Abstract
Purpose
Studies have shown that Mycoplasma pneumoniae (Mp) infection can aggravate symptoms in asthmatics. However, the mechanism by which Mp infection exacerbates asthma remains unclear. Metabolomics can help identify the mechanism of Mp aggravating asthma in children, thereby providing more a potential target for improving clinical treatment programs. In this article, we analyzed the metabolic level of patients to explain how Mp aggravates asthma in children.
Methods
We divided the subjects into the asthma, Mp infection, asthma combined with Mp infection and healthy groups. Patients’ peripheral blood was collected for metabolic and interaction analysis. Cytokine levels were measured via serum and exhaled breath condensate (EBC).
Results
A total of 150 participating subjects were divided into four groups after exclusion. We found out that there were different metabolic pathways between the healthy and disease groups. The major pathways of both asthma and asthma combined with Mp infection were valine, leucine and isoleucine biosynthesis; malate-aspartate shuttle was the main differential pathway for Mp infection. Moreover, even though three disease groups involved 81 metabolites at the same time, compared with asthma combined with Mp infection, 2 single disease groups still involved different amino acid pathways (phenylalanine, tyrosine and tryptophan biosynthesis; valine, leucine and isoleucine biosynthesis). Interaction analysis showed that Mp infection in asthmatic patients not only activated cytokines, but also activated Toll-like receptors (TLRs) 2 and 6. Finally, the levels of interleukin (IL)-4, IL-8, IL-13 and tumor necrosis factor-α in EBC with asthma combined with Mp infection were significantly higher than the 2 single disease groups.
Conclusions
Mp infection in asthmatic children can cause changes in the levels of various amino acids in the body, which were enriched in the pathways such as valine, leucine and isoleucine biosynthesis. Palmitic acid can activate TLR2, and iloprost reduces IL-10 levels, ultimately leading to the increased airway inflammation.
Keywords: Asthma, Mycoplasma pneumoniae, metabolomics, cytokines, Toll-like receptors
INTRODUCTION
Mycoplasma pneumoniae (Mp) is one of the common pathogens of respiratory tract infections in children. Seasonal infection is a characteristic of Mp, which is more common in winter and spring.1 Mp infection is mostly manifested as pneumonia, also known as primary atypical pneumonia. Moreover, Mp is also involved in the production of many extrapulmonary diseases.2
Asthma is defined as a chronic airway inflammatory disease characterized by airway hyperresponsiveness, which was manifested by cough, chest tightness and dyspnea.3 More and more research has shown that Mp infection may cause asthma aggravation and prolonged course of the disease. In a clinical study, 20% of children hospitalized with severe asthma were acutely infected with Mp.4 Furthermore, nearly 50% of children with frequent asthma attacks were related to Mp infection.5 The above studies all indicated that Mp may be the cause of repeated asthma attacks. Although it has been found that Mp infection can cause the corresponding performance of the organism, there is still a lack of consensus on the mechanism of Mp. Most of the asthmatic patients need to be hospitalized after being infected with Mp. Thus, it is important for the treatment of children to clarify the mechanism of its aggravation.
As a facultative anaerobic bacterium, Mp has its special growth conditions and metabolic pathways, but its effects on the organisms under different conditions were poorly understood. Exploring the role of Mp in children with asthma from the perspective of metabolism can help us identify how Mp aggravates the occurrence of asthma and provide targeted treatment in a timely manner; discover the pathways through which Mp infection affects the organism as a potential target for subsequent treatment. This article analyzed the changes of serum metabolism in children with Mp infection for the first time and combined the levels of cytokines in serum and exhaled breath condensate (EBC) to illustrate the mechanism of Mp in children with asthma.
MATERIALS AND METHODS
Participants
This study consecutively recruited 168 subjects (asthma: 50, Mp infection: 40, asthma combined with Mp infection: 33, and healthy: 45) in the First Affiliated Hospital of Guangzhou Medical University from June 2020 to January 2021. According to the inclusion and exclusion criteria, subjects were finally divided into the following four groups. First, the asthma group: subjects in this group were diagnosed with asthma according to the Global Initiative for Asthma 2020 asthma diagnostic recommendation criteria.6 Via regular follow-ups in the outpatient department of the First Affiliated Hospital of Guangzhou Medical University, their asthma symptoms were well controlled and Mp infection was confirmed negative by Mp DNA detection. Second, the Mp infection group: the children recruited in this group were all enrolled on the first day of hospitalization, and those who had asthma history were excluded. According to the guidelines, Mp infection were defined as that the detection of Mp DNA via throat swabs was greater than 103 copies/L or the serum mixture titer of IgM and IgG was greater than 1:160 by gelatin particle agglutination reaction.7 Third, the asthma combined with Mp infection group: in this group of children, the original asthma symptoms were well controlled at regular outpatient clinics in the First Affiliated Hospital of Guangzhou Medical University, and persisted for at least 1 month. After acute exacerbation of symptoms, they were confirmed positive for Mp infection by nucleic acid or serum immunology detections and hospitalized for treatment. They had not only respiratory symptoms specific to asthma, but also positive Mp infection. Finally, the healthy group: the participants in this group were from primary school physical examinations and had no allergies, infections, or related family history during the study period. In our research, we excluded subjects who had other airway diseases such as bronchiectasis, sinusitis, etc., or combined with other bacterial or viral infections. This research has been approved by the Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (reference number: GYFYY-2016-73) and the informed consent of all patients has been obtained.
