γ model” group (n = 10).
Group data showing the effects of IFN-γ instillation on the IL-2 concentrations (A), IL-4 concentrations (B), IL-5 concentrations (C), IL-6 concentrations (D), keratinocyte-derived chemokine concentrations (E) and tumor necrosis factor-α concentrations (B2) in the supernatant of BALF. There is no significant difference between the “Control” group and the “IFN-γ model” group (n = 10 per group).
Summarized data showing the number of cough events in the “Control” group (n = 8) and the “IFN-γ group” (n = 7) at the time of “Baseline” and “After treatment”. The number of cough events were recorded within 10 minutes of exposing guinea pigs to aerosolised citric acid (0.3 M). “Baseline” means the day before the first intratracheal instillation; “After treatment” means two days later after the last intratracheal instillation.