Clinical information
Information including participants’ basic information (name, age, sex, children asthma control test [CACT] and history of allergy or infection) and laboratory detect results (blood routine, C-reactive protein [CRP], erythrocyte sedimentation rate [ESR], total immunoglobulin E [IgE] and Mp pathogenic test) were collected. The CACT score in the asthma combined with Mp infection group was collected during asthma exacerbation triggered by Mp infection.
Serum and EBC acquisition
For serum collection, we took 3–5 mL of peripheral blood, which would be centrifuged at 3,000 rpm at room temperature for 5 minutes, and then collected the serum and stored it at −80°C. Serum separation was performed by Agilent 1290 UHPLC system (Agilent Technologies, Santa Clara, CA, USA).
Under the comfortable conditions, subjects breathed steadily for 10–15 minutes in the Turbo-14 EBC collection system (Medivac, Parma, Italy) to collect EBC. After allocating 1 mL into each tube, EBC was stored at −80°C and repetitive freezing-defreezing cycles were avoided. All subjects were asked to stop vigorous activities and avoid drinking caffeine or carbonated beverages one hour before sampling to reduce sample differences.
The specimens of outpatients were collected on the day of outpatients visited; the specimens of inpatients were collected on the second day after admission. The collections of serum and EBC were done at the same time and completed by technicians.
Derivatization
The supernatant was thawed at room temperature and mixed with shaking. A sample of 50 μL of supernatant was added with standard HETE-d8, and then extracted three times with 200 μL of pre-cooled methanol, which later was blown with nitrogen for about 30 minutes to see light yellow crystals or thin films. A mixture of hydroxybenzotriazole (5 μL), cholamine (5 μL) and 1-[Bis(dimethylamino)methylene]-1H-1,2,3-triazolo[4,5-b]pyridinium 3-oxid hexafluorophosphate in dimethyl sulfoxide (5 μL) were incubated at room temperature for 1 minute. Finally, acetonitrile was added to 50 μL.
Chromatographic and mass spectrometry conditions
A Waters HSS T3 column (2.1 × 100 mm, 1.8 μm) was used for serum metabolic profile separation. Chromatographic separation was performed using the mobile phases consisting of water (A) and acetonitrile (B) which contained 0.1% formic acid. The column temperature and the temperature of the autosampler were set at 30°C and 4°C. The flow rate was set to 0.3 mL/min.
Mass spectrometric analysis was performed on an Agilent 6545 UHD accurate mass Q-TOF/MS system (Agilent Technologies Singapore, Singapore) equipped with a dual jet electrospray ion source. The instrument was operated in positive full scan mode with m/z of 200–1,000 to measure all peaks. The parameters of the jet technology included a superheated nitrogen sheath gas temperature of 300°C and a flow rate of 11 L/min. The MS parameters were set as follows: drying gas flow 11 L/min, gas temperature 250°C, nebulizer pressure 22 psi, capillary voltage 3,500 V, nozzle voltage 500 V, and fragment voltage 175 V. A low-flow TOF reference mixture was atomized for continuous calibration in cation mode: 922.0098 (C18H18F24N3O6P3).
Cytokines
Meso Scale Discovery was used to separate and detect the levels of cytokines from all patients’ peripheral blood serum and EBC which was correlated with inflammation of the respiratory tract. The detection indicators included interferon (IFN)-γ, interleukin (IL)-4, IL-6, IL-8, IL-13 and tumor necrosis factor (TNF)-α.
Statistical analysis
The metabolic data was analyzed adopting Qualitative Analysis B.05.00 (Agilent Technologies) and used for chromatographic peak extraction, comparison and data normalization processing. MetaboAnalys 5.0 (https://www.metaboanalyst.ca) were applied to metabolite enrichment analysis and metabolic pathway analysis. The heat map and Venn map were produced using TBtools software (version 1.082) and E Venn website (http://www.ehbio.com/test/venn/), respectively.8 SIMCA-P 14.1(MKS Umetrics AB, Umeå, Sweden) was used for unsupervised Principal Component Analysis (PCA) score plot and presented by R 3.4.1 with R-Studio (RStudio, Boston, MA, USA). STRING website (https://string-db.org/) was used to complete the interaction mapping between main different metabolites and cells or cytokines, using Cytoscape software (version 3.8.2) for figure modification.
All the experimental results were analyzed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). For subject characteristics, categorical variables are described as counts. Normally distributed numerical variables and skewed numerical variables are described as mean ± standard deviation and median (P25, P75), respectively. Significance was evaluated using independent samples t-test or one-way analysis of variance for normally distributed numerical variables, the Kruskal-Wallis nonparametric test for skewed numerical variables, and the χ2 test for categorical variables. The Mann-Whitney U test and the Kruskal-Wallis test were used to analyze the differences in various test indicators of metabolites and cytokines. P values < 0.05 were considered statistically significant. Differential metabolites were defined as simultaneously satisfying P values < 0.05, Fold Change > 1.5 or < 0.67 and variable importance in projection (VIP) > 1.
RESULTS
Subject characteristics
A total of 150 participating children were divided into four groups after exclusion. In Table, except for CRP and ESR which were skewed, all the other indicators were normally distributed. For total IgE and CACT score, we found that the asthma combined with Mp infection group was significantly higher than the asthma group (both P < 0.001). Moreover, not only was the neutrophil level of the asthma combined with Mp infection group the highest among the 4 groups, but also the eosinophil level was second only to the asthma group (all P > 0.05). For CRP, the asthma combined with Mp infection group was slightly higher than the Mp infection group, but ESR showed that the latter was higher than the former (both P > 0.05).
Table. The characteristics of the participants.
| Parameter | Healthy (n = 40) | Asthma (n = 40) | Mp infection (n= 40) | Asthma combined with Mp infection (n = 30) | P value |
|---|---|---|---|---|---|
| Male (%) | 20 (50.0) | 16 (40.0) | 24 (60.0) | 12 (40.0) | 0.257 |
| Age (year) | 7 ± 1 | 7 ± 1 | 8 ± 1 | 7 ± 1 | 0.612 |
| Total IgE (kU/L) | NA | 394.70 ± 179.00 | NA | 3,165.12 ± 2,042.99 | < 0.001 |
| CACT score | NA | 23.67 ± 7.35 | NA | 43.92 ± 5.23 | < 0.001 |
| WBC (109/L) | 7.51 ± 1.31 | 7.07 ± 0.45 | 9.26 ± 2.83 | 10.03 ± 3.54 | 0.414 |
| Neutrophil (109/L) | 3.34 ± 1.11 | 3.27 ± 0.42 | 4.59 ± 2.22 | 7.05 ± 3.34 | 0.132 |
| Eosinophil (109/L) | 0.21 ± 0.20 | 0.40 ± 0.20 | 0.18 ± 0.18 | 0.33 ± 0.30 | 0.635 |
| CRP (mg/L) | NA | NA | 13.61 (4.35–44.18) | 18.57 (3.73–37.65) | 0.774 |
| ESR (mm/H) | NA | NA | 59.00 (22.50–66.75) | 28.00 (27.25–57.25) | 0.054 |
Values are presented as number (%), mean ± standard deviation, median (25th percentile to 75th percentile). Significance was evaluated using independent samples t-test or one-way analysis of variance for normally distributed numerical variables, Kruskal-Wallis nonparametric test skewed numerical variables, a χ2 test for categorical variables.
Mp, Mycoplasma pneumoniae; IgE, immunoglobulin E; CACT, childhood asthma control test; CRP, C-reactive protein; WBC, white blood cell; ESR, erythrocyte sedimentation rate.
Metabolism differences between the healthy and disease groups
We detected the serum metabolite levels of all participants and compared the levels of 84 common metabolites between the 3 disease groups and the healthy group. Related metabolites of lipid metabolism, amino acid metabolism and tricarboxylic acid cycle were included. First, we compared the metabolic levels of the 40 healthy participants to the 110 patients (including 40 asthma children, 40 Mp infection children, and 30 children with asthma combined with Mp infection) in PCA, with each point representing the metabolism levels of each child. In Fig. 1, we found that the 2 groups were clearly divided into groups, which suggested that the dominant metabolites were different between healthy children and children with diseases.
Fig. 1. The PCA scores scatter plots of the healthy and disease groups showed a clear discrimination between the 2 groups (D: diseases; H: healthy).
PCA, Principal Component Analysis.
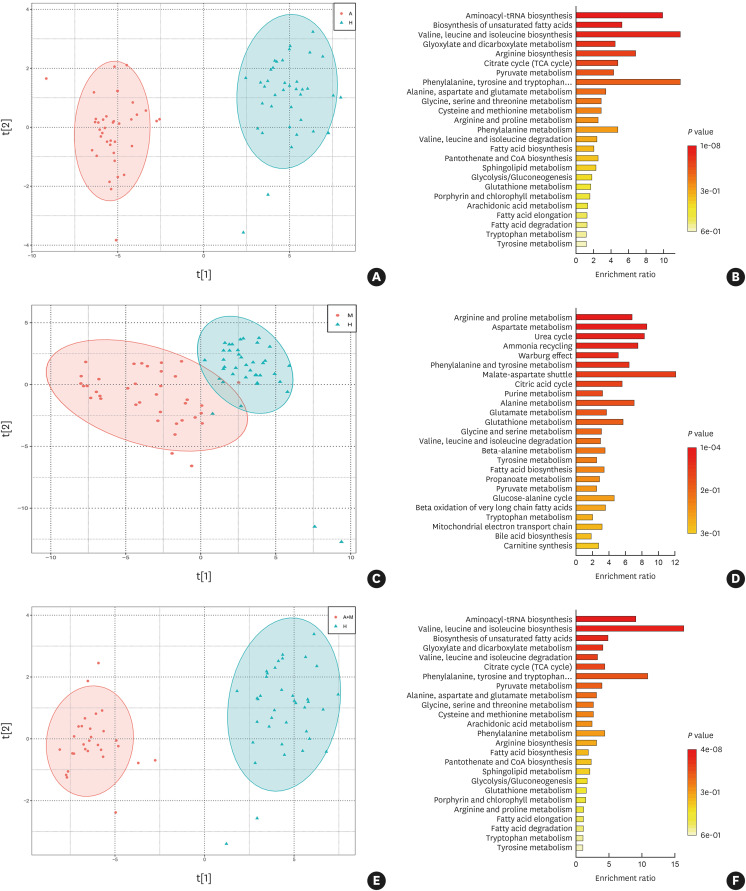
Furthermore, we also further compared the metabolic differences between different disease groups and the healthy group. According to our results, we found that the 3 disease groups had their own different metabolites and involved different metabolic pathways. The major pathways of both asthma and asthma combined with Mp infection were valine, leucine and isoleucine biosynthesis; malate-aspartate shuttle was the main differential pathway for Mp infection. The metabolic distribution and pathways of the three groups compared with the healthy group are presented in Fig. 2.
Fig. 2. The analysis of PCA showed that compared with the healthy group, the 3 disease groups all had some differences and involved different metabolic pathways. (A, B) Asthma compared with healthy. (C, D) Mp infection compared with healthy. (E, F) Asthma combined with Mp infection compared with healthy (A: asthma; M: Mp infection; A+M: asthma combined with Mp infection; H: healthy).
PCA, Principal Component Analysis; Mp, Mycoplasma pneumoniae.
Metabolism differences between the Mp infection and asthma combined with Mp infection groups
In order to find the metabolic association between the three disease groups, we compared the metabolites involved in the metabolic changes of the three groups of patients. It could be seen from the Wayne diagram that there were 81 metabolites included in the 3 groups at the same time (Fig. 3). Moreover, we compared both Mp infection and asthma combined with Mp infection groups to illustrate whether there were metabolites changes between the 2 groups. In this comparison, there were as many as 37 different metabolites between the 2 groups. Furthermore, the levels of the fatty acids (20 species including arachidonate, pentadecanoic acid, bovinic acid, palmitic acid, oleic acid and so on) were generally higher in the Mp infection group than in the asthma combined with Mp infection group (Fig. 4A). After enriching and analyzing these different metabolites, we found that there were 25 metabolic pathways, such as phenylalanine, tyrosine and tryptophan biosynthesis, D-glutamine and D-glutamate metabolism and Aminoacyl-tRNA biosynthesis, to produce changes. The specific metabolic pathways involved are shown in Fig. 4B.
Fig. 3. Venn diagram represents the interactions between metabolites among the 3 groups of asthma, Mp infection, and asthma combined with Mp infection (A: asthma; M: Mp infection; A+M: asthma combined with Mp infection).
Mp, Mycoplasma pneumoniae.
Fig. 4. Different metabolite levels (A) and metabolic pathways (B) between the Mp infection and asthma combined with Mp infection groups. The enrichment ratio was calculated as the number of hits within a particular metabolic pathway divided by the expected number of hits, which illustrated the proportion of different metabolites in each metabolic pathway. P values represent the significance of differential metabolites in the pathway. (M: Mp infection; A+M: asthma combined with Mp infection).
Mp, Mycoplasma pneumoniae.
Metabolism differences between the asthma and asthma combined with Mp infection groups
To assess the differences between the metabolic levels of asthma combined with Mp infection and the single disease, we also compared the metabolic differences between the asthma group and the asthma combined with Mp infection group. Comparing the levels of metabolites between the 2 groups, we found that there were 13 metabolites—12(S)- hydroxyeicosatetraenoic acid (HETE), palmitic acid, stearic acid, 12-hydroxy-8,10-octadeadienoic acid or isomer, 15-methyl palmitic acid, hexanoic acid isomer, behenic acid, tetracosanoic acid, isobutyric acid, l-leucine, l-methionine, l-serine, and l-tryptophan— identified as differential metabolites and all showed high levels in the asthma group (Fig. 5A). Among the enriched 12 metabolic pathways, the valine, leucine and isoleucine biosynthesis were the main influencing pathway (Fig. 5B).
Fig. 5. Different metabolite levels (A) and metabolic pathways (B) between the asthma and asthma combined with Mp infection groups. The enrichment ratio was calculated as the number of hits within a particular metabolic pathway divided by the expected number of hits, which illustrated the proportion of different metabolites in each metabolic pathway. P values represent the significance of differential metabolites in the pathway (A: asthma; A+M: asthma combined with Mp infection).
Mp, Mycoplasma pneumoniae.
To identify whether total IgE affected the fluctuation of metabolic levels in asthmatic children, we subdivided asthma combined with Mp infection groups by total IgE levels (< or ≥ 300 kU/L) and compared the differences in the original 13 differential metabolite levels. Only Behenic acid differed among these 13 metabolites (Supplementary Table S1). Furthermore, in the subgroup comparison of the asthma and asthma combined with Mp infection groups with similar IgE levels, only l-methionine of these 13 metabolites differed at low levels, but there were 7 overlap metabolites differed at high levels (12(S)-HETE, palmitic acid, stearic acid, 12-hydroxy-8,10-octadeadienoic acid or isomer, 15-methyl palmitic acid, behenic acid and l-methionine) (Supplementary Tables S2 and S3).
Interactions between metabolites and cells or cytokines, cytokine levels in serum and EBC
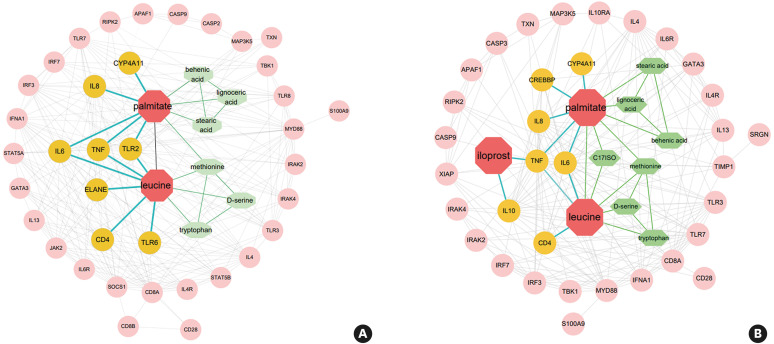
We analyzed the interaction between the selected metabolites from serum and inflammation-related cells or cytokines. Compared with the asthma combined with Mp infection group, palmitate and leucine screened out in the asthma and Mp infection groups were both related to the secretion of IL-4, IL-6, IL-8, IL-13 and TNF. However, there were some differences presenting as Toll-like receptors (TLRs) 2 and 6 were activated in the asthma group, whereas activation of IL-10 was found in the Mp infection group. This explains why the 2 diseases had their own characteristics. In Fig. 6, inflammation-related cells and cytokines were arranged from inside out according to their association with metabolites (differential metabolites: red; directly associated inflammatory factors and metabolites: yellow and green; indirectly related inflammatory factors: pink). Based on the results of the interactions, we measured the levels of cytokines in serum and EBC to verify the results of the interaction analysis. The number of serum and EBC samples in the asthma group was 11 cases each; the Mp infection group was 13 and 10, respectively; the asthma combined with Mp infection group was 9 and 10, respectively. Serum results showed that IL-13 was markedly expressed in the asthma group, and IL-6 and IFN-γ from Mp infection group showed high secretion (P < 0.05) (Fig. 7A). Interestingly, the levels of IL-4, IL-8, IL-13 and TNF-α in EBC from asthma combined with Mp infection were all significantly higher than the single disease, although they had no significantly differences in serum (P < 0.05) (Fig. 7B).
Fig. 6. Factors related to inflammation mediated by differential metabolites. (A) Asthma compared with asthma combined with Mp infection. (B) Mp infection compared with asthma combined with Mp infection. The colors were used to distinguish the differential metabolites (red) we screened, the directly associated inflammatory factors (yellow) and metabolites (green), and the pink one showed the indirectly related inflammatory factors.
Mp, Mycoplasma pneumoniae.
Fig. 7. The levels of cytokines (IFN-γ, IL-4, IL-6, IL-8, IL-13 and TNF-α) in serum and EBC. (A) The levels of cytokine in peripheral blood serum. (B) The levels of cytokine in EBC. All data are mean ± standard deviation of 9–13 samples from each group (A: asthma; M: Mp infection; A+M: asthma combined with Mp infection).
IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; EBC, exhaled breath condensate; Mp, Mycoplasma pneumoniae.
*P < 0.05.
DISCUSSION
Mp infection is particularly common in children and some preliminary research indicated that Mp infection may aggravate pre-existing asthma. However, the mechanism and pathway of action of Mp infection aggravating asthma is poorly understood, which increases the difficulty in treatment. Via metabolomics, we can understand the mechanism of Mp infection from another aspect. In our experiment, we found that all the 3 disease groups not only had significant differences in metabolic levels from the healthy group, but also had their own different types and metabolic pathways. The main changes belonged to amino acid metabolism, namely, phenylalanine, tyrosine and tryptophan biosynthesis as well as malate-aspartate shuttle and valine, leucine and isoleucine biosynthesis. It showed that the metabolic changes in amino acids in the body were closely related to the development of inflammation. There was a study pointing out that arginine was the substrates utilized as an energy source for mycoplasmas.9 Moreover, the discovery of these metabolic pathways would provide a new target for subsequent therapeutic research.
Furthermore, through the Venn diagram, most of the detected metabolites were concentrated in the middle area. However, when we further subdivided and compared the differences between the 2 groups, our results showed that when compared with the asthma combined with Mp infection group, there were differences between the asthma and the Mp infection groups in terms of quantity or metabolic pathways. In summary, it could be explained that although most of the metabolites were presented in the 3 groups, different diseases had their own main influences and changes in metabolic pathways and methods. Based on the identification of different metabolites, we wondered how metabolites were involved in inflammatory manifestations in asthmatics. After enriching the differential metabolites, it was found that they were mainly concentrated in the pathways such as valine, leucine and isoleucine biosynthesis. In addition, the levels of palmitate and leucine were significantly higher in the asthma group than in the asthma combined with Mp infection group, and directly affected TLRs, IL-6, IL-8 and other inflammatory factors. The activation of TLR2 was essential for the progression of Mp infection, which was reflected in the significant increase in TLR2 expression after Mp infection in asthma patients.10,11,12,13 Study has shown that both of palmitate and Mp lipoproteins (MPN611 and MPN162) can activate intracellular signaling pathways (NF- κB) through the TLR1 and TLR2 pathways to initiate inflammation.14,15,16 Therefore, it is inferred that after Mp infection in asthmatic patients, the changes in palmitate levels in vivo could prompt TLR2 to activate downstream signaling pathways and promote the aggravation of clinical symptoms.
A previous study has pointed out that IgE plays essential roles in the pathogenesis of Mp infection, especially in asthmatic patients.17 However, there is still no consensus on the relationship between total IgE and metabolites in children with asthma. Recent studies found that total IgE in asthmatic children was correlated with metabolites, such as isobutyric acid, but another study has suggested that there was no obvious association between total IgE and metabolites.18,19 To confirm that the changes in metabolites were indeed affected by Mp infection or total IgE, we divided the 2 groups of asthmatic children according to total IgE and analyzed the changes in the original 13 metabolites. After the comparison of the total IgE subgroups in the asthma combined with Mp infection group, there was a differential metabolite (behenic acid), representing that changes in total IgE may have caused changes in metabolite levels. Next, after comparing similar subgroups of total IgE between the asthma and asthma combined with Mp infection groups, metabolites that overlapped with the original 13 differential metabolites were found in both the low and high total IgE groups. In the high total IgE group, behenic acid is one of the overlap differential metabolites. It is speculated that the changes in metabolites in this study are more likely related to Mp infection. However, due to the difference in total IgE levels between the 2 groups, only 5 patients in the low IgE group with asthma complicated by Mp infection. In contrast, the high IgE group had only 9 patients in the asthma group, so there may be some bias in the results of this subgroup comparison.
Mp infection is usually a self-limiting disease that is resolved without specific treatment. However, under the premise of underlying disease, Mp infection significantly increases the risk of aggravating the underlying disease. Multiple studies demonstrated that cytokines, such as IL-6, IL-8 and TNF-α, were involved in the immunopathogenesis of Mp infection.20,21,22,23 From our results, differential metabolites enriched in pathways, such as D-Glutamine and D-glutamate metabolism, between the Mp infection and asthma combined with Mp infection groups were associated with IL-4, IL-6, IL-8 IL-10, IL-13 and TNF-α, which was consistent with the existing studies. Interestingly, unlike the asthma group, iloprost in the Mp infection group mediated the regulation of IL-10. Studies have found that iloprost inhabited the production of IL-10 in a dose-dependent manner as demonstrated by the significantly lower levels of IL-10 in children with asthma after Mp infection.24,25,26 In summary, we believed that Mp infection in asthmatic children can reduce the level of IL-10 in vivo by altering iloprost, thereby exacerbating asthma symptoms.
Since interaction analysis could only express the relationships between metabolites and inflammatory factors, the specific level could not be determined. Therefore, we performed further cytokine validation using IFN-γ, IL-4, IL-6, IL-8, IL-13 and TNF-α. Serum results showed that IL-13 was markably expressed in the asthma group, and the Mp infection group showed high secretion of IL-6 and IFN-γ. The high expression of IL-13 in asthmatic patients was related to eosinophilic inflammation and airway hyperresponsiveness, while IL-6 and IFN-γ prompted the severity of Mp infection.27,28,29 Furthermore, we found that while the 3 metabolites in the Mp infection group were associated with TNF-α in the interaction analysis, TNF-α in serum was not significantly different. Several studies have used EBC to assess inflammation in the upper respiratory tract of patients and have found elevated levels of various cytokines.30,31,32,33 Therefore, we further analyzed cytokine levels in EBC to describe inflammatory manifestations. In our results, the levels of asthma combined with Mp infection were higher in EBC than in a single disease although serum IL-4, IL-8 and TNF-α levels were not significantly different between the 3 groups. There was study finding that Mycoplasma pneumoniae lysate directly trigged airway epithelial cells to secrete IL-8.34 The adhesion of Mp to airway epithelial cells affected the balance of Th1/Th2 cells, ultimately leading to the high expression of IL-4.35 These results suggested that EBC were more sensitive to airway inflammation than serum. Furthermore, although there were no differences in serum IL-13 between the Mp infection and asthma combined with Mp infection groups, the latter had significantly higher levels in EBC than the former. This was because IL-13 increased Mp loads in well differentiated human airway epithelial cells under the air-liquid interface cultures, resulting in persistent Mp infection.36 Mp infection was a common respiratory disease in children, and serum cytokines could reflect the overall inflammatory state of the body. When children suffered from asthma and Mp infection at the same time, not only the upper and lower airway infections of patient was aggravated, but also non-respiratory symptoms can be developed. Therefore, by comprehensive serum and EBC cytokine validation, we believed that serum metabolites could directly reflect inflammatory changes in Mp infection.
However, there are some limitations in our study. First, since the Mp infected participants we recruited were all enrolled on the first day of admission, it is not sure whether the treatments they received before the admission would affect our results. Second, due to the fact that Mp infection has the characteristic of being able to infect repeatedly, we cannot determine whether the children we recruited have a history of Mp infection. Whether the first infection or re-infection will have different metabolic or inflammatory manifestations still needs further evaluation. Third, total IgE levels in asthmatics may be related to metabolite levels. Although we attempted subgroup metabolite profiling at different total IgE levels, small sample numbers in some subgroups may lead to inaccurate results. The association between total IgE and serum metabolism requires more systematic studies.
In conclusion, Mp infection in asthmatic children can cause changes in the levels of various amino acids in the body, which were enriched in the pathways such as valine, leucine and isoleucine biosynthesis. Palmitic acid can activate TLR2, and iloprost reduces IL-10 levels, ultimately leading to increased airway inflammation. However, the mechanism of action of Mp still needs more in-depth research to clarify.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (Project No.: 81871736), Guangzhou Science and Technology Foundation (Project No.: 201804020043) and The Open Project Funding Project of State Key Laboratory of Respiratory Disease of China (SKLRD-OP-202108). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding was received for this study.
Footnotes
Disclosure: There are no financial or other issues that might lead to conflict of interest.
SUPPLEMENTARY MATERIALS
Metabolite analysis of asthma combined with Mp infection with total IgE levels of < 300 and ≥ 300 kU/L
Metabolite results in asthma and asthma combined with Mp infection with total IgE < 300 kU/L
Metabolite results in asthma and asthma combined with Mp infection with total IgE ≥ 300 kU/L
References
- 1.Xing Y, Wang D, Sheng K, Xiao X, Wei H, Liu L, et al. Dynamic change of Mycoplasma pneumoniae pneumonia in hospitalized children in a general hospital: a 3-year retrospective analysis. Transl Pediatr. 2020;9:522–531. doi: 10.21037/tp-20-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cherry JD, Welliver RC. Mycoplasma pneumoniae infections of adults and children. West J Med. 1976;125:47–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Postma DS, Rabe KF. The asthma-COPD overlap syndrome. N Engl J Med. 2015;373:1241–1249. doi: 10.1056/NEJMra1411863. [DOI] [PubMed] [Google Scholar]
- 4.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, Heilbronner C, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 5.Kumar S, Roy RD, Sethi GR, Saigal SR. Mycoplasma pneumoniae infection and asthma in children. Trop Doct. 2019;49:117–119. doi: 10.1177/0049475518816591. [DOI] [PubMed] [Google Scholar]
- 6.Global Initiative for Asthma (GINA) Global strategy for asthma management and prevention [Internet] place unknown: GINA; 2020. [cited 2022 Jun 5]. Available from: https://ginasthma.org/ [Google Scholar]
- 7.Centers for Disease Control and Prevention. Mycoplasma pneumoniae infections [Internet] Atlanta (GA): Centers for Disease Control and Prevention; 2020. [cited 2022 Jun 5]. Available from: https://www.cdc.gov/pneumonia/atypical/mycoplasma/ [Google Scholar]
- 8.Chen T, Zhang H, Liu Y, Liu YX, Huang L. EVenn: Easy to create repeatable and editable Venn diagrams and Venn networks online. J Genet Genomics. 2021;48:863–866. doi: 10.1016/j.jgg.2021.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Awadh AA, Le Gresley A, Forster-Wilkins G, Kelly AF, Fielder MD. Determination of metabolic activity in planktonic and biofilm cells of Mycoplasma fermentans and Mycoplasma pneumoniae by nuclear magnetic resonance. Sci Rep. 2021;11:5650. doi: 10.1038/s41598-021-84326-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hawn TR, Smith KD, Aderem A, Skerrett SJ. Myeloid differentiation primary response gene (88)- and toll-like receptor 2-deficient mice are susceptible to infection with aerosolized Legionella pneumophila. J Infect Dis. 2006;193:1693–1702. doi: 10.1086/504525. [DOI] [PubMed] [Google Scholar]
- 11.Skerrett SJ, Wilson CB, Liggitt HD, Hajjar AM. Redundant Toll-like receptor signaling in the pulmonary host response to Pseudomonas aeruginosa. Am J Physiol Lung Cell Mol Physiol. 2007;292:L312–L322. doi: 10.1152/ajplung.00250.2006. [DOI] [PubMed] [Google Scholar]
- 12.Fuse ET, Tateda K, Kikuchi Y, Matsumoto T, Gondaira F, Azuma A, et al. Role of Toll-like receptor 2 in recognition of Legionella pneumophila in a murine pneumonia model. J Med Microbiol. 2007;56:305–312. doi: 10.1099/jmm.0.46913-0. [DOI] [PubMed] [Google Scholar]
- 13.Shao L, Cong Z, Li X, Zou H, Cao L, Guo Y. Changes in levels of IL-9, IL-17, IFN-γ, dendritic cell numbers and TLR expression in peripheral blood in asthmatic children with Mycoplasma pneumoniae infection. Int J Clin Exp Pathol. 2015;8:5263–5272. [PMC free article] [PubMed] [Google Scholar]
- 14.Barton GM, Medzhitov R. Toll-like receptor signaling pathways. Science. 2003;300:1524–1525. doi: 10.1126/science.1085536. [DOI] [PubMed] [Google Scholar]
- 15.Shimizu T, Kida Y, Kuwano K. Triacylated lipoproteins derived from Mycoplasma pneumoniae activate nuclear factor-kappaB through toll-like receptors 1 and 2. Immunology. 2007;121:473–483. doi: 10.1111/j.1365-2567.2007.02594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Snodgrass RG, Huang S, Choi IW, Rutledge JC, Hwang DH. Inflammasome-mediated secretion of IL-1β in human monocytes through TLR2 activation; modulation by dietary fatty acids. J Immunol. 2013;191:4337–4347. doi: 10.4049/jimmunol.1300298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang L, Chen Q, Shi C, Lv H, Xu X, Yu L. Changes of serum TNF-α, IL-5 and IgE levels in the patients of mycoplasma pneumonia infection with or without bronchial asthma. Int J Clin Exp Med. 2015;8:3901–3906. [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu CY, Cheng ML, Chiang MH, Wang CJ, Tsai MH, Lin G. Integrated metabolic and microbial analysis reveals host-microbial interactions in IgE-mediated childhood asthma. Sci Rep. 2021;11:23407. doi: 10.1038/s41598-021-02925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiu CY, Cheng ML, Chiang MH, Wang CJ, Tsai MH, Lin G. Metabolomic analysis reveals distinct profiles in the plasma and urine associated with IgE reactions in childhood asthma. J Clin Med. 2020;9:887. doi: 10.3390/jcm9030887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tanaka H, Narita M, Teramoto S, Saikai T, Oashi K, Igarashi T, et al. Role of interleukin-18 and T-helper type 1 cytokines in the development of Mycoplasma pneumoniae pneumonia in adults. Chest. 2002;121:1493–1497. doi: 10.1378/chest.121.5.1493. [DOI] [PubMed] [Google Scholar]
- 21.Lu A, Wang C, Zhang X, Wang L, Qian L. Lactate dehydrogenase as a biomarker for prediction of refractory Mycoplasma pneumoniae pneumonia in children. Respir Care. 2015;60:1469–1475. doi: 10.4187/respcare.03920. [DOI] [PubMed] [Google Scholar]
- 22.Narita M, Tanaka H. Cytokines involved in the severe manifestations of pulmonary diseases caused by Mycoplasma pneumoniae. Pediatr Pulmonol. 2007;42:397. doi: 10.1002/ppul.20445. [DOI] [PubMed] [Google Scholar]
- 23.Hardy RD, Jafri HS, Olsen K, Wordemann M, Hatfield J, Rogers BB, et al. Elevated cytokine and chemokine levels and prolonged pulmonary airflow resistance in a murine Mycoplasma pneumoniae pneumonia model: a microbiologic, histologic, immunologic, and respiratory plethysmographic profile. Infect Immun. 2001;69:3869–3876. doi: 10.1128/IAI.69.6.3869-3876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borish L, Aarons A, Rumbyrt J, Cvietusa P, Negri J, Wenzel S. Interleukin-10 regulation in normal subjects and patients with asthma. J Allergy Clin Immunol. 1996;97:1288–1296. doi: 10.1016/s0091-6749(96)70197-5. [DOI] [PubMed] [Google Scholar]
- 25.Ding S, Wang X, Chen W, Fang Y, Liu B, Liu Y, et al. Decreased interleukin-10 responses in children with severe Mycoplasma pneumoniae pneumonia. PLoS One. 2016;11:e0146397. doi: 10.1371/journal.pone.0146397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou W, Blackwell TS, Goleniewska K, O’Neal JF, Fitzgerald GA, Lucitt M, et al. Prostaglandin I2 analogs inhibit Th1 and Th2 effector cytokine production by CD4 T cells. J Leukoc Biol. 2007;81:809–817. doi: 10.1189/jlb.0606375. [DOI] [PubMed] [Google Scholar]
- 27.Borish LC, Steinke JW. 2. Cytokines and chemokines. J Allergy Clin Immunol. 2003;111:S460–S475. doi: 10.1067/mai.2003.108. [DOI] [PubMed] [Google Scholar]
- 28.Jin HL, Zhan L, Mei SF, Shao ZY. Serum cytokines and FeNO in school-aged children with Mycoplasma pneumoniae pneumonia. Med Sci Monit. 2020;26:e923449. doi: 10.12659/MSM.923449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin X, Zhu Y, Zhang Y, Chen J, Rong L, Zhao X. Assessment of levels of D-dimer and interferon-γ in pediatric patients with Mycoplasma pneumoniae pneumonia and its clinical implication. Exp Ther Med. 2018;16:5025–5030. doi: 10.3892/etm.2018.6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leung TF, Wong GW, Ko FW, Li CY, Yung E, Lam CW, et al. Analysis of growth factors and inflammatory cytokines in exhaled breath condensate from asthmatic children. Int Arch Allergy Immunol. 2005;137:66–72. doi: 10.1159/000085106. [DOI] [PubMed] [Google Scholar]
- 31.Shahid SK, Kharitonov SA, Wilson NM, Bush A, Barnes PJ. Increased interleukin-4 and decreased interferon-gamma in exhaled breath condensate of children with asthma. Am J Respir Crit Care Med. 2002;165:1290–1293. doi: 10.1164/rccm.2108082. [DOI] [PubMed] [Google Scholar]
- 32.Carpagnano GE, Resta O, Gelardi M, Spanevello A, Di Gioia G, Giliberti T, et al. Exhaled inflammatory markers in aspirin-induced asthma syndrome. Am J Rhinol. 2007;21:542–547. doi: 10.2500/ajr.2007.21.3066. [DOI] [PubMed] [Google Scholar]
- 33.Klaassen EM, van de Kant KD, Jöbsis Q, Penders J, van Schooten FJ, Quaak M, et al. Integrative genomic analysis identifies a role for intercellular adhesion molecule 1 in childhood asthma. Pediatr Allergy Immunol. 2014;25:166–172. doi: 10.1111/pai.12187. [DOI] [PubMed] [Google Scholar]
- 34.Lee KE, Kim KW, Hong JY, Kim KE, Sohn MH. Modulation of IL-8 boosted by Mycoplasma pneumoniae lysate in human airway epithelial cells. J Clin Immunol. 2013;33:1117–1125. doi: 10.1007/s10875-013-9909-y. [DOI] [PubMed] [Google Scholar]
- 35.Ye Q, Xu XJ, Shao WX, Pan YX, Chen XJ. Mycoplasma pneumoniae infection in children is a risk factor for developing allergic diseases. Sci World J. 2014;2014:986527. doi: 10.1155/2014/986527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gross CA, Bowler RP, Green RM, Weinberger AR, Schnell C, Chu HW. beta2-agonists promote host defense against bacterial infection in primary human bronchial epithelial cells. BMC Pulm Med. 2010;10:30. doi: 10.1186/1471-2466-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Metabolite analysis of asthma combined with Mp infection with total IgE levels of < 300 and ≥ 300 kU/L
Metabolite results in asthma and asthma combined with Mp infection with total IgE < 300 kU/L
Metabolite results in asthma and asthma combined with Mp infection with total IgE ≥ 300 kU/L